Abstract
The objective of this study was to evaluate the effects of heat treatment on the phenolics and antioxidant activity of rice hull. Heat treatment was performed at temperatures 80∼140°C for 1∼5 h, and the heated rice hull was extracted with 80% (v/v) methanol in an ultrasonic bath. The highest total polyphenol and flavonoid content (10.68 mg gallic acid equivalents/g and 1.83 mg catechin equivalents/g, respectively) occurred in rice hull heated at 130°C for 5 h. During heat treatment, the content of free phenolic acids increased compared with that of the bound phenolic acids. The highest 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity and reducing power was observed in rice hull heated at 140°C for 3 h. The highest OH radical scavenging activity was 75.30% in rice hull heated at 140°C for 5 h. These results suggested that heat treatment was an efficient method to enhance the antioxidant characteristics of rice hull.
Keywords: antioxidant activity, heat treatment, phenolic compound, rice hull
INTRODUCTION
Rough rice (Oryza sativa), one of the most produced and consumed grains in the world, is a rich source of bioactive compounds including many antioxidants, such as phenolic acid, vitamin E, and γ-oryzanol (de Mira et al., 2009). Antioxidants in whole cereal grains are considered major contributors to the health benefits of these foods. Whole grain rice is a good source of antioxidants, most of which are present in the bran and hull fractions of rice (Jariwalla, 2001).
Rice hull has low-cost value as feed stock due to its low digestibility, peculiar size, low bulk density, high ash/silica content, and abrasive characteristics, and hence, most of it gets wasted (Saha et al., 2005). Since rice hull is inedible, it is used in various non-food applications, such as the production of low value waste materials. However, rice hull contains an antioxidant defense system that protects the rice seed from oxidative stress, thus, offering valuable nutritional advantage (Ramarathnam et al., 1988). Most antioxidant phenolic compounds in rice hull have been found to be covalently bound to insoluble polymers (Niwa and Miyachi, 1986). Therefore, it is necessary to find a processing method to effectively release these phenolic compounds from rice hull.
Heat treatment of food is used extensively to destroy microorganisms and stop enzyme reactions. However, heated foods show a decrease in their nutritional values due to the loss of certain heat labile nutrients (Nicoli et al., 1999). Recent studies have reported an increase in the bioactivity of thermally processed foods. It has been reported that soluble phenolic compounds in foods are significantly increased after heat treatment due to the liberation and breakdown of the cellular matrix (Dewanto et al., 2002a; Beta and Hwang, 2018; Kim et al., 2019). Therefore, the objectives of this study were to investigate the changes in the content of the phenolic compounds and antioxidant activity of rice hull, induced by thermal processing at different temperatures and time. These results may aid in improving the antioxidant activity and quality of food for natural products by applying heat treatment.
MATERIALS AND METHODS
Materials
Rough rice (O. sativa L. cv. Ilpumbyeo) with hull was provided by the National Institute of Crop Science, Rural Development Administration, Wanju, Korea. Rice was milled into rice hull using a rice sweeper (Model MC-90A, Wakayama Co., Ltd., Wakayama, Japan). The rice hull was then ground by an ultrafine grinder (micro hammer cutter mill type-3, Culatti AG, Zürich, Switzerland) and passed through an 80-mesh sieve. The ground rice hull was stored in well-labeled polyethylene films (New Pack Korea Co., Ltd., Seoul, Korea) and placed in a deep freezer (Ultralow temperature freezer, MDF-393, Sanyo, Akaiwa, Japan) at −20°C until they were analyzed.
Heat treatment
The ground rice hull was subjected to heat treatment in a temperature-controlled autoclave apparatus (Jisico, Seoul, Korea) at 80°C, 100°C, 120°C, 130°C, and 140°C for 1 h, 3 h, and 5 h. The ground rice hull (10 g) was added to distilled water (100 mL) in an airtight container. The heated samples were freeze-dried (FD5508; Ilshin Bio-Base, Yangju, Korea) and stored in a deep freezer (ultra-low temperature freezer, MDF-393, Sanyo) at −20°C until they were analyzed to measure the various functional components and physiological activities of the rice hull.
Preparation of the rice hull extracts
The powdered samples (2 g) were extracted with 80% (v/v) methanol-water solution (40 mL). The extracts were sonicated thrice at room temperature for 1 h in an ultrasonic bath (frequency 40 Hz, power 300 W, SD-350H; Seong Dong, Seoul, Korea). The extracts were filtered (Whatman no. 4) and combined, and the solvent was evaporated at 40°C using a rotary evaporator (EYELA-1000; Eyela, Tokyo, Japan) under vacuum. The residue was dissolved in distilled water, amassed to 5 mL, and freeze-dried. The samples were dissolved in dimethyl sulfoxide (DMSO) to obtain a final concentration of 100 mg/mL.
Determination of total polyphenol and flavonoid content
Total polyphenol content was measured according to the method described by Dewanto et al. (2002b). The extract (50 μL) was appropriately diluted by adding 2% Na2CO3 (1 mL) and 50% of the Folin-Ciocalteu phenol reagent (Sigma-Aldrich Co., St. Louis, MO, USA) (50 μL), and the solution was mixed well. The absorbance at 750 nm was measured after 30 min, and the phenolic content was calculated from a calibration curve that was obtained using gallic acid as standard. All extracts were analyzed in triplicate measurements. The total flavonoid content was measured according to a slightly modified version of the method described by Choi et al. (2003). An aliquot (125 μL/mL) of extracts or the standard solution of catechin was added to distilled water (500 μL) in a microtube (1.5 mL), and then, 5% NaNO2 (37.5 μL) was added. After 5 min, 10% AlCl3 (75 μL) was added. After 6 min, 1 M NaOH (250 μL) was added, and the solution was mixed. After exactly 11 min, the absorbance at 510 nm was determined using a UV-Vis spectrophotometer (UV-1650PC; Shimadzu, Kyoto, Japan). The total flavonoid content was expressed as mg catechin equivalents (CE)/g dry weight. All extracts were analyzed in triplicate.
