Abstract
Background and Purpose
Nonketotic hyperglycemia often causes transient visual field defects, but only scattered anecdotes are available in the literature.
Methods
We report a patient with homonymous superior quadrantanopsia due to nonketotic hyperglycemia and provide a systematic literature review of the clinical features of 40 previously reported patients (41 in total, including our case) with homonymous visual field defects in association with nonketotic hyperglycemia.
Results
The typical visual field defect was congruous (84.6%), homonymous hemianopsia (87.8%) with macular splitting (61.5%) or sparing (38.5%). It was transient and repetitive in 54.5% of the patients, but it developed as a persistent form in the remainder. Positive visual symptoms such as hallucinations and phosphenes developed in 73.2% of patients. Brain MRI revealed corresponding abnormalities in most patients (84.8%), characterized by a low-intensity white-matter signal or a high-intensity gray-matter signal on T2-weighted or fluid-attenuated inversion recovery images with diffusion restriction or gadolinium enhancement. Most (97.0%) patients recovered completely, with 48.5% treated by glycemic control alone and the remainder also receiving antiepileptic agents.
Conclusions
Nonketotic hyperglycemia should be considered a possible cause of transient visual field defects, especially when it is associated with repetitive positive visual symptoms and typical MRI findings in hyperglycemic patients.
Keywords: visual fields, quadrantanopsia, hyperglycemia, diabetes mellitus
INTRODUCTION
Homonymous visual field defects usually result from a structural lesion involving the optic radiation to the visual cortex. Occasionally such defects can be observed in patients with head trauma, carbon monoxide poisoning, migraine, or occipital lobe epilepsy, with no lesions being discernible in neuroimaging.1 Recognizing the nature of homonymous visual field defects might be important for helping to localize lesions and determine their etiology.2,3
Nonketotic hyperglycemia typically develops in patients older than 50 years without proper glycemic control, and it can be the first clinical manifestation of asymptomatic diabetes mellitus. Nonketotic hyperglycemia is characterized by hyperglycemia, hyperosmolarity, and intracellular dehydration without ketoacidosis. It is attributed to partial insulin deficiency, in which the level of insulin is adequate for inhibiting alternative metabolic pathways such as gluconeogenesis or ketogenesis, but not for maintaining intracellular glucose transport. The consequent hyperglycemia, glucosuria, and osmotic diuresis lead to intracellular dehydration and neuronal dysfunction. Patients with nonketotic hyperglycemia usually manifest with coma, seizures, or generalized choreoathetosis,4,5 and they can also present with focal neurological deficits such as aphasia, somatosensory symptoms, or visual field defects.6
We recently encountered a patient presenting with transient homonymous quadrantanopsia as an isolated manifestation of nonketotic hyperglycemia. Although there have been numerous reports on nonketotic hyperglycemia and visual field defects, a systematic review has not been available in the literature. Here we therefore report our patient along with providing a systematic review of homonymous visual field defects in nonketotic hyperglycemia.
METHODS
Patient and evaluations
Our patient underwent a complete ophthalmic examination, including visual acuity assessment, automated refraction, slit-lamp, and dilated fundus examinations. The patient was also evaluated using standard automated perimetry (Humphrey Field Analyzer II 750, 30-2, Swedish interactive threshold algorithm, Carl Zeiss Meditec, Jena, Germany). Pupil light reflexes were evaluated at bedside using a dynamic infrared pupillometer (PLR-200, NeurOptics, Irvine, CA, USA). Brain MRI protocol included diffusion-weighted, T1-, and T2-weighted gradient echo axial imaging, gadolinium-enhanced T1-weighted axial imaging, and T1-weighted sagittal imaging using a 3.0-T scanner (Achieva, Philips, Amsterdam, The Netherlands).
This study followed the tenets of the Declaration of Helsinki and was performed according to the guidelines of Institutional Review Board of Seoul National University Bundang Hospital (B-1802-448-701).
