Abstract
Context:
Plant extracts are used in folklore medicine from time immemorial to treat different oral diseases. Chemical constituents extracted from these natural resources are gifted with huge opportunities.
Aim:
The aim of this study is to assess the antibacterial property of Azadirachta indica (Neem), Ocimum sanctum (Tulsi), and Vitex negundo (Pochotia) against oral microorganisms.
Materials and Methods:
Plant extract was prepared with hot continuous extraction method by the Soxhlet Apparatus. Microorganisms isolated from the oral cavity and identified by Vitek-2. Bacterial inoculums poured and spread into Mueller Hinton plates. Plant extract was poured into prepared wells taking ciprofloxacillin as the positive control and dimethyl sulfoxide as the negative control. The experiment was performed in duplicates with two different concentrations of the extract and mean value of inhibition zone was calculated.
Statistical Analysis:
Paired t-test, analysis of variance, and regression analysis.
Results:
Isolated microorganisms were Klebsiella oxytoca, Kochuria kristinae, Acinetobacter boumani, Sphingomonas paucimobilis, Pseudomonas fluorescens, Streptococcus gordonii, Enterococcus faecalis, and Bacillus subtilis. Higher zone of inhibition was observed against E. faecalis by V. negundo followed by A. indica. Among the aqueous and acetone group, in the aqueous group, the regression models of K. kristinae and B. subtilis have been found to be statistically significant (P= < 0.05), whereas, in the acetone group, the regression model of B. subtilis has been found to be statistically significant (P = < 0.05).
Conclusion:
All the three plants showed antibacterial potency against the isolated organisms. Acetone group showed better efficacy than the aqueous extract group.
Keywords: Antibacterial, microorganism, traditional medicine
INTRODUCTION
Plant extract either in standardized form or in pure form is endowed with vast opportunities for the introduction of a new medicine because of the presence of unlimited chemical diversity. In the last century, roughly 121 pharmaceuticals contributed significantly to the development of new drugs from the medicinal plants.[1] About 60%–90% of populations in the developing countries use crude plant extracts as herbal medicine for infectious diseases.[2]
Azadirachta indica (Neem) twigs as a toothbrush is an age-old method practiced by the Indian population.[3] It is the most revenue-releasing plant grown due to the presence of several phytoconstituents and pharmacological activities associated with it.
Ocimum sanctum (Tulsi) is from the Lamiaceae family and used both in aqueous and dried form and is considered as the “Mother medicine of nature.”
Vitex negundo (Pochotia) is considered as a tonic, vermifuge, and also used as an anti-inflammatory, and astringent in toothache.[2,4]
Oral microorganisms lead to several infectious diseases such as dental caries and periodontal disease. Medicinal plants are known for their antibacterial activity and are used against these microorganisms in various oral hygiene products.[5]
The aim of this study is to assess the antibacterial property of A. indica, O. sanctum, and V. negundo against isolated oral microorganisms.
MATERIALS AND METHODS
Plant material - Twigs from A. indica, O. sanctum, and V. negundo were collected separately and washed thoroughly with tap water followed by distilled water. The twigs were cut into small pieces and left for drying in shade for 5 days. Twigs of individual plants are grounded and sieved finely
Preparation of plant extract: Hot continuous extraction method by soxhlet apparatus was used to prepare the plant extract [Figure 1]. Dry powder of 2.5 g, 5 g dissolved in 10 ml distilled water, and acetone, respectively, to make them 25% and 50% concentration. The remaining part of the solvent was removed in the evaporator and stored in the refrigerator at 4°C until further use
-
Collection of samples -Samples were collected from the outpatient department aged between 18 and 60 years. Patients with diabetes, systemic illness, pregnant women, and those who used local or systemic antibiotics within 6 months were excluded from the study. Informed consent was obtained from the patient before sample collection.
Methods used for taking out samples were:
- Supragingival plaque taken in a sterile swab
- Curette used to collect the plaque and drawn coronally from the gingival margin
- Spoon excavator used to collect the material from the carious site
- Absorbent points to collect the samples from the root canal.
