Abstract
Brachytherapy BT), using low-dose-rate (LDR) permanent seed implantation or high-dose-rate (HDR) temporary source implantation, is an acceptable treatment option for select patients with prostate cancer of any risk group. The benefits of HDR-BT over LDR-BT include the ability to use the same source for other cancers, lower operator dependence, and — typically — fewer acute irritative symptoms. By contrast, the benefits of LDR-BT include more favourable scheduling logistics, lower initial capital equipment costs, no need for a shielded room, completion in a single implant, and more robust data from clinical trials. Prospective reports comparing HDR-BT and LDR-BT to each other or other treatment options (such as external beam radiotherapy (EBRT) or surgery) suggest similar outcomes (evidence level 1). The 5-year freedom from biochemical failure rates for patients with low-risk, intermediate-risk, and high-risk disease are >85%, 69–97%, and 63–80%, respectively. Brachytherapy with EBRT (versus brachytherapy alone) is an appropriate approach in select patients with intermediate-risk and high-risk disease (evidence level 1). The 10-year rates of overall survival, distant metastasis, and cancer-specific mortality are >85%, <10%, and <5%, respectively. Grade 3–4 toxicities associated with HDR-BT and LDR-BT are rare, at <4% in most series, and quality of life is improved in patients who receive brachytherapy, compared with those who undergo surgery (evidence level 1).
Prostate cancer is one of the most frequently diagnosed cancers diagnosed in the developed world, with 1.4 million incident cases and 293,000 deaths in 2013.1 Local tumour control is associated with improved outcomes in patients with organ-confined (T1 or T2) prostate cancer, even in the presence of high-risk features, which include PSA >20 ng/ml and Gleason score 8–10.2 Treatment options for nonmetastatic prostate cancer typically include active surveillance (AS), radical prostatectomy and radiotherapy.3 Radiotherapy can be administered in the form of external beam radiotherapy (EBRT) using various fractionation options, and brachytherapy (BT), either high-dose-rate (HDR-BT) or low-dose-rate (LDR-BT), given alone or combined with EBRT.4 The Prostate Testing for Cancer and Treatment (ProtecT) trial suggests that radiotherapy offers similar outcomes and improved toxicity and quality of life over surgery.5-7
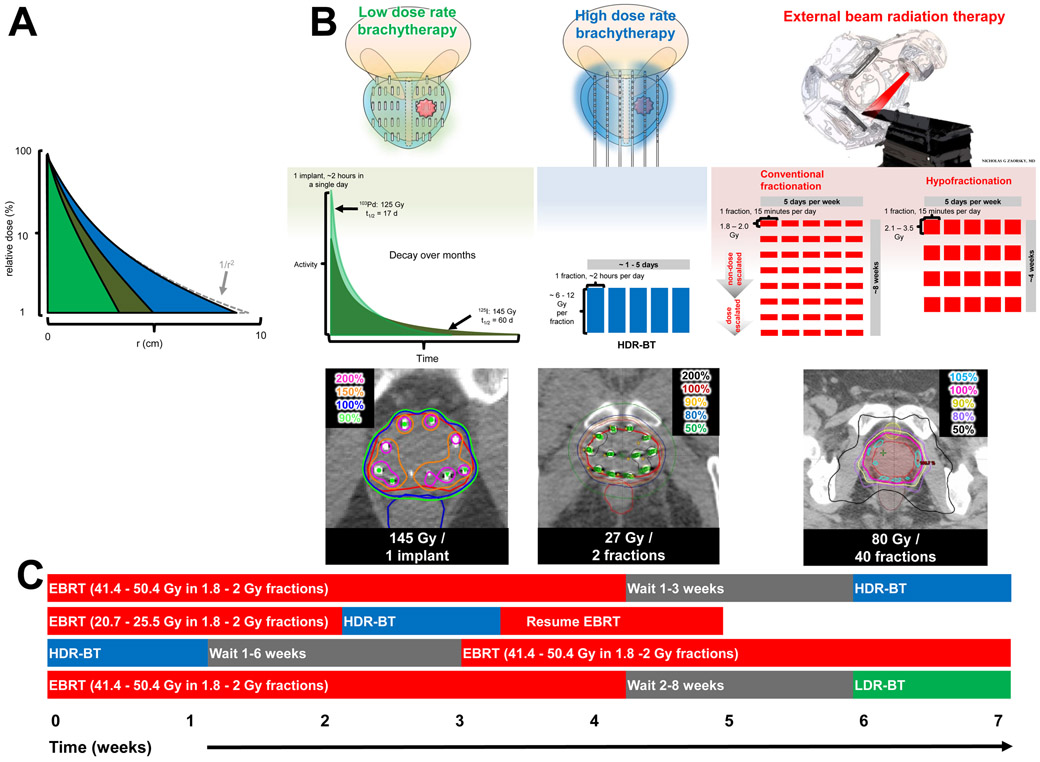
Brachytherapy is an excellent treatment option for patients of all disease groups, according to the American Brachytherapy Society (ABS) guidelines,8, 9 the Groupe Européen de Curiethérapie / European Society for Radiation Oncology (GEC/ESTRO), and the European Society for Radiation Oncology / European Urologic Association / European Organisation for Research and Treatment of Cancer (ESTRO/EAU/EORTC) guidelines.10, 11 Specifically, LDR-BT is defined as ≤2 Gy/h, and consists of the permanent implantation of sealed sources (seeds) in the prostate.8 HDR-BT is defined as ≥12 Gy/h and consists of insertion of a temporary source into a target volume (which contains cancer cells) via a remote afterloader using catheters implanted in the prostate and computer optimization to optimize dose distribution.9 The dose fall-off of both LDR- and HDR-BT is rapid, with < 10% of dose delivered to tissue > 4 cm away from the source (FIG 1A). LDR-BT and HDR-BT can be used as monotherapies for some patients with low-risk disease. Furthermore, EBRT combined with brachytherapy, also known as ‘LDR-BT boost’ or ‘HDR-BT boost,’ is hypothesized to further improve local control compared with monotherapy, and to improve outcomes in certain patients with intermediate-risk or high-risk disease (FIG 1B). Various boost schedules are used (FIG 1C): in HDR-BT boost, the implantation can be performed after EBRT, interdigitated with EBRT, or before EBRT; by contrast, in LDR-BT boost, EBRT is usually delivered before the implant is inserted.4
FIG 1 ∣. Comparison of LDR-BT, HDR-BT, and EBRT.
a∣ The dose distribution in water as a function of distance from a point source of three isotopes commonly used for prostate brachytherapy: LDR-BT with 103Pd (light green), LDR-BT with 125I (dark green), and HDR-BT with 192Ir (blue). The dose has been normalized to 100% at a distance of 1.0 cm (note log scale). The high energy 192Ir departs slightly from the inverse square law (1/r2), whereas the lower energy isotopes of LDR-BT have more absorption over a lower range. b∣ Comparison of LDR-BT, HDR-BT, and conventional or hypofractionated EBRT. The doses (with respect to the patient) are shown in colour (top panel). The dose fractionation of LDR-BT with decay using 103Pd (light green) or 125I (dark green) compared with HDR-BT (shown as blue fractions); and EBRT (red fractions) are shown in the middle panel. Notably, brachytherapy requires only 1–5 insertions compared with up to 40 fractions of EBRT. The isodose distributions (lower panel) of brachytherapy are superior to that of EBRT, as X-rays do not pass through the skin.
c∣ Brachytherapy boost is defined as the combination of EBRT with HDR-BT or LDR-BT. If EBRT is delivered first, HDR-BT is typically delivered 1–6 weeks later. A possible benefit of this method is to use the HDR-BT to account for suboptimal dosimetry of EBRT. Alternatively, EBRT can be interdigitated with HDR-BT. Finally, HDR-BT can be delivered first and EBRT delivered 1–3 weeks later.
This Review considers the evolution of brachytherapy from its inception to contemporary practice, including its historical background and the current indications and contraindications of brachytherapy use compared with EBRT and surgery. The underlying radiophysics and technical aspects, including dosimetric quality constraints, radiobiology, cost, clinical outcomes and toxicities, and appropriate follow-up monitoring will also be discussed.
Historical background
The history of LDR-BT
Radioactivity was accidentally discovered by Henri Becquerel in 1896.12 Radium was discovered by Marie and Pierre Curie just 3 years later in 1899, and was used for the treatment of malignant disease in 1901 — just 5 years after its discovery.13 The use of prostatic brachytherapy was first reported in 1911, when radium was administered temporarily via a urethral catheter.14, 15 In 1917, transperineal implantation of radium was performed in New York.16 Several cohorts of patients were treated with radium brachytherapy in the USA during the 1920s; however, this approach was not favoured because prostate cancer was believed to be a relatively radioresistant cancer and it was thought that local control could not be obtained without significant toxicity.17
At Iowa State University in the 1950s, LDR-BT was performed by injecting 198Au colloid solution intratumorally via open and closed approaches.18 In this series, the 5-year survival was 48%, which was similar to other reported techniques at the time.18, 19 Despite the encouraging results, brachytherapy was still not accepted for several reasons. Firstly, reports of experience with EBRT began to be published in the 1960s, and this technique did not require anaesthesia and also was also associated with promising results and minimal morbidity.20 Furthermore, 198Au is a relatively high-energy isotope and, therefore, more challenging radiation safety precautions were necessary.
In the 1970s, Dr. Willet F. Whitmore and colleagues at Memorial Sloan–Kettering Cancer Center began to use 125I seeds for prostate cancer implants via an open surgical procedure that included a bilateral pelvic lymphadenectomy.21, 22 This 125I technique was associated with several advantages over existing methods for EBRT and brachytherapy.14 Firstly, the open procedure permitted direct assessment of both the prostate and the lymph nodes. Secondly, the open approach permitted the precise placement of radioactive material, thus increasing the total tumour dose while minimizing exposure to the rectum and bladder. Thirdly, staging and implantation were performed at the same time. Notably, open procedures and intraprocedure staging are no longer performed in contemporary practice; by contrast, novel anatomical and functional imaging methods (including CT, MRI, and PET) have replaced invasive staging23 and implantations are now performed via a transperineal approach.
Finally, 125I has several benefits in comparison to 198Au, which had been used in previous decades.14 125I has a lower energy, requiring less challenging radioprotection and providing improved dosimetry. 125I has a low half-dose volume — that is, the volume of tissue receiving 50% of the minimum tumour dose — compared with 198Au: 2 cm versus 6 cm.24 125I also has a relatively long half-life of 60 days, providing extended periods of radiation to the target tissue. This extended duration of radiation was hypothesized to be radiobiologically favourable because of the long doubling time of prostate cancer cells, and because it would allow for the repair of normal tissues, thereby minimizing acute genitourinary toxicities.21
In the 1970s and 1980s, patients who were selected for 125I LDR-BT were anaesthetized, placed in a modified lithotomy position, and an infraumbiliical incision was made to perform the implantation.24-27 A bilateral pelvic lymph node dissection was performed, and the prostate was exposed. The prostate gland was mobilized by incising the endopelvic fascia bilaterally. Caliper measurements were used to assess the prostate dimensions: a needle was directed through the gland in the anteroposterior direction using a needle, with a finger placed in the rectum to prevent rectal perforation. Next, a nomogram was used to calculate the 3D volume. A computer was used to derive the matched peripheral dose, which was the absorbed dose at the periphery of an ellipsoid with the same dimensions. Doses at the centre of the gland were typically much higher than those at the periphery. Given the morbidity of incision, the limits of assessing prostate dimensions, and poor dose distribution and low total dose of 125I, clinical outcomes were poor in many patients.28
The application of transrectal ultrasonography (TRUS) to guide LDR-BT was successfully introduced in the 1980s.29 TRUS -guided LDR-BT was first developed in Denmark in the late 1970s, but with disappointing early clinical outcomes, likely related to suboptimal seed placement and dosimetry.30, 31 The lack of uniform dosimetry and the use of combination EBRT resulted in rectal ulcers.30-32
As techniques improved, 125I and 103Pd became the most commonly used isotopes in nonrandomized trials through the late 1980s.33 TRUS-guided LDR-BT became a standard technique in the USA and elsewhere by the late 1990s because of improved associated outcomes and use of a less-invasive procedure compared with laparotomy-based suprapubic approaches.34,35 In the USA, LDR-BT was subsequently endorsed for treatment of low-risk prostate cancer by numerous organizations, including the ABS and the American Society for Radiation Oncology (ASTRO).36
Although TRUS-guided LDR-BT is still a standard-of-care treatment option for men with prostate cancer, potential technical limitations of LDR-BT exist. Firstly, loose LDR-BT seeds — particularly those that are not held by a strand (termed ‘stranded’) — can migrate.37 Secondly, permanent implantation of radioactive material in the body, as is carried out for LDR-BT means that the implant emits a small but detectable dose of radiation for some months after implantation. Additionally, correct seed placement if highly operator-dependent, seed cannot be adjusted once they are deposited. Thus, dosimetry might among implants. Furthermore, the multiple radioactive seeds required — ~100 seeds might need to be custom made for a particular date — is costly. By contrast, HDR-BT procedures use a single, reusable, 192Ir source that lasts 3 months. However, relative cost is debatable, because in certain environments seeds are cheaper than HDR-BT, particularly if multiple HDR-BT fractions are used. Furthermore, LDR-BT exposures staff to radiation, although this exposure is very minimal.38 Finally, acute irritative urinary symptoms frequently occur as the radioactive sources of LDR-BT decay (FIG 1B). The acute toxicity period is more pronounced but shorter in duration for 131Cs, which has a half-life of 9.7 days; by contrast, the acute toxicity is less pronounced but longer in duration (by months) for 125I, which has a half-life of 59 days.
History of HDR-BT
In the late 1980s, analysis of CT-based dosimetry revealed that dose coverage of LDR-BT plans was often lower than in the preplans. Thus, investigators explored the use of HDR-BT with 192Ir to overcome these limitations39, theorizing that the higher energy 192Ir HDR-BT isotope would enable dose delivery to the periphery of the prostate in a highly conformal manner, assuring good tumour coverage and minimizing the dose to the adjacent bladder and rectum.39 Furthermore, this approach would enable the dose to differentially delivered within the peripheral zones of the prostate where the bulkier portions of carcinoma typically reside;40, 41 this dose distribution would also limit dose to the central region that contains the urethra.39
A TRUS-guided remote afterloading system (RALS) was first introduced in 1980 to deliver a high radiation dose to the prostate while limiting exposure of the surrounding tissues (FIG 1A) and to address some limitations of TRUS-guided LDR-BT, including high dependence on the operator for proper seed placement, inability to adjust seeds once they are deposited, and variability of dosimetry among implants.35 HDR-BT began to be used as a boost with EBRT in Sweden, Germany, Japan, the UK, and the USA in the 1980s and 1990s.35, 39, 42, 43
HDR-BT boost was attempted before HDR-BT monotherapy because it was theorized to be an improvement over LDR-BT boost. With HDR-BT boost, staff would be completely protected from radiation exposure, and the procedure could be more widely applied, because abdominal surgery could be avoided.42 Safety and efficacy of HDR-BT boost was subsequently illustrated in Phase I/II and Phase III trials.44-56
By the 1990s, HDR-BT boost had been evaluated in many studies worldwide. In Osaka, Japan, a trial of HDR-BT monotherapy was initiated after a year of using HDR-BT boost, and the reports on HDR-BT monotherapy have been published since the year 2000.57-60 According to the authors, HDR-BT monotherapy was felt to be more advantageous than HDR-BT boost because with monotherapy the high dose per fraction of brachytherapy would not have to be reduced. Furthermore, any extracapsular spread of disease could be covered by dose from the needles. In the USA, a trial of HDR-BT monotherapy was conducted from 1999 to 2000, and the results were published in 2001.61 Patients with low-intermediate-risk prostate cancer were treated with HDR-BT, 38 Gy in four fractions of 9.5 Gy each, delivered twice a day over 2 days. None of the patients developed severe acute toxic effects.
The use of HDR-BT addresses some of the issues associated with LDR-BT, but it has its own disadvantages. For example, HDR-BT must be performed in a shielded room instead of an operating room, as in the case of LDR-BT; thus, start-up costs are associated with ensuring an appropriate environment. HDR-BT is typically performed in 1–3 implants and the catheters are left inside the patient to deliver 1–6 fractions in total. Typically, fractions must be separated by >6 h in order to capture cancer cells during the radiosensitive G2/M phase and allow DNA repair of normal cells. As an increased number of implants are used, the risk of procedure-related events, including infection and bleeding, is also increased. 58, 62, 63 Furthermore, as many fractionation options are available for HDR-BT, no single dose for HDR-BT has been standardized, unlike for LDR-BT.
Current trends
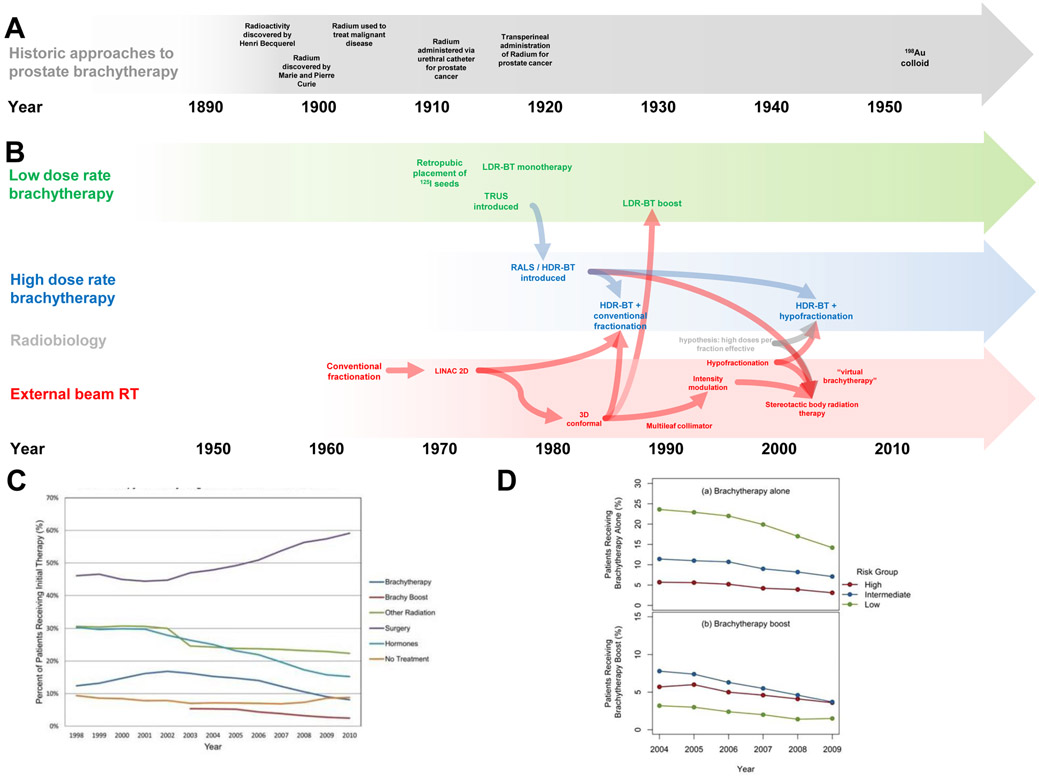
The use of brachytherapy to treat patients with localized prostate cancer in the USA and western Europe has been steadily declining since 2003. In the USA, brachytherapy use (either as monotherapy or boost) reached a peak in 2002, with 17% of all prostate cancer patients receiving the therapy; in 2010, the rate decreased to 8% (FIG 2).64 For intermediate-risk patients, use of brachytherapy boost decreased from 33% in 2004 to 12% in 2013; and for high-risk patients, use dropped from 27% to 11%.65
FIG 2 ∣. The evolution of brachytherapy for prostate cancer.
a∣ The use of prostatic brachytherapy was first reported in 1911, when radium was administered temporarily via a urethral catheter.14, 15 In 1917, a transperineal implantation of radium was performed in New York.16 During the 1920s, cohorts of patients were treated with radium brachytherapy in the US.
b∣ In the 1950s, LDR-BT was performed with 198Au,18 but by the 1970s 125I seeds were used for prostate cancer implants.21 A remote afterloading system (RALS) was developed in the 1980s and used for HDR-BT. With more advanced forms of external beam radiotherapy (EBRT) (for example, 3D conformal radiotherapy and then image-modulated radiotherapy (IMRT)),35 EBRT was combined with both forms of brachytherapy to deliver brachytherapy boost.56 EBRT developed in parallel over the 1990s and 2000s to become increasingly hypofractionated.75 In 2001, the development of the robotic arm linear accelerator, which delivers SBRT, led investigators to tout it as “virtual HDR-BT,” and they touted this treatment to be a superior and more advanced form of brachytherapy since it could be performed without anesthesia or needles entering the prostate.75 As of 2016, no trials comparing the two technologies have been performed,76 and the dosimetric distribution of HDR-BT (FIG 1B) is superior to any form of EBRT because X-rays do not pass through the skin of the patient.4
c∣ Trends in the use of brachytherapy compared with other modalities of therapy in the USA, from 1998–2013 show that use has declined over the time period. Brachytherapy use reached a high around 2002, when 18% of patients received the therapy. 64
d∣ Trends in the use of brachytherapy across risk groups (adapted form Martin, et al64). The percentage of patients treated with brachytherapy alone by year from 2004 to 2009 stratified by National Comprehensive Cancer Network (NCCN) risk grouping (upper panel), and percentage of patients treated with brachytherapy boost by year from 2004 to 2009 stratified by NCCN risk grouping (lower panel).64
The declining use of brachytherapy could be due to several reasons. Firstly, according to data from 2015, 30% of US medical school graduates are not aware of brachytherapy as a definitive treatment modality for cancer, and 10% do not believe that radiation therapy alone can be used to cure cancer.66, 67 Additionally, the declining rate of brachytherapy use in the USA might be due to the low and declining number of prostate brachytherapy procedures performed by residents.68-70 For example, the number of LDR-BT implantations performed in academic year 2006–2007 was 1,106 total, with a median of 14 per resident, and a range of 0–129. By academic year 2010–2011, the total number increased to 1,990, but the number of residents grew, and the median decreased to just 10 LDR-BT implantations per resident, with a range of 0–122.68-70
The number of HDR-BT prostate implantations performed by trainees in the USA as a whole has been negligible. In academic year 2006–2007, the total number was 234 (median of 0 per resident; range 0–38); in academic year 2010–2011, the total number of was 336 (median of 0 per resident, range 0–71).70 Graduates are unlikely to develop the necessary skill to do the procedures with such few cases, as the associated learning curve means that >20 cases are likely necessary to perform the procedure acceptably without supervision.71-73 By contrast, the number of Accreditation Council for Graduate Medical Education (ACGME)-accredited radiation oncology residency programmes has been growing: from 76 in 2011 to 89 in 2014. These data suggest that a growing number of graduating radiation oncology residents have a declining experience in brachytherapy for prostate cancer.
