Abstract
Background and Purpose
The aim of this study was to investigate the effects of multicomponent exercise on cognitive function, depression, and quality of life in elderly individuals.
Methods
This study prospectively recruited 605 participants, and constructed an exercise pyramid comprising even distributions of daily physical activities, aerobic exercise, muscle-strengthening exercise, flexibility exercise, balance exercise, and activities that subjects could perform while sitting down. The exercise program was divided into six stages according to the participant's level of frailty. The 12-week exercise program intervention was conducted once yearly.
Results
The exercise regimen was followed by 402 of the 605 enrolled participants, giving a dropout rate of 33.6%. The 27-month exercise program was completed by 60 participants. The scores for the Mini Mental State Examination for dementia screening (MMSE-DS), short form of the Geriatric Depression Scale, World Health Organization Quality of Life Assessment (WHOQOL-BREF), International Physical Activity Questionnaire (IPAQ), fear of falling, handgrip strength, and walking speed were improved after the exercise intervention. The analysis of frailty revealed that participants in the frail group showed greater improvements for the MMSE-DS, WHOQOL-BREF, IPAQ, fear of falling, handgrip strength, and walking speed.
Conclusions
Individually customized, multicomponent exercise programs lead to improved levels of cognitive function, depression, and quality of life, especially among those who are more frail.
Keywords: exercise, cognition, quality of life
INTRODUCTION
Healthy aging is the process of developing and maintaining the functional abilities to facilitate the well-being of older individuals, which is of great social interest in the aging population. Maintaining cognitive functional abilities is an important part of healthy aging, and strategies are needed to slow the age-related decline of cognitive function and reduce disease-related cognitive impairment in older adults. Exercise is one such strategy because it improves not only physical health, by reducing the risk of health issues such as cardiovascular disease, stroke, diabetes, and functional disability,1 but also mental health2,3 and cognitive function in cognitively healthy older adults4,5,6 as well as in older adults with cognitive impairment or dementia.7,8 Despite the benefits of exercise, many older people lead less-active and more-sedentary lives. An exercise program that can be easily implemented by that population in the community is needed.
There are various types of exercise, including aerobic, muscle-strengthening, balance, and flexibility exercise. Among these types of exercise, cognitive function is most commonly associated with aerobic exercise. Muscle-strengthening exercise is considered to have a positive effect on cognitive function, but this type of exercise was not as well-studied. There is a lack of evidence showing that exercise focused on flexibility and balance prevents cognitive decline. However, it is important to combine various types of exercise when attempting to promote healthy aging. Although aerobic exercise is occupies the largest portion, the American College of Sports Medicine (ACSM) and the American Heart Association (AHA)9 encourage all kinds of exercise. Muscle strengthening exercise prevents age-related loss of muscle mass and balance exercise is important for elderly people who are at a high risk of falling. Several previous studies suggested that multicomponent exercise may have a larger effect on cognition in older adults than aerobic exercise alone.5,10,11 However, the application of different exercise regimens in each study restricts the ability to identify the effects of multicomponent exercise.
An important aspect of aging that varies among individuals is frailty. Frailty is a geriatric syndrome characterized by increased vulnerability to possible stressors, and it is strongly predictive of mortality and disability.12,13 The presence of three or more of the following five dimensions indicates frailty: weight loss, weakness, poor endurance, low energy level, and a low level of physical activity.12 The severity of frailty can be divided into three levels: not frail, prefrail, and frail. Frailty has cognitive components as well as physical components, and these components exert adverse affects on each other.14,15 Although exercise is an important method of managing frailty,16 few studies have analyzed the effects of balanced multicomponent exercise on frailty in elderly people.
Based on the above-described situation, we developed individually customized multicomponent exercise programs that could be performed by elderly individuals in their homes. The present study analyzed the effectiveness of multicomponent exercise on the cognitive function, depression, and quality of life of elderly individuals. Our hypothesis was that a multicomponent exercise program would be effective at improving health-related problems in community-dwelling older individuals.
METHODS
Study participants
Participants who were older than 60 years and were followed by welfare centers for the elderly, Seongnam Center for Senior Health, and Seongnam Visiting Health Care Center were arbitrarily recruited from local communities. We visited these centers and advertised multicomponent exercise as a part of the public health care and welfare program. The participants were not compensated financially, and participated voluntarily. The exclusion criteria included subjects who had 1) participated in other clinical trials within the previous 4 weeks or had received a clinical trial drug treatment; 2) physical disabilities and could not walk independently; 3) signs of a severe or unstable physical illness, such as acute and severe asthma, severe and unstable cardiovascular disease, an active peptic ulcer, or renal disease to the extent of severe liver disease or requiring renal dialysis; 4) severe hearing or visual difficulties that inhibited the ability to evaluate the effectiveness of exercise; or 5) not agreed to participate in this study.
Applying the above criteria resulted in the recruitment of 605 subjects who were willing to participate voluntarily. Among them, 203 subjects were excluded from the analysis because they stopped performing the exercise before the first postexercise evaluation. Ethical approval was obtained from the Institutional Review Board of the Korea National Institute for Bioethics Policy (P01-201702-11-001). All participants provided written informed consent before enrollment.
The multicomponent exercise pyramid
To develop dementia prevention exercise regimens that are individually customized according to the physical strength of the individuals, we assembled a specialist team composed of two neurologists, a professor of medicine, a professor of biotechnology, social workers, physiotherapists, occupational therapists, and nurses. These specialists constructed an exercise pyramid model by referring to the ACSM/AHA recommendations for physical activity and public health in older adults.9 The exercise pyramid was designed to encourage the participants to perform an even distribution of daily physical activities, aerobic exercise, muscle-strengthening exercise, flexibility exercise, balance exercise, and activities while sitting down. The sedentary activity included simple cognitive activities (e.g., reciting the names of 15 cities). The exercise pyramid is in the form of a triangular diagram that includes the optimal amount of each type of exercise on each stage so as to create a balanced regimen. The pyramid consists of six stages that differ in intensity and frequency: the frail, prefrail, and not-frail groups were assigned to stages 1 and 2, 3 and 4, and 5 and 6, respectively (Supplementary Material 1 in the online-only Data Supplement). The pyramid is designed so that a participant can perform a customized exercise regimen according to their degree of frailty. The frail group was excluded from the balance exercises in order to prevent accidents such as falls.