Sample for aerobic culture was plated directly on blood agar and MacConkey agar and incubated at 37°C for 24 h. Sample for anaerobic culture was immediately inoculated in 5 ml Robertson cooked meat broth at the site of collection. Broth was incubated at 37°C for 48 h. Subculture was made from Robertson cooked meat broth in the blood agar media and incubated in an oxoid anaerobic culture jar with anaerobic gas pack at 37°C for 48–72 h. Smears were taken from the colony and staining done with Gram stain. Stained slides were examined under (X100) the light microscope. Microorganisms were identified with Vitek-2 (BioMerieux st louis, USA).
Antimicrobial assay - The antimicrobial assay was determined using the agar well-diffusion method. Pure isolates of bacteria (3–5 isolated colonies) were subcultured in peptone water and incubated for 4 h. The density of each microbial suspension was adjusted to 0.5 McFarland turbidity standards, which correspond to 106 (CFU/ml). Inoculums were spread on Mueller Hinton media plates and allowed to dry. Five wells were made with a tip of a sterile cork borer in each plate. Plant extract dissolved in 2% dimethyl sulfoxide (DMSO) and poured (100 μl) in the wells with the tip of a micropipette and incubated at 37°C. DMSO was used as a negative control and ciprofloxacillin (5 μg) disc was used as a positive control. Zone of inhibition around antibiotic disc and herbal extract was measured and recorded.
Figure 1.

(a) Soxhlet apparatus. (b) Plant extract
Ethics
Ethical clearance was obtained from the Ethical Committee of Institutional Review Board.
RESULTS
All organisms showed higher zone in 50% acetone extract. Highest zone of inhibition of 23 mm observed in Enterococcus faecalis by V. negundo, Streptococcus gordonii of 21 mm by A. indica, Bacillus subtilis of 19 mm by both A. indica, and V. negundo, Klebsiella oxytoca and Sphingomonas paucimobilis showed ZI of 16 mm in V. negundo. Pseudomonas fluorescence ZI of 14 mm by both A. indica and O. sanctu, Kochuria kristinae of 12 mm in A. indica 50% aqueous extract and also with acetone extract.
In order to understand the antimicrobial property of the plants with respect to each of the microorganisms, regression analysis was conducted. An analysis of variance (ANOVA) test was also done at 5% level of significance to test how significantly the regression model predicts the dependent variable.
In the aqueous group, the regression models of K. kristinae and B. subtilis have been found to be statistically significant (P values of the ANOVA test are <0.05). The regression equations are formulated for estimated zone of inhibition, Y for any given extract concentration level (%), X, as follows: For K. kristinae Regression estimate of zone of inhibition, Y = 8 + 0.07X, for B. subtilis: Y = 12.67 + 0.08X.
In the acetone group, the regression model of B. subtilis has been found to be statistically significant (P values of the ANOVA test are <0.05). The equations of the regression line for B. subtilis: Y = 14.67 + 0.08X. V. negundo (pochotia) showed best result against E. faecalis, A. indica (Neem) against S. gordini and Tulsi against B. subtilis.
