Abstract
The primary function of the arterial microvasculature is to ensure that regional perfusion of blood flow is matched to the needs of the tissue bed. This critical physiological mechanism is tightly controlled and regulated by a variety of vasoactive compounds that are generated and released from the vascular endothelium. Although these substances are required for modulating vascular tone, they also influence the surrounding tissue and have an overall effect on vascular, as well as parenchymal, homeostasis. Bioactive lipids, fatty acid derivatives that exert their effects through signaling pathways, are included in the list of vasoactive compounds that modulate the microvasculature. Although lipids were identified as important vascular messengers over three decades ago, their specific role within the microvascular system is not well defined. Thorough understanding of these pathways and their regulation is not only essential to gain insight into their role in cardiovascular disease but is also important for preventing vascular dysfunction following cancer treatment, a rapidly growing problem in medical oncology. The purpose of this review is to discuss how biologically active lipids, specifically prostanoids, epoxyeicosatrienoic acids, sphingolipids, and lysophospholipids, contribute to vascular function and signaling within the endothelium. Methods for quantifying lipids will be briefly discussed, followed by an overview of the various lipid families. The cross talk in signaling between classes of lipids will be discussed in the context of vascular disease. Finally, the potential clinical implications of these lipid families will be highlighted.
Keywords: epoxyeicosatrienoic acids, lysophospholipids, microvasculature, prostanoids, sphingolipids
INTRODUCTION
Bioactive lipids have emerged as critical signaling messengers involved in both the prevention and progression of disease (95). Over the last three decades, a vast amount of data has been generated regarding the role of various types of lipids within the vasculature, mostly in the large conduit arteries. This work has concentrated on identifying specific lipid products and their role in cellular metabolism, in both endothelial and smooth muscle cells. As more information is collected on downstream signaling events and how these lipids interact with one another, a picture is emerging that lipids have a significant impact on vascular formation, tone, and overall homeostasis (35, 38, 48). Despite the well-recognized role for lipids in conduit artery pathophysiology, there exists a large knowledge gap in our understanding of how lipids modulate vascular tone in the microcirculation, the network of small resistance arterioles, capillaries, and venules. One reason for this gap in knowledge is that unlike conduit arteries, which develop marked phenotypical changes with disease (e.g., atherosclerosis) and can easily be seen angiographically, the microvasculature does not develop clear lesions and is not visible by traditional imaging techniques. Interestingly, microvascular dysfunction precedes many chronic diseases, including coronary artery disease (CAD) and prominently contributes to the morbidity and prognosis of atherosclerosis, even more so than does the extent of large vessel disease (155). Revealing how bioactive lipids contribute to (dys)function of the microvasculature may bring new insights into the prevention, diagnosis, and treatment of cardiovascular disease.
Bioactive lipids have also been increasingly employed in cancer therapy. Several new cancer treatments, including some still in development, specifically target lipid mediators. While these novel treatments show promise for defeating cancer, the resulting alteration of the lipid profile can produce collateral damage to the vascular endothelium and other vascular cell types. To prevent an increase in the prevalence of cardiovascular disease among cancer survivors, efforts need to be directed toward discovering how lipid pathways can be manipulated to avoid replacing one disease with another. This will prove to be a difficult task, as identification and quantification of lipids are considered more challenging compared with measuring protein or DNA.
This review will focus on how bioactive lipids modulate the endothelium and microcirculation. Popular methods for measuring bioactive lipids will be discussed. We will then highlight various families of lipids, including arachidonic acid derivatives, lysophospholipids, and sphingolipids, concentrating on their contribution to vascular health and disease. Finally, interactions between lipid families will be reviewed as well as the clinical implications of biological lipids within the microvasculature.
QUANTIFICATION OF BIOACTIVE LIPIDS
The structural nature of lipids creates a challenge for precise quantification, particularly in small biological samples, such as arterioles. The lipid extraction process from tissue samples requires multiple fractionation steps and often results in a low yield and underestimation of lipid levels. Depending on the lipid of interest, the fractionation protocol may use organic solvents, inorganic solvents, or a combination of the two which often leads to the contamination and inaccuracy of results. The storage method used can also produce a source of error. For instance, activation of platelets upon contact with plastic tubes can artificially increase lipid levels (54). EDTA or citrate is needed to mitigate these effects.
Adding complexity to quantification are the multiple isoforms that exist for each individual lipid. A good example is ceramide, a sphingolipid that functions as a critical cell signaling molecule during times of stress. To date, over 28 enzymes are involved in the metabolism of ceramide, resulting in the possibility of generating more than 200 structurally distinct species (47). Likewise, lysophospholipids, such as lysophosphatidic acid (LPA), can vary by acyl chain length, as well as degree of saturation. While the term LPA typically refers to 18:1 oleoyl-LPA, multiple other isoforms have been identified in plasma and serum, most notably 18:2, 20:4, 16:0, and 16:1 (120). And finally, the four cis double bonds of arachidonic acid allow it to react with three oxygenases to form different subtypes of eicosanoids, including prostaglandins, epoxyeicosatrienoic acids, and leukotrienes. Therefore, while methods that do not require lipid extraction may result in higher yield, these methods often lack specificity to distinguish between isoforms within the same lipid family. For these reasons, the method of choice for lipid measurement should be chosen on the basis of the specific question being addressed.
Some of the earliest bioassays for lipid quantification relied on comparison of biological activity with the assumption that activity was directly correlated to concentration (147). These results were expressed as “lipid-equivalent levels.” Unfortunately, this methodology does not account for volume of distribution, activity, and extent of metabolite formation, binding affinity, and membrane permeability, each of which needs to be considered for precise measurement. Relevant to the study of the microcirculation, more recent methods have been developed that rely on radiolabeling, fluorescence detection, and measurement of absorbance (colorimetric assays) to quantify lipids of interest. While these methods will not be extensively reviewed here, brief explanations, as well as advances and pitfalls, for each of these methods will be briefly mentioned below and are summarized in Table 1. The reader interested in a more detailed explanation of strengths and weaknesses of these assays is referred to several excellent citations (1, 61, 86, 99, 145).
Table 1.
Various methods to measure bioactive lipids
| Method | Arachidonic Acid | Sphingolipids | (Lyso)phospholipids | Advantages | Disadvantages |
|---|---|---|---|---|---|
| 1. Radioenzymatic | Remesha et al. (5) Mauco et al. (31) |
Perry et al. (15) Olivera et al. (32)* Brizuela and Cuvilllier (33) |
Saulnier-Blache et al. (14) Kishimoto et al. (7) |
Linear detection, high sensitivity, available for different lipid families | Designated area for working with radioactive elements, no specificity |
| 2. Colorimetric | N/A | Brizuela and Cuvilllier (33) | Kishimoto et al. (7, 18) Hosogaya et al. (8) |
No lipid extraction before assay, linear detection | No specificity |
| 3. Fluorometric | Nakagawa and Waku (34)# | N/A | Alpturk et al. (16) | Sensitivity, wide detection range | Lipid extraction before assay, indirect measurements, low sample yields, no specificity |
| 4. ELISA | aSee note. | bSee note. | Balood et al. (35) dSee note. |
Readily available, low complexity | No specificity |
| 5. GC ± MS | Gerber et al. (36) de Silva et al. (37) Hammerstrom et al. (38) |
Raith et al. (39) Kuksis and Myher (40) |
Tokumura et al. (41) Bese et al. (42) Sugiura et al. (43) |
High sensitivity and specificity | Multistep, technically challenging |
| 6. TLC ± MS | Neufeld and Majerus (44) | Olivera et al. (32) Brizuela and Cuvilllier (33) |
Sutphen et al. (45) Xiao et al. (46) |
High sensitivity, rapid analysis time | Multistep, limited resolution capability |
| 7. LC ± MS | Perret et al. (47) Nakagawa and Waku (34)# |
cSee note. | Baker et al. (20, 48) | High sensitivity, minimizes ion suppression effect | Multi-step, expensive, technically hallenging, potential elution of targets |
| 8. LC + MS/MS | Gachet et al. (49) Bian et al. (50) |
Blachnio-Zabielska et al. (51) Mano et al. (52) |
Scherer et al. (53) Liebisch et al. (54) Shan et al. (22) |
Gold-standard, sensitive, specific | Multi-step, expensive, technically challenging, carryover contamination with crude samples |
| 9. MALDI – TOF + MS |
N/A | Fujiwaki et al. (28) Morishige et al. (29) |
Morishige et al. (29) | Rapid turnaround time, little to no fragmentation of species, small sample quantity required | Multistep lipid extraction process, labor-intensive |
Methods 1–4 indicate methods that do not distinguish/separate the different isoforms in the lipid family. ELISA, enzyme-linked immunosorbent assay; GC, gas chromatography; LC, liquid chromatography; MS, mass spectrometry; MS/MS, tandem mass spectrometry; MALDI-TOF, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; N/A, not applicable; TLC, thin-layer chromatography.
Enzyme-linked immunosorbent assay (ELISA) kits for AA measurements are available for purchase; no references found.
Antibodies for ceramide(s) and sphingosine(s) are available for purchase, and multiple publications are available where the antibody was used for ELISA, IHC, or flow cytometry.
High-performance liquid chromatography (HPLC) with single mass spectrometry analysis is rarely performed.
ELISA kits are available from commercial venders; no references found.
Radiolabeled element was used before thin-layer chromatography separation.
HPLC was combined with fluorescence measurements.
Radioenzymatic Assay
The radiometric assay labels the lipid by incorporation of a radioactive isotope, which is then typically measured using a scintillation counter. Once samples are separated using either thin-layer chromatography (TLC) or a two-dimensional TLC, autoradiography is used to localize the radiolabeled lipids, which are then removed and analyzed using the scintillation method. In 2000, Saulnier-Blache et al. (119) described a radioenzymatic assay to measure LPA. Using a recombinant LPA acetyl transferase (LPAAT) and [14C] oleoyl-CoA, [14C] phosphatidic acid could be measured with the assumption that formation of phosphatidic acid directly correlates to total amount of LPA. Although this method can detect lipids like LPA linearly at concentrations as low as 200 picomolar, this assay unfortunately does not distinguish between various isoforms, for instance alkyl- versus acyl-LPA. Likewise, following lipid extraction and TLC, ceramides can also be quantified using a scintillation counter (126). Unfortunately, as with LPA measurement, this approach fails to differentiate one ceramide isoform from another.
Fluorometric Assay
On the basis of the concept that substrates and products have a difference in fluorescence spectra, absorption of energy will cause an emittance of light at a different wavelength. In this fashion, the fluorometric method can be used to analyze the enzymatic formation of hydrogen peroxide (H2O2) as a readout to measure LPA (7, 10). The fluorometric assay requires a lipid extraction step before the reaction, which can result in a low sample yield. One advantage is that lipid extraction techniques are not necessary before using a colorimetric assay. Using the same approach, Kishimoto et al. (64, 65) demonstrated that H2O2 derived from LPA could also be measured colorimetrically and was shown to be a more sensitive assay compared with fluorometry.