The use of brachytherapy could also be declining owing to developments in EBRT. EBRT developed in parallel with brachytherapy during the 1990s and 2000s, and employed hypofractionated regimens that used doses similar to HDR-BT. In 2001, development of the robotic arm linear accelerator used to deliver stereotactic body radiotherapy (SBRT) in dose fractionation schemes similar to HDR-BT was reported.74, 75 SBRT is a type of EBRT delivered as a single fraction lasting up to 45 min per day, for a total of ~5 treatments, each about 6–9 Gy, over ~2 weeks. As of 2016, no randomized trials comparing brachytherapy with any form of EBRT, including SBRT, have been performed.76 Importantly, the dosimetric distribution of brachytherapy provides superior conformality and dose concentration to EBRT, in part because the X-rays do not pass through the bowel and bladder in order to reach the prostate (FIG 1B).4, 74
Indications and contraindications to brachytherapy for prostate cancer
All patients require a biopsy to determine tumour Gleason score, pretherapy serum PSA measurement ,and clinical tumour classification with digital rectal examination and possible imaging with a CT of the pelvis before initiation of any form of treatment, as these prognostic factors determine risk classification.3, 77 Over 80% of prostate cancer patients do not die of their disease;78 thus, maintaining quality of life is key in all patients. All patients should have their urinary and erectile function assessed with validated questionnaires, including the American Urologic Association (AUA), International Index of Erectile Function (IIEF-5), and/or Expanded Prostate Cancer Index Composite (EPIC), before treatment begins.3, 79
Indications
The National Comprehensive Cancer Network (NCCN) risk group classification for low-risk disease includes cancers with Gleason score ≤ 6, serum PSA < 10 ng/ml, and clinical tumour classification T1 or T2a. For intermediate-risk disease, patients have Gleason score 7, or PSA ≥10 ng/ml ≤ 20 ng/ml or clinical tumour classification of T2b or T2c. For high-risk disease, patients have Gleason score 8–10, serum PSA >20 ng/ml, or clinical tumour classification of T3a. Additionally, in more recent versions of the NCCN guidelines3 and in studies of HDR-BT boost,45, 46, 48, 50, 80-87 a ‘very-high’ risk classification is made. In the NCCN guidelines, this includes patients with T3b–T4 disease, primary Gleason score 5, or >4 cores with Gleason score 4–5. In studies of HDR-BT boost,45, 46, 48, 50, 80-87 the definition is more heterogeneous and typically includes patients with multiple high-risk features; for example, Gleason 8 and a serum PSA level >20 ng/ml.
The NCCN guidelines state that brachytherapy monotherapy and boost (that is, in combination with EBRT) can be used as first-line therapies in the management of men with prostate cancer of all risk groups (Table 1).3 Monotherapy is an option for those with low-risk disease and favourable intermediate-risk disease (evidence level 2, compared with EBRT). Brachytherapy boost is an option for patients with high-risk or very-high-risk disease. For high-risk patients, brachytherapy boost is preferred to brachytherapy or EBRT monotherapy because of improved outcomes (evidence level 1), based on retrospective evidence88 and prospective studies.54, 89 For purposes of this Review, we consider high-risk and very-high-risk disease as a single high-risk category because the outcomes and toxicities are typically reported without dichotomization.
Table 1∣.
Indications and contraindications of brachytherapy compared with external beam radiotherapy
| Indication or contraindication | LDR-BT or HDR-BT | EBRT | Radical prostatectomy |
|---|---|---|---|
| Low-risk disease (Gleason score ≤ 6, and PSA < 10 ng/ml, and clinical tumour classification T1, T2a) | Monotherapy | Monotherapy | Monotherapy |
| Intermediate-risk disease (Gleason score 7, or PSA ≥ 10 ng/ml ≤ 20 ng/ml or clinical tumour classification of T2b, T2c) | Boost or monotherapy | Monotherapy or boost | Monotherapy |
| High-risk disease (Gleason score 8–10, or PSA >20 ng/ml, or clinical tumour classification of T3a) | Boost usually preferred over monotherapy | Boost usually preferred over monotherapy | Monotherapy |
| Post-radical prostatectomy | Rarely performed | Adjuvant indications: pT3; positive surgical margins Salvage indications: suspected local recurrence (e.g. rising PSA, findings on imaging or biopsy) | NA |
| Ataxia telangiectasia | Contraindicated | Contraindicated | NA |
| Pre-existing rectal fistula | Contraindicated | Contraindicated | Possible contraindication or logistical difficulty |
| Unacceptable operative risks or medically unsuitable for anaesthesia | Contraindicated | Possible contraindication or logistical difficulty *, ‡, § | Contraindicated |
| Distant metastases | Contraindicated | Possible contraindication or logistical difficulty *, ‡, § | Possible contraindication or logistical difficulty |
| Absence of rectum such that TRUS-guidance is precluded | Contraindicated | Not a contraindication*, ‡ | |
| Large TURP defects that preclude seed placement and acceptable radiation dosimetry | Contraindicated | Possible contraindication or logistical difficulty *, ‡ | Possible contraindication or logistical difficulty |
| History of previous pelvic radiotherapy | Possible contraindication or logistical difficulty | Possible contraindication or logistical difficulty | Uncommon |
| Limited life expectancy (<10 years; patient will not realize benefit of radiotherapy in lifetime) | Possible contraindication or logistical difficulty | Possible contraindication or logistical difficulty | Possible contraindication or logistical difficulty |
| Moderate-to-severe urinary symptoms (such as high IPSS score, typically defined as >20) | Possible contraindication or logistical difficulty | Possible contraindication or logistical difficulty; consider conventional fractionation | Not a contraindication |
| Inflammatory bowel disease | Possible contraindication or logistical difficulty | Possible contraindication or logistical difficulty * | Not a contraindication |
| Risk of bleeding (from use of anticoagulant therapy) | Possible contraindication or logistical difficulty | Not a contraindication | Possible contraindication or logistical difficulty |
| Large median lobes | Possible contraindication or logistical difficulty | Not a contraindication | Not a contraindication |
| Pubic arch interference (from previous pelvic fracture, irregular pelvic anatomy, or a penile prosthesis) | Possible contraindication or logistical difficulty | Not a contraindication | Not a contraindication |
| Patient peak flow rate <10 cm3/s and postvoid residual volume prior to brachytherapy >100 cm3 | Possible contraindication or logistical difficulty | Not a contraindication | Not a contraindication |
| Large prostate (e.g. >60 cm3) | Possible contraindication or logistical difficulty; patient might have accompanying bother symptoms, but implant is still technically possible | Not a contraindication * | Not a contraindication |
| Concurrent androgen deprivation therapy use | Not a contraindication | Not a contraindication | Not a contraindication |
NA: not applicable; IPSS: International Prostate Symptom Score; TRUS: transrectal ultrasonography; TURP: transurethral resection of prostate.
Excluded on clinical trial: Radiation Therapy Oncology Group 0938 or 0534.
Placement of fiducials for IGRT might be difficult.
Depends on intraprostatic versus extraprostatic disease burden.
Contraindications
The presence of ataxia telangiectasia or pre-existing rectal fistula are absolute contraindications to any type of radiotherapy. Additionally, TRUS-guided brachytherapy has more contraindications than EBRT, including absence of a rectum meaning that TRUS guidance cannot be performed. Other relative contraindications include pubic arch interference, a large prostate or a urethral defect associated with previous transurethral resection of the prostate (TURP), a low peak urinary flow rate of <10 cm3/s, and a postvoid residual volume >100 cm3.4, 8, 9 In the past, a gland size of >60 cm3 was generally considered a relative contraindication for LDR-BT, but LDR-BT can now easily be performed for large prostates using 3D planning with CT or ultrasonography (rather than the caliper measurements performed in the 1950s).90 However, a large prostate can be accompanied by urinary bother symptoms, which would be exacerbated in the postimplant period. Additionally, the patient must be able to tolerate general anaesthesia. Multiple implantation procedures, typically 1–3 (to deliver 1–6 fractions), can be necessary for HDR-BT (FIG 1B). The procedure is typically performed under general anaesthesia; thus, a patient would need to be anaesthetized 1–3 times, and needles might need to be placed through the perineum 1–3 times, although one insertion followed by twice daily treatments is common.
Androgen deprivation therapy (ADT) can be used with either form of brachytherapy in certain patients with intermediate-risk and high-risk disease or as a means of prostate cytoreduction in any patient.91 With respect to risk group, ADT is almost always recommended for patients with high-risk disease because it has been shown to improve overall survival in prospective trials of EBRT; ADT is typically recommended in patients with ‘unfavorable’ intermediate-risk prostate cancer — that is, those with primary Gleason pattern 4, >50% positive biopsy cores, or multiple intermediate-risk factors.92 However, the effect of ADT–brachytherapy combination on survival has not been confirmed as it has been with EBRT.91, 93-99
Radiophysics and technical aspects
Target delineation
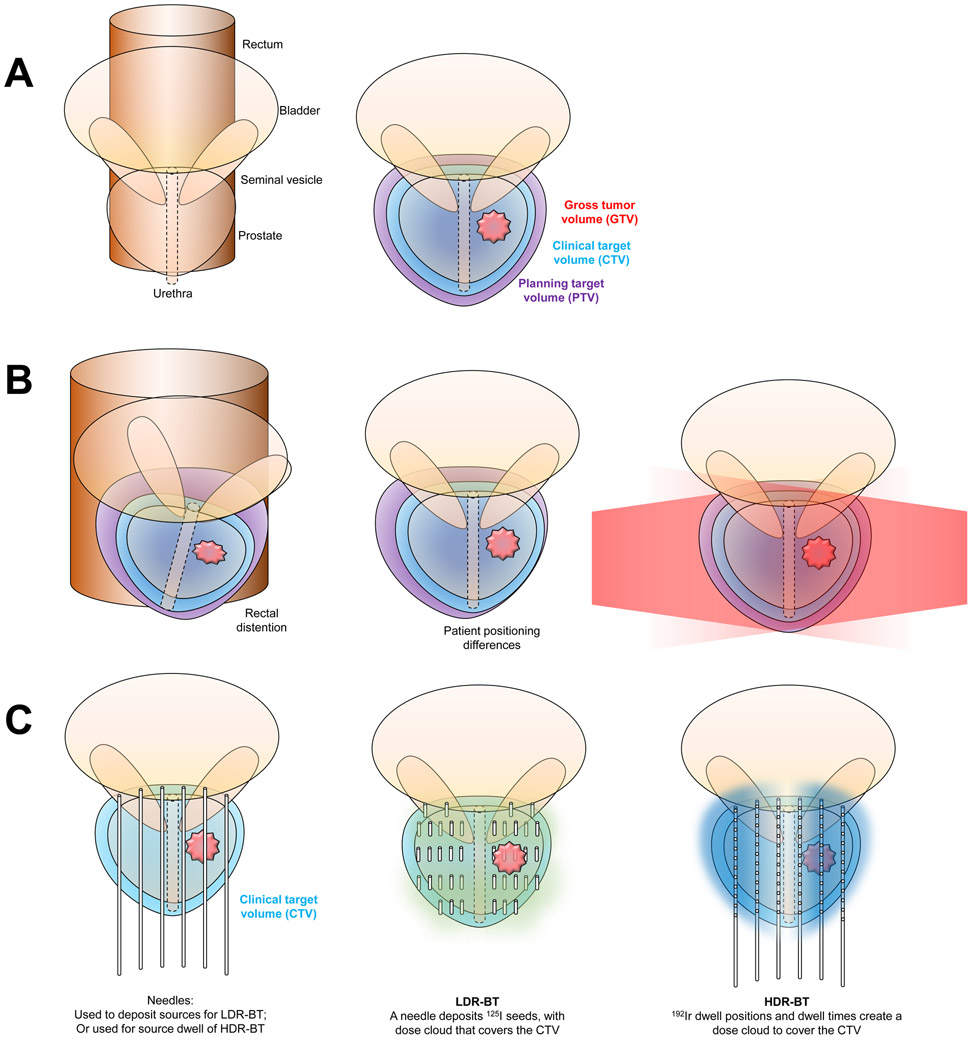
The GEC–ESTRO guidelines10, 11 provide detailed instructions for brachytherapy target delineation. Several differences exist in target volume expansions of brachytherapy 100 versus EBRT (FIG 3).101, 102 The gross tumour volume (GTV) is the gross demonstrable extent and location of the malignant growth; it consists of the primary tumour — which for prostate cancer has historically been defined as the entire gland as well as any visualized extension into surrounding normal tissues — the regional lymph nodes, or distant metastases based on clinical data (that is, physical examination, anatomical imaging with CT and MRI, and functional and molecular imaging).101, 102 For both forms of brachytherapy, GTV is typically not contoured, unless gross disease — either intraprostatic or extraprostatic — is noted on imaging. The clinical target volume (CTV) encompasses the GTV as well as areas at risk for subclinical cancer involvement. The CTV can include a margin around the prostate GTV, and it might include adjacent regions at risk of having subclinical disease. For example, this might include the seminal vesicles and expansion for extraprostatic extension (EPE). For brachytherapy, the CTV is equivalent to the entire prostate gland, including the prostate capsule plus any macroscopic extracapsular disease, and a 3D expansion of 3 mm. The CTV is typically constrained anatomically by the anterior rectal wall and bladder base.10, 11 This definition is similar to that used in EBRT planning guidelines.101, 102
FIG 3∣. Target volume definitions.
a∣ Anteroposterior image of the prostate and seminal vesicles (left). The principle organs at risk include the urethra, bladder, and rectum. The gross tumour volume (GTV; red) is the gross demonstrable extent and location of the malignant growth. The clinical target volume (CTV; light blue) encompasses the GTV as well as areas at risk for subclinical cancer involvement. The planning target volume (PTV; purple) encompasses the CTV plus an additional margin to account for patient movement, set-up error, and organ movement (right).
b∣ For prostate cancer treated with EBRT, the PTV is typically CTV + 0.5 – 1.0 cm.101, 102 The PTV expansion is necessary because the prostate can move owing to nearby organ changes, such as rectal distention (illustrated on the left), and because the patient might be positioned differently on the treatment table (on the right). Movements can be translational, rotational, and deformational. Note that the CTV (blue volume) stays inside of the PTV (purple volume). EBRT (shown in red) covers the PTV by passing through the soft tissues of the pelvis.
c∣ For brachytherapy, the expansion from a CTV to make a PTV can typically be because the set-up error is almost nonexistent. 10, 11 In the case of LDR-BT, once the needles are deposited, the dose cloud (green cloud) would cover the CTV, even if the patient were to move or if there were organ movement. In the case of HDR-BT, the needles (typically about 12 in count) anchor the prostate while the 192Ir source moves to the dwell positions, shown as circles inside of the needles, for prespecified dwell times to deliver the prescribed dose (blue cloud).
The planning target volume (PTV) encompasses the CTV plus an additional margin to account for patient movement, setup error, and organ movement (for example, bladder or rectal distention). For prostate cancer treated with EBRT, the PTV is typically CTV + 0.5 – 1.0 cm.101, 102 For brachytherapy, no further PTV expansion is required.10, 11 In the case of LDR-BT, once the needles are deposited, the dose cloud would cover the CTV, even if the patient were to move or if there were organ movement. In the case of HDR-BT, the needles (typically about 12 in number) anchor the prostate while the 192Ir source moves to the dwell positions for prespecified dwell times to deliver the prescribed dose. During the dose delivery, there should be no uncertainty regarding the position of the needles.
LDR-BT technical aspects
As of 2017, LDR-BT is typically performed with 125I or 103Pd radioisotopes; few centres use 131Cs, but this isotope is also an option (Table 2). The American Brachytherapy Society (ABS) does not recommend the use of one specific radionuclide.8 Both 125I and 103Pd have demonstrated excellent long-term outcomes; 131Cs was introduced in 2004.103 125I has the longest half-life of these three isotopes (59 days); hence, it tends to have a milder toxicity peak, and longer window of low-grade toxicity over 2–5 months. By contrast, 131Cs has the shortest half-life (10 days) and causes more irritative symptoms in the 2–5 weeks following the implantation.
Table 2∣.
Properties of radionuclides and quality planning constraints
| Radionuclide | t1/2 (days) | Average energy (keV) |
Prostate (CTV) | Urethra | Rectum | |||
|---|---|---|---|---|---|---|---|---|
| D90 | V100 | V150 | ||||||
| 125I | 59.4 | 28.4 | >100% of dose | >90-95% | <50-60% | UV150 ~0 (in volume) UV5 <150% UV30 <125% |
RV100 <1 cc on day 0; and < 1.3 cc on day 30 |
D2cc < prescribed dose; and D0.1cc < 200 Gy |
| 103Pd | 17.0 | 20.7 | ||||||
| 131Cs | 9.7 | 30.4 | ||||||
| 192Ir | 73.8 | 380 | >90-95% of dose | D0.1 ≤ 120 Gy EQD2 D10 ≤ 120 Gy EQD2 D30 ≤ 105 Gy EQD2 |
D2cc ≤ 75 Gy EQD2 | |||
| No normal tissue constraints from ABS due to wide range of fractionation options9 | ||||||||
Before implantation, CT or a TRUS planning study can be performed to permit treatment planning as well as to calculate prostate volume so that radioactive seeds for the implant can be ordered. Alternatively, an intraoperative treatment planning approach can be followed, whereby all radiation treatment planning and delivery occurs in real time in a single procedure.104 Additionally, real-time planning can be performed to overcome the limitations of preimplantation planning.104-106
With pre-implant planning, TRUS is performed a few weeks prior to the implantation, and the 3D placement of seeds, with resultant dose to cover the CTV, is modelled using a computer. However, preimplant dosimetry has limitations, including quality of the image: Distinguishing the density of the prostate parenchyma from the capsule and some of the pelvic floor musculature can be impossible. Moreover, seeds might not be deposited in the exact locations modelled by the programme. Thus, the intraoperative dose coverage might not be the same as that seen on the preoperative scan. With intraoperative planning using TRUS, the seeds are deposited as they would be with an intraoperative plan; if differences in anatomy or dose coverage are evaluated by TRUS-based computer planning, the deposition of additional seeds can be adjusted.