Assessment of frailty
To assign individually customized exercise programs, the participants were categorized into frail, prefrail, and not-frail groups according to the Fried frailty criteria.12 These criteria assess the five dimensions of frailty using self-reported and performance-based measures. This study modified some of those dimensions for the context-specific situations in Korea. The five dimensions are as follows: 1) unintentional weight loss of 4.5 kg during the previous year; 2) muscle weakness as assessed by handgrip strength measurements, while accounting for sex and body mass index; 3) exhaustion, which was considered to be indicated by a score of 8 or higher on the short form of the Geriatric Depression Scale (GDS-SF)17,18,19; 4) walking speed, which was evaluated by a 3-m walk based on the typical characteristics of actual living spaces in Korea; and 5) low physical activity, as evaluated using the International Physical Activity Questionnaire (IPAQ). The weekly rate of energy expenditure was calculated and compared with reference values according to sex. After evaluating the five dimensions, frail, prefrail, and not-frail individuals were defined as those who met at least three criteria, one or two criteria, and none of the criteria, respectively (Supplementary Material 2 in the online-only Data Supplement).
Components and methods of the exercise program
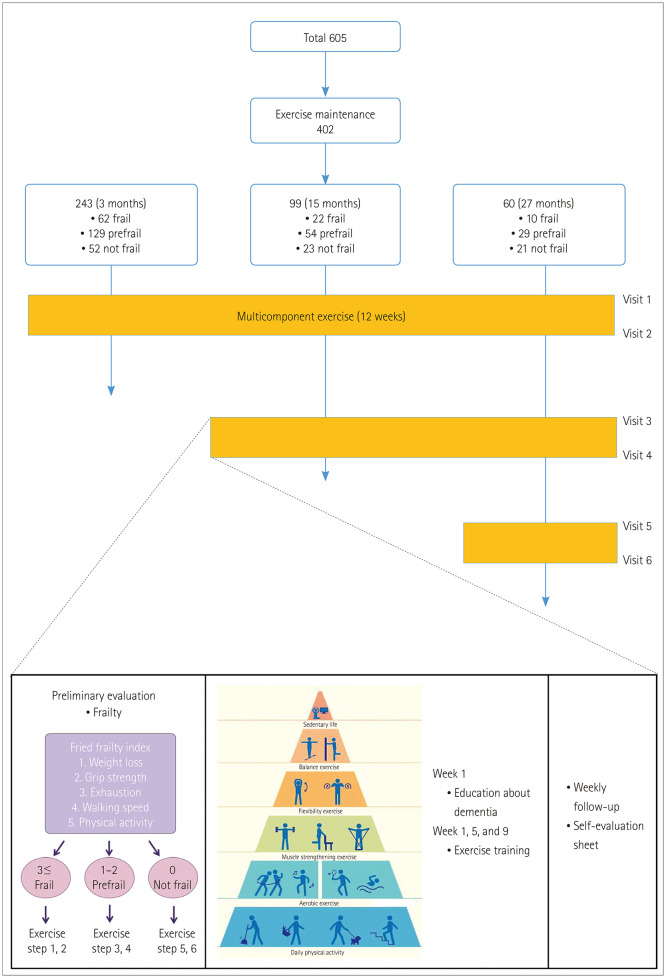
A 12-week exercise program intervention was conducted yearly from April 2015 to July 2017. Participants were interviewed about their current lifestyle before the exercise training, including their habitual physical activity and current medication. During the exercise training, 15-minute educational sessions were conducted to improve awareness of the benefits of exercise on dementia and to motivate the participants. This was followed by the instructor demonstrating the exercises to the participants in a step-by-step manner for 30 minutes. Exercise training was conducted once monthly for a total of three times during the 12-week period in groups or individually. The participants performed daily exercise individually and voluntarily at home. During the 12-week period, we kept track of the participants on a weekly basis by phone, ensuring that they were exercising according to their assigned level and that they completed a self-assessment form daily (Fig. 1 and Supplementary Material 3 in the online-only Data Supplement).
Fig. 1. Flow diagram of the study population and intervention.
Clinical assessment and outcomes
The following assessments were performed before and after each yearly implementation of the 12-week exercise program: cognitive status, using the Mini Mental State Examination for dementia screening (MMSE-DS); depression status, using the GDS-SF; quality of life, using the World Health Organization Quality of Life Assessment (WHOQOL-BREF); handgrip strength; 3-m walking speed; fear of falling, using a Visual Analog Scale for fear of falling (VAS-FOF); and level of physical activity, using the IPAQ. Depending on the participant's exercise duration, a maximum of six evaluations were conducted over the 27-month study period (Fig. 1).
The MMSE-DS is a screening test developed by Folstei et al. that is used for simple and rapid assessments of cognitive impairment.20 Higher scores indicate better cognitive function, and the maximum score is 30 points. Cognitive impairment was defined as any score lower than the cutoff score of the MMSE-DS based on the education level, age, and sex. The GDS-SF is designed to detect depression in elderly people.21,22 The original scale was developed as a 30-item test, but its 15-item short form was used in this study. Depression is characterized by a score of ≥8 on this test, with higher scores indicating more-severe depressive symptoms. The WHOQOL-BREF is a questionnaire developed by the World Health Organization for assessing the quality of life of individuals.23,24 This questionnaire consists of 26 items that measure 4 domains: physical health, psychological health, social relationships, and environment. Each WHOQOL-BREF item is rated on a 5-point Likert Scale from 1 to 5, with higher scores indicating a higher quality of life. Fear of falling was measured using a Visual Analog Scale25,26 ranging from 0 (no fear of falling) to 100 (extreme fear of falling). Finally, the IPAQ was used to measure health-related physical activity.27,28,29 This measure assesses the intensity of the physical activities that people engage in over 7 days, and then quantifies the total physical activity as the metabolic equivalent task minutes per week and also based on the total sitting time. The intensity of physical activity is classified into vigorous, moderate, walking, and sitting. The recorded physical activity time was converted into the calories used for physical activity per week based on the IPAQ value conversion guidelines.
Statistical analysis
Descriptive data are presented as frequencies and percentages for categorical variables and mean±standard-deviation values for continuous variables. The chi-square test, t-test, and ANOVA were used for group comparisons. Two-way repeated-measures ANOVA for the continuous variables and generalized estimating equations for the categorical variables were used to identify annual changes in variables before and after the exercise intervention. One factor (before and after the intervention) shows the short-term effect of multicomponent exercise, and the other factor (annual change) shows the long-term effect of multicomponent exercise. Probability values of p<0.05 were considered statistically significant. All statistical analyses were performed with SAS® software (version 9.4, SAS Institute, Cary, NC, USA).
