Abstract
Aim:
The purpose of this study was to determine the chemical composition of oregano essential oil, minimal inhibitory concentration (MIC) and to assess its antimicrobial efficiency against Enterococcus faecalis.
Material and Methods:
Gas Chromatography and Mass Spectrometry (GC-MS) was used to determine the chemical composition of essential oil from oreganum vulgare. Broth dilution and agar diffusion method was used to evaluate the MIC. For Broth dilution, 100 μL of different concentration of oil (6.25, 12.5, 25.0, 50.0, and 100 μg/ml) was tested. Agar diffusion method was utilized to evaluate the antimicrobial efficiency of different concentration of oil (25.0, 50.0, and 100 μg/mL) against E. faecalis.
Results:
GC-MS analysis revealed that oregano essential oil contained carvacrol (41.2%), γ-terpinene (12.68%), p-cymene (9.47%), α-terpinene (1.19%) as the major compounds and β–caryophyllene (0.83%), β-linalool (0.67%), β–bisabolene (0.601%), α-pinene (0.6%), β-pinene (0.5%), terpinen-4-ol (0.41%), borneol (0.4%), 3-thujene (0.4%), spathulenol (0.4%), myristicin (0.25%), and apiol (0.14%). The results of the present study reported Oregano essential oil possess antimicrobial activity against E. faecalis. The MIC was 25 μg/ml and the minimum bacterial concentration (MBC) was 50 μg/ml.
Conclusion:
Oregano essential oil was reported to be an effective antimicrobial agent against E. faecalis. The MIC was found to be 25 μg/ml and the MBC was found to be 50 μg/ml.
Keywords: Antimicrobial efficiency, minimal inhibitory concentration, oregano essential oil, oreganum vulgare
INTRODUCTION
Successful endodontic treatment aims at effective removal of microorganisms from the root canal. Gram-positive facultative bacteria, mainly Enterococcus faecalis survive in the root canal even after chemomechanical preparation and cause persistent infection.[1] Complete debridement of root canal system, including its ramification and anatomical irregularities is almost impossible with only hand or mechanical-driven instruments.[2] This should be accompanied by irrigation of the root canal system using antimicrobial agents.[3]
The commonly used conventional root canal irrigant, sodium hypochlorite is effective against E. faecalis and exhibit the tissue dissolving property, but the major disadvantage is the cytotoxicity of sodium hypochlorite.[4] In the recent years, there have been many studies on plant extract which possess antimicrobial activity against E. faecalis. Yet these plant compounds do not possess the tissue dissolving property, therefore it can be used only as an adjuctive root canal irrigant.
Essential oil from aromatic and medicinal plants has known to possess antimicrobial and antioxidant property.[5,6] Orieganum vulgare (Lamiaceae family), is an endemic plant found in India, Southern part of Iran and Mediterranean region, that has been used conventionally used for antiseptic purpose. Oreganum essential oil is edible plant oil used in food products. In the year 2014, it had obtained the approval from food and drug administration, Code of federal regulations, Title 21 part 582.[7] Reports have shown oregano, thyme, clove, and cinnamon to possess antimicrobial property.[8]
Many medicinal plant extracts have been claimed to have other additive properties along with antimicrobial activity, one of which being the antioxidant activity. Hence, it was not surprising for the present compound to have the above-mentioned properties. However, the interesting aspect of this oregano oil was revealed in our preliminary molecular level study (unpublished). This might have a significant impact on improvising the oil extract as an intracanal medicament.
Therefore, this pilot study is aimed to determine its chemical composition, minimal inhibitory concentration (MIC) and evaluate the antimicrobial efficiency of the oregano essential oil against E. faecalis.
MATERIALS AND METHODS
This vitro study was two-fold; we first collected and identified the plant materials. Then, we isolated the essential oil of the plant and subjected it to the gas chromatography and mass spectrometry (GC-MS) analysis to define the chemical compositions of the oil and also to have better understanding of its bioactivity. Second, we evaluated the antimicrobial efficiency against E. faecalis. The validation of the identified compound was confirmed based on the retention time at the peak area. Following the identification of antimicrobial bio constituent in essential oil, we validated its antimicrobial efficiency by assessing the MIC antimicrobial efficiency against E. faecalis.
MIC determines the susceptibility of microorganisms to antimicrobials. In this, the microorganisms are tested for their ability to produce visible growth in microtitration plate wells of broth containing serial dilutions of antimicrobial agents. MIC is defined as the minimum concentration of antimicrobial agents that caused inhibition in the growth of test microorganism.
