Abstract
This study describes and illustrates the jaws, teeth, and tooth microstructure of the Prickly Dogfish Oxynotus bruniensis. Detailed accounts of the dental morphology of O. bruniensis are rare and have not addressed the tissue arrangement or microstructure of the teeth. These features are documented and discussed in the contexts of interspecific comparisons with other elasmobranchs and the dietary specialization of O. bruniensis. The overall tooth morphology of O. bruniensis is similar to those of other closely related members in the order Squaliformes, as is the tissue arrangement, or histotype. Oxynotus bruniensis exhibits a simplified enameloid microstructure, which we compare with previously documented enameloid microstructures of other elasmobranchs. Though subtle interspecific differences in dental characters are documented, neither overall tooth morphology nor histotype and microstructure are unique to O. bruniensis. We conclude that in the case of O. bruniensis, dietary specialization is facilitated by behavioral rather than morphological specialization.
Keywords: elasmobranch, enameloid, histotype, specialization, teeth
The Prickly Dogfish is an understudied species of shark. We provide an illustrated account the tooth morphology and microstructure of this species and discuss them in the context of the Prickly Dogfish’s dietary specialization
1. INTRODUCTION
The prey‐capture abilities of predators reflect interactions between morphological phenotypes, the environment, and behavior (Phillips, 2000; Ferry‐Graham et al., 2002; Hulsey and García de León, 2005; Mori and Vincent, 2008). Predators that feed on a narrow range of prey species are often described as dietary specialists, but applications of the terms ‘specialist’ and ‘specialization’ are frequently open to debate and assigned relative to other taxa within a clade or community (Futuyma and Moreno, 1988; Ferry‐Graham et al., 2002; Irschick et al., 2005). So varied are the interpretations of the term ‘specialist’ that Ferry‐Graham et al. (2002: table 1) identified five types of specialists distinguished by the ecological, mechanistic, and evolutionary implications of their specializations. Based on Ferry‐Graham et al.’s (2002) scheme, functional and behavioral specialists are organisms whose morphology and behavior constrain them to a subset of resources. Despite previous attempts to demarcate specialists and to establish formalized definitions of ‘specialization’, much of the usage remains context‐dependent (Munroe et al., 2014).
The frequency with which specialization is invoked without a standardized definition to explain interspecific morphological variation complicates the exploration of correlations between form and function, which are not always clearcut (Lauder, 1995; Lauder, 1996). For example, across 14 species of Recent and extinct sharks (Chondrichthyes: Elasmobranchii), Whitenack and Motta (2010) found little difference in the puncture and draw performance of the species’ isolated teeth. This does not support the widely accepted scheme of classifying shark tooth morphotypes by presumed function (e.g. cutting type teeth in sharks that prey on large, fleshy marine mammals or piercing type teeth in predominantly piscivorous species of sharks). Furthermore, stomach contents of sharks with a tooth morphotype indicative of specialization on one type of prey may reveal a much broader diet than tooth morphology alone would indicate. For example, the Tiger Shark Galeocerdo cuvier (Péron and Lesueur, 1822) typifies the cutting tooth morphotype (Cappetta, 2012; Klimley, 2013). Authors have not shied from labeling this a case of specialization (Witzell, 1987; Hammerschlag et al., 2015). However, stomach contents suggest that G. cuvier is an opportunistic generalist. Castro (2011: p. 469) described the Tiger Shark as both specialized for preying on large sea turtles and the species with the most extensive diet known among sharks. Additionally, recent findings that some sharks may be regarded as generalists at the species level but exhibit specialist behaviors at the individual level complicate the distinction of specialist species. Matich et al. (2011) report that individual Bull Sharks Carcharhinus leucas (Müller and Henle, 1839) sampled off Florida exhibited steady stable isotope values over the course of their study and concluded that although the population may have the niche breadth of a generalist species, that breadth was explained by variation among individual specialist sharks.
Given the ambiguity surrounding the term ‘specialist’ and its differential application across organizational levels (i.e. individual, population, and species levels), exploring the evolutionary implications of dietary specialization on morphology in sharks is a complicated undertaking. However, it may be aided by a comparative approach in which a putative morphological or behavioral specialization is evaluated against the morphology and behaviors of related taxa. If, as Munroe et al. (2014) suggest, specialization is best thought of as a continuum, then by comparing the morphologies and behaviors of elasmobranchs in the middle or generalist end of the spectrum with those of a sister taxon on the highly specialized end, we may better understand whether selected morphological traits indicate dietary specialization. Here, we model this approach using morphological and microstructural characters of the teeth of a dietary specialist, the Prickly Dogfish Oxynotus bruniensis (Ogilby, 1893) and compare our findings to the teeth of other elasmobranchs.
The Prickly Dogfish Oxynotus bruniensis is a small (adults reach approximately 72 cm total length) squaliform shark found on outer continental and insular shelves and upper slopes of southern Australia and New Zealand and is most common at depths between 350 and 650 m (Compagno, 1984; Ebert et al., 2013). Oxynotus bruniensis is one of five extant species in the family Oxynotidae, all of which are readily identified by their triangular body shape, sail‐like dorsal fins, and enlarged dermal denticles (Ebert et al., 2013; Finucci et al., 2016). The diet of O. bruniensis was only recently described by Finucci et al. (2016), who used genetic analysis of the stomach contents of 53 specimens to determine that the Prickly Dogfish feeds exclusively on the egg capsules of holocephalans. The degree to which this dietary specialization is found among oxynotid sharks remains to be seen. However, a captive specimen of the Angular Roughshark Oxynotus centrina (Linnaeus, 1758), whose natural diet includes polychaete worms, crustaceans, and small teleosts (Capapé, 2008), was observed feeding on elasmobranch egg cases after caretakers initially offered live invertebrates that the shark did not consume (Guallart et al., 2015).
Detailed descriptions of tooth morphology and microstructure of O. bruniensis are sparse despite the utility of tooth morphology. Welton (1981) describes an extinct species of Oxynotus, †O. crochardi, based on two Meckelian teeth and proposes it as the sister taxon to O. bruniensis. Welton’s (1981) description does not describe the teeth of O. bruniensis in great detail or with respect to their functional significance, but it proved sufficient for some researchers to postulate, on the basis of shared dental characters with O. bruniensis, that dietary specialization in Oxynotidae may have evolved more than 20 million years ago (Welton, 1981; Flammensbeck et al., 2018). Cappetta (2012) offers an overview of oxynotid tooth morphology and arrangement remarking on the extreme heterodonty exhibited by oxynotids, but he does not address O. bruniensis explicitly. Bigelow and Schroeder (1957) offer an illustration of the palatoquadrate and Meckelian dentitions of O. centrina and note that there are interspecific differences in dental morphology within the genus, but their description of teeth of O. bruniensis is limited to a general overview of tooth shape and count. Following Bigelow and Schroeder (1957), Herman et al. (1989) provide an account of the arrangement of teeth in O. centrina but do not provide odontological details explicitly relating to O. bruniensis. Perhaps the most extensively illustrated descriptions of oxynotid dentitions to date are those of Herman et al. (2005) in which they compare the external morphologies of teeth of O. bruniensis, O. centrina, and the Sailfin Roughshark Oxynotus paradoxus Frade, 1929 but do not comment on functionality, comparisons with other squaliform taxa or microstructure. Much of what is known about squaliform tooth development, replacement, and microstructure comes from studies of the Spiny Dogfish Squalus acanthias Linnaeus, 1758 (Kerr, 1956; Grady, 1970; Nanci et al., 1983; Samuel et al., 1983; Slavkin et al., 1983). Only lately has the taxonomic scope of squaliform tooth development and microstructural studies been increased (e.g. Moyer and Bemis, 2016; Underwood et al., 2016). This is surprising given the use of external morphological characters of teeth in phylogenetic studies of Squaliformes and other clades of elasmobranchs (Casier, 1961; Adnet and Cappetta, 2001; Guinot et al., 2018).
In addition to descriptions of external tooth morphology, histological differences among shark teeth are reported in the literature, but not with respect to O. bruniensis. Differences in the arrangement of dental tissues in sharks have been documented since the mid‐19th century and continue to be an active field of study (Agassiz, 1833‐1843; Owen, 1840‐1845; Moyer et al., 2015; Schnetz et al., 2016; Moyer and Bemis, 2017; Jambura et al., 2020). The arrangement of orthodentine around a hollow pulp cavity and a root of osteodentine in orthodont teeth and a pulp cavity filled by osteodentine like that of the root, and the absence of orthodentine in osteodont teeth represent the two principal histotypes of elasmobranch teeth (see Moyer et al., 2015 and references therein for a more detailed account of histotype differentiation). Peyer (1968) noted that elasmobranch teeth may be arranged by histological complexity, and the orthodont and osteodont histotypes represent the extremes. Recently, the term pseudoosteodont has been reintroduced to the literature referring to a histotype in which osteodentine secondarily fills the majority of a pulp cavity surrounded by orthodentine (Jambura et al., 2018). In selachian fishes, crowns of teeth of all histotypes are covered by triple‐layered enameloid. Each layer is distinguished by the orientation of fluorapatite crystallite bundles (Reif, 1973; Cuny et al., 2017). The enameloid layers are the outermost, or shiny layer enameloid (SLE); the middle, or parallel fibered enameloid (PFE); and the innermost, or tangled fibered enameloid (TFE) (Cuny et al., 2017; Gillis and Donoghue, 2007).
We conducted the current study to document the arrangement, morphology, and microstructure of teeth of the Prickly Dogfish O. bruniensis, a dietary specialist for which no detailed record of dental morphology and histology exists beyond general tooth shape and tooth counts. By providing detailed morphological and histological documentation of the dentition of O. bruniensis, we are able to address two related issues concerning the comparative odontology of elasmobranchs and aspects of morphological specialization: (a) How do the morphology and arrangement of teeth in O. bruniensis compare with those of other selachians? (b) Does the dentition of O. bruniensis reflect either in gross morphology or microstructure the specialized diet of the species? Here, we not only provide the first in‐depth account of tooth morphology and microstructure in relation to the diet of O. bruniensis but also model an investigatory framework to document, identify, and analyze morphological traits of a specialist species.
2. METHODS
2.1. Specimen collection
We examined the jaws and teeth of six specimens of O. bruniensis, three males and three females ranging from 57.8 to 67.9 cm in total length (TL). Specimens were obtained during fisheries independent research trawl surveys conducted by the National Institute of Water and Atmospheric Research (NIWA; New Zealand) on board RV Tangaroa on Chatham Rise of New Zealand during January 2018. Specimens were frozen whole at sea and transported to the mainland for analysis. All but one specimen, a 64.4‐cm total length (TL) male (UMA F20812), had empty stomachs. The single specimen that did not, had the partially digested remains of a chondrichthyan egg case in its stomach. The head of each specimen was removed anterior to the pectoral girdle but posterior to the hyoid arch, fixed in 10% formalin, and then transferred to 70% ethanol. Specimens were shipped to the University of Massachusetts Amherst (UMA; Amherst, MA, USA) where they were accessioned into the university’s Natural History Collections. The jaws of a 57.8‐cm TL male (UMA F20813), 62.6‐cm TL female (UMA F20814), and a 67.9‐cm TL female (UMA F20815) were excised and prepared as skeletal specimens using a method modified from Enault et al. (2016). The jaws, chondrocranium, and hyoid arch of the 64.4‐cm male (UMA F20812) were skeletonized using methods described by Bemis et al. (2004). The heads of a 59.1‐cm TL male and a 52.6‐cm TL female (UMA F20810 and UMA F20811, respectively) were retained as whole heads with soft tissue intact and preserved in ethanol as fluid specimens.
To identify specific teeth with respect to position across the rami of the jaws and within tooth files, we used the tooth identification scheme employed by Moyer et al. (2015) and Bemis et al. (2015). In this identification scheme, the letter S denotes symphyseal teeth overlaying the palatoquadrate symphysis of the upper jaw or the Meckelian symphysis of the lower jaw. The letters R and L indicate the right or left side of the jaw, the letters P or M indicate whether the tooth is of the palatoquadrate or Meckelian cartilages, and a number indicates the position of the tooth relative to the symphysis of the jaw. For example, the fourth tooth from the Meckelian symphysis on the right side of the Mecklian cartilage would be identified as R (right) M (Meckel’s cartilage) 4 (fourth tooth from the center). If a tooth is fully erupted and in a functional position or if it is a replacement tooth, we append the letters F or R, respectively. In the case of replacement teeth or multiple functional teeth in a single tooth file, a number indicating the tooth’s position from the labial side of the jaw follows the letters R or F.