RESULTS
The exercise regimen was followed by 402 of the 605 enrolled participants. The duration of exercise varied with the voluntary will of individual participants. The 27-month exercise program (3 consecutive years) was completed by 60 subjects, while 243 subjects followed the regimen for 3 months and 99 subjects followed it for 15 months. Differences between the subjects who did and did not finish the program are listed in Table 1. The participants who maintained their exercise were aged 76.2±5.4 years, and 81.7% of them were female. The duration of education was 5.2±4.6 years. The MMSE-DS score was 24.2±4.9, and 16.7% of them had cognitive impairment. The scores on the GDS-SF, WHOQOL-BREF, and VAS-FOF were 3.5±3.4, 88.4±11.8, and 46.5±28.9, respectively. The values for the IPAQ and handgrip strength test were 1,372.4± 1,396.6 kcal and 19.6±8.2 kg, respectively; these values were within the normal range for 95.0% and 56.7% of the participants, respectively. The walking speed was 0.9±0.3 m/s, and 81.7% of the participants had a walking speed within the normal range (Table 1).
Table 1. Baseline characteristics of subjects who were included in the 1-year follow-up and who completed the 27-month exercise program.
| Total | Completed the study | One-year follow-up | p | |
|---|---|---|---|---|
| Number of subjects | 402 (100.0) | 60 (14.9) | 342 (85.1) | |
| Sex, female | 344 (85.6) | 49 (81.7) | 295 (86.3) | 0.351 |
| Age, years | 77.5±6.4 | 76.2±5.4 | 77.7±6.5 | 0.089 |
| Education, years | 5.2±4.6 | 5.2±4.6 | 5.3±4.6 | 0.974 |
| Literate | 321 (76.9) | 45 (75.0) | 276 (80.7) | 0.310 |
| Living alone | 161 (40.0) | 22 (36.7) | 139 (40.6) | 0.562 |
| Medical insurance | 306 (76.1) | 60 (100.0) | 246 (71.9) | <0.001 |
| Diseases | ||||
| Hypertension | 255 (64.4) | 40 (66.7) | 215 (62.9) | 0.573 |
| Diabetes | 102 (25.4) | 15 (25.0) | 87 (25.4) | 0.943 |
| Hyperlipidemia | 100 (24.9) | 17 (28.3) | 83 (24.3) | 0.502 |
| Heart disease | 59 (14.7) | 6 (10.0) | 53 (15.5) | 0.267 |
| Stroke | 24 (6.0) | 3 (5.0) | 21 (6.1) | 0.731 |
| Physical activity | ||||
| Vigorous | 8 (1.2) | 1 (1.7) | 7 (2.2) | 0.785 |
| Moderate | 152 (37.8) | 11 (18.3) | 141 (44.8) | <0.001 |
| Walking | 331 (82.3) | 59 (98.3) | 272 (86.4) | 0.008 |
| Sitting | 218 (54.2) | |||
| MMSE-DS score | 24.5±4.2 | 24.2±4.9 | 24.6±4.0 | 0.554 |
| Cognitive impairment | 38 (9.5) | 10 (16.7) | 28 (8.2) | 0.038 |
| GDS-SF score | 4.6±4.2 | 3.5±3.4 | 4.8±4.3 | 0.008 |
| Depression | 98 (24.4) | 9 (15.0) | 89 (26.0) | 0.067 |
| WHOQOL-BREF score | 82.9±15.6 | 88.4±11.8 | 81.9±16.0 | <0.001 |
| VAS-FOF score | 47.2±31.8 | 46.5±28.9 | 47.4±32.4 | 0.848 |
| 298 (74.1) | 46 (76.7) | 252 (73.7) | 0.627 | |
| IPAQ value, kcal | 1,258.3±1,339.1 | 1,372.4±1,396.6 | 1,238.3±1,329.8 | 0.475 |
| Normal | 337 (83.8) | 57 (95.0) | 280 (81.9) | 0.011 |
| Handgrip strength, kg | 18.5±7.2 | 19.6±8.2 | 18.3±7.0 | 0.208 |
| Normal | 174 (43.3) | 34 (56.7) | 140 (40.9) | 0.023 |
| Walking speed, m/s | 0.9±0.3 | 0.9±0.3 | 0.9±0.3 | 0.058 |
| Normal | 270 (67.2) | 49 (81.7) | 221 (64.6) | 0.010 |
The chi-square test and ANOVA were used for group comparisons. Data are n (%) or mean±standard-deviation values.
GDS-SF: short form of the Geriatric Depression Scale, IPAQ: International Physical Activity Questionnaire, MMSE-DS: Mini Mental State Examination for dementia screening, VAS-FOF: Visual Analogue Scale for fear of falling, WHOQOL-BREF: World Health Organization Quality of Life Assessment.
The 60 subjects who completed the exercise program comprised 10, 29, and 21 who were defined as frail, prefrail, and not frail according to the Fried frailty index. The subjects were older in the frail group (78.7±4.8 years in the frail group vs. 76.6 ± 4.8 years and 74.4 ± 6.1 years in the prefrail and not-frail groups, respectively, p<0.001), and the frail group included subjects with significant illiteracy (p<0.001). There was no significant intergroup difference in the disease status except for heart disease. Compared to the other groups, the frail group had significantly lower MMSE-DS scores (p=0.003) and handgrip strength (p=0.028), and significantly higher rates of cognitive impairment (p=0.002), depression (p<0.001), and subjects with abnormal walking speeds (p<0.001) (Table 2).
Table 2. Baseline characteristics of subjects in the three frailty groups who completed the 27-month study.
