Abstract
Background:
Urine provides a minimally invasive specimen that may allow for development of rapid tests to detect antiretroviral drugs and provide opportunities to improve individual adherence. This study sought to determine whether urine could provide a biomarker of adherence for currently approved pre-exposure prophylaxis and HIV treatment regimens.
Methods:
Urine and blood were collected from 34 HIV-negative men who have sex with men aged 18–49 years, enrolled in a clinical trial comparing 2 antiretroviral regimens. Specimens were collected 4 and 24 hours after a single oral dose of tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) (n = 10) or tenofovir alafenamide (TAF)/FTC/cobicistat (COBI)/elvitegravir (EVG) (n = 8), or after 4 and 10 days of daily oral TDF/FTC (n = 9) or TAF/FTC/ COBI/EVG (n = 7). Tenofovir (TFV), FTC, and EVG were measured by high-performance liquid chromatography-mass spectrometry.
Results:
Median urine FTC concentrations at 4 and 24 hours were similar between men receiving TDF/FTC (4 hours 147 μg/mL; 24 hours 10 μg/mL) and men receiving TAF/FTC/COBI/EVG (4 hours 333 μg/mL, P = 0.173; 24 hours 13 μg/mL, P = 0.681). Median urine TFV concentrations were lower among men receiving TAF/FTC/COBI/EVG (4 hours 1.2 μg/mL; 24 hours 0.8 μg/mL) compared with men receiving TDF/FTC (4 hours 17 μg/mL, P < 0.001; 24 hours 7 μg/mL, P = 0.001). Urine TFV concentrations remained reduced among men receiving TAF/FTC/COBI/EVG compared with men receiving TDF/FTC after daily dosing. EVG was not consistently measurable in urine.
Conclusions:
High urine FTC and TFV concentrations could provide an indication of adherence to daily oral dosing with TDF or TAF-based regimens used for treatment and prevention.
Keywords: antiretroviral agents, point-of-care testing, PrEP, urine, men who have sex with men, HIV
INTRODUCTION
Daily oral dosing with tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) is highly effective at preventing HIV infection and efficacy is strongly correlated with adherence.1–3 All currently approved antiretroviral (ARV) drug regimens for treatment of HIV infection require adherence to daily dosing regimens for effective control of viremia and prevention of the emergence of ARV-resistance4–6 Existing subjective methods to determine adherence such as self-report and pill count are considered unreliable.7–9 Therefore, assays that rapidly assess adherence using minimally invasive specimens could be used in clinical settings for immediate feedback and behavioral interventions to improve adherence among persons using ARVs for treatment or prevention.
Intracellular metabolites of tenofovir (TFV) and FTC in dried blood spots (DBS) correlate with adherence and protective efficacy among persons receiving TDF/FTC as pre-exposure prophylaxis (PrEP) in clinical trials.10–12 Likewise, hair drug concentrations correlate with cumulative exposure to ARVs.13–17 However, analysis of DBS and hair are often not collected in clinical settings and require specialized and time-consuming mass spectrometry assays generating results that do not reflect recent ARV exposure.18 Urine provides a minimally invasive specimen that could be amenable to development of rapid tests for ARV adherence. Previous studies showed urine TFV concentrations are more predictive of PrEP adherence than plasma and urine FTC concentrations correlated with those found in plasma.19–22 Low urine TFV concentrations were also associated with seroconversion among persons receiving PrEP.23 Although not a rapid test, real-time mass spectrometry analysis of urine TFV has been used to provide a measurement of adherence in some clinical settings.19,20 Therefore, defining urine ARV concentrations reflecting adherence to daily dosing regimens will provide guidance for development of rapid tests measuring adherence.