Determination of the free and bound individual phenolic compounds
The free phenolic compounds in the heated rice hull were extracted using the method described by Seo et al. (2011) and Jung et al. (2011) with slight modifications. The powdered samples (4 g) were extracted thrice with 80% methanol (40 mL) at room temperature for 1 h using an ultrasonic bath (SD-350H; Seong Dong). The extracts were then filtered, combined, and concentrated using a rotary evaporator under vacuum, and the residue was dissolved in distilled water (5 mL) and extracted thrice with diethyl ether:ethyl acetate solvent (50:50, v/v) (10 mL). The supernatant layer was evaporated using a rotary evaporator under vacuum. The residues were then dissolved in methanol and filtered through a 0.45 mm syringe filter (Millipore, Billerica, MA, USA). The bound phenolic compounds in rice hull were extracted according to the method described by Zielinski et al. (2001). After extraction of the bound phenolic compounds, 4 M NaOH (10 mL) was added directly to the flour residues, and the suspension was sonicated for 90 min at 40°C. After alkaline hydrolysis, the solution was acidified to pH 2.0 with concentrated HCl and centrifuged at 2,200 g for 10 min to remove any cloudy precipitates. The liberated phenolic compounds in the clear solution were extracted thrice with diethyl ether:ethyl acetate solvent (50:50, v/v) (20 mL). The supernatant layer was evaporated using a rotary evaporator under vacuum. The residues were dissolved in methanol and filtered through a 0.45 mm syringe filter (Millipore). The phenolic compounds composition of each extract was determined using a high performance liquid chromatography system. The analytical column was an ODS column (5 μm, 4.6 mm×250 mm, Agilent Technologies, Santa Clara, CA, USA). A gradient elution was employed using solvent A [water containing 0.1% (v/v) acetic acid] and solvent B [acetonitrile containing 0.1% (v/v) acetic acid]. The gradient program was as follows: 0∼2 min, 92∼90% A in B (gradient); 2∼27 min, 90 to 70% A in B (gradient); 27∼50 min, 70 to 10% A in B (gradient); 50∼51 min, 10 to 0% A in B (gradient); 51∼60 min, 0% A in B (isocratic); and 60∼70 min, 0 to 92% A in B (gradient). The flow rate was 1 mL/min, and the injection volume was 20 μL. The UV detector was set at 280 nm.
Measurement of the 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation scavenging activity
The ABTS radical cation scavenging activity was measured according to the method described by Choi et al. (2006). The ABTS radical cation was generated by adding 7 mM ABTS (Sigma-Aldrich Co.) to 2.6 mM potassium persulfate solution and leaving the mixture to stand overnight in the dark at room temperature. The ABTS radical cation solution was diluted with distilled water to obtain an absorbance of 1.4∼1.5 at 735 nm. An aliquot (1 mL) of the diluted ABTS radical cation solution was added to the samples or distilled water (50 μL). The absorbance at 735 nm was determined after 1 h using a spectrophotometer (UV-1650PC, Shimadzu). The ABTS radical cation scavenging activity was expressed as mg ascorbic acid equivalents (AAE)/g.
Measurement of the OH radical scavenging activity
The OH radical, formed by the Fenton reaction, oxidizes 2-deoxyribose to malondialdehyde (Gutteridge, 1984). A solution (0.2 mL) of FeSO4·7H2O (10 mM) and EDTA (10 mM) was prepared in a test tube, and a solution (0.2 mL) of 2-deoxyribose (10 mM), the sample solution, and phosphate buffer (pH 7.4, 0.1 M) were added to obtain a total volume of 1.8 mL. Finally, a solution (200 μL) of H2O2 (10 mM) was added to this reaction mixture, and the resultant solution was incubated at 37°C for 4 h. After incubation, a trichloroacetic acid solution (1 mL) (2.8%) and thiobarbituric acid solution (1 mL) (1.0%) were added to the reaction mixture. The resultant solution was boiled for 10 min and then cooled in ice, and its absorbance at 520 nm was measured.
Measurement of the reducing power
The reducing power of the extracts was determined according to the method described by Mau et al. (2002). The sample (250 mL) was added to 0.2 M sodium phosphate buffer (pH 6.6, 250 μL) and 1% potassium ferricyanide [K3Fe(CN)6] (250 μL). The mixture was shaken vigorously and then kept in the dark for protection from light at 50°C for 20 min. The mixture was then added to 1% trichloroacetic acid (w/v). The absorbance at 700 nm was determined using a UV-Vis spectrophotometer (UV-1650PC; Shimadzu). All extracts were analyzed in triplicate.
Statistical analysis
The results have been reported as mean±standard deviation (SD). The significance of differences among the treatment means was determined using the SPSS version 12 (SPSS Inc., Chicago, IL, USA) and a P-value <0.05 was considered significant.