| Total | Frail | Prefrail | Not frail | p | |
|---|---|---|---|---|---|
| Number of subjects | 60 (100.0) | 10 (16.7) | 29 (48.3) | 21 (35.0) | |
| Sex, female | 49 (81.7) | 10 (100.0) | 22 (75.9) | 17 (81.0) | 0.234 |
| Age, years | 76.2±5.4 | 78.7±4.8 | 76.6±4.8 | 74.4±6.1 | <0.001 |
| Education, years | 5.2±4.6 | 1.2±2.8 | 4.8±4.6 | 7.8±3.8 | 0.236 |
| Literate | 45 (75.0) | 3 (30.0) | 21 (72.4) | 21 (100.0) | <0.001 |
| Living alone | 22 (36.7) | 4 (40.0) | 13 (44.8) | 5 (23.8) | 0.458 |
| Medical insurance | 60 (100.0) | 10 (100.0) | 29 (100.0) | 21 (100.0) | |
| Diseases | |||||
| Hypertension | 40 (66.7) | 7 (70.0) | 18 (62.1) | 15 (71.4) | 0.763 |
| Diabetes | 15 (25.0) | 3 (30.0) | 8 (27.6) | 4 (19.1) | 0.729 |
| Hyperlipidemia | 17 (28.3) | 1 (10.0) | 9 (31.0) | 7 (33.3) | 0.365 |
| Heart disease | 6 (10.0) | 0 (0.0) | 1 (3.5) | 5 (23.8) | 0.031 |
| Stroke | 3 (5.0) | 0 (0.0) | 2 (6.9) | 1 (4.8) | 0.113 |
| Dementia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.790 |
| Physical activity | |||||
| Vigorous | 1 (1.7) | 0 (0.0) | 0 (0.0) | 1 (6.0) | |
| Moderate | 11 (18.3) | 0 (0.0) | 5 (17.2) | 6 (28.6) | |
| Walking | 59 (98.3) | 10 (100.0) | 29 (100.0) | 20 (95.2) | |
| MMSE-DS score | 24.2±5.0 | 17.5±5.3 | 24.1±4.0 | 27.4±2.0 | 0.003 |
| Cognitive impairment | 10 (16.7) | 5 (6.6) | 5 (3.9) | 0 (3.2) | 0.002 |
| GDS-SF score | 3.5±3.4 | 6.6±3.9 | 3.2±3.6 | 2.4±1.8 | 0.004 |
| Depression | 9 (15.0) | 6 (60.0) | 3 (10.3) | 0 (0.0) | <0.001 |
| WHOQOL-BREF score | 88.4±11.8 | 84.4±16.2 | 90.5±11.1 | 87.3±10.4 | 0.169 |
| VAS-FOF score | 46.5±28.9 | 65.0±30.3 | 44.1±31.1 | 41.0±22.1 | 0.105 |
| IPAQ value, kcal | 1,372.4±1,396.6 | 595.8±293.3 | 1,197.3±1,032.8 | 1,983.9±1,866.4 | 0.089 |
| Normal | 57 (95.0) | 9 (90.0) | 27 (93.1) | 21 (100.0) | 0.396 |
| Handgrip strength, kg | 19.6±8.2 | 11.8±4.1 | 19.6±9.1 | 23.3±5.6 | 0.028 |
| Normal | 34 (56.7) | 0 (0.0) | 13 (44.8) | 21 (100.0) | <0.001 |
| Walking speed, m/s | 0.9±0.2 | 0.7±0.2 | 1.0±0.2 | 1.1±0.1 | 0.223 |
| Normal | 49 (81.7) | 4 (40.0) | 24 (82.8) | 21 (100.0) | <0.001 |
The chi-square test and ANOVA were used for group comparisons. Data are n (%) or mean±standard-deviation values.
GDS-SF: short form of the Geriatric Depression Scale, IPAQ: International Physical Activity Questionnaire, MMSE-DS: Mini Mental State Examination for dementia screening, VAS-FOF: Visual Analogue Scale for fear of falling, WHOQOL-BREF: World Health Organization Quality of Life Assessment.
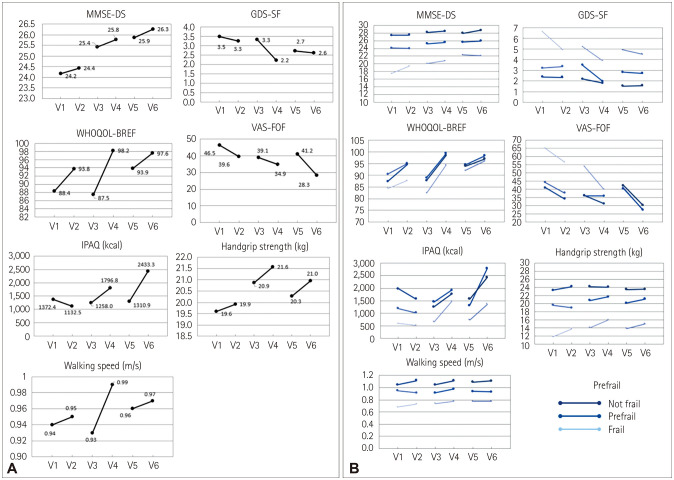
The scores for the MMSE-DS (p<0.001 for time), GDS-SF (p=0.017 for time and p=0.023 for intervention), and WHOQOL-BREF (p<0.001 for intervention) improved significantly after the multicomponent exercise. The VAS-FOF score (p=0.001 for intervention), handgrip strength (p=0.010 for time and p=0.037 for intervention), and walking speed (p=0.027 for intervention) also improved significantly. There were statistically significant improvements in the WHOQOL-BREF score (p=0.003 for time) and IPAQ value (p=0.002 for time and p=0.001 for intervention), but a significant interaction was observed across time and exercise intervention for WHOQOL-BREF (p=0.003) and IPAQ (p<0.001).
Analyzing the effects of multicomponent exercise according to the degree of frailty revealed that the frail group showed significant improvements in the MMSE-DS score (p=0.001 for time), WHOQOL-BREF score (p=0.049 for time and p=0.004 for intervention), VAS-FOF score (p=0.040 for time), IPAQ value (p=0.035 for intervention), handgrip strength (p=0.023 for time and p=0.009 for intervention), and walking speed (p=0.026 for intervention). However, the GDS-SF score did not change significantly, and there was no significant interaction across time and exercise for any of the variables. The prefrail group showed significant improvements in the MMSE-DS score (p<0.001 for time), WHOQOL-BREF score (p<0.001 for intervention), and handgrip strength (p=0.003 for time time), whereas the GDS-SF score, VAS-FOF score, IPAQ value, and walking speed did not change significantly. The not-frail group showed significant improvements in the MMSE-DS score (p=0.006 for time), WHOQOL-BREF score (p<0.001 for intervention), VAS-FOF score (p=0.009 for in tervention), and walking speed (p=0.027 for intervention), while the GDS-SF score, IPAQ value, and handgrip strength did not change significantly (Table 3 and Fig. 2).
