Abstract
The seeds of Citrus sinensis (L.) Osbeck (sweet orange) are waste products usually discarded. They may however contain phytochemicals that have potent bioactivities. In this study, the phenolic content, and antioxidant and antimicrobial activities of oil and non-oil (solid) extracts of C. sinensis seeds were evaluated using standard protocols. The seed oil contained significantly (P>0.05) higher contents of total phenol and total flavonoid when compared to the solid extract. However, the non-oil extract contained significantly (P<0.05) higher tannin contents than the seed oil. Ferric reducing antioxidant potential was not significantly different between both extracts. The antimicrobial activities of both extracts revealed that the seed oil possesses better antibacterial activities compared to the non-oil extract. The antifungal test revealed that the seed oil significantly inhibited the growth of Candida albicans (20 mm zone of inhibition at a concentration of 200 μg/mL), however, it did not inhibit the growth of Aspergillus niger and Penicillum sp. The minimum inhibitory concentration values against the bacterial and fungal strains were similar for both extracts in the range of 50∼100 μg/mL. Minimum bactericidal concentration and minimum fungicidal concentration values ranged from 100∼200 μg/mL for both extracts. The results in this study indicate that C. sinensis seed oil and non-oil extracts possess antioxidant, and antibacterial and antifungal properties that may be differentially exploited in the development of antimicrobial agents.
Keywords: antimicrobial, Citrus sinensis, minimum bactericidal concentration, minimum fungicidal concentration, minimum inhibitory concentration
INTRODUCTION
More than eight thousand phenolic compounds are known to exist in nature. These compounds chemically contain at least one aromatic ring with one or more attached hydroxyl (OH−) groups as well as other substituents. Phenolic derivatives are the largest group of secondary plant metabolites synthesized by higher plants and are believed to be essential for plant survival by providing defense against microbial attack and by making the plant unpalatable to herbivores (Apak et al., 2007; Tungmunnithum et al., 2018)
Several plant phenols have been identified as the bioactive principles of folk herbal medicine with reported antioxidant, antitumor, antiviral, and antibiotic activities. Flavonoids and tannins are among the 15 major structural classes of plant phenolics believed to be responsible for these health benefits (Mahmud et al., 2009). They have also been implicated as being useful in managing ailments such as cardiovascular disease, inflammatory conditions, liver disease, and macular degeneration (Apak et al., 2007; Ayoola et al., 2008). Tannins are polyphenolic metabolites of plants that possess the ability to precipitate gelatin and other proteins from solution (Rahman et al., 2011). Flavonoids are a special class of more than 4,000 members in which the 2 aromatic rings are linked by 3 carbons cyclized with oxygen. Flavonoids are known to be potent antioxidants with diverse mechanisms of action (Silva and Fernandes Júnior, 2010).
The existence of antimicrobial resistance of several pathogenic organisms is one of the issues that has continued to be of concern to scientists and the abuse of antimicrobial drugs is one factor that has contributed to this problem (Ekhaise et al., 2012). Scientists have sustained the search for new and effective medicines targeted against various microbial strains. Plant materials continue to be the preferred starting material for discovery of potential antimicrobial drugs (Akinsulire et al., 2007; Ekhaise et al., 2012) due to the presence of compounds such as phenolics, tannins, and essential oils synthesized in the secondary metabolism of medicinal plants (Nascimento et al., 2000).
The genus Citrus belong to the Rutaceae family and comprises trees, shrubs, and herbs of diverse sizes and uses. Citrus plants are among the world’s most important crops (Mahmud et al., 2009). Citrus fruits and juices are known to contain several bioactive compounds that are important to human health. They include ascorbic acid, pectin, flavonoids, and phenolic compounds (Ghasemi et al., 2009; Oikeh et al., 2015; Jannat et al., 2016; Sir Elkhatim et al., 2018). We have in earlier studies shown that wastes generated from citrus fruits possess useful phytochemicals with antioxidant properties (Oikeh et al., 2013; Oikeh et al., 2014). This study therefore aims to contribute to the drug discovery process by providing information on the phenolic content and antimicrobial activities of oil and non-oil extracts of sweet orange seeds.