Mass Spectrometry
The current gold standard for bioactive lipid quantification is liquid chromatography-electron spray ionization coupled to tandem mass spectrometry (LC-ESI-tandem MS) (12, 13, 88, 93, 94, 127, 151). The main advantage of mass spectrometry is the ability to differentiate complex lipid species. Further, this technique is able to detect lipids at orders of magnitude lower than traditional techniques (approximately femtomolar or less) and can be used on samples with as few as 106 cells, which is important when studying microvessels. The detection of different subspecies of lipids allows for identification of variations within specific lipid categories. This method, however, comes with caveats. First, proper calibration before sample analysis is crucial, considering there is often a high amount of variability between biological, as well as technical replicates. matrix assisted laser desorption ionization-time of flight (MALDI-TOF), as opposed to LC-MS/MS, has been proposed as another option to analyze different lipid species (41, 91). Here, samples are prepared as a mixture of analyte and a special matrix compound, which upon absorption of energy, places the analytes into a gas phase before tandem MS. The choice of matrix, which is based on the specific suspecies of lipid being analyzed, ensures minimal fragmentation and maximal sensitivity (91, 134). Unfortunately, the cost to analyze lipid samples using LC MS/MS is high and may require transfer of the sample to another institution that specializes in identification of lipids using this technique.
The ideal assay to quantify biological lipids would be affordable, high-throughput, easy to execute, and sensitive enough to detect not only target lipids in small sample volumes, but also capable of distinguishing each subspecies within a particular lipid family. An assay with all of these properties has yet to be developed. Thus, analysis of samples of limited volume may yield inconsistent results when using a sophisticated method like mass spectrometry, and show undetectable levels using other methods described above.
VASOACTIVE LIPIDS
Derivatives of Arachidonic Acid
Once released from membranes by phospholipase A2 (PLA2), arachidonic acid (AA) is rapidly metabolized to form metabolites collectively known as eicosanoids (50). Cyclooxygenase (COX) enzymes are capable of converting AA to prostanoids such as prostaglandin H2 (PGH2), prostacyclin (PGI2), or thromboxane A2 (TXA2). In a separate pathway, AA can be enzymatically converted by lipoxygenases (LOXs) to form hydroperoxyeicosatetraenoic acids (HPETEs), which are rapidly metabolized to hydroxyeicosatetraenoic acids (HETEs), dihydroxyeicosatetraenoic acids (DiHETEs), or leukotrienes. Cytochrome-P450 enzymes can also oxidize AA via two different reactions to form either epoxyeicosatrienoic acids (EETs), or HETEs. Olefin epoxidation promotes formation of EETs, (5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET), whereas hydroxylation favors generation of 16–20-HETEs. (see Fig. 1). All of these lipid products contribute to vascular tone by acting as either vasodilators (EETs) or constrictors (HETEs). Most of these enzymes are constitutively expressed in cells where AA concentrations are very low. Thus, production of the above described metabolites is regulated by activity of phospholipases to cleave the AA substrate from cell membranes.
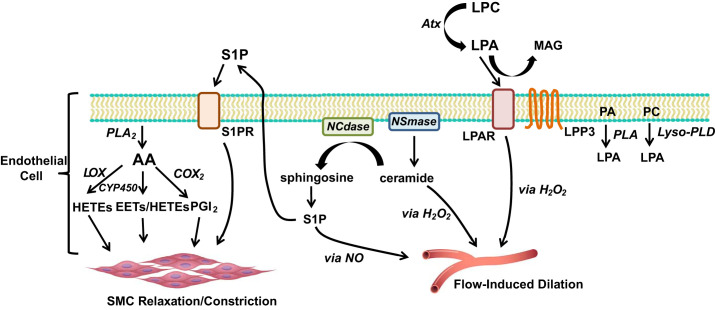
Fig. 1.
Role of bioactive lipids in vascular tone. AA is formed via PLA2, which can subsequently be converted to a HETE (via LOX), an EET (via CYP-450), or to PGI2 (via COX2). EETs can be further metabolized by soluble epoxide hydrolase (sEH) to dihydroxyeicosatrienoic acids (DHETs). AA derivatives are capable of causing either smooth muscle cell relaxation or constriction, depending on specific isoform and concentration. Ceramide, formed by NSmase, promotes H2O2-dependent flow-induced dilation (FID). However, if hydrolyzed to sphingosine by NCdase, it can be further converted to S1P, which promotes NO-dependent FID. Alternatively, S1P can transport to the extracellular space, where it can bind to S1PRs. Activation of S1PR results in SMC relaxation and vasodilation. PA and PC can be converted to LPA by PLA and lyso-PLD. Atx converts LPC to LPA, which then can activate LPARs, or, be degraded by LPP3 to form MAG. Downstream signaling from LPARs promotes H2O2-dependent FID in the microcirculation. AA, arachidonic acid; Atx, autotaxin; HETE, hydroxyeicosatetraenoic acid; LPAR, lysophosphatidic acid receptor; LPP3, lipid phosphate phosphatase 3; COX2 cyclooxygenase 2; CYP-450, cytochrome-P450; EETs, epoxyeicosatrienoic acids; LOX, lipoxygenase; LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; Lyso-PLD, lysophospholipase D; MAG, monoacylglycerol; NCdase, neutral ceramidase; NO, nitric oxide; NSmase, neutral sphingomyelinase; PA, phosphatidic acid; PC, phosphatidylcholine; PGI2, prostacyclin; PLA, phospholipase; SMC, smooth muscle cell; S1P, sphingosine-1-phosphate; S1PR, sphingosine-1-phosphate receptor.
Two COX isoforms exist (COX-1 and COX-2) that convert AA ultimately to PGI2 or TXA2. In endothelial cells, the majority of PGI2 is generated at the cell surface and in addition to activating cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA), in paracrine fashion can induce hyperpolarization of smooth muscle cells, resulting in vasodilation (104). The role of PGI2 in modulating vascular tone is age-dependent. Animal studies have shown that the primary mediator formed in response to shear stress changes from PGI2 to nitric oxide (NO) from infancy to adulthood, respectively (136). Beyer et al. (16) translated these findings to humans, showing that arterioles collected from children (ages 0 to 18 yr) lose the ability to vasodilate in response to an increase in flow (flow-induced dilation; FID) if first treated with the cyclooxygenase inhibitor, indomethacin, whereas inhibition of nitric oxide synthase (NOS) with l-NAME had little effect. Inhibition of PGI2 synthase with trans-2-phenyl cyclopropylamine (TPC) also resulted in a complete loss of dilation to flow, implicating PGI2 as necessary for FID in children. In contrast, L-NAME severely diminishes the ability of healthy human adult microvessels to dilate in response to flow. This suggests that although NO primarily mediates FID in healthy human adults, formation of PGI2 is mechanistically critical for FID in children and, therefore, may importantly regulate organ perfusion. Although the significance of differing vasodilator mediators at different stages of life is unclear, these data do speak to the versatility and importance of endothelium-dependent dilation in the microcirculation. The mechanism of this switch in dilators is an area for future investigation.
The antithrombotic effect of PGI2 through inhibition of platelet aggregation and its role as a vasodilator sharply contrasts to the vasoconstrictive, platelet-activating effects of TXA2 (100). Contributing factors that determine the fate of PGH2 (whether it is converted to PGI2 or TXA2) include the specific vascular bed and the oxidative state of the cell (143). Healthy endothelial cells constitutively express the COX-1 isoform that primarily forms PGI2, whereas with aging or disease, expression of the induced isoform, COX-2, predominates with the resulting generation of TXA2 (129). Prostanoids and TXA2 are also capable of binding to similar receptors, albeit with different affinities. Many of the biological effects of PGI2 can be attributed to activation of prostanoid PGI2 receptors located within the cell membranes of both endothelial and smooth muscle cells (36). PGI2, as well as other prostanoids, namely prostaglandin E2 (PGE2), also bind to one of four E series receptors (EP1–4) (111). Currently, only EP3 and EP4 have been identified within the vasculature (85).
Metabolites of the LOX pathway can also cause constriction or dilation (25). Depending on what major isoform of LOX is activated (5-LOX, 12-LOX, or 15-LOX) (67, 110, 152), a specific HPETE will undergo rapid reduction to form a HETE. 5-LOX metabolites tend to promote constriction as opposed to dilation. Activation of the 5-LOX pathway was shown to be necessary for ACh-induced aortic constriction in a hypertensive rat model (73). The 12-LOX pathway predominantly produces 12-HETE, which is capable of inducing smooth muscle hyperpolarization and relaxation by activating large-conductance KCa (BKCa) channels (89). This is in sharp contrast to 12-HETE-induced constriction that is observed in canine renal arcuate arteries (81). Within the vascular endothelium, 15-LOX metabolizes AA to 15-HPETE and then subsequently to 15-HETE. Interestingly, in canine coronary, splenic, femoral, and renal arteries, both 15-HETE and 15-HPETE cause dilation at low concentrations but induce constriction at elevated concentrations (140). It is thought that the constriction is due to direct activation of thromboxane A2 receptors on smooth muscle cells.
Other AA derivatives include epoxyeicosatrienoic acids, or EETs, produced via cytochrome-P450 (CYP-450) epoxygenase enzymes. Four regioisomers of EETs are produced (5,6-, 8,9-, 11,12-, and 14,15-EET) based on epoxidation of AA at one of four double bonds. Rosolowsky and Campbell (114) used reverse-phase- and HPLC to collect radioactive metabolites of AA from media that had been exposed to cultured bovine artery endothelial cells. All four types of EETs were identified, and it was concluded that CYP-450 enzymes were, in fact, present in endothelial cells and capable of producing these lipid derivatives. EETs have been demonstrated to induce vasodilation in an array of vascular beds, including, but not limited to, mesenteric (116), cerebral (32), renal (22), and coronary conduit arteries (37). Studies from animal models show that all regioisomers of EETs act as endothelium-derived hyperpolarizing factors (EDHF) in smooth muscle cells of bovine coronary arterioles (20). Our work has shown that EETs may be a critical regulator of human coronary vascular resistance, evidenced by the ability of three EET regioisomers (8,9-, 11,12-, and 14,15-EET) to induce a concentration-dependent dilation, which was blocked by use of iberiotoxin, suggesting a role for BKCa channels (70). CYP-450 derivatives may be of particular importance in the presence of CAD, as regulation of coronary perfusion becomes increasingly dependent on H2O2 rather than NO. Using a tandem-bioassay preparation, Larsen et al. (70) showed that bradykinin-induced relaxation of human coronary microvessels was reduced by catalase but was not reduced in the presence of 14,15-epoxyeicosa-5(Z)-enoic acid (EEZE), 6-(2-propargyloxyphenyl)hexanoic acid, an EET receptor blocker, sulfaphenazole, or iberiotoxin. However, during exposure to catalase, which partially reduces BKCa dilation, these EET inhibitors abolished the residual dilation, suggesting that H2O2 has an inhibitory effect on CYP epoxygenases and regulates bioavailability of EETs.