Table 3. Effects of multicomponent exercise according to the degree of frailty.
| Year 1 | Year 2 | Year 3 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | year | Intervention | Year×intervention | |
| MMSE-DS score | |||||||||
| Total (n=60) | 24.2±4.9 | 24.4±4.6 | 25.4±3.8 | 25.8±4.0 | 25.9±3.5 | 26.3±3.6 | <0.001 | 0.057 | 0.946 |
| Frail (n=10) | 17.5±5.3 | 19.4±4.4 | 20.1±2.9 | 20.8±3.7 | 22.3±4.1 | 22.1±4.6 | 0.001 | 0.072 | 0.309 |
| Prefrail (n=29) | 24.1±4.0 | 24.0±4.3 | 25.2±3.1 | 25.6±3.8 | 25.6±3.1 | 25.9±3.1 | <0.001 | 0.576 | 0.594 |
| Not frail (n=21) | 27.4±2.0 | 27.5±1.9 | 28.2±1.9 | 28.5±1.3 | 28.0±1.8 | 28.7±1.2 | 0.006 | 0.154 | 0.362 |
| Cognitive impairment | |||||||||
| Total | 10 (16.7) | 7 (11.7) | 3 (5.0) | 2 (3.3) | 5 (8.3) | 2 (3.3) | 0.153 | 0.010 | |
| Frail | 5 (50.0) | 4 (40.0) | 2 (20.0) | 1 (10.0) | 2 (20.0) | 1 (10.0) | 0.131 | <0.001 | |
| Prefrail | 5 (17.2) | 3 (10.3) | 1 (3.4) | 1 (3.4) | 2 (6.9) | 1 (3.4) | 0.276 | 0.249 | |
| Not frail | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.8) | 0 (0.0) | |||
| GDS-SF score | |||||||||
| Total | 3.5±3.4 | 3.3±3.5 | 3.3±3.7 | 2.2±3.0 | 2.7±3.1 | 2.6±3.3 | 0.017 | 0.023 | 0.082 |
| Frail | 6.6±3.9 | 4.9±4.0 | 5.2±4.2 | 3.9±3.8 | 4.9±4.0 | 4.5±3.5 | 0.309 | 0.074 | 0.622 |
| Prefrail | 3.2±3.6 | 3.3±3.9 | 3.5±3.9 | 2.0±2.8 | 2.8±3.1 | 2.7±3.5 | 0.288 | 0.140 | 0.061 |
| Not frail | 2.4±1.8 | 2.3±2.3 | 2.2±2.7 | 1.8±2.8 | 1.5±2.1 | 1.6±2.6 | 0.099 | 0.592 | 0.769 |
| Depression | |||||||||
| Total | 9 (15.0) | 8 (13.3) | 10 (16.7) | 6 (10.0) | 6 (10.0) | 7 (11.7) | 0.421 | 0.424 | |
| Frail | 6 (60.0) | 3 (30.0) | 3 (30.0) | 2 (20.0) | 3 (30.0) | 2 (20.0) | 0.357 | 0.162 | |
| Prefrail | 3 (10.3) | 5 (17.2) | 5 (17.2) | 2 (6.9) | 3 (10.3) | 4 (13.8) | 1.000 | 0.111 | |
| Not frail | 0 (0.0) | 0 (0.0) | 2 (9.5) | 2 (9.5) | 0 (0.0) | 1 (4.8) | 1.000 | 0.161 | |
| WHOQOL-BREF score | |||||||||
| Total | 88.4±11.8 | 93.8±13.7 | 87.5±13.8 | 98.2±15.5 | 93.9±16.1 | 97.6±14.7 | 0.003 | <0.001 | 0.003 |
| Frail | 84.4±16.2 | 87.8±12.9 | 82.4±17.4 | 94.4±17.9 | 92.0±18.6 | 96.2±12.4 | 0.049 | 0.004 | 0.081 |
| Prefrail | 90.5±11.1 | 95.1±13.4 | 88.9±13.9 | 99.3±16.3 | 94.5±17.9 | 98.4±16.4 | 0.270 | <0.001 | 0.111 |
| Not frail | 87.3±1.4 | 94.7±14.4 | 87.8±11.9 | 98.4±13.7 | 93.8±12.6 | 97.0±13.9 | 0.055 | <0.001 | 0.090 |
| VAS-FOF score | |||||||||
| Total | 46.5±28.9 | 39.6±33.9 | 39.1±33.7 | 34.9±34.5 | 41.2±32.6 | 28.3±30.3 | 0.128 | 0.001 | 0.193 |
| Frail | 65.0±30.3 | 56.5±30.4 | 54.0±31.0 | 40.0±35.9 | 41.0±31.5 | 27.0±31.3 | 0.040 | 0.114 | 0.821 |
| Prefrail | 44.1±31.1 | 37.8±32.5 | 36.0±36.6 | 35.9±36.7 | 40.3±34.4 | 27.3±29.5 | 0.530 | 0.088 | 0.229 |
| Not frail | 41.0±22.1 | 34.1±36.2 | 36.2±30.1 | 31.2±31.9 | 42.4±32.2 | 30.2±32.3 | 0.839 | 0.009 | 0.682 |
| IPAQ value, kcal | |||||||||
| Total | 1,372.4±1,396.6 | 1,132.5±1,302.1 | 1,258.0±1,030.0 | 1,796.8±1,302.4 | 1,310.9±1,194.5 | 2,433.3±2,190.7 | 0.002 | 0.001 | <0.001 |
| Frail | 595.8±293.3 | 526.9±700.1 | 660.0±385.0 | 1,479.4±1,258.4 | 739.5±563.1 | 1,365.3±1,007.4 | 0.118 | 0.035 | 0.225 |
| Prefrail | 1,198.3±1,032.8 | 1,025.5±1,408.3 | 1,461.0±1,041.4 | 1,925.2±1,479.4 | 1,315.8±1,043.6 | 2,798.4±2,366.7 | 0.001 | 0.016 | <0.001 |
| Not frail | 1,983.9±1,866.4 | 1,568.6±1,266.9 | 1,262.3±1,142.7 | 1,770.5±1,073.2 | 1,576.3±1,518.0 | 2,437.8±2,257.3 | 0.346 | 0.097 | 0.055 |
| Normal | |||||||||
| Total | 57 (95.0) | 49 (81.7) | 55 (91.7) | 58 (96.7) | 53 (88.3) | 57 (95.0) | 1.000 | 0.008 | |
| Frail | 9 (90.0) | 6 (60.0) | 9 (90.0) | 10 (100.0) | 7 (70.0) | 9 (90.0) | 0.714 | 0.025 | |
| Prefrail | 27 (93.1) | 23 (79.3) | 28 (96.6) | 27 (93.1) | 26 (89.7) | 28 (96.6) | 0.350 | 0.385 | |
| Not frail | 21 (100.0) | 20 (95.2) | 18 (85.7) | 21 (100.0) | 20 (95.2) | 20 (95.2) | 0.301 | 0.072 | |
| Handgrip strength, kg | |||||||||
| Total | 19.6±8.2 | 19.9±9.1 | 20.9±8.4 | 21.6±8.2 | 20.3±7.5 | 21.0±7.8 | 0.010 | 0.037 | 0.807 |
| Frail | 11.8±4.1 | 13.8±3.0 | 14.1±3.3 | 16.0±3.9 | 13.8±2.8 | 15.0±2.8 | 0.023 | 0.009 | 0.798 |
| Prefrail | 19.6±9.1 | 18.9±9.8 | 20.8±9.3 | 21.7±9.2 | 20.2±7.9 | 21.1±8.9 | 0.003 | 0.337 | 0.124 |
| Not frail | 23.3±5.6 | 24.2±8.1 | 24.2±6.9 | 24.1±6.9 | 23.5±6.5 | 23.5±6.1 | 0.830 | 0.535 | 0.689 |
| Normal | |||||||||
| Total | 34 (56.7) | 29 (48.3) | 31 (51.7) | 33 (55.0) | 31 (51.7) | 34 (56.7) | 0.960 | 0.324 | |
| Frail | 0 (0.0) | 1 (10.0) | 1 (10.0) | 2 (20.0) | 2 (20.0) | 2 (20.0) | 0.198 | 0.069 | |
| Prefrail | 13 (44.8) | 12 (41.4) | 11 (37.9) | 15 (51.7) | 12 (41.4) | 14 (48.3) | 0.696 | 0.117 | |
| Not frail | 21 (100.0) | 16 (76.2) | 19 (90.5) | 16 (76.2) | 17 (81.0) | 18 (85.7) | 0.749 | 0.410 | |
| Walking speed, m/s | |||||||||
| Total | 0.9±0.2 | 1.0±0.3 | 0.9±0.2 | 1.0±0.2 | 1.0±0.2 | 1.0±0.2 | 0.519 | 0.027 | 0.032 |
| Frail | 0.7±0.2 | 0.7±0.2 | 0.8±0.2 | 0.8±0.2 | 0.8±0.2 | 0.8±0.2 | 0.246 | 0.026 | 0.655 |