The MBC is defined as the lowest concentration of antimicrobial agent needed to kill 99.9% of the final inoculum after incubation for 24 h under a standardized set of conditions. MBC can be determined after broth microdilution by sub-culturing a sample from wells or tubes, yielding a negative microbial growth after incubation on the surface of nonselective agar plates to determine the number of surviving cells (CFU/mL) after 24 h of incubation.
Collection of plant material
The fresh leaves of O. vulgare were collected from the southern region of India (Tamil Nadu).
Isolation of essential oil
Oreganum leaves were dried in hot air oven at 60°C till a constant weight was obtained. The dried leaves were ground and placed inside a soxhlet apparatus. Petroleum ether was used as a solvent for the extraction of oil. Soxhlet apparatus was run at 60°C for 8 h. After which the solvent (40°C–60°C) was evaporated in a rotary evaporator to isolate the essential oil.
Gas chromatography and mass spectrometry analysis
A gas chromatography (GC) apparatus (Agilent 7890A; USA) with capillary column (DB-1MS; 30 m × 0.250 mm, 0.2 μL injection) was used to analyze the O. vulgare essential oil. The carrier gas in this assay was Helium (1.2 mL/min). The program of GC oven was set as follows: Initial temperature at 70°C for 5 min, the programmed rate at 3°C/min up to 280°C for 4 min and temperature of the injector at 250°C. An electron ionization system with an ionization energy of 70 eV was applied for the mass spectrometry (MS) detector (7000 Triple Quad). The temperature of MS transfer line was also adjusted to 280°C. To calculate Kovats indices (KI) for the detected compounds, n-alkanes were employed. Identification of the components was validated by comparing the relative retention time of components and mass spectra with those previously obtained from the Wiley 275 library and Adams data for GC-MS.
Bacterial strains
Bacterial strains Gram-positive (Enterococcus faecalis) ATCC 29212, was chosen based on their clinical and pharmacological importance. The bacterial microorganisms were cultured on nutrient agar by using the spread plate technique and were incubated for 24 h at 37°C. The bacterial strains were grown in Mueller-Hinton agar (MHA) plates at 37°C (the bacteria were grown in the nutrient broth at 37°C and maintained on nutrient agar slants - at 4°C. The stock cultures were maintained at 4°C. The sterile spreader was used for the inoculation of this organisms across respective media.
Stock solution
Stock solutions of 10 mg/ml of oregano oil were freshly diluted to working concentrations in 10% DMSO.
Preparation of test solutions
From this stock solution, different lower dilutions (25–200 μg/ml) were prepared. There were three experimental groups.
Determination of minimal inhibitory concentration
The MICs for the test solutions were determined by broth microdilution method [Figure 1] according to the Clinical Laboratory Standards Institute (CLSI) 2012 standard protocol.[9] The cultures were then incubated and subsequently, serially diluted to reach the density of 2 × 104 cells per ml. Cell counting was done using hemocytometer. Two milliliters of MHA broth were dispensed in tubes, and 100 μL of cell culture was inoculated in it. Then, 100 μL of different concentration of oil (6.25, 12.5, 25.0, 50.0, and 100 μg/ml) was added to each tube. Amoxicillin (10 μg/ml) used as a positive control for bacteria and Disc without plant extract was used as a negative control. Growth control was run in parallel with every experiment. All the experimental tubes were incubated in anaerobic jars for 48 h. After completion of the incubation period, the optical density of broth was measured at 600 nm.
Figure 1.

Broth dilution method
The percentage of bacterial inhibition by the oil was computed using the following equation:

Determination of minimum bacterial concentration
The antimicrobial efficiency of the oil was evaluated by the agar well diffusion method using Muller Hinton Agar. The microorganism was then inoculated (0.25 mL) into molten MHA and poured into Petri dishes. Wells of uniform diameter (6 mm) were then made on the solidified agar. The discs (6 mm in diameter) were impregnated with (25.0, 50.0, and 100 μg/mL) oil (100 μL/disc) placed on the inoculated agar. Amoxicillin (10 μg/ml) used as a positive control for bacteria and disc without plant extract was used as a negative control. Antimicrobial activity was evaluated by measuring the inhibition zone. Plates were then left at room temperature for 1 h to allow the solutions diffusion into the MHA; plates were then incubated at 37°C overnight. Finally, the zones of inhibition were measured from the base of the plates, and the experiments were performed in duplicate and repeated independently three times. Each experiment was carried out in a triplicate set.