In some vessels, EET-induced activation of BKCa channels on smooth muscle cells results from activation of transient receptor potential vanilloid type 4 (TRPV4) channels, which produce calcium sparks, leading to activation of BKCa channels (39). More recent investigations have focused on identifying the EET receptors responsible for these vascular effects. G protein-coupled receptor 40 (GPR40) has emerged as a potential candidate (80), although more recent studies in pancreatic β-cells suggest that this receptor is not specific, binding also to free fatty acids (79). Although GPR40 may not directly mediate the vasodilatory actions of EETs, this receptor potentially contributes to intercellular communication within the vasculature. Using photoaffinity labeling, Chen et al. demonstrated that a potential 47-kDa receptor candidate found on vascular smooth muscle cells, as well as endothelial cells, can bind EETs at nanomolar concentrations to relax preconstricted bovine coronary arteries, suggesting that EET receptors may exhibit varying affinities for ligand binding (26).
EETs undergo rapid metabolism by endothelial and smooth muscle cell-soluble epoxide hydrolase (sEH) to form dihydroxyeicosatrienoic acids (DHETs), which compared with EETs, are less potent vasodilators in large conduit arteries (37, 70) In larger arteries, EETs cause vasodilation in nanomolar concentrations, whereas micromolar concentrations of DHETs are required to see the same effect. Oltman et al. (103) highlighted the importance of DHETs in the coronary microcirculation as only picomolar amounts of both EETs and DHETs were necessary to induce dilation in canine coronary arterioles. Despite evidence that DHETs can contribute to dilation, sEH inhibitors (sEHIs) have emerged as a promising strategy to combat cardiovascular disease (58). The sEHI, N,N′-dicyclohexylurea, lowered ANG II-induced high blood pressure in the spontaneously hypertensive rat (59). When administered orally, the sEHI, 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA) also lowered blood pressure in both rat and the mouse models of hypertension (60). sEHIs may also mitigate brain damage from stroke-induced cerebral ischemia (30). While these inhibitors have emerged as a potential new therapy for the treatment of many cardiovascular-related diseases, EETs also promote tumor growth (150) and can exacerbate pulmonary hypertension (108), therefore limiting their therapeutic utility.
Sphingolipids
Sphingolipids, named after the Greek mythical creature, the Sphinx, due to its enigmatic nature, constitute one of the major classes of bioactive lipids (49). Sphingosine, an unsaturated hydrocarbon, is the backbone from which all sphingolipids are constructed. Sphingolipids can be generated de novo or recycled by enzymes known as sphingomyelinases (47, 56). Three sphingomyelinases have been discovered to date and are characterized on the basis of the pH of the environment in which they are active. Neutral sphingomyelinase is located within the plasma membrane, endoplasmic reticulum, Golgi, as well as the nucleus and is most efficient in neutral pH conditions (138). Acid sphingomyelinase is sequestered within acidic lysosomes (109), whereas alkaline sphingomyelinase is limited to expression in the intestines and bile ducts (31). Sphingomyelinases are mainly responsible for hydrolyzing sphingomyelin into ceramide and phosphorylcholine (84). An array of enzymes exists within the cytosol capable of altering the sphingolipid balance within the cell. Converting one type of sphingolipid to another may have profound effects on cellular physiology, as each lipid moiety elicits very different effects (135).
Neutral ceramidase, an enzyme that hydrolyzes ceramide into sphingosine, is a case in point. Ceramide is a bioactive sphingolipid that is elevated in the plasma of patients with cardiovascular disease, and elevated levels are predictive of major adverse cardiovascular events and even death (52, 69). Our laboratory has shown that ceramide exerts detrimental effects on the human microvasculature. When a human arteriole collected from a healthy patient is exposed to exogenous ceramide, a change in the mechanism of FID occurs similar to that observed in a patient with CAD (78). More specifically, ceramide changes the mediator of FID from NO to mitochondria-derived H2O2 (40). Conversely, inhibition of neutral sphingomyelinase restores NO-dependent vasodilation in microvessels collected from patients with coronary atherosclerosis, similar to what is observed in patients without CAD. Adverse effects of ceramide can be mitigated by conversion to sphingosine via neutral ceramidase. A role for neutral ceramidase in the development of microvascular dysfunction, and cardiovascular disease, is a potential area for investigation.
As an alternative to sphingomyelinase, sphingosine can be phosphorylated by sphingosine kinases (SpK1 and SpK2) to form sphingosine-1-phosphate (S1P). Of all the sphingolipids, S1P has been the most studied within the vasculature. Unlike ceramide and other sphingolipids, S1P participates in “inside-out signaling”, where upon secretion from an endothelial cell, it is able to bind and activate extracellular S1P receptors located within the endothelial plasma membrane (133). For instance, once S1P is exported out of an endothelial cell via ABC transporters (e.g., Spinster 2), it can then bind to carrier proteins, such as high-density lipoprotein (HDL), or albumin, allowing it to be recognized by S1P receptor and act in an autocrine or paracrine fashion throughout the vasculature (98). There are currently five known S1P receptors (S1PR1–5) that are coupled to heterotrimeric G proteins (83), allowing for regulation of multiple physiological processes, including but not limited to vascular maturation (6), angiogenesis (72), endothelial cell barrier function (87), and vasodilation (21). S1PR1 and S1PR3 are expressed in vascular endothelial cells (63), whereas S1PR2 and S1PR3 are primarily found on vascular smooth muscle cells (115). Tong et al. (139) demonstrated that PGI2 promotes binding of S1P to high-density lipoprotein (HDL) and exerts a protective effect along the endothelium.
Adding to the complexity of the sphingolipid pathway is the fact that while ceramide promotes mitochondrial dysfunction (42), formation of H2O2 as an FID mediator, and cell death (153), S1P has opposing effects and promotes cellular survival and increases intracellular levels of NO (132). The sphingolipid “rheostat” of a cell refers to the critical balance of prosurvival versus antisurvival sphingolipids, with the ratio ultimately deciding cellular fate (28). Our group has recently shown that this yin and yang effect occurs with respect to microvascular dilator mechanisms. Exogenous S1P restores NO-dependent FID in arterioles collected from patients with known CAD. Further, inhibition of sphingosine kinase transitions the mediator of FID from NO to H2O2 in vessels from healthy patients, similar to the effects of direct application of ceramide (123). On the basis of these findings, better understanding of how this lipid ratio is regulated within the vasculature could identify new approaches to improving vascular health.
Lysophospholipids
Lysophosphatidic acid (LPA) is composed of a glycerol backbone, a phosphate group, and an acyl chain that can vary in length and degree of saturation (12, 14). Generation of LPA can occur both intracellularly and extracellularly. LPA detected in biological fluids is the result of the action of one of two enzymes, phospholipases (PLAs), which convert phosphatidic acid to LPA, and lysophospholipase D (lyso-PLD), which removes the polar head group (choline) from lysophosphatidylcholine (LPC) to produce LPA (3, 141). The majority of LPA production in the circulation happens extracellularly during the process of mild oxidation of low-density lipoprotein (LDL) (51, 130). The dominant plasma isoform of lyso-PLD enzyme, also known as autotaxin (ATX), is a secreted form of lyso-PLD. This enzyme was originally discovered by Liotta and colleagues, who were studying factors that regulate cell motility in cancer (96).
The biological activity of LPA is mediated by six cognate receptors LPA1–LPA6 (27, 154). Although the expression of LPA receptors has been examined in different species and tissues, little information is available regarding the expression pattern of these receptors in the human macrovasculatures or microvasculatures. Aldi et al. (4) observed differential expression of LPA receptors in conduit vessels from patients with or without coronary plaque. LPA2, LPA5, and LPA6 receptors were all upregulated in vessels with plaques. LPA1 receptor was downregulated in plaques, while LPA3 and LPA4 showed no difference. Experiments from our laboratory have demonstrated that both the LPA1 and LPA2 receptors are expressed in human adipose arterioles from both healthy adults and those with CAD; however, LPA3 was not detected in these preparations (23). Future studies are needed to examine LPA receptor expression in specific cell types, as well as individual vascular beds. In endothelial cells, LPA stimulates proliferation and migration (154), chemokine secretion (75, 76), and adhesion molecule expression (71, 113), as well as stress fiber formation, cell contraction, and rounding (148). Additionally, LPA is a potent upregulator and downregulator of endothelial barrier function depending on vessel type and conditions (5, 33, 97, 118, 124, 142). Data on how LPA regulates vascular tone is somewhat conflicting. LPA can decrease eNOS expression in cultured endothelial cells, but is also capable of activating eNOS via phosphorylation of Ser-1179 (66). In animal models, intravenous administration of LPA has resulted in both increased and decreased blood pressure (62, 137).
We recently reported that acute (30 min) treatment of healthy human adipose arterioles with 10 μM of LPA, acts upon the LPA1 receptor to change the mediator of FID from NO to mitochondrial-derived H2O2 without a change in overall dilatory capacity, effectively mimicking the CAD phenotype. Interestingly, despite LPA2 receptors being identified in the human arterioles, they do not appear to be involved in this change in dilator mechanism (23). We also observed that prolonged inhibition of LPA1/3 receptors in healthy human microvessels can cause a similar functional phenotype and trigger the transition from NO to H2O2. (D. S. Chabowski and D. D. Gutterman, unpublished observations). Thus, it seems that basal levels of LPA receptor signaling are necessary for normal vascular function, but overstimulation of, or complete inhibition of the receptors can alter dilator mechanisms. This may be attributed to the state of the LPA receptor. GPCRs, which make up the LPA receptor family, are known to undergo rapid desensitization, which alters the downstream signaling cascade following receptor activation. While LPA may be critical in maintaining vascular homeostasis, the rapid metabolism of this lipid suggests that elevated or prolonged exposure may have adverse consequences by altering the signaling pathway, resulting in opposing effects.
Three isoforms of lipid phosphate phosphatases (LPP1, LPP2, LPP3) are tasked with degradation of the majority of extracellular LPA (24, 117). To a lesser degree, LPA acetyltransferases can also convert LPA to intermediate lipid species, namely phosphatidic acid (8, 9). Plasma levels of LPA increase in the presence of risk factors for CAD (122, 149), and genome-wide association studies have identified a risk allele that leads to decreased expression of LPP3 as being highly prevalent in the general population and highly predictive of atherosclerosis (125). For these reasons, targeting LPP3 may be a desirable approach to regulating LPA levels in individuals at risk for CAD.
