Abstract
The vomeronasal system (VNS) has been extensively studied within specific animal families, such as Rodentia. However, the study of the VNS in other families, such as Canidae, has long been neglected. Among canids, the vomeronasal organ (VNO) has only been studied in detail in the dog, and no studies have examined the morphofunctional or immunohistochemical characteristics of the VNS in wild canids, which is surprising, given the well‐known importance of chemical senses for the dog and fox and the likelihood that the VNS plays roles in the socio‐reproductive physiology and behaviours of these species. In addition, characterising the fox VNS could contribute to a better understanding of the domestication process that occurred in the dog, as the fox would represent the first wild canid to be studied in depth. Therefore, the aim of this study was to analyze the morphological and immunohistochemical characteristics of the fox VNO. Tissue dissection and microdissection techniques were employed, followed by general and specific histological staining techniques, including with immunohistochemical and lectin‐histochemical labelling strategies, using antibodies against olfactory marker protein (OMP), growth‐associated protein 43 (GAP‐43), calbindin (CB), calretinin (CR), α‐tubulin, Gαo, and Gαi2 proteins, to highlight the specific features of the VNO in the fox. This study found significant differences in the VNS between the fox and the dog, particularly concerning the expression of Gαi2 and Gαo proteins, which were associated with the expression of the type 1 vomeronasal receptors (V1R) and type 2 vomeronasal receptors (V2R), respectively, in the vomeronasal epithelium. Both are immunopositive in foxes, as opposed to the dog, which only expresses Gαi2. This finding suggests that the fox possesses a well‐developed VNO and supports the hypothesis that a profound transformation in the VNS is associated with domestication in the canid family. Furthermore, the unique features identified in the fox VNO confirm the necessity of studying the VNS system in different species to better comprehend specific phylogenetic aspects of the VNS.
Keywords: fox, G proteins, immunohistochemistry, lectins, pheromones, Ulex europaeus agglutinin , vomeronasal
We present the first morphofunctional study of the vomeronasal organ of a wild canid, the red fox, which highlights the importance of chemical communication in this species and opens a new perspective on the canid vomeronasal system.
1. INTRODUCTION
Although historically, chemical senses have not been viewed to be as important as other sensorial systems, they play key roles in animal behaviours, in part because chemical detection systems project to the limbic system, where they affect emotions and conducts (Iovino et al., 2019).
The two primary systems involved in chemical recognition are the main olfactory system (MOS) and the vomeronasal system (VNS). The MOS is mediated by the main olfactory epithelium (MOE) and is closely related to the limbic system, where it has been associated with both recall and conscious sensation (Reep et al., 2007). In contrast, the VNS, which consists of the vomeronasal organ (VNO) and the accessory olfactory bulb (AOB), has been shown to play unconscious roles in reproductive behaviours (Over et al., 1990; Signoret, 1991; Rekwot et al., 2001), maternal recognition (Del Cerro, 1998), and the detection of predators (Brechbühl et al., 2013), and is specialised for the detection of pheromones (Holy, 2018).
Despite their anatomical proximity, however, these two systems are anatomically independent and show large morphological and functional differences, indicating that these two systems likely evolved independently (Estes, 1972; Wysocki, 1979; Herrada and Dulac, 1997; Halpern and Martinez‐Marcos, 2003; Salazar and Sánchez‐Quinteiro, 2009). However, morphological independence has been questioned, based on the presence of vomeronasal receptors in the olfactory mucosa and vice versa (Rodriguez et al., 2000; Sam et al., 2001; Trinh and Storm, 2003; Swaney and Keverne, 2009).
Knowledge regarding the diversity of the VNS, at both anatomical and genetic levels, such as the expression patterns of the VNS receptors, has recently broadened. This diversity differs from the general pattern of evolutionary conservation observed among olfactory receptors in the MOS. The ‘differential tuning‘ hypothesis states that the VNO evolved to detect a limited group of ligands, such as pheromones, due to the specificity of these ligands within species, whereas MOE receptors evolved to detect a broad range of odours, such as environmental cues, which are expected to remain relatively stable within the environment (Grus and Zhang, 2008). Comparative sequence analyses have demonstrated that MOE receptor gene sequences are well‐conserved, whereas VNO receptors are associated with a wide range of genes, suggesting a more dynamic evolution and a potentially more important role within species (Grus and Zhang, 2004).
The Rodentia family has been used as the primary referent for the VNO studies in mammals (Salazar et al., 2013). In most of the species studied within this family, the neuroepithelium is organised into two layers: the apical layer and the basal layer. Each of these layers expresses distinct G proteins, which are inherent to the receptor transduction cascade: the Gαi2 protein and the Gαo protein. The Gαi2 protein is expressed in the apical neurosensorial layer and is associated with the expression of type 1 vomeronasal receptors (V1R), which project to the anterior region of the AOB. In contrast, the Gαo protein is expressed in the basal neurosensorial layer of the epithelium and is associated with the transduction cascade of type 2 vomeronasal receptors (V2R), which project to the posterior region of the AOB. However, in other mammals, such as the dog, cat, and sheep, the differential expression of G proteins and vomeronasal receptors has not been observed, as these species exclusively express the V1R receptor family (Dulac and Axel, 1995; Halpern and Martinez‐Marcos, 2003; Salazar et al., 2007; 2013; Salazar and Sánchez‐Quinteiro, 2011).
Chemical senses are well‐known to be important in the dog (Jezierski et al., 2016). However, little is known regarding the VNO in canids, aside from morphological and immunohistochemical studies performed in the dog (Salazar et al., 1984; 2013; Dennis et al., 2003). Moreover, the limited development of the VNO epithelium observed in the dog and the poor differentiation of the AOB glomerular and nervous layers in this species does not appear to reflect the importance of chemical senses in this species (Nakajima et al., 1998; Salazar et al., 2013).
In canids, such as the dog, the VNO is located on either side of the vomer bone, at the base of the nasal cavity. The organ is surrounded by incomplete cartilage and is laterally covered by the nasal cavity respiratory mucosa. The organ has a cul‐de‐sac caudal end, and at its rostral end, it connects with the incisive duct, which opens into the oral cavity through the incisive papilla (Salazar et al., 2013).
When determining the G‐protein distribution in dogs, and consequently the expression pattern of V1R and V2R receptors, immunohistochemical studies performed by Salazar et al. (2013) demonstrated that the Gαi2 protein is apically distributed within the sensorial epithelium and is expressed in the nervous and glomerular vomeronasal layers of the AOB.
Salazar et al. (2013) were unable to detect Gαo immunopositivity in either the VNO or the AOB, suggesting that the dog VNS is entirely dependent on V1R receptors. In contrast, Dennis et al. (2003) were able to observe immunopositive labelling in the sensorial epithelium for both Gαi2 and Gαo proteins. However, both authors indicated that obtaining these results required antigenic retrieval, which was accomplished by boiling tissue sections in a citrate buffer solution, prior to the addition of primary antibodies. The use of this antigenic retrieval technique could unintentionally amplify cross‐reactivity, promoting positive immunolabelling.
Regrettably, this issue has not been addressed in more recent studies performed in dogs. Therefore, the scientific community continues to accept the absence of V2R expression in studied domestic animals. The absence of V2R receptors has been theorised to be the result of the domestication process, during which artificial selection may have produced an involution of the VNS in canids, a hypothesis that has been proposed by several authors (Barrios et al., 2014; Jezierski et al., 2016). Therefore, studying the expression patterns of these receptors and the general anatomy of the VNO in a wild canid with phylogenetic proximity to the dog, such as the red fox, Vulpes vulpes, would allow interspecies comparisons that could broaden the understanding of this theory. Thus far, only a very elementary study has been performed examining the fox VNO (Karimi et al., 2016) and it did not include immunohistochemical characterisations, which will be key for understanding the organisation of the VNS.
The lack of information concerning the fox VNS is astounding, given that this system is thought to play a decisive role in the reproductive processes for this species. The red fox is generally a solitary animal, mating only during the reproductive season, which predominantly occurs during spring, for one cycle each year. Not all females in a specific population reproduce every year, and the proportion of females that do not reproduce is highly variable and correlates with population density, likely due to the social suppression of reproduction in large groups (Gentle, 2005). This suppression has recently been observed in other species, such as the naked mole‐rat (Dennis et al., 2020). The availability of food allows dominant females to subordinate sterile females, who help to raise the litter and assist with group formation (Cavallini and Santini, 1996).
De Miguel et al. (2005) correlated the smell of other carnivores (derived from faeces or urine) with behavioural changes in the fox (increased defecation). Because the stimuli used in this study were presented over a long period of time, in the open air, the observed behavioural changes in the foxes were likely caused by the stimulation of the VNS, evoked by pheromones in the faeces or urine, instead of specifically induced by the smells associated with these stimuli, which are primarily composed of volatile components, with an ephemeral presence in the external environment (González et al., 1991). McLean et al. (2019) characterised the chemical compounds excreted by fox tails, identifying several compounds (for example, sulcatone) that are used as semiochemicals in several mammal species, further illustrating the importance of the VNS for fox behaviours.
The aim of this study was to analyse the morphological and immunohistochemical characteristics of the fox VNO because of the importance of this system in canines and the need to study the VNO in wild canids to better understand the domestication process. Various tissue dissection and microdissection techniques were used, followed by general and specific histological staining, including immunohistochemical and lectin‐histochemical labelling techniques. For this study, three different lectins were used: Ulex europaeus agglutinin (UEA), a specific vomeronasal marker in several species, including the dog (Salazar et al., 2013); Bandeiraea simplicifolia isolectin B4 (BSI‐B4), which selectively marks the vomeronasal pathway in both rats (Salazar and Sánchez‐Quinteiro, 1998) and opossums (Shapiro et al., 1995); and Lycopersicon esculentum agglutinin (LEA), a specific marker for both the MOS and the VNS in several species of interest, such as the rabbit (Villamayor et al., 2018; 2020) and the dog (Salazar et al., 2013).
In addition, a variety of antibodies were used for the immunohistochemical study of the fox VNO. Among these, the anti‐olfactory marker protein (OMP) antibody was employed specifically to label mature neurons in the MOS and VNS pathways. The anti‐growth‐associated protein 43 (GAP‐43) antibody was used to identify neural growth. Antibodies against specific calcium‐binding proteins (calbindin [CB] and calretinin [CR]), which were previously characterised in other species, such as the rabbit (Villamayor et al., 2018), were also used. Lastly, antibodies against G proteins (Gαi2 and Gαo) were used to examine G protein expression patterns.
We aimed to improve our understanding of the VNS in canids, specifically in the fox. Because the fox is a wild canid, anatomical and morphofunctional resemblances and divergences compared with a domestic canid, such as the dog, can help us understand the intricacies of the domestication process, and several of the features in the fox that were observed in this study suggest the validity of this hypothesis.
2. METHODS
A total of nine foxes were used in this study, eight of which (Z1–Z7 and ZC1) were acquired through hunting activities, organised by the Galician Hunting Federation and authorised by the Galician Environment, Territory, and Tenement Council, with the necessary permissions. The ninth fox (Zx) was posteriorly incorporated into this lot, after being acquired by the Anatomy Department staff from a car accident, and preserved immediately following dissection in Bouin’s fixative (Bf).
Foxes Z1–3 and Z7 were also conserved in Bf. Foxes Z5 and ZC1 were preserved in formalin. Fox ZC1 head was frozen and transverse‐sectioned to construct a macroscopic photographic series. Foxes Z1–3, Z5, and Z7 were dissected and the VNOs extracted, embedded in paraffin, and cut by a microtome. The olfactory bulbs were extracted and conserved in either 10% formalin or Bf. Table 1 gives details of the foxes and how the samples were processed.