After soaking skeletonized jaws in a dilute solution of household ammonia, we excised the following individual teeth from UMA F20813: SMF1, SMR1, RM1F1, RM2F1, LM2F1, LM2R1, SP1F1, SP1F2, RP3F1, RP3F2, and LP3F2. We selected these teeth on the basis of condition and ease of extraction. Teeth were cleaned first in warm water and then in acetone before air‐drying. We used the same tooth extraction procedure to isolate teeth of the Shortfin Mako Isurus oxyrinchus Rafinesque, 1810 and the Cownose Ray Rhinoptera bonasus (Mitchill, 1815) from specimens UMA 00046 and UMA F20809, respectively. We use the same tooth numbering scheme to refer to the isolated teeth of I. oxyrinchus. As previously described by Sasko et al. (2006) and Berkovitz and Shellis (2016), teeth of R. bonasus are arranged in an imbricated, pavement‐like dentition with seven files of flattened teeth in the palatoquadrate and Meckelian cartilage. Consequently, the tooth numbering scheme we use for shark teeth is not applicable to R. bonasus. We refer to specific teeth of R. bonasus by their exact location within the jaw.
2.2. Histological preparation and specimen imaging
SMF1, RM1F1, and LM2R1 teeth of O. bruniensis specimen UMA F20813 were flat‐lapped using first 1,200 and then 2,000 grit carborundum paper to remove the lingual and labial surfaces of each tooth. Once tooth sections were approximately 70 μm thick they were washed again in acetone and allowed to air‐dry before being mounted using PermountTM mounting medium (Fisher Scientific) on glass slides for light microscopy. We applied the same specimen preparation procedure to the isolated LM4F1 tooth of I. oxyrinchus (UMA 00046) and the tooth plate sixth from the front of the central upper jaw tooth file in R. bonasus (UMA F20809). Micrographs of tooth sections were taken using a Leica M165 FC light microscope equipped with a Leica DFC450 C digital camera. Following Schmidt and Keil (1971) and Ripa et al. (1972), we imaged tooth sections under polarized light to enhance differentiation of dental tissues. We compared the microstructure of teeth in O. bruniensis with those of I. oxyrinchus and R. bonasus as well as previously published descriptions of tooth microstructure in other extant and extinct taxa (e.g. Enault et al., 2013; Moyer et al., 2015; Moyer and Bemis, 2016; Cuny et al., 2017). In our comparison, I. oxyrinchus serves as a representative of modern selachians and R. bonasus as a representative of batoids.
To obtain micro‐computed tomography (CT) datasets, we used a ZEISS Xradia 520 Versa 3D X‐Ray microscope operating at 110 kV source voltage, 10 W power, and using the LE3 propriety high pass ZEISS filter provided with the instrument (Carl Zeiss Microcopy Inc.). The LM2F1 and RP3F2 teeth of UMA F20813 were scanned at 2.7 μm resolution. The jaws of UMA F20810 were scanned in situ at 25.3 μm resolution. We used the open source biomedical image viewer Horos (based on osirixtm DICOM imaging software; Rosset et al., 2004) on an Apple Macintosh computer running OSX 10.13.6 to generate two‐ and three‐dimensional reconstructions of scanned specimens. In three‐dimensional reconstructions, tissue densities are represented by customized color‐lookup tables (CLUTs) with denser tissues appearing lighter shades of yellow or white and less dense tissues appearing darker shades of red or orange.
Excised jaws were photographed using a Canon EOS Rebel T7i digital camera. We corrected images for contrast and merged them using through‐focus stacking in Adobe photoshop CS5 (version 15.0.2) to make a single high‐resolution image with greater depth of field.
3. RESULTS
3.1. Jaw morphology and tooth arrangement
In situ, the jaws of Oxynotus bruniensis are surrounded by fleshy, papillose lips, and the functional dentition of the palatoquadrate and Meckelian cartilages are visible (Figure 1). The jaws are positioned sub‐terminally under the chondrocranium in an orbitostylic mode. Visible in excised and skeletonized jaw specimens, the quadrate process of the palatoquadrate is greatly enlarged (Figure 2a,b). When viewed from a posterior position, two separate quadratomandibular joints are visible behind the wing‐like quadrate processes (Figure 2c). These two joints form the points of articulation between the palatoquadrate and Meckelian cartilages. Though present, the orbital processes of the palatoquadrate are very small, and in dried skeletal specimens the orbital process tends to fold under a crest formed on the dorsal ridge of the palatoquadrate (Figure 2b,c). In manually manipulated specimens, the jaws open vertically to create an oral opening approximately twice as high as it is wide. Note that manual manipulation of postmortem material can only serve as a rough approximation of in vivo performance. However, the maximum dimensions allowed by the jaws, the relatively small and inflexible symphyses of the palatoquadrate and Meckelian cartilages (Figure 2c), and the short range of the suspensory ligaments suggest that jaw protrusion and gape expansion do not factor heavily in the predatory mode of O. bruniensis.
Figure 1.
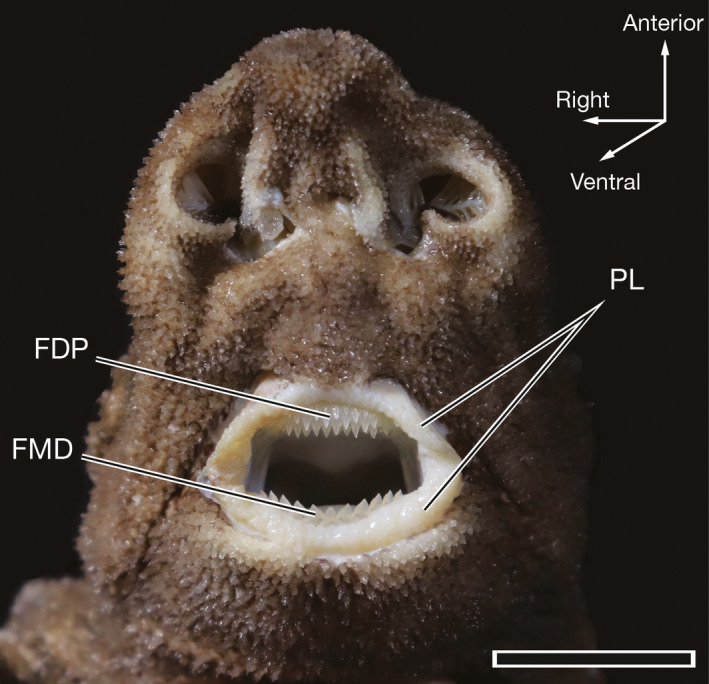
Ventral view of the mouth, functional dentition, and lips of 62.6‐cm TL female Oxynotus bruniensis (UMA F20811). FDP, functional dentition of the palatoquadrate; FMD, functional Meckelian dentition; PL, papillose lips. Scale bar: 2 cm
Figure 2.
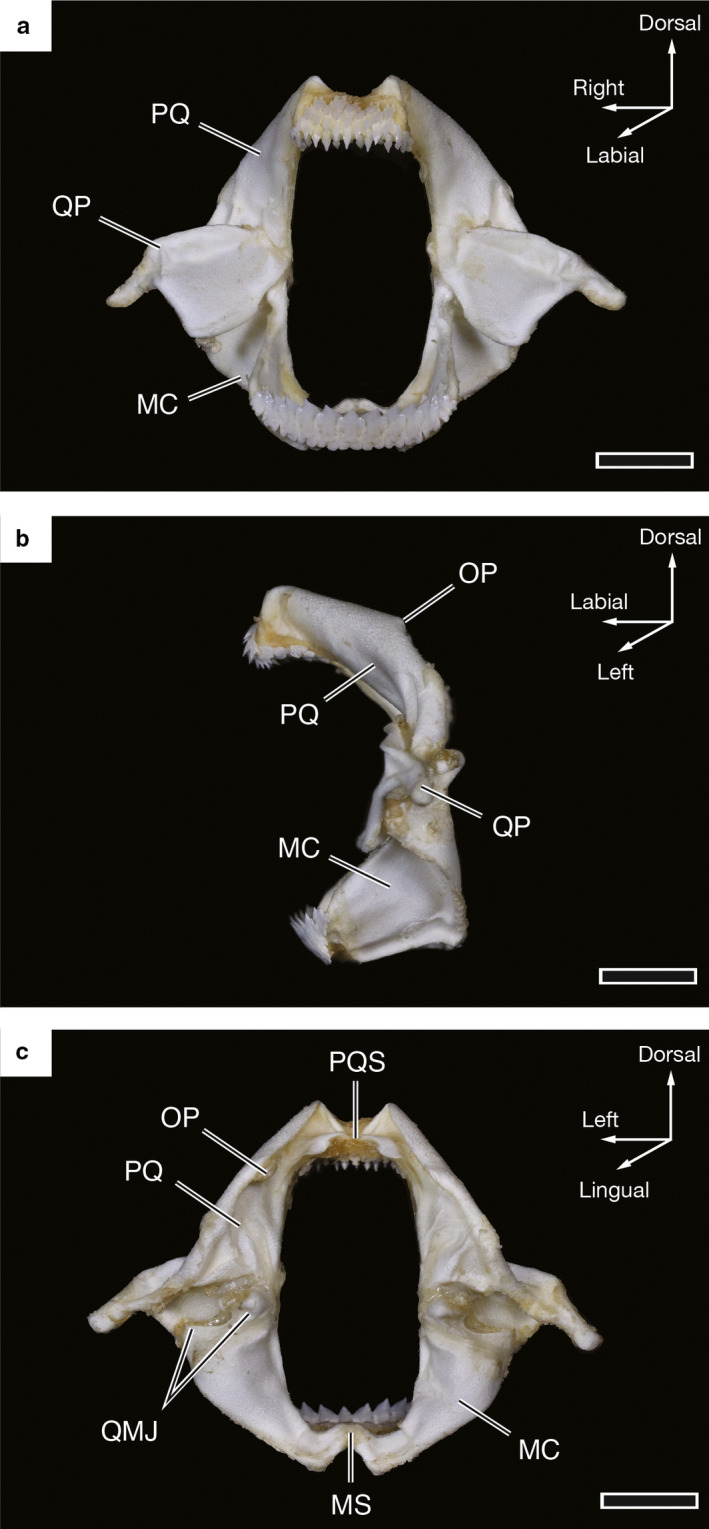
Photographs of the jaws of a 67.9‐cm TL female Oxynotus bruniensis (UMA F20815). (a) Labial view of the jaws. (b) Lateral view of the jaws shown in (a). (c) Lingual view of the jaws shown in (a). MC, Meckel’s cartilage; MS, Meckelian symphysis; OP, orbital process; PQ, palatoquadrate; PQS, palatoquadrate symphysis; QMJ, quadratomandibular joints; QP, quadrate process. Scale bars: 1 cm
The functional dentition of O. bruniensis displays monognathic and dignathic heterodonty in which tooth shape and size differs within and between the palatoquadrate and Meckelian dentitions (Figures 3a and S1). In anterior tooth files of the palatoquadrate, such as RP1 – 6, SP1, and LP1 – 6 of UMA F20810, the teeth are very similar morphologically and are positioned in an arrangement consistent with alternate file tooth replacement (see Underwood et al. 2016 for an in‐depth account of tooth replacement patterns in squaliform sharks). Teeth are offset across developmental rows, and this pattern extends across the anterior region of the palatoquadrate until reaching the lateral region of the rami of the palatoquadrate.
Figure 3.