Previous reports focused on urine TFV as a measure of adherence to TDF/FTC PrEP regimens; thus, data on additional classes of ARVs are lacking. Furthermore, treatment regimens are replacing TDF with tenofovir alafenamide (TAF) to reduce systemic TFV concentrations and unwanted toxicity with continued use, which may affect urine-based measures of TFV. This study analyzed urine and blood drug concentrations among HIV-negative men who have sex with men (MSM) receiving a single dose or daily dosing with TDF/FTC or a currently approved HIV treatment regimen containing TAF and the integrase inhibitor elvitegravir (EVG) to define ARV concentrations indicating adherence to treatment and prevention regimens.
METHODS
Study Design
This study was funded by the US Centers for Disease Control and Prevention (CDC) and approved by Emory University and CDC Institutional Review Boards. This study analyzed specimens collected during a trial registered at clinicaltrials.gov (NCT02985996) and written informed consent was obtained from all study participants. Thirty-four HIV-negative MSM between the ages of 18–49 were enrolled in a nonblinded, randomized 2-arm clinical trial at the Emory Hope Clinic (Atlanta, GA) (February-November 2017) to receive either TDF/FTC or TAF/FTC/COBI/EVG. Participants in each arm were randomized to receive an observed single oral dose of the indicated drug regimen (TDF/FTC n = 10, TAF/FTC/COBI/EVG n = 8) or daily oral dosing for 10 days (TDF/FTC n = 9, TAF/FTC/COBI/EVG n = 7). Urine and peripheral blood specimens were collected at 4 and 24 hours after a single dose, or at 4 and 10 days after initiation of self-administered daily dosing. Daily dosing time points were used to determine whether accumulation of analytes in urine occurs with subsequent dosing. Participants provided adherence to daily dosing through self-report. Blood was collected in sodium citrate cell preparation tubes (Becton Dickinson, Franklin Lakes, NJ) and separated into plasma and peripheral blood mononuclear cell (PBMC) fractions by centrifugation. Urine was collected in sterile specimen containers (Thermo Fisher Scientific, Waltham, MA). One participant receiving TDF/FTC and one receiving TAF/FTC/COBI/EVG provided specimens at 4 hours, but not at 24 hours after a single dose. This study conforms to the US Federal Policy for the Protection of Human Subjects.
Laboratory Measurements
FTC, TFV, and EVG concentrations in urine and plasma were measured using high-performance liquid chromatography-tandem mass spectrometry based on previously published methodology24,25 with a lower limit of quantification for each drug of 10 ng/mL. Drug concentrations were estimated using a standard curve with a range of 0.5–2000 ng/mL using the Analyst software (ABSciex, Foster City, CA). Urine specimens were diluted 1:10 or 1:100 in 0.2% formic acid to obtain values within the standard curve. Intracellular TFV diphosphate (TFV-DP) and FTC triphosphate (FTC-TP) were measured in PBMCs as previously described with a lower limit of quantification of 20 fmol/106 PBMC (TFV-DP) and 100 fmol/106 PBMC (FTC-TP).26 Laboratory staff were blinded to study arm assignments. Urinalysis for protein, blood, leukocytes, nitrite, glucose, ketone, pH, specific gravity, bilirubin, and urobilinogen was performed using the Multistix 10SG urinalysis strip (Siemens Healthcare, Norwood, MA) and read using a CLINITEK analyzer (Siemens). Drug concentrations were compared between study regimens using the Wilcoxon signed-rank test. Correlations between plasma and urine concentrations, or intracellular PBMC and urine concentrations, were determined using the Spearman correlation test. Associations between urine drug concentrations and semiquantitative urinalysis results were examined using analysis of variance on ranks using the Prism 7 software (GraphPad Software, San Diego, CA).