COMMUNICATION BETWEEN LIPID FAMILIES
Cross talk between different lipid families is common. One example is the interplay between sphingolipids and the eicosanoid pathway. Phosphorylation of ceramide by ceramide kinases produces ceramide-1-phosphate (C1P), a sphingolipid known to activate PLA2 resulting in release of AA from the plasma membrane (105). Further, S1P can trigger activation of COX-2, resulting in an increase in PGI2 (106). Evidence suggests that S1P-induced activation of COX-2 relies on S1PR2 or S1PR3, as both JTE-013 and suramin, S1PR2 and S1PR3 antagonists, respectively, inhibit S1P-induced expression of COX-2 (82). Tumor necrosis factor-α (TNF-α) is a stimulus for sphingolipid production via activation of sphingomyelinases as well as induction of COX-2 and PGH2 synthesis (48, 121). While inflammation cascades are complex, it is likely that both lipid families signal between one another acting in a synergistic fashion.
There is also significant overlap in signaling between the sphingolipid and LPA pathways. LPP3, the enzyme primarily responsible for hydrolysis of LPA (see Lysophospholipids) can also use sphingolipids as substrates. For instance, LPP3 is able to hydrolyze both C1P and S1P, which as described above, are capable of activating PLA2 and COX-2, respectively (19). It has been suggested that LPA may counteract the proapoptotic signals induced by ceramide and ultimately help regulate the sphingolipid rheostat by activating prosurvival pathways, such as ERK and PI3K (146). LPA triggers also production and promotes excretion of S1P into the vasculature (11). This combination of anticeramide and pro-S1P signaling from LPA would suggest a vasculoprotective effect of LPA. However, multiple lines of evidence have correlated hyperactive LPA signaling axes with vascular dysfunction (97). A possible explanation for this conundrum may involve activity of LPPs. LPPs are capable of dephosphorylating many bioactive lipids, including LPA, PA, S1P, and ceramide-1-phosphate (C1P) (18). The competition among these lipids for LPP may present an opportunity for cross talk. Interestingly, ceramides (e.g., C2- and C6-ceramides) increase activity of LPP and promote the breakdown of lipid mediators (44). The activity rate of lysophospholipids is much faster than for sphingolipids. For instance, our laboratory has shown that a 30-min exposure to LPA is sufficient to cause transition in FID mediator (23), whereas a longer incubation period (~4 h) is needed to see the same effect from the sphingolipid ceramide (J. K. Freed unpublished results).
Lysophosphatidylcholine (LPC) can also stimulate eicosanoid production. In bovine aortic endothelial cells, LPC phosphorylates p38 and activates ERK to stimulate CREB and ATF-1, which ultimately leads to increased COX-2 expression (112). Oguro and Imaoka (102) demonstrated that LPA, like EETs, is a substrate for sEH.
PLA2 not only liberates AA from organ membranes, but also releases lysophospholipids, including LPC, the precursor to LPA via autotaxin (3). Although the exact details of how these two lipid families communicate are unclear, one can infer that potentially interesting connections do exist, since they share the same precursor lipid. Autotaxin is believed to be mostly extracellular, whereas eicosanoid-forming enzymes are mainly thought to be intracellular. The effects of PLA2 activation will ultimately depend on whether AA or LPC is liberated from the membrane and where in the cell this occurs, as the enzymes that target these lipids are compartmentalized.
As mentioned above, there are likely many common pathways that communicate downstream of the GPCRs that interact with LPA and S1P. All of the lipids discussed here induce vasodilation at low levels, raising the possibility of a final common pathway. The redundancy of the more proximal initiating pathways suggests that the lipids provide a means for plasticity to ensure proper vascular function; however, prolonged or excessive exposure may cause detrimental changes in GPCR signaling. Intersections of these lipid families are illustrated in Fig. 2.
Fig. 2.
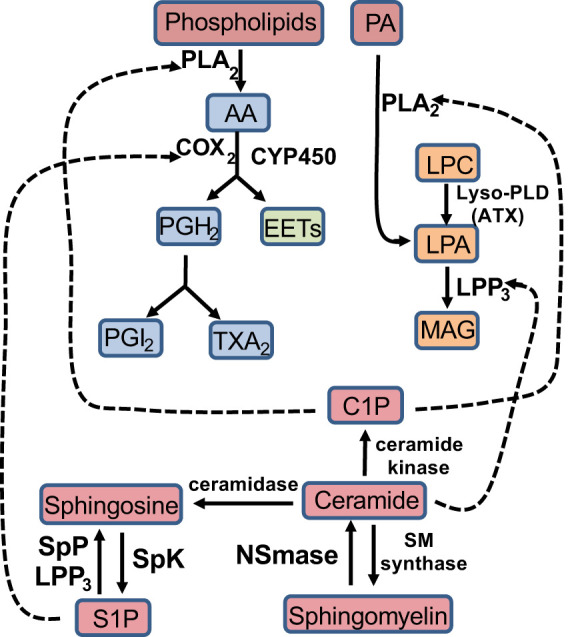
Cross talk between lipid families. C1P is capable of activating PLA2, which converts phospholipids to AA, or, PA to LPA. Ceramide can activate LPP3, which hydrolyzes LPA to MAG. S1P can activate COX2 to convert AA to PGH2. AA, arachidonic acid; ATX, autotaxin COX2, cyclooxygenase 2; CYP-450, cytochrome-P450; C1P, ceramide-1-phosphate; EETs, epoxyeicosatrienoic acids; LPC, lysosphatidylcholine; LPA, lysophosphatidic acid; LPP3, lipid phosphate phosphatase 3; Lyso-PLD, lysophospolipase D; MAG, monoacylglycerol; NSmase, neutral sphingomyelinase; PA, phosphatidic acid; PGH2, prostaglandin H2; PGI2, prostacyclin; PLA2, phospholipase A2; SM synthase, sphingomyelin synthase; SpK, sphingosine kinase; SpP, sphingosine phosphatase; S1P, sphingosine-1-phosphate; TXA2, thromboxane.
Activity of bioactive lipids and reactive oxygen species (ROS) are typically coupled. Bioactive lipids can trigger or inhibit the formation of ROS. For instance, work by Liu et al. (77) has demonstrated that 20-HETE, an eicosanoid produced from CYP-450, can increase ROS formation; however, 14,15-EET, also formed via CYP-450, is capable of decreasing ROS levels in neurons. Both LPA and ceramide can activate NADPH oxidase, thereby, increasing intracellular ROS levels; however, the relationship between these lipids and ROS is more complex. Ceramide-induced ROS formation occurs via activation of NADPH oxidase or through alteration of mitochondrial respiration (156). The resulting oxidative stress is capable of degrading ceramidases, which further elevate levels of ceramide, initiating a vicious circle (17).
CLINICAL IMPLICATIONS
A vast amount of data has been collected on how lipids can promote or prevent cardiovascular disease (74). It is also well accepted that microvascular dysfunction precedes the formation of atherosclerosis and predicts adverse cardiovascular events (46); therefore, understanding how these lipid mediators affect microvascular endothelial cell function may have important implications for prevention, diagnosis, and treatment of CAD and its resulting complications. Currently, lipid profiles used clinically to predict cardiovascular risk include measurement of total cholesterol, LDL, and HDL (128). Elevated plasma ceramides, specifically Cer (d18:1/16:0), Cer (d18:1/18:0), Cer (d18:1/24:0), and Cer (d18:1/24:1), are now considered independent risk factors for major adverse cardiac events (MACE) in otherwise healthy individuals. As mentioned previously, elevated levels of ceramide induce microvascular dysfunction. Likewise, LPA is increased in the plasma of patients presenting with acute coronary syndrome (29), and like ceramide, can elicit detrimental effects on the microvasculature. In contrast, S1P may prevent or reverse microvascular dysfunction. We recently discovered that exogenous S1P restores NO-dependent FID in arterioles collected from patients with CAD (123). It is currently unknown whether administration of S1P can reverse microvascular dysfunction and/or reduce an individual’s risk of MACE; however, S1P or S1P agonists may be a promising therapy based on vascular observations in vitro. Because of their anti-inflammatory and anti-thrombotic effects, EETs also have high translational potential. EET analogs, as well as inhibitors of sEH, protect against hypertension-induced organ damage, inflammation, and atherosclerosis in in vivo animal studies, as well as clinical trials, with next-generation compounds under development (34, 43, 57, 92, 131, 157).
Diabetes mellitus is a well-known risk factor for development of CAD, which is currently the leading cause of death in this patient population (45). Evidence suggests that coronary vascular dysfunction is observed in diabetic subjects despite the absence of other CAD risk factors, such as hypertension or dyslipidemia (107). Biologically active lipids may serve as a connection between diabetes and the well-described microvascular dysfunction associated with the disease. 5,6-EET regulates pancreatic β-cells by directly stimulating release of insulin, whereas 8,9-EET, 11,12-EET, and 14,15-EET initiate release of glucagon by α-cells (55). The use of sEHs to increase plasma concentrations of EETs may allow for treatment, and/or prevention, of obesity-induced diabetes, while at the same time protect against obesity-induced increases in blood pressure and diabetes-induced microvascular dysfunction. Similarly, sphingolipids, such as ceramide, are known to inhibit glucose uptake and induce apoptosis in pancreatic β-cells, whereas S1P receptor agonists (e.g., FTY720) exert beneficial effects on these cells by preventing infiltration of effector lymphocytes, thus preserving overall function (53). Strategies that decrease ceramide and increase S1P levels may also be effective in both treating diabetes, as well as preserving microvascular function.
Bioactive lipids are also a major area of investigation in cancer research. ATX expression is often increased in cancer and is linked to tumor progression and metastasis (96). As mentioned previously, LPA stimulates S1P formation and extracellular excretion. Since elevated plasma levels of S1P are associated with tumor metastasis (68), neutralizing S1P with a monoclonal antibody has emerged as a novel cancer treatment (144). Anticancer treatments, including both chemotherapy and radiation therapy, increase systemic ceramide, which is believed to be the key step in promoting tumor cell death and cancer remission. In fact, tumor resistance to chemotherapy is linked to an inability to elevate ceramide levels (90). Therefore, upregulation and downregulation of ceramide and S1P, respectively, has become the goal of many cancer treatments (90, 101). An as of yet untested hypothesis is that this combination of increased ceramide combined with decreased S1P levels may lead to accelerated microvascular function and premature cardiovascular disease, a known complication in cancer patients (157). These in vitro data raise concerns about therapies that rely on systemic activation of ceramide to combat cancer, since such an approach would be expected to aggravate vascular disease, and argue for a more tumor-targeted application of these anti-cancer molecules. Another potential strategy may be to activate the S1P pathway following cancer treatment to avoid or reverse vascular damage in an effort to prevent future cardiovascular disease.