Systematic review
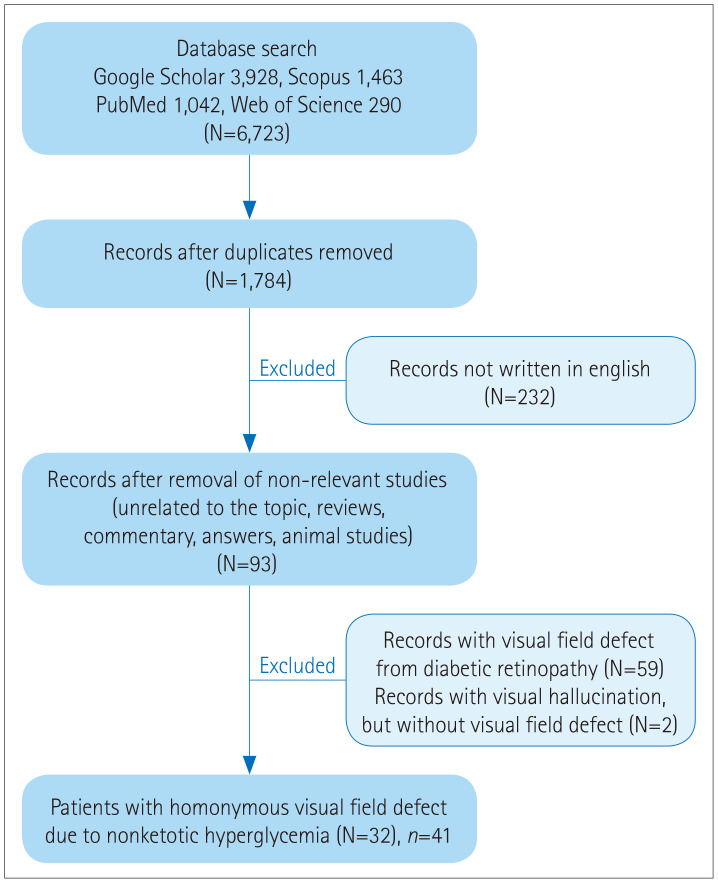
We searched PubMed, Scopus, Google Scholar, and ISI Web of Science for reports on visual field defects and hyperglycemia in January 2020 using the search terms ‘diabetes mellitus’ AND ‘visual field,’ ‘hyperglycemia’ AND ‘hemianop(s)ia,’ ‘diabetes mellitus’ AND ‘hemianop(s)ia,’ and ‘hyperglycemia’ AND ‘visual field.’ The references cited in the retrieved articles were also reviewed. Only articles written in English (at least with abstracts) were included, with no restriction for the date of publication (Fig. 1). The articles were reviewed independently by two authors. We reviewed the included reports to analyze the clinical features of the patients, type of visual field defect, electroencephalography (EEG), cerebrospinal fluid (CSF), and MRI findings, and serum glucose and HbA1c levels. Based on the findings of this analysis, we attempted to find correlations among those variables.
Fig. 1. Algorithm for the systematic review. N: number of articles, n: number of patients.
RESULTS
Case report
A 59-year-old male has experienced recurrent visual phosphenes lasting tens of seconds for 2 weeks. The phosphenes occurred congruently in the left superior visual fields of both eyes typically 20–30 times daily. The patient denied any headache, dizziness, nausea/vomiting, tonic eye deviation, eyelid fluttering, or other abnormal sensations during or after the events. Neurological examination revealed left homonymous superior quadrantanopsia. The Humphrey visual field test also documented the left superior quadrantanopsia with macular splitting (Fig. 2A). Pupillary light and near reflexes were symmetric in both eyes. All of the other findings of neurological and neuroophthalmological examinations were normal.