| Prefrail | 1.0±0.2 | 0.9±0.2 | 0.9±0.1 | 1.0±0.2 | 0.9±0.2 | 0.9±0.2 | 0.822 | 0.725 | 0.020 |
| Not frail | 1.1±0.1 | 1.1±0.2 | 1.0±0.1 | 1.1±0.2 | 1.1±0.2 | 1.1±0.2 | 0.760 | 0.027 | 0.317 |
| Normal | |||||||||
| Total | 49 (81.67) | 46 (76.67) | 50 (83.33) | 53 (88.33) | 47 (78.33) | 52 (86.67) | 0.910 | 0.015 | |
| Frail | 4 (40.0) | 3 (30.0) | 4 (40.0) | 7 (70.0) | 5 (50.0) | 6 (60.0) | 0.612 | 0.005 | |
| Prefrail | 24 (82.8) | 22 (75.9) | 25 (86.2) | 26 (89.7) | 22 (75.9) | 26 (89.7) | 0.796 | 0.034 | |
| Not frail | 21 (100.0) | 21 (100.0) | 21 (100.0) | 20 (95.2) | 20 (95.2) | 20 (95.2) | 0.282 | 0.544 | |
Data are from two-way repeated-measures ANOVA and generalized estimating equations. Data are n (%) or mean±standard-deviation values.
GDS-SF: short form of the Geriatric Depression Scale, IPAQ: International Physical Activity Questionnaire, MMSE-DS: Mini Mental State Examination for dementia screening, VAS-FOF: Visual Analogue Scale for fear of falling, WHOQOL-BREF: World Health Organization Quality of Life Assessment.
Fig. 2. Effects of multicomponent exercise according to the degree of frailty. A: All participants. B: According to the degree of frailty. GDS-SF: short form of the Geriatric Depression Scale, IPAQ: International Physical Activity Questionnaire, MMSE-DS: Mini Mental State Examination for dementia screening, VAS-FOF: Visual Analogue Scale for fear of falling, WHOQOL-BREF: World Health Organization Quality of Life Assessment.
DISCUSSION
Multicomponent exercise exerted significant beneficial effects on cognitive function, depression, quality of life, physical activity, fear of falling, handgrip strength, and walking speed in this study. Long-term effects were observed for cognitive function, depression, and handgrip strength, and short-term effects were observed for depression, quality of life, fear of falling, handgrip strength, and walking speed.
Many studies have investigated the effects of exercise on cognitive function, depression, and quality of life. There is a growing body of evidence from epidemiological studies showing that exercise and physical activity can delay the onset and progression of dementia in older adults.30,31 Aerobic exercise is known to be associated with reduced cardiovascular risk factors, resulting in a reduced risk of vascular dementia or small-vessel disease of the brain, and enhancing neurogenesis and neuroplasticity of the hippocampus by increasing the regional blood volume in the hippocampal dentate gyrus,32 brain-derived neurotrophic factors,33,34 and the volume of the hippocampus. Although less clear than the effects of aerobic exercise, muscle-strengthening exercise is considered to have a positive effect on cognitive function by promoting the secretion of insulin-like growth factor 135,36 in the brain. The results of the present study may be interpreted in the same context.
The effects of multicomponent exercise and the mechanisms of action on cognitive function are not well known. Few studies have investigated multicomponent exercise, and each of them has used a different exercise program. For example, only aerobic and muscle-strengthening exercises were performed in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability.37 Another example is a review study reported on in 201538 that analyzed six previous randomized controlled studies on the effects of multicomponent exercise on frailty. None of the six studies used a combination of aerobic and muscle-strengthening exercises, and those authors suggested that while frail older adults seemed to benefit from exercise interventions, the optimal exercise program remained unclear.
The present study is noteworthy due to its use of a well-balanced multicomponent exercise program. This program included various types of exercise (e.g., aerobic, muscle-strengthening, flexibility, and balance exercises) with different intensities and frequencies that depended on the degree of frailty of individual participants. We were able to confirm the effect of multicomponent exercise over a 27-month period, during which the subjects participated in repeated sessions of exercise training.
Our analysis of the different frailty groups showed that all of the evaluated outcomes other than depression were significantly improved with short- or long-term effects in the frail group. Multicomponent exercises showed a tendency to protect against cognitive decline and depression in the frail group. One of the particularly interesting results of the present study is that while depression was protected against during the exercise period, the depressive symptoms tended to deteriorate after the exercise period. This suggests that it is very important to maintain exercise programs when attempting to prevent depression, particularly in frail subjects.