RESULTS AND DISCUSSION
The total polyphenol and flavonoid content in the heated rice hull
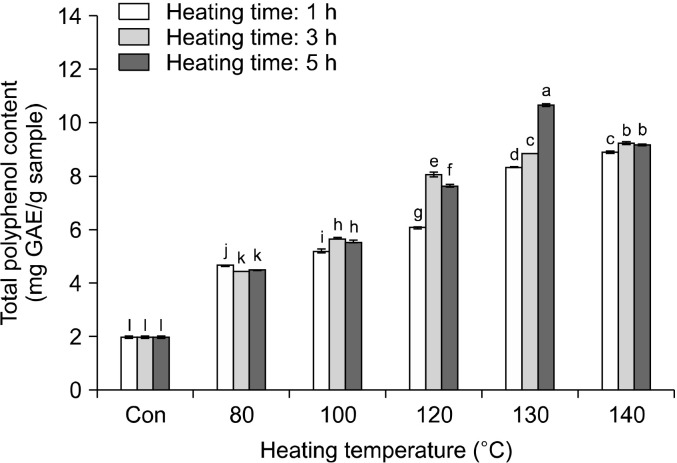
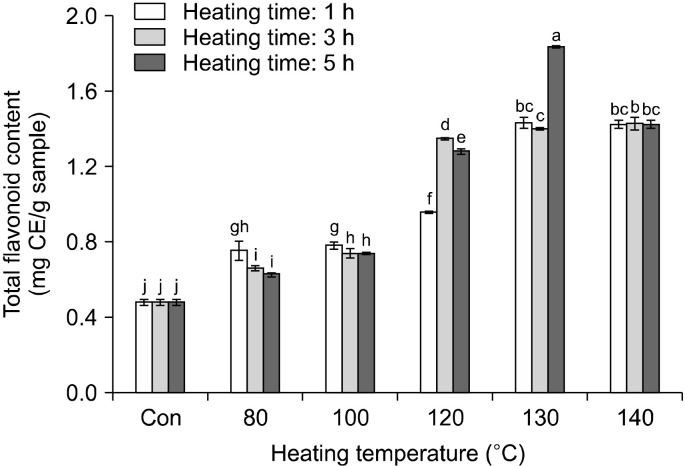
The change of the total polyphenol content in rice hull, caused by different heat treatments, is shown in Fig. 1. Total polyphenol content increased with increasing heating temperature and time. The total polyphenol content in the sample heated for 1 h increased from 1.99 mg GAE /g at room temperature (raw rice hull) to 8.90 mg GAE/g at 140°C. The total polyphenol content in the sample heated for 3 h increased to 9.23 mg GAE/g at 140°C, while that in the sample heated for 5 h increased to 10.68 mg GAE/g at 130°C and then decreased at 140°C. The change in the total flavonoid content in rice hull, caused by different heat treatments, is shown in Fig. 2. The total flavonoid content increased on increasing the heating temperature and time. The total flavonoid content in the sample heated for 1 h increased from 0.41 mg CE/g at room temperature (raw rice hull) to 1.43 mg CE/g at 130°C and then decreased to 1.42 mg CE/g at 140°C. The total flavonoid content in the sample heated for 3 h increased to 1.43 mg CE/g at 140°C, while that in the sample heated for 5 h increased to 1.83 mg CE/g at 130°C and then decreased to 1.42 mg CE/g at 140°C. Thus, the highest content of total flavonoid was 1.83 mg CE/g in rice hull heated at 130°C for 5 h.
Fig. 1.
Changes in total polyphenol content (mg GAE/g) of rice hull by different heating temperatures and times. Different letters (a-l) indicate a significant difference (P<0.05) among different heat treatments by Duncan’s multiple range test. GAE, gallic acid equivalents.
Fig. 2.
Changes in total flavonoid content (mg CE/g) of rice hull by different heating temperatures and times. Different letters (a-j) indicate a significant difference (P<0.05) among different heat treatments by Duncan’s multiple range test. CE, catechin acid equivalents.
These results, on the antioxidant activity of different parts of the germinated rough rice, closely agreed with those reported by Kim et al. (2011), who observed that the total polyphenol content in rice hull was 2.32 mg∼ 2.45 mg GAE/g. Additionally, Wanyo et al. (2014) reported that among different treatments such as hot-air, far-infrared (FIR) radiation, and cellulase, FIR radiation provided the highest values of polyphenol and flavonoid content in the samples. The phenolic content increased because FIR heat could break the covalent bonds of polymerized polyphenols and subsequently transform the high molecular phenolic compounds to low molecular phenolic compounds (Niwa and Miyachi, 1986). In this study, heat treatment damaged the cell structure of the rice hull matrix, resulting in the extraction of phenolic compounds. This effect may be due to the softening or disruption of the plant cell walls and destruction of the complex phenolic compounds during thermal treatment (Huang et al., 2006). Therefore, we postulated that thermal processing may also cause the breakdown of complex compounds in rice hull, resulting in the easier release of some individual phenolic substances.
Individual free and bound phenolic compounds in heated rice hull
Phenolic acids are the most abundant form of phenolic compounds in various food crops (Mattila et al., 2005), and have been hypothesized to combat oxidative stress in humans by maintaining a balance between oxidants and antioxidants (Temple, 2000). Changes in the content of the free and the bound phenolics in rice hull, caused by different heat treatments, have been presented in Table 1 and 2. The phenolic acid standard mixture contained gallic acid, homogentisic acid, protocatechuic acid, gentisic acid, chlorogenic acid, (+)-catechin, vanillic acid, caffeic acid, phloretic acid, p-coumaric acid, ferulic acid, naringin, hesperidin, salicylic acid, quercetin, trans-cinnamic acid, naringenin, hesperetin, and biochanin. The profile of the free form revealed the presence of 17 phenolics except for salicylic acid and quercetin, of the 19 phenolics that comprised the standard (280 nm). In contrast, the profile of the bound form revealed the presence of 15 phenolics except homogentisic acid and protocatechuic acid, which were revealed in the profile of the free form. The free phenolics content generally increased during heat treatment, whereas the bound phenolics content in heated rice hull decreased compared with the raw rice hull. The highest content of free phenolics, like that of the total polyphenol, was 5,698.98 μg/g in rice hull heated at 140°C for 5 h. However, the highest content of the bound phenolics was 5,552.35 μg/g in raw rice hull. The free forms of (+)-catechin, phloretic acid, p-coumaric acid, and ferulic acid, which are the major phenolic compounds in rice hull, increased about ten-fold to twenty-fold compared with the phenolics in the raw rice hull. Therefore, it may be considered that the increase in free phenolics content was due to their extraction from the bound phenolics. Therefore, we can conclude that thermal processing is effective in generating more antioxidant phenolic compounds from rice hull. Soluble phenolic compounds significantly increased after heat treatment due to the liberation and breakdown of the cell matrix (Dewanto et al., 2002a). Temperature has been found to play an important role in the extraction process since high extraction temperatures increased the solubility of phenolic compounds and the diffusion coefficient, which reduced extraction times (Cacace and Mazza, 2003). Ferulic and p-coumaric acids in plants may be linked to the cell wall polysaccharides, lignin, suberin, and cutin, and diferulic acid served as a cross-link between pentosan chains (Herrmann, 1989). Additionally heat treatment of rice hull liberated ferulic acid by breaking the covalent bonds between the carboxyl group of ferulic acid and the proteoglycan (Yamagishi et al., 1984).