RESULTS
Median urine FTC concentrations at 4 and 24 hours after a single observed dose were consistent among men receiving TDF/FTC (4 hours: 146,875 ng/mL; 24 hours: 10,045 ng/mL) compared with men receiving TAF/FTC/COBI/EVG (4 hours: 333,250 ng/mL, P = 0.173; 24 hours: 12,800 ng/mL, P = 0.681) (Fig. 1). Urine FTC concentrations were significantly lower at 24 hours compared with 4 hours for men receiving both regimens (P < 0.001). Median urine TFV concentrations were reduced more than 8-fold at both 4 and 24 hours among men receiving TAF/FTC/COBI/EVG (4 hours: 1207 ng/mL; 24 hours: 805 ng/mL) compared with men receiving TDF/FTC (4 hours: 17,287 ng/mL, P < 0.001; 24 hours: 6628 ng/mL, P = 0.001) (Fig. 1). Median urine TFV concentrations were reduced at 24 hours compared with 4 hours for men receiving TDF/FTC (P = 0.025), but not for men receiving TAF/FTC/COBI/EVG (P > 0.200). EVG was only detected in 7/15 urine specimens from men receiving a single dose of TAF/FTC/COBI/EVG (Fig. 1).
FIGURE 1.
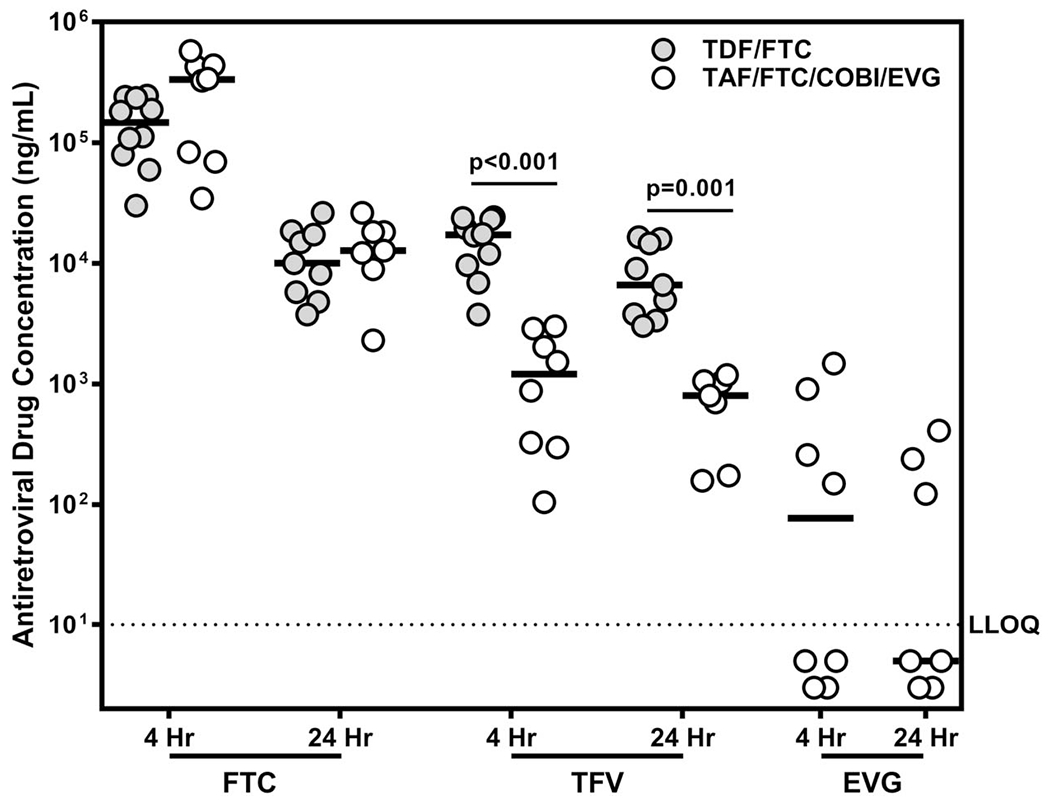
Antiretroviral drug concentrations in urine, 4 and 24 hours after a single oral dose of TDF/FTC or TAF/FTC/COBI/EVG. FTC, TFV, and EVG concentrations were measured in urine collected 4 or 24 hours after a single oral dose of the indicated regimen. Median values are indicated by solid lines. The lower limit of quantification for the assay is indicated by the dotted line (10 ng/ mL). All P-values were determined for median differences between drug regimens by Wilcoxon rank sum test.