The standard procedure for implantation of brachytherapy seeds uses a transperineal approach under TRUS guidance with a template in place. Efforts are taken to ensure that patient position and TRUS-probe alignment closely replicates the preimplant planning study, if this has been performed. A high-resolution biplanar ultrasonography system operating at 5–12 MHz with dedicated prostate brachytherapy software is used. Fluoroscopy can be used as a complementary imaging modality to TRUS, and is typically used to check seed deposition. Fluoroscopy can be enhanced by the use of differential concentrations of contrast in the bladder and a Foley balloon partially radio-opaque catheter to identify the urethra, and gold fiducial markers implanted at the prostatic base and apex.107 In some centres, fluoroscopy is used for intraoperative dose calculation using image fusion.108 However, this approach is not considered mandatory for successful LDR-BT.8
The ABS8 recommends that CT-based postimplant dosimetry be performed within 60 days of implantation, in order to achieve good quality assurance.109 A planning system generates dose–volume histograms, dose–volume statistics, and 2D and 3D isodose curves superimposed on CT and other images, including ultrasonographic and MRI. Careful postimplant assessment provides objective measures of implant quality. Postimplant dosimetry is typically performed on the day of LDR-BT and/or within 30 days after the implant, once the initial oedema has resolved. If logistically feasible and consistent with LDR-BT clinical trials, dosimetry performed at 3–4 weeks postimplant is preferred.109
The time required for oedema to resolve and, therefore, the optimal time to perform the postimplant scan, depends on the radionuclide used: 16 ± 4 days for 103Pd and 30 ± 7 days for 125I and seems likely less for 131Cs, though evidence for the use of 131Cs is limited.8, 9, 110 Reproducibility of postimplant dosimetry can be improved by using MR–CT image fusion.111, 112 The principle benefit of fusing MRI to the CT is for improved delineation of soft tissue, including the prostate, seminal vesicles, urethra, rectum, bladder, and penile bulb.23, 113-116 Additionally, multiparametric MRI can help to delineate the GTV, where a focal boost could be administered.117, 118 The principal drawback is that many radiation oncology departments do not own a dedicated MRI unit, imaging is expensive (although the overall cost might be the same119), some patients might have contraindications to imaging (such as a pacemaker), and the MRI might not substantially improve tissue delineation at the prostatic apex, which is blurred by trauma after catheter insertion.117-119
LDR-BT dosimetric quality constraints
Several dosimetric quality constraints must be achieved (Table 2). The ABS,8 ESTRO/EAU/EORTC,10, 11 and the American Association of Physicists in Medicine (AAPM)120, 121 recommend specific postbrachytherapy dosimetric parameters, according to the anatomy. The following terminology is used: the D(percent) is the minimum dose to the hottest percentage of the volume. The V(percent) is the percentage volume receiving a particular percent of the dose. The D(cc) is the dose to a specified cubic centimetres of a volume. The dose for any of these parameters can be described as a percent of the prescribed dose or in the Equivalent Dose in 2 Gy fractions (that is, the EQD2), which is converted using a radiobiological formula to approximate the dose of conventionally fractionated EBRT.
The ABS and AAPM recommend reporting dosimetric values in the prostate, bladder, and rectum. In the prostate D90, the minimum dose to the hottest 90% of the prostate volume in Gy, should be >100%. The prostate V100, the percentage volume receiving 100% of the dose, should be >90–95%. The prostate V150, the volume receiving 150% of the dose, should be <50–60%
According to the ASTRO, ABS, and AAPM guidelines, the urethra UV150,122, 123 the percentage of the urethra that receives 150% of the prescription dose, should be 0. The UD5, or the average dose to the 5% of the hottest urethra volume, should be <150% of the prescribed dose. The urethra UD30 or the average dose to the 30% of the hottest urethra volume, should be <125% of the prescribed dose. The GEC–ESTRO guidelines10 provide similar recommendations with slightly different terminology. In the prostatic urethra, the D10, or the dose to 10% of the urethra volume, should be <150% of the prescription dose. A secondary parameter, the D30, or the dose to 30% of the urethra, should be <130% of the prescription dose.
In the rectum, the RV100, the volume receiving 100% of the dose, should be <1 cc on day 0 dosimetry and <1.3 cc on day 30 dosimetry. According to GEC–ESTRO guidelines,10 the D2cc, or the dose to hottest 2 cubic centimetres of the rectum should be less than the prescribed dose; and the D0.1cc, the hotspot in the rectum, should be <200 Gy EQD2.
No agreement has been reached regarding the critical structures and dose constraints for postimplant erectile function, although the internal pudendal artery, penile bulb, and neurovascular bundles have been studied.124-126 Gillan et al.124 calculated the dose from LDR-BT to the internal pudendal arteries. An increased dose to these arteries would purportedly place patients at higher risk of erectile dysfunction. The authors reported that the internal pudendal arteries can be visualized and receive a low but calculable dose from LDR-BT, but the clinical significance of this dose is unknown.124 Conversely, Merrick et al.125 report that radiation doses to the proximal penis are predictive of brachytherapy-induced erectile dysfunction; the authors recommend penile bulb D50 and D20 should be maintained <40% and 60%, respectively, of the minimum peripheral dose. Buyyounouski et al.126 recommend the use of MRI for better delineation of erectile tissues but do not provide dose constraints.
LDR-BT fractionation and sequencing
According to the ABS and GEC–ESTRO guidelines,8, 10 the recommended dose of LDR-BT monotherapy using 125I is 145 Gy. For LDR-BT boost, the dose is 108–110 Gy. The recommended dose of LDR-BT monotherapy using 103Pd is 125 Gy, and that for 131Cs is 120 Gy. For LDR-BT boost, the dose is 90–100 Gy. The EBRT dose is 41.4–50.4 Gy at 1.8–2 Gy fractions per day. Optimal 125I prostate implants should deliver a D90 of 140–180 Gy, based on postimplant dosimetry. Doses of >140 Gy for 125I and > 125 Gy for 103Pd seem to result in similar outcomes in retrospective studies.127, 128 For 125I, doses >180 Gy are associated with a slight increase in long-term urinary symptoms.129
EBRT is generally performed before LDR-BT, with a 2–8 week interval between the two therapies.8 No studies have been published investigating either the sequencing of LDR-BT and EBRT or the time interval between the modalities. The downside of delivering LDR-BT before EBRT is that it exposes tissues to radiation simultaneously from both treatments and can theoretically increase toxic effects on normal tissue. By contrast, performing LDR-BT first enables physician assessment of the dose distribution8 and the seeds can be used as fiducial markers for daily image guidance during EBRT (FIG 1c).
Technical aspects of HDR-BT
During the HDR-BT procedure, a RALS automatically deploys and retracts a single small radioactive source of 192Ir along the implant needle at specific positions delivering ≥12 Gy/h, compared with 0.4–2.0 Gy/h with LDR-BT. The RALS enables a physician to control the position where the HDR source stops (the dwell position) for a predetermined time period (the dwell time). The 192Ir used in HDR-BT is contained within the needles placed in the prostate during this temporary implant; thus, the target does not move during radiation, and seed migration, is not possible, as it is with LDR-BT.130, 131 Moreover, the treating clinicians are not exposed to radiation, and source preparation is not required, unlike the case with LDR-BT.132 Furthermore, ultrasonography-based planning minimizes catheter displacement.55, 56, 63
HDR-BT has a number of benefits compared with EBRT and LDR-BT, some of which are theoretical and not yet validated in the clinic. Firstly, HDR-BT has the potential to increase prostate-cancer-cell death and minimize radiation-related toxicity by widening the therapeutic ratio, depending on the fractionation, α/β ratio, and relative biologically equivalent dose (BED).35, 63, 75, 133
Secondly, dosimetry is improved, as a range of dwell times can be employed at each dwell position, with better dose distribution than EBRT (FIG 1B).134, 135 Thirdly, the treatment is completed in a few fractions over 1–4 days, which is more convenient for the patient than a protracted course of conventional EBRT.11, 35, 55, 63, 136-140
Notably, both LDR-BT and HDR-BT have excellent dosimetry. Additionally, both forms of brachytherapy are similarly economically favourable versus EBRT. Costs of LDR-BT and HDR-BT take into account initial investment cost, including shielding necessary for HDR-BT, the cost for each implant, as new sources must be used for LDR-BT, the cost of the number of implants per patient, and the number of patients treated with the device, as well as other potential uses for the modality, such as gynaecological implants to additionally treat these cancers using HDR-BT (Table 3).
Table 3 ∣.
Comparison of physician and patient considerations of LDR-BT and HDR-BT
| Considerations | LDR-BT | HDR-BT |
|---|---|---|
| Provider and/or technical aspects | ||
| Need to use shielded room | No, can be performed in OR | Yes; thus, associated costs to build room |
| Initial capital equipment costs | Relatively low | Relatively high |
| Radioactive source can be used for other cancers | No | Yes; most commonly used for gynaecological,, breast, advanced head and neck, skin, lung |
| Recurring cost with each implant | Relatively high, to pay for seeds for each implant | Relatively low, as source is unchanged |
| Recurring costs related to source | Seeds must be custom made for each implant before date of implantation; if 125I implant delayed by one week, seed activity would decrease by 7.8% | Same source used for different patients over 3–4 months; if implant delayed by one week, treatment time relatively unchanged |
| Operator dependence | Relatively high | High, though not as high as LDR-BT |
| Planning | Preimplant or intraoperative ultrasonography planning then CT postimplant dosimetry checks | Ultrasonography or CT: inverse planning at the time of implant, postimplant dosimetry not necessary |
| Cost for treatment | Relatively low (<$12,000) compared with EBRT | Relatively low (<$12,000) compared to EBRT |
| Dose conformality (versus EBRT) | Superior, similar to HDR-BT | Superior, similar to LDR-BT |
| Radiation dose to clinician | Some, though extremely low | None |
| Use for large prostates (>60 cm3) | Technically possible | Yes |
| Use as monotherapy | Yes, particularly for patients with low-risk or select intermediate-risk disease | Yes, particularly for patients with low-risk or select intermediate-risk disease |
| Body of evidence | Excellent | Good, though not as robust as LDR-BT |
| Uniform consensus dose | Yes, e.g. 145 Gy for 125I, 125 Gy for 103Pd, and 120 Gy for 131Cs implants | Typically, no, multiple fractionation options |
| Potential use with EBRT | Yes, typically after EBRT | Yes: before, interdigitated with, or after EBRT |
| Potential use to salvage local recurrence | Yes | Yes |
| Patient | ||
| Number of implants | One | Typically, 1–3 (to deliver 1–6 fractions); potentially increasing risk of infection, anaesthesia complications |
| Convenient for patients who live far from cancer centre | Yes | Yes; however, depending on implantation schedule, might require hospitalization for 1–2 days |
| General anaesthesia used | Yes, but can also be performed under spinal or local anaesthesia | Yes, but can also be performed under spinal or local anaesthesia |
| Incisions, sutures | No | No |
| Outcomes: FFBF, DM, PCSS, OS | Similar to HDR-BT, EBRT, surgery (evidence level 1) | Similar to LDR-BT, EBRT, surgery (evidence level 1) |
| Acute toxicities |
|
|
| Chronic toxicities |
|
|
ADT, androgen deprivation therapy; DM, distant metastases; LDR-BT, low-dose-rate brachytherapy; EBRT, external beam radiation therapy; FFBF, freedom from biochemical failure; HDR-BT, high-dose-rate brachytherapy; OR, operating room; PCSS, prostate-cancer-specific survival; OS, overall survival.
HDR-BT dosimetric quality constraints
During an HDR-BT procedure, the physician implants the needles, and a dosimetrist or physicist prepares a plan. Next, the physician reviews the plan, and the dosimetrist or physicist mcan make requested changes to the plan. Once the plan is optimized, the physician approves the plan, and the treatment is delivered. The ABS9 and GEC–ESTRO11 guidelines state that the CTV V100 should be >90% . The ABS does not provide normal tissue constraints given the heterogeneity in dose fractionation.9 The GEC–ESTRO11 guidelines provide constraints, with conversion into the EQD2 (Table 2).
Comparison of dosimetry among EBRT, LDR-BT, and HDR-BT
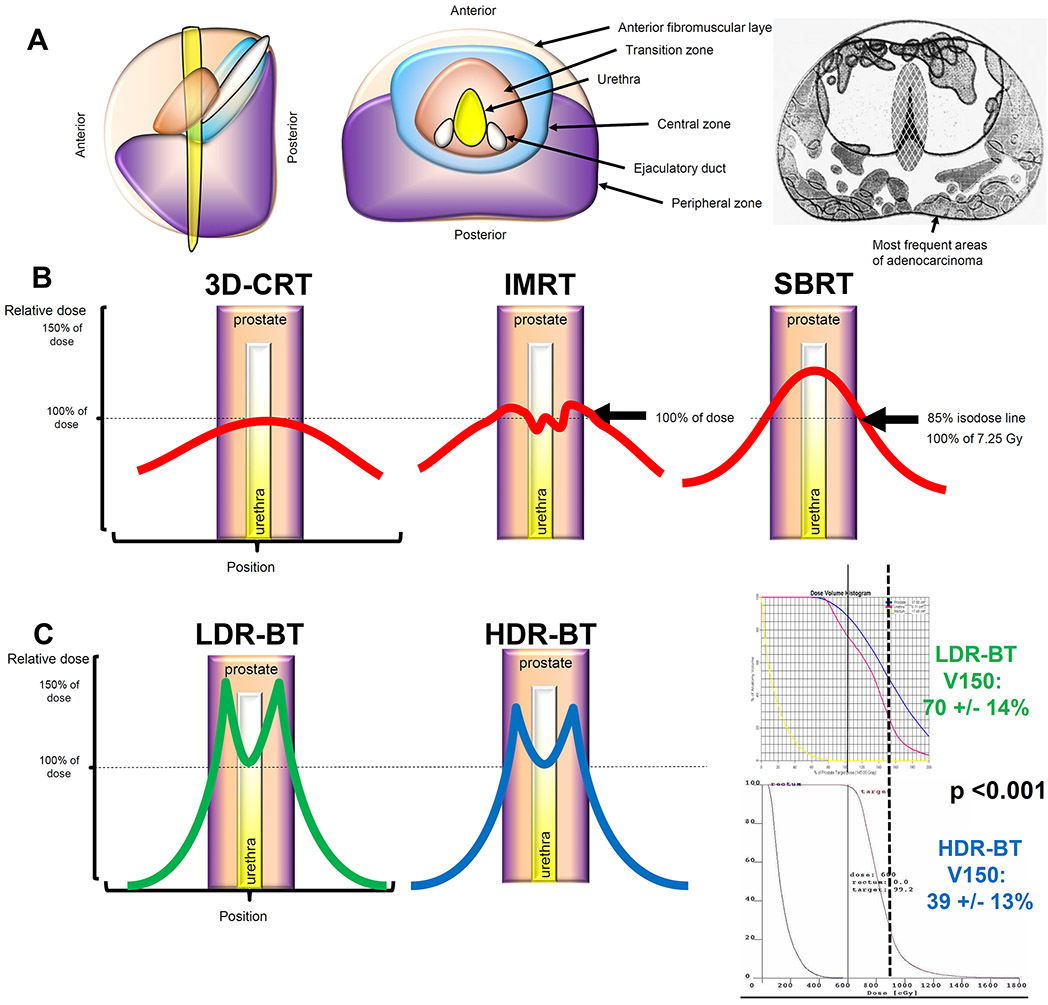
Both LDR-BT and HDR-BT have favourable dosimetry compared with EBRT. The majority of prostate cancers develop in the peripheral zone of the gland, and brachytherapy plans can be tailored to deposit the highest dose in this zone (FIG 4a).40 Furthermore, several caveats to dose prescriptions differentiate EBRT from brachytherapy.
FIG 4∣. Dosimetric comparison of LDR-BT versus HDR-BT.
a∣ The prostate gland is composed of a peripheral zone, central zone, transitional zone, and an anterior fibromuscular layer. The majority of prostate cancers develop in the peripheral zone. 40
b∣ Relative dose versus position in tissue. With 3D-CRT, the maximal point dose is towards the centre of the prostate, near the urethra; the dose decreases gradually toward the periphery of the prostate. IMRT (centre) ensures coverage of the entire prostate gland with dose and minimizes hotspots within the gland. Nonetheless, with IMRT, the prescription dose must be delivered to the PTV, which expands outside of the prostate. With SBRT, radiotherapy is prescribed to an isodose line to cover the PTV. Furthermore, the hotspot is again in the centre of the prostate toward the urethra; although protocols include a urethra dose constraint,101, 102 the higher dose toward the centre of the gland with SBRT is sometimes unavoidable.74, 75
c∣ Both LDR-BT and HDR-BT provide excellent dosimetric coverage of the prostate, particularly because they provide excellent coverage of the peripheral zone with a low dose to the urethra. HDR-BT might, in some instances, be superior to LDR-BT because the hot-spots assessed by looking at the V150 (or volume receiving 150% of the prescribed dose), are typically smaller in a HDR-BT plan than in the LDR-BT plan.
In the 1990s, EBRT was delivered using 3D conformal radiation therapy (3D-CRT), whereby multiple beams were centred on the prostate (FIG 4B)141. With 3D-CRT, the maximal point dose was toward the centre of the prostate, near the urethra; the dose decreased gradually toward the periphery of the prostate. Intensity modulated radiation therapy (IMRT) was introduced in the early 1990s as a further refinement in the delivery of highly-conformal radiation because it increases the dose delivered to the tumour volume and minimizes the dose delivered to surrounding organs.35 IMRT was made possible by use of a multileaf collimator (MLC), a device made up of individual leaves of a high atomic numbered material that can move independently in and out of the path of a photon beam to contour its shape to a tumour, and advanced treatment planning calculation algorithms, which enable inverse optimization of MLC positioning for complex dose delivery.35
The dose distribution created by IMRT is characterized by a concavity or invagination of the edge of the higher doses away from the rectum, rather than a straight edge through the rectum as seen with 3D-CRT. IMRT ensures coverage of the entire prostate gland with dose and minimizes hotspots within the gland. Nonetheless, with IMRT, the prescription dose must be delivered to the PTV, which expands outside of the prostate (FIG 4b). With SBRT (FIG 4b), radiotherapy is prescribed to an isodose line to cover the PTV. Furthermore, the hotspot is in the centre of the prostate toward the urethra; although a urethra dose constraint is provided by clinical trials and guidelines,101, 102 the higher dose toward the centre of the gland with SBRT is sometimes unavoidable.74, 75
With LDR-BT and HDR-BT, the dose can be differentially delivered within the peripheral zones of the prostate, and the dose towards the centre of the gland can be minimized (FIG 4c). Furthermore, as the CTV is equivalent to the PTV for brachytherapy, the dose is not prescribed to a large volume outside of the gland. With HDR-BT, the hotspots, assessed by looking at the V150 (or volume receiving 150% of the prescribed dose), are typically smaller than in LDR-BT (FIG 4c); whether this is an advantage or disadvantage is unclear.
HDR-BT fractionation and sequencing
Monotherapy
For HDR-BT monotherapy, various options are available according to GEC–ESTRO11: 34 Gy in four fractions at 8.5 Gy per fraction, 36–38 Gy in four fractions at about 9.25 Gy per fraction, 31.5 Gy in 3 fractions at 10.5 Gy per fraction, and 26 Gy in two fractions at 13.5 Gy per fraction.
Boost
The standard doses used in HDR-BT boost vary among institutions.9 Based on systematic reviews,55, 56 EBRT is usually delivered to a total dose of 36–54 Gy in 1.8–2.0 Gy fractions and HDR-BT is typically delivered to a total dose of 12–30 Gy in 1–4 fractions. GEC-ESTRO recommends: 45 Gy in 25 fractions over 5 weeks; 46 Gy in 23 fractions over 4.5 weeks, 35.7 Gy in 13 fractions over 2.5 weeks, or 37.5 Gy in 15 fractions over 3 weeks.
A particular brachytherapy dose fractionation schedule has not been recommended by the ABS9 and various options are available according to GEC–ESTRO: 15 Gy in three fractions at 5 Gy per fraction, 11–22 Gy in two fractions at 5.5–11 Gy fractions; or 12–15 Gy in a single fraction.11
Thus, for HDR-BT boost, the dose is typically delivered in 1–2 implants, using 2–6 fractions. Each fraction of radiation is 9–15 Gy: a lower dose-per-fraction is used if more fractions are delivered (for example four fractions of 8.5 Gy), or a higher dose-per-fraction is used if fewer fractions are used (for example a single fraction of 15 Gy).60, 142-156 The separate insertion schedule results in a greater workload of resource-intensive procedures and a greater anaesthesia time than the single-insertion procedure, which can require overnight hospital admission and is associated with risks of interfractional catheter displacement.62
Two HDR-BT boost fractionation schedules have evolved most likely because of preference by the physician, centre, and reimbursement models.55, 56 A separate procedure for catheter insertion for each fraction is typically used in European countries. A single insertion followed by 1–4 fractions delivered over 1–2 days is favoured in North American centres.51 Currently, 15 Gy in one fraction is the dose schedule in use in the Radiation Therapy Oncology Group 0924 Phase III trial of dose-escalated radiation therapy with or without pelvic nodal irradiation in HDR-BT.63
Three temporal approaches for combining EBRT and HDR-BT have been described (FIG 1c).55, 56 If EBRT is delivered first, HDR-BT is typically delivered 1–6 weeks later. One benefit of this method is to dose escalate a particular part of the prostate that contains the GTV, though this approach is still investigational. One disadvantage of this method is the oedema caused by EBRT, which can make the implant technically challenging and worsen the toxicity of HDR-BT. Centres are using this method in the USA,47, 82, 152, 157-159 Australia,152, 160-162 Europe,50, 54, 163, 164 Japan,165-167 Canada,168 and the UK.54
Alternatively, HDR-BT can be delivered first, with EBRT delivered 1–3 weeks later. With this method, EBRT can be used to compensate for suboptimal implant dosimetry of HDR-BT; furthermore, preimplant radiation-induced oedema and genitourinary symptoms that typically follow EBRT are minimized.169 A disadvantage of this method is the delayed application of radiotherapy to pelvic lymph nodes (if these are included in the treatment volume). This method is in use in Europe (including the UK), 152, 170-172 the USA,47, 52, 152, 173-176 Australia,44, 177, 178 China,179, 180 Brazil,181 Canada.51
Finally, EBRT can be interdigitated with HDR-BT. This technique combines some of the advantages and disadvantages of the other temporal approaches. EBRT is delivered on days when HDR-BT is not delivered, thus minimizing treatment time prolongation and possible accelerated repopulation that would be present with a split course of radiotherapy. Centres are using the interdigitated method in Europe,45, 46, 80, 182-185 the USA,45, 53, 183, 186 and Japan.166, 187
Radiobiology
Fractionation — which refers to dividing a radiation dose into smaller doses given at least 6 h apart — has several theoretical radiobiological advantages including repair of normal tissue damage, redistribution of cancer cells into radiosensitive phases of the cycle (G2–M), and reoxygenation of the tumour. Thus, fractionation might increase the efficacy of radiotherapy. As the total fractionated radiation dose delivered increases, the number of surviving cells within the treated volume decreases.188 However, the benefits of an increased total dose are offset by increased toxicity to the surrounding normal tissue.