MATERIALS AND METHODS
Raw materials
Fresh oranges were purchased from New Benin Market in Benin City, Nigeria. The fruits were washed with distilled water and the peels were removed with the aid of a sharp knife. A juice extractor was used to extract the juice and then the seeds collected. The seeds were pulverized immediately after collection using a sterile mortar and pestle.
Preparation of extract
The pulverized seeds were subjected to Soxhlet extraction for a period of 12 h with 500 mL of ethanol and then concentrated using a rotary evaporator at reduced pressure. The extracts were stored in the refrigerator till required for use.
Determination of total phenolic content
Total phenolic content was determined according to Folin-Ciocalteu reagent method (Cicco et al., 2009). Different concentrations (0.2∼1.0 mg/mL) of gallic acid were prepared in methanol. Concentrations of the extracts were also prepared in methanol. Distilled water (4.5 mL) was added to 0.5 mL of the extract and mixed with 0.5 mL of a 10-fold diluted Folin-Ciocalteu reagent. Subsequently, 5 mL of 7% sodium carbonate and 2 mL of distilled water were added. The mixture stood for 90 min at room temperature before the absorbance was read at 760 nm. All determinations were performed in triplicates with gallic acid utilized as the positive control. The total phenolic content was expressed as gallic acid equivalents (GAE).
Determination of total flavonoid content
Total flavonoid contents were estimated using the method described in Ebrahimzadeh et al. (2008). The extract (0.5 mL of 1 mg/mL) was mixed with 1.5 mL of methanol. To this mixture, 0.1 mL of 10% aluminum chloride was added, followed by 0.1 mL of 1 M potassium acetate and 2.8 mL of distilled water. The mixture was incubated at room temperature for 30 min. The absorbance was measured by a spectrophotometer at 420 nm. The results were expressed as milligrams quercetin equivalents (QE) per gram of extract (mg QE/g extract).
Determination of total tannin content
The total tannin content was determined by the modified method of Polshettiwar et al. (2007). The sample (0.1 mL) was mixed with 0.5 mL of Folin-Denis reagent and 1 mL of 0.5% sodium carbonate (w/v) solution. The mixture was then made up to 5 mL with distilled water. The absorbance was measured at 755 nm within 30 min of reaction. The total tannin in the extract was expressed as the equivalent to tannic acid.
Ferric reducing antioxidant power (FRAP) assay
The Benzie and Strain (1996) protocol with slight modification was employed for this assay. Different concentrations (100∼500 mg/mL) of the concentrates and the standard were serially diluted with distilled water. Then 1 mL of FRAP reagent [200 mL of 300 mM sodium acetate buffer at pH 3.6, 20 mL of 10.0 mM 2,4,6-tripyridyl-S-triazine (TPTZ) solution, 20 mL of 20.0 mM FeCl3・6H2O solution and 24 mL of distilled water] was added to each test tube. The resulting mixture was vigorously shaken and then incubated at 37°C for 4 min. The increase in absorbance at 593 nm was measured and compared with the standard ascorbic acid.
Antimicrobial assay
Test microorganisms: Seven microorganisms were used in this study: consisting of 4 bacterial and 3 fungal strains. Two of the 4 bacterial strains were Gram-positive (Staphylococcus aureus and Enterococcus faecalis) and the other 2 were Gram-negative (Escherichia coli and Salmonella sp). The 3 fungal strains used were Candida albicans, Aspergillus niger, and Penicillum sp. All microorganisms were obtained from Lahor Research and Diagnostic Laboratories, Benin City, Nigeria.