Table 1.
Changes in free phenolics content of rice hull by different heating temperatures and times (unit: μg/g)
| Treatment | Phenolics content of free form | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gallic acid | Homogentisic acid | Protocatechuic acid | Gentisic acid | Chlorogenic acid | (+)-Catechin | Vanillic acid | Caffeic acid | Phloretic acid | ||
| Control | 0.38k | 2.99g | 34.04l | 7.75k | 9.98j | 39.72n | 3.94j | 2.84n | 183.48m | |
| 80°C | 1 h | 1.10j | 4.41g | 41.26k | 9.56k | 12.59i | 50.64m | 5.57i | 4.47m | 255.88l |
| 3 h | 2.45i | 5.02g | 46.55j | 17.63j | 16.32h | 66.68l | 5.87i | 5.70l | 384.27k | |
| 5 h | 3.26h | 5.15g | 51.59i | 21.51ij | 19.93g | 82.20k | 6.78h | 8.15k | 425.04k | |
| 100°C | 1 h | 5.10gh | 6.02g | 59.19i | 19.18ij | 21.88g | 104.25j | 6.82h | 8.06k | 519.38j |
| 3 h | 8.09gh | 5.71g | 65.15h | 31.98fg | 25.77f | 122.62i | 8.46g | 11.65j | 586.28i | |
| 5 h | 6.73e | 6.03g | 67.77gh | 47.62e | 28.51e | 134.70h | 11.60e | 14.63i | 822.77h | |
| 120°C | 1 h | 3.74g | 8.28g | 67.36fg | 27.84gh | 33.08d | 166.33g | 7.45h | 16.33h | 1,184.18g |
| 3 h | 3.73f | 34.03f | 63.94f | 28.49gh | 41.33c | 222.93f | 10.20f | 19.37g | 1,419.42f | |
| 5 h | 4.32f | 43.15f | 62.36f | 24.92hi | 26.93ef | 229.59f | 10.38f | 23.62f | 1,581.75e | |
| 130°C | 1 h | 3.74e | 48.36f | 94.16e | 35.60f | 27.73ef | 246.82e | 10.04f | 25.07e | 1,762.93c |
| 3 h | 5.71d | 82.92e | 103.76d | 62.20d | 33.67d | 243.76e | 12.36de | 29.74d | 1,885.47b | |
| 5 h | 15.46c | 314.06d | 130.65c | 131.31b | 60.45b | 316.86c | 12.46d | 47.73b | 2,073.81a | |
| 140°C | 1 h | 11.75b | 122.00c | 115.98b | 97.47c | 40.24c | 296.72d | 14.00c | 43.54c | 1,847.59b |
| 3 h | 15.43b | 226.02b | 129.90b | 101.30c | 41.92c | 326.85b | 15.53b | 47.04b | 1,664.55d | |
| 5 h | 22.10a | 247.73a | 258.88a | 198.40a | 136.24a | 431.56a | 28.21a | 83.30a | 1,394.47f | |
| Treatment | Phenolics content of free form | |||||||||
| p-Coumaric acid | Ferulic acid | Naringin | Hesperidin | Cinnamic acid | Naringenin | Hesperitin | Biochanin | Total | ||
| Control | 57.73k | 8.52l | 32.10l | 14.89l | 4.17l | 51.89h | 63.28k | 16.66ij | 534.36n | |
| 80°C | 1 h | 102.37j | 22.28k | 39.80jk | 18.25l | 4.68l | 57.83g | 69.81j | 16.50ij | 716.99m |
| 3 h | 193.71i | 29.97j | 54.26i | 23.02k | 7.65k | 82.63bc | 94.55ef | 19.47h | 1,055.73l | |
| 5 h | 206.52i | 34.56i | 38.20k | 25.14jk | 7.85k | 81.43cd | 86.97h | 17.54i | 1,121.82l | |
| 100°C | 1 h | 212.57i | 46.06h | 39.51jk | 25.98jk | 8.51j | 81.48cd | 91.51g | 11.43l | 1,266.92k |
| 3 h | 264.20h | 68.44f | 44.20j | 28.18ij | 9.43i | 85.95b | 110.36b | 12.89k | 1,489.37j | |
| 5 h | 343.47g | 58.46g | 44.15j | 30.02i | 11.18h | 85.23bc | 91.25g | 12.85k | 1,816.95i | |
| 120°C | 1 h | 549.45f | 59.53g | 99.19h | 38.06h | 12.41g | 68.55ef | 96.76e | 15.29j | 2,453.83h |
| 3 h | 730.81e | 89.45e | 126.82g | 43.01g | 15.31d | 71.52e | 107.25c | 38.72f | 3,066.32g | |
| 5 h | 758.84d | 70.06f | 135.30f | 48.25f | 14.21e | 67.17f | 92.51fg | 36.01g | 3,229.37f | |
| 130°C | 1 h | 879.59c | 94.81d | 133.05f | 58.69e | 16.76c | 90.17a | 114.44a | 38.27f | 3,680.22e |
| 3 h | 903.65c | 93.47d | 166.97e | 66.55d | 18.29b | 72.21e | 115.15a | 43.22e | 3,939.10d | |
| 5 h | 991.26b | 96.07d | 214.83b | 98.23b | 21.78a | 69.39ef | 63.82k | 46.21d | 4,704.39b | |
| 140°C | 1 h | 1,110.76a | 115.48a | 196.67d | 82.00c | 12.25g | 78.67d | 79.76i | 47.89c | 4,312.78c |
| 3 h | 997.70b | 109.05b | 316.94b | 85.56c | 13.40f | 84.70bc | 91.25g | 54.09b | 4,321.23c | |
| 5 h | 980.39b | 102.87c | 348.06a | 193.78a | 17.95b | 91.50a | 100.03d | 62.14a | 5,698.98a | |
Mean of triplicate determinations.