Fig. 2. Visual field tests and brain MRIs of the patient. A: The Humphrey visual field test performed at the initial presentation showed left homonymous superior quadrantanopsia with macular splitting. B and C: Brain MRI performed at the initial presentation. Fluid-attenuated inversion recovery images contained a low-intensity signal in the right temporal white matter (yellow arrowheads) and swelling (white arrowheads) of the parahippocampal and middle and inferior temporal gyri (B), with T1-weighted gadolinium enhancement (arrows) in the corresponding area (C). D: A follow-up visual field test one 1 month later showed that the field defect had improved completely.
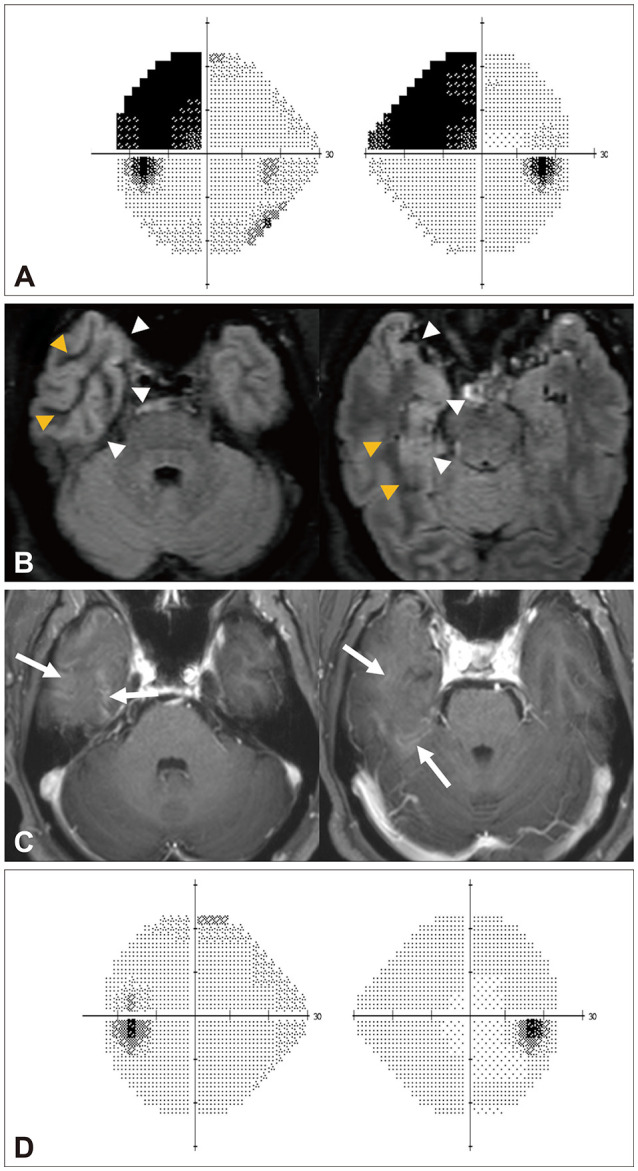
T2-weighted and fluid-attenuated inversion recovery (FLAIR) MRIs revealed decreased white-matter signals and gyral swelling in the right anteromedial region of the temporal lobe. Gadolinium-enhanced T1-weighted images showed contrast enhancement in the corresponding area (Fig. 2B and C). The findings of MR angiography were normal. EEG did not produce any discernible findings. CSF analyses of viral polymerase chain reactions, bacterial and tuberculosis stains and cultures, and cytology evaluation for malignancy were negative. Autoimmune and tumor markers were also negative. The serum glucose and HbA1c levels were 307 mg/dL and 13.2%, respectively, and the calculated serum osmolality was 307 mOsm/kg (normal range=280–290 mOsm/kg). Urinalyses were negative for ketone bodies.
Under the suspicion of focal epilepsy associated with nonketotic hyperglycemia, the patient was administered intravenous insulin and 600 mg of oxcarbazepine per day. The superior quadrantanopsia disappeared 1 month later, along with the normalization of serum glucose (Fig. 2D). The gyral swelling and gadolinium enhancement in the temporal lobe also decreased in the follow-up MRI.