Approximately half of the adult population in the United States carries the diagnosis of some form of cardiovascular disease (15). It remains the number one cause of mortality. Deaths due to cardiovascular disease are expected to rise over the next decade, mainly due an increase in both the elderly population, as well as those who are obese. The microcirculation plays a large role in maintaining vascular homeostasis, and dysfunction of this vascular network occurs before the development of large artery disease. Bioactive lipids have emerged as key modulators of microvascular health and have the potential to promote or prevent diseases, such as atherosclerosis. However, manipulation of one lipid pathway may alter levels of other lipids from a different family of compounds. To enhance signaling of bioactive lipids that have beneficial effects on the microvasculature or suppress the actions of those that cause harm, a sophisticated understanding of the complex interplay among these lipids must be attained.
SUMMARY AND CONCLUSIONS
Bioactive lipids are among the key endogenous substances that regulate vascular tone and suppress or accelerate vascular dysfunction. A vast amount of knowledge regarding how biologically active lipids are formed, metabolized, and function as messengers within the vascular wall has been generated over the last three decades. Not only do arachidonic acid derivatives, sphingolipids, and lysophospholipids all act as vasodilators, they also participate in a closely collaborative manner, creating substantial interdependent feedback and compensation. Evidence is mounting that bioactive lipids are key modulators of the microcirculation, which once dysfunctional, often leads to chronic disease. The interplay among these lipid pathways has not been extensively explored and holds potential for a more comprehensive and targeted systems approach to maintaining cardiovascular homeostasis in health and disease.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.S.C., K.E.C., and J.K.F. prepared figures; D.S.C. and J.K.F. drafted manuscript; D.S.C., K.E.C., O.A.-H., D.D.G., and J.K.F. edited and revised manuscript; D.S.C., K.E.C., O.A.-H., D.D.G., and J.K.F. approved final version of manuscript.
REFERENCES
- 1.Aaltonen N, Laitinen JT, Lehtonen M. Quantification of lysophosphatidic acids in rat brain tissue by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 878: 1145–1152, 2010. doi: 10.1016/j.jchromb.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Albers HM, Dong A, van Meeteren LA, Egan DA, Sunkara M, van Tilburg EW, Schuurman K, van Tellingen O, Morris AJ, Smyth SS, Moolenaar WH, Ovaa H. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc Natl Acad Sci USA 107: 7257–7262, 2010. doi: 10.1073/pnas.1001529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldi S, Matic LP, Hamm G, van Keulen D, Tempel D, Holmstrøm K, Szwajda A, Nielsen BS, Emilsson V, Ait-Belkacem R, Lengquist M, Paulsson-Berne G, Eriksson P, Lindeman JH, Gool AJ, Stauber J, Hedin U, Hurt-Camejo E. Integrated human evaluation of the lysophosphatidic acid pathway as a novel therapeutic target in atherosclerosis. Mol Ther Methods Clin Dev 10: 17–28, 2018. doi: 10.1016/j.omtm.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander JS, Patton WF, Christman BW, Cuiper LL, Haselton FR. Platelet-derived lysophosphatidic acid decreases endothelial permeability in vitro. Am J Physiol Heart Circ Physiol 274: H115–H122, 1998. doi: 10.1152/ajpheart.1998.274.1.H115. [DOI] [PubMed] [Google Scholar]
- 6.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 102: 3665–3667, 2003. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- 7.Alptürk O, Rusin O, Fakayode SO, Wang W, Escobedo JO, Warner IM, Crowe WE, Král V, Pruet JM, Strongin RM. Lanthanide complexes as fluorescent indicators for neutral sugars and cancer biomarkers. Proc Natl Acad Sci USA 103: 9756–9760, 2006. doi: 10.1073/pnas.0603758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol 15: 477–489, 2004. doi: 10.1016/j.semcdb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Aoki J, Inoue A, Okudaira S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta 1781: 513–518, 2008. doi: 10.1016/j.bbalip.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, Mizuno K, Saku K, Taguchi R, Arai H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem 277: 48737–48744, 2002. doi: 10.1074/jbc.M206812200. [DOI] [PubMed] [Google Scholar]
- 11.Avraham-Davidi I, Grunspan M, Yaniv K. Lipid signaling in the endothelium. Exp Cell Res 319: 1298–1305, 2013. doi: 10.1016/j.yexcr.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Baker DL, Desiderio DM, Miller DD, Tolley B, Tigyi GJ. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Anal Biochem 292: 287–295, 2001. doi: 10.1006/abio.2001.5063. [DOI] [PubMed] [Google Scholar]
- 13.Baker DL, Morrison P, Miller B, Riely CA, Tolley B, Westermann AM, Bonfrer JM, Bais E, Moolenaar WH, Tigyi G. Plasma lysophosphatidic acid concentration and ovarian cancer. JAMA 287: 3081–3082, 2002. doi: 10.1001/jama.287.23.3081. [DOI] [PubMed] [Google Scholar]
- 14.Baker DL, Umstot ES, Desiderio DM, Tigyi GJ. Quantitative analysis of lysophosphatidic acid in human blood fractions. Ann N Y Acad Sci 905: 267–269, 2000. doi: 10.1111/j.1749-6632.2000.tb06557.x. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UK, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke satistics-2019 update: a report from the American Heart Association. Circulation 139: e56–e528, 2019. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 16.Beyer AM, Zinkevich N, Miller B, Liu Y, Wittenburg AL, Mitchell M, Galdieri R, Sorokin A, Gutterman DD. Transition in the mechanism of flow-mediated dilation with aging and development of coronary artery disease. Basic Res Cardiol 112: 5, 2017. doi: 10.1007/s00395-016-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhat OM, Yuan X, Li G, Lee R, Li PL. Sphingolipids and redox signaling in renal regulation and chronic kidney diseases. Antioxid Redox Signal 28: 1008–1026, 2018. doi: 10.1089/ars.2017.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brindley DN. Lipid phosphate phosphatases and related proteins: signaling functions in development, cell division, and cancer. J Cell Biochem 92: 900–912, 2004. doi: 10.1002/jcb.20126. [DOI] [PubMed] [Google Scholar]
- 19.Brindley DN, Lin FT, Tigyi GJ. Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim Biophys Acta 1831: 74–85, 2013. doi: 10.1016/j.bbalip.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell WB, Falck JR, Gauthier K. Role of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factor in bovine coronary arteries. Med Sci Monit 7: 578–584, 2001. [PubMed] [Google Scholar]
- 21.Cantalupo A, Gargiulo A, Dautaj E, Liu C, Zhang Y, Hla T, Di Lorenzo A. S1PR1 (sphingosine-1-phosphate receptor 1) signaling regulates blood flow and pressure. Hypertension 70: 426–434, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carroll MA, Balazy M, Margiotta P, Falck JR, McGiff JC. Renal vasodilator activity of 5,6-epoxyeicosatrienoic acid depends upon conversion by cyclooxygenase and release of prostaglandins. J Biol Chem 268: 12260–12266, 1993. [PubMed] [Google Scholar]
- 23.Chabowski DS, Kadlec AO, Ait-Aissa K, Hockenberry JC, Pearson PJ, Beyer AM, Gutterman DD. Lysophosphatidic acid acts on LPA1 receptor to increase H2O2 during flow-induced dilation in human adipose arterioles. Br J Pharmacol 175: 4266–4280, 2018. doi: 10.1111/bph.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandra M, Escalante-Alcalde D, Bhuiyan MS, Orr AW, Kevil C, Morris AJ, Nam H, Dominic P, McCarthy KJ, Miriyala S, Panchatcharam M. Cardiac-specific inactivation of LPP3 in mice leads to myocardial dysfunction and heart failure. Redox Biol 14: 261–271, 2018. doi: 10.1016/j.redox.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chawengsub Y, Gauthier KM, Campbell WB. Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone. Am J Physiol Heart Circ Physiol 297: H495–H507, 2009. doi: 10.1152/ajpheart.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Falck JR, Manthati VL, Jat JL, Campbell WB. 20-Iodo-14,15-epoxyeicosa-8(Z)-enoyl-3-azidophenylsulfonamide: photoaffinity labeling of a 14,15-epoxyeicosatrienoic acid receptor. Biochemistry 50: 3840–3848, 2011. doi: 10.1021/bi102070w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol 50: 157–186, 2010. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 28.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381: 800–803, 1996. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 29.Dohi T, Miyauchi K, Ohkawa R, Nakamura K, Kishimoto T, Miyazaki T, Nishino A, Nakajima N, Yaginuma K, Tamura H, Kojima T, Yokoyama K, Kurata T, Shimada K, Yatomi Y, Daida H. Increased circulating plasma lysophosphatidic acid in patients with acute coronary syndrome. Clin Chim Acta 413: 207–212, 2012. doi: 10.1016/j.cca.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 30.Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol 46: 842–848, 2005. doi: 10.1097/01.fjc.0000189600.74157.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan RD. Alkaline sphingomyelinase: an old enzyme with novel implications. Biochim Biophys Acta 1761: 281–291, 2006. doi: 10.1016/j.bbalip.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Ellis EF, Police RJ, Yancey L, McKinney JS, Amruthesh SC. Dilation of cerebral arterioles by cytochrome P-450 metabolites of arachidonic acid. Am J Physiol Heart Circ Physiol 259: H1171–H1177, 1990. doi: 10.1152/ajpheart.1990.259.4.H1171. [DOI] [PubMed] [Google Scholar]
- 33.English D, Kovala AT, Welch Z, Harvey KA, Siddiqui RA, Brindley DN, Garcia JG. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. J Hematother Stem Cell Res 8: 627–634, 1999. doi: 10.1089/152581699319795. [DOI] [PubMed] [Google Scholar]