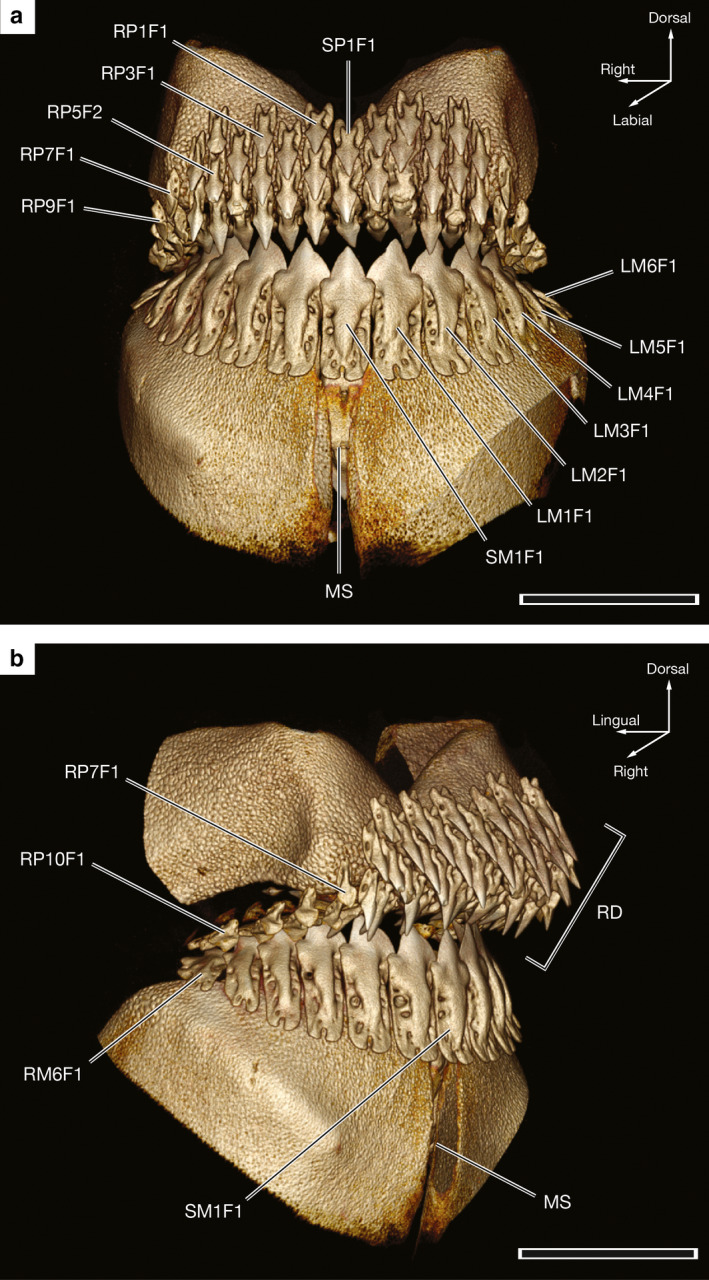
Three‐dimensional reconstructions of the dentition of a 59.1‐cm TL male Oxynotus bruniensis (UMA F20810) generated from CT data. (a) Labial view of upper and lower jaw dentitions with landmark tooth loci labeled. (b) Oblique view from the right side of the same specimen shown in (a) with rasping dentition in the anterior region of the upper jaw identified. MS, Meckelian symphysis; RD, rasping dentition. See the text for tooth numbering scheme. Scale bars: 1 cm
Teeth at the 6th or 7th through 10th loci on the left and right sides of the palatoquadrate are markedly smaller and more asymmetrical than teeth of the anterior region. The crowns of teeth in these lateral loci are less pointed, not as tall, and the structures of the tooth roots are poorly defined. When viewed from a lateral or oblique angle, it is apparent that teeth of the lateral region of the palatoquadrate are not closely associated with the jaw cartilage. Rather, they are held in connective tissue not visible in CT reconstructions when settings are optimized for hard tissue visualization and are not as close to the tessellated cartilage of the palatoquadrate as the more anterior teeth (Figure 3b). The restricted range of the dentition of the palatoquadrate in O. bruniensis is noteworthy as the functional dentition is confined to the anterior‐most regions of the jaw and does not extend distally across the rami of the palatoquadrate cartilages.
All specimens examined displayed retention of functional teeth in the following tooth files: RP1 – 5 or 6, SP1, and LP 1 – 5 or 6. As a result, each of these tooth files contained up to four functional teeth starting at the margin of the jaw and around to its labial surface. Collectively, these cuspidate teeth make a rasping dentition on the anterior of the palatoquadrate (Figure 3b). This arrangement would likely be suitable for gripping and scratching through food items similar to the anterior Meckelian dentition of Heterodontus spp., based on previously published descriptions of the latter (e.g. Garman, 1913; Reif, 1976; Huber et al., 2005).
The Meckelian teeth of O. bruniensis are asymmetrical with the exception of the single symphyseal tooth (SM1), which is symmetrical (Figure 3a). Unlike the dentition of the palatoquadrate, only a single functional tooth is present at each tooth locus in all specimens examined. In specimen UMA F20814, the LM2F1 and LM3F1 tooth crowns were broken, leaving a gap in the functional dentition; however, the tooth roots were retained. Presence of the roots indicates that the gap in the dentition was likely due to mechanical breakage of tooth crowns and not variable rates of replacement between tooth files. From mesial to distal Meckelian tooth loci, teeth become shorter, and their angulation relative to the jaw margin changes such that more distal teeth are reclined lingually and point backwards into the mouth (e.g. tooth RM6F1 in Figure 3b). More anterior teeth retain an upright position.
Three‐dimensional reconstructions of CT data illustrating the functional and replacement teeth of O. bruniensis show dignathic differences in tooth development and replacement (Figure 4). In these reconstructions, tissue density serves as an indicator of mineralization. When the lingual side of the palatoquadrate is viewed, teeth in early stages of development are visible as partial crowns and lack any mineralized root (e.g. SP1R3, LP1R4, and LP2R3 in Figure 4a). Root mineralization appears to take place as the tooth progresses from the R2 to R1 positions in the tooth files of the palatoquadrate. Sequentially added teeth (sensu Underwood et al., 2016) progress from replacement to functional positions, giving rise to the alternate tooth replacement pattern observed in the functional teeth. Replacement teeth become functional as their angle relative to the margin of the jaw changes. For example, the SP1F3 tooth depicted in Figure 4a is deemed functional as it is angled downward into the oral cavity, whereas the neighboring LP1R1 tooth remains approximately perpendicular to the margin of the jaw. Tooth files of the palatoquadrate each have three or four replacement teeth.
Figure 4.
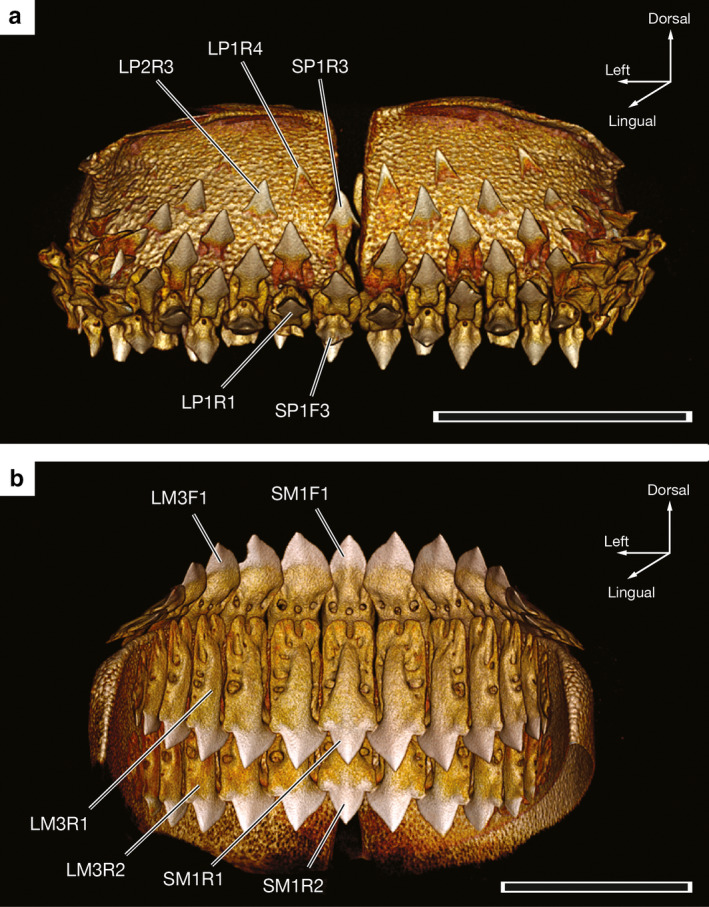
Three‐dimensional reconstructions generated from CT data depicting replacement teeth of a 59.1‐cm TL male Oxynotus bruniensis (UMA F20810). (a) Lingual view of the tooth‐bearing region of the palatoquadrate with landmark tooth loci labeled to illustrate alternate tooth replacement. (b) Lingual view of the tooth‐bearing region of Meckel’s cartilage with landmark tooth loci labeled. Scale bars: 1 cm
In contrast to the tooth files of the palatoquadrate, Meckelian tooth files bear a single functional tooth and two replacement teeth per file (Figure 4b). Replacement teeth lay flush with the lingual side of the jaw (e.g. SM1R1, SM1R2, LM3R1, and LM3R2 in Figure 4b). Like the functional Meckelian teeth, replacement teeth remain in close contact and form a single serrated dentition. This indicates a ‘single file’ pattern of tooth replacement (sensu Underwood et al., 2016). Crowns of the earliest replacement Meckelian teeth, designated R2, are complete and connected to roots that are almost completely mineralized. The exceptions to this trend are replacement teeth of the most distal loci, which do not complete mineralization of the root until they transition to a functional position.
Including the small lateral teeth of the palatoquadrate, the dental formula of O. bruniensis is U: 9 or 10 – 1 – 9 or 10, L: 6 or 7 – 1 – 6 or 7. Whether there is an ontogenetic difference in tooth number in O. bruniensis remains to be determined and would require larger sample sizes spanning multiple ontogenetic stages. However, based on the six specimens observed in the current study, variability in tooth number does not appear to be correlated with sex.
3.2. Tooth morphology
In O. bruniensis the symphyseal teeth of the palatoquadrate (SP1) and teeth of the first six or seven tooth files on either side of the symphysis (LP1 through LP6 or 7 and RP1 through RP6 or 7) are small and, in the specimens examined, do not exceed 3.5 mm in height when measured from the tip of the longest root lobe to the apex of the enameloid‐covered crown. The crowns of these teeth appear lanceolate, or spear‐shaped, and widen before tapering into narrow aprons (sensu Cappetta, 2012) of enameloid at the base of the crown (Figure 5a). LP1 and RP1 through LP7 and RP7 teeth are nearly symmetrical. Only in more distal loci are the root lobes uneven in length. Labial marginal foramina are present on both sides of the apron. In CT‐scanned teeth, a single marginal foramen is visible at the base of the apron inside the root notch and is refered to as the root notch foramen (Figure 5b). Labial marginal foramina and root notch foramina are small and are difficult to observe without the use of repeated cleanings and micro‐CT reconstructions, as the foramina of individually excised teeth are frequently covered by connective tissue or filled with what is presumably vascular tissue. LP1 and RP1 through LP7 and RP7 teeth are linguo‐labially robust, such that the distance from the lingual to labial‐most points on the teeth equals almost a third of the overall tooth height (Figure 5c). When viewed laterally, the extent of the overlap between the enameloid‐covered portions of the tooth and the root becomes more apparent. This arrangement results in the crown of the tooth being situated not under the root when the tooth is in a functional position but overhanging the root (Figure 5c). In contrast, the distal‐most teeth of the palatoquadrate are less well defined and are more linguo‐labially compressed. None of the teeth of the palatoquadrate exhibit appreciable serrations, although multiple teeth examined during this study bear chipped cutting edges.
Figure 5.