DISCUSSION
The Indian subcontinent is a vast storehouse of medicinal plants that are used in traditional medical treatments, which also form a rich source of knowledge.[6] These products proved scientifically to be beneficial for health as well as social and financial benefit. According to the World Health Organization, 65% of the Indian population of the rural areas use Ayurveda and medicinal plants to meet their health-care needs.[7] There are about 1250 Indian plants used as the medicine for treating various ailments. The researchers reported that natural phytochemical could offer an alternative approach in the prevention and cure for dental caries and other oral infections.[8]
In the present study, all three plants exhibited moderate to good antibacterial property against all isolated microbes [Table 1]. Plant extract of all 50% acetone groups showed better results than the 50% aqueous group [Table 2]. A. indica showed efficacy against all the isolated microbes, but the zone of inhibition is found to be highest against S. gordonii with 21 mm, followed by E. faecalis with 20 mm, and B. subtilis with 19 mm. Koona and Budida reported a larger ZI of methanol extract of neem tree extract against E. faecalis of 23 mm and smaller zone in B. subtilis of 10 mm [Table 1 and Figure 2] compared to the present study.[9] E. faecalis is most frequently associated with root canal infection with periapical lesion.[10] It may remain in a well debrided and coronally well-sealed canal and survive as it needs only the serum component of the dentinal fluid as the source of energy and nutrition.[11,12] A. indica contains the alkaloid margosine, resins, gum, chloride, fluoride, silica, sulfur, oils, tannins, saponins, flavonoids, sterols, and calcium.[13] The antibacterial activity of A. indica might be due to the presence of triterpenoids, phenolic compounds, carotenoids, steroids, ketones, and tetra-triterpenoids azadirachtin.[10] Neem shows the antibacterial role by inhibiting microbial growth and cell wall breakdown.[14] Toothpastes-containing neem leaf extract reduces oral plaque index and bacterial count.[15]
Table 1.
Antibacterial activity of plant extracts against different microorganisms
| Name of microbes | ZI (mm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aqueous | Acetone | |||||||||||
| A. indica | V. negundo | O. sanctum | A. indica | V. negundo | O. sanctum | |||||||
| 50% | 25% | 50% | 25% | 50% | 25% | 50% | 25% | 50% | 25% | 50% | 25% | |
| KO | 12 | 10 | 15 | 12 | 11 | 8 | 13 | 11 | 16 | 14 | 12 | 10 |
| KK | 12 | 10 | 11 | 9 | 11 | 10 | 12 | 12 | 11 | 10 | 11 | 11 |
| AB | 10 | 8 | 9 | 7 | 8 | 7 | 11 | 10 | 12 | 10 | 10 | 9 |
| SP | 10 | 8 | 14 | 12 | 10 | 8 | 11 | 10 | 16 | 13 | 11 | 9 |
| PF | 12 | 11 | 11 | 10 | 13 | 11 | 14 | 13 | 12 | 12 | 14 | 12 |
| SG | 19 | 17 | 10 | 8 | 15 | 12 | 21 | 19 | 11 | 9 | 17 | 13 |
| EF | 18 | 16 | 20 | 17 | 15 | 13 | 20 | 18 | 23 | 19 | 17 | 15 |
| BS | 17 | 15 | 16 | 14 | 17 | 15 | 19 | 17 | 19 | 16 | 18 | 17 |
ZI: Zone of inhibition, KO: Klebsiella oxytoca, KK: Kocuria kristinae, AB: Acinetobacter boumanni, SP: Sphingomonas paucimobilis, PF: Pseudomonas fluorescens, SG: Streptococcus gordonii, EF: Enterococcus faecalis, BS:Bacillus subtilis, A. indica: Azadirachta indica, V. negundo: Vitex negundo, O. sanctum: Ocimum sanctum
Table 2.
Difference between the mean values of the two groups through paired samples t-test at 5% confidence level
| Name of microbes | ZI of AQ (mm) | SD | ZI of AE (mm) | SD | P |
|---|---|---|---|---|---|
| KO | 11.33 | 2.34 | 12.67 | 2.16 | 0.001 |
| KK | 10.50 | 1.05 | 11.17 | 0.75 | 0.102 |
| AB | 8.17 | 1.17 | 10.33 | 1.03 | 0.000 |
| SP | 10.33 | 2.34 | 11.67 | 2.50 | 0.001 |
| PF | 11.33 | 1.03 | 12.83 | 0.98 | 0.001 |
| SG | 13.50 | 4.23 | 15.00 | 4.73 | 0.001 |
| EF | 16.50 | 2.43 | 18.67 | 2.73 | 0.000 |
| BS | 15.67 | 1.21 | 17.67 | 1.21 | 0.000 |
ZI: Zone of inhibition, AQ: Aqueous extract, AE: Acetone extract, SD: Standard deviation, KO: Klebsiella oxytoca, KK: Kocuria kristinae, AB: Acinetobacter boumanni, SP: Sphingomonas paucimobilis, PF: Pseudomonas fluorescens, SG: Streptococcus gordonii, EF: Enterococcus faecalis, BS: Bacillus subtilis
Figure 2.
