Abstract
The aim of this work was to evaluate the inhibitory activities of organic acids identified from commercial vinegars on α-amylase and α-glucosidase. Six organic acids (acetic, citric, lactic, malic, succinic, and tartaric) were identified in nine commercial vinegars, whose contents varied considerably depending on the raw materials. Most of the fruit vinegars, comprised of various organic acids, were found to be more effective inhibitors against digestive enzymes than grain vinegars containing mainly acetic acid. Citric acid had the lowest IC50 values for α-amylase and α-glucosidase activities 0.64±0.04 μM/mL and 8.95±0.05 μM/mL, respectively, and thus exhibited the strongest antidiabetic effect. Mulberry fruit vinegar containing the highest content of total organic acid (111.02±1.50 mg/mL) showed the strongest digestive enzyme inhibitory impact. The results indicate that vinegars with higher contents of various organic acids hold strong potential against digestive enzymes.
Keywords: digestive enzymes inhibition, organic acid, vinegar
INTRODUCTION
Vinegars contain several bioactive compounds that are characterized according to the type of raw materials, such as grain vinegars and fruit vinegars (Xia et al., 2020). In the past 20 years, there have been several reports in which intake of vinegar with a meal was shown to reduce postprandial glucose concentrations in clinical trials featuring healthy adults and patients with diabetes (Johnston and Gaas, 2006; Shishehbor et al., 2017; Santos et al., 2019). This therapeutic effect of vinegars might be due to the presence of bioactive components including organic acids, amino acids, and polyphenolics, capable of maintaining glucose homeostasis (Xia et al., 2020).
Organic acids in vinegars have been shown to have beneficial effects, such as antimicrobial activity, suppres-sion of fat accumulation and hyperlipidemia, improvement of insulin resistance and metabolic abnormalities, and reduction of hypertension and anti-fatigue activity (Ho et al., 2017; Xia et al., 2020). Acetic acid, one of the major components of vinegar, has been reported to be a potential modulator of glucose metabolism in horses and rodents (Johnston and Gaas, 2006; Santos et al., 2019). However, individual organic acids in vinegars have long been neglected in terms of research, and their pharmacological actions have not been sufficiently studied. Based on the available evidence, we hypothesize one pathway through which organic acids in vinegar may improve blood glucose: inhibition of α-amylase and α-glucosidase activities. α-Glucosidase and α-amylase are digestive enzymes released into the intestinal lumen in response to food stimuli for the digestion of carbohydrates to glucose (Kao et al., 2006; Zhang et al., 2011). Inhibition of these digestive enzymes, either α-glucosidase or α-amylase reduces the available amount of monosaccharides, particularly glucose, that can be absorbed by the body (Kao et al., 2006). Interestingly, vinegar does not have an antiglycemic effect when the vinegar was neutralized with sodium bicarbonate, a finding that implies a mechanism related to acidity (Santos et al., 2019). It has been known that daily vinegar intake in amounts of 10∼30 mL (2∼6 tablespoons) appear to improve the glycemic response to carbohydrate-rich meals (Johnston and Gaas, 2006). Therefore, the present study is designed to evaluate the in vitro antidiabetic activities of six organic acids derived from nine commercial vinegars and understand their mechanisms of action against α-glucosidase and α-amylase.
MATERIALS AND METHODS
Vinegar samples
Two grain vinegars and six fruit vinegars of different brands, produced by traditional fermentation, were purchased from a local market in Seoul, Korea. All commercial vinegars were stored in the laboratory at a temperature of 4±1°C prior to analysis. Characteristics of the commercial vinegars such as the raw materials and acidity (%) are shown in Table 1.
Table 1.