Antimicrobial susceptibility assay: This was carried out using the protocol described by Owoseni and Ajayi (2010). Test organisms were sub-cultured onto fresh suitable broth medium. Broth cultures were then incubated at 37°C till the turbidity of 0.5 McFarland’s standard (1.5×108 colony forming unit/mL) was obtained. Mueller-Hinton agar was used as bacterial medium and Sabouraud agar as fungal medium. All were incubated appropriately as specified for each test organism. The turbidity of the actively growing broth culture was then adjusted with sterile saline to obtain 0.5 McFarland’s standard turbidity. This was used to flood and then drain dry the surface of solid Mueller-Hinton agar plates. Wells of 5 mm in diameter and about 2 cm apart were punched in the culture media with a sterile cork borer. The extracts were thereafter used to fill the boreholes. Each plate was kept in the refrigerator at 4°C for 1 h before incubating at 37°C for 24 h (bacteria) and 72 h (fungi). Zones of inhibition around the wells, measured in millimeters, were used as positive bioactivity.
Minimum inhibitory concentration (MIC)
The organisms that showed susceptibility to the different solvent extracts were introduced into the broths containing different concentrations of each extract (serial dilutions of the extracts corresponding to 200, 100, 50, 25, and 12.5 μg/mL). The tubes were then incubated for 24 h at 37°C. The MIC was taken as the lowest concentration of the extracts that did not permit any visible growth (Owoseni and Ajayi, 2010).
Minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC)
The tubes that showed no turbidity in the MIC test were taken and a loopfull from each tube was streaked onto Mueller-Hinton agar. The plates were incubated for 24 h at 37°C and the absence of growth was observed. The concentration of the extracts that showed no growth was recorded as the MBC/MFC (CLSI, 2008a; CLSI, 2008b; CLSI, 2012).
Statistical analysis
The data were expressed as mean±standard error of mean for three replicates. The data were subjected to one-way analysis of variance and differences between means were determined by Duncan’s multiple range test using SPSS Statistics version 17.0 (SPSS Inc., Chicago, IL, USA) where applicable. P-values <0.05 were regarded as significant.
RESULTS AND DISCUSSION
Phytochemicals possessing antioxidant activities are important for different reasons. They are known to be useful against diseases linked to free radicals, DNA damage and carcinogenesis as well as in the prevention of oxidative reactions in foods. They are also believed to possess anti-inflammatory and antimicrobial properties (Zhang et al., 2015; Lee et al., 2017; Mintie et al., 2020). Several plants and plant parts hold promise as rich reservoirs of antimicrobial compounds and thus, require sustained pharmacological investigations. Citrus sinensis seeds contain phytoconstituents such as flavonoids, saponins, tannins, cardiac glycoside alkaloids, and steroids (Oikeh et al., 2013). Some of these phytoconstituents are known to contain diverse antimicrobial properties (Owoseni and Ajayi, 2010; Dhiman et al., 2012).
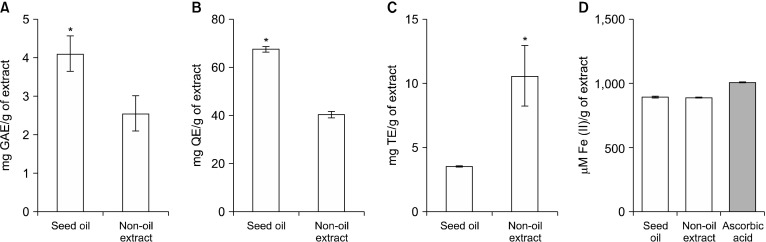
Upon completion of the Soxhlet extraction process, a yield of 15.69% was obtained (Table 1). This consisted predominantly of oil (13.05%) and a denser non-oil portion (2.64%). Both components were separated by draining off the oil and then used for further analysis. The C. sinensis oil seed extract was observed to contain significantly higher amounts of total flavonoids and total phenols compared to the non-oil extract (Fig. 1A and 1B). Total tannins were however significantly higher in the non-oil extract compared to the oil extract (Fig. 1C). The result of the ferric reducing antioxidant potential assay revealed that there was no significant difference between the FRAP values of both seed extracts and the reference antioxidant, ascorbic acid (Fig. 1D). This result suggests that the seeds may possess good reductive properties comparable to ascorbic acid and this could be attributed to the presence of phenolics such as flavonoids and tannins present in both extracts.