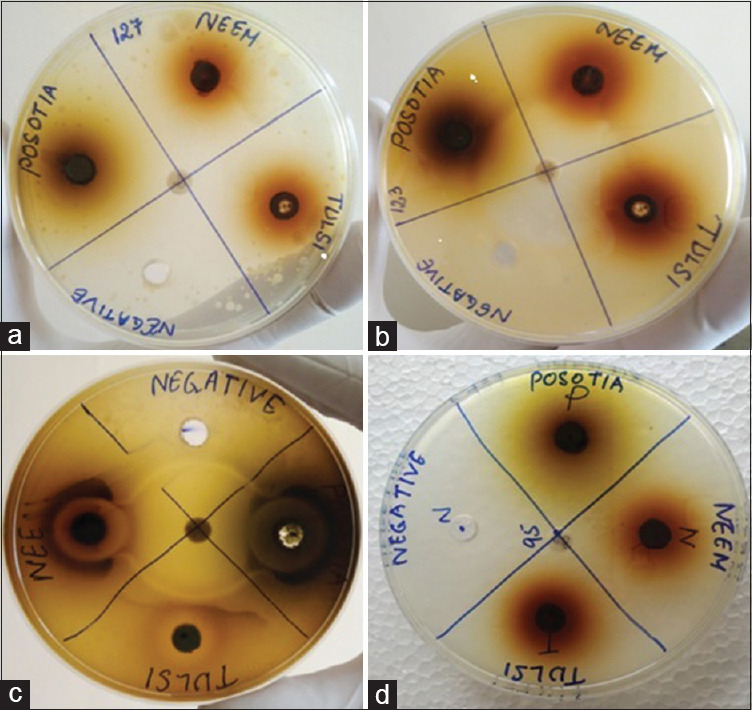
(a) Zone of inhibition of Kochuria kristinii, (b) Zone of inhibition of Streptococcus gordonii, (c) Zone of inhibition of Enterococcus faecalis, (d) ZI of Bacillus subtilis
O. sanctum (Tulsi) is a good source of beta carotene, calcium, Vitamin C, tannins, camphor, and flavonoids. It is used in the therapeutic applications such as asthma, bronchitis, catarrhal fever, vomiting, and ringworm.[16,17] In the present study, it showed highest efficacy against B. subtilis with 18 mm and least in Acinetobacter baumannii with 10 mm inhibition zone in 50% acetone extract. Studies reported that O. sanctum has antibacterial efficacy against B. subtilis and E. faecalis.[18,19]
V. negundo (Pochotia) contains many polyphenolic compounds such as terpenoids, glycosidic iridoids, flavonoids, and alkaloids. It showed potent antibacterial activity against E. faecalis with 23 mm zone and least in S. gordonii followed by K. kristinae with 11 mm ZI. Acinetobacter boumanni showed the highest ZI of 12 mm in V. negundo 50% Acetone extract. A. boumanni is one of the critical pathogens because of its antibiotic resistance.[20] Among all the organism, B. subtilis and K. kristinae showed significantly potent antibacterial activity than the others [Table 3]. Singh et al. reported antibacterial activity of the oil extracted from the leaves of V. negundo against B. subtilis in different concentrations.[21,22] The rich source of secondary metabolites in the plant such as tannin, flavonoids, terpenoids, and alkaloids is thought to be responsible for antibacterial properties.[23] Khan et al. inferred that methanolic extract of V. negundo is highly effective against Gram-positive organism.[24] Nagarsekar et al. observed that antimicrobial property of V. negundo may be to monoterpene constituent, which has cytoplasmic membrane-damaging effect.[25]
Table 3.