Characteristics and organic acid contents of Korean commercial vinegars
| Characteristics | Acidity (%) | Organic acids (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ID | Main materials | Acetic | Citric | Lactic | Malic | Succinic | Tartaric | Total | |
| Fruit vinegars | |||||||||
| CV | Jeju citrus concentrates 60% | 4.65 | 16.46±0.27f | 34.14±0.22a | 0.96±0.29de | 3.52±0.03b | 0.30±0.03b | 18.90±2.14b | 74.28±0.51b |
| YV | Citron 32% | 5.89 | 43.89±1.42c | 11.36±0.63d | 7.03±0.54a | 1.08±0.01c | 0.23±0.01cd | ND | 63.59±0.52c |
| MV | Plum 30% | 5.54 | 38.77±0.06d | 17.88±0.84c | 0.22±0.01e | 1.24±0.08c | 0.13±0.01e | ND | 58.24±0.20d |
| AV | Apple juice 100% | 5.12 | 46.22±0.55b | 0.08±0.00e | 1.23±0.20de | 5.41±0.14a | 0.22±0.01cd | ND | 53.16±0.18e |
| PV | Persimmon 98% | 5.36 | 53.31±0.17a | 0.02±0.00e | 2.95±0.01c | 0.06±0.00e | 0.15±0.00e | ND | 56.49±0.04d |
| OV | Mulberry fruit 80% | 5.20 | 9.16±0.06g | 27.49±1.15b | 4.58±0.22b | 0.50±0.06d | 0.26±0.01bc | 69.02±7.59a | 111.02±1.50a |
| Grain vinegars | |||||||||
| HV | Brown rice 50% | 4.11 | 36.72±0.34e | 0.005±0.00e | 1.94±0.24cd | 0.07±0.01e | 0.39±0.02a | ND | 39.12±0.12f |
| RV | Five kinds1) of cereals 32.7% | 5.60 | 53.63±0.67a | 0.01±0.00e | 4.91±0.44b | 0.07±0.01e | 0.25±0.04bc | ND | 58.86±0.23d |
| GV | Black garlic 20% | 5.62 | 53.27±0.25a | 0.03±0.00e | 1.96±0.91cd | 0.11±0.04e | 0.18±0.01de | ND | 55.54±0.24de |
Values are presented as mean±SE (n=3).
Means with different letters (a-g) within the same column indicate significant differences at P<0.05.
ND, not detected.
1)Brown rice, millet, barley, wheat, and sorghum.
Chemicals and reagents
Six high-performance liquid chromatography (HPLC) grade organic acids: acetic, citric, lactic, malic, succinic, and tartaric acids, and α-amylase from porcine pancreas (EC 3.2.1.1), α-glucosidase from Saccharomyces cerevisiae (EC 3.2.1.20), acarbose, sodium carbonate (100%), p-nitrophenyl-α-D-glucopyranoside (α-pNPG), and dinitro-salicylic acid (DNS) were acquired from Sigma-Aldrich Co. (St. Louis, MO, USA). All chemicals used in the study were of analytical grade.
Determination of organic acids
An Agilent 1260 HPLC system (Agilent Technologies, Santa Clara, CA, USA) was employed for organic acid analysis (Sanarico et al., 2003; Cocchi et al., 2006). The chromatographic separation used for organic acid detec-tion employed H2SO4 in pure ultrapure water (4.0 mM/L) as the mobile phase, following an isocratic elution procedure at a flow rate of 0.5 mL/min. The samples were injected into an Agilent Hi-Plex H (300×7.7 mm) with internal particles (8.0 μm) protected by a PL Hi-Plex H (5×3 mm) guard column (Agilent Technologies). The temperature of the column compartment was maintained at 70°C, and the injection volume was 20 μL. Organic acids were detected with a UV-Visible diode array detector at 210 nm. The organic acids were quantified using external calibration curves. The data were described as micrograms of organic acid per milliliter of vinegar samples.
Inhibition of α-amylase enzyme
α-Amylase activity was determined according to a previous method with slight modifications (Zhang et al., 2011). Briefly, 40 mL of α-amylase (5 U/mL) was mixed with 0.36 mL of 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) and 0.2 mL of sample or acarbose. Acarbose was used as the positive control. After incubation for 20 min at 37°C, 300 mL of starch solution (1%) in 0.02 M sodium phosphate buffer was added, after which the mixture was reincubated for 20 min, followed by the addition of 0.2 mL DNS. The contents were mixed well and kept in a boiling water bath for 5 min. The reaction mixture was diluted by adding 6 mL of distilled water, and the absorbance was measured at 540 nm in a UV-Visible spectrophotometer (Biotek Power, Biotek Instruments Inc., Winooski, VT, USA). All assays were carried out in triplicate. The α-amylase inhibitory activity was calculated as follows:
where As and Ac are the absorbance of the sample and control, respectively. The half maximal inhibitory concentrations (IC50) values of pure compounds were calculated by logarithmic repression analysis.
