Abstract
The use of thermography for the identification of cutaneous “hot spots” that coincide with perforators is not a new concept, but the required professional cameras may be prohibitively expensive. Only relatively recently, incredibly cheap but adequate thermal imaging cameras have become available that work in concert with the ubiquitous cell phone. This can now serve as a rapid, accurate, and complementary method for finding a perforator sufficient to serve as the hub for a perforator pedicled propeller flap. In addition, the preferred direction of rotation about that hub, effect of flap insetting on perfusion, and then postoperative monitoring are possible by proper interpretation of corresponding thermograms. Every reconstructive surgeon should be able to obtain this device, and then easily learn what potential attributes for them are available when planning a propeller flap.
Keywords: smartphone, thermography, thermal imaging camera, perforator pedicled propeller flap
The principal mechanism for maintenance of body temperature in a state of equilibrium relies on radiative heat loss from the skin to the environment. 1 This is perceived as infrared radiation, whose wavelength (700–1 mm) is found in the nonvisible range of the electromagnetic spectrum. 2 Increments of emitted infrared radiation are determined by variations of the skin temperature. 2 3 Since blood is the medium for heat transport throughout the body, skin temperature in turn will be directly related to any variations of local blood flow or perfusion. 1 4 Thus, thermal imaging cameras can assess skin temperature based on the quantity of infrared radiation observed. A thermogram so obtained will detect “hot spots” on the skin surface that correspond to zones of greater heat expelled by a dominant perforator, and over time depict the extent of the associated surrounding vascular network currently called the “perforasome.” 1 2 3 4 5 6 Theuvenet et al 7 some 30 years ago proved that thermography could be accurate for identifying perforator arteries of both fasciocutaneous and musculocutaneous flaps! Thermal images have since been found to quite accurately complement other preoperative studies for perforator identification such as computed tomography (CT) angiography 3 or auditory 6 and flow Doppler ultrasound. 5
Advances in technology have now made available incredibly inexpensive miniature thermal imaging cameras (FLIR ONE Pro [FLIR Systems, Inc., Wilsonville, OR], FLIR.com/FLIRONE/Start) that may be attached to the charging plug of any type of smartphone. Real-time thermogram still images or videos can rapidly be digitally merged with a visible light camera photograph on the smartphone using an app the manufacturer provides. 8 Hardwicke et al 8 have used this capability to make preoperative, intraoperative, and postoperative thermograms that identify the requisite perforator to assist the design of the appropriate flap, assess flap viability during the surgery itself, and provide a means for eventual monitoring of continued flap perfusion. Now anyone with a smartphone has the ability to use the concept of thermal imaging to more safely harvest and transfer a propeller flap.
Methodology
Thermal Challenge
The smartphone application system has a lower resolution image and narrower temperature detection range than the vastly more expensive professional camera. 9 10 Therefore, dynamic infrared thermography (DIRT) must be a preferable preoperative adjunct whereby first a “cold challenge” of the potential donor site must be invoked. 1 2 4 11 12 Muntean et al's method to create this thermal stress is to spray isopropyl alcohol on the skin, while simultaneously accelerating evaporation with a high-speed portable fan. 2 A simpler way is to soak towels in ice water that are then laid directly upon the donor site. As a bedside test, either option may be completed in minutes to then allow serial observation of rewarming as “hot spots” emerge. Each spot is marked, and provides a valuable guide for further perforator identification using the ubiquitous audible Doppler probe if warranted. The perforator pedicled propeller flap is designed appropriately about the suspected perforator to be found at the “hot spot.”
Operative
An exploratory incision is always necessary to confirm the exact site of the perforator hub, and the flap redesigned as necessary. 13 Upon complete elevation of the flap in the customary fashion, a thermal image will show any limitations of the extent of perfusion. This should be compared in contrast to the flap when turned in a clockwise or counterclockwise direction about its hub to determine the preferred direction of axial rotation of the flap ( Fig. 1 ). Once chosen, the flap is inset again with the evaluation of perfusion using a thermogram to determine if there is excessive suture tension, pedicle twisting, or other compromise that must be overcome, including the need to consider a flap delay ( Fig. 2 ). 14
Fig. 1.