Regression model of antibacterial property of plant extract
| Name of microbes | Aqueous extract | Acetone extract | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Slope | SE | ANOVA P | Inference | Intercept | Slope | SE | ANOVA P | Inference | |
| KO | 7.33 | 0.11 | 2.04 | 0.18 | NS | 9.67 | 0.08 | 2.08 | 0.30 | NS |
| KK | 8.00 | 0.07 | 0.58 | 0.02 | S | 10.67 | 0.01 | 0.82 | 0.64 | NS |
| AB | 5.67 | 0.07 | 0.82 | 0.06 | NS | 8.33 | 0.05 | 0.82 | 0.11 | NS |
| SP | 7.33 | 0.08 | 2.31 | 0.34 | NS | 8.67 | 0.08 | 2.52 | 0.38 | NS |
| PF | 9.33 | 0.05 | 0.82 | 0.11 | NS | 11.33 | 0.04 | 0.91 | 0.25 | NS |
| SG | 10.00 | 0.09 | 4.51 | 0.56 | NS | 11.00 | 0.11 | 5.03 | 0.55 | NS |
| EF | 13.00 | 0.09 | 2.31 | 0.28 | NS | 14.67 | 0.11 | 2.58 | 0.27 | NS |
| BS | 12.67 | 0.08 | 0.58 | 0.01 | S | 14.67 | 0.08 | 0.58 | 0.01 | S |
KO: Klebsiella oxytoca, KK: Kocuria kristinae, AB: Acinetobacter boumanni, SP: Sphingomonas paucimobilis, PF: Pseudomonas fluorescens, SG: Streptococcus gordonii, EF: Enterococcus faecalis, BS: Bacillus subtilis, SE: Standard error, ANOVA: Analysis of variance
The antibiotic potential of parts of these trees may be utilized and incorporated in different oral health-care products to treat various oral diseases.
CONCLUSION
Injudicious use of antibiotics has led to an increase in resistance to antibiotics globally. Traditional medicine has always been preferred among the common mass because of its availability and cost-effectiveness. As newer techniques sometimes pose a barrier for dental treatment, traditional medicine presents a better alternative which is already time tested. However, metabolism in plants is variable in nature, and hence, the plant extract must be validated scientifically for medicinal use. Three plants used for the present study showed good antibacterial activity, but large numbers of herbs used traditionally are yet to be scientifically proved for its application in oral health care.
Financial support and sponsorship
This study was financially supported by Grant received by Srimanta Sankardeva University of Health Science.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The author would like to acknowledge Srimanta Sankardeva University of Health Science for the financial support.
REFERENCES
- 1.Anesini C, Perez C. Screening of plants used in Argentine folk medicine for antimicrobial activity. J Ethnopharmacol. 1993;39:119–28. doi: 10.1016/0378-8741(93)90027-3. [DOI] [PubMed] [Google Scholar]
- 2.Keerti G, Padma K. Evaluation of phytochemical and antimicrobial study of extracts of Vitex negundo Linn. Am J Respir Med. 2012;4:192–9. [Google Scholar]
- 3.Kalita C, Saikia AK, Saikia K. Antibacterial property of few plants used as chewing stick. IJHRMLP. 2017;3:9–11. [Google Scholar]
- 4.Chandramu C, Manohar RD, Krupadanam DG, Dashavantha RV. Isolation, characterization and biological activity of betulinic acid and ursolic acid from Vitex negundo L. Phytother Res. 2003;17:129–34. doi: 10.1002/ptr.1088. [DOI] [PubMed] [Google Scholar]
- 5.Prasanth M. Antimicrobial efficacy of different toothpastes and mouthrinses: Anin vitro study. Dent Res J (Isfahan) 2011;8:85–94. [PMC free article] [PubMed] [Google Scholar]
- 6.Asolkar LV, Kakkar KK, Chakre OJ, Chopra IC, Chopra RN, Nayar SL. Glossary of Indian Medicinal Plants New Delhi: Council of Scientific & Industrial Research: (A-K) 1992:414. [Google Scholar]
- 7.WHO. Traditional Medicine Strategy: 2014-2023. World Health Organization; 2013. p. 76. [Google Scholar]