Statistical analysis
For statistical analysis of data, multiple comparisons were performed using one-way analysis of variance (ANOVA) followed by the LSD test for post hoc analysis. Statistical significance was accepted at a level of P < 0.05. Data were analyzed using SPSS (version 11, (IBM SPSS Predictive Analytics Community, Bangalore, Karnataka, India)).
RESULTS
Gas chromatography and mass spectrometry analysis
The results obtained by GC-MS analysis are presented in Table 1. A total of 15 constituents were identified. The analysis revealed that oregano essential oil contained carvacrol (41.2%), γ-terpinene (12.68%), p-cymene (9.47%), α-terpinene (1.19%) as the major compounds and β–caryophyllene (0.83%), β-linalool (0.67%), β–bisabolene (0.601%), α-pinene (0.6%), β-pinene (0.5%), terpinen-4-ol (0.41%), borneol (0.4%), 3-thujene (0.4%), spathulenol (0.4%), myristicin (0.25%), and apiol (0.14%).
Table 1.
Chemical Constituent
| Compound Name | Retention time | Peak area % |
|---|---|---|
| 3-thujene | 4.9 | 0.4 |
| α-pinene | 7.6 | 0.6 |
| β-pinene | 10.15 | 0.5 |
| α -Terpinene | 10.47 | 1.19 |
| p-cymene | 12.04 | 9.47 |
| γ -Terpinene | 12.8 | 12.68 |
| β -linalool | 14.035 | 0.67 |
| Borneol | 14.767 | 0.4 |
| Terpinen-4-ol | 15.94 | 0.41 |
| Carvacrol | 2.0672 | 41.2 |
| β -caryophyllene | 21.84 | 0.83 |
| β -Bisabolene | 22.47 | 0.601 |
| Myristicin | 22.573 | 0.25 |
| Spathulenol | 25.28 | 0.4 |
| Apiol | 27.16 | 0.14 |
Minimal inhibitory concentration
Table 2 depicts the MIC. Each value is expressed as mean ± standard deviation (SD) (n = 3), and the results showed a statistically significant difference as compared with negative control (disc without test solution) (P < 0.001). MIC of the test sample is 25 μg/ml.
Table 2.
Determination of minimal inhibitory concentration
| Samples | Zone of Inhibition (mm) | % of Inhibition |
|---|---|---|
| Conc(μg/ml) | ||
| 6.25 | 0.387±0.02* | 7.6 |
| 12.5 | 0.356±0.06 | 15.0 |
| 25.0 | 0.317±0.03* | 24.3 |
| 50.0 | 0.187±0.96* | 55.3 |
| 100 | 0.125±0.89* | 70.1 |
| Positive Control | 0.035±0.01* | 91.6 |
| Negative control | 0.419 ±0.31 |
Minimum bacterial concentration
Figure 2 depicts the zone of inhibition at different concentration, and Table 3 depicts the MBC of oregano essential oil. NI means no inhibition zone. Each value is expressed as mean ± SD (n = 3) and the results showed a statistically significant difference as compared with negative control (Disc without test solution) (P < 0.05). MBC of the test sample is 50 μg/ml.
Figure 2.
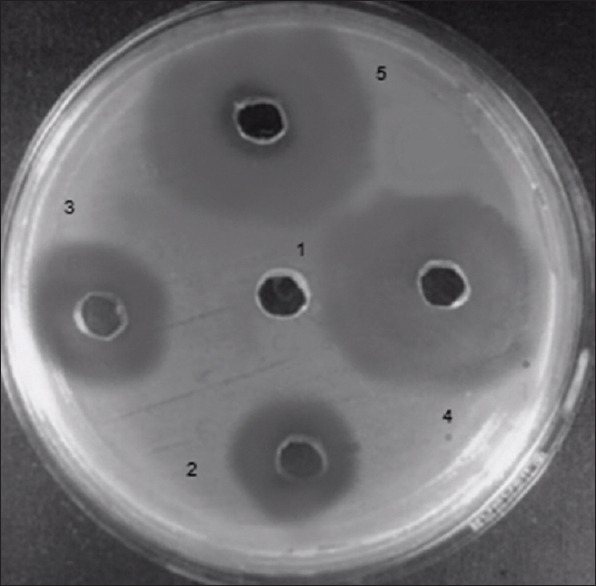
Zone of inhibition
Table 3.