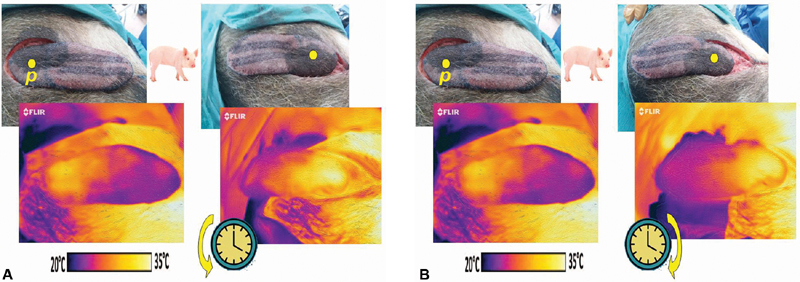
( A ) Pig model propeller flap pedicled on intercostal perforator “ p ,” with corresponding in situ thermogram below (left), flap appearance after counterclockwise rotation, with thermogram below predicting superior perfusion (right). ( B ) Same pig model propeller flap (left), with instead clockwise rotation, with thermogram below that had similar darker colored surface area implying that a counterclockwise rotation would be preferable (right).
Fig. 2.
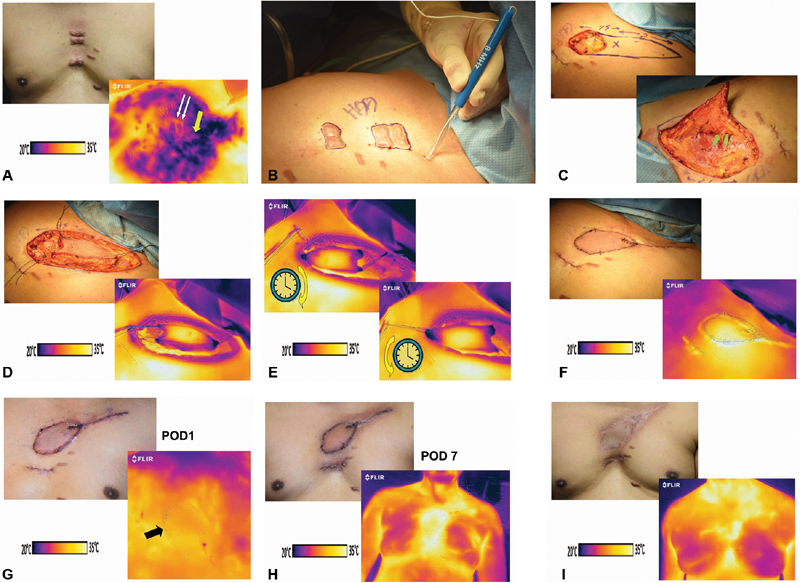
( A ) Painful midsternal keloids for planned excision (above) with “cold challenge” encompassing left second intercostal space that revealed a “hot spot” (yellow arrow) between left 2nd and 3rd ribs (white arrows show location of keloids; below). ( B ) Audible Doppler-confirmed presence of perforator at the marked “hot spot.” ( C ) Propeller flap designed using the identified perforator as hub (above), then precisely identified on green microgrid via exploratory incision (below). ( D ) Propeller flap in situ (above) and corresponding thermogram show dark color only at tips on either end, suggesting limited diminished perfusion (below). ( E ) Comparison of thermograms after clockwise rotation (above) and counterclockwise (below). The overall appearance revealed perhaps slightly larger area of darker colors after the clockwise rotation, so a counterclockwise rotation was performed. ( F ) Flap upon insetting had a slightly violaceous hue on clinical examination, suggesting venous congestion (above), but the thermogram had brilliant heterogenous colors throughout implying adequate perfusion at this time (below). ( G ) Minimal resolution of skin color on first postoperative day (above) with thermogram not quite as brilliant perhaps, but adequate (arrow = medial border of flap). ( H ) Skin color reflected a significant radiation effect at postoperative day 7 (above), yet with excellent sign of total flap perfusion on thermogram (below). ( I ) Satisfactory surgical defect coverage with left 2nd intercostal internal mammary artery perforator pedicled propeller flap at 3 months (above), and adequacy of perfusion of flap in midsternal region confirmed as expected by thermogram (below).
Follow-up
As with any similar microsurgical flap transfer, 15 monitoring for untoward sequela is advantageous, for as long as considered necessary ( Fig. 2 ). A smartphone series of thermal images over time can each be rapidly obtained even by nursing staff with their cell phone, and then transmitted to the responsible practitioner for proper comparison of events and their evaluation.