Inhibition of α-glucosidase enzyme
α-Glucosidase inhibition was from the modified method of Zhang et al. (2011). A mixture of 50 mL of sample and 50 μL of enzyme (0.57 U/mL) was incubated at 37°C for 10 min. Then, 50 μL of α-pNPG (5 mM) as substrate was added into the mixture and incubated at 37°C for 20 min, after which. 50 μL of 1 M Na2CO3 solution was added to stop the reaction. Measurement of absorbance at 405 nm using the UV-Vis absorbance spectrophotometer was carried out. α-Glucosidase inhibition was expressed as the percentage inhibition and was calculated as follows:
where Acontrol is the absorbance of the control and Asample is the absorbance of the sample. Acarbose was used as the positive control. IC50 values of pure compounds were determined.
Statistical analysis
Experimental results were expressed as mean±standard error (SE). Data were analyzed by ANOVA using 2014 SAS (version 9.3, SAS Inc., Cary, NC, USA). Duncan’s multiple range test was carried out to determine any significant differences between samples (P<0.05). Evaluation of the associations between parameters was carried out using Pearson’s correlation.
RESULTS AND DISCUSSION
Organic acid contents in commercial vinegars
The profiles and concentrations of organic acids are important parameters for evaluating the fermentation processing and chemical compositions of vinegars (Ho et al., 2017). Of the examined commercial vinegars, six (acetic, citric, lactic, malic, succinic, and tartaric acid) organic acids were identified. As shown in Table 1, the total acid content quantified in the vinegars ranged from 39.12 to 111.02 mg/mL. Acetic acid was the main organic acid present in the vinegars, with values ranging from 9.16± 0.06 to 53.63±0.67 mg/mL, and represented over 67% of the total quantifiable acid content, with the exception of mulberry fruit vinegar (OV) and Jeju citrus vinegar (CV). The content of acetic acid (53.63±0.67) in five cereals vinegar (brown rice, millet, barley, wheat, and sorghum; RV) was the highest of the nine vinegar samples, followed by persimmon vinegar (PV), black garlic vinegar (GV), and apple juice vinegar (AV) in descending order. The content of citric acid was very low (0.005 to 0.01 mg/mL) in grain vinegars [brown rice vinegar (HV) and RV] but varied from 11.36 to 34.14 mg/mL in fruit vinegars [CV, plum vinegar (MV), OV, and citron vinegar (YV)]. Interestingly, the contents of citric acid (27.49 and 34.14 mg/ mL) and tartaric acid (18.90 and 69.02 mg/mL) in OV and CV, respectively are higher than that of acetic acid (9.16 to 16.46 mg/mL). The content of tartaric acid in OV was the highest (69.02±7.59 mg/mL), accounting for 62.2% of the total organic acid content. This result is consistent with previous reports in which the concentrations of tartaric acid and citric acid were found to be higher than that of acetic acid in both wine vinegar and lemon vinegar (Cocchi et al., 2006). Tartaric acid was not detected in most of the commercial vinegars except for CV and OV, as shown in Table 1. Tartaric acid and citric acid are derived from grapes and citrus fruits, respectively. Both are used as souring agents and add a pleasantly sour taste to dishes (Ho et al., 2017). The total organic acid contents in grain vinegars (39.12 to 58.86 mg/mL) were lower than those in fruit vinegars (53.16 to 111.02 mg/mL). Among the vinegars tested, OV had the highest concentration of total organic acids (110.02±1.50 mg/ mL), whereas HV showed the lowest content (39.12± 0.12 mg/mL). The contents of citric, malic, and tartaric acids were higher in fruit vinegars than in grain vinegars. On the other hand, grain vinegars were composed mostly of acetic acid (91.1 to 93.9%) and lactic acid (1.94 to 4.91 mg/mL). In general, acetic acid is derived from oxidizing alcohol during acetic acid fermentation while lactic acid is produced by proliferating lactic acid bacteria at the beginning of alcoholic fermentation (Xia et al., 2020). It has been reported that the level and composition of organic acids in vinegars depend on the raw materials, fermentation technique, and rate of microbiological growth (Ho et al., 2017; Xia et al., 2020).