Several epidemiological studies have found that frailty increases the risk of cognitive decline and that cognitive impairment increases the risk of frailty, with this cycle being associated with aging.15,39,40,41 Although further experimental data are needed to elucidate the mechanisms linking frailty to cognitive impairment,42,43,44 possible mechanisms include underlying Alzheimer's disease,45 hormonal effects,46,47 nutritional problems, and sarcopenia.15 In the present study, the frail group showed lower MMSE-DS scores and higher rates of cognitive impairment compared with the other groups (Table 2). The handgrip strength was weak in the frail group, suggesting that sarcopenia was more likely to be involved in the frail group than in the other groups. This in the frail group might already have had Alzheimer's disease, which is the most common cause of dementia, or sarcopenia, which has adverse effects on both frailty and cognition. Furthermore, the age of the participants—which is an important risk factor for frailty—obviously increased during the 27-month study period.
Frailty is a multidimensional concept that influences several domains, such as gait, mobility, balance, muscle strength, motor processing, cognition, nutrition, endurance, and physical activity.48 Therefore, a multidimensional approach may be needed for the management of frailty. A few studies of the management of frailty49,50 have led to improvements in the cognitive function of frail subjects. In addition to exercise therapy, these studies have controlled frailty by applying multidomain interventions, including diet, cognitive interventions, and the management of cardiovascular risk factors. As in the present study, the frail subjects were old, and many of them had a low socioeconomic status and lived alone. Considering these unique characteristics of frail subjects, the management of this population may require attention to various risk factors for frailty, including by applying exercise therapy.
This study was conducted in the community based on public projects, and it was subject to some limitations. First, we had to rely on voluntary participation, and so the follow-up periods were not consistent and there was a high rate of dropouts (i.e., not completing the first postexercise assessment). At the end of 27-month follow-up, the remaining subjects were additionally analyzed based on their frailty, which involved a relatively small sample. There is a possibility of attribution bias due to small number of subjects who completed the 27-month follow-up. However, considering that no significant differences were found between the remaining 1-year follow-up subjects and the 27-month follow-up subjects, it is unlikely that the attribution bias was large. We unfortunately did not investigate the cause of participant dropout or whether participants had kept performing multicomponent exercises during the intervention period. However, to encourage participation, we promoted exercise as a lifestyle habit by distributing posters in stages, organizing training sessions before and after the exercise programs to improve the awareness of the relationship between dementia and exercise, preparing self-evaluation sheets, and regularly following up individuals after each exercise period. The second limitation was that the study was conducted without a control group. However, we can infer the effects of multicomponent exercise by performing comparisons with elderly subjects who do not exercise by referring to other studies. Although there was no control group, this study is still meaningful since we compared the effects of multicomponent exercise among the frail, prefrail, and not-frail groups. We also provided multicomponent exercise in various stages according to the degree of frailty in individual subjects. Finally, we did not analyze the change in frailty after the exercise program. The effects of multicomponent exercise should therefore be confirmed more precisely by future studies addressing these limitations and conducting larger-scale investigations.
This study evaluated the effects of long-term exercises on cognition, depression, and the quality of life using a well-balanced multicomponent exercise pyramid that was consistent with the degree of frailty of individual participants. The application of individually customized, multicomponent exercise programs led to improvements in cognitive function, depression, and quality of life in the elderly subjects, especially among those who were more frail.
Acknowledgements
This study was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI18C0479).
Footnotes
- Conceptualization: Yeon-Jung Kim, Hyuntae Park, Jong Hwan Park, Kyung Won Park, Kiheon Lee, Moon Ho Park, Seong-Ho Koh, Hae Ri Na.
- Data curation: Yeon-Jung Kim, Hae Ri Na.
- Formal analysis: Yeon-Jung Kim, Sukil Kim, Kyunghee Chae.
- Funding acquisition: Hae Ri Na.
- Investigation: Yeon-Jung Kim, Hae Ri Na.
- Methodology: Yeon-Jung Kim, Hyuntae Park, Jong Hwan Park, Kyung Won Park, Kiheon Lee, Moon Ho Park, Seong-Ho Koh, Hae Ri Na.
- Project administration: Hae Ri Na.
- Resources: Yeon-Jung Kim, Hae Ri Na.
- Supervision: Hae Ri Na.
- Validation: Sukil Kim, Kyunghee Chae, Hae Ri Na.
- Visualization: Yeon-Jung Kim, Sukil Kim, Kyunghee Chae.
- Writing—original draft: Yeon-Jung Kim.
- Writing—review & editing: all authors.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2020.16.4.612.
References
- 1.Martínez-Velilla N, Casas-Herrero A, Zambom-Ferraresi F, Sáez de Asteasu ML, Lucia A, Galbete A, et al. Effect of exercise intervention on functional decline in very elderly patients during acute hospitalization: a randomized clinical trial. JAMA Intern Med. 2019;179:28–36. doi: 10.1001/jamainternmed.2018.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42–51. doi: 10.1016/j.jpsychires.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Windle G, Hughes D, Linck P, Russell I, Woods B. Is exercise effective in promoting mental well-being in older age? A systematic review. Aging Ment Health. 2010;14:652–669. doi: 10.1080/13607861003713232. [DOI] [PubMed] [Google Scholar]
- 4.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 5.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 6.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 7.Eggermont L, Swaab D, Luiten P, Scherder E. Exercise, cognition and Alzheimer's disease: more is not necessarily better. Neurosci Biobehav Rev. 2006;30:562–575. doi: 10.1016/j.neubiorev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86:876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 10.Sáez de Asteasu ML, Martínez-Velilla N, Zambom-Ferraresi F, Casas-Herrero Á, Izquierdo M. Role of physical exercise on cognitive function in healthy older adults: a systematic review of randomized clinical trials. Ageing Res Rev. 2017;37:117–134. doi: 10.1016/j.arr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Jung HW, Kim K. Multimorbidity in older adults. J Korean Geriatr Soc. 2014;18:65–71. [Google Scholar]