Table 1.
Percentage yield of oil and non-oil extracts of dry Citrus sinensis seed
| Yield (%) | |
|---|---|
| Seed oil | 13.05 |
| Non-oil extract | 2.64 |
Fig. 1.
(A) Total phenolic content, (B) flavonoid content, (C) tannin content, and (D) ferric reducing antioxidant potential of oil and non-oil extracts of dry Citrus sinensis seed. Values are mean±SEM (n=3). *Significantly different from the other group at P<0.05. GAE, gallic acid equivalents; QE, quercetin equivalents; TE, tannic equivalents.
Phenolic compounds are known to play important roles in plant defense against pathogens and herbivore predators. They are effective in the control of human pathogenic infections (Jimoh et al., 2008). The antioxidant properties of phenolic compounds are mainly attributed to their redox properties, allowing them to act as reducing agents, hydrogen donors, etc. (Firoozi et al., 2016; Filip, 2017). Flavonoids interfere with the activities of enzymes involved in reactive oxygen species generation and quenching of free radicals (Doughari, 2012). Tannins are astringent in nature. Their antioxidant capacity is linked to the presence of readily oxidizable hydroxyl groups and their degree of polymerization (Smeriglio et al., 2017; Huang et al., 2018).
The antibacterial susceptibility test for oil and non-oil extracts of dry C. sinensis seed against some bacterial strains is shown in Table 2. The observed zones of inhibition varied from one organism to another and at different concentrations of either extract. The Gram-positive strain Staphylococcus aureus showed concentration-dependent susceptibility to both extracts at 200, 100, and 50 μg/mL, the zone of microbial inhibition corresponded to 6, 4, and 2 mm, respectively. However, Enterococcus faecalis was insensitive to either extract at 50 μg/mL but at 200 and 100 μg/mL, the degree of susceptibility of the organism to the seed oil was 8 mm and 6 mm, respectively. Susceptibility was however lower for the non-oil extract (2 and 1 mm, respectively). Inhibition of Escherichia coli and Salmonella sp. growth was observed at 100 and 200 μg/mL for both extracts. Zones of inhibition observed for Salmonella sp. were 16 and 18 mm, respectively for the oil extract and 2 and 4 mm, respectively for the non-oil extract. E. coli inhibition zones were much smaller (2 and 4 mm, respectively for the oil extract; 1 and 2 mm, respectively for the non-oil extract).
Table 2.
Zones of inhibition for some bacterial strains at various concentrations of dry Citrus sinensis seed oil and non-oil extracts (unit: mm)
| Concentration (μg/mL) |
Staphylococcus aureus | Enterococcus faecalis | Escherichia coli | Salmonella sp. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seed oil | Non-oil extract | Seed oil | Non-oil extract | Seed oil | Non-oil extract | Seed oil | Non-oil extract | ||||
| 200 | 6 | 6 | 8 | 2 | 4 | 2 | 18 | 4 | |||
| 100 | 4 | 4 | 6 | 1 | 2 | 1 | 16 | 2 | |||
| 50 | 2 | 2 | − | − | − | − | − | − | |||
Several studies have reported lower zones of inhibition in Gram-negative organisms compared to Gram-positive organisms. This is attributed to the Gram-negative outer membrane functioning as a barrier that prevents the penetration of antimicrobial compounds directed against the bacteria. The presence of hydrolytic enzymes that break down foreign molecules in the periplasmic space is also another factor that limits the susceptibility of Gram-negative bacteria to antimicrobial substances (Holetz et al., 2002; Oikeh et al., 2015). Our results however show that the C. sinensis oil has good inhibitory action against Salmonella sp. The C. sinensis seed oil may therefore be a potent source of antimicrobials targeted against Salmonella sp. The effectiveness of the seed oil against Salmonella sp. is particularly important due to the prevalence of Salmonella sp. in food-borne infections.