Inhibitory effects of commercial vinegars on α-amylase and α-glucosidase
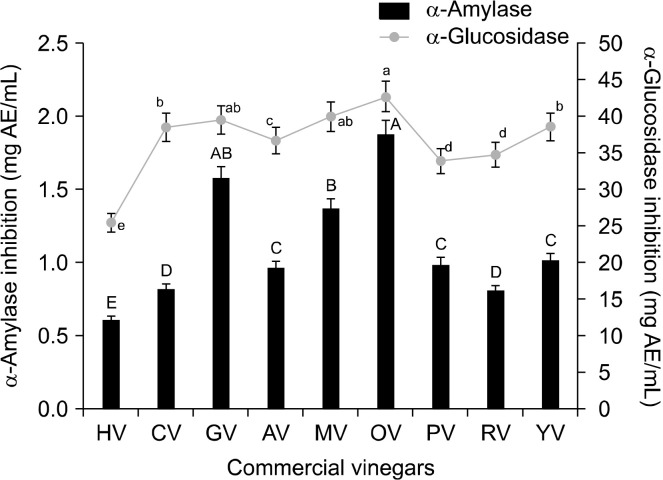
The suppression of glucose production from carbohydrates in the gut or glucose absorption from the intestine has been previously investigated using vinegar resources (Johnston and Gaas, 2006; Shishehbor et al., 2017; Santos et al., 2019). As shown in Fig. 1, data from the present study provide evidence of the inhibitory effect of commercial vinegars on α-amylase and α-glucosidase activities. The inhibitory potency of the vinegar samples was expressed as mg acarbose (positive control) equivalents per mL of individual vinegar [mg acarbose equivalent (AE)/mL]. The highest inhibitory activity against α-amylase was detected in OV containing 1.87 mg AE/mL, followed by GV (1.57 mg AE/mL), MV (1.36 mg AE/mL), and YV (1.01 mg AE/mL). This pattern was similar to that of α-glucosidase inhibitory activity (OV> MV> GV> YV=CV). The highest AE value was detected in OV, which confirms that OV had the highest α-amylase (1.87 mg AE/mL) and α-glucosidase (42.55 mg AE/mL) inhibitory activities. As an explanation (Bao et al., 2016), OV was found to contain more bioactive components such as organic acids, amino acids, and polyphenol compounds than the other vinegars. Ogawa et al. (2000) demonstrated that acetic acid treatment for 15 days suppressed sucrase, lactase, maltase, and trehalase activities in concentration- and time-dependent manners. The acid properties of vinegar can result in hindered absorption when consumed with a carbohydrate-rich meal. This is supported by in vitro data showing that a decrease in pH below 4.0 inactivates α-amylase (Marunaka, 2018). Therefore, given that the pH of commercially marketed vinegars is about 2∼3, its consumption may inactivate the salivary α-amylase action and decrease its release until nutrients reach the small intestine, which results in lower blood glucose levels (Marunaka, 2018; Santos et al., 2019). The study suggested that regular ingestion of vinegar can modestly improve glycemic control. Pancreatic α-amylase, a key enzyme in the digestive system, catalyzes the initial step in the hydrolysis of starch, which is a principal source of dietary glucose (Kao et al., 2006). Meanwhile, α-glucosidase has been recognized as a therapeutic target for the modulation of postprandial hyperglycemia, which is the earliest metabolic abnormality in type 2 diabetes mellitus (Santos et al., 2019). As shown in Fig. 1, OV containing the highest total organic acid content also showed the best digestive enzyme inhibitory rate. This finding indicates that the observed inhibitory effect of vinegars on digestive enzymes might be more dependent on the total organic acid content than individual organic acid content. Overall, fruit vinegars (OV, CV, YV, and AV) containing a higher organic acid content were more effective inhibitors against digestive enzymes than grain vinegars such as HV and RV. Therefore, the inhibitory effect of organic acids against digestive enzymes should be further investigated.