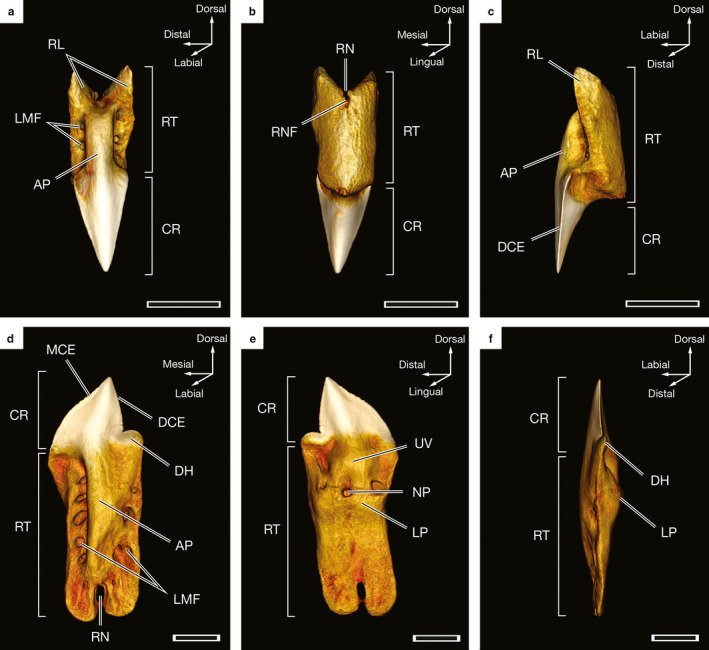
Three‐dimensional reconstructions of individual teeth of a 57.8‐cm TL male Oxynotus bruniensis (UMA F20813) generated from CT data. (a) Labial view of RP3F2 tooth. (b) Lingual view of the tooth shown in (a). (c) Lateral view from the distal side of the tooth shown in (a). (d) Labial view of LM2F1 tooth. (e) Lingual view of the tooth shown in (d). (f) Lateral view from the distal side of the tooth shown in (d). AP, apron; CR, crown; DCE, distal cutting edge; DH, distal heel; LMF, labial marginal foramina; LP, lingual protuberance; MCE, mesial cutting edge; NP, nutritive pore; RL, root lobe; RN, root notch; RNF, root notch foramen; RT, root; UV, uvula. Scale bars: 1 mm
The Meckelian teeth of O. bruniensis are larger than the teeth of the palatoquadrate. Among the specimens examined, the largest Meckelian teeth are LM and RM2F1 and measure 5 mm from crown apex to root lobe. Tooth height decreases to approximately 3 mm in the distal‐most loci. With the exception of the symphyseal tooth (SM1), Meckelian teeth are asymmetrical with a single distal heel at the base of a distal cutting edge on one side of the crown and a curved mesial cutting edge on the opposite side of the crown, (Figure 5d). The SM1 tooth of the Meckelian dentition is symmetrical, with an erect crown and a distal heel on either side (Figures 3 and 4b). Serrations on Meckelian teeth, particularly on the mesial cutting edge, are very light. At 2.7 μm resolution, they are barely visible in CT reconstructions. The apices of functional Meckelian teeth point distally. The exception to this trend is the SM1 tooth, which bears a distal heel on both sides and points dorsally rather than distally. Meckelian teeth therefore have a discernible distal inclination of the crown apex that indicates the side of the jaw from which they originate. We follow Moyer and Bemis (2016) in referring to this directionality as handedness. The handedness of teeth at a given locus remains consistent within the tooth file. On the labial surfaces of Meckelian teeth, labial marginal foramina penetrate the root down the lengths of each root lobe on either side of the apron, a central ridge covered by a very thin layer of enameloid that extends from the base of the crown to the root notch (Figure 5d). Marginal foramina are not visible on the lingual side of Meckelian teeth. Rather, there is a large nutritive pore beneath a lingual extension of the crown termed the uvula (sensu Cappetta, 2012) and above a lingual protuberance of the root (Figure 5e). Meckelian teeth are linguo‐labially compressed. Even with the lingual protuberance, LM and RM1 and 2 teeth are more than five times taller than they are linguo‐labially wide (Figure 5f).
3.3. Histotype and microstructure
Sectioned reconstructions generated from CT data allow visualization of the pulp cavities of palatoquadrate and Meckelian teeth. The pulp cavities of teeth of the palatoquadrate in tooth files LP1 through LP6 or 7, SP1 and RP1 through RP6 or 7 are best visualized when reconstructions of teeth are sectioned medially (Figure 6a). The extent of pulp cavities in the more linguo‐labially compressed Meckelian teeth is more apparent when the labial surfaces of tooth reconstructions are virtually removed (Figure 6b). Figure 6 shows sectioned reconstructions of the RP3F2 and LM2F1 teeth of UMA F20813. In both teeth, the pulp cavities extend into the root, beyond the base of the crown. Labial marginal foramina connect to the pulp cavity, as do the root notch foramen of RP3F2 (Figure 6a) and the nutritive pore of LM2F1 (Figure 6b). The presence of a hollow pulp cavity is indicative of the orthodont histotype, an identification confirmed by light microscopy (Figure 7).
Figure 6.
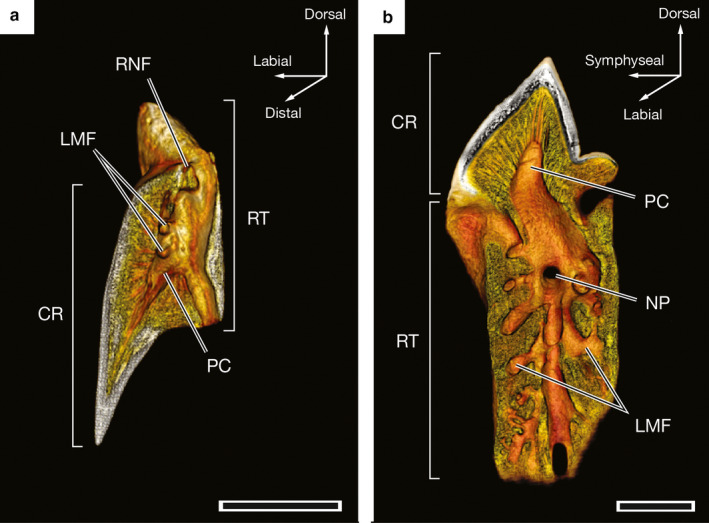
Virtually sectioned three‐dimensional reconstructions of individual teeth of a 57.8‐cm TL male Oxynotus bruniensis (UMA F20813) generated from CT data to illustrate internal anatomy of the teeth. (a) RP3F2 tooth sectioned by virtually removing the distal half of the tooth reconstruction. (b) LM2F1 sectioned by virtually removing the labial surface of the tooth reconstruction. CR, crown; LMF, labial marginal foramina; NP, nutritive pore; PC, pulp cavity; RNF, root notch foramen; RT, root. Scale bars: 1 mm
Figure 7.
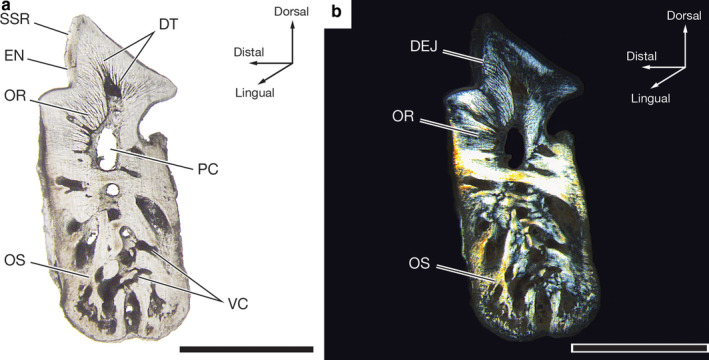
Light micrographs of the flat‐lapped LM2R1 tooth of a 57.8‐cm TL male Oxynotus bruniensis (UMA F20813). (a) Entire tooth section under unpolarized white light illustrating tissue arrangement and internal structures of the tooth. (b) Same tooth section shown in (a) viewed under polarized light. EN, enameloid; DEJ, dentine‐enameloid junction; DT, dentine tubules; OR, orthodentine; OS, osteodentine; PC, pulp cavity; SSR, secondary serration; VC, vascular canal. Scale bars: 2 mm
Light microscopy of the fully formed flat‐lapped LM2R1 tooth of UMA F20813 reveals three tissue types (Figure 7a). Osteodentine, discernible by the presence of vascular canals, or denteones (sensu Smith and Sanson, 2000; formally osteons), forms the root. Orthodentine surrounds much of the pulp cavity in the crown and is distinguished from osteodentine by dentine tubules radiating from the pulp cavity and the absence of denteones. Enameloid covers the outer surface of the crown. Due to differing birefringence indices of the dental tissues, polarized light microscopy makes the junctions between these tissues, especially the dentine‐enameloid junction, more apparent (Figure 7b). Based on the uniform dentine‐enameloid junction, serrations of the Meckelian teeth of O. bruniensis are superficial secondary serrations (sensu Moyer and Bemis, 2017). The underlying orthodentine does not show signs of a serrated dentine‐enameloid junction that would mirror the serrated enameloid.
Higher magnification of the region of the dentine‐enameloid junction in the same LM2R1 tooth reveals a simplified enameloid microstructure (Figure 8a,b). The outermost layer of enameloid is the shiny layer enameloid (SLE). Beneath the shiny layer enameloid, crystallites appear bundled in an inner enameloid layer (IEL; Figure 8a). In some regions of the crown, the crystallites are oriented more or less perpendicular to the underlying orthodentine. Elsewhere within the same enameloid layer, crystallites are oriented more obliquely. None of the teeth examined showed an enameloid layer in which crystallites were arranged with a shared and consistent orientation (e.g. parallel or perpendicular to the central axis of the tooth). The homology of these enameloid layers to those in other taxa is currently unknown. Therefore, we follow Enault et al. (2013) and identify enameloid layers based on location relative to each other. Consequently, O. bruniensis does not exhibit the triple‐layer enameloid found in other neoselachians, represented in this study by the Shortfin Mako Isurus oxyrinchus in concert with the findings of previous documentation of enameloid microstructure in other neoselachians (e.g. the Gulper Shark Centrophorus granulosus (Bloch and Schneider, 1801) studied by Moyer and Bemis, 2016; Heterodontus spp. studied by Reif, 1973; the Blue Shark Prionace glauca (Linnaeus, 1758) studied by Moyer et al., 2015; †Squalicorax spp. studied by Andreev, 2010).
Figure 8.
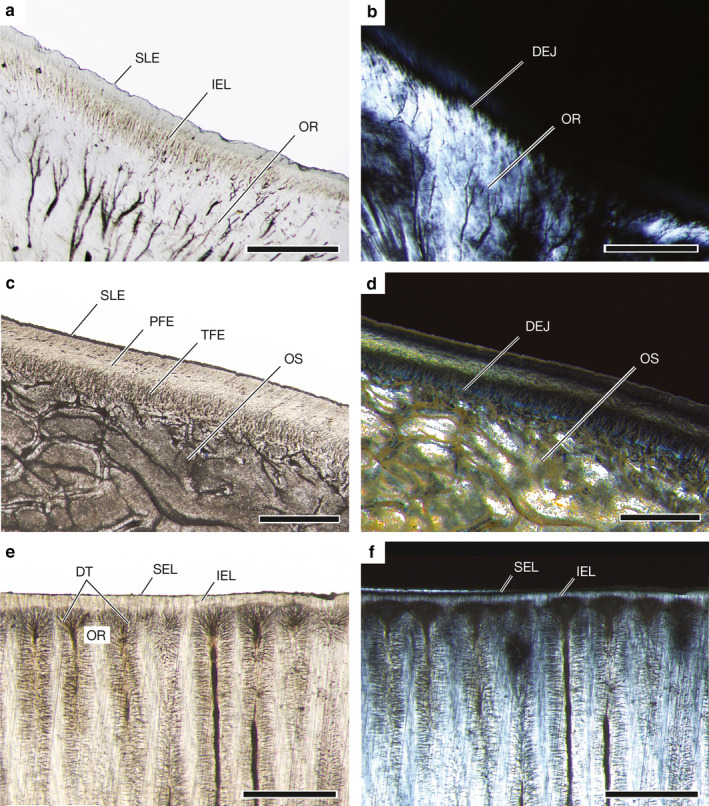
Enameloid microstructure in teeth of Oxynotus bruniensis (UMA F20813), Isurus oxyrinchus (UMA 00046), and Rhinopetera bonasus (UMA F20809). (a) Simplified enameloid microstructure of LM2R1 tooth of O. bruniensis with underlying orthodentine. (b) Same tooth and region shown in (a) viewed under polarized light to distinguish dentine‐enameloid junction. (c) Triple‐layer enameloid of LM4F1 tooth of I. oxyrinchus (UMA 00046). (d) Same tooth and region shown in (c) viewed under polarized light. (e) Tissue arrangement and simplified enameloid of the sixth central tooth plate of the upper jaw of R. bonasus (UMA F20809). (f) Same tooth and region shown in (e) viewed under polarized light. DEJ, dentine‐enameloid junction; DT, dentine tubules; IEL, inner enameloid layer; OS, osteodentine; OR, orthodentine; PFE, parallel fibered enameloid; SEL, superficial enameloid layer; SLE, shiny layer enameloid; TFE, tangled fibered enameloid. Scale bars: (a,b) 200 μm; (c‐f) 500 μm
Isurus oxyrinchus exhibits three layers of enameloid: the outermost SLE, the parallel fibered enameloid discernible by the parallel orientation of its crystallite bundles (PFE), and the tangled fibered enameloid (TFE), which appears as a meshwork of fibers adjacent to the dentine‐enameloid junction (Figure 8c,d). Osteodentine fills the crown beneath the dentine‐enameloid junction. The arrangement of three enameloid layers in the teeth of I. oxyrinchus exemplifies the triple‐layer enameloid that is common but not ubiquitous among neoselachians. Thus, it is a useful point of comparison when evaluating the simplified enameloid microstructure of O. bruniensis. Another neoselachian, the Cownose Ray Rhinoptera bonasus, exhibits a modified tooth microstructure and simplified enameloid (Figure 8e,f). In R. bonasus a thin outer layer of enameloid, here termed the superficial enameloid layer, and an inner enameloid layer covers orthodentine, which is discernible by the rhizoid‐like arrangement of dentine tubules. This region of densely packed dentine tubules appears as a dark band beneath the enameloid under unpolarized light (Figure 8e). The orthodentine‐enameloid junction is marked by the termination of the rhizoid‐like dentine tubules where they abut an inner layer of enameloid. The inner layer of enameloid is discernible as a faint glow under polarized light as the positively birefringent orthodentine and enameloid form a progressive rather than abrupt dentine‐enameloid junction. The superficial enameloid layer is negatively birefringent and therefore not as visible under polarized light (Figure 8f). As is the case with the enameloid of O. bruniensis, the homology (or lack thereof) of enameloid layers in R. bonasus with those of the triple‐layer enameloid exemplified by I. oxyrinchus has yet to be determined. We again follow Enault et al. (2013) in identifying the layers by their positions relative to each other and not the orientation of their crystallite bundles. The overall tissue arrangement we observe in R. bonasus agrees with previous descriptions of tooth histology in myliobatiform rays (note Radinsky, 1961 who identified orthodentine consisting of pallial dentine and circumpulpar dentine noted the presence of both in Myliobatis sp.; Berkovitz and Shellis, 2016). Collectively, these results indicate that although simplification of enameloid microstructure is not unique to O. bruniensis, the Prickly Dogfish does represent a variant of microstructural modification of enameloid not typically associated with extant selachian fishes or, more broadly, modern neoselachians.