TABLE 1.
Summary of the foxes, samples, and fixation techniques used
| Fox | Description | Samples | Fixative |
|---|---|---|---|
| Z1 | ♀ Adult | VNO, brain | Bouin’s |
| Z2 | ♂ Elderly | VNO, brain | Bouin’s |
| Z3 | ♂ Adult | VNO, brain | Bouin’s |
| Z5 | ♂ Young | VNO, brain | Formalin |
| Z7 | ♀ Young | Nasal cavity, brain | Bouin’s |
| ZC1 | Adult | Macroscopic transverse sections | Formalin |
| ZX | Young | Nasal cavity | Bouin’s |
The VNO samples were embedded in paraffin, in a gradual manner, and posteriorly sectioned by a microtome at a thickness of 6–7 µm. The VNO was serially transverse‐sectioned along its entire length, from caudal to rostral, or after previously dividing the organ into two sections, posterior and anterior.
Sample sections were stained using Haematoxylin‐Eosin, Periodic acid‐Schiff (PAS), Alcian Blue, and Gallego’s Trichome stains (Ortiz‐Hidalgo, 2011).
2.1. Lectin histochemistry protocol
LEA and BSI‐B4 were obtained as biotinylated conjugates. First, a 3% hydrogen peroxide solution was added to deparaffinised and rehydrated slides, to inactivate the endogenous peroxidase activity. Sections were then incubated with 2% bovine serum albumin (BSA) in 0.1 m phosphate‐buffered (pH 7.2) solution (PB). LEA and BSI‐B4 lectins were added, separately, and incubated overnight in 0.5% BSA solution. The next day, after two 2‐min washes in PB, the samples were incubated for 1.5 h at room temperature with an avidin‐biotin‐peroxidase complex (ABC) reagent (ABC complex; Vector Laboratories). A 0.003% hydrogen peroxide solution, in 0.2 m Tris‐HCl buffer, and a 0.05% 3,3‐diaminobenzidine (DAB) chromogen solution were used to visualise the reaction. The DAB reagent developed into a brown precipitate, which enabled visualisation of the reaction.
For the UEA lectin, we followed the same protocol described for LEA and BSI‐B4 lectins for the two first steps. Then, the slides were incubated for 1 hr in a 0.5% BSA–UEA solution. The samples were then washed for 3 × 5 min in a PB solution. An anti‐UEA peroxidase‐conjugated antibody was added and the slides were incubated overnight. The samples were washed with a PB solution and the reaction was visualised by adding a DAB solution, as described for the LEA and BSI‐B4 lectins.
Controls were performed both without the addition of lectins and with the preabsorption of lectins, using an excess amount of the corresponding sugar.
2.2. Immunohistochemical protocol
All samples were first incubated in a 3% hydrogen peroxide solution to inactivate endogenous peroxidase activity, prior to the immunohistochemical reaction. Then, samples were incubated with either 2.5% horse serum, for the ImmPRESS reagent kit anti‐mouse IgG/anti‐rabbit IgG (Vector Laboratories), or 2% BSA for 30 min, to block non‐specific binding sites. The primary antibody was then added and the incubation was performed at 4ºC overnight. The next day the samples blocked with the ImmPRESS kit were incubated with either the ImmPRESS VR Polymer HRP anti‐Rabbit IgG or the anti‐mouse IgG reagent (see Table 2), with the exception of the samples incubated with the anti‐OMP antibody, which was raised in goat. anti‐OMP‐labelled samples were incubated with a biotinylated anti‐goat IgG. All samples were then incubated for 1.5 hr in an ABC reagent (Vectastain, Vector Laboratories). All antibodies were maintained under humid conditions. In all cases, three successive 5‐min PB rinses were performed in‐between steps. All samples were rinsed in 0.2 m Tris‐HCl, pH 7.61, for 10 min, prior to visualising the reaction with a DAB chromogen, following the same protocol described for the lectin histochemical labelling. Samples for which the primary antibody was omitted were used as negative controls (Figures S1 and S2).
TABLE 2.
Summary of the antibodies and lectins used, including species of elaboration, dilution, catalogue number, and manufacturer
| Antibody | 1st Ab species/dilution | 1st Ab catalogue number | 2nd Ab species/dilution, catalogue number |
|---|---|---|---|
| Anti ‐ Gαo | Rabbit 1:200 | Santa Cruz Biotechnology sc‐387 | ImmPRESS VR HRP anti‐rabbit IgG Reagent MP‐6401‐15 |
| Anti ‐ Gαo | Rabbit 1:400 | MBL 551 | ImmPRESS VR HRP anti‐rabbit IgG Reagent MP‐6401‐15 |
| Anti ‐ Gαi2 | Rabbit 1:200 | Santa Cruz Biotechnology sc‐7276 | ImmPRESS VR HRP anti‐rabbit IgG Reagent MP‐6401‐15 |
| Anti ‐ OMP | Goat 1:400 | Wako 544‐10001 | Horse anti‐goat IgG 1:250 Vector BA‐9500 |
| Anti ‐ GAP‐43 | Mouse 1:400‐1:4,000 | Sigma G9264 | ImmPRESS VR HRP anti‐mouse IgG Reagent MP‐6402‐15 |
| Anti ‐ CB | Rabbit 1:6,000 | Swant CB38 | ImmPRESS VR HRP anti‐rabbit IgG Reagent MP‐6401‐15 |
| Anti ‐ CR | Rabbit 1:6,000 | Swant 7697 | ImmPRESS VR HRP anti‐rabbit IgG Reagent MP‐6401‐15 |
| Anti‐α‐tubulin | Rabbit 1:500 | Abcam 7291 | ImmPRESS VR HRP anti‐mouse IgG Reagent MP‐6402‐15 |
| UEA | 60 µg/ml | Vector L‐1060 | Rabbit 1:50 DAKO P289 |
| LEA | 20 µg/ml | Vector B‐1175 | Vectastain ABC reagent PK‐4000 |
| BSI‐B4 | 100 µg/ml | Sigma L‐2140 | Vectastain ABC reagent PK‐4000 |
ABC, avidin‐biotin‐complex; BSI‐B4, Bandeiraea simplicifolia isolectin B4; CB, calbindin; CR, calretinin; GAP‐43, growth‐associated protein 43; Gαi2, subunit αi2 of G protein; Gαo, subunit αo of G protein; HRP, horseradish peroxidase; IgG, Immunoglobulin G; LEA, Lycopersicum esculentum agglutinin; OMP, olfactory marker protein; UEA, Ulex europaeus agglutinin.
2.3. Image acquisition and digital processing
Digital images were captured using a Carl Zeiss Axiocam MRc5 digital camera connected to a Zeiss Axiophot microscope. Adobe photoshop CS4 (Adobe Systems) was used to adjust for brightness, contrast, and balance levels. However, no features of the image were enhanced in any way, moved or introduced. Additionally, an image‐stitching software (ptguipro) was used for those figures composed of several photographs.
3. RESULTS
The study of the VNS will be addressed at both macroscopic and microscopic levels. For each of these levels, descriptions of the VNO and the vomeronasal nerves are presented in detail.
3.1. Macroscopic study of the VNS
3.1.1. Vomeronasal organ
To access the VNO, it was necessary to expose the nasal septum (Figure 1). First, the lateral wall of the cavity formed by the maxillary bone was removed and the large ventral nasal concha extracted. In the rostroventral area of the septum, covered by a thin respiratory mucosa layer, a slightly prominent tubular formation was observed (Figure 2a,b), which extended in a rostrocaudal direction through the nasal cavity. After a meticulous dissection, hindered by the hidden position of the organ, the tubular structure was successfully extracted in its entirety (Figure 2c).
FIGURE 1.
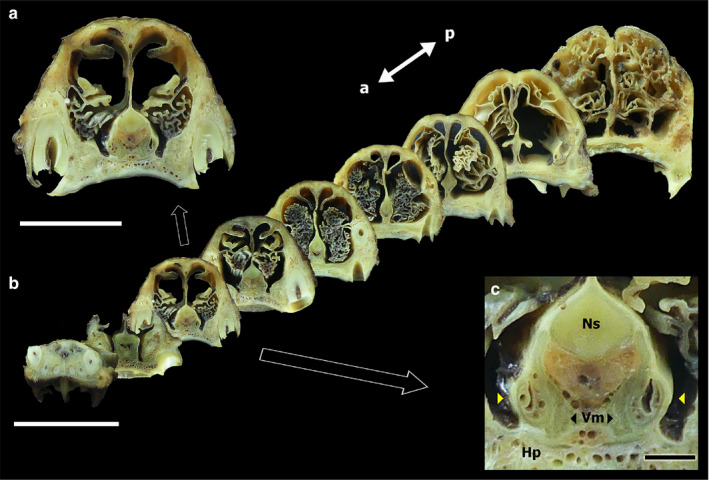
(a) Transverse sections of the fox nasal cavity. Enlarged view of a central transverse section of the nasal cavity, in which the VNO can be identified, from the macroscopic series (b), which extends from the incisive papilla to the coanas. (c) Enlarged view of the central section, showing the following elements: VNO (yellow arrowheads), nasal septum (Ns), vomer bone (Vm), hard palate (Hp). Scale bars: (a) 2.5 cm, (b) 5 cm, (c) 2 mm
FIGURE 2.
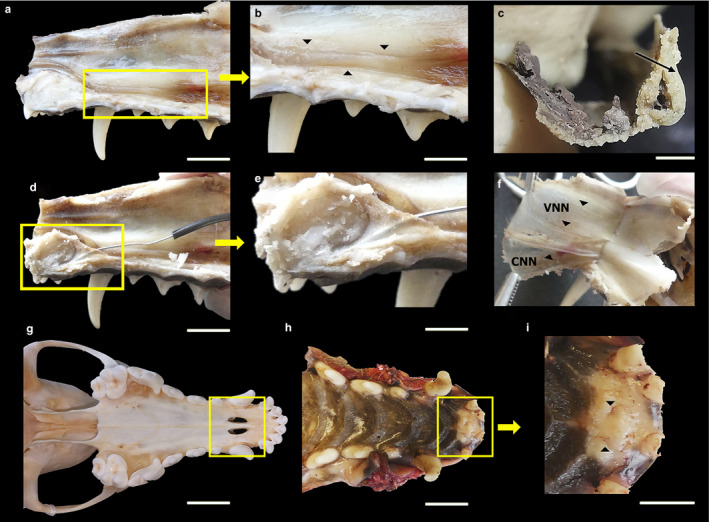
Dissection of the VNO and its innervation, showing the cannulation of the incisive duct and its opening in the incisive papilla, in the fox. (a) Lateral view of the nasal septum, where the VNO is framed. (b) Enlarged view of the nasal septum. The arrowheads delimit the VNO. (c) VNO extracted and cross‐sectioned, showing the lumen of the duct and the cartilaginous capsule (arrowhead). (d) Lateral view of the nasal septum. Cannulation of the incisive duct is framed. (e) Enlarged view of the cannulation. (f) Medial view of the nasal cavity mucosa after separation from the nasal septum. Innervation is indicated by arrowheads. VNN, vomeronasal nerves; CNN, caudal nasal nerve. (g) Ventral view of the skull of a fox. The palatine fissure is framed. (h) Ventral view of the hard palate. The rostral area is framed, and the higher magnification view shows that the incisive papilla can be found here, demarcated by arrowheads (i). Scale bars: (a,b,d,e,g,h) 1 cm, (c) 2 mm, (i) 0.5 cm.