Results of the systematic review
Our systematic literature review additionally identified 40 patients from 32 studies, and so 41 patients (including our patient) were analyzed. They included 26 male, and were aged 57.3±13.7 years [mean±standard deviation; median=60 years, range=28–83 years, interquartile range (IQR)=49–67 years]. The clinical findings of the literature review are summarized in Table 1, and the neuroophthalmological findings of the individual patients are presented in the Supplementary Table 1 (in the online-only Data Supplement).
Table 1. Findings of the systematic review of visual field defects in nonketotic hyperglycemia.
| Value | |
|---|---|
| Demographic and clinical findings | |
| Age, years | 57.3±13.7 |
| Sex, male | 26/41 (63.4) |
| Side of visual field defect, right | 16/38 (42.1) |
| Frequency of visual field defect | |
| Continuous (not countable) | 10/22 (45.5) |
| ≥10/day | 8/22 (36.4) |
| >3/day to <10/day | 2/22 (9.1) |
| ≤3/day | 2/22 (9.1) |
| Duration of visual field defect | |
| Continuous | 10/22 (45.5) |
| <5 min | 12/22 (54.5) |
| Topography of visual field defect | |
| Hemianopsia | 36/41 (87.8) |
| Inferior quadrantanopsia | 4/41 (9.8) |
| Superior quadrantanopsia | 1/41 (2.4) |
| Other features of visual field defect | |
| Congruency | 33/39 (84.6) |
| Macular involvement | 8/13 (61.5) |
| Positive visual symptoms | |
| Visual hallucination | 20/41 (48.7) |
| Visual phosphene | 17/41 (41.5) |
| Total | 30/41 (73.2) |
| Other visual symptoms | |
| Diplopia or polyopia | 2/41 (4.9) |
| Palinopsia | 1/41 (2.4) |
| Other associated nonvisual symptoms | |
| Headache | 15/33 (45.5) |
| Limb tonic–clonic movement or weakness | 11/41 (26.8) |
| Gaze deviation | 10/41 (24.4) |
| Head version | 7/41 (17.1) |
| Nystagmus | 6/41 (14.6) |
| Sensory changes | 2/41 (4.9) |
| Neglect | 2/41 (4.9) |
| Aphasia, acalculia, or agraphia | 2/41 (4.9) |
| Dysphagia, chorea-ballismus, or eyelid twitching | 1/41 (2.4) |
| Laboratory and imaging findings | |
| Serum glucose, mg/dL | 479 [366–600] |
| HbA1c, % | 12.1 [10.9–13.5] |
| CSF abnormalities | 0/10 (0.0) |
| MRI abnormalities | |
| Low-intensity white-matter signal on T2-weighted or FLAIR image | 23/33 (69.7) |
| High-intensity gray-matter signal on T2-weighted or FLAIR image | 19/33 (57.6) |
| Gyral enhancement | 14/33 (42.4) |
| Diffusion restriction | 17/31 (54.8) |
| Total | 28/33 (84.8) |
| EEG abnormalities | |
| Epileptiform discharges | 22/36 (61.1) |
| Focal or generalized slow waves | 10/36 (27.8) |
| Total | 32/36 (88.9) |
| PET or SPECT abnormalities | |
| Hypermetabolism or hyperperfusion | 6/10 (60.0) |
| Hypometabolism or hypoperfusion | 2/10 (20.0) |
| Total | 8/10 (80.0) |
| Treatment and prognosis | |
| Treatment | |
| Insulin | 24/25 (96.0) |
| Antiepileptic drug | 17/33 (51.5) |
| Complete recovery | 32/33 (97.0) |
| Time to recovery, days* | 3 [2–16] |
Data are mean±standard deviation, n (%), or median [interquartile range] values. The study included 41 patients, but the number of analyzed patients for each finding differed with the data availability. *Time to resolution of the visual field defect after achieving blood glucose control.