Fig. 1.
Effect of commercial vinegars on the inhibitory activities of digestive enzymes. Values are presented as means±SE (n=3). Values with different letters (A-E and a-e) are significantly different at P<0.05. ID of commercial vinegars are presented in Table 1. AE, acarbose equivalent.
Inhibitory effects of pure organic acids on α-amylase and α-glucosidase
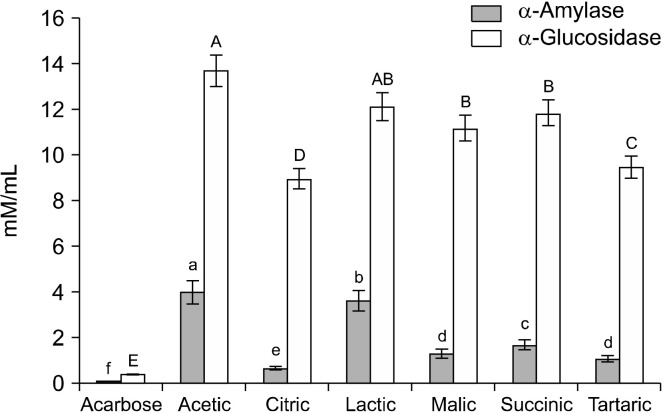
It has been reported that organic acids in vinegars can not only be considered as nutrients but also as bioactive compounds, which have beneficial effects such as antimicrobial activity, suppression of fat accumulation and glucose production, as well as improvement of insulin resistance and metabolic abnormalities (Ho et al., 2017; Xia et al., 2020). However, information concerning the detailed chemical composition of these organic acids remains extremely limited. As shown above, nine commercial vinegars were revealed to have inhibitory activities against α-glucosidase and α-amylase. Therefore, major organic acids identified from commercial vinegars were evaluated for their inhibitory activities against α-amylase and α-glucosidase (Fig. 2). In the digestive enzymes inhibitory assay, organic acids showed a dose-dependent inhibitory effect on α-amylase and α-glucosidase activities. The mean IC50 of organic acids was estimated to be 6.62±0.14 μM/mL, whereas acarbose (standard drug) exhibited an IC50 value of 0.21±0.05 μM/mL. As shown in Fig. 2, acarbose exhibited remarkably higher inhibitory activity compared to other organic acids tested. It has been previously reported that weak acid has a weak inhibitory effect on digestive enzyme activity (Marunaka, 2018; Heitor et al., 2019), which is consistent with our results. In the present study, citric acid had the lowest IC50 values against α-amylase and α-glucosidase activities (0.64±0.04 and 8.95±0.05 μM/mL, respectively), which suggests citric acid had the strongest antidiabetic effect. In comparison, moderate inhibitory activities were exhibited by tartaric acid, malic acid, and succinic acid (mean IC50 values of 5.26±0.41, 6.22±0.38, and 6.74± 0.34 μM/mL, respectively) (Fig. 2). Although citric acid exhibited much stronger inhibition of digestive enzymes than acetic acid, the contribution of acetic acid to digestive enzyme inhibition was greater due to its exceptionally high concentration. Thus, we can assume that the digestive enzyme inhibitory activities of the vinegars are related more to the organic acid content than to other minor compounds such as polyphenolics and amino acids. The antidiabetic effects of these organic acids are in the following descending order: citric acid> tartaric acid> malic acid> succinic acid> lactic acid> acetic acid. Hence, it could be concluded that the in vitro inhibitory effects of organic acids on α-glucosidase and α-amylase are one mechanism of action through which vinegars can control post-prandial hyperglycemia.