This observation was complemented by the study of the transversal section series (Figure 1b), which allowed the assessment of the topographical relationships between the organ and the vomer bone, the nasal septum, and the hard palate. The medial cartilage of the cartilaginous capsule of the organ contacts the vomer bone, extending its most dorsal projection onto the cartilaginous portion of the nasal septum (Figure 1a). Laterally, the organ relates to the ventral meatus recess formed within the nasal cavity. The organ is covered by the respiratory mucosa. Through these transversal sections, the organ can be observed to rest on the hard palate, which is profusely irrigated and forms a mucous pad for the organ (Figure 1c).
Additionally, the vomeronasal duct can be distinguished. Its two primary components, the parenchyma and the lumen of the organ, can be discriminated. In the medial portion of the parenchyma, numerous vessels can be macroscopically distinguished (Figure 1c). The rostrocaudal variation of the VNO can be appreciated in the transverse section series. The organ extends from the level of the canine tooth root to the second premolar level (Figure 1b).
The communication between the VNO and the external environment allows the molecules responsible for chemical communications to access the neurosensory epithelium. This connection is established in the fox indirectly, through the incisive duct (or nasopalatine duct). The incisive duct establishes a communication between the nasal and oral cavities, and with the vomeronasal duct, which occupies the midpoint between these cavities. Macroscopically, using cannulation with a metal rod, we demonstrated that the incisive duct communicates with the oral cavity through the incisive papilla (Figure 2e). The incisive duct was observed to extend in the fox, from a very rostral portion, caudal to the incisors, to the beginning of the tubular formation (Figure 2d,e). It remains to be determined whether the vomeronasal duct opens into the incisive duct, as this could not be established macroscopically; this will be assessed on the microscopic level.
Finally, the VNO was extracted from the bone tissue, to which it is tightly bound by dense connective tissue. A transverse cut was made to the sample to ensure the identity of the VNO. At first glance, the vomeronasal duct, the cartilaginous capsule, and the parenchyma of the organ can be recognised (Figure 2c).
3.1.2. Vomeronasal nerves
The connection between the VNO and the AOB is established through the vomeronasal nerves (Figure 2). Macroscopically, these nerves were relatively easy to identify because they course along the nasal cavity submucosa (Figure 2f). After leaving the organ through the dorsal cleft formed by the cartilage, the vomeronasal nerves course in a caudodorsal direction to the cribriform ethmoidal plate. Approximately five to six vomeronasal nerve branches were identified, with a fine appearance, which were slightly translucent, suggesting their unmyelinated nature. This sensory innervation is complemented by autonomic caudal nasal nerve fibres, which can be observed to have larger calibres and a white appearance, coursing independently from the sensory nerves along the nasal cavity floor (Figure 2f).
3.2. Microscopic study of the VNO
3.2.1. Communication with the external environment
The VNO establishes indirect communication with both the nasal and oral cavities through the incisive duct (or nasopalatine duct; Figures 3 and 4).
FIGURE 3.
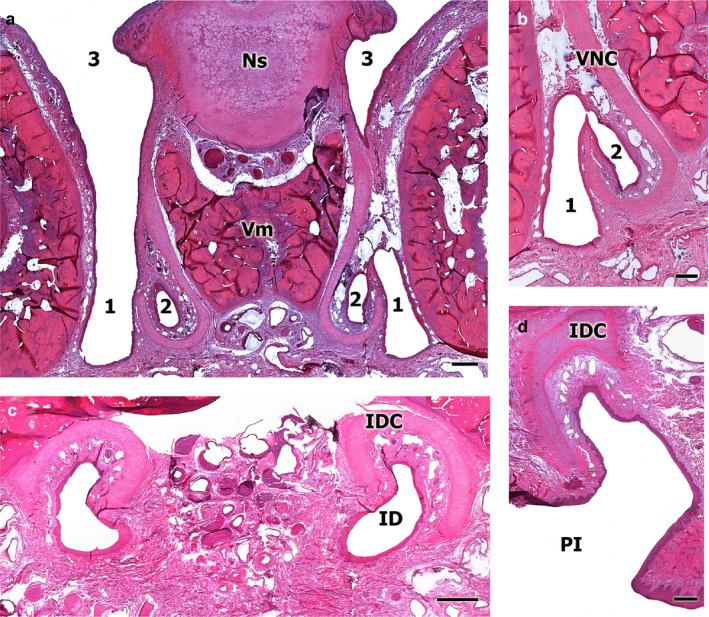
Sections of the decalcified fox nasal cavity for study of the communication between the VNO and the external environment. (a) Transverse section at the level of the anterior area of the nasal cavity, the incisive duct (ID) can be observed (1) running parallel to the vomeronasal duct (2) and opening into the ventral meatus of the nasal cavity (3, left‐hand). Ns, nasal septum; Vm, vomer bone. (b) View at higher magnification of the exact point where communication between the vomeronasal duct (2) and the ID (1) is established. VNC, vomeronasal cartilage. (c) Transverse section of the ID at the level of the palatine fissure, which shows the cartilage of its capsule (IDC) in a dorsal position to the ID. (d) Enlarged view of the rostral transverse section of the nasal cavity, which shows how the ID opens to the incisive papilla. Scale bars: (a) 500 µm, (b–d) 250 µm
FIGURE 4.
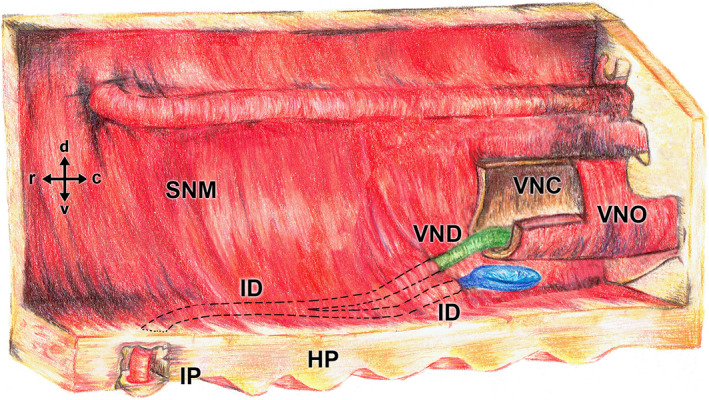
Drawing of the nasal cavity showing a schematic of the fox VNO, the confluence of the vomeronasal duct (VND, coloured in green) into the incisive duct (ID), and the opening of the ID into the oral cavity through the incisive papilla (IP). The opening of the ID into the nasal cavity is coloured in blue. HP, hard palate; SNM, septal nasal mucosa; VNC, vomeronasal cartilage
The incisive duct opens to the nasal cavity through an aperture located on the floor of the ventral recess, lateral to the VNO (Figures 2, 3 and 2, 3). Furthermore, the duct establishes communication with the oral cavity, through the incisive papilla (Figure 3d). The incisive duct meets the VNO at a central point of its course (Figures 3b and 4).
The vomeronasal cartilage surrounds both the VNO and the incisive duct. This cartilage modifies both its position and its length along its course from a caudal to a rostral position. In the caudal section, it is more elongated in its medial axis (Figure 3a), whereas in the more rostral levels, the cartilage situates on a dorsal position, relative to the nasopalatine duct (Figure 3c) and, at the most rostral portion, warps sideways at the opening through the incisive papilla (Figure 3d).
3.2.2. Vomeronasal organ
As a first approximation, the organ is divided into a vomeronasal capsule and a vomeronasal duct, which is associated with the parenchyma, where blood vessels, nerves, and vomeronasal glands are located (Figure 5a,d,e).
FIGURE 5.
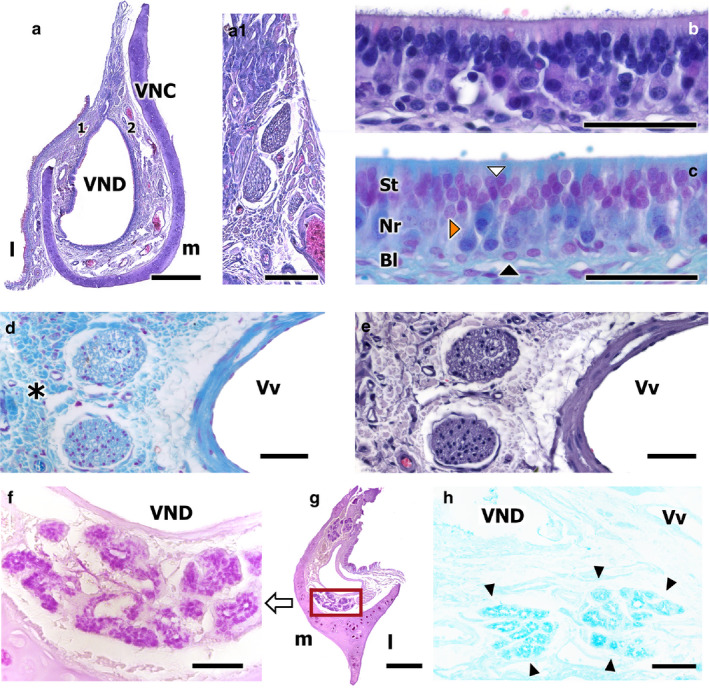
Transverse sections of the fox VNO. (a) Haematoxylin‐Eosin (HE)‐stained transverse section of the VNO. Higher magnification views of the neuroreceptor epithelium, after HE (b), and Gallego’s Trichrome (c) staining, showing the three layers of the neuroepithelium, the sustentacular layer (St), the neuroreceptor layer (Nr), and the basal cell layer (Bl). The white arrowhead points to a sustentacular cell, the orange arrowhead to a neuroreceptor cell, and the black arrowheads to basal cells. Higher magnification of the unmyelinated nerves in (a.1) (HE‐stained). (d,e) Vomeronasal nerves. Gallego’s Trichrome and HE stains, respectively. Loose connective tissue is clearly identified (asterisk). (f–h) Disposition and morphology of the fox vomeronasal glands: ventral transversal section of the organ (f), which shows acinar, tubular, and acinotubular glands, serose and PAS+ in nature. PAS stain. VND, vomeronasal duct. (g) Transverse caudal section of the VNO, showing the PAS+ glandular tissue disposition. (h) Transverse caudodorsal section of the VNO. Tubular, serose, Alcian Blue+ glands. Alcian Blue stain. Scale bars: (a) 500 µm, (b–e) 50 µm, (f,h) 50 µm, (g) 250 µm
The capsule surrounds the parenchyma, protecting it and preventing the lumen from collapsing. The capsule is composed of hyaline cartilage and has a ‘J’ shape, due to being incomplete in its dorsolateral portion (Figure 5a).
The vomeronasal duct consists of parenchyma and a lumen. The lumen generally has a kidney‐like shape and is lined internally with a pseudostratified epithelium. The lateral portion of this epithelium is respiratory in nature, the medial portion is neurosensory in nature, and both epithelia appear to be equally developed in size (Figure 5a).
The vomeronasal neurosensory epithelium is a columnar, pseudostratified, and non‐ciliated epithelium, composed primarily of three different cell types: the neuroreceptor cells, the sustentacular cells, and the basal cells. The first two cell types are arranged in a stratified manner, giving rise to an apical and a basal portion. The neuroreceptor cell dendrites have a terminal button, on which they present microvilli (Figure 5b,c).