Determination of minimum bacterial concentration
| Samples | Zone of Inhibition (mm) | % of Inhibition |
|---|---|---|
| Conc(μg/ml) | - | |
| 25.0 | 8.6 ±0.84* | 58.5 |
| 50.0 | 11.5 ±1.0* | 78.2 |
| 100.0 | 13.8 ±1.4* | 93.8 |
| Positive control | 14.7 ±1.4* | 100.0 |
| Negative control | NI | - |
DISCUSSION
Essential oil mainly composed of oxygenated monoterpenes and monoterpene hydrocarbon. The major constituent of the oregano essential oil is carvacrol (41%). Oxygenated monoterpenes carvacrol causes inhibition of ATPase activity and increase the nonselective permeability of bacterial cell membrane. It inhibits the microbial colonization and also makes the microbes more sensitive to antibacterial agents.[10] Carvacrol is known to possess strong antioxidant properties and also exhibit antibacterial activity against several bacteria.[11,12,13,14,15]
High monoterpene hydrocarbons such as, α-pinene, and linalool, which were found in oregano essential oil in the current study, have been extensively proven as strong antimicrobial substances[5] and therefore might also be responsible for the antibacterial activity of the experimented essential oil.
Seghatoleslami et al. assessed the antibacterial effects of essential oil, Satureja Khuzistanica Jamzad, which belongs to the same Lamiaceae family as that of Oregano essential oil. Antimicrobial activity and MIC evaluation were carried out by agar dilution and well diffusion method. At 0.31 mg/mL it proved to be is effective against E. faecalis.[16] Furthermore, the previous in vitro study has assessed the cytotoxicity of oreganum extract solution and reported that at 0.5% showed the least cytotoxic effect.[17]
Mostly the studies on Oregano oil extract were restricted to the medicinal field, especially where its effectiveness was evaluated in gastrointestinal and respiratory disorders. The study by Brđanin et al.[18] tested the antimicrobial activity against various organisms, including the enterococcus species, stated that the oreganum extract had a maximal antimicrobial activity on the tested pathogens, especially on Gram-positive organisms. The MIC in that study ranged from 62.5–125 μg/ml, whereas the MIC obtained from the present study on the tested pathogen was only 25 μg/ml.
A study by Mellencamp et al.[19] demonstrated the antimicrobial activity of oregano extract on various pathogens enterococcus species and concluded that extract had a maximal antibacterial activity on both Gram-positive and Gram-negative organisms with highest antioxidant activity. The tested MIC and MBC ranged from 1.25–10 μg/ml. The other quite interesting point discussed was, the oregano extract demonstrated synergistic activity with the commonly used antibiotics. Especially when the extract is used alone or in the combination of antibiotic use. This could be beneficial and applicable in endodontic infections, where especially antibiotic resistance is a major concern.
A previous report[20] demonstrated antimicrobial activity of the oregano extract on Gram-negative organisms and demonstrated the mean zone of inhibition of the oregano oil ranged from 9.5–24.5 mm. Kumari et al.,[21] demonstrated the MIC of 120–160 μg/ml on Gram-positive organisms and 175–200 μg/ml on Gram-negative organisms. However in all the above-mentioned studies, the tested pathogens were quite unrelated in causing oral diseases. Hence, a clear-cut demarcation or concluding remarks on the present compound cannot be made. The reason for these varied results could be the tested pathogens in the previous study were quite different from the present study.
A study done by Botelho et al.[22] have evaluated the antimicrobial activity of carvacrol along with other essential oils on oral pathogens. The GC-MS method used for analyzing the essential oil in the above-mentioned study was similar to the present study for the identification of specific compounds. They concluded an interesting point that the observed antimicrobial potential of the isolated phenolic compounds such as carvacrol was higher single than in combination with other essential oils or components. When observed, they lowered the antimicrobial potential and had an antagonistic effect on the activity of the essential oil, suggesting that minor components contribute to antagonistic activity on the essential oils. Hence, future studies have to be much concentrated on isolating specific carvacrol components and assessing their beneficial activity.