- 14.Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer's disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 2019;18:177–184. doi: 10.1016/S1474-4422(18)30371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment--a review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12:840–851. doi: 10.1016/j.arr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 17.Sergi G, Veronese N, Fontana L, De Rui M, Bolzetta F, Zambon S, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol. 2015;65:976–983. doi: 10.1016/j.jacc.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 18.Solfrizzi V, Scafato E, Frisardi V, Seripa D, Logroscino G, Maggi S, et al. Frailty syndrome and the risk of vascular dementia: the Italian Longitudinal Study on Aging. Alzheimers Dement. 2013;9:113–122. doi: 10.1016/j.jalz.2011.09.223. [DOI] [PubMed] [Google Scholar]
- 19.Bae JN, Cho MJ. Development of the Korean version of the Geriatric Depression Scale and its short form among elderly psychiatric patients. J Psychosom Res. 2004;57:297–305. doi: 10.1016/j.jpsychores.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Han JW, Kim TH, Jhoo JH, Park JH, Kim JL, Ryu SH, et al. A normative study of the Mini-Mental State Examination for dementia screening (MMSE-DS) and its short form (SM MSE-DS) in the Korean elderly. J Korean Geriatr Psychiatry. 2010;14:27–37. [Google Scholar]
- 21.Fountoulakis KN, Tsolaki M, Iacovides A, Yesavage J, O'Hara R, Kazis A, et al. The validation of the short form of the Geriatric Depression Scale (GDS) in Greece. Aging (Milano) 1999;11:367–372. doi: 10.1007/BF03339814. [DOI] [PubMed] [Google Scholar]
- 22.Jang Y, Small BJ, Haley WE. Cross-cultural comparability of the Geriatric Depression Scale: comparison between older Koreans and older Americans. Aging Ment Health. 2001;5:31–37. doi: 10.1080/13607860020020618. [DOI] [PubMed] [Google Scholar]
- 23.Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28:551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 24.Skevington SM, Lotfy M, O'Connell KA WHOQOL Group. The World Health Organization's WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL Group. Qual Life Res. 2004;13:299–310. doi: 10.1023/B:QURE.0000018486.91360.00. [DOI] [PubMed] [Google Scholar]
- 25.Scheffer AC, Schuurmans MJ, van Dijk N, van der Hooft T, de Rooij SE. Fear of falling: measurement strategy, prevalence, risk factors and consequences among older persons. Age Ageing. 2008;37:19–24. doi: 10.1093/ageing/afm169. [DOI] [PubMed] [Google Scholar]
- 26.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13:227–236. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]
- 27.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 28.Hallal PC, Victora CG. Reliability and validity of the International Physical Activity Questionnaire (IPAQ) Med Sci Sports Exerc. 2004;36:556. doi: 10.1249/01.mss.0000117161.66394.07. [DOI] [PubMed] [Google Scholar]
- 29.Oh JY, Yang YJ, Kim BS, Kang JH. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) short form. J Korean Acad Fam Med. 2007;28:532–541. [Google Scholar]
- 30.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 31.Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 32.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 34.Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borst SE, De Hoyos DV, Garzarella L, Vincent K, Pollock BH, Lowenthal DT, et al. Effects of resistance training on insulin-like growth factor-I and IGF binding proteins. Med Sci Sports Exerc. 2001;33:648–653. doi: 10.1097/00005768-200104000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Cassilhas RC, Viana VA, Grassmann V, Santos RT, Santos RF, Tufik S, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. 2007;39:1401–1407. doi: 10.1249/mss.0b013e318060111f. [DOI] [PubMed] [Google Scholar]
- 37.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 38.de Labra C, Guimaraes-Pinheiro C, Maseda A, Lorenzo T, Millán-Calenti JC. Effects of physical exercise interventions in frail older adults: a systematic review of randomized controlled trials. BMC Geriatr. 2015;15:154. doi: 10.1186/s12877-015-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armstrong JJ, Stolee P, Hirdes JP, Poss JW. Examining three frailty conceptualizations in their ability to predict negative outcomes for home-care clients. Age Ageing. 2010;39:755–758. doi: 10.1093/ageing/afq121. [DOI] [PubMed] [Google Scholar]
- 40.Solfrizzi V, Scafato E, Lozupone M, Seripa D, Giannini M, Sardone R, et al. Additive role of a potentially reversible cognitive frailty model and inflammatory state on the risk of disability: the Italian Longitudinal Study on Aging. Am J Geriatr Psychiatry. 2017;25:1236–1248. doi: 10.1016/j.jagp.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Montero-Odasso MM, Barnes B, Speechley M, Muir Hunter SW, Doherty TJ, Duque G, et al. Disentangling cognitive-frailty: results from the gait and brain study. J Gerontol A Biol Sci Med Sci. 2016;71:1476–1482. doi: 10.1093/gerona/glw044. [DOI] [PubMed] [Google Scholar]
- 42.Kelaiditi E, Cesari M, Canevelli M, van Kan GA, Ousset PJ, Gillette-Guyonnet S, et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. 2013;17:726–734. doi: 10.1007/s12603-013-0367-2. [DOI] [PubMed] [Google Scholar]
- 43.Panza F, Seripa D, Solfrizzi V, Tortelli R, Greco A, Pilotto A, et al. Targeting cognitive frailty: clinical and neurobiological roadmap for a single complex phenotype. J Alzheimers Dis. 2015;47:793–813. doi: 10.3233/JAD-150358. [DOI] [PubMed] [Google Scholar]
- 44.Ruan Q, Yu Z, Chen M, Bao Z, Li J, He W. Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res Rev. 2015;20:1–10. doi: 10.1016/j.arr.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Buchman AS, Schneider JA, Leurgans S, Bennett DA. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology. 2008;71:499–504. doi: 10.1212/01.wnl.0000324864.81179.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maggio M, Dall'Aglio E, Lauretani F, Cattabiani C, Ceresini G, Caffarra P, et al. The hormonal pathway to cognitive impairment in older men. J Nutr Health Aging. 2012;16:40–54. doi: 10.1007/s12603-012-0002-7. [DOI] [PubMed] [Google Scholar]
- 47.Muller M, Grobbee DE, Thijssen JH, van den Beld AW, van der Schouw YT. Sex hormones and male health: effects on components of the frailty syndrome. Trends Endocrinol Metab. 2003;14:289–296. doi: 10.1016/s1043-2760(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 48.Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM. Toward a conceptual definition of frail community dwelling older people. Nurs Outlook. 2010;58:76–86. doi: 10.1016/j.outlook.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Langlois F, Vu TT, Kergoat MJ, Chassé K, Dupuis G, Bherer L. The multiple dimensions of frailty: physical capacity, cognition, and quality of life. Int Psychogeriatr. 2012;24:1429–1436. doi: 10.1017/S1041610212000634. [DOI] [PubMed] [Google Scholar]
- 50.Ng TP, Ling LHA, Feng L, Nyunt MSZ, Feng L, Niti M, et al. Cognitive dffects of multi-domain interventions among pre-frail and frail community-living older persons: randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2018;73:806–812. doi: 10.1093/gerona/glx207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.