Table 3 shows the minimum inhibitory and bacteriocidal concentrations of oil and non-oil extracts for dry C. sinensis seed against some bacterial strains. The MIC is the lowest concentration of a drug that is bacteriostatic, preventing the visible growth of bacteria. MICs are used by diagnostic laboratories mainly to confirm resistance, but mostly as a research tool to determine the activity of novel antimicrobial agents. On the other hand, MBC is the minimum concentration of an antimicrobial agent that can kill 99.9% of bacteria (Andrews, 2001). The MIC values ranged from 50 to 100 μg/mL, while MBC values ranged from 100 to 200 μg/mL for both extracts studied. The results obtained showed that the MIC values for both the oil and non-oil extract were lower than their MBC values. This suggests that both extracts possess bacteriostatic effect at lower concentrations and bacteriocidal effect at higher concentrations.
Table 3.
Minimum inhibitory and bacteriostatic concentrations of dry Citrus sinensis seed oil and non-oil extract against microbial strains (unit: μg/mL)
| Gram-positive | Gram-negative | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | Enterococcus faecalis | Escherichia coli | Salmonella sp. | ||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||||
| Seed oil | 50 | 100 | 100 | 200 | 100 | 200 | 100 | 200 | |||
| Non-oil extract | 50 | 100 | 100 | 200 | 100 | 200 | 100 | 200 | |||
MIC, minimum inhibitory concentration; MBC, minimum bactericidal concentration.
The antifungal activities of the extracts for dry C. sinensis seed revealed that C. albicans was more susceptible to the seed oil compared to non-oil extract with observed zones of inhibition ranging from 4 mm to 20 mm for seed oil extract at different concentrations while non-oil extract recorded 1 mm and 2 mm at 100 and 200 μg/mL, respectively (Table 4). However, A. niger and Penicillum sp. were not susceptible to seed oil at all concentrations studied. The bioactivity of the seed oil against C. albicans is important due to the disease-causing potential of C. albicans. Previous studies of the authors have also shown good susceptibility of C. albicans to some citrus fruit concentrates (Oikeh et al., 2015). Citrus fruits may hold promise as a starting material for the discovery of new anti-Candidiasis agents.
Table 4.
Zones of inhibition of some fungal strains at various concentrations of dry Citrus sinensis seed oil and non-oil extracts (unit: mm)
| Concentration (μg/mL) | Candida albicans | Aspergillus niger | Penicillum sp. | |||||
|---|---|---|---|---|---|---|---|---|
| Seed oil | Non-oil extract | Seed oil | Non-oil extract | Seed oil | Non-oil extract | |||
| 200 | 20 | 2 | − | 8 | − | 4 | ||
| 100 | 8 | 1 | − | 4 | − | 2 | ||
| 50 | 4 | − | − | 2 | − | 1 | ||
Table 5 shows the inhibitory and fungicidal concentrations of oil and non-oil extracts for dry C. sinensis seed against some fungi. MIC values ranged from 50 to 100 μg/mL, while MFC values ranged from 100 to 200 μg/ mL for both extracts studied against the different fungal strains. The seed oil was more effective in inhibiting the growth of C. albicans with MIC and MFC values of 50 μg/mL and 100 μg/mL, respectively, compared to 100 μg/mL and 200 μg/mL observed for the non-oil extract. This result suggests that C. sinensis oil extract is a better fungicidal agent against C. albicans compared to the non-oil extract. MIC and MFC values for the non-oil extract against A. niger and Penicillum sp. were both 50 μg/mL and 100 μg/mL, respectively. MIC and MFC values could not be obtained for the C. sinensis seed oil as these fungal species were not susceptible to the oil.
Table 5.
Minimum inhibitory and fungicidal concentrations of fresh and dry Citrus sinensis seed oil and non-oil extracts against some fungal strains (unit: μg/mL)
| Candida albicans | Aspergillus niger | Penicillum sp. | ||||||
|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | |||
| Seed oil | 50 | 100 | − | − | − | − | ||
| Non-oil extract | 100 | 200 | 50 | 100 | 50 | 100 | ||
MIC, minimum inhibitory concentration; MFC, minimum fungicidal concentration.