A previous study evaluated the antimicrobial and antibiofilm activity of the isolated extracts thymol and carvacrol from the essential oil of oregano vulgare on oral pathogen streptococcus mutans and the inhibitory concentration of the carvacrol was around 65 μg/ml. The study demonstrated an interesting aspect that carvacrol has a good antibiofilm activity, along with antimicrobial activity on the tested pathogen.[23]
In infected root canals, where the host defense mechanisms are altered, at the molecular level, various genes such as MMPs play an important role in beneficiary regulation of host defenses and at a times may be pathological in inflammatory disease.[24] There comes the importance of the antimicrobial external agent used, which should have a maximal inhibitory activity as compared to bactericidal. Because the extent of inflammatory damage induced by infection is mainly because of pathogenicity and virulence of the organism, rather than the number of organisms involved in the infectious activity. Hence, the determination of MIC is of utmost importance in determining the extent of antimicrobial activity. Although MIC is the most reliable and easily interpreted method for comparison of different formulations, it also has limitations such as unpredictable interaction of medium components with one or more of the test medicaments and the instability of some essential constituent of the test agents.[25]
In the present study, broth dilution and agar diffusion methods were used for antimicrobial susceptibility testing. Broth dilution is one of the most basic antimicrobial susceptibility testing methods.[26] The limitation of the broth dilution method is that the material tested might mask the detection of microbial growth with its color.[26] Moreover, this method may not be accurate for testing fastidious anaerobic organisms. In such a condition, agar dilution method is often preferred to broth dilution for the MIC determination. The other advantage of the agar diffusion is that a large number of bacterial isolates can be tested simultaneously under exactly identical conditions.
Hence, more studies have to be explored and concentrated in endodontics, especially on the specific tested pathogens as the microenvironment in root canal infections is different, and the specific pathogenic organisms are quite difficult to eradicate with the currently available agents. To prevent antimicrobial resistance due to the continuous usage of antimicrobial agents, which is also applicable to root canal infections, the research has to concentrate more on the natural extracts, which has maximal antimicrobial activity. Hence, the present study would be appropriate and reliable.
When limitations are taken into consideration, the present study has evaluated only a single pathogen, which might not translate a clinical scenario. Although E. faecalis is a persistent organism in root canal infections and difficult to eradicate, in clinical situations, various other factors also play an important role in the prognosis of the specific case. Especially when we consider the microflora of the root canal environment, which is quite complex, it is not justifiable to say the beneficial antimicrobial effect of the extract by testing a single species alone. Hence, future studies have to concentrate more on multiple species rather than a single species alone and standardized in vitro studies have to be formulated on the specific extract to determine other beneficiary effects.
When implications of oregano extract, pertaining to endodontics are considered, the future scope is more on this specific oil extract, especially research has to concentrate on isolated compound carvacrol and its implications on endodontic pathogens. Molecular-level studies, have to concentrate on the antioxidant potential and other beneficiary effects of the extract and its isolated components. Conclusive remarks cannot be made from the preliminary assessment made from the present study.
CONCLUSION
The main ingredient that is responsible for antibacterial activity was found to be carvacrol. At the concentration of 25 μg/ml was found to be more effective against E. faecalis. Within the limitations of the study, it can be concluded that oregano essential oil is an effective antimicrobial agent against E. faecalis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pinheiro ET, Gomes BP, Ferraz CC, Sousa EL, Teixeira FB, Souza-Filho FJ. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J. 2003;36:1–11. doi: 10.1046/j.1365-2591.2003.00603.x. [DOI] [PubMed] [Google Scholar]
- 2.Young GR, Parashos P, Messer HH. The principles of techniques for cleaning root canals. Aust Dent J. 2007;1(Suppl 52):S52–63. doi: 10.1111/j.1834-7819.2007.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 3.Teja KV, Ramesh S. Shape optimal and clean more. Saudi Endod J. 2019;9:235–6. [Google Scholar]
- 4.Spangberg L, Engström B, Langeland K. Biologic effects of dental materials 3 Toxicity and antimicrobial effect of endodontic antiseptics in vitro. Oral Surg Oral Med Oral Pathol. 1973;36:856–71. doi: 10.1016/0030-4220(73)90338-1. [DOI] [PubMed] [Google Scholar]
- 5.Daferera DJ, Tarantilis PA, Polissiou MG. Characterization of essential oils from Lamiaceae species by fourier transform raman spectroscopy. J Agric Food Chem. 2002;50:5503–7. doi: 10.1021/jf0203489. [DOI] [PubMed] [Google Scholar]
- 6.Mockute D, Bernotiene G, Judzentiene A. The essential oil of Origanum vulgare L ssp vulgare growing wild in PA Vilnius district (Lithuania) Phytochemistry. 2001;57:65–9. doi: 10.1016/s0031-9422(00)00474-x. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration. Code of Federal Regulations, Title 21 Part 582. [Last accessed on 2014 Jan 01]. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm? CFRPart=582 .