4. DISCUSSION
Our study presents a detailed treatment of the jaws, dentition, tooth morphology, and microstructure of the teeth of the Prickly Dogfish Oxynotus bruniensis. Consequently, we are afforded the opportunity to revisit the utility of morphological descriptions of the macro‐ and microscopic dental morphologies of squaliform sharks and to assess their novelty in O. bruniensis. We present illustrated qualitative data on the microstructure of enameloid and histotype in O. bruniensis, which is germane to the recent revivals of comparative microstructural studies of chondrichthyan enameloid (e.g. Gillis and Donoghue, 2007; Enault et al., 2015; Cuny et al., 2017) and tissue arrangement (e.g. Moyer et al., 2015; Moyer and Bemis, 2016; Schnetz et al., 2016; Jambura et al., 2020). By evaluating our results in a comparative framework, taking into account phylogenetic relationships and the ubiquity of odontological characteristics exhibited by O. bruniensis among neoselachians, we may revisit the topics of specialization and the challenge of evaluating morphological ‘specializations’ in ecological and evolutionary contexts.
4.1. Comparison of tooth morphology and microstructure
Squaliform teeth are well represented in the fossil record (Ledoux, 1970; 1972; Welton, 1981; Suzuki, 2008; Cappetta, 2012). Accordingly, several authors present phylogenies of the order Squaliformes based heavily or, in some cases, exclusively on dental characters of extinct and Recent species (Adnet and Cappetta, 2001; Kriwet and Klug, 2009; Klug and Kriwet, 2010; Flammensbeck et al., 2018). Adnet and Cappetta (2001) place the genus Oxynotus within the Somniosinae at the base of the clade [[[Centroscymnus + Scymnodon] + Scymnodalatias] + Oxynotus]. The authors reason that in the absence of any apomorphic odontological characters to support this clade, the placement of Oxynotus could be the result of morphological convergence. Klug and Kriwet (2010) present a supertree combining odontological, morphological, and molecular data from extant and extinct squaliform taxa with slightly different results, notably the recovery of four monophyletic clades within Squaliformes. Nevertheless, inclusion of Oxynotidae on the basis of molecular or morphological data often renders whichever clade it is nested within paraphyletic (e.g. Somniosidae as reported by Flammensbeck et al., 2018). Another trend in phylogenetic analysis of Squaliformes is that Oxynotus is consistently recovered in close phylogenetic proximity to the genera Somniosus, Centroscymnus, and Scymnodon in multiple studies but with exact relationships differing slightly (Shirai, 1996; Klug and Kriwet, 2010; Da Silva et al., 2018). The molecular (mtDNA) study conducted by Naylor et al. (2012) recovered Oxynotus as the sister taxon to two of three species of a paraphyletic Centroscymnus.
Though a complete review and revision of these genera and ‘Somniosinae’ (as used by Adnet and Cappetta, 2011) is beyond the scope of our study, tooth morphology in O. bruniensis does bear a striking similarity to the tooth morphologies of Centroscymnus spp. (Suzuki, 2008: table 1; Cappetta, 2012: fig. 111; Underwood et al., 2016: fig. 2d). In particular, the lanceolate morphology of teeth in the anterior region of the palatoquadrate of O. bruniensis resembles that of teeth in the palatoquadrate of the Portuguese Dogfish Centroscymnus coelolepis Barboza du Bocage and de Brito Capello, 1864 and Roughskin Dogfish Centroscymnus owstonii Garman 1906. Cappetta (2012: p. 124) notes in Centroscymnus the presence of long, prominent aprons and numerous labial marginal foramina on teeth of the palatoquadrate. This description also matches the teeth of the palatoquadrate in O. bruniensis (Figure 5a). Suzuki (2008) notes that in Meckelian teeth of C. coelolepis and C. owstoniii, labial marginal foramina are horizontally expanded and sit alongside a long apron. Our study demonstrates that the same is true in Meckelian teeth of O. bruniensis (Figure 5d). Additional morphological similarities between the teeth of C. coelolepis and C. owstonii and the teeth of O. bruniensis include distal inclination of the Meckelian teeth (with the exception of symphyseal teeth of O. bruniensis) with elongated root lobes. Although overall tooth morphology and the arrangement of foramina are not themselves sufficient diagnostic characters to resolve the phylogenetic placement of Oxynotus, in this case they are consistent with the phylogenetic proximity of Oxynotus and Centroscymnus hypothesized by Naylor et al. (2012).
There is interspecific variation in tooth morphology within the genus Oxynotus. Bigelow and Schroeder (1957: fig. 2f), Compagno (1984: p. 127), Cappetta (2012: fig. 118A), and Ebert et al. (2013: p. 163) offer line diagrams of either individual symphyseal teeth or the anterior‐most regions of the Meckelian dentition of O. centrina. When compared with previous illustrations, our results indicate that the distal heels on the Meckelian symphyseal teeth of O. bruniensis are more pronounced than in O. centrina. Furthermore, the very weak secondary serrations of Meckelian teeth of O. bruniensis are far less prominent than the serrations of O. centrina as described and illustrated by Herman et al. (2005). Whether the serrations of O. centrina are primary serrations that include the underlying orthodentine or secondary serrations, such as those of O. bruniensis, remains to be seen. It is noteworthy, however, that both O. bruniensis and O. centrina bear teeth with a pulp cavity that extends well into the root (Herman et al., 1989; 2003), displaying a similar gross arrangement of dental tissues. We call on future researchers to quantify microstructural differences, such as vascular canals and serration composition, between the two species.
The enameloid microstructure that we report in O. bruniensis represents a variant of simplified enameloid among elasmobranchs (used here to refer to neoselachians sensu Compagno, 1977, along with sister taxa as described by Grogan et al., 2012; for an alternate taxonomic conclusion see Maisey, 2012). Other neoselachian elasmobranchs (sensu Grogan et al., 2012) with fewer than three enameloid layers include †Hybodus spp. (Enault et al., 2015; Cuny et al., 2017), rays of the order Myliobatiformes and other batoids such as Rhynchobatus (Cappetta, 2012; Enault et al., 2015) and, in the lateral teeth, Heterodontus spp. (Reif, 1973). Figure 9 summarizes the phylogenetic relationships of representative elasmobranch taxa and their enameloid microstructure. Enameloid simplifications among neoselachians are attributed to dietary specialization. For example, in durophagous Heterodontus spp., lateral teeth lack a PFE layer and have SLE covering TFE, the latter being more resistant to compressive forces than PFE (Preuschoft et al., 1974; Cuny et al., 2017). In batoids, a simplified double‐layer enameloid has also been attributed to durophagy and, in the case of filter‐feeding taxa, a single enameloid layer likely represents the loss of mechanical constraints (Enault et. al., 2013). It is noteworthy that teeth with simplified enameloid may not necessarily represent the same character state variation. That is to say, the homology of the enameloid layers that are present should not be taken for granted.
Figure 9.
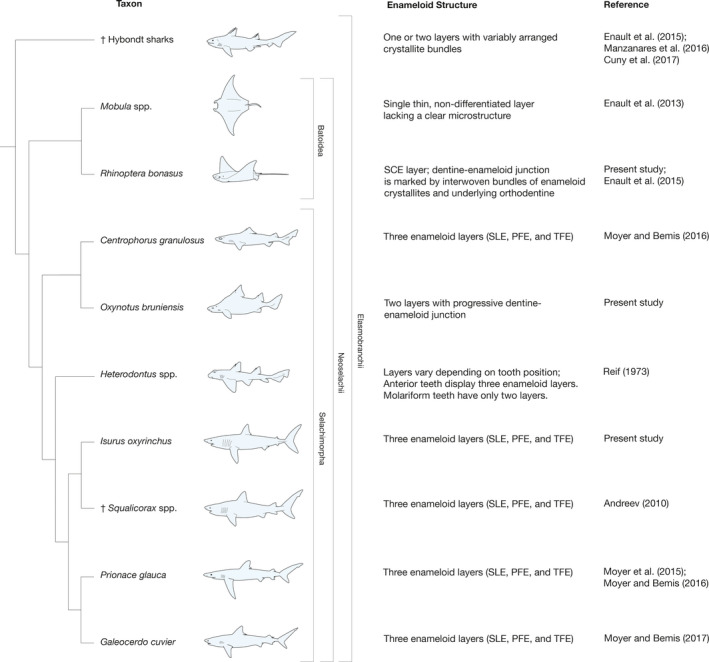
Phylogenetic relationships of elasmobranch taxa for which enameloid microstructure has been documented. Description of enameloid microstructure and the documenting reference(s) are provided next to each taxon. Placement of †hybodont sharks follows Maisey et al. (2004). †Squalicorax placement within Lamniformes follows Andreev (2010) and Cappetta (2012). Interrelationships of extant species follow Naylor et al. (2012)
In O. bruniensis, enameloid fibers under the SLE form a single layer, with bundled fibers exhibiting oblique and perpendicular orientation to the underlying orthodentine (Figure 8a). This may be homologous to the TFE of the more common selachian triple‐layer enameloid; however, the identification should be confirmed using electron microscopy. If the second enameloid layer of O. bruniensis were a modified TFE, then the most parsimonious explanation for the distribution of this variant of simplified enameloid would be its convergent evolution in Heterodontus spp. and O. bruniensis. As this is, to our knowledge, the first examination of enameloid microstructure in an extant oxynotid, we cannot comment on the commonality of this enameloid microstructure among the other members of the family. We therefore suggest a systematic study of tooth histology and microstructure in the five extant species of Oxynotus.
4.2. Histotypes as descriptors and indicators of performance
Oxynotus bruniensis represents a variant of the orthodont histotype similar to previously studied squaliform sharks (Moyer and Bemis, 2016; Jambura et al., 2020). The classification of O. bruniensis as an orthodont species necessitates a brief overview of the terminology associated with histotypes of shark teeth and comparison with other squaliform species, the histotype classifications of which are matters of debate. The term pseudoosteodonty, coined by Herman et al. (1991) to reference a histotype wherein osteodentine completely fills the pulp cavity and is surrounded by orthodentine in fully developed teeth, applies to modern and extinct species of the genus Hemipristis as well as multiple extinct taxa, such as †Notorynchus kempi, †Synechodus sp., and †Stethacanthus sp. (Lund, 1985; Compagno, 1988; Herman et al., 1991; Jambura et al., 2020). However, the descriptor ‘pseudoosteodont’ has also been applied to the histotypes of many other species including modern and extinct representatives of the orders Squaliformes and Orectolobiformes (Jambura et al., 2020). In some cases, species that retain a hollow, albeit reduced pulp cavity surrounded by orthodentine and a portion of root osteodentine within the crown, such as Centrophorus granulosus, are also labeled as pseudoosteodonts (Jambura et al., 2020). In many species of the aforementioned orders, the interpretation differs from the species’ previous designations as orthodonts (Compagno, 2002; Moyer and Bemis, 2016). At the center of the debate appears to be the size and location of the pulp cavity.