Different letters (a-n) indicate a significant difference (P<0.05) among different heat treatments by Duncan’s multiple range test.
Table 2.
Changes in bound phenolics content of rice hull by different heating temperatures and times (unit: μg/g)
| Treatment | Phenolics content of free form | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gallic acid | Homogentisic acid | Protocatechuic acid | Gentisic acid | Chlorogenic acid | (+)-Catechin | Vanillic acid | Caffeic acid | Phloretic acid | ||
| Control | 10.94d | ND | ND | 43.02g | 4.99c | 127.20a | 37.78abc | 37.49a | 486.44a | |
| 80°C | 1 h | 7.15g | ND | ND | 57.20d | 2.87f | 73.65g | 36.25c | 33.18c | 324.80c |
| 3 h | 7.15g | ND | ND | 84.90a | 5.16c | 95.94e | 39.04a | 34.93b | 319.85c | |
| 5 h | 4.74i | ND | ND | 50.44ef | 2.28ij | 75.86g | 20.76h | 22.17j | 221.41i | |
| 100°C | 1 h | 3.28j | ND | ND | 45.97g | 5.77a | 120.51b | 38.06ab | 38.16a | 355.11b |
| 3 h | 5.56h | ND | ND | 57.78d | 4.55d | 107.65c | 37.09bc | 31.14d | 303.51d | |
| 5 h | 9.00ef | ND | ND | 77.37b | 5.37b | 103.29d | 31.80d | 29.23e | 281.46ef | |
| 120°C | 1 h | 9.33e | ND | ND | 43.77g | 2.42hi | 107.89c | 32.03d | 32.31cd | 327.76c |
| 3 h | 8.90ef | ND | ND | 52.72e | 2.48gh | 93.51e | 28.10e | 28.78e | 311.82cd | |
| 5 h | 12.72c | ND | ND | 61.30c | 3.74e | 106.03cd | 31.48d | 31.83d | 283.94e | |
| 130°C | 1 h | 5.27h | ND | ND | 39.41h | 1.50k | 84.78f | 26.32f | 27.18f | 245.56h |
| 3 h | 8.58f | ND | ND | 43.78g | 2.20j | 91.88e | 28.22e | 28.89e | 262.29g | |
| 5 h | 20.75b | ND | ND | 57.68d | 2.95f | 92.12e | 22.36g | 23.62gh | 216.41ij | |
| 140°C | 1 h | 22.32a | ND | ND | 24.63i | 0.75l | 60.96h | 23.35g | 22.65hi | 230.89hi |
| 3 h | 20.51b | ND | ND | 44.16g | 2.37hij | 117.54b | 30.61d | 22.82hi | 266.47fg | |
| 5 h | 20.41b | ND | ND | 49.73f | 2.63g | 85.09f | 22.44g | 24.72g | 203.94j | |
| Treatment | Phenolics content of free form | |||||||||
| p-Coumaric acid | Ferulic acid | Naringin | Hesperidin | Cinnamic acid | Naringenin | Hesperitin | Biochanin | Total | ||
| Controls | 4,212.06b | 405.43c | 49.83a | 23.37b | 4.62g | 38.52b | 38.53d | 32.15a | 5,552.35b | |
| 80°C | 1 h | 3,075.49g | 370.99d | 40.99d | 17.00k | 7.90b | 37.26bc | 35.50e | 29.28b | 4,149.50i |
| 3 h | 2,657.61i | 259.64ij | 46.42bc | 18.04ij | 7.39c | 20.97h | 47.10a | 22.91d | 3,667.05k | |
| 5 h | 1,931.35j | 175.37k | 32.52e | 11.93l | 4.39g | 18.12i | 34.57e | 9.65j | 2,615.57l | |
| 100°C | 1 h | 3,294.18f | 308.22f | 51.93a | 20.15ef | 8.27a | 32.24f | 30.74f | 9.64j | 4,362.21gh |
| 3 h | 2,969.32gh | 270.72hi | 44.48c | 18.80h | 7.24c | 36.28cd | 43.28c | 11.94h | 3,949.32j | |
| 5 h | 2,943.50h | 262.33hi | 44.90bc | 21.04d | 6.74d | 34.56e | 35.07e | 10.65i | 3,896.30j | |
| 120°C | 1 h | 3,455.36e | 289.79g | 45.24bc | 17.42jk | 6.03e | 35.24de | 38.61d | 9.39j | 4,452.60fg |
| 3 h | 3,681.33d | 346.82e | 40.20e | 19.03gh | 6.10e | 31.95f | 43.41c | 12.81h | 4,707.96e | |
| 5 h | 4,503.00a | 517.48a | 47.23b | 22.18c | 7.84b | 40.04a | 39.68d | 12.85h | 5,721.37a | |
| 130°C | 1 h | 4,039.32c | 461.87b | 44.21c | 20.57de | 6.30e | 34.83de | 42.37c | 5.82k | 5,085.30d |
| 3 h | 4,153.18bc | 470.51b | 43.93c | 20.97d | 5.14f | 36.37cd | 45.46b | 16.77f | 5,258.18c | |
| 5 h | 3,373.26ef | 279.73gh | 28.32f | 19.56fg | 4.04h | 22.64g | 28.56g | 14.87g | 4,206.87hi | |
| 140°C | 1 h | 3,663.95d | 242.31j | 16.03g | 11.14m | 1.78i | 13.49j | 13.21h | 6.09k | 4,353.56gh |
| 3 h | 3,655.11d | 242.07j | 17.41g | 24.21a | 2.01i | 38.44b | 48.17a | 28.16c | 4,560.06f | |
| 5 h | 3,456.7e | 258.25ij | 29.64f | 18.41hi | 5.38f | 23.76g | 33.97e | 18.99c | 4,254.12hi | |
Mean of triplicate determinations.