Minimum values indicating adherence to daily dosing were calculated as 80% of the lowest urine FTC concentration (1844 ng/mL) observed 24 hours after a single dose to account for 20% assay variability using mass spectrometry methods. TFV values indicating daily dosing adherence were calculated according to the regimen (TDF/FTC: 2424 ng/mL; TAF/FTC/COBI/EVG: 126 ng/mL). Urine collected after 4 and 10 days of daily dosing with TDF/FTC or TAF/FTC/COBI/EVG was evaluated for adherence based on the above values. Urine FTC concentrations from all men receiving daily dosing indicated adherence to FTC-containing regimens (Fig. 2A). Although 15/18 specimens collected from men receiving TDF/FTC contained TFV concentrations indicating adherence according to values determined by a single dose of TDF/FTC, only 2/14 specimens collected from men receiving TAF/FTC/COBI/EVG contained TFV concentrations greater than 2424 ng/mL (Fig. 2B). All urine specimens collected from men receiving daily dosing contained TFV concentrations indicating adherence according to values determined by a single dose of TAF/FTC/COBI/EVG (Fig. 2B). Urine TFV concentrations remained reduced among men receiving TAF/FTC/COBI/EVG compared with men receiving TDF/FTC after 4 days (P = 0.021) and 10 days (P = 0.016) of daily dosing. EVG was only detectable in 8/14 specimens collected from men receiving daily dosing of TAF/FTC/COBI/EVG (Fig. 2).
FIGURE 2.

Antiretroviral drug concentrations in urine after 4 or 10 days of daily oral dosing with TDF/FTC or TAF/FTC/COBI/EVG. Urine concentrations of FTC (A), TFV (B), and EVG (C) are presented for specimens collected after 4 or 10 days of daily oral dosing with TDF/FTC or TAF/FTC/COBI/EVG. Dashed lines indicate values suggesting adherence and were determined as 80% of the lowest antiretroviral drug measurement 24 hours after a single oral dose. The value for FTC was calculated using data from men receiving TDF/FTC or TAF/FTC/CBOI/EVG (α). Values for TFV were calculated using data from men receiving TDF/FTC (β) or TAF/FTC/COBI/EVG (δ). The lower limit of quantification for the assay is indicated by the dotted line (10 ng/mL).
Urine FTC concentrations in specimens from all study participants at all visits were highly correlated with those measured in plasma (r = 0.766, P < 0.0001) (see Figure, Supplemental Digital Content, http://links.lww.com/QAI/B354). Neither urine TFV (r = 0.238, P > 0.15) nor EVG (r = 0.276, P > 0.29) concentrations correlated with corresponding plasma concentrations (see Figure, Supplemental Digital Content, http://links.lww.com/QAI/B354). Urine FTC concentrations correlated weakly with FTC-TP concentrations in PBMCs among men receiving either dosing regimen (r = 0.271, P = 0.029). Urine TFV concentrations correlated with TFV-DP concentrations in PBMCs among men receiving TAF/FTC/COBI/EVG (r = 0.491, P = 0.007), but not among men receiving TDF/FTC (r = −0.022, P > 0.500) (data not shown).
Among men receiving daily dosing, increased urine FTC concentrations were associated with higher urine specific gravity (P = 0.022) among men receiving both regimens (see Figure, Supplemental Digital Content, http://links.lww.com/QAI/B354). TFV concentrations among specimens collected from men receiving TDF/FTC (P = 0.039), but not from men receiving TAF/FTC/COBI/EVG, were associated with specific gravity (P > 0.12) (see Figure, Supplemental Digital Content, http://links.lww.com/QAI/B354). ARV concentrations were not significantly associated with urine total protein, hemoglobin, leukocyte esterase, nitrite ion, glucose, aceto-acetic acid (ketone), pH, bilirubin, or urobilinogen (data not shown).