The α/β ratio is used to describe the dose response of radiation on different tissues. The α/β ratio is thought to be ≥10 Gy for early-responding tissues, including skin, mucosa, and most malignant tumours, and 3–5 Gy for late-responding tissues, including connective tissues and muscles. Clinical radiobiological models suggest that prostate cancer has a low α/β ratio (~1.5), compared with most other malignancies.189 The α/β ratio is an important component of dose equivalent formulae used to convert different fractionation schedules into a common currency. It includes other assumptions in relation to repair and repopulation. A simplified form of the BED formula is often used to relate different fractionation schedules, where n is the number of radiation fractions and d is dose size per fraction: BED = (nd[1 + d/(α/β)])
If the α/β ratio for the tumour is lower than that of the surrounding tissues, as is hypothesized for prostate cancer, increasing the dose per fraction increases the BED more for the tumour than for the normal tissues; that is, the BED1.5 increases more than BED10.35 The diverging BED values result in an increase in the therapeutic ratio.190, 191 Radiobiological models approximate cell death due to DNA damage from radiation therapy using conventional fractionation, that is, 1.8–2.0 Gy per fraction. However, the models do not account for cell death due to other mechanisms (as seen with >5 Gy per fraction), including lipid membrane phosphorylation, necroptosis, or immune-mediated death,192-195 but, they do account for vasculogenesis secondary to mesenchymal stem cell infiltration.196 Thus, the higher BED of hypofractionated approaches suggests a theoretical benefit of HDR-BT versus conventionally fractionated EBRT. Similar estimates for the BED of LDR-BT show that optimal 125I prostate implants should deliver a D90 of 140–180 Gy, based on postimplant dosimetry. Doses of <140 Gy are associated with increased biochemical failure rates and doses >180 Gy with a slight increase in long-term urinary symptoms.129, 197
Cost
Brachytherapy is typically much more efficient, in terms of the resources it consumes,198 than EBRT.35 For radiotherapy, calculation models show that wage costs outweigh the cost of machines, owing to the labour-intensive nature of radiotherapy planning and delivery.139, 140 For treatment of a patient with prostate cancer using 40 fractions of EBRT, staffing of radiotherapy facilities accounts for an estimated 50% of the cost.199 Additionally, although IMRT treatment planning is complex, the planning is only done at the beginning of therapy, while cost builds with the delivery of each fraction.136 Thus, changing to a hypofractionated schedule or brachytherapy could decrease the number of work-hours and overall cost of treating each patient.75 By contrast, brachytherapy treatment is substantially more efficient — based on American Medicare reimbursements, per-patient costs of conventionally fractionated EBRT with intensity modulation, LDR-BT, and HDR-BT with four fractions, are estimated at $29,356, $9,938, and $17,514 respectively.137 LDR-BT might have a lower initial capital expense requirement than HDR-BT, in part because the kV energy isotopes used for LDR-BT do not require use of a shielded room and the procedure can be performed in an operating room, unlike that of mV energy 192Ir used for HDR-BT, which requires a special vault. Nonetheless, both forms of brachytherapy are relatively inexpensive.
Clinical outcomes and toxicities
Level 2 evidence regarding outcomes for HDR-BT and LDR-BT suggests they are similar in patients with low-risk and favourable intermediate-risk disease.4 Furthermore, level 1 evidence shows improved quality of life with brachytherapy over prostatectomy, based on the results of the Surgical Prostatectomy Versus Interstitial Radiation Intervention Trial (SPIRIT).200 For both types of brachytherapy, the 5-year freedom from biochemical failure (FFBF) outcomes for patients with low-risk, intermediate-risk, and high-risk disease are >85%, 69–97%, and 63–80%, respectively.4, 56
Brachytherapy plus EBRT (as opposed to brachytherapy alone) is an appropriate approach in select patients with intermediate-risk and high-risk disease (evidence level 1)4 using either LDR-BT89 or HDR-BT, 54, 201 (Table 1;Box 1). Briefly, patients who benefit from brachytherapy boost are typically those with unfavourable-intermediate risk and high-risk disease. Patients receiving boost should have no absolute contraindications to the treatment, including ataxia telangiectasia, a pre-existing rectal fistula, unacceptable operative risks, distant metastases, absence of a rectum such that TRUS-guidance is precluded, or large TURP defect that would result in unacceptable dosimetry. Other factors, including history of previous pelvic radiotherapy, limited life expectancy, and moderate-to-severe urinary symptoms are relative contraindications.
Box 1 ∣. The ideal patient for definitive brachytherapy.
Patients should have the following characteristics:
For brachytherapy monotherapy: Low-risk disease (Gleason score ≤6, and PSA <10 ng/ml, and clinical tumour classification T1, T2a), or favorable intermediate-risk disease (Gleason score 7, or PSA ≥10 ng/ml ≤ 20 ng/ml or clinical tumour classification of T2b, T2c) with primary Gleason score 3+4, <50% percent positive biopsy cores, and only a single intermediate-risk feature.
For brachytherapy boost: High-risk disease (Gleason score 8–10, or PSA >20 ng/ml, or clinical tumour classification of T3a), or unfavourable intermediate-risk disease (Gleason score 7, or PSA ≥10 ng/ml ≤ 20 ng/ml or clinical tumour classification of T2b, T2c) with primary Gleason score 4+3, ≥50% percent positive biopsy cores, and multiple intermediate-risk features.
Patients should have none of the following
Ataxia telangiectasia
Pre-existing rectal fistula
Unacceptable operative risks or medically unsuitable for anaesthesia
Distant metastases
Absence of rectum such that TRUS-guidance is precluded
Large TURP defects that preclude seed placement and acceptable radiation dosimetry
Patients should preferably not have the following
History of previous pelvic radiotherapy
Limited life expectancy (<10 years)
Moderate-to-severe urinary symptoms (for example, high IPSS score, typically defined as >20)
Inflammatory bowel disease
Increased risk of bleeding
Large median lobes
Pubic arch interference
Patient peak urinary flow rate <10 cm3/s and postvoid residual volume prior to brachytherapy >100 cm3
Large prostate (>60 cm3)
LDR-BT
LDR-BT monotherapy for low-risk disease
Patients with low-risk features deemed suitable candidates for LDR-BT can be appropriately treated with LDR-BT monotherapy. Published studies demonstrate that excellent long-term outcomes can be expected when optimal dosimetric parameters are achieved.202-204 According to a review published by the Prostate Cancer Results Study Group, the 10-year rates of FFBF for patients with low-risk disease receiving LDR-BT monotherapy have been estimated at >86%.205 Rates of prostate cancer distant metastasis, prostate-cancer-specific mortality (PCSM), and overall survival (OS) in these patients are estimated to be <10%, <5%, and >85%, respectively, at 10 years after treatment.205 and grade 3–4 toxicities occur in <4% of patients (Table 4).
Table 4∣.
Percentage of late Grade 3–4 toxicities associated with HDR-BT, LDR-BT, and EBRT
| Brachytherapy | Boost | External beam radiotherapy (with intensity modulation) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low-dose-rate | High-dose-rate | Brachytherapy + conventional fractionation |
Conventional fractionation |
Hypofractionation | Stereotactic body radiotherapy (SBRT) |
|||||||
| Quality of life domain | Early | Late | Early | Late | Early | Late | Early | Late | Early | Late | Early | Late |
| Sexual | 1–5% | 1–5% | 1–5% | Inconclusive | 1–5% | >5% | 1–5% | 1–5% | 1–5% | 1–5% | 1–5% | Inconclusive |
| Urinary incontinence | 1–5% | <1% | 1–5% | <1% | 1–5% | <1% | <1% | <1% | <1% | <1% | <1% | Inconclusive |
| Urinary irritative and/or obstructive | >5% | 1–5% | <1% | Inconclusive | >5% | 1–5% | 1–5% | <1% | 1–5% | <1% | 1–5% | Inconclusive |
| Bowel and/or rectal | 1–5% | <1% | <1% | Inconclusive | 1–5% | 1–5% | 1–5% | 1–5% | 1–5% | 1–5% | <1% | Inconclusive |
LDR-BT monotherapy or boost for intermediate-risk disease
For patients with intermediate-risk disease, the appropriateness of LDR-BT monotherapy depends on many factors including the required margin of treatment. In pathological series of whole-mount radical prostatectomy specimens of <pT3 disease defined as being organ-confined,206-209 the radial extraprostatic extension (EPE) rarely extends >5 mm; the posterolateral region, which is near the seminal vesicles, is at highest risk of EPE.210 Many intermediate-risk tumours have equivalent or even lower risk of adverse pathological features such as EPE, seminal vesicle invasion, or lymph node involvement. Thus, they can be treated with LDR-BT monotherapy.211 A recommended margin of 3 mm around the prostate is used as the planning target volume in all directions, except posteriorly because this would expose the rectum to high doses of radiation. This expansion usually encompasses all EPE in intermediate-risk disease.8
In the RTOG 0232 study, which ran from 2003 to 2012, patients with intermediate-risk prostate cancer were randomized to receive LDR-BT alone (with either 103Pd or 125I) or EBRT (45 Gy, partial pelvis, 1.8 Gy/fraction), followed by LDR-BT. Based on the report released in 2016, the addition of EBRT to LDR-BT did not result in superior FFBF (80% at 5 years for LDR-BT and LDR-BT + EBRT), overall survival, distant metastasis rate, or PCSM212. Additionally, participants in the LDR-BT-alone arm experienced fewer late events than the EBRT group: 53% versus 37% for any late Grade 2+ effects and 12% versus 7% for any late Grade 3+ effects.
The 10-year rates of FFBF for patients with intermediate-risk disease receiving LDR-BT monotherapy is estimated to be >65%, with most reports around 90%.205 In a multi-institutional analysis of about 3,000 patients with prostate cancer, including 960 men with intermediate-risk disease, the 8-year FFBF rate was 70%.204 Notably, the majority of these patients were treated before 1999 and fewer than 25% had formal postimplantation quality assurance.109 A survey of 18 brachytherapy practitioners who treated over 10,000 patients with LDR-BT suggested that practitioners employ monotherapy carefully, on a case-by-case basis. 213
LDR-BT boost for high-risk disease
LDR-BT boost is an accepted treatment modality for patients with high-risk disease.8, 9 Outcomes and toxicities of LDR-BT boost have been reported from Radiation Therapy Oncology Group (RTOG) 0019214, Cancer, Leukemia Group B (CALGB) 99809,215 and the Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (ASCENDE-RT, clinical trial number NCT00175396).89 In ASCENDE-RT, LDR-BT boost was shown to be superior to EBRT in terms of FFBF, bodily pain, general health, sexual function, and urinary function.89 Additionally, in a retrospective cohort of 245 patients receiving LDR-BT (with or without a boost) rates of Grade ≥2 and ≥3 rectal toxicities were estimated to be 7% and 3%, respectively.216 The risk of Grade ≥2 rectal toxicity was 2.8-fold higher in patients receiving supplemental EBRT compared with LDR-BT alone, and the risk of Grade ≥3 rectal toxicity was 11.9-fold higher (Table 4).216 Toxic effects are likely to be due, in part, to patient comorbidities, including increased BMI and hyperglycaemia or hyperinsulinaemia.217, 218 Incontinence is not a common toxic effect after brachytherapy.
ADT in combination with EBRT (in the neoadjuvant, concurrent, and adjuvant settings) has been shown to improve patient outcomes over the use EBRT alone in multiple clinical trials.91, 93-99, 219-221 However, the data regarding the addition of ADT to LDR-BT patients is less robust than for results regarding EBRT. Merrick et al.222 reported that 10-year FFBF was improved with the addition of ADT, but overall survival and PCSM were not. In addition, a multi-institutional series reported by Stone et al.223 showed that patients with Gleason score 8–10 tumours had improved overall survival and freedom from distant metastasis with higher doses (BED10 > 220 Gy) of LDR-BT.
HDR-BT
HDR-BT monotherapy for low-risk and intermediate-risk disease
HDR-BT monotherapy can be used in select (Table 1; Box 1) patients with low-risk and intermediate-risk disease.4, 63 The ABS and GEC–ESTRO guidelines recommend monotherapy for patients with high-risk disease only in a clinical trial or in case-by-case circumstances.9, 11 In a systematic review of HDR-BT monotherapy, FFBF rates for patients with low-risk, intermediate-risk, and high-risk disease are ≥85% at up to 5 years.60, 142-156 Overall survival, prostate cancer specific mortality, local recurrence, and distant metastasis rates are typically > 95%, < 4%, < 4%, and < 4%, respectively.63 The highest rate of distant metastasis was reported by Yoshioka and colleagues153, 154 but was secondary to their inclusion of a greater number of patients with high-risk disease than other studies. Based on a systematic review, grade 3–4 gastrointestinal or genitourinary toxicity is seen in <5% of patients.63
In one of the largest series of HDR-BT monotherapy from the University of California, Los Angeles (UCLA),224 the authors reported encouraging outcomes with 6.5 years of follow-up monitoring, which was some of the longest follow-up available at that time, but likely not long enough to draw long-term outcome conclusions. Similar to LDR-BT, HDR-BT monotherapy is associated with excellent and comparable rates of biochemical control, patient survival, treatment toxicity, and erectile preservation.63
HDR-BT boost for intermediate-risk and high-risk disease
HDR-BT boost beneficial in patients with intermediate-risk and high-risk disease because it combines the benefits of conventionally fractionated EBRT or hypofractionated EBRT monotherapy to cover extraprostatic disease with the high radiation dose delivered to the CTV by HDR-BT.56, 225 Thus, the BED achieved with HDR-BT boost is typically much higher than can be achieved with EBRT alone; at an α/β ratio of 1.5, the BEDs are 200–300 for HDR-BT boost versus ~187 for EBRT monotherapy of 80 Gy in 2.0 Gy fractions (FIG 5).56, 226
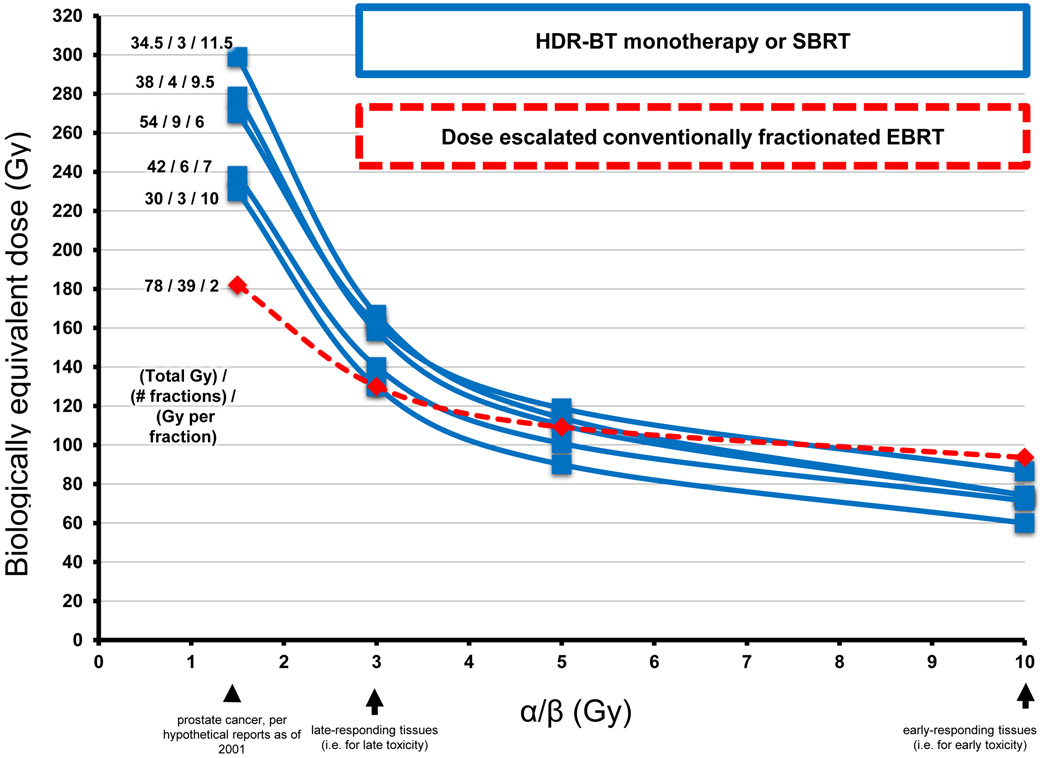
FIG 5 ∣. Biologically equivalent dose (BED) versus α/β curves for fractionated radiotherapy for prostate cancer.
BEDs for several major studies of HDR-BT (blue line) are shown compared with the regimen of dose-escalated conventionally fractionated EBRT monotherapy (dashed red line). The use of higher doses per fraction (for example with HDR-BT versus conventionally fractionated EBRT) results in a higher BED at α/β of 1.5 (for prostate cancer) than at α/β of 3.0 (for late toxicity), thereby increasing the therapeutic ratio.
Hoskin et al.54 published one of the few randomized trials performed, including 220 patients randomized to HDR-BT boost versus EBRT alone. After a median follow-up duration of 85 months, a noticeable improvement in was observed in recurrence-free survival for patients who received HDR-BT boost, with a median time to relapse of 116 months compared with 74 months for EBRT alone. The 5-year, 7-year and 10-year estimates of FFBF were 75%, 66% and 46%, respectively, for HDR-BT boost, compared with 61%, 48% and 39%, respectively, for EBRT alone (log rank P = 0.04). T3 disease was present in 27% of their population and Gleason score ≥ 7 in 58%. The 5-year and 7-year incidences for patients with any severe urinary symptom were 26% and 31%, respectively for those treated with HDR-BT boost compared with 26% and 30%,respectively, for those given EBRT alone (log rank P = 0.5). The authors concluded that HDR-BT boost could have an important role in the treatment of patients with intermediate-risk and high-risk disease.
A number of other prospective studies have been performed using HDR-BT boost.44-46, 48, 51, 53, 54, 161, 174, 175, 183 In a systematic review of these studies,56 the reported 5-year prostate-cancer-specific mortality, overall survival, local recurrence rates, and distant metastasis rates were 99–100%, 85–100%, 0–8%, and 0–12%, respectively. These outcomes are similar to those of LDR-BT, EBRT, and LDR-BT boost55, 56 and better than EBRT alone, as reported in ASCENDE-RT.54 Studies reporting outcomes outside of these ranges typically include patients with high-risk and locally advanced disease or exclude patients who have received ADT.45, 48, 49, 53, 183
Based on systematic reviews,55, 56 the rates of Grade 3–4 genitourinary and gastrointestinal toxicities are 0–12% and 0–8%, respectively, in phase I/II studies with ≥4-year median follow-up duration44, 46, 48, 52, 53. Furthermore, among studies that compare HDR-BT boost with EBRT alone, the rate of stricture occurrence is considerably higher in the boost arm.54, 160, 162 RTOG 032147, the first multi-institutional prospective trial using HDR-BT boost in the USA, provided detailed reports of toxicity and stricture occurrence. With a median follow-up period of 2.5 years, RTOG 0321 reported a rate of 2.6% for Grade 3–4 toxic effects, and a rate of urinary stricture of 0.7%47.