In conclusion, the results of this study show differences in the antimicrobial activities of the oil and non-oil extracts against the bacterial and fungal strains studied. This study has provided preliminary in vitro data on the antimicrobial potential of extracts obtained from seeds of C. sinensis. The results in this study provide information on potential antibacterial and antifungal leads. More extensive studies for the potential identification and isolation of lead compounds as well as confirmatory in vivo studies will be carried out.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Akinsulire OR, Aibinu IE, Adenipekun T, Adelowotan T, Odugbemi T. In vitro antimicrobial activity of crude extracts from plants Bryophyllum pinnatum and Kalanchoe crenata. Afr J Tradit Complement Altern Med. 2007;4:338–344. doi: 10.4314/ajtcam.v4i3.31227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Apak R, Güçlü K, Demirata B, Ozyürek M, Celik SE, Bektaşoğlu B, et al. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007;12:1496–1547. doi: 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoola GA, Folawewo AD, Adesegun SA, Abioro OO, Adepoju-Bello AA, Coker HAB. Phytochemical and antioxidant screening of some plants of apocynaceae from South West Nigeria. Afr J Plant Sci. 2008;2:124–128. [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Cicco N, Lanorte MT, Paraggio M, Viggiano M, Lattanzio V. A reproducible, rapid and inexpensive Folin-Ciocalteu micromethod in determining phenolics of plant methanol extracts. Microchem J. 2009;91:107–110. doi: 10.1016/j.microc.2008.08.011. [DOI] [Google Scholar]
- CLSI, author. CLSI document M07-A9. 9th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard; pp. 1–63. [Google Scholar]
- CLSI, author. CLSI document M38-A2. 2nd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2008a. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard; pp. 1–35. [Google Scholar]
- CLSI, author. CLSI document M27-A3. 3rd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2008b. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard; pp. 1–24. [Google Scholar]
- Dhiman A, Nanda A, Ahmad S, Narasimhan B. In vitro antimicrobial status of methanolic extract of Citrus sinensis Linn. fruit peel. Chron Young Sci. 2012;3:204–208. doi: 10.4103/2229-5186.99573. [DOI] [Google Scholar]
- Doughari JH. Phytochemicals: extraction methods, basic structures and mode of action as potential chemotherapeutic agents. In: Rao N, editor. Phytochemicals: A Global Perspective of Their Role in Nutrition and Health. IntechOpen; London, UK: 2012. pp. 1–32. [Google Scholar]
- Ebrahimzadeh MA, Pourmorad F, Bekhradnia AR. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr J Biotechnol. 2008;7:3188–3192. [Google Scholar]
- Ekhaise FO, Ofoezie VG, Enobakhare DA. Antibacterial properties and preliminary phytochemical analysis of methanolic extract of mistletoe (Tapinanthus bangwensis) Bayero J Pure Appl Sci. 2010;3:65–68. doi: 10.4314/bajopas.v3i2.63223. [DOI] [Google Scholar]
- Filip S. Basil (Ocimum basilicum L.) a source of valuable phytonutrients. Int J Clin Nutr Diet. 2017;3:118. doi: 10.15344/2456-8171/2017/118. https://doi.org/10.15344/2456-8171/2017/118 . [DOI] [Google Scholar]
- Firoozi S, Jamzad M, Yari M. Biologically synthesized silver nanoparticles by aqueous extract of Satureja intermedia C.A. Mey and the evaluation of total phenolic and flavonoid contents and antioxidant activity. J Nanostruct Chem. 2016;6:357–364. doi: 10.1007/s40097-016-0207-0. [DOI] [Google Scholar]
- Ghasemi K, Ghasemi Y, Ebrahimzadeh MA. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak J Pharm Sci. 2009;22:277–281. [PubMed] [Google Scholar]