Different letters (a-m) indicate a significant difference (P<0.05) among different heat treatments by Duncan’s multiple range test.
ND, not detected.
Antioxidant activity of the heated rice hull
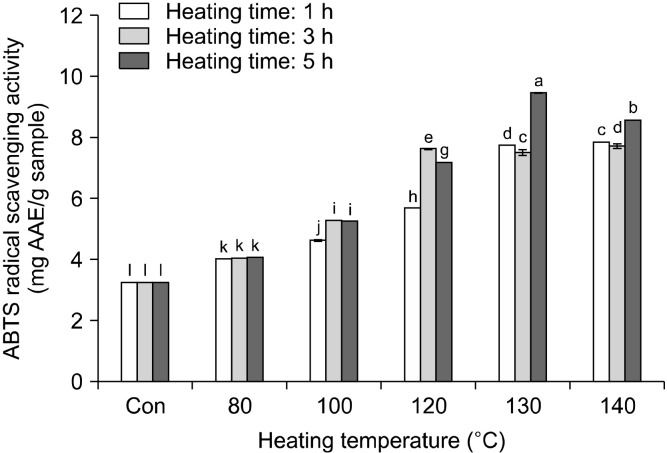
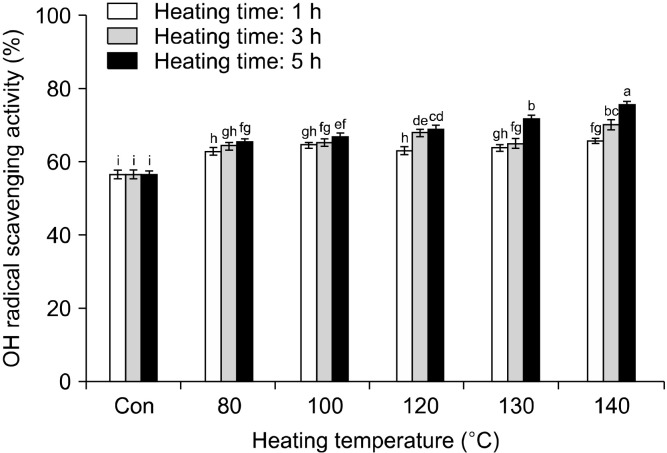
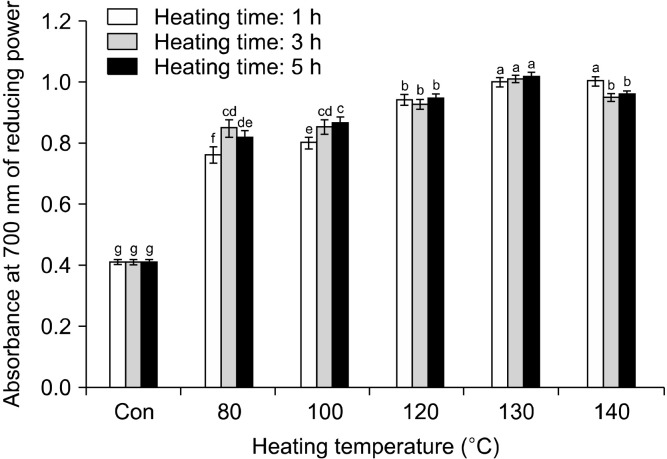
The ABTS and the OH radical scavenging and reducing power assays were used to evaluate the antioxidant activity of rice hull at different heating conditions. The change in the ABTS radical cation scavenging activity of rice hull, caused by different heat treatments, is shown in Fig. 3. The ABTS radical cation scavenging activity generally increased with increasing temperature and time to 140°C and 3 h, respectively. The ABTS radical cation scavenging activity of the rice hull heated for 1 h increased from 3.26 mg AAE/g at room temperature (raw rice hull) to 7.86 mg AAE/g at 140°C. The ABTS radical cation scavenging activity of the sample heated for 3 h increased to 7.72 mg AAE/g at 140°C, while that of the sample heated for 5 h increased to 9.46 mg AAE/g at 130°C and then decreased to 8.58 mg AAE/g at 140°C. Therefore, the highest ABTS radical cation scavenging activity (9.46 mg AAE/g) was of the sample heated at 130°C for 5 h. The differences in the ABTS radical cation scavenging activity between the samples subjected to various treatments were directly proportional to the values of the total polyphenol content, including the phenolic compound content in the respective samples. Similarly, Siddhuraju (2006) reported elevated ABTS radical cation scavenging activity of a dry-heated moth bean. This radical cation scavenging activity of the raw and the heated rice hull may be related to the substitution of the hydroxyl groups in the aromatic rings of phenolic compounds, thus contributing to their hydrogen-donating ability (Brand-Williams et al., 1995). The change in the OH radical scavenging activity of rice hull, caused by different heat treatments, is shown in Fig. 4. The OH radical scavenging activity of the sample heated for 1 h increased from 56.44% at room temperature (raw rice hull) to 65.62% at 140°C. The OH radical scavenging activity of the sample heated for 3 h increased to 69.86% at 140°C, while that of the sample heated for 5 h increased to 75.30% at 140°C. Thus, the highest OH radical scavenging activity (75.30 %) was of the sample heated at 140°C for 5 h. Many studies have reported that thermal processing positively affects the radical scavenging activity; strongly lowering the signal amplitude of the DMSO-OH radical adduct in comparison with the reference spectrum in fruits and vegetables (Gazzani et al., 1998; Juániz et al., 2016). The reducing power of rice hull increased significantly on heat treatment (Fig. 5). The reducing power of the sample heated for 1 h increased from 0.41 at room temperature (raw rice hull) to 1.00 at 140°C. The reducing power of the sample heated for 3 h increased to 1.01 at 130°C and then decreased to 0.95 at 140°C, while that of the sample heated for 5 h increased to 1.01 at 130°C and then decreased to 0.96 at 140°C. The highest reducing power (1.01) was of the samples heated at 130°C for 3 h and 5 h. Many studies have included the measurement of the antioxidant activity, including the radical scavenging activity, reducing power, and antioxidant enzyme activity, after heat treatment; the heated products exhibited elevated chain-breaking and oxygen-scavenging activities (Jeong et al., 2004; Olivares-Tenorio et al., 2017). Previous studies have reported that the antioxidant properties of garlic (Kwon et al., 2006) and tomato (Dewanto et al., 2002a) were elevated on thermal processing due to the disruption of the cell wall and liberation of phenolic compounds from their insoluble forms. In this study, heat treatment with distilled water as the soaking solvent increased the breakdown of the cell wall matrix and caused liberation of the enhanced individual phenolic compounds, which had strong antioxidant activities.