- 8.Fabian D, Sabol M, Domaracká K, Bujnáková D. Essential oils-their antimicrobial activity against Escherichia coli and effect on intestinal cell viability. Toxicol In Vitro. 2006;20:1435–45. doi: 10.1016/j.tiv.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing CLSI M100-S21. Wayne: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 10.Gill AO, Holley RA. Disruption of Escherichia coli, Listeria Monocytogenes, and Lactobacillus Sakei cellular membranes by plant oil aromaticus. Int J Food Microbiol. 2006;108:1–9. doi: 10.1016/j.ijfoodmicro.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Ruberto G, Baratta MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69:167–74. [Google Scholar]
- 12.Safaei-Ghomi J, Ebrahimabadi AH, Djafari-Bidgoli Z, Batooli H. GC/MS analysis and in vitro antioxidant activity of essential oil and methanol extracts of Thymus caramanicusJalas and its main constituent carvacrol. Food Chem. 2009;115:1524–8. [Google Scholar]
- 13.Yanishlieva NV, Marinova EM, Gordon MH, Raneva VG. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999;64:59–66. [Google Scholar]
- 14.Aligiannis N, Kalpoutzakis E, Mitaku S, Chinou IB. Composition and antimicrobial activity of the essential oils of two Origanum species. J Agric Food Chem. 2001;49:4168–70. doi: 10.1021/jf001494m. [DOI] [PubMed] [Google Scholar]
- 15.Chorianopoulos N, Kalpoutzakis E, Aligiannis N, Mitaku S, Nychas GJ, Haroutounian SA. Essential oils of Satureja, Origanum, and thymus species: Chemical composition and antibacterial activities against foodborne pathogens. J Agric Food Chem. 2004;52:8261–7. doi: 10.1021/jf049113i. [DOI] [PubMed] [Google Scholar]
- 16.Seghatoleslami S, Samadi N, Salehnia A, Azimi S. Antibacterial activity of endemic Satureja Khuzistanica Jamzad essential oil against oral pathogens. Iran Endod J. 2009;4:5–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Ok E, Adanir N, Hakki S. Comparison of cytotoxicity of various concentrations oreganum extract solution with 2% chlorhexidine gluconate and 5.25% sodium hypochlorite. Eur J Dent. 2015;9:6–10. doi: 10.4103/1305-7456.149630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brđanin S, Bogdanović N, Kolundžić M, Milenković M, Golić N, Kojić M, et al. Antimicrobial activity of oregano (Origanum vulgare L): And basil (Ocimum basilicum L): Extracts. Adv Technol. 2015;4:5–10. [Google Scholar]
- 19.Mellencamp MA, Koppien-Fox J, Lamb R, Dvorak R. Antibacterial and antioxidant activity of oregano essential oil 122nd International Conference on the Epidemiology and Control of Biological, Chemical and Physical Hazards in Pigs and Pork. 2011:354–7. [Google Scholar]
- 20.Chaudhry NM, Saeed S, Tariq P. Antibacterial effects of oregano (Origanum vulgare) against gram negative bacilli. Pakistan J Botany. 2007;39:609. [Google Scholar]
- 21.Kumari P, Joshi GC, Tewari LM, Singh BK. Quantitative assessment and antibacterial activity of Origanum vulgare L. Journal of Phytology. 2011;3:15–21. [Google Scholar]
- 22.Botelho MA, Nogueira NA, Bastos GM, Fonseca SG, Lemos TL, Matos FJ, et al. Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Braz J Med Biol Res. 2007;40:349–56. doi: 10.1590/s0100-879x2007000300010. [DOI] [PubMed] [Google Scholar]
- 23.Khan ST, Khan M, Ahmad J, Wahab R, Abd-Elkader OH, Musarrat J, et al. Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. AMB Express. 2017;7:49. doi: 10.1186/s13568-017-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teja KV, Ramesh S, Priya V. Regulation of matrix metalloproteinase-3 gene expression in inflammation: A molecular study. J Conserv Dent. 2018;21:592–6. doi: 10.4103/JCD.JCD_154_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haffajee AD, Yaskell T, Socransky SS. Antimicrobial effectiveness of an herbal mouth rinse compared with an essential oil and a chlorhexidine mouth rinse. J Am Dent Assoc. 2008;139:606–11. doi: 10.14219/jada.archive.2008.0222. [DOI] [PubMed] [Google Scholar]
- 26.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6:71–9. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