- 34.Falck JR, Kodela R, Manne R, Atcha KR, Puli N, Dubasi N, Manthati VL, Capdevila JH, Yi XY, Goldman DH, Morisseau C, Hammock BD, Campbell WB. 14,15-Epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates containing epoxide bioisosteres: influence upon vascular relaxation and soluble epoxide hydrolase inhibition. J Med Chem 52: 5069–5075, 2009. doi: 10.1021/jm900634w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Félétou M, Huang Y, Vanhoutte PM. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol 164: 894–912, 2011. doi: 10.1111/j.1476-5381.2011.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Félétou M, Verbeuren TJ, Vanhoutte PM. Endothelium-dependent contractions in SHR: a tale of prostanoid TP and IP receptors. Br J Pharmacol 156: 563–574, 2009. doi: 10.1111/j.1476-5381.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 38.Fleming I. Cytochrome p450 and vascular homeostasis. Circ Res 89: 753–762, 2001. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- 39.Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, Busse R. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol 27: 2612–2618, 2007. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- 40.Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res 115: 525–532, 2014. doi: 10.1161/CIRCRESAHA.115.303881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujiwaki T, Yamaguchi S, Sukegawa K, Taketomi T. Application of delayed extraction matrix-assisted laser desorption ionization time-of-flight mass spectrometry for analysis of sphingolipids in tissues from sphingolipidosis patients. J Chromatogr B Biomed Sci Appl 731: 45–52, 1999. doi: 10.1016/S0378-4347(99)00190-5. [DOI] [PubMed] [Google Scholar]
- 42.García-Ruiz C, Colell A, Marí M, Morales A, Fernández-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem 272: 11369–11377, 1997. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 43.Gauthier KM, Falck JR, Reddy LM, Campbell WB. 14,15-EET analogs: characterization of structural requirements for agonist and antagonist activity in bovine coronary arteries. Pharmacol Res 49: 515–524, 2004. doi: 10.1016/j.phrs.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 44.Gómez-Muñoz A, Waggoner DW, O’Brien L, Brindley DN. Interaction of ceramides, sphingosine, and sphingosine 1-phosphate in regulating DNA synthesis and phospholipase D activity. J Biol Chem 270: 26318–26325, 1995. doi: 10.1074/jbc.270.44.26318. [DOI] [PubMed] [Google Scholar]
- 45.Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. JAMA 281: 1291–1297, 1999. doi: 10.1001/jama.281.14.1291. [DOI] [PubMed] [Google Scholar]
- 46.Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM. The human microcirculation: regulation of flow and beyond. Circ Res 118: 157–172, 2016. doi: 10.1161/CIRCRESAHA.115.305364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hannun YA, Obeid LM. Many ceramides. J Biol Chem 286: 27855–27862, 2011. doi: 10.1074/jbc.R111.254359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9: 139–150, 2008. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 49.Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol 19: 175–191, 2018. [Erratum in Nat Rev Mol Cell Biol 19: 6732018, 2018.] doi: 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med 14: 461–469, 2008. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Haserück N, Erl W, Pandey D, Tigyi G, Ohlmann P, Ravanat C, Gachet C, Siess W. The plaque lipid lysophosphatidic acid stimulates platelet activation and platelet-monocyte aggregate formation in whole blood: involvement of P2Y1 and P2Y12 receptors. Blood 103: 2585–2592, 2004. doi: 10.1182/blood-2003-04-1127. [DOI] [PubMed] [Google Scholar]
- 52.Havulinna AS, Sysi-Aho M, Hilvo M, Kauhanen D, Hurme R, Ekroos K, Salomaa V, Laaksonen R. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler Thromb Vasc Biol 36: 2424–2430, 2016. doi: 10.1161/ATVBAHA.116.307497. [DOI] [PubMed] [Google Scholar]
- 53.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 29: 381–402, 2008. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hosogaya S, Yatomi Y, Nakamura K, Ohkawa R, Okubo S, Yokota H, Ohta M, Yamazaki H, Koike T, Ozaki Y. Measurement of plasma lysophosphatidic acid concentration in healthy subjects: strong correlation with lysophospholipase D activity. Ann Clin Biochem 45: 364–368, 2008. doi: 10.1258/acb.2008.007242. [DOI] [PubMed] [Google Scholar]
- 55.Huang H, Weng J, Wang MH. EETs/sEH in diabetes and obesity-induced cardiovascular diseases. Prostaglandins Other Lipid Mediat 125: 80–89, 2016. doi: 10.1016/j.prostaglandins.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Huang X, Withers BR, Dickson RC. Sphingolipids and lifespan regulation. Biochim Biophys Acta 1841: 657–664, 2014. doi: 10.1016/j.bbalip.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imig JD, Dimitropoulou C, Reddy DS, White RE, Falck JR. Afferent arteriolar dilation to 11, 12-EET analogs involves PP2A activity and Ca2+-activated K+ channels. Microcirculation 15: 137–150, 2008. doi: 10.1080/10739680701456960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov 8: 794–805, 2009. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension 39: 690–694, 2002. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 60.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension 46: 975–981, 2005. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jesionowska A, Cecerska E, Dolegowska B. Methods for quantifying lysophosphatidic acid in body fluids: a review. Anal Biochem 453: 38–43, 2014. doi: 10.1016/j.ab.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 62.Kano K, Matsumoto H, Inoue A, Yukiura H, Kanai M, Chun J, Ishii S, Shimizu T, Aoki J. Molecular mechanism of lysophosphatidic acid-induced hypertensive response. Sci Rep 9: 2662, 2019. doi: 10.1038/s41598-019-39041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kimura T, Watanabe T, Sato K, Kon J, Tomura H, Tamama K, Kuwabara A, Kanda T, Kobayashi I, Ohta H, Ui M, Okajima F. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem J 348: 71–76, 2000. doi: 10.1042/bj3480071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kishimoto T, Matsuoka T, Imamura S, Mizuno K. A novel colorimetric assay for the determination of lysophosphatidic acid in plasma using an enzymatic cycling method. Clin Chim Acta 333: 59–67, 2003. doi: 10.1016/S0009-8981(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 65.Kishimoto T, Soda Y, Matsuyama Y, Mizuno K. An enzymatic assay for lysophosphatidylcholine concentration in human serum and plasma. Clin Biochem 35: 411–416, 2002. doi: 10.1016/S0009-9120(02)00327-2. [DOI] [PubMed] [Google Scholar]
- 66.Kou R, Igarashi J, Michel T. Lysophosphatidic acid and receptor-mediated activation of endothelial nitric-oxide synthase. Biochemistry 41: 4982–4988, 2002. doi: 10.1021/bi016017r. [DOI] [PubMed] [Google Scholar]
- 67.Kuhn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases: structure, function, and biological implications. Prostaglandins Other Lipid Mediat 68-69: 263–290, 2002. doi: 10.1016/S0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 68.Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov 12: 688–702, 2013. doi: 10.1038/nrd4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, Suoniemi M, Hurme R, März W, Scharnagl H, Stojakovic T, Vlachopoulou E, Lokki ML, Nieminen MS, Klingenberg R, Matter CM, Hornemann T, Jüni P, Rodondi N, Räber L, Windecker S, Gencer B, Pedersen ER, Tell GS, Nygård O, Mach F, Sinisalo J, Lüscher TF. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J 37: 1967–1976, 2016. doi: 10.1093/eurheartj/ehw148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol 290: H491–H499, 2006. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee H, Lin CI, Liao JJ, Lee YW, Yang HY, Lee CY, Hsu HY, Wu HL. Lysophospholipids increase ICAM-1 expression in HUVEC through a Gi- and NF-κB-dependent mechanism. Am J Physiol Cell Physiol 287: C1657–C1666, 2004. doi: 10.1152/ajpcell.00172.2004. [DOI] [PubMed] [Google Scholar]
- 72.Lee OH, Kim YM, Lee YM, Moon EJ, Lee DJ, Kim JH, Kim KW, Kwon YG. Sphingosine 1-phosphate induces angiogenesis: its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem Biophys Res Commun 264: 743–750, 1999. doi: 10.1006/bbrc.1999.1586. [DOI] [PubMed] [Google Scholar]
- 73.Lefebvre B, Caron F, Bessard G, Stanke-Labesque F. Effect of 5-lipoxygenase blockade on blood pressure and acetylcholine-evoked endothelium-dependent contraction in aorta from spontaneously hypertensive rats. J Hypertens 24: 85–93, 2006. doi: 10.1097/01.hjh.0000198027.76729.b8. [DOI] [PubMed] [Google Scholar]
- 74.Li X, Becker KA, Zhang Y. Ceramide in redox signaling and cardiovascular diseases. Cell Physiol Biochem 26: 41–48, 2010. doi: 10.1159/000315104. [DOI] [PubMed] [Google Scholar]
- 75.Lin CI, Chen CN, Chen JH, Lee H. Lysophospholipids increase IL-8 and MCP-1 expressions in human umbilical cord vein endothelial cells through an IL-1-dependent mechanism. J Cell Biochem 99: 1216–1232, 2006. doi: 10.1002/jcb.20963. [DOI] [PubMed] [Google Scholar]
- 76.Lin CI, Chen CN, Lin PW, Chang KJ, Hsieh FJ, Lee H. Lysophosphatidic acid regulates inflammation-related genes in human endothelial cells through LPA1 and LPA3. Biochem Biophys Res Commun 363: 1001–1008, 2007. doi: 10.1016/j.bbrc.2007.09.081. [DOI] [PubMed] [Google Scholar]
- 77.Liu X, Davis CM, Alkayed NJ. P450 eicosanoids and reactive oxygen species interplay in brain injury and neuroprotection. Antioxid Redox Signal 28: 987–1007, 2018. doi: 10.1089/ars.2017.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 79.Lu J, Byrne N, Wang J, Bricogne G, Brown FK, Chobanian HR, Colletti SL, Di Salvo J, Thomas-Fowlkes B, Guo Y, Hall DL, Hadix J, Hastings NB, Hermes JD, Ho T, Howard AD, Josien H, Kornienko M, Lumb KJ, Miller MW, Patel SB, Pio B, Plummer CW, Sherborne BS, Sheth P, Souza S, Tummala S, Vonrhein C, Webb M, Allen SJ, Johnston JM, Weinglass AB, Sharma S, Soisson SM. Structural basis for the cooperative allosteric activation of the free fatty acid receptor GPR40. Nat Struct Mol Biol 24: 570–577, 2017. doi: 10.1038/nsmb.3417. [DOI] [PubMed] [Google Scholar]
- 80.Ma SK, Wang Y, Chen J, Zhang MZ, Harris RC, Chen JK. Overexpression of G-protein-coupled receptor 40 enhances the mitogenic response to epoxyeicosatrienoic acids. PLoS One 10: e0113130, 2015. doi: 10.1371/journal.pone.0113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma YH, Harder DR, Clark JE, Roman RJ. Effects of 12-HETE on isolated dog renal arcuate arteries. Am J Physiol Heart Circ Physiol 261: H451–H456, 1991. doi: 10.1152/ajpheart.1991.261.2.H451. [DOI] [PubMed] [Google Scholar]