Fig. 3.
Changes in 2,2’-azino-bis(3-ethylbenzothiazoline-6-sul-fonic acid) (ABTS) radical scavenging activity (mg AAE/g) in rice hull by different heating temperatures and times. Different letters (a-l) indicate a significant difference (P<0.05) among different heat treatments by Duncan’s multiple range test. AAE, ascorbic acid equivalents.
Fig. 4.
Changes in OH radical scavenging activity in rice hull by different heating temperatures and times. Different letters (a-i) indicate a significant difference (P<0.05) among different heat treatments by Duncan’s multiple range test.
Fig. 5.
Changes in reducing power of rice hull by different heating temperatures and times. Different letters (a-g) indicate a significant difference (P<0.05) among different heat treatments by Duncan’s multiple range test.
In conclusion, by using rice hull (O. sativa L. cv. ilpumbyeo) as a functional food material and developing effective conditions of heat treatment (time and temperature), we used thermal processing to analyze the content of the antioxidant components and activity of rice hull. Total polyphenol and flavonoid content of the heated rice hull were quantified using a spectrophotometer, and the antioxidant activity of the heated rice hull was determined by analyzing the ABTS and OH radical scavenging activities and reducing power. Improvements in the content of the antioxidant components and activity occurred on increasing the heating temperature and time. The major phenolic compounds of rice hull are (+)-catechin, phloretic acid, p-coumaric acid, and ferulic acid. The free phenolics content in the present study showed an increase on heat treatment, whereas the bound phenolics content in the heated rice hull decreased compared to the raw rice hull. We concluded that thermal processing was effective in liberating free phenolic compounds from the rice hull matrix. Therefore, based on the increase in the total polyphenol and flavonoid content and antioxidant effects of rice hull, we reported that heat treatment was a significantly efficient method, considering time and expenditure, for the industrial development of functional feed, food, and drug materials.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Beta T, Hwang T. Influence of heat and moisture treatment on carotenoids, phenolic content, and antioxidant capacity of orange maize flour. Food Chem. 2018;246:58–64. doi: 10.1016/j.foodchem.2017.10.150. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Cacace JE, Mazza G. Mass transfer process during extraction of phenolic compounds from milled berries. J Food Eng. 2003;59:379–389. doi: 10.1016/S0260-8774(02)00497-1. [DOI] [Google Scholar]
- Choi Y, Kim M, Shin JJ, Park JM, Lee J. The antioxidant activites of the some commercial teas. J Korean Soc Food Sci Nutr. 2003;32:723–727. doi: 10.3746/jkfn.2003.32.5.723. [DOI] [Google Scholar]
- Choi Y, Lee SM, Chun J, Lee HB, Lee J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006;99:381–387. doi: 10.1016/j.foodchem.2005.08.004. [DOI] [Google Scholar]
- de Mira NVM, Massaretto IL, Pascual CSCI, Lanfer-Marquez UM. Comparative study of phenolic compounds in different Brazilian rice (Oryza sativa L.) genotypes. J Food Compos Anal. 2009;22:405–409. doi: 10.1016/j.jfca.2008.06.012. [DOI] [Google Scholar]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002a;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Dewanto V, Wu X, Liu RH. Processed sweet corn has higher antioxidant activity. J Agric Food Chem. 2002b;50:4959–4964. doi: 10.1021/jf0255937. [DOI] [PubMed] [Google Scholar]
- Gazzani G, Papetti A, Massolini G, Daglia M. Anti- and prooxidant activity of water soluble components of some common diet vegetables and the effect of thermal treatment. J Agric Food Chem. 1998;46:4118–4122. doi: 10.1021/jf980300o. [DOI] [Google Scholar]
- Gutteridge JM. Streptonigrin-induced deoxyribose degradation: inhibition by superoxide dismutase, hydroxyl radical scavengers and iron chelators. Biochem Pharmacol. 1984;33:3059– 3062. doi: 10.1016/0006-2952(84)90609-9. [DOI] [PubMed] [Google Scholar]
- Herrmann K. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit Rev Food Sci Nutr. 1989;28:315–347. doi: 10.1080/10408398909527504. [DOI] [PubMed] [Google Scholar]
- Huang YC, Chang YH, Shao YY. Effects of genotype and treatment on the antioxidant activity of sweet potato in Taiwan. Food Chem. 2006;98:529–538. doi: 10.1016/j.foodchem.2005.05.083. [DOI] [Google Scholar]