DISCUSSION
Development of rapid minimally invasive tests for adherence to daily dosing with ARV regimens could allow health care workers to assess adherence, thus providing opportunities to improve adherence through immediate behavioral interventions. In this study, we assessed urine ARV concentrations after a single dose and daily dosing with the currently approved PrEP regimen (TDF/FTC) or an approved HIV treatment regimen (TAF/FTC/COBI/EVG). EVG was not consistently detectable in urine of men receiving EVG, which is unsurprising, as EVG is not cleared primarily through the kidneys.27 However, FTC and TFV are routinely measured at high concentrations in urine from men receiving FTC in combination with either TDF or TAF, suggesting they provide potential markers of adherence and are good targets for development of rapid assays for adherence.
Urine FTC concentrations were routinely measured at μg/mL concentrations suggesting assays detecting FTC in urine may not need to be extremely sensitive to provide valuable information. Although urine FTC concentrations declined substantially from 4 to 24 hours after a single dose, concentrations among men receiving daily dosing were consistent with adherence. In addition, urine and plasma FTC concentrations were highly correlated in this study and a previous one suggesting urine FTC could provide a surrogate measure for plasma FTC concentrations and may result from the combination of a short plasma half-life and a rapid clearance through urine.28 Together, these results suggest FTC may be amenable to development of an assay to measure urine that accurately reflects recent dosing.
Urine TFV has been shown to provide a potential marker for adherence among individuals receiving TDF/FTC.19–21 In the results presented here, urine TFV concentrations were consistently lower among men receiving TAF compared with men receiving TDF, which could be expected with TAF producing lower concentrations of TFV in plasma.29–31 Urine TFV concentrations among men receiving daily TDF/FTC were not consistently above the lowest values observed 24 hours after a single dose in this study. However, urine TFV concentrations among all men receiving TDF/FTC were above 1000 ng/mL, a value reported to indicate dosing within the previous 48–72 hours.19 Urine TFV concentrations observed here were greater than 1000 ng/mL in only 16/29 specimens collected from participants receiving TAF/FTC/COBI/EVG, suggesting further studies to define TFV concentrations representing recent dosing for persons receiving TAF-based regimens.
This study indicates urine FTC and TFV concentrations are amenable to rapid test development, yet it also has several limitations. This study included a small number of participants and larger studies are likely to provide more refined urine drug concentrations that reflect adherence. This study was performed in HIV-negative MSM, so it is unclear whether these results can be extended to women or to HIV-positive persons on treatment regimens. We evaluated urine drug concentrations for TDF/FTC and TAF/FTC/COBI/EVG; therefore, direct comparisons between urine TFV concentrations among men receiving TDF and TAF may be affected by the presence of the booster COBI. Future studies comparing TAF/FTC to TDF/FTC will be able to determine the difference in urine drug concentrations of TFV with newer TAF-containing regimens. Our observation of associations between urine FTC and TFV concentrations and urine specific gravity suggests additional biological factors, such as hydration, may influence urine drug concentrations. However, specific gravity did not seem to affect the ability of urine drug concentrations to predict recent dosing in a qualitative or semiquantitative manner. High urine drug concentrations after a single dose and the lack of drug accumulation after repeat dosing observed here suggest it will be difficult to use urine to measure cumulative exposure to ARVs and detect “white coat dosing” among individuals (ie, taking medication only before medical appointments). In addition, as daily dosing was not observed in this study, it is possible that persons did not take all doses and we underestimated accumulation of urine drug concentrations. ARV measures from DBS or hair10,13,14 will likely provide a better measure of cumulative drug exposure and studies such as the TARGET study will provide valuable information regarding the limitations of different minimally invasive methods to measure ARV adherence.32 Comparison of urine drug concentrations to more established measures of adherence, such as TFV-DP in DBS or TFV in hair, will provide information regarding the ability of urine to predict efficacy.