CSF: cerebrospinal fluid, EEG: electroencephalography, FLAIR: fluid-attenuated inversion recovery, PET: positron-emission tomography, SPECT: single-photon-emission CT.
Visual field defects in nonketotic hyperglycemia
The pattern of visual field defects was in the order of homonymous hemianopsia (36/41, 87.8%), inferior quadrantanopsia (4/41, 9.8%), and superior quadrantanopsia (1/41, 2.4%). Among the patients with available information, the visual field defects were mostly congruent in both eyes (33/39, 84.6%), and macular splitting was reported in more than half of them (8/13, 61.5%). Thirty (73.2%) of the 41 patients had associated positive visual symptoms defined as hallucinations and phosphenes. Other visual symptoms were polyopia/diplopia or palinopsia. The visual field defect was transient and repetitive in approximately half of the patients (12/22, 54.5%), but it developed as a persistent form in the other half (10/22, 45.5%).
Evaluations
The median serum glucose level was 479 mg/dL (range=249–1,000 mg/dL, IQR=366–600 mg/dL), and three patients had visual field defects even with a glucose level less than 300 mg/dL. HbA1c levels ranged from 9.4% to 17.4%, with a median of 12.1% (IQR=10.9–13.5%). The serum glucose level was higher in those with than without positive visual symptoms [median=501 mg/dL (IQR=379–620 mg/dL) vs. 404 mg/dL (310–469 mg/dL), p=0.023, Mann-Whitney's U test], whereas the serum HbA1c level did not differ between these two groups [median=11.4% (IQR=10.9–13.4%) vs. 13.0% (11.7–14.8%), p=0.330, Mann-Whitney U test)]. The CSF findings were normal in all 10 of the patients who were evaluated. Twenty-eight (84.8%) of the 33 patients who underwent brain MRI had minor but obvious abnormalities, including a low-intensity white-matter signal (n=23, 69.7%) or a high-intensity in gray-matter signal (n=19, 57.6%) on T2-weighted and FLAIR images, and gyral gadolinium enhancement (n=14, 42.4%). Diffusion-weighted images showed a focal area with diffusion restriction in 17 of 31 patients (54.8%). EEG was performed in 36 patients, which identified epileptiform discharges in 22 (61.1%). Additionally, 6 (60.0%) of the 10 patients who received positron-emission CT or single-photon-emission CT evaluations had hypermetabolism or hyperperfusion, and 2 (20.0%) had hypometabolism or hypoperfusion in the areas corresponding to MRI lesions.
Treatment and prognosis
The prognoses were reported for 33 patients. Strict glycemic control was applied to all of the patients, while 17 (51.5%) also received antiepileptic drugs. Most patients (32/33, 97.0%) recovered completely, with a median remission interval of 3 days (IQR=2–16 days).
DISCUSSION
The notable findings in this study can be summarized as follows:
1) Our newly reported patient was a representative case that showed transient homonymous superior quadrantanopsia and repetitive visual phosphenes in association with typical MRI findings for nonketotic hyperglycemia.
2) The systematic review revealed that visual field defects in nonketotic hyperglycemia were mostly congruent homonymous hemianopsia associated with positive visual symptoms, and macular vision was involved more than half of the cases.
3) Patients with positive visual symptoms showed higher serum glucose levels than those without.
4) Visual field defects were mostly reversible, recovering within a few days when the glucose level was normalized and without requiring the administration of antiepileptic agents.
5) Typical MRI abnormalities are mostly observed in nonketotic hyperglycemia patients presenting with visual field defects.