Fig. 2.
Effect of individual organic acid on the inhibitory activities of digestive enzymes. Values are presented as means±SE (n=3). Values with different letters (A-E and a-f) are significantly different at P<0.05. IC50, half maximal inhibitory concentrations.
Pearson’s correlation coefficient analysis
Many previous studies have indicated that the acetic acid content of vinegar is one of the most important parameters affecting antidiabetic activity (Johnston and Gaas, 2006; Mitrou et al., 2015; Santos et al., 2019). Therefore, Pearson’s correlation analysis among the digestive enzyme inhibitory activities of the nine commercial vinegars and content of individual organic acids derived from vinegars was carried out. As shown in Table 2, Pearson’s test showed a moderately positive correlation (mean; r= 0.4737) between total organic acid content (mg/mL) in the commercial vinegars and digestive enzyme inhibitory activity (mg AE/mL), which could be ascribed to tartaric acid, succinic acid, and citric acid (P<0.05). Based on the correlation coefficient, vinegars showed more promising effects against α-glucosidase (r=0.4989) than against α-amylase (r=0.4485) (Table 2). Among the organic acids tested, tartaric acid showed a significant correlation (P< 0.05) with digestive enzyme inhibition. This result indicates that tartaric acid not only is a main determinant of the special flavor of vinegar but also is highly correlated with anti-diabetic capacity. This result is consistent with the above data that OV manufactured from mulberry fruits, which contains the highest content of tartaric acid (69.02±7.59 mg/mL), showed the most potent digestive enzyme inhibitory activity. This supportive evidence further indicates that the inhibitory effect of vinegar on digestive enzymes is closely related to both the profiles and concentrations of the organic acids in vinegars. Strictly speaking, correlations cannot prove a cause-effect relationship between organic acid content and digestive enzyme inhibitory activity since the organic acids in vinegars are not pure single compounds and combinations of specific structures might have synergistic or antagonistic effects (Marunaka, 2018). Generally, the types and concentrations of organic acids in vinegars are closely related to the raw materials used and production technologies employed, in addition to the chemical reactions, physical changes, and microbial fermentation process during brewing (Chen et al., 2016; Ho et al., 2017).
Table 2.
Pearson’s correlation coefficients (r) between organic acids contents and digestive enzyme inhibition of commercial vinegars
| Organic acids | α-Amylase inhibition | α-Glucosidase inhibition |
|---|---|---|
| Acetic acid | 0.0928 | 0.1098 |
| Citric acid | 0.1044 | 0.3308* |
| Lactic acid | 0.0039 | 0.0115 |
| Malic acid | 0.0414 | 0.0393 |
| Succinic acid | 0.2181* | 0.3096* |
| Tartaric acid | 0.3860* | 0.2383* |
| Total organic acids | 0.4485* | 0.4989* |
*P<0.05 while the other correlation coefficients present P>0.05.
In conclusion, this study represents the first assessment of the in vitro antidiabetic potential of organic acids derived from commercial vinegars, with a focus on their inhibitory effects against α-glucosidase and α-amylase. Six organic acids (acetic, citric, lactic, malic, succinic, and tartaric acid) were identified in nine commercial vinegars. Fruit vinegars containing various organic acids (acetic, citric, tartaric, and malic acids, etc.) were more effective inhibitors against digestive enzymes than grain vinegars. The inhibitory effects of organic acids against α-glucosidase and α-amylase were in the following order: citric acid> tartaric acid> malic acid> succinic acid> lactic acid> acetic acid. The total organic acid content of commercial vinegars was found to have a higher positive correlation (mean; r=0.4737) with digestive enzyme inhibitory activity than the content of individual organic acids. Collectively, this study suggests that vinegars having high concentrations of various organic acids may improve the blood glucose level through inhibition of α-amylase and α-glucosidase activities.