Discussion
The use of a thermal imaging camera attached to a smartphone has become a cheap option now available to any reconstructive surgeon who desires to use the concept of thermography, with virtually no learning curve. 3 8 Pereira et al, 3 at least in a preoperative concordance study for free flaps, compared detection of perforators using a smartphone with CT angiography and had an accuracy with a sensitivity of 100% and specificity of 98%. However, experience with thermograms for local perforator flaps and propeller flaps in particular is limited, although our small experience has shown that the same accuracy should be expected for determining the hub of a propeller flap. 16
With the FLIR system (FLIR ONE Pro [FLIR Systems, Inc.], FLIR.com/FLIRONE/Start), during the “cold challenge” phase darker colors will be observed. During rewarming, “hot spots” usually coinciding with arterial perforators appear on the color palette of the device as a white or yellow hue, whereas orange or slightly darker colors may be equivocal. A similar bright appearance within the harvested flap itself consistently implies adequate perfusion. The presence of venous congestion in a propeller flap can be a common and indeterminate sequela that according to Chaput et al 14 can vary from individual to individual even over 48 to 72 hours after flap elevation due to variations in their “venous perforasome.” The thermal image of venous congestion, since there is usually some persistent arterial inflow, will show a diffusely homogeneous thermogram, in comparison to a normal perfusion pattern that should always have color variations representing the expected subtle differences in flow between captured vascular networks, but, of course, this real-time picture cannot be expected to predict future events.
As Wong et al 17 have determined in simulation models, the angle of twist of the propeller flap is a major factor for affected perforator patency, with venous occlusion more likely to occur than arterial compromise. Schonauer et al 18 advocated clinical evaluation after the twist for assessing the preference for clockwise versus counterclockwise rotation. For darker pigmented individuals, thermography would be a better option as color assessment alone may be difficult. Song et al 19 have used color Duplex ultrasound to obtain physiologic flow data on pedicle flow and volume following the rotation. They concluded that if the direction with higher values is always chosen, no major complications will occur, whereas if based solely on clinical signs in their experience, total flap loss or partial necrosis was a risk that can now be avoided. The use of indocyanine green angiography could be another alternative, but a far simpler and more available option now would be to compare thermograms after each twist and then chose the manipulation that appears to have better perfusion ( Fig. 1 ).
Conclusion
The relative value and therefore ultimate role of smartphone thermography for assisting in the identification of an adequate perforator hub to allow the appropriate design of a perforator pedicled propeller flap, selection of the proper direction for axial rotation, and finally ensuring the adequacy of proper insetting and monitoring has yet to be fully recognized. Yet, this indeed is a cheap, rapid, and objective device with no side effects that should be further investigated to prove how valuable it can be to allow the safer use of the versatile propeller flap.
Acknowledgments
The author thanks David C. Rice, BS, PE, St. Luke's Hospital–Sacred Heart Division, Allentown, PA, for assistance with thermograms and flap harvest.
Footnotes
Conflict of Interest None.