Herman et al.’s (1991) definition of pseudoosteodonty was introduced with respect to Hemprisitis, in which osteodentine completely, not partially, fills the pulp cavity. In 2003, the same team of researchers noted that in many squaliform taxa (e.g. Centrophorus and Somniosus) the pulp cavity is reduced to a few denteones in the apical region of the crown (Herman et al., 2003). Reduced pulp cavities are not limited to squalid sharks. In orthodont teeth of some carcharhiniforms, the pulp cavity may be so reduced that Compagno (1988) acknowledged it might be referred to as a central pulp ‘canal’. Herman et al. (2003: p. 19) refer to pulp cavities made of large denteones (osteons in the original reference) in taxa such as Etmopterus, Centroscyllium, and Oxynotus. In other taxa, they reference the absence of a ‘true’ pulp cavity but do not define it (Herman et al. 2003: p. 19). This raises the question of what a ‘true’ pulp cavity is.
Based on previous studies and the results observed in O. bruniensis, we propose that a true pulp cavity is defined not by size but by the role it plays in the development of the tooth. A true orthodont pulp cavity receives retreating odontoblasts that are responsible for the development of orthodentine, a tissue that can be distinguished visually from osteodentine by the arrangement and size of dentine tubules and often by the presence of lines of Owen, which are the result of periodic changes in dentine deposition (Peyer, 1968; Moyer et al., 2015; Nanci, 2017). With a developmental criterion separating orthodont pulp cavities or ‘canals’ (sensu Compagno, 1988) from vascular canals or osteons such as those found in osteodentine, we offer an amendment to the definition of the orthodont histotype shared by O. bruniensis and other squaliformes: Orthodont teeth retain a true pulp cavity or canal into which orthodentine‐depositing odontoblasts retreat that renders the junction between orthodentine in the crown and osteodentine of the root discontinuous.
In contrast, pseudoosteodont teeth in which osteodentine fills the pulp cavity have continuous osteodentine‐orthodentine junctions that are not interrupted by a true pulp cavity or canal (see Compagno, 1988; fig 3.6D; Jambura et al., 2018). This indicates that pseudoosteodont teeth during development have a true pulp cavity, but that it is filled completely by osteodentine as development progresses. By extension, the crowns of osteodont teeth display a modified and likely derived developmental pattern in keeping with the findings of Jambura et al. (2020). Our distinctions are consistent with the taxon Herman et al. (1991) used to introduce the term pseudoosteodonty. Additionally, they support histological accounts offered by authors describing fossil and Recent orthodont teeth, including those of the well‐studied species Squalus acanthias, which resembles in histotype the teeth of O. bruniensis (e.g. Kemp and Park, 1974; Moyer et al., 2015; Shimada et al., 2016; Moyer and Bemis, 2017; Jambura et al., 2020).
Distinguishing between histotypes is not simply a matter of semantics. The functional and evolutionary implications of tissue arrangement in shark teeth are active areas of research and have yet to be fully resolved. Therefore, an accurate and standardized nomenclature is essential when articulating tooth mineralization patterns and their significance. Jambura et al. (2020) assert that orthodont, osteodont, and pseudoosteodont are overused descriptors applied to a continuum of tissue arrangements first noted by Peyer (1968) and echoed by Moyer and Bemis (2016). We agree that within each histotype there is a gamut of relative structure (e.g. vascular canals, pulp cavities, etc.) and tissue arrangements. Nevertheless, if the traditional histotype terminology is to be used, even as references to histological extremes, consistent definitions that include the presence or absence of observable features, such as a continuous orthodentine‐osteodentine junction or direct contact of orthodentine with a true pulp cavity, should be observed. Given the importance of distinguishing between true pulp cavities, especially those that are greatly reduced, and vascular canals, we call for enhanced study of tooth histology and development with particular focus on odontoblast activity in taxa for which histotype differentiation is a matter of debate.
Despite the restricted diet of O. bruniensis, it exhibits a histotype shared by many species including dietary generalists (Compagno, 2002). Consequently, we conclude that histotype alone is not a sufficient indicator of dietary specialization. Thus we are left with an as yet unanswered question: what is the functional significance of different histotypes? Regarding the material properties of the dentinous tissues specifically, osteodentine is significantly harder than orthodentine (Whitenack et al., 2010), and the orthodont histotype is shared by species with a relatively low theoretical bite forces, such as the Bonnethead Shark Sphyrna tiburo (Linnaeus, 1758) and the Spiny Dogfish Squalus acanthias (Huber and Motta, 2004; Mara et al., 2010). However, perhaps counterintuitively, orthodonty is not restricted to low‐force biters. The theoretical maximum bite force of the Blacktip Shark Carcharhinus limbatus (Müller and Henle, 1839) and Bull Shark Carcharhinus leucas exceeds 1,000 N, and both species share the orthodont histotype (Huber et al., 2006; Habeggar et al., 2012). Despite the differences in hardness between osteodentine and orthodentine, review of the literature indicates that there is no singular link between histotype and bite force. Likely, enameloid thickness, tooth crown morphology, and histotype‐related variations in a tooth’s second moment of area all contribute to how that tooth reacts mechanically to loading. Therefore, estimations of the relative bite force of O. bruniensis in the absence of functional data should not be disproportionally influenced by tooth histotype.
4.3. The prickly dogfish as a specialist
Oxynotus bruniensis has a very narrow dietary breadth, exclusively reliant upon the eggs of other chondrichthyans for food (Finucci et al., 2016). However, based solely on the macroscopic morphology of its jaws and dentition as reported here, the extreme dietary specialization of the Prickly Dogfish is not self‐evident. The mode of feeding is more easily deduced. A Meckelian dentition that forms a serrated blade‐like cutting edge, the offset jaw joints, and papillose lips in O. bruniensis resemble features found in other squaliform species such as the Cookiecutter Shark Isistius brasiliensis (Quoy and Gaimard, 1824) and Squalus acanthias (Strasburg, 1963; Wilga and Motta, 1998). Suction feeding or, in the case of I. brasiliensis, a ‘strong oral vacuum’ (Shirai and Nakaya, 1992) factor prominently in these species’ prey acquisition (Wilga and Motta, 1998). Another squaliform and sister taxon to O. bruniensis, the Angular Roughshark O. centrina, feeds on chondrichthyan eggs but not exclusively. Oxynotus centrina leaves a distinct semicircular bite mark, and although there are interspecific variations in tooth morphology (e.g. the size of distal heels on symphyseal teeth), the overall dentition and oral anatomy appears very similar to that of O. bruniensis (Guallart et al., 2015). As illustrated by Guallart et al. (2015), teeth of the upper jaw in O. centrina scratch the surface of egg cases and, if they puncture the egg case, leave small holes. Based on morphological similarities in dentition and jaw position, we posit that O. bruniensis displays a similar feeding performance.
Assessment of the simplified enameloid microstructure of O. bruniensis in the context of specialization is complicated by several factors. Comparison of simplified enameloid within Neoselachii is limited to a few disparate taxa (Figure 9). Therefore, we may say with certainty that the enameloid microstructure of O. bruniensis is uncommon among studied taxa, but that is not sufficient to label it a functional specialization or, by extension, claim that it reflects the species’ ecological specialization following the definitions of specialization presented by Ferry‐Graham et al. (2002). Herein lies another complicating factor of evaluating the enameloid of O. bruniensis. A review of the chondrichthyan taxa known to exhibit simplified enameloid in some or all of their teeth reveals durophagous (e.g. Rhinoptera bonasus, Mitchell, 1815, and Heterodontus spp.) and filter feeding (e.g. Mobula spp.) species, as well as species with varied diets and teeth putatively suited for clutching (e.g. †Hybodus spp. of the late Jurassic; Cappetta, 2012; Cuny et al., 2017). Thus, a clear functional signal is not apparent. To support or refute functional advantages of simplified enameloid, we call for further research into the mechanical properties of enameloid crystallite orientation in a broader range of neoselachian species, including the four other species of extant oxynotids.
In the absence of a clear interspecific pattern of functional benefit related to diet, the simplified enameloid of O. bruniensis may represent not an evolved adaptation to the mechanical stresses of a specialized diet but the lack of a mechanical stress as seen in planktivorous rays (Cuny et al., 2017). Egg cases are non‐motile, and the yolk and embryonic tissues within them likely do not provide the same mechanical resistance to teeth as do fully developed prey, which may attempt to escape from the teeth. Therefore, preying exclusively on chondrichthyan eggs may have led to a secondary loss of the triple‐layer enameloid typically found in neoselachian fishes. In this scenario, the secondary loss of putatively neoselachian triple‐layered enameloid in the absence of a selective pressure may resemble a reversion to a plesiomorphic state. However, given recent findings that two species within the batoid family †Archaeobatidae exhibit complex triple‐layered enameloid, the ancestral state of neoselachian tooth enameloid is most likely triple‐layered (Manzanares et al., 2018). Clearly, the evolution of enameloid microstructure is a field in which continued research is required, and a more thorough survey of the enameloid microstructures of planktivorous and durophagous species, both extinct and extant, is a prerequisite to confirm the evolutionary origins of single‐ or double‐layered enameloid in elasmobranchs.
Given the previously reported dietary specificity of O. bruniensis, we conclude that the Prickly Dogfish is an ecological specialist, specifically a behavioral specialist. In other words, the behaviors associated with an ecologically relevant task, such as prey selection and acquisition, in O. bruniensis fall within a narrow range (Ferry‐Graham et al., 2002). Irschick et al. (2005: p. 405) emphasize that this definition of behavioral specialization assumes that ‘morphological adaptations are necessarily linked to behavior’. Indeed, they likely are as form and function are interconnected with behavior (Irschick and Higham, 2016). However, as our findings suggest, a behavior may be specialized, but it is not a foregone conclusion that the morphologies enabling that behavior represent physical specializations as well.
5. CONCLUSION
Two related questions in the field of ecological morphology are whether ecological specialists can be recognized from their morphology and whether ecological specialization allows predictions of morphological specialization (Ricklefs and Miles, 1994). The morphology of teeth and their microstructure in O. bruniensis, when compared with those of other elasmobranchs, demonstrate that there is no universally applicable answer to either question. In reality, the answer likely depends on the scale of inquiry (i.e. specialization at the individual, population, species or higher level) and necessitates a relatively complete library of relevant points of comparison. For example, in the study of neoselachian dentitions, much work remains to be done concerning odontological microstructure and tissue arrangement. Only after a suitably broad frame of reference including numerous taxa is established, can researchers determine whether behavioral or morphological changes precipitated specialization and to what extent. In this way, the search for specializations, both morphological and behavioral, is confirmation of the utility of comparative and descriptive studies of anatomy and performance.
AUTHOR CONTRIBUTIONS
J.K.M. and B.F. conceived the study, and B.F. supplied specimens of Oxynotus bruniensis. Computed tomography (CT) datasets were generated by M.L.R. Specimen dissection and preparation, CT dataset reconstruction, and tooth histology were performed by J.K.M. Manuscript and figures were drafted by J.K.M. and reviewed by B.F., M.L.R., and D.J.I. Funding was procured by J.K.M.
Supporting information
Figure S1
ACKNOWLEDGEMENTS
This study was made possible by a grant from the Jane H. Bemis Fund for Research in Natural History awarded to J.K.M. The first author thanks R. C. Albertson for use of his lab’s microscopy equipment. K. Doyle facilitated specimen accession in the University of Massachusetts Amherst Natural History Collection. B.F. thanks the scientific staff and crew of the RV Tangaroa who facilitated specimen collection. The authors have no conflict of interest that would compromise the scientific integrity of this work.
Moyer JK, Finucci B, Riccio ML, Irschick DJ. Dental morphology and microstructure of the Prickly Dogfish Oxynotus bruniensis (Squaliformes: Oxynotidae). J Anat. 2020;237:916–932. 10.1111/joa.13251
DATA AVAILABILITY STATEMENT
The CT datasets that support the findings of this study are available upon request to the corresponding author. Anatomical specimens used in this study are housed in the University of Massachusetts Amherst Natural History Collection.