Non‐sensory epithelium, which can be found in both the nasal mucosa and the respiratory epithelium of the VNO, is a pseudostratified columnar epithelium that presents cilia on its surface. The epithelium contains numerous mucus‐producing goblet cells and numerous glands that open into the duct, primarily on the dorsal and ventral commissures (Figure 5f–h). Four primary cell types can be identified in this epithelium, as shown in Figure 6a.2: sustentacular cells (white arrowheads), solitary cells (orange arrowheads), basal cells (black arrowheads), and goblet cells (green arrowheads).
FIGURE 6.
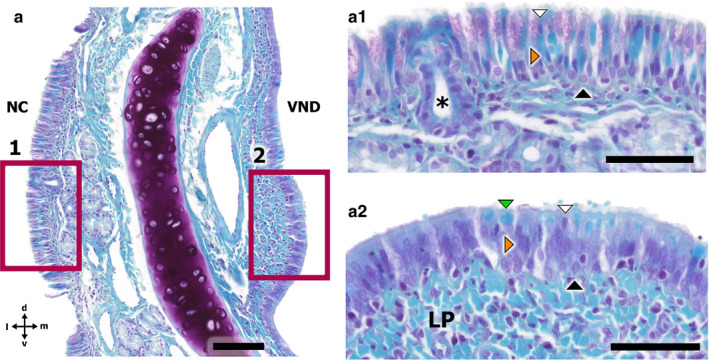
Gallego’s Trichome‐stained transverse section of the fox VNO and the nasal cavity respiratory mucosa, showing the two types of respiratory epithelia. (a) Transverse section of the VNO. NC, nasal cavity; VND, vomeronasal duct. (a.1) Higher magnification of the respiratory mucosa epithelium. The white arrowhead points to a sustentacular cell, the orange arrowhead to a columnar cell, and the black arrowhead to a basal cell. The asterisk shows a tubular gland duct. (a.2) Higher magnification of the respiratory epithelium of the VNO. The white arrowhead points to a sustentacular cell, the orange arrowhead to a columnar cell, the black arrowhead to a basal cell, and the green arrowhead to a goblet cell. LP, lamina propria. Scale bars: (a) 100 µm, (a.1,a.2) 50 µm
Additionally, the immunohistochemical study of the fox VNO, using the anti‐Gαo antibody, enabled the identification of a subpopulation of cells, in both the respiratory mucosa and VNO respiratory epithelia (Figure 7). A Gallego trichrome‐stained section of the VNO accompanies this immunohistochemical study (Figure 6).
FIGURE 7.
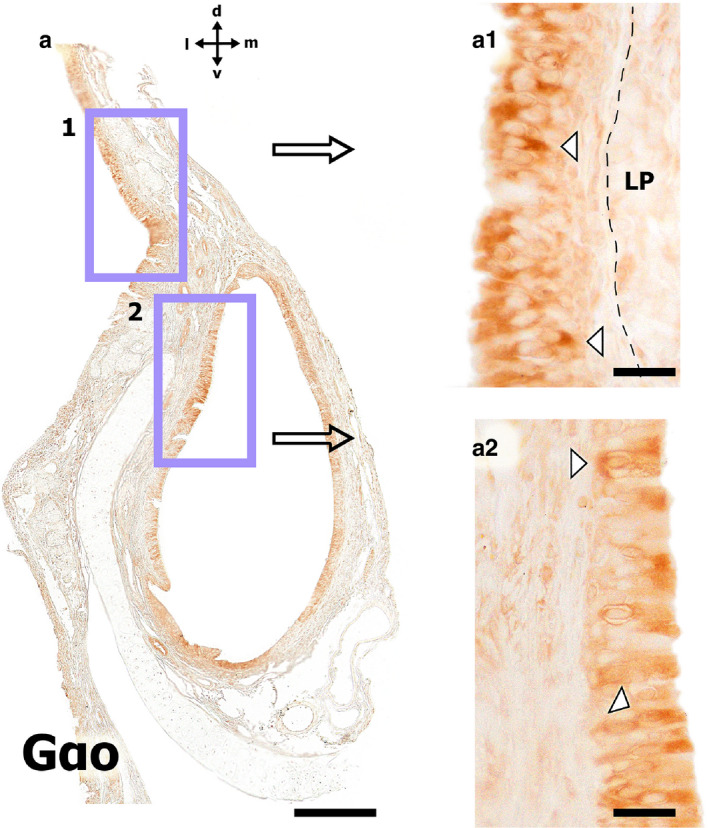
Immunohistochemical study using the anti‐Gαo antibody in the fox VNO. (a) Transverse section of the VNO. (a.1) Higher magnification view of the respiratory mucosa epithelium. The white arrowhead points to more intensely labelled cells. The dashed line shows the limits of the epithelium. LP, lamina propria. (a.2) Higher magnification of the respiratory epithelium of the VNO. The white arrowhead points to more intensely labelled cells. Scale bars: (a) 250 µm, (a.1,a.2) 50µm
Blood vessels are located in both the lateral and medial portions of the parenchyma; they are very numerous and form a large vascular network, providing the organ with an erectile tissue function.
The neuroreceptor cell axons converge to form unmyelinated nerve bundles, primarily located in the dorsomedial portion of the parenchyma (Figure 5a.1). In the lateral portion of the organ, less abundant, myelinated nerve bundles were also observed (Figure 5d,e).
The organ contains numerous glands that supply mucus to the lumen of the duct, known as serous glands, with tubular, acinar or tubuloacinar morphologies. Serous glands secrete a PAS‐positive and Alcian Blue‐positive secretion into the duct (Figure 5f–h). Surprisingly, in the central and middle portions of the VNO, very few glands were observed, associated almost exclusively with the dorsal and ventral duct commissures. As the organ progresses caudally, these glands increase in number and concentrate on the medial parenchyma. Finally, in the most caudal portion of the organ, the glands are arranged in the dorsal and ventral portions. The glands open through the respiratory epithelium into the duct (Figure 5a,g).
3.2.3. Immunohistochemical study of the VNO
An immunohistochemical study was performed in the VNO, using a panel of antibodies (Figure 8). The anti‐Gαo antibody, which specifically labels the αo subunit of the G‐protein transduction cascade, associated to the V2R receptor, labelled a subpopulation of neurons in the VNO epithelium, especially the dendrites and the soma. The dendrites can be identified from the soma to the lumen of the duct (Figure 8a,b). The anti‐Gαi2 antibody, which labels the αi2 subunit of the G‐protein transduction cascade associated to the V1R receptors, shows a more conspicuous immunopositive labelling of neuroreceptor cells compared with the anti‐Gαo immunolabelling, although not the entire neuroreceptor cell population. This labelling was more intense in the dendritic knobs of the neurons (Figure 8c,e). The labelling patterns displayed by the anti‐Gαo and anti‐Gαi2 antibodies are comparable with those displayed by the anti‐CB and anti‐CR antibodies, respectively, although calcium‐binding proteins appear to be less ubiquitous than the G‐proteins (Figure 8d,g).
FIGURE 8.
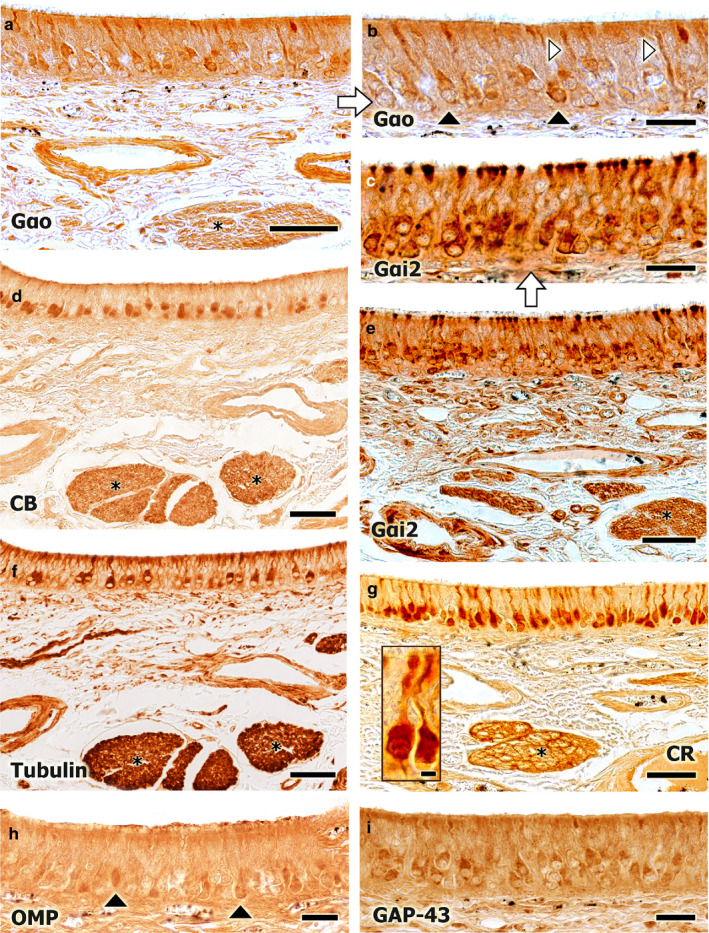
Immunohistochemical study of the fox VNO neuroepithelium. (a) Immunopositive labelling with an anti‐Gαo antibody. A subpopulation of neurons is labelled, with labelling concentrated in the soma (black arrowheads, b) and in the dendrites (white arrowheads, b). A similar pattern is observed for the anti‐CB antibody (d). Widespread immunopositive labelling is observed for the anti‐Gαi2 antibody. All neuron components are strongly labelled: the soma, the terminal button, the axon, and the dendrites. The asterisks (d–g) indicate the vomeronasal nerve fascicles in the parenchyma (e, at higher magnification in c). This labelling pattern is similar to that observed for the anti‐CR antibody (g, an immunopositive neuroreceptor cell is framed). (f) Immunopositive labelling for the anti‐α‐tubulin antibody, showing an intermediate pattern between those observed for the anti‐Gαo and anti‐Gαi2 antibodies. (h,i) Immunopositive labelling for the anti‐OMP and anti‐GAP‐43 antibodies are shown, respectively. In comparison with the anti‐OMP antibody, broader labelling is observed for the anti‐GAP‐43 antibody, which suggests the regenerative ability of the neuroepithelium. Scale bars: (a,d–g) 50 µm, (b,c,h,i) 25 µm
The anti‐α‐tubulin antibody, which specifically stains the cell soma and processes, shows an alternative labelling pattern, where the axons and dendrites of a subpopulation of neuroreceptor cells were positively labelled. This pattern represents a mix between the patterns resulting from the anti‐Gαi2 and the anti‐Gαo antibodies (Figure 8f).
The anti‐OMP antibody, which binds to OMP, a protein that acts as a marker of neuronal maturation, and the anti‐GAP43 antibody, which binds to GAP43, a protein associated with neuronal axonal growth, both identified neuronal subpopulations with less defined morphological patterns than the other antibodies (Figure 8d,g).
3.2.4. Lectin histochemical study of the VNO
Both the sensory epithelium neuroreceptor cells and the vomeronasal nerves of the VNO showed histochemically positive labelling for both the UEA and LEA lectins (Figure 9). UEA labelling was restricted to neuroreceptor cells (Figure 9a,b), whereas with LEA labelling was conspicuous (Figure 9c), and both neuroreceptor cells and the lamina propria were labelled.
FIGURE 9.