Persistent homonymous visual field defects mostly arise from stroke or tumor,3 whereas other etiologies such as migraine, transient ischemic attack, or seizures should be suspected in cases of transient visual field defects.7 Given that the superior quadrantanopsia associated with visual phosphenes disappeared along with the normalization of serum glucose level in our patient, his positive and negative visual symptoms may have been associated with nonketotic hyperglycemia. Similarly, most neurological abnormalities other than visual field defects can normalize along with correction of the hyperglycemic and hyperosmolar state.8 The findings for our patient imply that nonketotic hyperglycemia should be considered in hyperglycemic patients presenting with reversible visual field defects and positive visual symptoms, especially when they are associated with typical MRI abnormalities.
Homonymous superior quadrantanopsia has mostly been reported in discrete lesions involving the optic radiation.9 Since the geniculocalcarine tract is widely spread across the temporal, parietal, and occipital lobes, analyzing the pattern of the visual field defect aids in localizing the responsible lesion.9,10 The optic radiation forms Meyer's loop in the anterior temporal lobe before coursing to the occipital lobe, and focal lesions involving Meyer's loop can cause superior quadrantanopsia. The superior quadrantanopsia in occipital lesions is mostly characterized by macular sparing, since the calcarine cortex contains an extensive representation of the macula.11,12 In contrast, those from a lesion in the temporal lobe may present with macular splitting,13 as shown in our patient.
The mechanism of visual field defects in nonketotic hyperglycemia may be explained by postepileptic suppression of surrounding visual fields following focal awareness sensory epilepsy. Patients with nonketotic hyperglycemia are prone to seizures due to the reduced production of gamma aminobutyric acid (GABA), which is a neurotransmitter that normally stabilizes neural activity. However, a decrease in glucose utilization in the brain and a suppression of the Krebs cycle in diabetic patients cause a decrease in GABA levels in the central nervous system, which lowers the seizure threshold.14 However, EEG rarely produces discernible findings in patients with focal epilepsy presenting with visual field defects.15 Furthermore, although not attributed to seizures, diabetes can by itself result in neuronal dysfunction secondary to metabolic derangement.16 Indeed, our systematic review found that only half of the patients showed epileptic discharges.
It has been reported that the development of occipital seizures or hemianopsia can be affected more by the duration than the level of hyperglycemia.17 Likewise, acute fluctuation of serum glucose is known to exert little effect on the activity of the visual cortex.18 However, in our review we found that the serum glucose level was higher in patients with positive visual symptoms, suggesting that the degree of glycemia affects the development of positive symptoms. Our review also found that the HbA1c level did not differ between those with and without positive visual symptoms, which was probably due to HbA1c being evaluated in only a small number of patients (13/41, 31.7%).
The application of hydration and strict glucose control resulted in most patients with nonketotic hyperglycemia recovering within a few days even without the administration of antiepileptic drugs.19 Certain antiepileptic agents such as phenytoin and valproic acid can aggravate hyperglycemia by inhibiting insulin release or inducing insulin resistance,20,21 and seizures can be refractory to antiepileptic drugs in nonketotic hyperglycemia unless the hyperglycemia is properly controlled.8,14 Therefore, ensuring glycemic control is of paramount importance for the treatment of nonketotic hyperglycemia.
Since neurological prognoses can be favorable with appropriate treatment, early diagnosis of nonketotic hyperglycemia is important. In addition to the clinical presentation and laboratory findings, MRI can aid in diagnosing nonketotic hyperglycemia. Our patient exhibited a decreased white-matter signal intensity while the adjacent cortical signal intensity was increased on T2-weighted or FLAIR images. These subtle abnormal MRI findings can often be overlooked or missed.22 It was previously reported that less than one-fourth of patients showed abnormal MRI findings.23 However, a systematic review revealed that abnormal MRI findings were underestimated in more than 80% of patients. It is particularly important to detect the decreased T2-weighted signal in the white matter, since the differential diagnosis is limited to viral encephalitis, meningitis, leptomeningeal metastasis, hemorrhagic infarct, and hypoxic insult.24 This importance of white-matter signals is explained by the intracellular dehydration of glial and supporting tissue, and possibly also by the accumulation of free radicals in nonketotic hyperglycemia.23
In conclusion, nonketotic hyperglycemia should be considered a possible cause of transient visual field defects when associated with repetitive positive visual symptoms and typical MRI findings in hyperglycemic patients.