- 82.Machida T, Matamura R, Iizuka K, Hirafuji M. Cellular function and signaling pathways of vascular smooth muscle cells modulated by sphingosine 1-phosphate. J Pharmacol Sci 132: 211–217, 2016. doi: 10.1016/j.jphs.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 83.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296: 346–349, 2002. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 84.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol 82: 27–44, 2004. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- 85.Markovič T, Jakopin Ž, Dolenc MS, Mlinarič-Raščan I. Structural features of subtype-selective EP receptor modulators. Drug Discov Today 22: 57–71, 2017. doi: 10.1016/j.drudis.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 86.Masoodi M, Eiden M, Koulman A, Spaner D, Volmer DA. Comprehensive lipidomics analysis of bioactive lipids in complex regulatory networks. Anal Chem 82: 8176–8185, 2010. doi: 10.1021/ac1015563. [DOI] [PubMed] [Google Scholar]
- 87.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem 92: 1075–1085, 2004. doi: 10.1002/jcb.20088. [DOI] [PubMed] [Google Scholar]
- 88.Merrill AH Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 36: 207–224, 2005. doi: 10.1016/j.ymeth.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 89.Miller AW, Katakam PV, Lee HC, Tulbert CD, Busija DW, Weintraub NL. Arachidonic acid-induced vasodilation of rat small mesenteric arteries is lipoxygenase-dependent. J Pharmacol Exp Ther 304: 139–144, 2003. doi: 10.1124/jpet.102.041780. [DOI] [PubMed] [Google Scholar]
- 90.Morad SA, Cabot MC. Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer 13: 51–65, 2013. doi: 10.1038/nrc3398. [DOI] [PubMed] [Google Scholar]
- 91.Morishige J, Urikura M, Takagi H, Hirano K, Koike T, Tanaka T, Satouchi K. A clean-up technology for the simultaneous determination of lysophosphatidic acid and sphingosine-1-phosphate by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using a phosphate-capture molecule, Phos-tag. Rapid Commun Mass Spectrom 24: 1075–1084, 2010. doi: 10.1002/rcm.4484. [DOI] [PubMed] [Google Scholar]
- 92.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol 53: 37–58, 2013. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morris KE, Schang LM, Brindley DN. Lipid phosphate phosphatase-2 activity regulates S-phase entry of the cell cycle in Rat2 fibroblasts. J Biol Chem 281: 9297–9306, 2006. doi: 10.1074/jbc.M511710200. [DOI] [PubMed] [Google Scholar]
- 94.Murph M, Tanaka T, Pang J, Felix E, Liu S, Trost R, Godwin AK, Newman R, Mills G. Liquid chromatography mass spectrometry for quantifying plasma lysophospholipids: potential biomarkers for cancer diagnosis. Methods Enzymol 433: 1–25, 2007. doi: 10.1016/S0076-6879(07)33001-2. [DOI] [PubMed] [Google Scholar]
- 95.Nagao K, Yanagita T. Bioactive lipids in metabolic syndrome. Prog Lipid Res 47: 127–146, 2008. doi: 10.1016/j.plipres.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 96.Nam SW, Clair T, Kim YS, McMarlin A, Schiffmann E, Liotta LA, Stracke ML. Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Res 61: 6938–6944, 2001. [PubMed] [Google Scholar]
- 97.Neidlinger NA, Larkin SK, Bhagat A, Victorino GP, Kuypers FA. Hydrolysis of phosphatidylserine-exposing red blood cells by secretory phospholipase A2 generates lysophosphatidic acid and results in vascular dysfunction. J Biol Chem 281: 775–781, 2006. doi: 10.1074/jbc.M505790200. [DOI] [PubMed] [Google Scholar]
- 98.Nishi T, Kobayashi N, Hisano Y, Kawahara A, Yamaguchi A. Molecular and physiological functions of sphingosine 1-phosphate transporters. Biochim Biophys Acta 1841: 759–765, 2014. doi: 10.1016/j.bbalip.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 99.Nithipatikom K, Laabs ND, Isbell MA, Campbell WB. Liquid chromatographic-mass spectrometric determination of cyclooxygenase metabolites of arachidonic acid in cultured cells. J Chromatogr B Analyt Technol Biomed Life Sci 785: 135–145, 2003. doi: 10.1016/S1570-0232(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 100.Oates JA, FitzGerald GA, Branch RA, Jackson EK, Knapp HR, Roberts LJ 2nd. Clinical implications of prostaglandin and thromboxane A2 formation (2). N Engl J Med 319: 761–767, 1988. doi: 10.1056/NEJM198809223191206. [DOI] [PubMed] [Google Scholar]
- 101.Ogretmen B, Hannun YA. Updates on functions of ceramide in chemotherapy-induced cell death and in multidrug resistance. Drug Resist Updat 4: 368–377, 2001. doi: 10.1054/drup.2001.0225. [DOI] [PubMed] [Google Scholar]
- 102.Oguro A, Imaoka S. Lysophosphatidic acids are new substrates for the phosphatase domain of soluble epoxide hydrolase. J Lipid Res 53: 505–512, 2012. doi: 10.1194/jlr.M022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res 83: 932–939, 1998. doi: 10.1161/01.RES.83.9.932. [DOI] [PubMed] [Google Scholar]
- 104.Parkington HC, Coleman HA, Tare M. Prostacyclin and endothelium-dependent hyperpolarization. Pharmacol Res 49: 509–514, 2004. doi: 10.1016/j.phrs.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 105.Pettus BJ, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC, Evans JH, Freiberg J, Roddy P, Hannun YA, Chalfant CE. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem 279: 11320–11326, 2004. doi: 10.1074/jbc.M309262200. [DOI] [PubMed] [Google Scholar]
- 106.Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-α. FASEB J 17: 1411–1421, 2003. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- 107.Pitkänen OP, Nuutila P, Raitakari OT, Rönnemaa T, Koskinen PJ, Iida H, Lehtimäki TJ, Laine HK, Takala T, Viikari JS, Knuuti J. Coronary flow reserve is reduced in young men with IDDM. Diabetes 47: 248–254, 1998. doi: 10.2337/diab.47.2.248. [DOI] [PubMed] [Google Scholar]
- 108.Pokreisz P, Fleming I, Kiss L, Barbosa-Sicard E, Fisslthaler B, Falck JR, Hammock BD, Kim IH, Szelid Z, Vermeersch P, Gillijns H, Pellens M, Grimminger F, van Zonneveld AJ, Collen D, Busse R, Janssens S. Cytochrome P450 epoxygenase gene function in hypoxic pulmonary vasoconstriction and pulmonary vascular remodeling. Hypertension 47: 762–770, 2006. doi: 10.1161/01.HYP.0000208299.62535.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quintern LE, Schuchman EH, Levran O, Suchi M, Ferlinz K, Reinke H, Sandhoff K, Desnick RJ. Isolation of cDNA clones encoding human acid sphingomyelinase: occurrence of alternatively processed transcripts. EMBO J 8: 2469–2473, 1989. doi: 10.1002/j.1460-2075.1989.tb08382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rådmark O. Arachidonate 5-lipoxygenase. Prostaglandins Other Lipid Mediat 68-69: 211–234, 2002. doi: 10.1016/S0090-6980(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 111.Reader J, Holt D, Fulton A. Prostaglandin E2 EP receptors as therapeutic targets in breast cancer. Cancer Metastasis Rev 30: 449–463, 2011. doi: 10.1007/s10555-011-9303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rikitake Y, Hirata K, Kawashima S, Takeuchi S, Shimokawa Y, Kojima Y, Inoue N, Yokoyama M. Signaling mechanism underlying COX-2 induction by lysophosphatidylcholine. Biochem Biophys Res Commun 281: 1291–1297, 2001. doi: 10.1006/bbrc.2001.4510. [DOI] [PubMed] [Google Scholar]
- 113.Rizza C, Leitinger N, Yue J, Fischer DJ, Wang DA, Shih PT, Lee H, Tigyi G, Berliner JA. Lysophosphatidic acid as a regulator of endothelial/leukocyte interaction. Lab Invest 79: 1227–1235, 1999. [PubMed] [Google Scholar]
- 114.Rosolowsky M, Campbell WB. Synthesis of hydroxyeicosatetraenoic (HETEs) and epoxyeicosatrienoic acids (EETs) by cultured bovine coronary artery endothelial cells. Biochim Biophys Acta 1299: 267–277, 1996. doi: 10.1016/0005-2760(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 115.Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, Matsui O, Takuwa Y. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ Res 90: 325–332, 2002. doi: 10.1161/hh0302.104455. [DOI] [PubMed] [Google Scholar]
- 116.Sacerdoti D, Bolognesi M, Di Pascoli M, Gatta A, McGiff JC, Schwartzman ML, Abraham NG. Rat mesenteric arterial dilator response to 11,12-epoxyeicosatrienoic acid is mediated by activating heme oxygenase. Am J Physiol Heart Circ Physiol 291: H1999–H2002, 2006. doi: 10.1152/ajpheart.00082.2006. [DOI] [PubMed] [Google Scholar]
- 117.Salous AK, Panchatcharam M, Sunkara M, Mueller P, Dong A, Wang Y, Graf GA, Smyth SS, Morris AJ. Mechanism of rapid elimination of lysophosphatidic acid and related lipids from the circulation of mice. J Lipid Res 54: 2775–2784, 2013. doi: 10.1194/jlr.M039685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sarker MH, Hu DE, Fraser PA. Regulation of cerebromicrovascular permeability by lysophosphatidic acid. Microcirculation 17: 39–46, 2010. doi: 10.1111/j.1549-8719.2010.00001.x. [DOI] [PubMed] [Google Scholar]
- 119.Saulnier-Blache JS, Girard A, Simon MF, Lafontan M, Valet P. A simple and highly sensitive radioenzymatic assay for lysophosphatidic acid quantification. J Lipid Res 41: 1947–1951, 2000. [PMC free article] [PubMed] [Google Scholar]
- 120.Scherer M, Schmitz G, Liebisch G. High-throughput analysis of sphingosine 1-phosphate, sphinganine 1-phosphate, and lysophosphatidic acid in plasma samples by liquid chromatography-tandem mass spectrometry. Clin Chem 55: 1218–1222, 2009. doi: 10.1373/clinchem.2008.113779. [DOI] [PubMed] [Google Scholar]
- 121.Schmitz H, Fromm M, Bode H, Scholz P, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha induces Cl− and K+ secretion in human distal colon driven by prostaglandin E2. Am J Physiol Gastrointest Liver Physiol 271: G669–G674, 1996. doi: 10.1152/ajpgi.1996.271.4.G669. [DOI] [PubMed] [Google Scholar]
- 122.Schober A, Siess W. Lysophosphatidic acid in atherosclerotic diseases. Br J Pharmacol 167: 465–482, 2012. doi: 10.1111/j.1476-5381.2012.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schulz ME, Katunaric B, Hockenberry JC, Gutterman DD, Freed JK. Manipulation of the sphingolipid rheostat influences the mediator of flow-induced dilation in the human microvasculature. J Am Heart Assoc 8: e013153, 2019. doi: 10.1161/JAHA.119.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schulze C, Smales C, Rubin LL, Staddon JM. Lysophosphatidic acid increases tight junction permeability in cultured brain endothelial cells. J Neurochem 68: 991–1000, 1997. doi: 10.1046/j.1471-4159.1997.68030991.x. [DOI] [PubMed] [Google Scholar]