The outcomes of HDR-BT boost are encouraging, particularly for high-risk prostate cancer. One of the most important factors associated with outcome analyses is the role of ADT, which is associated with improved rates of FFBF, distant metastasis-free survival, and prostate-cancer-specific survival.91, 227 However, the role of ADT is often overlooked and in the systematic reviews of published studies, no multivariate analysis is performed to evaluate the effect of ADT on outcomes.55, 56 In the prospective study by Hoskin and colleagues54, treatment arm, risk category, and ADT use were significant covariates for biochemical recurrence.
The toxic effects associated with of HDR-BT boost are similarly encouraging, and in systematic reviews of prospective studies, the late Grade 3–4 toxicity rate is <5% (Table 4).55, 56, 62 By contrast, Grade 3–4 toxicities, including stricture, occur in <3% of patients receiving EBRT alone.4, 56, 228 LDR-BT boost RTOG Grade 3–4 genitourinary toxicities, including stricture, from two phase II studies were observed in 13%214 and 3%215 of patients, and gastrointestinal toxicities were seen in 3%214 and 0%215 of patients. Besides dosimetric quality constraints, characteristics predicting late toxicity include initial presence of symptoms,157 ADT use,157, 180 older age (> ~65 years),157, 181 high-risk status,180 previous transurethral resection of the prostate,152, 171 hypertension,152 and diabetes.229 Based on these data, HDT-BT is not an ideal treatment modality for all patients with high-risk prostate cancer, and clinicians might choose to use another modality, for example EBRT alone, in elderly patients with comorbidities.
In summary, HDR-BT boost is now a well-established treatment modality for patients with intermediate-risk and high-risk prostate cancer, particularly those without contraindications (Box 1). Similarly, HDR-BT monotherapy is associated with excellent outcomes and toxicity profiles in men with low-risk and favourable intermediate-risk disease. HDR-BT monotherapy studies tend to have a shorter follow-up time and include fewer patients than studies of LDR-BT and EBRT; thus, studies with >10–15 years of follow-up duration that report efficacy and toxicity will not be published until the 2020s.
Salvage for local recurrence after EBRT
Brachytherapy with either LDR-BT or HDR-BT monotherapy are possible treatments for local recurrence after EBRT or LDR-BT.230-234 Salvage brachytherapy is a promising option, particularly for patients who are not deemed fit for salvage prostatectomy. The NCCN guidelines include few recommendations regarding the approach;3 thus, referral to a specialty centre with salvage experience is recommended.
Nguyen et al.235 published a prospective phase II study of MRI-guided salvage brachytherapy for 24 men with a rising serum PSA and biopsy-proven, intraprostatic cancer at least 2 years after initial radiotherapy (EBRT or brachytherapy), who had favourable clinical features: Gleason score <8, serum PSA < 0 ng/ml, and negative pelvic and bone imaging studies. They achieved biochemical control in 70% of patients at 4 years after the salvage procedure. The 4-year estimate of grade 3+ gastrointestinal or genitourinary toxicity was 30% and 13 patients required a colostomy and/or urostomy to repair a fistula.
The ideal salvage brachytherapy patient (Box 2)235-238 should have biopsy-proven local-only recurrence, preferably with no evidence of widely metastatic disease, or none that cannot be controlled with focal therapy at other sites (for example, SBRT for oligometastatic recurrence).239, 240 Factors that suggest a local-only recurrence are the presence of a nadir after initial therapy, PSA doubling time >1–2 years, recurrent serum PSA measurements <10 ng/mL, and no Gleason 8–10 disease, which is more likely to disseminate241. For patients with widely metastatic disease, the excessive morbidity of local therapy and likely lack of a survival benefit obviate the need for salvage brachytherapy. Next, the patient should have minimal, and preferably no, morbidity from previous radiotherapy, and that treatment should have been performed >4.5 years before the date of salvage brachytherapy.235 Patients with ongoing toxic sequlae, such as a nonhealing ulcer, are unlikely to benefit from salvage brachytherapy and are more likely to develop further toxic effects, including infection, bleeding, pain, necrosis, and blood clots. Additionally, the patient should have no contraindications to prevent use of pelvic MRI, which is used to delineate the region of recurrence and for brachytherapy planning.235 Finally, the use of a rectal spacer can be considered to minimize radiation dose to the rectum.238
Box 2 ∣. The ideal patient for salvage brachytherapy.
- Local-only recurrence
- PSA nadir after initial therapy that did not rise
- PSA doubling time >1–2 years
- recurrent PSA <10 ng/ml
- no Gleason 8–10 disease, which has a higher propensity to disseminate
Biopsy-proven disease
No or minimal morbidity from prior radiotherapy
> 4.5 years since prior radiotherapy
Ability to tolerate MRI, which would be used for brachytherapy planning
Possible insertion of a rectal spacer to minimize dose to the rectum
Ongoing trials
Several studies are currently in progress to directly compare LDR-BT with HDR-BT (Table 5). Additionally, several studies are evaluating the efficacy of HDR-BT for local recurrence. The use of brachytherapy as a focal boost to treat part of the prostate in the area identified to have the cancer focus, either with biopsy or novel imaging, is also under investigation.
Table 5∣.
Ongoing studies evaluating brachytherapy for prostate cancer
| Study identifier | Location | Phase | Arms / randomization | Outcomes |
|---|---|---|---|---|
| NCT02628041264 | Université de Montréal, Sunnybrook Health Sciences Centre, Quebec, Canada | 1–2 | Active comparator: LDT-BT with 125I Experimental: HDR-BT |
Quality of life |
| NCT02346253265 | Stanford University, Palo Alto, California, USA | 1 | Experimental: Treatment (HDR-BT, ADT and LHRH agonist therapy) | Acute toxicities |
| NCT02258087266 | National Institute of Oncology, Hungary | 1–2 | Active Comparator: LDT-BT with 125I Experimental: HDR-BT |
Acute and chronic toxicities |
| NCT02560181267 | Sunnybrook Health Sciences Centre, Quebec, Canada | 1–2 | Experimental: HDR-BT for recurrent prostate cancer | Acute toxicities |
| NCT02322931268 | British Columbia Cancer Agency, Vancouver, British Columbia, Canada | 2 | Experimental: Imaging interventions. Patients are randomized to one of four arms with different imaging procedures for LDR-BT | Feasibility of replacing Day 0 CT with intraoperative 3D C-arm imaging |
| NCT02225925269 | Johns Hopkins University, Baltimore, Maryland, USA | 2 | Experimental: Dynamic dosimetry with intraoperative LDR-BT | Procedure time |
| NCT00450411270 | British Columbia Cancer Agency | 1–2 | Experimental: US-guided LDR-BT for prostate cancer local recurrence after EBRT | Acute toxicities |
| NCT02632669271 | Royal Surrey County Hospital NHS Foundation Trust | 1 | Experimental: Hemigland focal LDR-BT using permanent 125I seed implantation | Acute toxicities |
| NCT02290366272 | University of Pittsburgh, Pittsburgh, Pennsylvania, USA | 2 | Experimental: Hemigland focal LDR-BT using permanent 131Cs seed implantation | Biochemical outcomes at 5 years |
| NCT00913939273 | Princess Margaret Hospital, Toronto, Canada | 2 | Experimental arm 1: MRI-guided HDR-BT as salvage after EBRT Experimental arm 2: MRI-guided HDR-BT as boost to EBRT |
Favourable measures of technical performance |
| NCT01936883274 | British Columbia Cancer Agency, Vancouver, British Columbia, Canada | 3 | Active comparator: LDT-BT with 125I Experimental: HDR-BT |
Acute toxicities, quality of life, biochemical outcomes |
| NCT01909388275 | Alfonso Gomez-Iturriaga, Hospital de Cruces, Bizkaia, Spain | 1–2 | Active comparator: MRI–TRUS-fusion-guided real-time HDR-BT | Feasibility based on dosimetry |
| NCT02790216276 | Sheba Medical Center, Tel Hashomer, Israel | 2 | Experimental: Deformable registration of multiparametric MRI to intraoperative transrectal ultrasound for LDR-BT with 125I | Feasibility, based on absence of tumour outside of brachytherapy volume (from biopsy) |
| NCT02652000277 | University of Zurich, Germany | 3 | LDR-BT with 125I | Acute toxicities, quality of life |
| NCT02597894278 | University of California Los Angeles, California, USA | 1 | Experimental: Targeted biopsies in determining response to ADT and HDR-BT | Feasibility of obtaining adequate biopsies from intraprostatic index lesion prior to ADT and then at time of HDR-BT |
| NCT02805894279 | Brigham and Women's Hospital, Boston, Massachusetts, USA | 1 | Experimental: NBTXR3 ((R), a radiosensitizing nanoparticle) activated by IMRT Experimental: NBTXR3 activated by brachytherapy and IMRT |
Maximum tolerated dose and early dose limiting toxicities |
| NCT01437085280 | Cross Cancer Institute, Alberta, Canada | Not available | Experimental: Observational study quantifying needle deflection and tissue deformation in prostate brachytherapy | Not available |
| NCT02623933281 | Sunnybrook Health Sciences Centre, Ontario, Canada | 1 | Experimental: MRI-assisted focal boost with HDR-BT monotherapy | Acute toxicities |
Abbreviations: ADT: androgen deprivation therapy; EBRT: external beam radiation therapy; HDR-BT: high dose rate brachytherapy; LDR-BT: low dose rate brachytherapy; MRI: magnetic resonance imaging.
Focal therapy aims to minimize the volume of normal tissue irradiated (rectum, bladder, urethra) and maximize the dose to tumour, which is typically identified using imaging studies. In a systematic review of focal therapy in the management of localized prostate cancer that included 2,350 patients across 30 studies, brachytherapy was used in only two of these studies, and one was in the setting of recurrent disease.235 Thus, the ideal patient and dose for focal therapy are not well established; the meta-analysis states that focal therapy was mainly delivered to men with low-risk and intermediate-risk disease.242
Follow-up monitoring
After brachytherapy, close follow-up monitoring with serum PSA measurements at regular intervals is recommended, as well as a digital rectal examination (DRE). The optimal surveillance frequency following brachytherapy has not been established. According to the NCCN and ABS guidelines, an interval of every 6–12 months is appropriate for most patients, but those with high-risk disease should be monitored more frequently.3, 8 Quality of life (QOL) measurements can include the Short Form-36 (SF-36), the Client Satisfaction Questionnaire-8 (CSQ-8), the Functional Assessment of Cancer Therapy-General (FACT-G), the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)–C30 (EORTC QLQ–C30), the University of California, Los Angeles Prostate Cancer Index (UCLA-PCI), and the Expanded Prostate Cancer Index Composite (EPIC).79 All of these forms capture the recommended quality-of-life components: urinary incontinence, urinary obstruction and irritation, bowel-related symptoms, sexual dysfunction, and hormonal symptoms.243 Among the various metrics, the EPIC, UCLA-PCI, and EORTC are the most frequently used and are preferred.243-247,248, 249
Other QOL metrics available include the AUA International Prostate Symptom Score (I-PSS) and the Sexual Health Inventory for Men (SHIM).250 The IPSS is intended to evaluate men with benign prostatic hypertrophy, whereas the SHIM was designed by industry to evaluate therapies for erectile dysfunction251, 252 Although these are sometimes used for QOL comparison, they are not preferred as they do not capture such a wide range of data as is collected in the EPIC, UCLA-PCI, or EORTC QLQ-C30.253 For example, the IPSS score does not collect information about incontinence.
PSA kinetics should be monitored after brachytherapy (FIG 6). Biochemical failure can be defined in a number of ways: the ABS and ASTRO favour the use of the Phoenix definition (PSA nadir + 2 ng/ml) following treatment. After LDR-BT, PSA decreases to < 0.3 ng/ml in most men with localized disease.254 Patients should also be monitored for biochemical failure, keeping in mind that the features of benign PSA bounces and biochemical failure overlap substantially, although benign bounce is uncommon after 36 months.255
FIG 6 ∣. Characteristic PSA curves after treatment.
Different radiotherapy modalities are associated with different trends in post-treatment PSA.4 The Phoenix definition (PSA nadir + 2 ng/mL, circled on the right) following treatment (in this case, EBRT) is the preferred protocol to define biochemical failure. EBRT typically induces a gradual and inconsistent decrease in PSA to levels that are still detectable. After LDR-BT, PSA has been noted to decrease to <0.3 ng/ml in most men with localized disease, although a much lower decrease is possible. After HDR-BT, PSA nadir is usually very low (< 0.05 ng/ml). All radiotherapy modalities can induce a PSA bounce — a temporary elevation in PSA without disease recurrence, circled on the left, which are common and do not automatically represent cancer recurrence.
PSA bounce can occur after any form of radiotherapy, and the clinician must appreciate that PSA bounces are common, do not automatically represent cancer recurrence, and are associated with various patient, cancer, and dosimetric factors (for example, the urethra D90).256 A routine biopsy is not usually recommended; however, before any treatment is recommended or initiated, cancer recurrence needs to be documented by biopsy or imaging.23, 241 If a rising PSA is noted and prostate biopsy is performed, the biopsy should be done ≥30 months after completion of LDR-BT, or it might be uninterpretable, and a false positive result can be mistakenly determined when actually a benign PSA bounce is likely.257
PSA bounces >1.0 ng/ml are rare after LDR-BT with or without neoadjuvant ADT, occurring in <10% of patients.258 However, as PSA bounce amplitude increases, the biochemical failure rate also increases.258 By contrast, patients with Gleason score 6 disease are more likely to experience a PSA bounce and have improved FFBF.259 An increased prostatic urethra D90 seems to correlate with the likelihood of having a PSA bounce, and PSA levels typically take 2–3 years to reach the nadir.256
The PSA nadir is typically a very low after HDR-BT, <0.05ng/ml.260 The rate of PSA bounce seems to be more frequent in patients treated with LDR-BT than those who received HDR-BT or EBRT, at 42%, 23%, and 20%, respectively.261 In a series of 114 men treated with HDR-BT boost with hypofractionated EBRT, PSA bounce occurred in 39% of patients after a median of 16 months. The median magnitude of bounce was 0.45 ng/ml and biochemical failure occurred in 11% of patients who experienced a bounce.262 In a separate series of 67 patients, 43% experienced a PSA bounce; among all patients treated with HDR-BT monotherapy, 28% of patients have a bounce < 1 ng/mL and 15% have a bounce higher than 1 ng/mL .263 Patients who experienced a PSA bounce typically had a lower Gleason score tumour than those who did not experience a bounce and were aged < 55 years.263 The authors provided several hypotheses for these findings, including more frequent sexual activity among younger patients.
Conclusions
Brachytherapy and brachytherapy boost are excellent first-line therapies in the management of men with prostate cancer. LDR-BT monotherapy is acknowledged as a standard option in low-risk prostate cancer by health organizations internationally. HDR-BT monotherapy can be used as a first-line treatment option in patients with low-risk and intermediate-risk prostate cancer, whereas HDR-BT boost is a well-established treatment modality for certain intermediate-risk and high-risk prostate cancer. ADT can be used in conjunction with either form of brachytherapy.
The outcomes from either form of brachytherapy are generally excellent and superior to EBRT, and the risk of grade 3–4 toxicities is <5% according to most studies. Patients should be followed up every 6–12 months with serum PSA measurement and DRE. Several QOL questionnaires can be used to gauge urinary incontinence, urinary obstruction and irritation, bowel-related symptoms, sexual dysfunction, and hormonal symptoms.
The ideal brachytherapy patient should have no absolute contraindications to the therapy, including ataxia telangiectasia, pre-existing rectal fistula, comorbidity precluding anaesthesia, distant metastases, absence of a rectum, or large TURP defects. Clinicians considering LDR-BT versus HDR-BT should consider the need for a shielded room, initial capital equipment and recurring costs, and operator dependence.
Salvage brachytherapy can be used to treat local recurrence after prior radiation therapy. The ideal patient for salvage therapy has biopsy-proven local-only recurrence with no morbidity from prior radiotherapy. Salvage brachytherapy is best if performed >4.5 years after prior radiation treatment, with the use of advanced image guidance, and with a rectal spacer. Brachytherapy is an excellent treatment option for definitive and salvage treatment of prostate cancer; its continued evolution provides excellent outcomes, limited toxicity, generally excellent quality of life, and a low cost.
Key points.
Brachytherapy and brachytherapy boost with low-dose-rate brachytherapy (LDR-BT) or high-dose-rate (HDR)-BT can be used as first-line therapies in the management of prostate cancer patients of all National Comprehensive Cancer Network (NCCN)-defined risk groups.
LDR-BT, consisting of a single implant, typically uses 125I or 103Pd; by contrast, HDR-BT consists of 1–3 implants and uses 192Ir.
Benefits of HDR-BT over LDR-BT include the ability to use the same source for other cancers, lower operator dependence, and fewer acute irritative symptoms.
Benefits of LDR-BT include more favourable scheduling logistics, lower initial capital equipment costs, non-requirement of a shielded room, completion in a single implant, and more robust data from clinical trials.
Outcomes of HDR-BT and LDR-BT are similar to those of other treatment options, including external beam radiotherapy (EBRT) and surgery, and brachytherapy can also be used in combination with EBRT in intermediate-risk and high-risk disease.
Severe toxicities of HDR-BT and LDR-BT are rare, although the rate of urethral stricture is increased when brachytherapy boost is performed; incontinence is not associated with any radiotherapy modality.
GLOSSARY
- α/β ratio
The α/β ratio describes the shape of the cell surviva curve and the gradient of the two components of cell kill, α and β. The α/β ratio is used to describe the dose response of radiation on different tissues. Prostate cancer cells have a relatively low α/β ratio of 1.5, implying that those cells are more sensitive to doses delivered in larger fraction size. In the radiobiological linear quadratic equation, it is the dose at which cell killing due to the linear and quadratic components are equal.
- Biologically equivalent dose (BED)
A more conceptually useful measure of biological damage to cells than physical dose. It takes into account the α/β ratio, number of radiation fractions, and fraction size. BED = (nd[1 + d/(α/β)]). In this formula, n is the number of radiation fractions and d is dose size per fraction.
- Clinical target volume (CTV)
This volume encompasses the GTV as well as areas at risk for subclinical cancer involvement. The CTV can include a margin around the prostate GTV and adjacent regions at risk of having subclinical disease.
- D0.1cc or Dmax
The average dose to the hottest point of a volume. The term “D0.1cc” is sometimes used because this approximates the maximum dose to the smallest volume that can be calculated on a computer.
- D2cc
The average dose to 2cc of a volume.
- D10
The average dose to 10% of a volume, in Gy. The urethra D10 should be <150% of the prescribed dose. This constraint limits the dose to the urethra.
- D30
The average dose to 30% of a volume, in Gy. The urethra D30 should be <130% of the prescribed dose. This constraint limits the dose to the urethra.
- D90
In prostate cancer brachytherapy, this is the minimum dose in the hottest 90% of a volume, in Gy. The prostate D90% should be >100%. This constraint ensures the prostate volume receives adequate dose.
- Dwell time
The time that the 192Ir source spends in a predetermined dwell position during HDR-BT. A longer dwell time in a position translates to a greater dose deposited in the volume around the position.
- Dwell position
The position where a 192Ir source is located during HDR-BT. A combination of dwell positions in different needles allows the delivery of a predetermined dose to the CTV (FIG 1C, right panel).
- Equivalent dose in 2 Gy fractions (EQD2)
The “2 Gy-per-fraction equivalent dose.” EQD2 = n*d*((d+ α/β )/(2+ α/β )), where The EQD2 uses a mathematical conversion of fractions and dose per fraction, similar to the BED. In this formula, n is the number of radiation fractions and d is dose size per fraction..
- Gross tumour volume (GTV)
This is the demonstrable extent and location of the malignant growth; it consists of the primary tumor, which for prostate cancer has historically been defined as the entire gland as well as any visualized extension into surrounding normal tissues, the regional lymph nodes, or distant metastases based on clinical data.
- hot spot
A colloquialism used to describe volume outside the PTV which receives dose larger than 100% of the specified PTV dose.
- Hypofractionated radiation therapy
A type of EBRT that is delivered as a single 2.1–3.5 Gy fraction lasting 15 minutes per day, five days per week, for about four weeks
- intensity modulated radiation therapy (IMRT)
An advanced form of high-precision radiation that conforms the treatment volume to the shape of the tumor. The dose distribution created by IMRT is characterized by a concavity or invagination of the edge of the higher doses away from the rectum, rather than a straight edge through the rectum as seen with 3D-CRT.
- multi-leaf collimator (MLC)
A device made up of individual leaves of a high atomic numbered material that can move independently in and out of the path of an X-ray beam to contour its shape to a tumour.
- Phoenix definition
Used for measuring biochemical failure after radiotherapy for prostate cancer, defined as the PSA nadir value plus 2 ng/ml
- Planning target volume (PTV)
This volume encompasses the CTV plus an additional margin to account for patient movement, setup error, and organ movement
- Remote afterloading system (RALS)
Integral to HDR-BT, a RALS automatically deploys and retracts a single small radioactive source along the implant needle at specific positions delivering ≥ 12 Gy/hr. The RALS allows a physician to control the position where the HDR source stops for a predetermined time periods (the dwell position and dwell time).