Acknowledgements
This study was supported by 2018 Academic Research Funds of the Korean Society of Clinical Neurophysiology, and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2020R1A2C4002281).
Footnotes
- Conceptualization: Jeong-Yoon Choi.
- Formal analysis: all authors.
- Funding acquisition: Sun-Uk Lee, Ji-Soo Kim.
- Visualization: Hyo-Jung Kim.
- Writing—original draft: Sun-Uk Lee.
- Writing—review & editing: all authors.
Conflicts of Interest: Drs. SU Lee, J Lee, JE Yoon, HJ Kim, JY Choi, and CH Yun report nothing to disclose. Dr. JS Kim serves as an associate editor of Frontiers in Neuro-otology and on the editorial boards of the Journal of Clinical Neurology, Frontiers in Neuro-ophthalmology, Journal of Neuro-ophthalmology, Journal of Vestibular Research, Journal of Neurology, and Medicine.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2020.16.4.599.
Literature review for visual field defects in nonketotic hyperglycemia
References
- 1.Brazis PW, Lee AG, Graff-Radford N, Desai NP, Eggenberger ER. Homonymous visual field defects in patients without corresponding structural lesions on neuroimaging. J Neuroophthalmol. 2000;20:92–96. doi: 10.1097/00041327-200020020-00005. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. Homonymous hemianopias: clinical-anatomic correlations in 904 cases. Neurology. 2006;66:906–910. doi: 10.1212/01.wnl.0000203913.12088.93. [DOI] [PubMed] [Google Scholar]
- 3.Trobe JD, Lorber ML, Schlezinger NS. Isolated homonymous hemianopia. A review of 104 cases. Arch Ophthalmol. 1973;89:377–381. doi: 10.1001/archopht.1973.01000040379005. [DOI] [PubMed] [Google Scholar]
- 4.Brick JF, Gutrecht JA, Ringel RA. Reflex epilepsy and nonketotic hyperglycemia in the elderly: a specific neuroendocrine syndrome. Neurology. 1989;39:394–399. doi: 10.1212/wnl.39.3.394. [DOI] [PubMed] [Google Scholar]
- 5.Oh SH, Lee KY, Im JH, Lee MS. Chorea associated with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neurol Sci. 2002;200:57–62. doi: 10.1016/s0022-510x(02)00133-8. [DOI] [PubMed] [Google Scholar]
- 6.Maccario M. Neurological dysfunction associated with nonketotic hyperglycemia. Arch Neurol. 1968;19:525–534. doi: 10.1001/archneur.1968.00480050095009. [DOI] [PubMed] [Google Scholar]
- 7.López-Amorós A, Medrano-Martínez V, Francés-Pont I, Hernández-Rubio L, González-Fernández L, Fernández-Izquierdo S, et al. Reversible homonymous inferior quadrantanopia in a nonketotic hyperglycemic patient. Neuroophthalmology. 2018;44:45–48. doi: 10.1080/01658107.2018.1547914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson SF, Loge RV. Palinopsia due to nonketotic hyperglycemia. West J Med. 1988;148:331–332. [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson DM. The localizing value of a quadrantanopia. Arch Neurol. 1997;54:401–404. doi: 10.1001/archneur.1997.00550160045014. [DOI] [PubMed] [Google Scholar]
- 10.Baier B, de Haan B, Mueller N, Thoemke F, Birklein F, Dieterich M, et al. Anatomical correlate of positive spontaneous visual phenomena: a voxelwise lesion study. Neurology. 2010;74:218–222. doi: 10.1212/WNL.0b013e3181cb3e64. [DOI] [PubMed] [Google Scholar]