- Holetz FB, Pessini GL, Sanches NR, Cortez DA, Nakamura CV, Filho BP. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem Inst Oswaldo Cruz. 2002;97:1027–1031. doi: 10.1590/S0074-02762002000700017. [DOI] [PubMed] [Google Scholar]
- Huang Q, Liu X, Zhao G, Hu T, Wang Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr. 2018;4:137–150. doi: 10.1016/j.aninu.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannat S, Ali MY, Kim HR, Jung HA, Choi JS. Protective effects of sweet orange, unshiu mikan, and mini tomato juice powders on t-BHP-induced oxidative stress in HepG2 cells. Prev Nutr Food Sci. 2016;21:208–220. doi: 10.3746/pnf.2016.21.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimoh FO, Adedapo AA, Aliero AA, Afolayan AJ. Polyphenolic contents and biological activities of Rumex ecklonianus. Pharm Biol. 2008;46:333–340. doi: 10.1080/13880200801887765. [DOI] [Google Scholar]
- Lee MT, Lin WC, Yu B, Lee TT. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—a review. Asian-Australas J Anim Sci. 2017;30:299–308. doi: 10.5713/ajas.16.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud S, Saleem M, Siddique S, Ahmed R, Khanum R, Perveen Z. Volatile components, antioxidant and antimicrobial activity of Citrus acida var. sour lime peel oil. J Saudi Chem Soc. 2009;13:195–198. doi: 10.1016/j.jscs.2009.03.001. [DOI] [Google Scholar]
- Mintie CA, Singh CK, Ahmad N. Whole fruit phytochemicals combating skin damage and carcinogenesis. Transl Oncol. 2020;13:146–156. doi: 10.1016/j.tranon.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento GGF, Locatelli J, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz J Microbiol. 2000;31:247–256. doi: 10.1590/S1517-83822000000400003. [DOI] [Google Scholar]
- Oikeh EI, Omoregie ES, Oviasogie FE, Oriakhi K. Phytochemical, antimicrobial, and antioxidant activities of different citrus juice concentrates. Food Sci Nutr. 2016;4:103–109. doi: 10.1002/fsn3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikeh EI, Oriakhi K, Omoregie ES. Phenolic content and in vitro antioxidant activities or sweet orange (Citrus sinensis L.) fruit wastes. Arch Bas App Med. 2014;2:119–126. [Google Scholar]
- Oikeh EI, Oriakhi K, Omoregie ES. Proximate analysis and phytochemical screening of Citrus sinensis fruit wastes. The Bioscientist. 2013;1:164–170. [Google Scholar]
- Owoseni AA, Ajayi A. Antimicrobial properties of ethanolic and aqueous extracts of Cymbopogon citratus on selected bacteria and fungi. J Med Appl Biosci. 2010;2:64–73. [Google Scholar]
- Polshettiwar SA, Ganjiwale RO, Wadher SJ, Yeole PG. Spectrophotometric estimation of total tannins in some ayurvedic eye drops. Indian J Pharm Sci. 2007;69:574–576. doi: 10.4103/0250-474X.36949. [DOI] [Google Scholar]
- Rahman S, Parvez AK, Islam R, Khan MH. Antibacterial activity of natural spices on multiple drug resistant Escherichia coli isolated from drinking water, Bangladesh. Ann Clin Microbiol Antimicrob. 2011;10:10. doi: 10.1186/1476-0711-10-10. https://doi.org/10.1186/1476-0711-10-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NCC, Fernandes Júnior A. Biological properties of medicinal plants: a review of their antimicrobial activity. J Venom Anim Toxins Incl Trop Dis. 2010;16:402–413. doi: 10.1590/S1678-91992010000300006. [DOI] [Google Scholar]
- Sir Elkhatim KA, Elagib RAA, Hassan AB. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci Nutr. 2018;6:1214– 1219. doi: 10.1002/fsn3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeriglio A, Barreca D, Bellocco E, Trombetta D. Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. Br J Pharmacol. 2017;174:1244–1262. doi: 10.1111/bph.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. 2018;5:93. doi: 10.3390/medicines5030093. https://doi.org/10.3390/medicines5030093 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20:21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]