- RV100
In prostate cancer brachytherapy, this is the volume of the rectum receiving 100% of the dose, and should be <1 cc.
- Stereotactic body radiation therapy (SBRT)
A type of EBRT delivered as a single 3.5–15.0 Gy fraction lasting up to 45 minutes per day, for a total of about five treatments over about 2 weeks
- V100
In prostate cancer brachytherapy, this is the percentage of a structure receiving 100% of the dose. For example, the V100 for the prostate should be > 90%, meaning that 100% of the prostate CTV should receive more than 90% of the prescribed dose.
- V150
In prostate cancer brachytherapy, this is the percentage of a structure receiving 150% of the dose. The V150 for the prostate CTV should be <50–60%, meaning that <50–60% of the CTV should receive >150% of the prescribed dose.
- UV5
In prostate cancer brachytherapy, this is the average dose to 5% of the urethral volume receiving the highest dose. The UV5 should receive <150% of the dose.
- UV30
In prostate cancer brachytherapy, this is the average dose to 30% of the urethral volume receiving the highest dose. The UV30 should be <125% of the dose.
- UV150
In prostate cancer brachytherapy, this is the volume of the urethra receiving 150% of the prescribe dose. UV150 of the urethra should be 0%, meaning that 0% of the volume should receive 150% of the prescribed dose.
Footnotes
Competing interests statement
The authors declare no competing interests.
Review criteria
The MEDLINE and PubMed databases were searched, using PICOS/PRISMA methods for original articles and guidelines focusing on prostate cancer brachytherapy published between 1970 and 2016. The search terms used were “prostate cancer” and “brachytherapy” combined with any of: “high dose rate” or “low dose rate.” All papers identified were English-language full-text articles. The reference lists of identified articles were searched for further papers. Additionally, the ClinicalTrials.gov database was searched for ongoing clinical trials.
Publishers note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
REFERENCES
- 1.Global Burden of Disease Cancer, C. et al. The Global Burden of Cancer 2013. JAMA oncology 1, 505–527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cahlon O et al. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int. J. Radiat. Oncol. Biol. Phys 71, 330–337 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Mohler JL et al. Prostate Cancer, Version 1.2016. J. Natl. Compr. Canc. Netw 14, 19–30 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Zaorsky NG et al. Comparison of outcomes and toxicities among radiation therapy treatment options for prostate cancer. Cancer Treat. Rev 48, 50–60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donovan JL et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N. Engl. J. Med 375, 1425–1437 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamdy FC et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med 375, 1415–1424 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Shen X et al. Comparative effectiveness research for prostate cancer radiation therapy: current status and future directions. Future Oncol. 8, 37–54 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Davis BJ et al. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy 11, 6–19 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y et al. American Brachytherapy Society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy 11, 20–32 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Salembier C et al. Tumour and target volumes in permanent prostate brachytherapy: a supplement to the ESTRO/EAU/EORTC recommendations on prostate brachytherapy. Radiother. Oncol 83, 3–10 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Hoskin PJ et al. GEC/ESTRO recommendations on high dose rate afterloading brachytherapy for localised prostate cancer: an update. Radiother. Oncol 107, 325–332 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Case JT The early history of radium therapy and the American Radium Society. Am. J. Roentgenol. Radium Ther. Nucl. Med 82, 574–585 (1959). [PubMed] [Google Scholar]
- 13.Zeitlin SI, Sherman J, Raboy A, Lederman G & Albert P High dose combination radiotherapy for the treatment of localized prostate cancer. J. Urol 160, 91–95; discussion 95–96 (1998). [PubMed] [Google Scholar]
- 14.Garzotto M & Fair WR Historical perspective on prostate brachytherapy. J. Endourol 14, 315–318 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Lederman M The early history of radiotherapy: 1895–1939. Int. J. Radiat. Oncol. Biol. Phys 7, 639–648 (1981). [DOI] [PubMed] [Google Scholar]
- 16.Young HH The Use of Radium and the Punch Operation in Desperate Cases of Enlarged Prostate. Ann. Surg 65, 633–641 (1917). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deming CL Results in one hundred cases of cancer of prostate and seminal vesicles treated with radium. Surg. Gynecol. Obstet 34, 99–118 (1922). [Google Scholar]
- 18.Flocks RH, Kerr HD, Elkins HB & Culp DA The treatment of carcinoma of the prostate by interstitial radiation with radioactive gold (Au198); a follow-up report. J. Urol 71, 628–633 (1954). [DOI] [PubMed] [Google Scholar]
- 19.Flocks RH Interstitial Irradiation Therapy with a Solution of Au198 as Part of Combination Therapy for Prostatic Carcinoma. J. Nucl. Med 5, 691–705 (1964). [PubMed] [Google Scholar]
- 20.Bagshaw MA, Kaplan HS & Sagerman RH Linear accelerator supervoltage VII. Carcinoma of the prostate. Radiology 85, 121–129 (1965). [DOI] [PubMed] [Google Scholar]
- 21.Whitmore WF Jr., Hilaris B & Grabstald H Retropubic implantation to iodine 125 in the treatment of prostatic cancer. J. Urol 108, 918–920 (1972). [DOI] [PubMed] [Google Scholar]
- 22.Hilaris BS, Whitmore WF Jr., Batata MA & Grabstald H Radiation therapy and pelvic node dissection in the management of cancer of the prostate. Am. J. Roentgenol. Radium Ther. Nucl. Med 121, 832–838 (1974). [DOI] [PubMed] [Google Scholar]
- 23.Zaorsky NG et al. A paradigm shift from anatomic to functional and molecular imaging in the detection of recurrent prostate cancer. Future Oncol. 10, 457–474 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitmore WF Jr., Hilaris B, Grabstald H & Batata M Implantation of 125I in prostatic cancer. Surg. Clin. North Am 54, 887–895 (1974). [DOI] [PubMed] [Google Scholar]
- 25.Whitmore WF Jr., Hilaris B & Grabstald H Retropubic implantation of iodine 125 in the treatment of prostatic cancer. Trans. Am. Assoc. Genitourin. Surg 64, 55–57 (1972). [PubMed] [Google Scholar]
- 26.Batata MA et al. Radiation therapy in adenocarcinoma of the prostate with pelvic lymph node involvement on lymphadenectomy. Int J Radiat Oncol 6, 149–153 (1980). [DOI] [PubMed] [Google Scholar]
- 27.Whitmore WF Jr. Interstitial radiation therapy for carcinoma of the prostate. The Prostate 1, 157 (1980). [DOI] [PubMed] [Google Scholar]
- 28.Zelefsky MJ & Whitmore WF Jr. Long-term results of retropubic permanent 125iodine implantation of the prostate for clinically localized prostatic cancer. J. Urol 158, 23–29; discussion 29–30 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Ragde H et al. Interstitial iodine-125 radiation without adjuvant therapy in the treatment of clinically localized prostate carcinoma. Cancer 80, 442–453 (1997). [DOI] [PubMed] [Google Scholar]
- 30.Gottesman JE, Tesh DG & Weissman WD Failure of open radioactive 125iodine implantation to control localized prostate cancer: a study of 41 patients. J. Urol 146, 1317–1319; discussion 1319–1320 (1991). [DOI] [PubMed] [Google Scholar]
- 31.Kuban DA, el-Mahdi AM & Schellhammer PF I-125 interstitial implantation for prostate cancer. What have we learned 10 years later? Cancer 63, 2415–2420 (1989). [DOI] [PubMed] [Google Scholar]
- 32.Holm HH, Juul N, Pedersen JF, Hansen H & Stroyer I Transperineal 125iodine seed implantation in prostatic cancer guided by transrectal ultrasonography. 1983. J. Urol 167, 985–988; discussion 988–989 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Blasko JC et al. Should brachytherapy be considered a therapeutic option in localized prostate cancer? Urol. Clin. North Am 23, 633–650 (1996). [DOI] [PubMed] [Google Scholar]
- 34.Heysek RV Modern brachytherapy for treatment of prostate cancer. Cancer Control 14, 238–243 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Zaorsky NG et al. Evolution of advanced technologies in prostate cancer radiotherapy. Nat Rev Urol 10, 565–579 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Thompson I et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 177, 2106–2131 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Tapen EM et al. Reduction of radioactive seed embolization to the lung following prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys 42, 1063–1067 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Schwartz DJ et al. Radiation exposure to operating room personnel during transperineal interstitial permanent prostate brachytherapy. Brachytherapy 2, 98–102 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Mate TP, Gottesman JE, Hatton J, Gribble M & Van Hollebeke L High dose-rate afterloading 192Iridium prostate brachytherapy: feasibility report. Int. J. Radiat. Oncol. Biol. Phys 41, 525–533 (1998). [DOI] [PubMed] [Google Scholar]
- 40.McNeal JE, Redwine EA, Freiha FS & Stamey TA Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am. J. Surg. Pathol 12, 897–906 (1988). [DOI] [PubMed] [Google Scholar]
- 41.McNeal JE et al. Patterns of progression in prostatic carcinoma. Lancet 1, 60–63 (1986). [DOI] [PubMed] [Google Scholar]
- 42.Stromberg J et al. Ultrasound-guided high dose rate conformal brachytherapy boost in prostate cancer: treatment description and preliminary results of a phase I/II clinical trial. Int. J. Radiat. Oncol. Biol. Phys 33, 161–171 (1995). [DOI] [PubMed] [Google Scholar]
- 43.Kovacs G et al. Prostate preservation by combined external beam and HDR brachytherapy in nodal negative prostate cancer. Strahlenther. Onkol 175 Suppl 2, 87–88 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Duchesne GM, Williams SG, Das R & Tai KH Patterns of toxicity following high-dose-rate brachytherapy boost for prostate cancer: mature prospective phase I/II study results. Radiother. Oncol 84, 128–134 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Galalae RM et al. Hypofractionated conformal HDR brachytherapy in hormone naive men with localized prostate cancer. Is escalation to very high biologically equivalent dose beneficial in all prognostic risk groups? Strahlenther. Onkol 182, 135–141 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Kalkner KM et al. Clinical outcome in patients with prostate cancer treated with external beam radiotherapy and high dose-rate iridium 192 brachytherapy boost: a 6-year follow-up. Acta Oncol. 46, 909–917 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Hsu IC et al. Phase II trial of combined high-dose-rate brachytherapy and external beam radiotherapy for adenocarcinoma of the prostate: preliminary results of RTOG 0321. Int. J. Radiat. Oncol. Biol. Phys 78, 751–758 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez A et al. Conformal high dose rate brachytherapy improves biochemical control and cause specific survival in patients with prostate cancer and poor prognostic factors. J. Urol 169, 974–979; discussion 979–980 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Martinez AA et al. High-dose-rate prostate brachytherapy: an excellent accelerated-hypofractionated treatment for favorable prostate cancer. Am. J. Clin. Oncol 33, 481–488 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Martinez-Monge R et al. External-beam radiation therapy and high-dose rate brachytherapy combined with long-term androgen deprivation therapy in high and very high prostate cancer: preliminary data on clinical outcome. Int. J. Radiat. Oncol. Biol. Phys 82, e469–476 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Morton G et al. Is single fraction 15 Gy the preferred high dose-rate brachytherapy boost dose for prostate cancer? Radiother. Oncol 100, 463–467 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Myers MA et al. Phase I/II trial of single-fraction high-dose-rate brachytherapy-boosted hypofractionated intensity-modulated radiation therapy for localized adenocarcinoma of the prostate. Brachytherapy 11, 292–298 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Vargas CE et al. High-dose irradiation for prostate cancer via a high-dose-rate brachytherapy boost: results of a phase I to II study. Int. J. Radiat. Oncol. Biol. Phys 66, 416–423 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Hoskin PJ et al. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother. Oncol 103, 217–222 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Zaorsky NG, Den RB, Doyle LA, Dicker AP & Hurwitz MD Combining theoretical potential and advanced technology in high-dose rate brachytherapy boost therapy for prostate cancer. Expert Rev. Med. Devices 10, 751–763 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Zaorsky NG et al. High dose rate brachytherapy boost for prostate cancer: A systematic review. Cancer Treat. Rev 40, 414–425 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Yoshioka Y et al. High-dose-rate interstitial brachytherapy as a monotherapy for localized prostate cancer: treatment description and preliminary results of a phase I/II clinical trial. Int. J. Radiat. Oncol. Biol. Phys 48, 675–681 (2000). [DOI] [PubMed] [Google Scholar]
- 58.Yoshioka Y, Yoshida K, Yamazaki H, Nonomura N & Ogawa K The emerging role of high-dose-rate (HDR) brachytherapy as monotherapy for prostate cancer. J Radiat Res 54, 781–788 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshioka Y et al. Nationwide, Multicenter, Retrospective Study on High-Dose-Rate Brachytherapy as Monotherapy for Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys 97, 952–961 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Yoshioka Y et al. High-Dose-Rate Brachytherapy as Monotherapy for Intermediate- and High-Risk Prostate Cancer: Clinical Results for a Median 8-Year Follow-Up. Int. J. Radiat. Oncol. Biol. Phys 94, 675–682 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Martinez AA et al. Phase II prospective study of the use of conformal high-dose-rate brachytherapy as monotherapy for the treatment of favorable stage prostate cancer: a feasibility report. Int. J. Radiat. Oncol. Biol. Phys 49, 61–69 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Morton GC The emerging role of high-dose-rate brachytherapy for prostate cancer. Clin. Oncol. (R. Coll. Radiol.) 17, 219–227 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Zaorsky NG, Doyle LA, Hurwitz MD, Dicker AP & Den RB Do theoretical potential and advanced technology justify the use of high-dose rate brachytherapy as monotherapy for prostate cancer? Expert Rev. Anticancer Ther 14, 39–50 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Martin JM et al. The rise and fall of prostate brachytherapy: use of brachytherapy for the treatment of localized prostate cancer in the National Cancer Data Base. Cancer 120, 2114–2121 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glaser SM et al. Brachytherapy boost for prostate cancer: Trends in care and survival outcomes. Brachytherapy 16, 330–341 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Zaorsky NG et al. What Are Medical Students in the United States Learning About Radiation Oncology? Results of a Multi-Institutional Survey. Int. J. Radiat. Oncol. Biol. Phys 94, 235–242 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zaorsky NG et al. Impact of a radiation oncology elective on the careers of young physicians: update on a prospective cohort study. Int. J. Radiat. Oncol. Biol. Phys 86, 214–215 (2013). [DOI] [PubMed] [Google Scholar]
- 68.Gondi V et al. Results of the 2005–2008 Association of Residents in Radiation Oncology survey of chief residents in the United States: clinical training and resident working conditions. Int. J. Radiat. Oncol. Biol. Phys 81, 1120–1127 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Nabavizadeh N et al. Results of the 2013–2015 Association of Residents in Radiation Oncology Survey of Chief Residents in the United States. Int. J. Radiat. Oncol. Biol. Phys 94, 228–234 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Compton JJ et al. Resident-reported brachytherapy experience in ACGME-accredited radiation oncology training programs. Brachytherapy 12, 622–627 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Battermann JJ & van Es CA The learning curve in prostate seed implantation. Cancer Radiother. 4 Suppl 1, 119s–122s (2000). [PubMed] [Google Scholar]
- 72.Lee WR, deGuzman AF, Bare RL, Marshall MG & McCullough DL Postimplant analysis of transperineal interstitial permanent prostate brachytherapy: evidence for a learning curve in the first year at a single institution. Int. J. Radiat. Oncol. Biol. Phys 46, 83–88 (2000). [DOI] [PubMed] [Google Scholar]
- 73.Acher P et al. Permanent prostate brachytherapy: Dosimetric results and analysis of a learning curve with a dynamic dose-feedback technique. Int. J. Radiat. Oncol. Biol. Phys 65, 694–698 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Avkshtol V et al. A comparison of robotic arm versus gantry linear accelerator stereotactic body radiation therapy for prostate cancer. Research and reports in urology 8, 145–158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaorsky NG, Studenski MT, Dicker AP, Gomella L & Den RB Stereotactic body radiation therapy for prostate cancer: is the technology ready to be the standard of care? Cancer Treat. Rev 39, 212–218 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Zaorsky NG, Hurwitz MD, Dicker AP, Showalter TN & Den RB Is robotic arm stereotactic body radiation therapy "virtual high dose ratebrachytherapy" for prostate cancer? An analysis of comparative effectiveness using published data [corrected]. Expert Rev. Med. Devices 12, 317–327 (2015). [DOI] [PubMed] [Google Scholar]
- 77.Zaorsky NG, Li T, Devarajan K, Horwitz EM & Buyyounouski MK Assessment of the American Joint Committee on Cancer staging (sixth and seventh editions) for clinically localized prostate cancer treated with external beam radiotherapy and comparison with the National Comprehensive Cancer Network risk-stratification method. Cancer 118, 5535–5543 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zaorsky NG et al. Causes of death among cancer patients. Ann. Oncol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kleinmann N et al. The effect of ethnicity and sexual preference on prostate-cancer-related quality of life. Nat Rev Urol 9, 258–265 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Astrom L, Pedersen D, Mercke C, Holmang S & Johansson KA Long-term outcome of high dose rate brachytherapy in radiotherapy of localised prostate cancer. Radiother. Oncol 74, 157–161 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Ghilezan M et al. 10-year results in 1577 intermediate/high risk prostate cancer patients treated with external beam RT (EBRT) and hypofractionated high dose rate (HDR) brachytherapy boost. Int J Rad Biol Phys 69, S83–84 (2007). [Google Scholar]
- 82.Kaprealian T et al. High-dose-rate brachytherapy boost for prostate cancer: comparison of two different fractionation schemes. Int. J. Radiat. Oncol. Biol. Phys 82, 222–227 (2012). [DOI] [PubMed] [Google Scholar]
- 83.Kestin LL et al. Pathologic evidence of dose-response and dose-volume relationships for prostate cancer treated with combined external beam radiotherapy and high-dose-rate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys 54, 107–118 (2002). [DOI] [PubMed] [Google Scholar]
- 84.Mohammed N et al. Comparison of acute and late toxicities for three modern high-dose radiation treatment techniques for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys 82, 204–212 (2012). [DOI] [PubMed] [Google Scholar]
- 85.Prada PJ et al. Biochemical outcome after high-dose-rate intensity modulated brachytherapy with external beam radiotherapy: 12 years of experience. BJU Int. 109, 1787–1793 (2012). [DOI] [PubMed] [Google Scholar]
- 86.Vicini F, Vargas C, Gustafson G, Edmundson G & Martinez A High dose rate brachytherapy in the treatment of prostate cancer. World J. Urol 21, 220–228 (2003). [DOI] [PubMed] [Google Scholar]
- 87.Vicini FA, Vargas C, Edmundson G, Kestin L & Martinez A The role of high-dose rate brachytherapy in locally advanced prostate cancer. Semin. Radiat. Oncol 13, 98–108 (2003). [DOI] [PubMed] [Google Scholar]
- 88.Shen X, Keith SW, Mishra MV, Dicker AP & Showalter TN The impact of brachytherapy on prostate cancer-specific mortality for definitive radiation therapy of high-grade prostate cancer: a population-based analysis. Int. J. Radiat. Oncol. Biol. Phys 83, 1154–1159 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Morris WJ et al. ASCENDE-RT: A multicenter, randomized trial of dose-escalated external beam radiation therapy (EBRT-B) versus low-dose-rate brachytherapy (LDR-B) for men with unfavorable-risk localized prostate cancer. J. Clin. Oncol (2015). [Google Scholar]