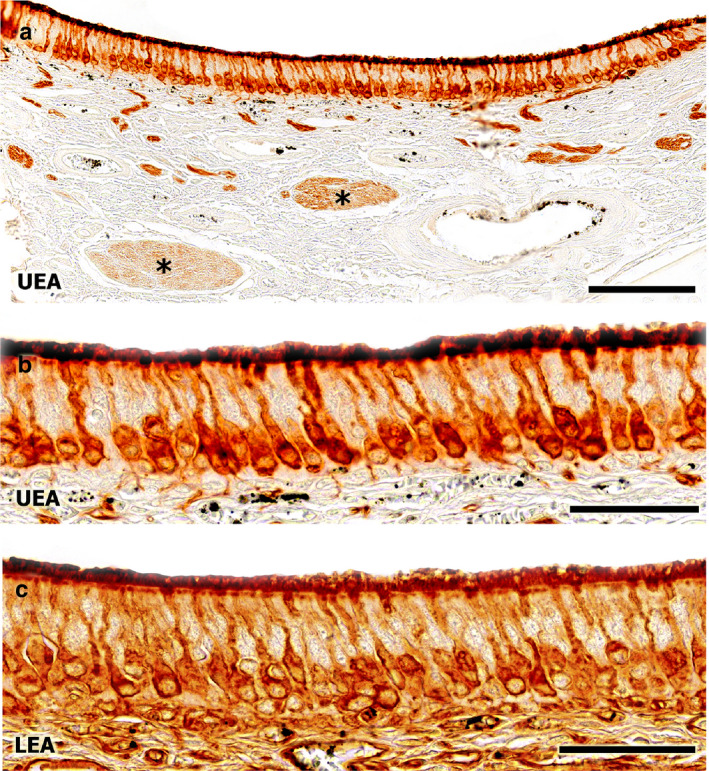
Lectin histochemical staining of the fox VNO. Both the neuroepithelium and the vomeronasal nerves (asterisk) show immunopositive labelling, with lectin UEA (a, at higher magnification in b) and with lectin LEA (c). Scale bars: (a) 250 µm, (b,c) 50 µm
BSI‐B4 lectin staining did not result in any positive labelling in either the neurosensory epithelium or in the respiratory epithelium.
4. DISCUSSION
An astonishing level of diversity exists among the structural, physiological, behavioural, and morphological aspects of the VNS in mammals (Meisami and Bhatnagar, 1998; Salazar and Sánchez‐Quinteiro, 2009), which differs from the extensive conservation demonstrated for the MOS. The VNO evolved to increase its specificity within species, compromising the ability to detect a broader group of ligands (Luo and Katz, 2004). The MOE receptors, however, can detect a wider range of odours but lack the specificity of the VNO. This difference can also be observed at the genetic level, where MOE receptor gene sequences are well‐conserved and the VNO contains a wider range of genes (Swaney and Keverne, 2009). Because of this wide morphological diversity, the interspecies extrapolation of information relative to the anatomy and histology of the VNS can be difficult and risky (Salazar et al., 2007).
Within the Canidae family, VNO studies have only been performed in the dog (Dennis et al., 2003; Salazar et al., 2013), except for an elementary study on the VNO of the fox (Vulpes vulpes) performed by Karimi et al. (2016). The apparent absence of V2R receptors (Salazar et al., 2013) in the dog has been proposed to be attributed to the domestication process, during which artificial selection induced an involution of the VNS in domesticated individuals of the Canidae family (Jezierski et al., 2016). Therefore, studying a wild species within the Canidae family would likely shed light on the implications of the domestication process on the VNO and elucidate the characteristics of this system among the Canidae family.
4.1. Macroscopic features
The topography of the VNO that was observed in the fox was similar to that reported for the dog and other domestic ungulates, like the cow or the horse (Adams and Wiekamp, 1984; Salazar et al., 1995). However, the fox VNO disposition differs from that described for Rodentia and Lagomorpha, which present VNOs located in a more dorsal position within the nasal cavity and are supported and encapsulated by the vomer bone (Vacarezza et al., 1981) and the palatine process of the incisive bone (Villamayor et al., 2018), respectively.
Similarities between the dog and the fox can also be observed when studying the communication between the vomeronasal duct with the outside world. In both the dog and the fox, as well as in other mammals, such as domestic ungulates (cow and horse), this communication is established indirectly, through the incisive duct, which communicates with the vomeronasal duct through the ventral recess of the nasal cavity, on one end, and with the oral cavity, through the incisive papilla, on the other (Adams and Wiekamp, 1984; Salazar et al., 1997). This morphology differs from what that observed in rodents or lagomorphs, in which the vomeronasal duct opens directly to the nasal cavity and the incisive duct communicates independently both cavities (Salazar and Sánchez‐Quinteiro, 1998; Villamayor et al., 2018).
Several authors have used the morphology and extension of the vomeronasal cartilage, which forms the capsule of the VNO, as a phylogenetic classification element of the VNS (Wöhrmann‐Repenning, 1984; Bhatnagar & Meisami 1998). In mammals, three different models have been observed for the vomeronasal capsule: this capsule can be entirely made of bone, as in the rat or the mouse (Salazar and Sánchez‐Quinteiro, 1998; 2003); in other species, such as the chinchilla (Oikawa et al., 1994) and the rabbit (Villamayor et al., 2018), the capsule is composed of a mixture of bone and cartilage; and in species such as the cat or the dog (Suarez et al., 2011; Salazar et al., 2013), the capsule is entirely made of cartilage. In the fox, the capsule is entirely made of cartilage, similar to those in cats and dogs. However, the cartilage in the fox has a ‘J’ shape, which differs from the prevailing ‘U‘ shape observed in the dog (Salazar et al., 1984).
4.2. Histological study
The neuroepithelium of the duct, where odour molecules are recognised, is morphologically less developed in the fox than in rodents or the rabbit (Luckhaus, 1969; Zuri et al., 1998). In the fox, this neuroepithelium is composed of three easily distinguishable layers: a basal layer, with distinct, round cells, a neuroreceptor cell layer, and a sustentacular cell layer. This layer definition, as well as the development of the neuroepithelium, is similar to the observations made in the dog by Salazar et al. (1984; 2013) and Dennis et al. (2003).
The sustentacular cell layer in the fox is well‐developed, with a tight cell configuration. Compared with the sustentacular layer in the dog, this layer is more apically located in the fox (Adams and Wiekamp, 1984; Salazar et al., 1984; 2013).
The placement of the vomeronasal epithelium greatly differs from the MOE, which is located in the nasal cavity, allowing olfactory receptors to be directly exposed to the outside world (Moran et al., 1982). To be functional, the VNS must possess a medium that allows pheromones and environmental molecules to access receptors located in the mucomicrovillar complex of the neurosensory epithelium. The VNO provides glandular and vascular components (Salazar and Sánchez‐Quinteiro, 1998), which facilitate efficient neuroreception, through separate mechanisms (Takami et al., 1994;1995).
The glandular component of the VNO provides mucus to the duct, which molecules of can diffuse through (Halpern and Martinez‐Marcos, 2003). In the fox, few glands can be observed in the central and medium portion of the organ. These glands become progressively more numerous in caudal portions, where they concentrate in the medial parenchyma, indicating unequal glandular secretion production between the rostral and caudal sections of the organ.
The PAS and Alcian Blue stains allowed us to characterise the nature of the glandular secretions in the fox VNO, which were PAS‐positive. Similarly, in the dog, Kondoh et al. (2020) observed that the glandular content is PAS‐positive. However, in the fox, the glands were also AB‐positive. The dual nature PAS‐ and AB‐positive vomeronasal glands in the fox is remarkable. Kondoh et al. (2020) observed that the vomeronasal glands in almost all studied species in the Laurasiatheria clade have a double‐acidic and neutral nature, except for Carnivora. However, these observations are somewhat controversial. In addition to our own observations in the fox, Tomiyasu et al. (2017) also found PAS‐ and AB‐positive glands in the bear, whereas in the cat and in the dog, Salazar et al. (1996) and Kondoh et al. (2020), respectively, only identified PAS‐positive glands.
These differences could be due to the region of the VNO studied, because most studies have only evaluated the central region of the VNO, where we have observed that the density of glandular tissue is much lower than in other regions. Further specific studies examining the nature of vomeronasal gland secretion in carnivores should help elucidate this issue.
Around the vomeronasal duct of the fox, many blood vessels were found in the parenchyma, both medially and laterally. In the dog, this disposition was similar (Adams and Wiekamp, 1984; Salazar et al., 1984; 2013). The abundant vascularisation of the VNO reveals the importance of its erectile function and the relevance of the vomeronasal pump for the physiology of the system. This pump, as stated by Broman (1920) and Meredith and O’Connell (1979) and Meredith et al. (1980), is required to transport fluids inside the vomeronasal duct, renewing their mucous contents, which contain molecules received by the organ.
The VNO has a double innervation, composed of myelinated and unmyelinated fibres, the latter emerging from the convergence of neuroreceptor cell axons projecting from the medial sensory epithelium of the organ. These unmyelinated nerves occupy a dorsomedial arrangement in the parenchyma, similar to the arrangements observed in carnivores (Salazar et al., 1996; 2013), ungulates (Salazar et al., 1997), and the rabbit (Villamayor et al., 2018). In the fox, the number and thickness of these nervous bundles are striking, given the small size of the organ and the reduced thickness of the neuroepithelium in this species, suggesting that, despite its small size, the VNO has a large functionality.
4.3. Immunohistochemical expression of G proteins alpha‐subunits
Specific antibodies against the α‐subunit of Gi and Go proteins identified two different zones within the rat AOB (Shinohara et al., 1992). Later, in 1995, Dulac and Axel genetically identified a family of possible pheromone receptors that were expressed in the rat VNO. Afterwards, a second type of vomeronasal receptor gene was identified (V2R) (Herrada and Dulac, 1997; Ryba and Tirindelli, 1997). V1Rs and V2Rs on the neuroepithelium were apically and basally distributed, respectively. The Gαi2 protein was thought to be expressed in the transduction cascade of V1Rs, whereas the Gαo protein co‐expressed with V2Rs (Dulac and Axel, 1995; Herrada and Dulac, 1997; Matsunami and Buck,1997; Ryba and Tirindelli, 1997). In conclusion, Gαi2 protein was associated to V1Rs and Gαo protein to V2R expression.
Afterwards, an examination of G protein expression and distribution in the goat VNS (Takigami et al., 2000), showed, for the first time, that in some species, the Gαo pathway had disappeared, which was subsequently confirmed in different mammals, including Laurasiatheria and Primates (Suarez et al., 2011). The few studies regarding G protein expression in the VNS of Carnivora have been controversial. Dennis et al. (2003) observed immunopositive labelling in the neurosensory epithelium using both anti‐Gαi2 and anti‐Gαo antibodies. Compared with the finding reported by Takigami et al. (2000) in the goat, Dennis et al. attributed this unexpected positiveness to an undesirable effect of the antigen retrieval procedure, a hypothesis that was supported by the subsequent study reported by Salazar et al. (2013), who observed, without applying antigen retrieval, immunonegative labelling when using the anti‐Gαo antibody. Therefore, the observed immunopositivity against the Gαo protein observed in the fox VNO is striking and of utmost importance because it may be analogous to the immunopositivity observed in the dog by Dennis et al. (2003). Aware of the importance of this finding, and to confirm this result, we performed the immunochemical study of Gαo protein expression in the fox VNO using two different anti‐Gαo antibodies and two different fixation techniques: formalin and Bf. Despite not performing any antigen retrieval procedures, immunopositivity against the Gαo protein persisted in all cases.