- 8.Chatterjee A, Pakrashi SC. The Treatise on Indian Medicinal Plants National Institute of Science Communication and Information Resources. 1991:1013. [Google Scholar]
- 9.Koona S, Budida S. Antibacterial potential of the extracts of the leaves of Azadirachta indica Linn. Not Sci Biol. 2011;3:65–9. [Google Scholar]
- 10.Pinheiro ET, Mayer MP. Enterococcus faecalis in oral infections. J Interdiscipl Med Dent Sci. 2014;3:1–5. [Google Scholar]
- 11.Gomes BP, Pinheiro ET, Jacinto RC, Zaia AA, Ferraz CC, Souza-Filho FJ. Microbial analysis of canals of root-filled teeth with periapical lesions using polymerase chain reaction. J Endod. 2008;34:537–40. doi: 10.1016/j.joen.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Kayaoglu G, Ørstavik D. Virulence factors of Enterococcus faecalis: Relationship to endodontic disease. Crit Rev Oral Biol Med. 2004;15:308–20. doi: 10.1177/154411130401500506. [DOI] [PubMed] [Google Scholar]
- 13.Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica) Curr Sci Bangalore. 2002;82:1336–45. [Google Scholar]
- 14.Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid Based Complement Alternat Med. 2016;2016:7382506. doi: 10.1155/2016/7382506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaur J, Chandra J, Chaudhry S, Vaish S, Dodwad V. Assessment of 4% ocimum sanctum and 0.2% chlorhexidine irrigation as an adjunct to scaling & root planing in management of chronic periodontitis-a randomized controlled trial. J Dent Specialities. 2015;3:217–9. [Google Scholar]
- 16.Godhwani S, Godhwani JL, Vyas DS. Ocimum sanctum: An experimental study evaluating its anti-inflammatory, analgesic and antipyretic activity in animals. J Ethnopharmacol. 1987;21:153–63. doi: 10.1016/0378-8741(87)90125-5. [DOI] [PubMed] [Google Scholar]
- 17.Gupta SK, Prakash J, Srivastava S. Validation of traditional claim of Tulsi, Ocimum sanctum Linn. as a medicinal plant. Indian J Exp Biol. 2002;40:765–73. [PubMed] [Google Scholar]
- 18.Mistry KS, Sanghvi Z, Parmar G, Shah S. The antimicrobial activity of Azadirachta indica, Mimusops elengi, Tinospora cardifolia, Ocimum sanctum and 2% chlorhexidine gluconate on common endodontic pathogens: Anin vitro study. Eur J Dent. 2014;8:172–7. doi: 10.4103/1305-7456.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subbiya A, Mahalakshmi K, Pushpangadan S, Padmavathy K, Vivekanandan P, Sukumaran VG. Antibacterial efficacy of Mangifera indica L kernel and Ocimum sanctum L leaves against Enterococcus faecalis dentinal biofilm. J Conserv Dent. 2013;16:454–7. doi: 10.4103/0972-0707.117507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–27. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 21.Singh P, Mishra G, Jha KK, Garg VK, Khosa RL. Chemical composition and antimicrobial activity of essential oil of leaves of Vitex negundo Linn.(Verbenaceae) Int J Chem Technol Res. 2010;2:1686–90. [Google Scholar]
- 22.Gautam LM, Shrestha SL, Wagle P, Tamrakar BM. Chemical constituents from Vitex negundo (Linn.) of nepalese origin. Sci World. 2008;6:27–32. [Google Scholar]
- 23.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan AM, Qureshi RA, Gilani SA, Ullah F. Antimicrobial activity of selected medicinal plants of Margalla Hills, Islamabad, Pakistan. J Med Plant Res. 2011;5:4665–70. [Google Scholar]
- 25.Nagarsekar KS, Nagarsenker MS, Kulkarni SR. Evaluation of composition and antimicrobial activity of supercritical fluid extract of leaves of Vitex negundo. Indian J Pharm Sci. 2010;72:641–3. doi: 10.4103/0250-474X.78537. [DOI] [PMC free article] [PubMed] [Google Scholar]