REFERENCES
- Adnet, S. and Cappetta, H. (2001) A palaeontological and phylogenetical analysis of squaliform sharks (Chondrichthyes: Squaliformes) based on dental characters. Lethaia, 34, 234–248. [Google Scholar]
- Agassiz, L. (1833–1843) Recherche sur les Poissons Fossiles, 5 vols. Neuchâtel: Imprimerie de Petitpierre. [Google Scholar]
- Andreev, P.S. (2010) Enameloid microstructure of the serrated cutting edges in certain fossil Carcharhiniform and Lamniform Sharks. Microscopy Research and Technique, 73, 704–713. [DOI] [PubMed] [Google Scholar]
- Bemis, W.E. , Hilton, E.J. , Brown, B. , Arrindell, R. , Richmond, A.M. , Little, C.D. , et al (2004) Methods for preparing dry, partially articulated skeletons of Osteichthyans, with notes on making Ridewood dissections of the cranial skeleton. Copeia, 2004, 603–609. [Google Scholar]
- Bemis, W.E. , Moyer, J.K. and Riccio, M. (2015) Homology of lateral cusplets in the teeth of lamnid sharks (Lamniformes: Lamnidae). Copeia, 103, 961–972. [Google Scholar]
- Berkovitz, B. and Shellis, P. (2016) Teeth of Non‐mammalian Vertebrates. London: Academic Press. [Google Scholar]
- Bigelow, H.B. and Schroeder, W.C. (1957) A study of the sharks of the suborder Squaloidea. Bulletin of the Museum of Comparative Zoology, 117, 1–150. [Google Scholar]
- Capapé, C. (2008) Diet of the angular rough shark Oxynotus centrina (Chondrichthyes: Oxynotidae) off the Languedocian coast (southern France, north‐western Mediterranean). Vie et Milieu, 58, 57–61. [Google Scholar]
- Cappetta, H. (2012) Chondrichthyes—Mesozoic and Cenozoic Elasmobranchii: Teeth In: Schultze H.‐P. (Ed.): Handbook of Paleoichthyology (Vol. 3E). Munich: Pfeil. [Google Scholar]
- Casier, E. (1961) Transformation des systèmes de fixation et de vascularisation dentaires dans l’évolution des sélaciens du sous‐ordre des Squaliformes. Mémoires de l’Institut Royal des Sciences Naturelles de Belgique, 65, 1–65. [Google Scholar]
- Castro, J.I. (2011) The Sharks of North America. New York: Oxford University Press. [Google Scholar]
- Compagno, L.J.V. (1977) Phyletic relationships of living sharks and rays. American Zoologist, 17, 303–322. [Google Scholar]
- Compagno, L.J.V. (1984). Sharks of the world: An annotated and illustrated catalogue of shark species known to date. FAO Species Catalogue. FAO Fisheries Synopsis 125, Vol. 4 Part 1. Rome: FAO. [Google Scholar]
- Compagno, L.J.V. (1988) Sharks of the Order Carcharhiniformes. Princeton: Princeton University Press. [Google Scholar]
- Compagno, L.J.V. (2002) Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Vol. 2. Bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes). FAO Species Catalogue for Fishery Purposes. Rome: FAO. [Google Scholar]
- Cuny, G. , Guinot, G. and Enault, S. (2017) Evolution of Dental Tissues and Paleobiology in Selachians. London: ISTE Press and Elsevier. [Google Scholar]
- Da Silva, J.P.C. , Vaz, D.F. and De Carvalho, D.F. (2018) Phylogenetic inferences on the systematics of squaliform sharks based on elasmobranch scapular morphology (Chondrichthyes: Elasmobranchii). Zoological Journal of the Linnean Society, 182, 614–630. [Google Scholar]
- Ebert, D.A. , Fowler, S. and Compagno, L. (2013) Sharks of the World: A Fully Illustrated Guide. Plymouth: Wild Nature Press. [Google Scholar]
- Enault, S. , Cappetta, H. and Adnet, S. (2013) Simplification of the enameloid microstructure of the large stingrays (Chondrichthyes: Myliobatiformes): a functional approach. Zoological Journal of the Linnean Society, 169, 144–155. [Google Scholar]
- Enault, S. , Guinot, G. , Koot, M.B. and Cuny, G. (2015) Chondrichthyan tooth enameloid: past, present, and future. Zoological Journal of the Linnean Society, 174, 549–570. [Google Scholar]
- Enault, S. , Auclair, A. , Adnet, S. and Debiais‐Thibaud, M. (2016) A complete protocol for the preparation of chondrichthyan skeletal specimens. Journal of Applied Ichthyology, 32, 409–415. [Google Scholar]
- Ferry‐Graham, L.A. , Bolnick, D.I. and Wainwright, P.C. (2002) Using functional morphology to examine the ecology and evolution of specialization. Integrative and Comparative Biology, 42, 265–277. [DOI] [PubMed] [Google Scholar]
- Finucci, B. , Bustamante, C. , Jones, E.G. and Dunn, M.R. (2016) Reproductive biology and feeding habits of the prickly dogfish Oxynotus bruniensis . Journal of Fish Biology, 89, 2326–2344. [DOI] [PubMed] [Google Scholar]
- Flammensbeck, C.K. , Pollerspöck, J. , Schedel, F.D. , Matzke, N.J. and Straube, N. (2018). Of teeth and trees: A fossil tip‐dating approach to infer divergence times of extinct and extant squaliform sharks. Zoologica Scripta, 47, 539–557. [Google Scholar]
- Futuyma, D.J. and Moreno, G. (1988) The evolution of ecological specialization. Annual Review of Ecology and Systematics, 19(1), 207–233. [Google Scholar]
- Garman, S. (1913) The Plagiostomia (sharks, skates, and rays). Memoirs of the Museum of Comparative Zoology at Harvard College, 36, 1–515. [Google Scholar]
- Gillis, J.A. and Donoghue, P.C.J. (2007) The homology and phylogeny of chondrichthyan tooth enameloid. Journal of Morphology, 268, 33–49. [DOI] [PubMed] [Google Scholar]
- Grady, J.E. (1970) Tooth development in sharks. Archives of Oral Biology, 15, 613–619. [DOI] [PubMed] [Google Scholar]
- Grogan, E.D. , Lund, R. and Greenfest‐Allen, E. (2012) The origin and relationships of early chondrichthyans In: Carrier J.C., Musick J.A. and Heithaus M.R. (Eds.) The Biology of Sharks and Their Relatives. Boca Raton: CRC Press, pp. 3–29. [Google Scholar]
- Guallart, J. , García‐Salinas, P. , Ahuir‐Baraja, A.E. , Guimerans, M. , Ellis, J.R. and Roche, M. (2015) Angular roughshark Oxynotus centrina (Squaliformes: Oxynotidae) in captivity feeding exclusively on elasmobranch eggs: an overlooked feeding niche or a matter of individual taste? Journal of Fish Biology, 87, 1072–1079. [DOI] [PubMed] [Google Scholar]
- Guinot, G. , Adnet, S. , Shimada, K. , Shimada, K. , Underwood, C.J. , Siversson, M. , et al (2018) On the need of providing tooth morphology in descriptions of extant elasmobranch species. Zootaxa, 446, 118–126. [DOI] [PubMed] [Google Scholar]
- Habegger, M.L. , Motta, P.J. , Huber, D.R. and Dean, M.N. (2012) Feeding biomechanics and theoretical calculations of bite force in bull sharks (Carcharhinus leucas) during ontogeny. Zoology, 115, 354–364. [DOI] [PubMed] [Google Scholar]
- Hammerschlag, N. , Broderick, A.C. , Coker, J.W. , Coyne, M.S. , Dodd, M. , Frick, M.G. , et al (2015) Evaluating the landscape of fear between apex predatory sharks and mobile sea turtles across a large dynamic seascape. Ecology, 96, 2117–2136. [DOI] [PubMed] [Google Scholar]
- Herman, J. , Hovestadt‐Euler, M. and Hovestadt, D.C. (1989) Contributions to the study of the comparative morphology of teeth and other relevant ichthyodorulites in living supraspecific taxa of Chondrichtyan fishes. Part A: Selachii. No 3: Order : Squaliformes Families : Echinorhinidae, Oxynotidae and Squalidae. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Biologie, 59, 101–157. [Google Scholar]
- Herman, J. , Hovestadt‐Euler, M. and Hovestadt, D.C. (1991) Contributions to the study of the comparative morphology of teeth and other relevant ichthyodorulites in living supraspecific taxa of chondrichthyan fishes. Part A: Selachii. No.2c: Order: Carcharhiniformes, Families Proscylliidae, Hemigaleidae, Pseudotriakidae, Leptochariidae and Carcharhinidae. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Biologie, 61, 73–120. [Google Scholar]
- Herman, J. , Hovestadt‐Euler, M. and Hovestadt, D.C. (2003) Contributions to the study of the comparative morphology of teeth and other relevant ichthyodorulites in living supraspecific taxa of chondrichthyan fishes. Part A: Selachii. Addendum to 1: Order Hexanchiformes‐Family Hexachidae, 2: Order Carcharhiniformes, 2a: Family Triakidae, 2b: Family Scyliorhinidae, 2c: Family Carcharhinidae, Hemigaleidae, Leptochariidae, Sphyrnidae, Proscylliidae and Pseudotriakidae, 3: Order Squaliformes: Family Echinorhinidae, Oxynotidae and Squalidae. Tooth vascularization and phylogenetic interpretation. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Biologie, 73, 5–26. [Google Scholar]
- Herman, J. , Hovestadt‐Euler, M. and Hovestadt, D.C. (2005) Contributions to the odontological study of living Chondrichthyes. 2. The genus Oxynotus Rafinesque, 1810. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Biologie, 75, 5–20. [Google Scholar]
- Huber, D.R. and Motta, P.J. (2004) Comparative analysis of methods for determining bite force in the Spiny Dogfish Squalus acanthias . Journal of Experimental Zoology, 301A, 26–37. [DOI] [PubMed] [Google Scholar]
- Huber, D.R. , Eason, T.G. , Hueter, R.E. and Motta, P.J. (2005) Analysis of the bite force and the mechanical design of the feeding mechanism of the durophagous horn shark Heterodontus francisci . Journal of Experimental Biology, 208, 3553–3571. [DOI] [PubMed] [Google Scholar]
- Huber, D.R. , Weggelaar, C.L. and Motta, P.J. (2006) Scaling of bite force in the blacktip shark Carcharhinus limbatus . Zoology, 109, 109–119. [DOI] [PubMed] [Google Scholar]
- Hulsey, C.D. and García de León, F.J. (2005) Cichlid jaw mechanics: linking morphology to feeding specialization. Functional Ecology, 19(3), 487–494. [Google Scholar]
- Irschick, D.J. , Dyer, L. and Sherry, T.W. (2005) Phylogenetic methodologies for studying specialization. Oikos, 110, 404–408. [Google Scholar]
- Irschick, D.J. and Higham, T. (2016) Animal Athletes: An ecological and evolutionary approach. New York: Oxford University Press. [Google Scholar]
- Jambura, P.L. , Pfaff, C. , Underwood, C.J. , Ward, D.J. and Kriwet, J. (2018) Tooth mineralization and histology patterns in extinct and extant snaggletooth sharks, Hemipristis (Carcharhiniformes, Hemigaleidae)—Evolutionary significance or ecological adaptation? PLoS One, 13, e0200951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambura, P.L. , Türtscher, J. , Kindlimann, R. , Metscher, B. , Pfaff, C. , Stumpf, S. , et al (2020) Evolutionary trajectories of tooth histology patterns in modern sharks (Chondrichthyes, Elasmobranchii). Journal of Anatomy, 236, 753–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp, N.E. and Park, J.H. (1974) Ultrastructure of the enamel layer in developing teeth of the shark Carcharhinus menisorrah . Archives of Oral Biology, 19, 633–644. [DOI] [PubMed] [Google Scholar]
- Kerr, T. (1956) Development and structure of the teeth in the Dog Fish, Squalus acanthias L. and Scyliorhynus caniculus (L.). Proceedings of the Zoological Society of London, 125, 95–114. [Google Scholar]
- Klimley, A.P. (2013) The Biology of Sharks and Rays. Chicago: University of Chicago Press. [Google Scholar]