- 11.McFadzean R, Brosnahan D, Hadley D, Mutlukan E. Representation of the visual field in the occipital striate cortex. Br J Ophthalmol. 1994;78:185–190. doi: 10.1136/bjo.78.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strowd RE, Wabnitz A, Balakrishnan N, Craig J, Tegeler CH. Clinical reasoning: acute-onset homonymous hemianopia with hyperglycemia: seeing is believing. Neurology. 2014;82:e129–e133. doi: 10.1212/WNL.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 13.Krolak-Salmon P, Guenot M, Tiliket C, Isnard J, Sindou M, Mauguiere F, et al. Anatomy of optic nerve radiations as assessed by static perimetry and MRI after tailored temporal lobectomy. Br J Ophthalmol. 2000;84:884–889. doi: 10.1136/bjo.84.8.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harden CL, Rosenbaum DH, Daras M. Hyperglycemia presenting with occipital seizures. Epilepsia. 1991;32:215–220. doi: 10.1111/j.1528-1157.1991.tb05247.x. [DOI] [PubMed] [Google Scholar]
- 15.Shaw S, Kim P, Millett D. Status epilepticus amauroticus revisited: ictal and peri-ictal homonymous hemianopsia. Arch Neurol. 2012;69:1504–1507. doi: 10.1001/archneurol.2012.317. [DOI] [PubMed] [Google Scholar]
- 16.Burde RM. Neuro-ophthalmic associations and complications of diabetes mellitus. Am J Ophthalmol. 1992;114:498–501. doi: 10.1016/s0002-9394(14)71865-3. [DOI] [PubMed] [Google Scholar]
- 17.Hung WL, Hsieh PF, Lee YC, Chang MH. Occipital lobe seizures related to marked elevation of hemoglobin A1C: report of two cases. Seizure. 2010;19:359–362. doi: 10.1016/j.seizure.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Gruetter R, Uğurbil K, Seaquist ER. Effect of acute hyperglycemia on visual cortical activation as measured by functional MRI. J Neurosci Res. 2000;62:279–285. doi: 10.1002/1097-4547(20001015)62:2<279::AID-JNR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JP, Yancy A, Saint Louis L, Rosberger DF. Reversible hyperglycemic homonymous hemianopia. J Natl Med Assoc. 2009;101:373–376. doi: 10.1016/s0027-9684(15)30888-9. [DOI] [PubMed] [Google Scholar]
- 20.Verrotti A, la Torre R, Trotta D, Mohn A, Chiarelli F. Valproate-induced insulin resistance and obesity in children. Horm Res. 2009;71:125–131. doi: 10.1159/000197868. [DOI] [PubMed] [Google Scholar]
- 21.Al-Rubeaan K, Ryan EA. Phenytoin-induced insulin insensitivity. Diabet Med. 1991;8:968–970. doi: 10.1111/j.1464-5491.1991.tb01539.x. [DOI] [PubMed] [Google Scholar]
- 22.Lavin P, Donahue S. Magnetic resonance imaging changes associated with transient homonymous hemianopia in patients with nonketotic hyperglycemia. Arch Ophthalmol. 2008;126:1467–1468. doi: 10.1001/archopht.126.10.1467-a. [DOI] [PubMed] [Google Scholar]
- 23.Lavin PJ. Hyperglycemic hemianopia: a reversible complication of non-ketotic hyperglycemia. Neurology. 2005;65:616–619. doi: 10.1212/01.wnl.0000173064.80826.b8. [DOI] [PubMed] [Google Scholar]
- 24.Raghavendra S, Ashalatha R, Thomas SV, Kesavadas C. Focal neuronal loss, reversible subcortical focal T2 hypointensity in seizures with a nonketotic hyperglycemic hyperosmolar state. Neuroradiology. 2007;49:299–305. doi: 10.1007/s00234-006-0189-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Literature review for visual field defects in nonketotic hyperglycemia