- 90.Yamoah K et al. Large prostate gland size is not a contraindication to low-dose-rate brachytherapy for prostate adenocarcinoma. Brachytherapy 13, 456–464 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Zaorsky NG, Trabulsi EJ, Lin J & Den RB Multimodality therapy for patients with high-risk prostate cancer: current status and future directions. Semin. Oncol 40, 308–321 (2013). [DOI] [PubMed] [Google Scholar]
- 92.Zumsteg ZS et al. A New Risk Classification System for Therapeutic Decision Making with Intermediate-risk Prostate Cancer Patients Undergoing Dose-escalated External-beam Radiation Therapy. Eur. Urol (2013). [DOI] [PubMed] [Google Scholar]
- 93.D'Amico AV, Chen MH, Renshaw AA, Loffredo M & Kantoff PW Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA 299, 289–295 (2008). [DOI] [PubMed] [Google Scholar]
- 94.Jones CU et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N. Engl. J. Med 365, 107–118 (2011). [DOI] [PubMed] [Google Scholar]
- 95.Laverdiere J et al. The efficacy and sequencing of a short course of androgen suppression on freedom from biochemical failure when administered with radiation therapy for T2-T3 prostate cancer. J. Urol 171, 1137–1140 (2004). [DOI] [PubMed] [Google Scholar]
- 96.Roach M 3rd et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J. Clin. Oncol 26, 585–591 (2008). [DOI] [PubMed] [Google Scholar]
- 97.Denham JW et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 6, 841–850 (2005). [DOI] [PubMed] [Google Scholar]
- 98.Bolla M et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 360, 103–106 (2002). [DOI] [PubMed] [Google Scholar]
- 99.Lawton CA et al. Updated results of the phase III Radiation Therapy Oncology Group (RTOG) trial 85–31 evaluating the potential benefit of androgen suppression following standard radiation therapy for unfavorable prognosis carcinoma of the prostate. Int. J. Radiat. Oncol. Biol. Phys 49, 937–946 (2001). [DOI] [PubMed] [Google Scholar]
- 100.Merrick GS, Wallner KE & Butler WM Permanent interstitial brachytherapy for the management of carcinoma of the prostate gland. J. Urol 169, 1643–1652 (2003). [DOI] [PubMed] [Google Scholar]
- 101.Zaorsky NG et al. ACR Appropriateness Criteria® External beam Radiation Therapy treatment planning for Clinically localized Prostate Cancer, Part II of II. Advances in Radiation Oncology (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zaorsky NG et al. ACR Appropriateness Criteria® External beam Radiation Therapy treatment planning for Clinically localized Prostate Cancer, Part I of II. Advances in Radiation Oncology 2, 62–84 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bice WS et al. Recommendations for permanent prostate brachytherapy with (131)Cs: a consensus report from the Cesium Advisory Group. Brachytherapy 7, 290–296 (2008). [DOI] [PubMed] [Google Scholar]
- 104.Polo A, Salembier C, Venselaar J, Hoskin P & ESTRO, P.g.o.t.G. Review of intraoperative imaging and planning techniques in permanent seed prostate brachytherapy. Radiother. Oncol 94, 12–23 (2010). [DOI] [PubMed] [Google Scholar]
- 105.Stone NN, Hong S, Lo YC, Howard V & Stock RG Comparison of intraoperative dosimetric implant representation with postimplant dosimetry in patients receiving prostate brachytherapy. Brachytherapy 2, 17–25 (2003). [DOI] [PubMed] [Google Scholar]
- 106.Zauls AJ, Ashenafi MS, Onicescu G, Clarke HS & Marshall DT Comparison of intraoperatively built custom linked seeds versus loose seed gun applicator technique using real-time intraoperative planning for permanent prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys 81, 1010–1016 (2011). [DOI] [PubMed] [Google Scholar]
- 107.Prestidge BR et al. A survey of current clinical practice of permanent prostate brachytherapy in the United States. Int J Radiat Oncol Biol Phys 40, 461–465 (1998). [DOI] [PubMed] [Google Scholar]
- 108.Orio PF 3rd et al. Intraoperative ultrasound-fluoroscopy fusion can enhance prostate brachytherapy quality. Int J Radiat Oncol Biol Phys 69, 302–307 (2007). [DOI] [PubMed] [Google Scholar]
- 109.Shaikh T et al. Is it necessary to perform week three dosimetric analysis in low-dose-rate brachytherapy for prostate cancer when day 0 dosimetry is done? A quality assurance assessment. Brachytherapy 14, 316–321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Expert Panel on Radiation, O.-P. et al. American College of Radiology Appropriateness Criteria permanent source brachytherapy for prostate cancer. Brachytherapy 10, 357–362 (2011). [DOI] [PubMed] [Google Scholar]
- 111.Crook J, McLean M, Yeung I, Williams T & Lockwood G MRI-CT fusion to assess postbrachytherapy prostate volume and the effects of prolonged edema on dosimetry following transperineal interstitial permanent prostate brachytherapy. Brachytherapy 3, 55–60 (2004). [DOI] [PubMed] [Google Scholar]
- 112.Tanaka O et al. Comparison of MRI-based and CT/MRI fusion-based postimplant dosimetric analysis of prostate brachytherapy. Int J Radiat Oncol Biol Phys 66, 597–602 (2006). [DOI] [PubMed] [Google Scholar]
- 113.Soni PD, Berlin A, Venkatesan AM & McLaughlin PW MRI-guided functional anatomy approach to prostate brachytherapy. Brachytherapy (2016). [DOI] [PubMed] [Google Scholar]
- 114.Kataria T et al. Simple diagrammatic method to delineate male urethra in prostate cancer radiotherapy: an MRI based approach. Br. J. Radiol 89, 20160348 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rylander S, Buus S, Pedersen EM, Bentzen L & Tanderup K Dosimetric impact of contouring and needle reconstruction uncertainties in US-, CT- and MRI-based high-dose-rate prostate brachytherapy treatment planning. Radiother. Oncol (2017). [DOI] [PubMed] [Google Scholar]
- 116.Buus S et al. Learning curve of MRI-based planning for high-dose-rate brachytherapy for prostate cancer. Brachytherapy 15, 426–434 (2016). [DOI] [PubMed] [Google Scholar]
- 117.Hosni A et al. Dosimetric feasibility of ablative dose escalated focal monotherapy with MRI-guided high-dose-rate (HDR) brachytherapy for prostate cancer. Radiother. Oncol 122, 103–108 (2017). [DOI] [PubMed] [Google Scholar]
- 118.Venkatesan AM et al. Prostate MRI for brachytherapists: Anatomy and technique. Brachytherapy (2017). [DOI] [PubMed] [Google Scholar]
- 119.Thaker NG, Orio PF & Potters L Defining the value of magnetic resonance imaging in prostate brachytherapy using time-driven activity-based costing. Brachytherapy (2017). [DOI] [PubMed] [Google Scholar]
- 120.Yu Y et al. Permanent prostate seed implant brachytherapy: report of the American Association of Physicists in Medicine Task Group No. 64. Med. Phys 26, 2054–2076 (1999). [DOI] [PubMed] [Google Scholar]
- 121.Williamson JF et al. Recommendations of the American Association of Physicists in Medicine on 103Pd interstitial source calibration and dosimetry: implications for dose specification and prescription. Med. Phys 27, 634–642 (2000). [DOI] [PubMed] [Google Scholar]
- 122.Crook JM, Potters L, Stock RG & Zelefsky MJ Critical organ dosimetry in permanent seed prostate brachytherapy: defining the organs at risk. Brachytherapy 4, 186–194 (2005). [DOI] [PubMed] [Google Scholar]
- 123.Nath R et al. AAPM recommendations on dose prescription and reporting methods for permanent interstitial brachytherapy for prostate cancer: report of Task Group 137. Med Phys 36, 5310–5322 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gillan C et al. Radiation dose to the internal pudendal arteries from permanent-seed prostate brachytherapy as determined by time-of-flight MR angiography. Int. J. Radiat. Oncol. Biol. Phys 65, 688–693 (2006). [DOI] [PubMed] [Google Scholar]
- 125.Merrick GS et al. The importance of radiation doses to the penile bulb vs. crura in the development of postbrachytherapy erectile dysfunction. Int. J. Radiat. Oncol. Biol. Phys 54, 1055–1062 (2002). [DOI] [PubMed] [Google Scholar]
- 126.Buyyounouski MK et al. The radiation doses to erectile tissues defined with magnetic resonance imaging after intensity-modulated radiation therapy or iodine-125 brachytherapy. Int. J. Radiat. Oncol. Biol. Phys 59, 1383–1391 (2004). [DOI] [PubMed] [Google Scholar]
- 127.Wallner K et al. 125I versus 103Pd for low-risk prostate cancer: preliminary PSA outcomes from a prospective randomized multicenter trial. Int. J. Radiat. Oncol. Biol. Phys 57, 1297–1303 (2003). [DOI] [PubMed] [Google Scholar]
- 128.Peschel RE, Colberg JW, Chen Z, Nath R & Wilson LD Iodine 125 versus palladium 103 implants for prostate cancer: clinical outcomes and complications. Cancer J. 10, 170–174 (2004). [DOI] [PubMed] [Google Scholar]
- 129.Stock RG, Stone NN, Dahlal M & Lo YC What is the optimal dose for 125I prostate implants? A dose-response analysis of biochemical control, posttreatment prostate biopsies, and long-term urinary symptoms. Brachytherapy 1, 83–89 (2002). [DOI] [PubMed] [Google Scholar]
- 130.Yoshioka Y et al. External-beam radiotherapy for clinically localized prostate cancer in Osaka, Japan, 1995–2006: time trends, outcome, and risk stratification. Strahlenther. Onkol 185, 446–452 (2009). [DOI] [PubMed] [Google Scholar]
- 131.Kovacs G et al. GEC/ESTRO-EAU recommendations on temporary brachytherapy using stepping sources for localised prostate cancer. Radiother. Oncol 74, 137–148 (2005). [DOI] [PubMed] [Google Scholar]
- 132.Pisansky TM et al. High-dose-rate brachytherapy in the curative treatment of patients with localized prostate cancer. Mayo Clin. Proc 83, 1364–1372 (2008). [DOI] [PubMed] [Google Scholar]
- 133.Fowler J, Chappell R & Ritter M Is alpha/beta for prostate tumors really low? Int. J. Radiat. Oncol. Biol. Phys 50, 1021–1031 (2001). [DOI] [PubMed] [Google Scholar]
- 134.Yoshioka Y et al. Evaluation of anatomy-based dwell position and inverse optimization in high-dose-rate brachytherapy of prostate cancer: a dosimetric comparison to a conventional cylindrical dwell position, geometric optimization, and dose-point optimization. Radiother. Oncol 75, 311–317 (2005). [DOI] [PubMed] [Google Scholar]
- 135.Sumida I et al. Optimization of dose distribution for HDR brachytherapy of the prostate using Attraction-Repulsion Model. Int. J. Radiat. Oncol. Biol. Phys 64, 643–649 (2006). [DOI] [PubMed] [Google Scholar]
- 136.Van de Werf E, Lievens Y, Verstraete J, Pauwels K & Van den Bogaert W Time and motion study of radiotherapy delivery: Economic burden of increased quality assurance and IMRT. Radiother. Oncol 93, 137–140 (2009). [DOI] [PubMed] [Google Scholar]
- 137.Shah C et al. Brachytherapy provides comparable outcomes and improved cost-effectiveness in the treatment of low/intermediate prostate cancer. Brachytherapy 11, 441–445 (2012). [DOI] [PubMed] [Google Scholar]
- 138.Parthan A et al. in ASCO: Genitourinary Cancers Symposium. Journal of Clinical Oncology suppl 7:abstr 87 (2011). [Google Scholar]
- 139.Lievens Y, van den Bogaert W & Kesteloot K Activity-based costing: a practical model for cost calculation in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys 57, 522–535 (2003). [DOI] [PubMed] [Google Scholar]
- 140.Norlund A Costs of radiotherapy. Acta Oncol. 42, 411–415 (2003). [DOI] [PubMed] [Google Scholar]
- 141.Bauman G, Rumble RB, Chen J, Loblaw A & Warde P Intensity-modulated Radiotherapy in the Treatment of Prostate Cancer. Clin. Oncol. (R. Coll. Radiol.) 24, 461–473 (2012). [DOI] [PubMed] [Google Scholar]
- 142.Demanes DJ et al. High-dose-rate monotherapy: safe and effective brachytherapy for patients with localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys 81, 1286–1292 (2011). [DOI] [PubMed] [Google Scholar]
- 143.Barkati M et al. High-dose-rate brachytherapy as a monotherapy for favorable-risk prostate cancer: a Phase II trial. Int. J. Radiat. Oncol. Biol. Phys 82, 1889–1896 (2012). [DOI] [PubMed] [Google Scholar]
- 144.Ghadjar P et al. Toxicity and early treatment outcomes in low- and intermediate-risk prostate cancer managed by high-dose-rate brachytherapy as a monotherapy. Brachytherapy 8, 45–51 (2009). [DOI] [PubMed] [Google Scholar]
- 145.Ghilezan M et al. High-dose-rate brachytherapy as monotherapy delivered in two fractions within one day for favorable/intermediate-risk prostate cancer: preliminary toxicity data. Int. J. Radiat. Oncol. Biol. Phys 83, 927–932 (2012). [DOI] [PubMed] [Google Scholar]
- 146.Grills IS et al. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared to low dose rate palladium seeds. J. Urol 171, 1098–1104 (2004). [DOI] [PubMed] [Google Scholar]
- 147.Hayes J et al. Post-treatment PSA Kinetics of Three Prostate Cancer Treatment Regimens Involving Brachythe of Three Prostate Cancer Treatment Regimens Involving Brachytherapy. Brachytherapy 5, P106 (2006). [Google Scholar]
- 148.Mark RJ et al. Interstitial high dose rate (HDR) brachtherapy as monotherapy for early stage prostate cancer: A report of 206 cases. Int J Radiat Oncol 69, S329–S329 (2007). [Google Scholar]
- 149.Mark R, Anderson PJ, Akins RS & Nair M High-Dose-Rate Brachytherapy Under Local Anesthesia for Early Stage Prostate Cancer: A Report of 546 Cases. Brachytherapy 10, S93 (2011). [Google Scholar]
- 150.Prada PJ et al. High-dose-rate interstitial brachytherapy as monotherapy in one fraction and transperineal hyaluronic acid injection into the perirectal fat for the treatment of favorable stage prostate cancer: treatment description and preliminary results. Brachytherapy 11, 105–110 (2012). [DOI] [PubMed] [Google Scholar]
- 151.Rogers CL et al. High dose brachytherapy as monotherapy for intermediate risk prostate cancer. J. Urol 187, 109–116 (2012). [DOI] [PubMed] [Google Scholar]
- 152.Sullivan L et al. Urethral stricture following high dose rate brachytherapy for prostate cancer. Radiother. Oncol 91, 232–236 (2009). [DOI] [PubMed] [Google Scholar]
- 153.Yoshioka Y et al. High-dose-rate brachytherapy without external beam irradiation for locally advanced prostate cancer. Radiother. Oncol 80, 62–68 (2006). [DOI] [PubMed] [Google Scholar]
- 154.Yoshioka Y et al. Monotherapeutic high-dose-rate brachytherapy for prostate cancer: five-year results of an extreme hypofractionation regimen with 54 Gy in nine fractions. Int. J. Radiat. Oncol. Biol. Phys 80, 469–475 (2011). [DOI] [PubMed] [Google Scholar]
- 155.Zamboglou N et al. High-Dose-Rate Interstitial Brachytherapy as Monotherapy for Clinically Localized Prostate Cancer: Treatment Evolution and Mature Results. Int. J. Radiat. Oncol. Biol. Phys (2012). [DOI] [PubMed] [Google Scholar]
- 156.Hoskin P et al. High-dose-rate brachytherapy with two or three fractions as monotherapy in the treatment of locally advanced prostate cancer. Radiother. Oncol 112, 63–67 (2014). [DOI] [PubMed] [Google Scholar]
- 157.Kotecha R et al. Clinical outcomes of high-dose-rate brachytherapy and external beam radiotherapy in the management of clinically localized prostate cancer. Brachytherapy 12, 44–49 (2013). [DOI] [PubMed] [Google Scholar]
- 158.Phan TP, Syed AM, Puthawala A, Sharma A & Khan F High dose rate brachytherapy as a boost for the treatment of localized prostate cancer. J. Urol 177, 123–127; discussion 127 (2007). [DOI] [PubMed] [Google Scholar]
- 159.Vicini FA, Kestin LL & Martinez AA Use of conformal high-dose rate brachytherapy for management of patients with prostate cancer: optimizing dose escalation. Tech. Urol 6, 135–145 (2000). [PubMed] [Google Scholar]
- 160.Khor R et al. Direct 2-arm comparison shows benefit of high-dose-rate brachytherapy boost vs external beam radiation therapy alone for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys 85, 679–685 (2013). [DOI] [PubMed] [Google Scholar]
- 161.Tang JI, Williams SG, Tai KH, Dean J & Duchesne GM A prospective dose escalation trial of high-dose-rate brachytherapy boost for prostate cancer: Evidence of hypofractionation efficacy? Brachytherapy 5, 256–261 (2006). [DOI] [PubMed] [Google Scholar]
- 162.Zwahlen DR, Andrianopoulos N, Matheson B, Duchesne GM & Millar JL High-dose-rate brachytherapy in combination with conformal external beam radiotherapy in the treatment of prostate cancer. Brachytherapy 9, 27–35 (2010). [DOI] [PubMed] [Google Scholar]
- 163.Ares C et al. Hypofractionated boost with high-dose-rate brachytherapy and open magnetic resonance imaging-guided implants for locally aggressive prostate cancer: a sequential dose-escalation pilot study. Int. J. Radiat. Oncol. Biol. Phys 75, 656–663 (2009). [DOI] [PubMed] [Google Scholar]
- 164.Pistis F et al. External beam radiotherapy plus high-dose-rate brachytherapy for treatment of locally advanced prostate cancer: the initial experience of the Catalan Institute of Oncology. Brachytherapy 9, 15–22 (2010). [DOI] [PubMed] [Google Scholar]
- 165.Nohara T et al. Clinical results of iridium-192 high dose rate brachytherapy with external beam radiotherapy. Jpn. J. Clin. Oncol 40, 677–683 (2010). [DOI] [PubMed] [Google Scholar]
- 166.Sato M et al. High-dose-rate brachytherapy of a single implant with two fractions combined with external beam radiotherapy for hormone-naive prostate cancer. Int. J. Radiat. Oncol. Biol. Phys 72, 1002–1009 (2008). [DOI] [PubMed] [Google Scholar]
- 167.Akimoto T et al. Rectal bleeding after high-dose-rate brachytherapy combined with hypofractionated external-beam radiotherapy for localized prostate cancer: impact of rectal dose in high-dose-rate brachytherapy on occurrence of grade 2 or worse rectal bleeding. Int. J. Radiat. Oncol. Biol. Phys 65, 364–370 (2006). [DOI] [PubMed] [Google Scholar]
- 168.Bachand F, Martin AG, Beaulieu L, Harel F & Vigneault E An eight-year experience of HDR brachytherapy boost for localized prostate cancer: biopsy and PSA outcome. Int. J. Radiat. Oncol. Biol. Phys 73, 679–684 (2009). [DOI] [PubMed] [Google Scholar]
- 169.Hurwitz MD Technology Insight: Combined external-beam radiation therapy and brachytherapy in the management of prostate cancer. Nat. Clin. Pract. Oncol 5, 668–676 (2008). [DOI] [PubMed] [Google Scholar]
- 170.Aluwini S et al. High-dose-rate brachytherapy and external-beam radiotherapy for hormone-naive low- and intermediate-risk prostate cancer: a 7-year experience. Int. J. Radiat. Oncol. Biol. Phys 83, 1480–1485 (2012). [DOI] [PubMed] [Google Scholar]
- 171.Deger S et al. High dose rate (HDR) brachytherapy with conformal radiation therapy for localized prostate cancer. Eur. Urol 47, 441–448 (2005). [DOI] [PubMed] [Google Scholar]
- 172.Martin T et al. 3D conformal HDR brachytherapy and external beam irradiation combined with temporary androgen deprivation in the treatment of localized prostate cancer. Radiother. Oncol 71, 35–41 (2004). [DOI] [PubMed] [Google Scholar]
- 173.Curran MJ, Healey GA, Bihrle W 3rd, Goodman N & Roth RA Treatment of high-grade low-stage prostate cancer by high-dose-rate brachytherapy. J. Endourol 14, 351–356 (2000). [DOI] [PubMed] [Google Scholar]
- 174.Demanes DJ, Brandt D, Schour L & Hill DR Excellent results from high dose rate brachytherapy and external beam for prostate cancer are not improved by androgen deprivation. Am. J. Clin. Oncol 32, 342–347 (2009). [DOI] [PubMed] [Google Scholar]
- 175.Demanes DJ, Rodriguez RR, Schour L, Brandt D & Altieri G High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy's 10-year results. Int. J. Radiat. Oncol. Biol. Phys 61, 1306–1316 (2005). [DOI] [PubMed] [Google Scholar]
- 176.Yamada Y et al. Favorable clinical outcomes of three-dimensional computer-optimized high-dose-rate prostate brachytherapy in the management of localized prostate cancer. Brachytherapy 5, 157–164 (2006). [DOI] [PubMed] [Google Scholar]
- 177.Izard MA et al. Six year experience of external beam radiotherapy, brachytherapy boost with a 1Ci (192)Ir source, and neoadjuvant hormonal manipulation for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys 66, 38–47 (2006). [DOI] [PubMed] [Google Scholar]
- 178.Whalley D et al. HDR brachytherapy combined with external beam radiation for localised prostate cancer: early experience from the Sydney Cancer Centre. J. Med. Imaging Radiat. Oncol 56, 220–226 (2012). [DOI] [PubMed] [Google Scholar]
- 179.Chen YC et al. High-dose-rate brachytherapy plus external beam radiotherapy for T1 to T3 prostate cancer: an experience in Taiwan. Urology 70, 101–105 (2007). [DOI] [PubMed] [Google Scholar]
- 180.Luo HL, Fang FM, Kang CH, Chuang YC & Chiang PH Can high-dose-rate brachytherapy prevent the major genitourinary complication better than external beam radiation alone for patients with previous transurethral resection of prostate? Int. Urol. Nephrol 45, 113–119 (2013). [DOI] [PubMed] [Google Scholar]