- 125.Schunkert H, König IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M, Gieger C, Absher D, Aherrahrou Z, Allayee H, Altshuler D, Anand SS, Andersen K, Anderson JL, Ardissino D, Ball SG, Balmforth AJ, Barnes TA, Becker DM, Becker LC, Berger K, Bis JC, Boekholdt SM, Boerwinkle E, Braund PS, Brown MJ, Burnett MS, Buysschaert I, Carlquist JF, Chen L, Cichon S, Codd V, Davies RW, Dedoussis G, Dehghan A, Demissie S, Devaney JM, Diemert P, Do R, Doering A, Eifert S, Mokhtari NE, Ellis SG, Elosua R, Engert JC, Epstein SE, de Faire U, Fischer M, Folsom AR, Freyer J, Gigante B, Girelli D, Gretarsdottir S, Gudnason V, Gulcher JR, Halperin E, Hammond N, Hazen SL, Hofman A, Horne BD, Illig T, Iribarren C, Jones GT, Jukema JW, Kaiser MA, Kaplan LM, Kastelein JJP, Khaw KT, Knowles JW, Kolovou G, Kong A, Laaksonen R, Lambrechts D, Leander K, Lettre G, Li M, Lieb W, Loley C, Lotery AJ, Mannucci PM, Maouche S, Martinelli N, McKeown PP, Meisinger C, Meitinger T, Melander O, Merlini PA, Mooser V, Morgan T, Mühleisen TW, Muhlestein JB, Münzel T, Musunuru K, Nahrstaedt J, Nelson CP, Nöthen MM, Olivieri O, Patel RS, Patterson CC, Peters A, Peyvandi F, Qu L, Quyyumi AA, Rader DJ, Rallidis LS, Rice C, Rosendaal FR, Rubin D, Salomaa V, Sampietro ML, Sandhu MS, Schadt E, Schäfer A, Schillert A, Schreiber S, Schrezenmeir J, Schwartz SM, Siscovick DS, Sivananthan M, Sivapalaratnam S, Smith A, Smith TB, Snoep JD, Soranzo N, Spertus JA, Stark K, Stirrups K, Stoll M, Tang WHW, Tennstedt S, Thorgeirsson G, Thorleifsson G, Tomaszewski M, Uitterlinden AG, van Rij AM, Voight BF, Wareham NJ, Wells GA, Wichmann HE, Wild PS, Willenborg C, Witteman JCM, Wright BJ, Ye S, Zeller T, Ziegler A, Cambien F, Goodall AH, Cupples LA, Quertermous T, März W, Hengstenberg C, Blankenberg S, Ouwehand WH, Hall AS, Deloukas P, Thompson JR, Stefansson K, Roberts R, Thorsteinsdottir U, O’Donnell CJ, McPherson R, Erdmann J, Samani NJ,; CARDIoGRAM Consortium . Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet 43: 333–338, 2011. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Selvam R, Radin NS. Quantitation of lipids by charring on thin-layer plates and scintillation quenching: application to ceramide determination. Anal Biochem 112: 338–345, 1981. doi: 10.1016/0003-2697(81)90302-X. [DOI] [PubMed] [Google Scholar]
- 127.Shan L, Jaffe K, Li S, Davis L. Quantitative determination of lysophosphatidic acid by LC/ESI/MS/MS employing a reversed phase HPLC column. J Chromatogr B Analyt Technol Biomed Life Sci 864: 22–28, 2008. doi: 10.1016/j.jchromb.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 128.Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W; Atherosclerosis Risk in Communities Study Group . Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 104: 1108–1113, 2001. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 129.Shi Y, Man RY, Vanhoutte PM. Two isoforms of cyclooxygenase contribute to augmented endothelium-dependent contractions in femoral arteries of 1-year-old rats. Acta Pharmacol Sin 29: 185–192, 2008. doi: 10.1111/j.1745-7254.2008.00749.x. [DOI] [PubMed] [Google Scholar]
- 130.Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci USA 96: 6931–6936, 1999. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, Kim DH, Manthati VL, Koduru SR, Falck JR, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther 331: 906–916, 2009. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4: 397–407, 2003. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 133.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 60: 181–195, 2008. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tanaka T, Tsutsui H, Hirano K, Koike T, Tokumura A, Satouchi K. Quantitative analysis of lysophosphatidic acid by time-of-flight mass spectrometry using a phosphate-capture molecule. J Lipid Res 45: 2145–2150, 2004. doi: 10.1194/jlr.D400010-JLR200. [DOI] [PubMed] [Google Scholar]
- 135.Taniguchi M, Kitatani K, Kondo T, Hashimoto-Nishimura M, Asano S, Hayashi A, Mitsutake S, Igarashi Y, Umehara H, Takeya H, Kigawa J, Okazaki T. Regulation of autophagy and its associated cell death by “sphingolipid rheostat”: reciprocal role of ceramide and sphingosine 1-phosphate in the mammalian target of rapamycin pathway. J Biol Chem 287: 39898–39910, 2012. doi: 10.1074/jbc.M112.416552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Toda N. Age-related changes in endothelial function and blood flow regulation. Pharmacol Ther 133: 159–176, 2012. doi: 10.1016/j.pharmthera.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 137.Tokumura A, Fukuzawa K, Tsukatani H. Effects of synthetic and natural lysophosphatidic acids on the arterial blood pressure of different animal species. Lipids 13: 572–574, 1978. doi: 10.1007/BF02533598. [DOI] [PubMed] [Google Scholar]
- 138.Tomiuk S, Hofmann K, Nix M, Zumbansen M, Stoffel W. Cloned mammalian neutral sphingomyelinase: functions in sphingolipid signaling? Proc Natl Acad Sci USA 95: 3638–3643, 1998. doi: 10.1073/pnas.95.7.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tong X, Peng H, Liu D, Ji L, Niu C, Ren J, Pan B, Hu J, Zheng L, Huang Y. High-density lipoprotein of patients with type 2 diabetes mellitus upregulates cyclooxgenase-2 expression and prostacyclin I-2 release in endothelial cells: relationship with HDL-associated sphingosine-1-phosphate. Cardiovasc Diabetol 12: 27, 2013. doi: 10.1186/1475-2840-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Van Diest MJ, Verbeuren TJ, Herman AG. 15-lipoxygenase metabolites of arachidonic acid evoke contractions and relaxations in isolated canine arteries: role of thromboxane receptors, endothelial cells and cyclooxygenase. J Pharmacol Exp Ther 256: 194–203, 1991. [PubMed] [Google Scholar]
- 141.van Meeteren LA, Moolenaar WH. Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res 46: 145–160, 2007. doi: 10.1016/j.plipres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 142.van Nieuw Amerongen GP, Vermeer MA, van Hinsbergh VW. Role of RhoA and Rho kinase in lysophosphatidic acid-induced endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol 20: E127–E133, 2000. doi: 10.1161/01.ATV.20.12.e127. [DOI] [PubMed] [Google Scholar]
- 143.Vanhoutte PM. Endothelium-dependent contractions in hypertension: when prostacyclin becomes ugly. Hypertension 57: 526–531, 2011. doi: 10.1161/HYPERTENSIONAHA.110.165100. [DOI] [PubMed] [Google Scholar]
- 144.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 9: 225–238, 2006. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 145.Watts JD, Aebersold R, Polverino AJ, Patterson SD, Gu M. Ceramide second messengers and ceramide assays. Trends Biochem Sci 24: 228, 1999. doi: 10.1016/S0968-0004(99)01411-5. [DOI] [PubMed] [Google Scholar]
- 146.Weiner JA, Chun J. Schwann cell survival mediated by the signaling phospholipid lysophosphatidic acid. Proc Natl Acad Sci USA 96: 5233–5238, 1999. doi: 10.1073/pnas.96.9.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Westermann AM, Havik E, Postma FR, Beijnen JH, Dalesio O, Moolenaar WH, Rodenhuis S. Malignant effusions contain lysophosphatidic acid (LPA)-like activity. Ann Oncol 9: 437–442, 1998. doi: 10.1023/A:1008217129273. [DOI] [PubMed] [Google Scholar]
- 148.Wu C, Huang RT, Kuo CH, Kumar S, Kim CW, Lin YC, Chen YJ, Birukova A, Birukov KG, Dulin NO, Civelek M, Lusis AJ, Loyer X, Tedgui A, Dai G, Jo H, Fang Y. Mechanosensitive PPAP2B regulates endothelial responses to atherorelevant hemodynamic forces. Circ Res 117: e41–e53, 2015. doi: 10.1161/CIRCRESAHA.117.306457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu YJ, Aziz OA, Bhugra P, Arneja AS, Mendis MR, Dhalla NS. Potential role of lysophosphatidic acid in hypertension and atherosclerosis. Can J Cardiol 19: 1525–1536, 2003. [PubMed] [Google Scholar]
- 150.Yang S, Wei S, Pozzi A, Capdevila JH. The arachidonic acid epoxygenase is a component of the signaling mechanisms responsible for VEGF-stimulated angiogenesis. Arch Biochem Biophys 489: 82–91, 2009. doi: 10.1016/j.abb.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yoon HR, Kim H, Cho SH. Quantitative analysis of acyl-lysophosphatidic acid in plasma using negative ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 788: 85–92, 2003. doi: 10.1016/S1570-0232(02)01031-0. [DOI] [PubMed] [Google Scholar]
- 152.Yoshimoto T, Takahashi Y. Arachidonate 12-lipoxygenases. Prostaglandins Other Lipid Mediat 68-69: 245–262, 2002. doi: 10.1016/S0090-6980(02)00034-5. [DOI] [PubMed] [Google Scholar]
- 153.Yu J, Novgorodov SA, Chudakova D, Zhu H, Bielawska A, Bielawski J, Obeid LM, Kindy MS, Gudz TI. JNK3 signaling pathway activates ceramide synthase leading to mitochondrial dysfunction. J Biol Chem 282: 25940–25949, 2007. doi: 10.1074/jbc.M701812200. [DOI] [PubMed] [Google Scholar]
- 154.Yung YC, Stoddard NC, Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res 55: 1192–1214, 2014. doi: 10.1194/jlr.R046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zeiher AM, Drexler H, Wollschläger H, Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation 83: 391–401, 1991. doi: 10.1161/01.CIR.83.2.391. [DOI] [PubMed] [Google Scholar]
- 156.Zhang DX, Zou AP, Li PL. Ceramide-induced activation of NADPH oxidase and endothelial dysfunction in small coronary arteries. Am J Physiol Heart Circ Physiol 284: H605–H612, 2003. doi: 10.1152/ajpheart.00697.2002. [DOI] [PubMed] [Google Scholar]
- 157.Zhang G, Panigrahy D, Hwang SH, Yang J, Mahakian LM, Wettersten HI, Liu JY, Wang Y, Ingham ES, Tam S, Kieran MW, Weiss RH, Ferrara KW, Hammock BD. Dual inhibition of cyclooxygenase-2 and soluble epoxide hydrolase synergistically suppresses primary tumor growth and metastasis. Proc Natl Acad Sci USA 111: 11127–11132, 2014. doi: 10.1073/pnas.1410432111. [DOI] [PMC free article] [PubMed] [Google Scholar]