- Klug, S. and Kriwet, J. (2010) Timing of deep‐sea adaptation in dogfish sharks: insights from a supertree of extinct and extant taxa. Zoologica Scripta, 39, 331–342. [Google Scholar]
- Kriwet, J. and Klug, S. (2009) Fossil record and origin of squaliform Sharks In: Gallucci V.F., McFarlane G.A. and Bargmann G.G. (Eds.) Biology and Management of Dogfish Sharks. Bethesda, MD: American Fisheries Society, pp. 19–38. [Google Scholar]
- Lauder, G.V. (1995) On the inference of function from structure In: Thomason J. (Ed.) Functional Morphology in Vertebrate Paleontology. Cambridge: Cambridge University Press, pp. 1–18. [Google Scholar]
- Lauder, G.V. (1996) The argument from design In: Rose M.R. and Lauder G.V. (Eds.) Adaptation. San Diego: Academic Press, pp. 55–91. [Google Scholar]
- Ledoux, J.‐C. (1970) Les dents de squalidés de la Méditerranée occidentale et de l’Atlantique nord‐ouest africain. Vie et Milieu, 21(2A), 309–362. [Google Scholar]
- Ledoux, J.‐C. (1972) Les Squalidae (Euselachii) miocènes des environs d’Avignon (Vaucluse). Documents du Laboratoire de Géologie de la Faculté des Sciences de Lyon. Notes et Mémoires, 52, 133–175. [Google Scholar]
- Lund, R. (1985) Stethacanthid elasmobranch remains from the Bear Gulch Limestone (Namurian E2b) of Montana. American Museum Novitates, 2828, 1–24. [Google Scholar]
- Maisey, J.G. (2012) What is an ‘elasmobranch’? The impact of palaeontology in understanding elasmobranch phylogeny and evolution. Journal of Fish Biology, 80, 918–951. [DOI] [PubMed] [Google Scholar]
- Maisey, J.G. , Naylor, G.J.P. and Ward, D.J. (2004) Mesozoic elasmobranchs, neoselachian phylogeny and the rise of modern elasmobranch diversity In: Arratia G. and Tintori A. (Eds.) Mesozoic Fishes 3 – Systematics, Paleoenvironments and Biodiversity. Munich: Verlag Dr. Friedrich Pfeil, pp. 17–56. [Google Scholar]
- Manzanares, E. , Pla, C. , Martínez‐Pérez, C. , Ferrón, H. and Botella, H. (2016) Lonchidion derenzii, sp. nov., a new lonchidiid shark (Chondrichthyes, Hybodontiforms) from the Upper Triassic of Spain, with remarks on lonchidiid enameloid. Journal of Vertebrate Paleontology, DOI: 10.1080/02724634.2017.1253585 [DOI] [Google Scholar]
- Manzanares, E. , Botella, H. and Delsate, D. (2018) On the enameloid microstructure of Archaeobatidae (Neoselachii, Chondrichthyes). Journal of Iberian Geology, 44, 67–74. [Google Scholar]
- Mara, K.R. , Motta, P.J. and Huber, D.R. (2010) Bite Force and Performance in the Durophagous Bonnethead Shark, Sphyrna tiburo . Journal of Experimental Zoology, 313A, 95–105. [DOI] [PubMed] [Google Scholar]
- Matich, P. , Heithaus, M.R. and Layman, C.A. (2011) Contrasting patterns of individual specialization and trophic coupling in two marine apex predators. Journal of Animal Ecology, 80, 294–305. [DOI] [PubMed] [Google Scholar]
- Mori, A. and Vincent, S.E. (2008) An integrative approach to specialization: relationships among feeding morphology, mechanics, behaviour, performance and diet in two syntopic snakes. Journal of Zoology, 275(1), 47–56. [Google Scholar]
- Moyer, J.K. and Bemis, W.E. (2016) Tooth microstructure and replacement in the gulper shark, Centrophorus granulosus (Squaliformes: Centrophoridae). Copeia, 104, 529–538. [Google Scholar]
- Moyer, J.K. and Bemis, W.E. (2017) Shark teeth as edged weapons: serrated teeth of three species of selachians. Zoology, 120, 101–109. [DOI] [PubMed] [Google Scholar]
- Moyer, J.K. , Riccio, M. and Bemis, W.E. (2015) Development and microstructure of tooth histotypes in the blue shark, Prionace glauca (Carcharhiniformes: Carcharhinidae) and the great white shark, Carcharodon carcharias (Lamniformes: Lamnidae). Journal of Morphology, 276, 797–817. [DOI] [PubMed] [Google Scholar]
- Munroe, S.E.M. , Simpfendorfer, C.A. and Heupel, M.R. (2014) Defining shark ecological specialisation: concepts, context, and examples. Reviews in Fish Biology and Fisheries, 24, 317–331. [Google Scholar]
- Nanci, A. , Bringas, P. Jr , Samuel, N. and Slavkin, H.C. (1983) Selachian tooth development: Ill. Ultrastructural features of secretory amelogenesis in Squalus acanthias . Journal of Craniofacial Genetics and Developmental Biology, 3, 53–73. [PubMed] [Google Scholar]
- Nanci, A. (2017) Ten Cate’s Oral Histology: Development, Structure, and Function, 9th edn. St. Louis: Elsevier. [Google Scholar]
- Naylor, G.J. , Caira, J.N. , Jensen, K. , Rosana, K.A. , Straube, N. and Lakner, C. (2012) Elasmobranch phylogeny: A mitochondrial estimate based on 595 species In: Carrier J.C., Musick J.A. and Heithaus M.R. (Eds.) The Biology of Sharks and Their Relatives. Boca Raton: CRC Press, pp. 31–56. [Google Scholar]
- Owen, R. (1840–1845) Odontography. 2 London: Hippolyte Bailliere. [Google Scholar]
- Peyer, B. (1968) Comparative Odontology. Chicago: University of Chicago Press. [Google Scholar]
- Phillips, C.J. (2000) A theoretical consideration of dental morphology, ontogeny, and evolution in bats In: Adams R.A. and Pederson S.C. (Eds.) Ontogeny, Functional Ecology and Evolution of Bats. Cambridge: Cambridge University Press, pp. 247–274. [Google Scholar]
- Preuschoft, H. , Reif, W.‐E. and Müller, W.H. (1974) Funktionsanpassungen in Form und Struktur an Haifischzähnen. Zeitschrift für Anatomie und Entwicklungsgeschichte, 143, 315–344. [PubMed] [Google Scholar]
- Radinsky, L. (1961) Tooth histology as a taxonomic criterion for cartilaginous fishes. Journal of Morphology, 109, 73–92. [DOI] [PubMed] [Google Scholar]
- Reif, W.E. (1973) Morphologie und Ultrastruktur des Hai‐‘Schmelzes’. Zoologica Scripta, 2, 231–250. [Google Scholar]
- Reif, W.E. (1976) Morphogenesis, pattern formation and function of the dentition of heterodontus (Selachii). Zoomorphologie, 83, 1–47. [Google Scholar]
- Ricklefs, R.E. and Miles, D.B. (1994) Ecology and evolutionary inference from morphology: an ecological perspective In: Wainwright P.C. and Reilly S.M. (Eds.) Ecological Morphology: Integrative Organismal Biology. Chicago: University of Chicago Press, pp. 13–41. [Google Scholar]
- Ripa, L.W. , Gwinnett, A.J. , Guzman, C. and Legler, D. (1972) Microstructural and microradiographic qualities of lemon shark enameloid. Archives of Oral Biology, 17, 165–172. [DOI] [PubMed] [Google Scholar]
- Rosset, A. , Spadola, L. and Ratib, O. (2004) OsiriX: An open‐source software for navigating in multidimensional DICOM images. Journal of Digital Imaging, 17, 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel, N. , Bringas, P. Jr , Santos, V. , Nanci, A. and Slavkin, H.C. (1983) Selachian tooth development: I. Histogenesis, morphogenesis, and anatomical features in Squalus acanthias . Journal of Craniofacial Genetics and Developmental Biology, 3, 29–41. [PubMed] [Google Scholar]
- Sasko, D.E. , Dean, M.N. , Motta, P.J. and Hueter, R.E. (2006) Prey capture behavior and kinematics of the Atlantic cownose ray, Rhinoptera bonasus . Zoology, 109, 171–181. [DOI] [PubMed] [Google Scholar]
- Schmidt, W.J. and Keil, A. (1971) Polarizing Microscopy of Dental Tissues. New York: Pergamon Press. [Google Scholar]
- Schnetz, L. , Pfaff, C. and Kriwet, J. (2016) Tooth development and histology patterns in Lamniform Sharks (Elasmobranchii, Lamniformes) revisited. Journal of Morphology, 277, 1584–1598. [DOI] [PubMed] [Google Scholar]
- Shimada, K. , Egi, N. , Tsubamoto, T. , Maung‐Maung, M.M. , Thaung‐Htike, T.H. , Zin‐Maung‐Maung‐Thein, Z.M. , Nishioka, Y. , Sonoda, T. and Takai, M. (2016) The extinct river shark Glyphis pagoda from the Miocene of Myanmar and a review of the fossil record of the genus Glyphis (Carcharhiniformes: Carcharhinidae). Zootaxa, 4161, 237–251. [DOI] [PubMed] [Google Scholar]
- Shirai, S. (1996) Phylogenetic interrelationships of Neoselachians (Chondrichthyes: Euselachii) In: Stiassny A.L.J., Parenti L.R. and Johnson G.D. (Eds.) Interrelationships of Fishes. San Diego: Academic Press, pp. 9–34. [Google Scholar]
- Shirai, S. and Nakaya, K. (1992) Functional morphology of feeding apparatus in the Cookie‐Cutter Shark, Isistius brasiliensis (Elasmobranchii, Dalatiinae). Zoological Science, 9, 811–821. [Google Scholar]
- Slavkin, H.C. , Samuel, N. , Bringas, P. Jr , Nanci, A. and Santos, V. (1983) Selachian tooth development: II. lmmunolocalization of amelogenin polypeptides in epithelium during secretory amelogenesis in Squalus acanthias . Journal of Craniofacial Genetics and Developmental Biology, 3, 43–52. [PubMed] [Google Scholar]
- Smith, M.M. and Sansom, I.J. (2000) Evolutionary origins of dentine in the fossil record of early vertebrates: diversity, development and function In: Teaford M.F., Smith M.M. and Ferguson M.W.J. (Eds.) Development, Function and Evolution of Teeth. Cambridge: Cambridge University Press, pp. 65–81. [Google Scholar]
- Strasburg, D.W. (1963) The diet and dentition of Isistius brasiliensis, with remarks on tooth replacement in other sharks. Copeia, 1963, 33–40. [Google Scholar]
- Suzuki, H. (2008) Squaliform shark teeth of the genus Centroselachus from the Miocene of Japan. Journal of the Geological Society of Japan, 114, 536–539. [Google Scholar]
- Underwood, C. , Johanson, Z. and Smith, M.M. (2016) Cutting blade dentitions in squaliform sharks form by modification of inherited alternate tooth ordering patterns. Royal Society Open Science, 3, 160385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton, B.J. (1981) A new species of Oxynotus Rafinesque 1810 (Chondrichthyes: Squalidae) from the Early Miocene (Saucesian) Jewett Sand, Kern County, California, U.S.A. Teritary Research, 3, 141–152. [Google Scholar]
- Whitenack, L.B. and Motta, P.J. (2010) Performance of shark teeth during puncture and draw: implications for the mechanics of cutting. Biological Journal of the Linnean Society, 100, 271–286. [Google Scholar]
- Whitenack, L.B. , Simkins, D.C. , Motta, P.J. , Hirai, M. and Kumar, A. (2010) Young’s modulus and hardness of shark tooth biomaterials. Archives of Oral Biology, 55, 203–209. [DOI] [PubMed] [Google Scholar]
- Wilga, C.D. and Motta, P.J. (1998) Conservation and variation in the feeding mechanism of the spiny dogfish Squalus acanthias . Journal of Experimental Biology, 201, 1345–1358. [DOI] [PubMed] [Google Scholar]
- Witzell, W.N. (1987) Selective predation on large Cheloniid Sea turtles by tiger sharks (Galeocerdo cuvier). Japanese Journal of Herpetology, 12, 22–29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Data Availability Statement
The CT datasets that support the findings of this study are available upon request to the corresponding author. Anatomical specimens used in this study are housed in the University of Massachusetts Amherst Natural History Collection.