- 181.Pellizzon AC et al. The relationship between the biochemical control outcomes and the quality of planning of high-dose rate brachytherapy as a boost to external beam radiotherapy for locally and locally advanced prostate cancer using the RTOG-ASTRO Phoenix definition. Int J Med Sci 5, 113–120 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Agoston P et al. Moderate dose escalation with single-fraction high-dose-rate brachytherapy boost for clinically localized intermediate- and high-risk prostate cancer: 5-year outcome of the first 100 consecutively treated patients. Brachytherapy 10, 376–384 (2011). [DOI] [PubMed] [Google Scholar]
- 183.Galalae RM et al. Health-related quality of life measurement in long-term survivors and outcome following radical radiotherapy for localized prostate cancer. Strahlenther. Onkol 180, 582–589 (2004). [DOI] [PubMed] [Google Scholar]
- 184.Prada PJ et al. Long-term biochemical results after high-dose-rate intensity modulated brachytherapy with external beam radiotherapy for high risk prostate cancer. Radiat. Oncol 7, 31–39 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Borghede G et al. Combined treatment with temporary short-term high dose rate iridium-192 brachytherapy and external beam radiotherapy for irradiation of localized prostatic carcinoma. Radiother. Oncol 44, 237–244 (1997). [DOI] [PubMed] [Google Scholar]
- 186.Kestin LL et al. Matched-pair analysis of conformal high-dose-rate brachytherapy boost versus external-beam radiation therapy alone for locally advanced prostate cancer. J. Clin. Oncol 18, 2869–2880 (2000). [DOI] [PubMed] [Google Scholar]
- 187.Hiratsuka J et al. Clinical results of combined treatment conformal high-dose-rate iridium-192 brachytherapy and external beam radiotherapy using staging lymphadenectomy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys 59, 684–690 (2004). [DOI] [PubMed] [Google Scholar]
- 188.Fertil B & Malaise EP Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: analysis of 101 published survival curves. Int. J. Radiat. Oncol. Biol. Phys 11, 1699–1707 (1985). [DOI] [PubMed] [Google Scholar]
- 189.Miralbell R, Roberts SA, Zubizarreta E & Hendry JH Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: alpha/beta = 1.4 (0.9–2.2) Gy. Int J Radiat Oncol Biol Phys 82, e17–24 (2012). [DOI] [PubMed] [Google Scholar]
- 190.Fowler JF, Ritter MA, Chappell RJ & Brenner DJ What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys 56, 1093–1104 (2003). [DOI] [PubMed] [Google Scholar]
- 191.Brenner DJ et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 52, 6–13 (2002). [DOI] [PubMed] [Google Scholar]
- 192.Park C, Papiez L, Zhang S, Story M & Timmerman RD Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys 70, 847–852 (2008). [DOI] [PubMed] [Google Scholar]
- 193.Kwilas AR, Donahue RN, Bernstein MB & Hodge JW In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Frontiers in oncology 2, 104 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Meng MB et al. Necroptosis in tumorigenesis, activation of anti-tumor immunity, and cancer therapy. Oncotarget 7, 57391–57413 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Meng MB et al. Pericytes: a double-edged sword in cancer therapy. Future Oncol., 1–11 (2014). [DOI] [PubMed] [Google Scholar]
- 196.Wang HH et al. Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett 375, 349–359 (2016). [DOI] [PubMed] [Google Scholar]
- 197.Stock RG, Stone NN, Tabert A, Iannuzzi C & DeWyngaert JK A dose-response study for I-125 prostate implants. Int. J. Radiat. Oncol. Biol. Phys 41, 101–108 (1998). [DOI] [PubMed] [Google Scholar]
- 198.Haynes B Can it work? Does it work? Is it worth it? The testing of healthcareinterventions is evolving. BMJ 319, 652–653 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kesteloot K, Lievens Y & van der Schueren E Improved management of radiotherapy departments through accurate cost data. Radiother. Oncol 55, 251–262 (2000). [DOI] [PubMed] [Google Scholar]
- 200.Crook JM et al. Comparison of health-related quality of life 5 years after SPIRIT: Surgical Prostatectomy Versus Interstitial Radiation Intervention Trial. J. Clin. Oncol 29, 362–368 (2011). [DOI] [PubMed] [Google Scholar]
- 201.Hoskin P et al. High-dose-rate brachytherapy alone for localized prostate cancer in patients at moderate or high risk of biochemical recurrence. Int. J. Radiat. Oncol. Biol. Phys 82, 1376–1384 (2012). [DOI] [PubMed] [Google Scholar]
- 202.Potters L et al. 12-year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer.[Reprint in J Urol. 2008 May;179(5 Suppl):S20–4; PMID: 18405743]. J Urol 173, 1562–1566 (2005). [DOI] [PubMed] [Google Scholar]
- 203.Stone NN et al. Customized dose prescription for permanent prostate brachytherapy: insights from a multicenter analysis of dosimetry outcomes. Int J Radiat Oncol Biol Phys 69, 1472–1477 (2007). [DOI] [PubMed] [Google Scholar]
- 204.Zelefsky MJ et al. Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys 67, 327–333 (2007). [DOI] [PubMed] [Google Scholar]
- 205.Grimm P et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 109 Suppl 1, 22–29 (2012). [DOI] [PubMed] [Google Scholar]
- 206.Davis BJ et al. The radial distance of extraprostatic extension of prostate carcinoma: implications for prostate brachytherapy. Cancer 85, 2630–2637 (1999). [PubMed] [Google Scholar]
- 207.Sohayda C, Kupelian PA, Levin HS & Klein EA Extent of extracapsular extension in localized prostate cancer. Urology 55, 382–386 (2000). [DOI] [PubMed] [Google Scholar]
- 208.Teh BS, Bastasch MD, Mai W-Y, Butler EB & Wheeler TM Predictors of extracapsular extension and its radial distance in prostate cancer: implications for prostate IMRT, brachytherapy, and surgery. Cancer J 9, 454–460 (2003). [DOI] [PubMed] [Google Scholar]
- 209.Chao KK et al. Clinicopathologic analysis of extracapsular extension in prostate cancer: should the clinical target volume be expanded posterolaterally to account for microscopic extension? Int J Radiat Oncol Biol Phys 65, 999–1007 (2006). [DOI] [PubMed] [Google Scholar]
- 210.Chao KK et al. Clinicopathologic analysis of extracapsular extension in prostate cancer: should the clinical target volume be expanded posterolaterally to account for microscopic extension? Int J Radiat Oncol Biol Phys 65, 999–1007 (2006). [DOI] [PubMed] [Google Scholar]
- 211.Cosset JM et al. Selecting patients for exclusive permanent implant prostate brachytherapy: the experience of the Paris Institut Curie/Cochin Hospital/Necker Hospital group on 809 patients. Int. J. Radiat. Oncol. Biol. Phys 71, 1042–1048 (2008). [DOI] [PubMed] [Google Scholar]
- 212.Prestige BR et al. Initial Report of NRG Oncology/RTOG 0232: A Phase III Study Comparing Combined External Beam Radiation and Transperineal Interstitial Permanent Brachytherapy with Brachytherapy Alone for Selected Patients with Intermediate Risk Prostatic Carcinoma Int. J. Radiat. Oncol. Biol. Phys 96, S4 (2016). [Google Scholar]
- 213.Frank SJ et al. Interstitial implant alone or in combination with external beam radiation therapy for intermediate-risk prostate cancer: a survey of practice patterns in the United States. Brachytherapy 6, 2–8 (2007). [DOI] [PubMed] [Google Scholar]
- 214.Lee WR et al. Late toxicity and biochemical recurrence after external-beam radiotherapy combined with permanent-source prostate brachytherapy: analysis of Radiation Therapy Oncology Group study 0019. Cancer 109, 1506–1512 (2007). [DOI] [PubMed] [Google Scholar]
- 215.Hurwitz MD et al. Combination external beam radiation and brachytherapy boost with androgen deprivation for treatment of intermediate-risk prostate cancer: long-term results of CALGB 99809. Cancer 117, 5579–5588 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Serrano N et al. Comparative study of late rectal toxicity in prostate cancer patients treated with low-dose-rate brachytherapy: With or without supplemental external beam radiotherapy. Brachytherapy 15, 435–441 (2016). [DOI] [PubMed] [Google Scholar]
- 217.Wang LS et al. Impact of obesity on outcomes after definitive dose-escalated intensity-modulated radiotherapy for localized prostate cancer. Cancer 121, 3010–3017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zaorsky NG et al. Prostate Cancer Patients With Unmanaged Diabetes or Receiving Insulin Experience Inferior Outcomes and Toxicities After Treatment With Radiation Therapy. Clin. Genitourin. Cancer (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Horwitz EM et al. Ten-year follow-up of radiation therapy oncology group protocol 92–02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 26, 2497–2504 (2008). [DOI] [PubMed] [Google Scholar]
- 220.Bolla M et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. The Lancet Oncology 11, 1066–1073. [DOI] [PubMed] [Google Scholar]
- 221.Granfors T, Modig H, Damber JE & Tomic R Long-term followup of a randomized study of locally advanced prostate cancer treated with combined orchiectomy and external radiotherapy versus radiotherapy alone. J. Urol 176, 544–547 (2006). [DOI] [PubMed] [Google Scholar]
- 222.Merrick GS et al. Androgen deprivation therapy does not impact cause-specific or overall survival in high-risk prostate cancer managed with brachytherapy and supplemental external beam. Int J Radiat Oncol Biol Phys 68, 34–40 (2007). [DOI] [PubMed] [Google Scholar]
- 223.Stone NN et al. Multicenter analysis of effect of high biologic effective dose on biochemical failure and survival outcomes in patients with Gleason score 7–10 prostate cancer treated with permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 73, 341–346 (2009). [DOI] [PubMed] [Google Scholar]
- 224.Hauswald H et al. High-Dose-Rate Monotherapy for Localized Prostate Cancer: 10-Year Results. Int. J. Radiat. Oncol. Biol. Phys 94, 667–674 (2016). [DOI] [PubMed] [Google Scholar]
- 225.Yoshioka Y Current status and perspectives of brachytherapy for prostate cancer. Int. J. Clin. Oncol 14, 31–36 (2009). [DOI] [PubMed] [Google Scholar]
- 226.Zaorsky NG et al. What is the ideal radiotherapy dose to treat prostate cancer? A meta-analysis of biologically equivalent dose escalation. Radiother. Oncol 115, 295–300 (2015). [DOI] [PubMed] [Google Scholar]
- 227.Nomiya T et al. Management of high-risk prostate cancer: Radiation therapy and hormonal therapy. Cancer Treat. Rev 39, 872–878 (2013). [DOI] [PubMed] [Google Scholar]
- 228.Zaorsky NG et al. Impact of Radiation Therapy Dose Escalation on Prostate Cancer Outcomes and Toxicities. Am. J. Clin. Oncol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zaorsky NG et al. Prostate Cancer Patients With Unmanaged Diabetes or Receiving Insulin Experience Inferior Outcomes and Toxicities After Treatment With Radiation Therapy. Clin. Genitourin. Cancer 15, 326–335 e323 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Allen GW, Howard AR, Jarrard DF & Ritter MA Management of prostate cancer recurrences after radiation therapy-brachytherapy as a salvage option. Cancer 110, 1405–1416 (2007). [DOI] [PubMed] [Google Scholar]
- 231.Tetreault-Laflamme A & Crook J Options for Salvage of Radiation Failures for Prostate Cancer. Semin. Radiat. Oncol 27, 67–78 (2017). [DOI] [PubMed] [Google Scholar]
- 232.Crook JM NCT00450411: A Prospective Phase II Trial of Transperineal Ultrasound-Guided Brachytherapy for Locally Recurrent Prostate Adenocarcinoma Following External Beam Radiotherapy. https://clinicaltrials.gov/ct2/show/NCT00450411 [DOI] [PMC free article] [PubMed]
- 233.Tharp M et al. Prostate high-dose-rate brachytherapy as salvage treatment of local failure after previous external or permanent seed irradiation for prostate cancer. Brachytherapy 7, 231–236 (2008). [DOI] [PubMed] [Google Scholar]
- 234.Lee B et al. Feasibility of high-dose-rate brachytherapy salvage for local prostate cancer recurrence after radiotherapy: the University of California-San Francisco experience. Int. J. Radiat. Oncol. Biol. Phys 67, 1106–1112 (2007). [DOI] [PubMed] [Google Scholar]
- 235.Nguyen PL et al. Magnetic resonance image-guided salvage brachytherapy after radiation in select men who initially presented with favorable-risk prostate cancer: a prospective phase 2 study. Cancer 110, 1485–1492 (2007). [DOI] [PubMed] [Google Scholar]
- 236.Nguyen PL, D'Amico AV, Lee AK & Suh WW Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure: a systematic review of the literature. Cancer 110, 1417–1428 (2007). [DOI] [PubMed] [Google Scholar]
- 237.Nguyen PL et al. Patient-reported quality of life after salvage brachytherapy for radio-recurrent prostate cancer: A prospective Phase II study. Brachytherapy 8, 345–352 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Nguyen PL et al. High-dose-rate brachytherapy for prostate cancer in a previously radiated patient with polyethylene glycol hydrogel spacing to reduce rectal dose: case report and review of the literature. Brachytherapy 12, 77–83 (2013). [DOI] [PubMed] [Google Scholar]
- 239.Tree AC et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 14, e28–37 (2013). [DOI] [PubMed] [Google Scholar]
- 240.Yao HH, Hong M, Corcoran NM, Siva S & Foroudi F Advances in local and ablative treatment of oligometastasis in prostate cancer. Asia Pac. J. Clin. Oncol 10, 308–321 (2014). [DOI] [PubMed] [Google Scholar]
- 241.Zaorsky NG, Raj GV, Trabulsi EJ, Lin J & Den RB The dilemma of a rising prostate-specific antigen level after local therapy: what are our options? Semin. Oncol 40, 322–336 (2013). [DOI] [PubMed] [Google Scholar]
- 242.Valerio M et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur. Urol 66, 732–751 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chen RC et al. Recommended patient-reported core set of symptoms to measure in prostate cancer treatment trials. J. Natl. Cancer Inst 106 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wilt TJ et al. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Annals of internal medicine 148, 435–448 (2008). [DOI] [PubMed] [Google Scholar]
- 245.Efficace F et al. Patient-reported outcomes in randomised controlled trials of prostate cancer: methodological quality and impact on clinical decision making. European urology 66, 416–427 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Punnen S, Cowan JE, Chan JM, Carroll PR & Cooperberg MR Long-term health-related quality of life after primary treatment for localized prostate cancer: results from the CaPSURE registry. Eur. Urol 68, 600–608 (2015). [DOI] [PubMed] [Google Scholar]
- 247.Chen RC, Clark JA & Talcott JA Individualizing quality-of-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, bowel, and sexual function. J. Clin. Oncol 27, 3916–3922 (2009). [DOI] [PubMed] [Google Scholar]
- 248.Schmidt S et al. Assessing quality of life in patients with prostate cancer: a systematic and standardized comparison of available instruments. Qual. Life Res 23, 2169–2181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lloyd AJ, Kerr C, Penton J & Knerer G Health-Related Quality of Life and Health Utilities in Metastatic Castrate-Resistant Prostate Cancer: A Survey Capturing Experiences from a Diverse Sample of UK Patients. Value Health 18, 1152–1157 (2015). [DOI] [PubMed] [Google Scholar]
- 250.Barry MJ et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. The Journal of urology 148, 1549–1557; discussion 1564 (1992). [DOI] [PubMed] [Google Scholar]
- 251.Rosen RC et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 49, 822–830 (1997). [DOI] [PubMed] [Google Scholar]
- 252.NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA 270, 83–90 (1993). [PubMed] [Google Scholar]
- 253.Johnson ME et al. Patient reported outcomes among treatment modalities for prostate cancer. The Canadian journal of urology 23, 8535–8545 (2016). [PubMed] [Google Scholar]
- 254.Reis LO, Sanches BC, Zani EL, Castilho LN & Monti CR PSA-nadir at 1 year as a sound contemporary prognostic factor for low-dose-rate iodine-125 seeds brachytherapy. World J. Urol 32, 753–759 (2014). [DOI] [PubMed] [Google Scholar]
- 255.Hackett C et al. Distinguishing prostate-specific antigen bounces from biochemical failure after low-dose-rate prostate brachytherapy. Journal of contemporary brachytherapy 6, 247–253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tanaka N et al. Minimal percentage of dose received by 90% of the urethra (%UD90) is the most significant predictor of PSA bounce in patients who underwent low-dose-rate brachytherapy (LDR-brachytherapy) for prostate cancer. BMC Urol. 12, 28 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Reed D, Wallner K, Merrick G, Buskirk S & True L Clinical correlates to PSA spikes and positive repeat biopsies after prostate brachytherapy. Urology 62, 683–688 (2003). [DOI] [PubMed] [Google Scholar]
- 258.McGrath SD et al. PSA bounce after prostate brachytherapy with or without neoadjuvant androgen deprivation. Brachytherapy 9, 137–144 (2010). [DOI] [PubMed] [Google Scholar]
- 259.Naghavi AO et al. Clinical implications of a prostate-specific antigen bounce after radiation therapy for prostate cancer. Int. J. Clin. Oncol (2014). [DOI] [PubMed] [Google Scholar]
- 260.Fuller DB, Naitoh J & Mardirossian G Virtual HDR CyberKnife SBRT for Localized Prostatic Carcinoma: 5-Year Disease-Free Survival and Toxicity Observations. Frontiers in oncology 4, 321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pinkawa M et al. Prostate-specific antigen kinetics following external-beam radiotherapy and temporary (Ir-192) or permanent (I-125) brachytherapy for prostate cancer. Radiother. Oncol 96, 25–29 (2010). [DOI] [PubMed] [Google Scholar]
- 262.Patel N et al. Prostate-specific antigen bounce after high-dose-rate prostate brachytherapy and hypofractionated external beam radiotherapy. Brachytherapy 13, 450–455 (2014). [DOI] [PubMed] [Google Scholar]
- 263.Mehta NH, Kamrava M, Wang PC, Steinberg M & Demanes J Prostate-specific antigen bounce after high-dose-rate monotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys 86, 729–733 (2013). [DOI] [PubMed] [Google Scholar]
- 264.NCT02628041: HDR Brachytherapy vs. LDR Brachytherapy Monotherapy in Localized Prostate Cancer (HDRvsLDR). https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 265.NCT02346253: High-Dose Brachytherapy in Treating Patients With Prostate Cancer. https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 266.NCT02258087: HDR vs LDR Brachytherapy as Monotherapy in the Treatment of Localized Prostate Cancer. (PROMOBRA). https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 267.NCT02560181: Pilot Study of Whole Gland Salvage HDR Prostate Brachytherapy for Locally Recurrent Prostate Cancer. https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 268.NCT02322931: C-arm Cone-beam CT in Prostate Brachytherapy (C-arm). https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 269.NCT02225925: Intraoperative Dosimetry for Prostate Brachytherapy Using Fluoroscopy and Ultrasound. https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 270.NCT00450411: Ultrasound-Guided Implant Radiation Therapy in Treating Patients With Locally Recurrent Prostate Cancer Previously Treated With External-Beam Radiation Therapy. https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 271.NCT02632669: Hemi-Ablative Prostate Brachytherapy (HAPpy). https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 272.NCT02290366: Prospective Evaluation of Focal Brachytherapy Using Cesium-131 For Patients With Low Risk Prostate Cancer. https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 273.NCT00913939: High-Dose-Rate Brachytherapy. https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 274.NCT01936883: Improving Quality of Life After Prostate Brachytherapy: a Comparison of HDR and LDR Brachytherapy (BrachyQOL). https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 275.NCT01909388: Dose Escalation to Dominant Intraprostatic Lesions (DIL) With MRI-TRUS Fusion High Dose Rate (HDR) Prostate Brachytherapy (BRAPROST). https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 276.NCT02790216: Deformable Registration of Multi-parametric MRI to Intra-operative Transrectal Ultrasound for Prostate Brachytherapy. https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 277.NCT02652000: Quality of Life After Permanent Interstitial Iodine Seed Prostate Brachytherapy. https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 278.NCT02597894: Targeted Biopsies in Determining Response in Patients With Prostate Cancer Undergoing High-Dose-Rate Brachytherapy. https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 279.NCT02805894: NBTXR3 Nanoparticles and EBRT or EBRT With Brachytherapy in the Treatment of Prostate Adenocarcinoma. https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 280.NCT01437085: A Study to Measure Needle Bending and Changes in Prostate Shape During a Prostate Seed Implant. https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
- 281.NCT02623933: MRI Assisted Focal Boost With HDR Monotherapy for Prostate Cancer Patients (MARS). https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).