Although the immunohistochemical characterisation of Gαo has widely been considered an excellent phenotypic indicator of V2R expression in the VNO, we are aware that this conclusion is not well‐supported by the currently available genomic studies, which assume that functional V2R genes have regressed in many groups of mammals, including carnivores, showing a high rate of pseudogenisation (Young and Trask, 2007). No specific information regarding the fox V2R genes exists, except for the recent first attempt to sequence and assemble the red fox genome, in which neither functional nor pseudogene V2R genes were reported (Kukekova et al., 2018). However, the complete lack of V2R genes would represent a unique case among all mammals whose vomeronasal genes have been studied, as even primates, including humans, and all Laurasiatheria possess V2R pseudogenes. Even the dog, which is closely related to the fox, possesses nine V2R pseudogenes. It is likely that new sequencing studies performed in the fox, particularly those that are more focused on olfactory and vomeronasal genes, will result in the identification of additional genes, which can be added to the initially annotated repertory of vomeronasal and olfactory genes that were detected using a whole‐genome approach.
Translating sequencing studies into neuroanatomical terms is not always easy. A recent study on TRPC2 gene regression stated: ‘The results of our present study invite more in‐depth neuro‐anatomical investigation in mammals for which VNO function remains equivocal’ (Zhang and Nikaido, 2020). The high degree of pseudogenisation observed among vomeronasal receptors represents an unresolved issue which could explain the discrepancies observed between sequencing and neuroanatomical studies. For instance, an olfactory receptor gene containing a premature stop codon was found to encode a functional protein, due to efficient translational read‐through (Prieto‐Godino et al., 2016). Transcriptomic studies have identified the expression of vomeronasal pseudogenes in the mouse VNO (Oboti et al., 2015). In addition, the growing interest in the olfactory effects of long noncoding RNA and their transcripts is likely to lead to a better understanding of the molecular processes underlying olfaction (Camargo et al., 2019).
We cannot exclude the possibility that the Gαo protein is performing cell‐to‐cell contact functions in the fox neuroepithelium, unrelated to transduction. However, such a finding would represent the first instance of this function in the vomeronasal neuroepithelium of a mammal and does not fit the immunolabelling pattern detected in the fox VNO, which extends along the dendritic processes, the soma, and the axons that form the vomeronasal nerves. Instead, the immunolabelling pattern correlates with the typical pattern observed in those species in which both G proteins are involved in the transduction cascade (Halpern and Martinez‐Marcos, 2003).
Our study reports a very specific finding, in the context of also being the first study to examine the VNO of the fox; therefore, further studies must be performed examining the expression of V2R family receptors, including immunohistochemistry studies and in situ hybridisation studies, to determine the expression patterns of these receptors. Moreover, the immunohistochemical study of the fox accessory olfactory bulb, which has not yet been described, could also clarify these issues.
When Gαi2 and Gαo protein expression has been observed in rodents, such as the rat, two distinct zones were observed: an apical layer of neurosensory cells, which was immunopositive for anti‐Gαi2 antibodies, and a basal layer, which was immunopositive to anti‐Gαo antibodies (Halpern et al., 1998). In the fox, as in the rabbit (Villamayor et al., 2018), two distinct interspersed patterns were observed for the immunopositivity of both Gα proteins in the fox, which may be associated with two distinct subpopulations of neuroreceptor cells in the sensory epithelium of the VNO.
Although Gαo immunoreactivity is absent from the microvilli of the fox neurosensory epithelium, the pattern found in this study, comprising immunopositivity in the cellular soma, dendritic processes, and vomeronasal axons, is consistent with the pattern found in all species in which immunoreactivity has been associated with Gαo transduction. Among those species in which Gαo immunopositivity in the VNO has been excluded, immunolabelling is absent from dendrites, somas, and vomeronasal axons, as observed for the goat (Takigami et al., 2000) and the cat (Salazar and Sánchez‐Quinteiro, 2011).
The detection of immunolabelling in dendritic buttons and epithelial microvilli differs among studies, likely due to either variance in receptor density or to the sensitivity of the techniques used. Studies reported by Dennis et al. (2003) in the dog, by Jia and Halpern (1996) in the mouse, and by Villamayor et al. (2018) in the rabbit have shown that even when Gαo immunolabelling was clearly visible in the axons, somas, and dendrites, labelling was reduced in the dendritic buttons; however, overall Gαo marking was considered to be positive in all cases. The immunohistochemical identification of dendritic buttons and microvilli is, therefore, not consistent among species, unlike the labelling of dendritic processes, somas, and axons. Ultrastructural studies have confirmed that Gαo in neurons is primarily distributed to cell bodies and the neuronal cytoplasm, as demonstrated in the ultrastructural localisation study reported by Gabrion et al. (1989). In VNO ultrastructural studies performed in rats, a species with a large family of V1R and V2R neuroreceptors, Matsuoka et al. (2001) reported immunolabelling in dendritic knobs and microvilli, but also observed that a fraction of receptor cells were not immunopositive for any G protein subtypes.
The presence of both Gαi2 and Gαo proteins in the sensory epithelium of the fox VNO, in contrast to the reported expression of Gαi2 protein alone in other carnivores such as the dog or the cat (Salazar and Sánchez‐Quinteiro, 2011; Salazar et al., 2013), raises new questions regarding the domestication process. The absence of Gαo protein expression in the VNS of domesticated animals, such as the goat (Takigami et al., 2000), the sheep (Salazar et al., 2007), the dog (Salazar et al., 2013), and the cat (Salazar and Sánchez‐Quinteiro, 2011), has been theorised to be attributable to the domestication process (Jezierski et al., 2016), which may have caused an involution of the VNS. Indeed, the results of this study, showing the immunopositive labelling for the anti‐Gαo antibody in the fox VNO, strengthens this theory, as the fox is a wild, non‐domesticated animal.
4.4. Other immunohistochemical and lectin histochemical markers
Additionally, the fox VNO was immunohistochemically studied using other complementary antibodies, such as anti‐CB, anti‐CR, anti‐GAP‐43, anti‐OMP, and anti‐α‐tubulin antibodies, and histochemically examined using with Ulex europaeus agglutinin (UEA), Lycopersicum esculentum agglutinin (LEA), and Bandeiraea simplicifolia (BSI‐B4) isolectin.
Anti‐CB and anti‐CR antibodies were used to characterise specific neuronal components, as they display distinct expression patterns in the VNS of each species (Kishimoto et al., 1993; Jia and Halpern, 2003). In the fox, the anti‐CB antibody showed a characteristic labelling pattern in the neurosensory epithelium, revealing a subpopulation of neuroreceptor cells, primarily in the basal portion of the epithelium, with concentrated labelling in the soma. This labelling pattern resembles that observed for the anti‐Gαo antibody. Similarly, the anti‐CR antibody displayed a labelling pattern that was complementary to that observed for the anti‐CB antibody and similar to the pattern observed for the anti‐Gαi2 antibody. Similarly, the anti‐α‐tubulin antibody showed a unique labelling pattern, which evokes the patterns observed for the anti‐CB and the anti‐Gαo antibodies. Interestingly, CB and CR appear to be less ubiquitously expressed in the examined tissues than the G proteins, which are mediators of a wide spectrum of intracellular effects, including enzymes and ion channels (Wettschureck and Offermanns, 2005). Therefore, the CR and CB immunolabelling showed more defined patterns.
Additionally, immunolabelling was performed using the anti‐GAP43 antibody, which detects GAP43, a protein expressed by neurons experiencing axon growth and synaptogenesis (Skene, 1989; Verhaagen et al., 1989; Gispen et al., 1992; Ramakers et al., 1992). The GAP‐43 immunopositive labelling observed in the fox VNO is consistent with the immunopositive labelling previously observed by Dennis et al. (2003) in the dog. This labelling suggests that the VNS experiences intense neuronal regeneration, which may be due to the high level of VNO exposure to a variety of substances in the environment, many of which have the potential to damage cellular structures (Ogura et al., 2010), indicating the importance of the VNS for reproductive behaviours (Osakada et al., 2018) and, therefore, for the survival of the species.
To protect itself from exposure to harmful substances, the VNS possesses a regulatory system that can modulate access to these substances, based on the presence of solitary chemosensory cells (CSSs) in the respiratory tract. These specialised cells are situated at an appropriate location to detect chemical substances in the environment that are able to access the VNO. CSSs are generally innervated by the trigeminal nerve and respond to a variety of irritants and bitter substances. These CSSs play key roles in the regulation of VNO access, limiting the entrance of these potentially harmful molecules (Ogura et al., 2010).
Braun et al. (2011) described these CSSs as expressing G‐protein coupled receptors and found these cells to be present in the human nose. In our study in the fox, the positive detection of G‐protein receptors was confirmed using the anti‐Gαo antibody to immunolabel cells in the respiratory epithelia of both the VNO and the respiratory mucosa. Additionally, Gallego’s Trichome stain was performed in these epithelia, which provided information regarding the morphology of these anti‐Gαo‐positive cells. The morphology, immunoreactivity, and location of the cells observed in the fox epithelia generally coincide with those described by Ogura et al. (2010) and Braun et al. (2011).
By employing the anti‐OMP antibody, we specifically labelled OMP proteins, which are expressed in mature neurons in both the MOS and the VNS (Farbman and Margolis, 1980; Rodewald et al., 2016). The information provided by this marker complements the information obtained using the anti‐GAP43 antibody. Like other markers, OMP has been extensively used in the literature to study the VNS (Halpern et al., 1998), showing immunopositive labelling for both the MOS and the VNS in different species, such as the rat (Weiler and Benali, 2005) or the mouse (Monti Graziadei et al., 1977).
The UEA pattern observed in the fox was similar to that observed in the dog (Salazar et al., 2013). In the nervous system of the dog, the UEA labelling is specific to the VNS (Salazar et al., 1992). We observed a similar specificity in the fox, confirmed by the labelling of both the neuroepithelium and the nerves. UEA is a marker for the α‐fucose pathway (Kondoh et al., 2017). In contrast, LEA displays a non‐specific labelling pattern in the vomeronasal epithelium, labelling other components of the olfactory pathway, including olfactory nerves and the olfactory mucosa. This observation is consistent with the study performed on the VNO of the dog (Salazar et al., 2013).
The findings presented in this study highlight the importance of chemical communication in the fox and the subtle, but significant, differences between the VNO structures of the fox and the dog. These two closely related species only diverged approximately 10 million years ago, within the Canidae family. However, they occupy two substantially different ecological niches. Kukekova et al. (2018) suggested that the red fox may be an extraordinarily promising model for the study of the genetic foundations involved in social behaviours, domestication, genetics, and human behaviours.
The differences in the VNS between the fox and the dog suggested that domestication, beyond resulting in behavioural changes, may directly influence certain morphofunctional features discussed in this study, such as the double expression of Gαi2 and Gαo proteins.
After the exhaustive anatomical and morphofunctional description presented here, we can conclude that the fox has a well‐developed VNO, with all components necessary to assess the reception and recognition of pheromones and other chemical cues involved in chemical communication. Further anatomical studies, however, are necessary to better characterise the VNS in this species and to address the unforeseen outcome of this study. Specifically, future studies should address the results regarding G protein expression patterns and be extended to the AOB and the vomeronasal amygdala.
AUTHOR CONTRIBUTIONS
P.S.Q. and I.O.L. designed the research and wrote the paper. P.S.Q., I.O.L., P.V., M.T., and A.L.B performed the work, and analysed and discussed the results.
Supporting information
Figure S1
Figure S2
ACKNOWLEDGEMENTS
The red foxes used in this study were provided by the Wildlife Recovery Centres of Galicia, Dirección Xeral de Patrimonio Natural (Xunta de Galicia, Spain) and by Federación Galega de Caza. Special thanks to Jennifer Ríos Caamaño for her dedication and accuracy in her drawing of the VNO topography.