References
- 1.de Weerd L, Mercer J B, Weum S. Dynamic infrared thermography. Clin Plast Surg. 2011;38(02):277–292. doi: 10.1016/j.cps.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Muntean M V, Strilciuc S, Ardelean F, Georgescu A V. Dynamic infrared mapping of cutaneous perforators. J Xiangya Med. 2018;3:16. doi: 10.11152/mu.2013.2066.174.dyn. [DOI] [PubMed] [Google Scholar]
- 3.Pereira N, Valenzuela D, Mangelsdorff G, Kufeke M, Roa R. Detection of perforators for free flap planning using smartphone thermal imaging: a concordance study with computed tomographic angiography in 120 perforators. Plast Reconstr Surg. 2018;141(03):787–792. doi: 10.1097/PRS.0000000000004126. [DOI] [PubMed] [Google Scholar]
- 4.de Weerd L, Weum S, Mercer J B. The value of dynamic infrared thermography (DIRT) in perforator selection and planning of free DIEP flaps. Ann Plast Surg. 2009;63(03):274–279. doi: 10.1097/SAP.0b013e3181b597d8. [DOI] [PubMed] [Google Scholar]
- 5.Tenorio X, Mahajan A L, Elias B. Locating perforator vessels by dynamic infrared imaging and flow Doppler with no thermal cold challenge. Ann Plast Surg. 2011;67(02):143–146. doi: 10.1097/SAP.0b013e3181ef6da3. [DOI] [PubMed] [Google Scholar]
- 6.Sheena Y, Jennison T, Hardwicke J T, Titley O G. Detection of perforators using thermal imaging. Plast Reconstr Surg. 2013;132(06):1603–1610. doi: 10.1097/PRS.0b013e3182a80740. [DOI] [PubMed] [Google Scholar]
- 7.Theuvenet W J, Koeyers G F, Borghouts M H. Thermographic assessment of perforating arteries. A preoperative screening method for fasciocutaneous and musculocutaneous flaps. Scand J Plast Reconstr Surg. 1986;20(01):25–29. doi: 10.3109/02844318609006287. [DOI] [PubMed] [Google Scholar]
- 8.Hardwicke J T, Osmani O, Skillman J M. Detection of perforators using smartphone thermal imaging. Plast Reconstr Surg. 2016;137(01):39–41. doi: 10.1097/PRS.0000000000001849. [DOI] [PubMed] [Google Scholar]
- 9.Muntean M V, Achimas-Cadariu P A. Detection of perforators for free flap planning using smartphone thermal imaging: a concordance study with computed tomographic angiography in 120 perforators. Plast Reconstr Surg. 2018;142(04):604e. doi: 10.1097/PRS.0000000000004751. [DOI] [PubMed] [Google Scholar]
- 10.Pereira N. Reply: detection of perforators for free flap planning using smartphone thermal imaging: a concordance study with computed tomographic angiography in 120 perforators. Plast Reconstr Surg. 2018;142(04):605e. doi: 10.1097/PRS.0000000000004752. [DOI] [PubMed] [Google Scholar]
- 11.de Weerd L, Mercer J B, Setså L B. Intraoperative dynamic infrared thermography and free-flap surgery. Ann Plast Surg. 2006;57(03):279–284. doi: 10.1097/01.sap.0000218579.17185.c9. [DOI] [PubMed] [Google Scholar]
- 12.Itoh Y, Arai K. Use of recovery-enhanced thermography to localize cutaneous perforators. Ann Plast Surg. 1995;34(05):507–511. doi: 10.1097/00000637-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Pignatti M, Pinto V, Dokerty Skogh A C, Giorgini F A, Cipriani R, De Santis G, Hallock G G. How to design and harvest a propeller flap. Semin Plast Surg. 2020;34(03):152–160. doi: 10.1055/s-0040-1714271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaput B, Grolleau J L, Garrido I. Delayed procedure in propeller perforator flap: defining the venous perforasome. J Plast Reconstr Aesthet Surg. 2017;70(02):286–289. doi: 10.1016/j.bjps.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Georgescu A V, Matei I, Ardelean F, Capota I. Microsurgical nonmicrovascular flaps in forearm and hand reconstruction. Microsurgery. 2007;27(05):384–394. doi: 10.1002/micr.20376. [DOI] [PubMed] [Google Scholar]
- 16.Pereira N, Hallock G G.Smartphone thermography for lower extremity local flap perforator mapping J Reconstr Microsurg 2020 10.1055/s-0039-3402032(ePub ahead of print) [DOI] [PubMed] [Google Scholar]
- 17.Wong C H, Cui F, Tan B K. Nonlinear finite element simulations to elucidate the determinants of perforator patency in propeller flaps. Ann Plast Surg. 2007;59(06):672–678. doi: 10.1097/SAP.0b013e31803df4e9. [DOI] [PubMed] [Google Scholar]
- 18.Schonauer F, La Rusca I, Di Monta G, Molea G. Choosing the correct sense of rotation in 180° propeller flaps. J Plast Reconstr Aesthet Surg. 2008;61(12):1492. doi: 10.1016/j.bjps.2008.04.073. [DOI] [PubMed] [Google Scholar]
- 19.Song S, Jeong H H, Lee Y. Direction of flap rotation in propeller flaps: Does it really matter? J Reconstr Microsurg. 2019;35(08):549–556. doi: 10.1055/s-0039-1688408. [DOI] [PubMed] [Google Scholar]