Urine provides a minimally invasive specimen type amenable to development of rapid assays for FTC and TFV that assess adherence to oral ARV regimens. Development of competitive immunoassays and lateral flow assays for TFV as well as aptamer sensing technologies to detect small molecules suggest these methods could be used to provide qualitative or semiquantitative assays of urine ARVs to evaluate recent exposure to ARVs.33–36 The combination of urine as a minimally invasive specimen with these new methods could allow health care workers to rapidly assess recent adherence to ARV regimens and provide appropriate behavioral interventions to improve individual adherence.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the study participants for their time and commitment to this study as well as Helen Koenig, Linden Lalley-Chareczko, and Walid Heneine for helpful discussions.
Supported by the United States Centers for Disease Control and Prevention.
Footnotes
Presented at the 2019 Conference on Retroviruses and Opportunistic Infections (CROI); March 4–7, 2019; Seattle, WA.
The authors have no conflicts of interest to disclose.
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the United States Centers for Disease Control and Prevention or the Department of Health and Human Sendees.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
REFERENCES
- 1.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010; 363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haberer JE, Bangsberg DR, Baeten JM, et al. Defining success with HIV pre-exposure prophylaxis: a prevention-effective adherence paradigm. AIDS. 2015;29:1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376:49–62. [DOI] [PubMed] [Google Scholar]
- 7.Fogarty L, Roter D, Larson S, et al. Patient adherence to HIV medication regimens: a review of published and abstract reports. Patient Educ Couns. 2002;46:93–108. [DOI] [PubMed] [Google Scholar]
- 8.Haberer JE, Kiwanuka J, Nansera D, et al. Multiple measures reveal antiretroviral adherence successes and challenges in HIV-infected Ugandan children. PLoS One. 2012;7:e36737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kagee A, Nel A. Assessing the association between self-report items for HIV pill adherence and biological measures. AIDS Care. 2012;24:1448–1452. [DOI] [PubMed] [Google Scholar]
- 10.Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng JH, Guida LA, Rower C, et al. Quantitation of tenofovir and emtricitabine in dried blood spots (DBS) with LC-MS/MS. J Pharm Biomed Anal. 2014;88:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo-Mancilla JR, Searls K, Caraway P, et al. Short communication: tenofovir diphosphate in dried blood spots as an objective measure of adherence in HIV-infected women. AIDS Res Hum Retroviruses. 2015; 31:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baxi SM, Liu A, Bacchetti P, et al. Comparing the novel method of assessing PrEP adherence/exposure using hair samples to other pharmacologic and traditional measures. J Acquir Immune Defic Syndr. 2015;68:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi M, Glidden DV, Liu A, et al. Strong correlation between concentrations of tenofovir (TFV) emtricitabine (FTC) in hair and TFV diphosphate and FTC triphosphate in dried blood spots in the iPrEx open label extension: implications for pre-exposure prophylaxis adherence monitoring. J Infect Dis. 2015;212:1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koss CA, Bacchetti P, Hillier SL, et al. Differences in cumulative exposure and adherence to tenofovir in the VOICE, iPrEx OLE, and PrEP demo studies as determined via hair concentrations. AIDS Res Hum Retroviruses. 2017;33:778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koss CA, Hosek SG, Bacchetti P, et al. Comparison of measures of adherence to human immunodeficiency virus preexposure prophylaxis among adolescent and young men who have sex with men in the United States. Clin Infect Dis. 2018;66:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koss CA, Natureeba P, Mwesigwa J, et al. Hair concentrations of antiretrovirals predict viral suppression in HIV-infected pregnant and breastfeeding Ugandan women. AIDS. 2015;29:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garrison LE, Haberer JE. Technological methods to measure adherence to antiretroviral therapy and preexposure prophylaxis. Curr Opin HIV AIDS. 2017;12:467–74. [DOI] [PubMed] [Google Scholar]