Ortiz‐Leal I, Torres MV, Villamayor PR, López‐Beceiro A, Sanchez‐Quinteiro P. The vomeronasal organ of wild canids: the fox (Vulpes vulpes) as a model. J Anat. 2020;237:890–906. 10.1111/joa.13254
Torres and Villamayor are joint second authors.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- Adams, D.R. and Wiekamp, M.D. (1984) The canine vomeronasal organ. Journal of Anatomy, 138, 771–787. [PMC free article] [PubMed] [Google Scholar]
- Barrios, A.W. , Sánchez‐Quinteiro, P. and Salazar, I. (2014) Dog and mouse: toward a balanced view of the mammalian olfactory system. Frontiers in Neuroanatomy, 8, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar, K.P. and Meisami, E. (1998) Vomeronasal organ in bats and primates: extremes of structural variability and its phylogenetic implications. Microscopy Research and Technique, 43, 465–475. [DOI] [PubMed] [Google Scholar]
- Braun, T. , Mack, B. and Kramer, M.F. (2011) Solitary chemosensory cells in the respiratory and vomeronasal epithelium of the human nose: a pilot study. Rhinology, 49, 507–512. [DOI] [PubMed] [Google Scholar]
- Brechbühl, J. , Moine, F. , Klaey, M. , Nenniger‐Tosato, M. , Hurni, N. , Sporkert, F. et al (2013) Mouse alarm pheromone shares structural similarity with predator scents. Proceedings of the National Academy of Sciences of the United States of America, 110, 4762–4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman, I. (1920) Das Organon vomeronasale ein Wassergeruchorgan. Anatomy Wiesbaden, 137–191. [Google Scholar]
- Camargo, A.P. , Nakahara, T.S. , Firmino, L.E.R. , Netto, P.H.M. , do Nascimento, J.B.P. , Donnard, E.R. et al (2019) Uncovering the mouse olfactory long non‐coding transcriptome with a novel machine‐learning model. DNA Research, 26, 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallini, P. and Santini, S. (1996) Reproduction of the red fox Vulpes vulpes in Central Italy. Annales Zoologici Fennici, 33, 267–274. [Google Scholar]
- De Miguel, F.J. , Marques, I.J. and Monclús, R. (2005) Respuesta de los zorros (Vulpes vulpes Linnaeus, 1758) al olor de otros carnívoros. Galemys, 17, 113–121. [Google Scholar]
- Del Cerro, M.C.R. (1998) Role of the vomeronasal input in maternal behavior. Psychoneuroendocrinology, 23, 905–926. [DOI] [PubMed] [Google Scholar]
- Dennis, J.C. , Allgier, J.G. , Desouza, L.S. , Eward, W.C. and Morrison, E.E. (2003) Immunohistochemistry of the canine vomeronasal organ. Journal of Anatomy, 202, 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis, J.C. , Stilwell, N.K. , Smith, T.D. , Park, T.J. , Bhatnagar, K.P. and Morrison, E.E. (2020) Is the mole rat vomeronasal organ functional? Anatomical Record, 303, 318–329. [DOI] [PubMed] [Google Scholar]
- Dulac, C. and Axel, R. (1995) A novel family of genes encoding putative pheromone receptors in mammals. Cell, 83, 195–206. [DOI] [PubMed] [Google Scholar]
- Estes, R.D. (1972) The role of the vomeronasal organ in mammalian reproduction. Mammalia, 36, 316–319. [Google Scholar]
- Farbman, A.I. and Margolis, F.L. (1980) Olfactory marker protein during ontogeny: immunohistochemical localization. Developmental Biology, 74, 205–215. [DOI] [PubMed] [Google Scholar]
- Gabrion, J. , Brabet, P. , Nguyen Than Dao, B. , Homburger, V. , Dumuis, A. , Sebben, M. et al (1989) Ultrastructural localization of the GTP‐binding protein Go in neurons. Cellular Signalling, 1, 107–123. [DOI] [PubMed] [Google Scholar]
- Gentle, M.N. (2005) Factors affecting the efficiency of fox (Vulpes vulpes) baiting practices on the central tablelands of new south wales. Thesis, School of Biological Sciences, University of Sydney; https://ses.library.usyd.edu.au/handle/2123/890 [Google Scholar]
- Gispen, W.H. , Nielander, H.B. , De Graan, P.N.E. , Oestreicher, A.B. , Schrama, L.H. and Schotman, P. (1992) Role of the growth‐associated protein B‐50/GAP‐43 in neuronal plasticity. Molecular Neurobiology, 5, 61–85. [DOI] [PubMed] [Google Scholar]
- González, R. , Levy, F. , Orgeur, P. , Poindron, P. and Signoret, J.P. (1991) Female effect in sheep. II. Role of volatile substances from the sexually receptive female; implication of the sense of smell. Reproduction, Nutrition, Development, 31, 103–109. [DOI] [PubMed] [Google Scholar]
- Grus, W.E. and Zhang, J. (2004) Rapid turnover and species‐specificity of vomeronasal pheromone receptor genes in mice and rats. Gene, 340, 303–312. [DOI] [PubMed] [Google Scholar]
- Grus, W.E. and Zhang, J. (2008) Distinct evolutionary patterns between chemoreceptors of 2 vertebrate olfactory systems and the differential tuning hypothesis. Molecular Biology and Evolution, 25, 1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern, M. , Shapiro, L.S. and Jia, C. (1998) Heterogeneity in the accessory olfactory system. Chemical Senses, 23, 477–481. [DOI] [PubMed] [Google Scholar]
- Halpern, M. and Martinez‐Marcos, A. (2003) Structure and function of the vomeronasal system: an update. Progress in Neurobiology, 70, 245–318. [DOI] [PubMed] [Google Scholar]
- Herrada, G. and Dulac, C. (1997) A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell, 90, 763–773. [DOI] [PubMed] [Google Scholar]
- Holy, T.E. (2018) The accessory olfactory system: innately specialized or microcosm of mammalian circuitry? Annual Review of Neuroscience, 41, 501–525. [DOI] [PubMed] [Google Scholar]
- Iovino, M. , Messana, T. , Iovino, E. , et al. (2019) Neuroendocrine Mechanisms Involved in Male Sexual and Emotional Behavior. Endocrine, Metabolic & Immune Disorders‐Drug Targets, 19, 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezierski, T. , Ensminger, J. and Papet, L.E. (2016) Canine olfaction science and law. Advances in Forensic Science, Medicine, Conservation, and Environmental Remediation. Boca Ratón, FL: CRC Press/Taylor & Francis. [Google Scholar]
- Jia, C. and Halpern, M. (1996) Subclasses of vomeronasal receptor neurons: differential expression of G proteins (Gi alpha 2 and G(o alpha)) and segregated projections to the accessory olfactory bulb. Brain Research, 719, 117–128. [DOI] [PubMed] [Google Scholar]
- Jia, C. and Halpern, M. (2003) Calbindin D28K immunoreactive neurons in vomeronasal organ and their projections to the accessory olfactory bulb in the rat. Brain Research, 977, 261–269. [DOI] [PubMed] [Google Scholar]
- Karimi, H. , Hassanzadeh, B. and Razmaraii, N. (2016) Structure of vomeronasal organ (Jacobson) in the male red fox (Vulpes vulpes). Anatomical Science, 13, 47–54. [Google Scholar]
- Kishimoto, J. , Keverne, E.B. and Emson, P.C. (1993) Calretinin, calbindin‐D28k and parvalbumin‐like immunoreactivity in mouse chemoreceptor neurons. Brain Research, 610, 325–329. [DOI] [PubMed] [Google Scholar]
- Kondoh, D. , Kamikawa, A. , Sasaki, M. and Kitamura, N. (2017) Localization of α1‐2 fucose glycan in the mouse olfactory pathway. Cells Tissues Organs, 203, 20–28. [DOI] [PubMed] [Google Scholar]
- Kondoh, D. , Tomiyasu, J. , Itakura, R. , Sugahara, M. , Yanagawa, M. and Watanabe, K. et al (2020) Comparative histological studies on properties of polysaccharides secreted by vomeronasal glands of dogs, minks, cattle, goats, deer, musk shrews and bats. Acta Histochemica, 122, 151515 10.1016/j.acthis.2020.151515 [DOI] [PubMed] [Google Scholar]
- Kukekova, A. , Johnson, J. , Xiang, X. , Feng, S. , Liu, S. , Rando, H.M. et al (2018) Red fox genome assembly identifies genomic regions associated with tame and aggressive behaviours. Nature Ecology & Evolution, 2, 1479–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhaus, G. (1969) Licht‐ und elektronenmikroskopische Befunde an der Lamina epithelialis des Vomeronasalorgans vom Kaninchen. Anatomischer Anzeiger, 124, 477–489. [PubMed] [Google Scholar]
- Luo, M. and Katz, L.C. (2004) Encoding pheromonal signals in the mammalian vomeronasal system. Current Opinion in Neurobiology, 14, 428–434. [DOI] [PubMed] [Google Scholar]
- Matsunami, H. and Buck, L.B. (1997) A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell, 90, 775–784. [DOI] [PubMed] [Google Scholar]
- Matsuoka, M. , Yoshida‐Matsuoka, J. , Iwasaki, N. , Norita, M. , Costanzo, R.M. and Ichikawa, M. (2001) Immunocytochemical study of G(i)2alpha and G(o)alpha on the epithelium surface of the rat vomeronasal organ. Chemical Senses, 26, 161–166. [DOI] [PubMed] [Google Scholar]
- McLean, S. , Davies, N.W. and Nichols, D.S. (2019) Scent chemicals of the tail gland of the Red Fox, Vulpes vulpes . Chemical Senses, 44, 215–224. [DOI] [PubMed] [Google Scholar]
- Meisami, E. and Bhatnagar, K.P. (1998) Structure and diversity in mammalian accessory olfactory bulb. Microscopy Research and Technique, 43, 476–499. [DOI] [PubMed] [Google Scholar]
- Meredith, M. , Marques, D.M. and O’Connell, R.J. (1980) Vomeronasal pump: significance for male hamster. Sexual Behaviour Science, 207, 1224–1226. [DOI] [PubMed] [Google Scholar]
- Meredith, M. and O’Connell, R.J. (1979) Efferent control of stimulus access to the hamster vomeronasal organ. Journal of Physiology, 286, 301–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti Graziadei, G.A. , Margolis, F.L. , Harding, J.W. and Graziadei, P.P. (1977) Immunocytochemistry of the olfactory marker protein. Journal of Histochemistry and Cytochemistry, 25, 1311–1316. [DOI] [PubMed] [Google Scholar]
- Moran, D.T. , Rowley, J.C. 3rd , Jafek, B.W. and Lovell, M.A. (1982) The fine structure of the olfactory mucosa in man. Journal of Neurocytology, 11, 721–746. [DOI] [PubMed] [Google Scholar]
- Nakajima, T. , Sakaue, M. , Kato, M. , Saito, S. , Ogawa, K. and Taniguchi, K. (1998) Immunohistochemical and enzyme‐histochemical study on the accessory olfactory bulb of the dog. Anatomical Record, 252, 393–402. [DOI] [PubMed] [Google Scholar]
- Oboti, L. , Ibarra‐Soria, X. , Pérez‐Gómez, A. , Schmid, A. , Pyrski, M. , Paschek, N. et al (2015) Pregnancy and estrogen enhance neural progenitor‐cell proliferation in the vomeronasal sensory epithelium. BMC Biology, 13, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura, T. , Krosnowski, K. , Zhang, L. , Bekkerman, M. and Lin, W. (2010) Chemoreception regulates chemical access to mouse vomeronasal organ: role of solitary chemosensory cells. PLoS One, 5, e11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa, T. , Shimamura, K. , Saito, T.R. and Taniguchi, K. (1994) Fine structure of the vomeronasal organ in the Chinchilla (Chinchilla laniger). Experimental Animals, 43, 487–497. [DOI] [PubMed] [Google Scholar]
