Dear Editor,
Stroke is the most-common neurological complication of infective endocarditis (IE), occurring in about 35% of cases.1 Ischemic stroke occurs due to the embolization of infected valve material to the brain.
Recognizing the possibility of IE within the very short time window available for intravenous thrombolysis in acute ischemic stroke is often difficult. The possible clinical indications of IE are being young with no risk factors, multiple ischemic or hemorrhagic brain lesions, valve abnormalities, preceding weight loss, and fever.1 The difficulty that clinicians experience identifying IE may increase in the presence of another cause of fever.
We present the case of a 59-year-old male with a biological prosthetic aortic valve that had been placed 5 years previously. He was admitted to the emergency department due to sudden-onset aphasia and right facial deficit. Low-grade fever over the previous 20 days and moderate dry cough were reported, and the patient had been treated with azithromycin and hydroxychloroquine.
A neurological evaluation confirmed global aphasia and slight right lower facial deficit, while the findings of brain CT were normal and CT angiography was negative for large-vessel occlusions. A chest CT scan disclosed features that were highly consistent with COVID-19 interstitial pneumopathy. The diagnosis was also supported by low oxygen saturation levels at baseline, promptly reaching normal values under noninvasive oxygen administration. Laboratory examinations detected neutrophilic leucocytosis with a normal lymphocyte count, slightly elevated international normalized ratio, and normal creatine phosphokinase levels.
Systemic recombinant tissue plasminogen activator administration was applied 130 minutes after the clinical onset of aphasia. At the end of the infusion, the patient developed nasal bleeding that was successfully managed with mechanical compression. Therapy with lopinavir/ritonavir (100/25 mg) was started.
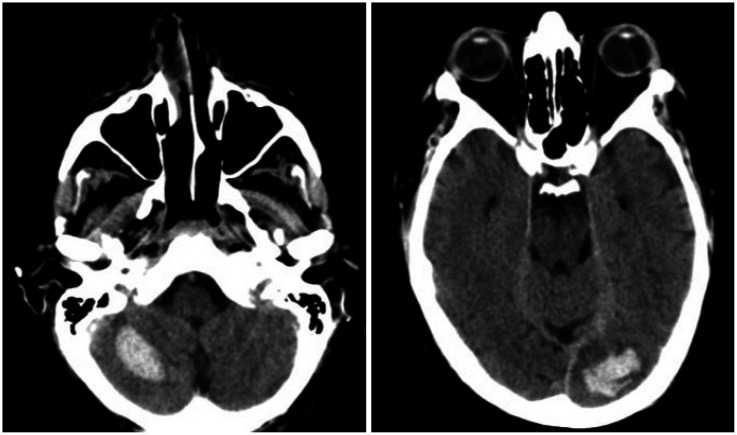
Brain CT performed at 24 hours after the intravenous thrombolysis showed foci of hemorrhagic transformation in the left frontal lobe, left occipital lobe, and right cerebellar hemisphere (Fig. 1), but the neurological condition of the patient had significantly improved.
Fig. 1. Brain CT scan showing foci of hemorrhagic transformation in the right cerebellar hemisphere and left occipital lobe.
Transthoracic echocardiography performed on day 3 after the stroke showed IE at the level of the biological prosthetic valve and a suspected periprosthetic abscess. Blood-specimen cultures showed growth of Enterococcus faecalis, which responded to ampicillin at 3 g four times daily and ceftriaxone at 2 g twice daily. A brain CT scan performed 10 days later showed reabsorption of the hemorrhagic foci.
Intravenous thrombolysis is the standard acute treatment for patients with ischemic stroke, but it is contraindicated in cerebral septic embolism arising from endocarditic lesions due to the high risk of hemorrhage. Although successful cases of thrombolysis have been reported, the overall outcomes are likely to be poor.2 The rate of postthrombolytic hemorrhagic transformation is significantly higher in patients with IE, and the rate of favorable outcomes is a significantly lower in these patients.3 However, excluding this diagnostic hypothesis requires both a high index of clinical suspicion and the emergency availability of echocardiography, which is not considered in current guidelines as mandatory before thrombolysis. Thus, interpreting the clinical and chest-CT picture of the present patient as an expression of ischemic stroke in the context of COVID-19 infection led to underestimation of the alternative hypothesis of embolic stroke in IE.
In this patient, COVID-19 infection was a confounding element and we think that it was not connected to IE. COVID-19 infection may lead both to superimposed bacterial disease and to increased activation of procoagulant cascade. Actually, due to the increasing awareness of the thromboembolic risk in these patients, the current practice in our institution includes prophylactic low-molecular-weight heparin for at least 14 days even in ambulatory COVID-19 patients who are dismissed from the hospital and waiting isolated at home to receive the negative result for a nasal swab.
To conclude, clinical scenarios in acute-ischemic-stroke patients with fever are often complex, due to both the rapid decisions required and the difficulty in performing echocardiography on an urgent basis. The present case emphasizes the need for echocardiography in urgent practice to ensure the correct differential diagnosis on etiologies when deciding whether a patient with ischemic stroke should be subjected to thrombolytic therapy. In the present era of the COVID-19 pandemic, we think that the risk-and-benefit equation in feverish patients questions any rigid guidelines, also considering that there is evidence that fibrinolytic therapy in acute lung injury and ARDS improves survival.4
Acknowledgements
None.
Footnotes
- Conceptualization: Vittorio Mantero.
- Data curation: Vittorio Mantero, Andrea Rigamonti, Paola Basilico, Davide Sangalli, Chiara Scaccabarozzi.
- Writing—original draft: all authors.
- Writing—review & editing: Andrea Salmaggi.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Jiad E, Gill SK, Krutikov M, Turner D, Parkinson MH, Curtis C, et al. When the heart rules the head: ischaemic stroke and intracerebral haemorrhage complicating infective endocarditis. Pract Neurol. 2017;17:28–34. doi: 10.1136/practneurol-2016-001469. [DOI] [PubMed] [Google Scholar]
- 2.Ong E, Mechtouff L, Bernard E, Cho TH, Diallo LL, Nighoghossian N, et al. Thrombolysis for stroke caused by infective endocarditis: an illustrative case and review of the literature. J Neurol. 2013;260:1339–1342. doi: 10.1007/s00415-012-6802-1. [DOI] [PubMed] [Google Scholar]
- 3.Asaithambi G, Adil MM, Qureshi AI. Thrombolysis for ischemic stroke associated with infective endocarditis: results from the nationwide inpatient sample. Stroke. 2013;44:2917–2919. doi: 10.1161/STROKEAHA.113.001602. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18:1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]