ACKNOWLEDGEMENTS
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2019-0233).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Bao T, Xu Y, Gowd V, Zhao J, Xie J, Liang W, et al. Systematic study on phytochemicals and antioxidant activity of some new and common mulberry cultivars in China. J Funct Foods. 2016;25:537–547. doi: 10.1016/j.jff.2016.07.001. [DOI] [Google Scholar]
- Chen H, Chen T, Giudici P, Chen F. Vinegar functions on health: constituents, sources, and formation mechanisms. Compr Rev Food Sci Food Saf. 2016;15:1124–1138. doi: 10.1111/1541-4337.12228. [DOI] [PubMed] [Google Scholar]
- Cocchi M, Durante C, Grandi M, Lambertini P, Manzini D, Marchetti A. Simultaneous determination of sugars and organic acids in aged vinegars and chemometric data analysis. Talanta. 2006;69:1166–1175. doi: 10.1016/j.talanta.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Ho CW, Lazim AM, Fazry S, Zaki UKHH, Lim SJ. Varieties, production, composition and health benefits of vinegars: a review. Food Chem. 2017;221:1621–1630. doi: 10.1016/j.foodchem.2016.10.128. [DOI] [PubMed] [Google Scholar]
- Johnston CS, Gaas CA. Vinegar: medicinal uses and antiglycemic effect. MedGenMed. 2006;8(2):61. PMCID: PMC1785201. [PMC free article] [PubMed] [Google Scholar]
- Kao YH, Chang HH, Lee MJ, Chen CL. Tea, obesity, and diabetes. Mol Nutr Food Res. 2006;50:188–210. doi: 10.1002/mnfr.200500109. [DOI] [PubMed] [Google Scholar]
- Marunaka Y. The proposal of molecular mechanisms of weak organic acids intake-induced improvement of insulin resistance in diabetes mellitus via elevation of interstitial fluid pH. Int J Mol Sci. 2018;19:3244. doi: 10.3390/ijms19103244. https://doi.org/10.3390/ijms19103244 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrou P, Petsiou E, Papakonstantinou E, Maratou E, Lambadiari V, Dimitriadis P, et al. The role of acetic acid on glucose uptake and blood flow rates in the skeletal muscle in humans with impaired glucose tolerance. Eur J Clin Nutr. 2015;69:734–739. doi: 10.1038/ejcn.2014.289. [DOI] [PubMed] [Google Scholar]
- Ogawa N, Satsu H, Watanabe H, Fukaya M, Tsukamoto Y, Miyamoto Y, et al. Acetic acid suppresses the increase in disaccharidase activity that occurs during culture of Caco-2 cells. J Nutr. 2000;130:507–513. doi: 10.1093/jn/130.3.507. [DOI] [PubMed] [Google Scholar]
- Sanarico D, Motta S, Bertolini L, Antonelli A. HPLC determination of organic acids in traditional balsamic vinegar of Reggio Emilia. J Liq Chromatogr Relat Technol. 2003;26:2177–2187. doi: 10.1081/JLC-120022402. [DOI] [Google Scholar]
- Santos HO, de Moraes WMAM, da Silva GAR, Prestes J, Schoenfeld BJ. Vinegar (acetic acid) intake on glucose metabolism: a narrative review. Clin Nutr ESPEN. 2019;32:1–7. doi: 10.1016/j.clnesp.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Shishehbor F, Mansoori A, Shirani F. Vinegar consumption can attenuate postprandial glucose and insulin responses; a systematic review and meta-analysis of clinical trials. Diabetes Res Clin Pract. 2017;127:1–9. doi: 10.1016/j.diabres.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Xia T, Zhang B, Duan W, Zhang J, Wang M. Nutrients and bioactive components from vinegar: a fermented and functional food. J Funct Foods. 2020;64:103681. doi: 10.1016/j.jff.2019.103681. https://doi.org/10.1016/j.jff.2019.103681 . [DOI] [Google Scholar]
- Zhang L, Hogan S, Li J, Sun S, Canning C, Zheng SJ, et al. Grape skin extract inhibits mammalian intestinal α-glucosidase activity and suppresses postprandial glycemic response in streptozocin-treated mice. Food Chem. 2011;126:466–471. doi: 10.1016/j.foodchem.2010.11.016. [DOI] [Google Scholar]