- Jariwalla RJ. Rice-bran products: phytonutrients with potential applications in preventive and clinical medicine. Drugs Exp Clin Res. 2001;27:17–26. [PubMed] [Google Scholar]
- Jeong SM, Kim SY, Kim DR, Jo SC, Nam KC, Ahn DU, et al. Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J Agric Food Chem. 2004;52:3389–3393. doi: 10.1021/jf049899k. [DOI] [PubMed] [Google Scholar]
- Juániz I, Ludwig IA, Huarte E, Pereira-Caro G, Moreno-Rojas JM, Cid C, et al. Influence of heat treatment on antioxidant capacity and (poly)phenolic compounds of selected vegetables. Food Chem. 2016;197:466–473. doi: 10.1016/j.foodchem.2015.10.139. [DOI] [PubMed] [Google Scholar]
- Jung KH, Hong HD, Cho CW, Lee MY, Choi UK, Kim YC. Phenolic acid composition and antioxidative activity of red ginseng prepared by high temperature and high pressure process. Korean J Food Nutr. 2012;25:827–832. doi: 10.9799/ksfan.2012.25.4.827. [DOI] [Google Scholar]
- Kim HY, Hwang IG, Kim TM, Park DS, Kim JH, Kim DJ, et al. Antioxidant and angiotensin converting enzyme I inhibitory activity on different parts of germinated rough rice. J Korean Soc Food Sci Nutr. 2011;40:775–780. doi: 10.3746/jkfn.2011.40.6.775. [DOI] [Google Scholar]
- Kim MY, Lee BW, Lee HU, Lee YY, Kim MH, Lee JY, et al. Phenolic compounds and antioxidant activity in sweet potato after heat treatment. J Sci Food Agric. 2019;99:6833–6840. doi: 10.1002/jsfa.9968. [DOI] [PubMed] [Google Scholar]
- Kwon OC, Woo KS, Kim TM, Kim DJ, Hong JT, Jeong HS. Physicochemical characteristics of garlic (Allium sativum L.) on the high temperature and pressure treatment. Korean J Food Sci Technol. 2006;38:331–336. [Google Scholar]
- Mattila P, Pihlava JM, Hellström J. Contents of phenolic acids, alkyl- and alkenylresorcinols, and avenanthramides in commercial grain products. J Agric Food Chem. 2005;53:8290– 8295. doi: 10.1021/jf051437z. [DOI] [PubMed] [Google Scholar]
- Mau JL, Lin HC, Song SF. Antioxidant properties of several specialty mushrooms. Food Res Int. 2002;35:519–526. doi: 10.1016/S0963-9969(01)00150-8. [DOI] [Google Scholar]
- Nicoli MC, Anese M, Parpinel M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci Technol. 1999;10:94–100. doi: 10.1016/S0924-2244(99)00023-0. [DOI] [Google Scholar]
- Niwa Y, Miyachi Y. Antioxidant action of natural health products and Chinese herbs. Inflammation. 1986;10:79–91. doi: 10.1007/BF00916043. [DOI] [PubMed] [Google Scholar]
- Olivares-Tenorio ML, Verkerk R, van Boekel MAJS, Dekker M. Thermal stability of phytochemicals, HMF and antioxidant activity in cape gooseberry (Physalis peruviana L.) J Funct Foods. 2017;32:46–57. doi: 10.1016/j.jff.2017.02.021. [DOI] [Google Scholar]
- Ramarathnam N, Osawa T, Namiki M, Kawakishi S. Chemical studies on novel rice hull antioxidants. 1. Isolation, fractionation, and partial characterization. J Agric Food Chem. 1988;36:732–737. doi: 10.1021/jf00082a014. [DOI] [Google Scholar]
- Saha BC, Iten LB, Cotta MA, Wu YV. Dilute acid pretreatment, enzymatic saccharification, and fermentation of rice hulls to ethanol. Biotechnol Prog. 2005;21:816–822. doi: 10.1021/bp049564n. [DOI] [PubMed] [Google Scholar]
- Seo MC, Ko JY, Song SB, Lee JS, Kang JR, Kwak DY, et al. Antioxidant compounds and activities of foxtail millet, proso millet and sorghum with different pulverizing methods. J Korean Soc Food Sci Nutr. 2011;40:790–797. doi: 10.3746/jkfn.2011.40.6.790. [DOI] [Google Scholar]
- Siddhuraju P. The antioxidant activity and free radical-scavenging capacity of phenolics of raw and dry heated moth bean (Vigna aconitifolia) (Jacq.) Marechal seed extracts. Food Chem. 2006;99:149–157. doi: 10.1016/j.foodchem.2005.07.029. [DOI] [Google Scholar]
- Temple NJ. Antioxidants and disease: more questions than answers. Nutr Res. 2000;20:449–459. doi: 10.1016/S0271-5317(00)00138-X. [DOI] [Google Scholar]
- Wanyo P, Meeso N, Siriamornpun S. Effects of different treatments on the antioxidant properties and phenolic compounds of rice bran and rice husk. Food Chem. 2014;157:457–463. doi: 10.1016/j.foodchem.2014.02.061. [DOI] [PubMed] [Google Scholar]
- Yamagishi T, Matsuda K, Watanabe T. Covalently attached ferulic acid in a proteoglycan from rice bran. Phytochemistry. 1984;23:2231–2232. doi: 10.1016/S0031-9422(00)80525-7. [DOI] [Google Scholar]
- Zielinski H, Kozlowska H, Lewczuk B. Bioactive compounds in the cereal grains before and after hydrothermal processing. Innov Food Sci Emerg Technol. 2001;2:159–169. doi: 10.1016/S1466-8564(01)00040-6. [DOI] [Google Scholar]