- Ortiz‐Hidalgo, C. (2011) Abelardo Gallego (1879–1930) and his contributions to histotechnology: the Gallego stains. Acta Histochemica, 113, 189–193. [DOI] [PubMed] [Google Scholar]
- Osakada, T. , Ishii, K.K. , Mori, H. , Eguchi, R. , Ferrero, D.M. , Yoshihara, Y. et al (2018) Sexual rejection via a vomeronasal receptor‐triggered limbic circuit. Nature Communications, 9, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Over, R. , Cohen‐Tannoudji, J. , Dehnhard, M. , Claus, R. and Signoret, J.P. (1990) Effect of pheromones from male goats on LH‐secretion in anoestrous ewes. Physiology & Behavior, 48, 665–668. [DOI] [PubMed] [Google Scholar]
- Prieto‐Godino, L.L. , Rytz, R. , Bargeton, B. , Abuin, L. , Arguello, J.R. , Peraro, M.D. et al (2016) Olfactory receptor pseudo‐pseudogenes. Nature, 539, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers, G.J. , Verhaagen, J. , Oestreicher, A.B. , Margolis, F.l. , Van Bergen En Henegouwen, P.M.P. and Gispen, W.H. (1992) Immunolocalization of B‐50 (GAP‐43) in the mouse olfactory bulb: predominant presence in preterminal axons. Journal of Neurocytology, 21, 853–869. [DOI] [PubMed] [Google Scholar]
- Reep, R.L. , Finlay, B.L. and Darlington, R.B. (2007) The limbic system in mammalian brain evolution. Brain, Behavior and Evolution, 70, 57–70. [DOI] [PubMed] [Google Scholar]
- Rekwot, P.I. , Ogwu, D. , Ovedipe, E.O. and Sekoni, V.O. (2001) The role of pheromones and biostimulation in animal reproduction. Animal Reproduction Science, 65, 157–170. [DOI] [PubMed] [Google Scholar]
- Rodewald, A. , Gisder, D. , Gebhart, V.M. , Oehring, H. and Jirikowski, G.F. (2016) Distribution of olfactory marker protein in the rat vomeronasal organ. Journal of Chemical Neuroanatomy, 77, 19–23. [DOI] [PubMed] [Google Scholar]
- Rodriguez, I. , Greer, C.A. , Mok, M.Y. and Mombaerts, P. (2000) A putative pheromone receptor gene expressed in human olfactory mucosa. Nature Genetics, 26, 18–19. [DOI] [PubMed] [Google Scholar]
- Ryba, N.P.N. and Tirindelli, R. (1997) A new multigene family of putative pheromone receptors. Neuron, 19, 371–379. [DOI] [PubMed] [Google Scholar]
- Salazar, I. , Barber, P.C. and Cifuentes, J.M. (1992) Anatomical and immunohistological demonstration of the primary neural connections of the vomeronasal organ in the dog. Anatomical Record, 233, 309–313. [DOI] [PubMed] [Google Scholar]
- Salazar, I. , Cifuentes, J.M. and Sánchez‐Quinteiro, P. (2013) Morphological and inmunohistochemical features of the vomeronasal system in dogs. Anatomical Record, 296, 146–155. [DOI] [PubMed] [Google Scholar]
- Salazar, I. , Rueda, A. and Cifuentes, J.M. (1984) Anatomy of the vomeronasal organ in the dog. Folia Morphologica, 32, 331–341. [PubMed] [Google Scholar]
- Salazar, I. and Sánchez‐Quinteiro, P. (1998) Supporting tissue and vasculature of the mammalian vomeronasal organ: the rat as a model. Microscopy Research and Technique, 41, 492–505. [DOI] [PubMed] [Google Scholar]
- Salazar, I. and Sánchez‐Quinteiro, P. (2003) Differential development of binding sites for four lectins in the vomeronasal system of juvenile mouse: from the sensory transduction site to the first relay stage. Brain Research, 979, 15–26. [DOI] [PubMed] [Google Scholar]
- Salazar, I. and Sánchez‐Quinteiro, P. (2009) The risk of extrapolation in neuroanatomy: The case of the mammalian vomeronasal system. Frontiers in Neuroanatomy, 3, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar, I. and Sánchez‐Quinteiro, P. (2011) A detailed morphological study of the vomeronasal organ and the accessory olfactory bulb of cats. Microscopy Research and Technique, 74, 1109–1120. [DOI] [PubMed] [Google Scholar]
- Salazar, I. , Sánchez‐Quinteiro, P. , Alemañ, N. , Cifuentes, J.M. and Troconiz, P.F. (2007) Diversity of the vomeronasal system in mammals: the singularities of the sheep model. Microscopy Research and Technique, 70, 752–762. [DOI] [PubMed] [Google Scholar]
- Salazar, I. , Sánchez‐Quinteiro, P. and Cifuentes, J.M. (1995) Comparative anatomy of the vomeronasal cartilage in mammals: mink, cat, dog, pig, cow and horse. Annals of Anatomy – Anatomischer Anzeiger, 177, 475–481. [DOI] [PubMed] [Google Scholar]
- Salazar, I. , Sánchez‐Quinteiro, P. and Cifuentes, J.M. (1997) The soft‐tissue components of the vomeronasal organ in pigs, cows and horses. Anatomia Histologia and Embryologia, 26, 179–186. [DOI] [PubMed] [Google Scholar]
- Salazar, I. , Sánchez Quinteiro, P. , Cifuentes, J.M. , Garcia Caballero, T. (1996) The vomeronasal organ of the cat. Journal of Anatomy, 188, 445–454. [PMC free article] [PubMed] [Google Scholar]
- Sam, M. , Vora, S. , Malnic, B. , Ma, W. , Novotny, M.V. and Buck, L.B. (2001) Odorants may arouse instinctive behaviours. Nature, 412, 142. [DOI] [PubMed] [Google Scholar]
- Shapiro, L.S. , Halpern, M. and Ee, P.‐L. (1995) Lectin histochemical identification of carbohydrate moieties in opossum chemosensory systems during development, with special emphasis on VVA‐identified subdivisions in the accessory olfactory bulb. Journal of Morphology, 224, 331–349. [DOI] [PubMed] [Google Scholar]
- Shinohara, H. , Asano, T. and Kato, K. (1992) Differential localization of G‐proteins Gi and Go in the accessory olfactory bulb of the rat. Journal of Neuroscience, 12, 1275–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoret, J.P. (1991) Sexual pheromones in the domestic sheep: importance and limits in the regulation of reproductive physiology. Journal of Steroid Biochemistry and Molecular Biology, 39, 639–645. [DOI] [PubMed] [Google Scholar]
- Skene, J.H.P. (1989) Axonal growth‐associated proteins. Annual Review of Neuroscience, 12, 127–156. [DOI] [PubMed] [Google Scholar]
- Suarez, R. , Fernandez‐Aburto, P. , Manger, P.R. and Mpodozis, J. (2011) Deterioration of the Gαo vomeronasal pathway in sexually dimorphic mammals. PLoS One, 6, e26436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney, W.T. and Keverne, E.B. (2009) The evolution of pheromonal communication. Behavioral Brain Research, 200, 239–247. [DOI] [PubMed] [Google Scholar]
- Takami, S. , Getchell, M.L. and Getchell, T.V. (1994) Lectin histochemical localization of galactose, N‐acetylgalactosamine, and N‐acetylglucosamine in glycoconjugates of the rat vomeronasal organ, with comparison to the olfactory and septal mucosae. Cell and Tissue Research, 277, 211–230. [DOI] [PubMed] [Google Scholar]
- Takami, S. , Getchell, M.L. and Getchell, T.V. (1995) Resolution of sensory and mucoid glycoconjugates with terminal alpha‐galactose residues in the mucomicrovillar complex of the vomeronasal sensory epithelium by dual confocal laser scanning microscopy. Cell and Tissue Research, 280, 211–216. [DOI] [PubMed] [Google Scholar]
- Takigami, S. , Mori, Y. and Ichikawa, M. (2000) Projection pattern of vomeronasal neurons to the accessory olfactory bulb in goats. Chemical Senses, 25, 387–393. [DOI] [PubMed] [Google Scholar]
- Tomiyasu, J. , Kondoh, D. , Sakamoto, H. , Matsumoto, N. , Sasaki, M. , Kitamura, N. et al (2017) Morphological and histological features of the vomeronasal organ in the brown bear. Journal of Anatomy, 231, 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh, K. and Storm, D.R. (2003) Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nature Neuroscience, 6, 519–525. [DOI] [PubMed] [Google Scholar]
- Vacarezza, O.L. , Sepich, L.N. and Tramezzani, J.H. (1981) The vomeronasal organ of the rat. Journal of Anatomy, 132, 167–185. [PMC free article] [PubMed] [Google Scholar]
- Villamayor, P.R. , Cifuentes, J.M. , Fdz‐de‐Troconiz, P. and Sanchez‐Quinteiro, P. (2018) Morphological and immunohistochemical study of the rabbit vomeronasal organ. Journal of Anatomy, 233, 814–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamayor, P.R. , Cifuentes, J.M. , Quintela, L. , Barcia, R. and Sanchez‐Quinteiro, P. (2020) Structural, morphometric and immunohistochemical study of the rabbit accessory olfactory bulb. Brain Structure and Function, 225, 203–226. [DOI] [PubMed] [Google Scholar]
- Verhaagen, J. , Oestreicher, A.B. , Gispen, W.H. and Margolis, F.L. (1989) The expression of the growth associated protein B50/GAP43 in the olfactory system of neonatal and adult rats. Journal of Neuroscience, 9, 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler, E. and Benali, A. (2005) Olfactory epithelia differentially express neuronal markers. Journal of Neurocytology, 34, 217–240. [DOI] [PubMed] [Google Scholar]
- Wettschureck, N. and Offermanns, S. (2005) Mammalian G proteins and their cell type specific functions. Physiological Reviews, 85, 1159–1204. [DOI] [PubMed] [Google Scholar]
- Wöhrmann‐Repenning, A. (1984) Comparative anatomical studies of the vomeronasal complex and the rostral palate of various mammals. Anatomischer Anzeiger, 157, 137–149.6507883 [Google Scholar]
- Wysocki, C.J. (1979) Neurobehavioral evidence for the involvement of the vomeronasal system in mammalian reproduction. Neuroscience and Biobehavioral Reviews, 3, 301–341. [DOI] [PubMed] [Google Scholar]
- Young, J.M. and Trask, B.J. (2007) V2R gene families degenerated in primates, dog and cow, but expanded in opossum. Trends in Genetics, 23, 212–215. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. and Nikaido, M. (2020) Inactivation of ancV1R as a predictive signature for the loss of vomeronasal system in mammals. Genome Biology and Evolution, 10.1093/gbe/evaa082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuri, I. , Fishelson, L. and Terkel, J. (1998) Morphology and cytology of the nasal cavity and vomeronasal organ in juvenile and adult blind mole rats (Spalax ehrenbergi). Anatomical Record, 251, 460–471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Data Availability Statement
Data available on request from the authors.