- 19.Koenig HC, Mounzer K, Daughtridge GW, et al. Urine assay for tenofovir to monitor adherence in real time to tenofovir disoproxil fumarate/emtricitabine as pre-exposure prophylaxis. HIV Med. 2017;18:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalley-Chareczko L, Clark D, Conyngham C, et al. Delivery of TDF/FTC for pre-exposure prophylaxis to prevent HIV-1 acquisition in young adult men who have sex with men and transgender women of color using a urine adherence assay. J Acquir Immune Defic Syndr. 2018;79:173–178. [DOI] [PubMed] [Google Scholar]
- 21.Lalley-Chareczko L, Clark D, Zuppa AF, et al. A case study of chewed Truvada(R) for PrEP maintaining protective drug levels as measured by a novel urine tenofovir assay. Antivir Ther. 2017;22:639–641. [DOI] [PubMed] [Google Scholar]
- 22.Haaland RE, Martin A, Holder A, et al. Urine tenofovir and emtricitabine concentrations provide biomarker for exposure to HIV preexposure prophylaxis. AIDS. 2017;31:1647–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinelli MA, Glidden DV, Rodrigues WC, et al. Low tenofovir level in urine by a novel immunoassay is associated with seroconversion in a preexposure prophylaxis demonstration project. AIDS. 2019;33:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuklenyik Z, Martin A, Pau CP, et al. Effect of mobile phase pH and organic content on LC-MS analysis of nucleoside and nucleotide HIV reverse transcriptase inhibitors. J Chromatogr Sci. 2009;47:365–372. [DOI] [PubMed] [Google Scholar]
- 25.Aouri M, Calmy A, Hirschel B, et al. A validated assay by liquid chromatography-tandem mass spectrometry for the simultaneous quantification of elvitegravir and rilpivirine in HIV positive patients. J Mass Spectrom. 2013;48:616–625. [DOI] [PubMed] [Google Scholar]
- 26.Kuklenyik Z, Martin A, Pau CP, et al. On-line coupling of anion exchange and ion-pair chromatography for measurement of intracellular triphosphate metabolites of reverse transcriptase inhibitors. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3659–3666. [DOI] [PubMed] [Google Scholar]
- 27.Ramanathan S, Mathias AA, German P, et al. Clinical pharmacokinetic and pharmacodynamic profile of the HIV integrase inhibitor elvitegravir. Clin Pharmacokinet. 2011;50:229–244. [DOI] [PubMed] [Google Scholar]
- 28.TRUVADA (Emtricitabine/Tenofovir Disoproxil Fumarate) Tablets, for Oral Use, Package Insert. Foster City, CA: Gilead Sciences Inc; 2016. [Google Scholar]
- 29.Sax PE, Zolopa A, Brar I, et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. 2014;67:52–58. [DOI] [PubMed] [Google Scholar]
- 30.Markowitz M, Zolopa A, Squires K, et al. Phase I/II study of the pharmacokinetics, safety and antiretroviral activity of tenofovir alafenamide, a new prodrug of the HIV reverse transcriptase inhibitor tenofovir, in HIV-infected adults. J Antimicrob Chemother. 2014;69:1362–1369. [DOI] [PubMed] [Google Scholar]
- 31.Ruane PJ, DeJesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63:449–55. [DOI] [PubMed] [Google Scholar]
- 32.Cressey TR, Siriprakaisil O, Klinbuayaem V, et al. A randomized clinical pharmacokinetic trial of Tenofovir in blood, plasma and urine in adults with perfect, moderate and low PrEP adherence: the TARGET study. BMC Infect Dis. 2017;17:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aliakbarinodehi N, Jolly P, Bhalla N, et al. Aptamer-based field-effect biosensor for tenofovir detection. Sci Rep. 2017;7:44409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Özalp VC, Çam D, Hernandez FJ, et al. Small molecule detection by lateral flow strips via aptamer-gated silica nanoprobes. Analyst. 2016; 141:2595–2599. [DOI] [PubMed] [Google Scholar]
- 35.Pratt GW, Fan A, Melakeberhan B, et al. A competitive lateral flow assay for the detection of tenofovir. Anal Chim Acta. 2018;1017:34–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gandhi M, Bacchetti P, Spinelli IM, et al. Validation of a urine tenofovir immunoassay for adherence monitoring to PrEP and ART and establishing the cut-off for a point-of-care test. J Acquir Immune Defic Syndr. 2019;81:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


