Microbes infect a diversity of species, influencing the performance and fitness of their hosts. Maternally transmitted Wolbachia bacteria infect most insects and other arthropods, making these bacteria some of the most common endosymbionts in nature. Despite their global prevalence, it remains mostly unknown how Wolbachia influence host physiology and behavior to proliferate. We demonstrate pervasive effects of Wolbachia on Drosophila temperature preference. Most hosts infected with A-group Wolbachia prefer cooler temperatures, whereas the one host species infected with divergent B-group Wolbachia prefers warmer temperatures, relative to uninfected genotypes. Changes to host temperature preference generally do not alter Wolbachia abundance in host tissues, but for some A-group strains, adult males have increased Wolbachia titer when shifted to a cooler temperature. This suggests that Wolbachia-induced changes to host behavior may promote bacterial replication. Our results help elucidate the impact of endosymbionts on their hosts amid the global Wolbachia pandemic.
KEYWORDS: Drosophila, host-microbe interaction, symbiosis, thermal adaptation, thermoregulation, wMel
ABSTRACT
Heritable symbionts can modify a range of ecologically important host traits, including behavior. About half of all insect species are infected with maternally transmitted Wolbachia, a bacterial endosymbiont known to alter host reproduction, nutrient acquisition, and virus susceptibility. Here, we broadly test the hypothesis that Wolbachia modifies host behavior by assessing the effects of eight different Wolbachia strains on the temperature preference of six Drosophila melanogaster subgroup species. Four of the seven host genotypes infected with A-group Wolbachia strains (wRi in Drosophila simulans, wHa in D. simulans, wSh in Drosophila sechellia, and wTei in Drosophila teissieri) prefer significantly cooler temperatures relative to uninfected genotypes. Contrastingly, when infected with divergent B-group wMau, Drosophila mauritiana prefers a warmer temperature. For most strains, changes to host temperature preference do not alter Wolbachia titer. However, males infected with wSh and wTei tend to experience an increase in titer when shifted to a cooler temperature for 24 h, suggesting that Wolbachia-induced changes to host behavior may promote bacterial replication. Our results indicate that Wolbachia modifications to host temperature preference are likely widespread, which has important implications for insect thermoregulation and physiology. Understanding the fitness consequences of these Wolbachia effects is crucial for predicting evolutionary outcomes of host-symbiont interactions, including how Wolbachia spreads to become common.
INTRODUCTION
Heritable symbionts have diverse ecological effects on their hosts. In insects, microbial symbionts influence host reproduction (e.g., cytoplasmic incompatibility) (1, 2), acquisition of nutrients (3–5), tolerance of extreme temperatures (6, 7), and susceptibility to viruses (8, 9). Much less is known about symbionts’ effects on host behavior and their ecological consequences (10–13). On the one hand, symbionts may induce behavioral changes that promote the spread of infection through host populations. Because symbiotic relationships can span a continuum from mutualism to parasitism, behavioral modifications that promote infection spread may not necessarily benefit hosts (2, 14). Parasites, for example, can induce behaviors that are detrimental or lethal to hosts, such as altering host locomotor behavior to increase the probability of parasite transmission (15–20). On the other hand, infected hosts may modify their own behavior in ways that mitigate negative aspects of the infection (16, 21–23), such as a “behavioral chill” thermoregulatory response in which hosts seek cool temperatures to increase their survival probability (24). These behavioral effects represent an important component of how symbionts impact host fitness, which ultimately dictates the evolutionary trajectory of host-symbiont interactions.
Maternally transmitted Wolbachia bacteria are the most common endosymbionts in nature, infecting the cells of about half of all insect species, as well as other arthropods (2, 25, 26). Wolbachia and host phylogenies are often discordant (27–29), and most Drosophila hosts have recently acquired Wolbachia via introgressive and/or horizontal transfer (30–32). Maternal transmission occurs in the host germ line, but Wolbachia also infects a variety of host somatic cells, including metabolic, digestive, and nervous system tissue (33–35). The fitness consequences of Wolbachia in host tissues ultimately determine infection spread, and initial spread from low frequencies requires positive Wolbachia effects on host fitness (36–38). Exactly how Wolbachia alters components of host fitness is poorly understood (39), even though theoretical and population-level analyses indicate pervasive positive effects on host fitness (1, 31, 37, 40–42).
Symbionts are known to influence host thermal tolerance (7, 43–46), and two recent studies found that Drosophila melanogaster lines infected with the wMelCS or wMel Wolbachia strain tend to prefer cooler temperatures than uninfected genotypes (47, 48). Modifications to host temperature preference (Tp) have important implications for insects, because ectothermic performance and fitness explicitly depend on temperature (49–55). Because Wolbachia infects most insects (2, 25, 26), it is crucial to understand how infections alter host thermoregulation. Few past analyses of insect behavioral thermoregulation have accounted for Wolbachia (51, 55, 56).
Differences in Tp between infected and uninfected flies could arise from conflicting physiological requirements of Wolbachia and their hosts. Wolbachia titer in host bodies is sensitive to temperature fluctuations (57), such that exceedingly cool (<20°C) and warm (>25°C) temperatures can reduce titer and the efficiency of maternal Wolbachia transmission (42, 57–63). Wolbachia-induced changes to Tp could provide more favorable thermal conditions for bacterial replication in hosts. Alternatively, host-induced changes to Tp could represent a host behavioral response that reduces Wolbachia titer to mitigate negative aspects of infection (e.g., behavioral chill). It is still unknown whether observed changes to Tp increase or decrease Wolbachia titer (47, 48).
Here, we broadly test for Wolbachia effects on host Tp across the D. melanogaster subgroup of flies. Our experiments include seven A-group Wolbachia-infected genotypes (wRi in Drosophila simulans, wHa in D. simulans, wMelCS in D. melanogaster, wMel in D. melanogaster, wSh in Drosophila sechellia, wYak in Drosophila yakuba, and wTei in Drosophila teissieri) and one B-group Wolbachia-infected genotype (wMau in Drosophila mauritiana), which diverged from A-group strains 6 to 46 million years ago (41). We find that hosts infected with four of the A-group Wolbachia strains (wRi, wHa, wSh, and wTei) prefer a significantly cooler Tp than uninfected flies of the same host genotype. In contrast, D. mauritiana infected with B-group wMau have a significantly warmer Tp. Unlike previous reports (47, 48), we find no evidence for wMelCS or wMel effects on Tp of D. melanogaster, indicating host effects on Tp. Shifting infected adults from an intermediate temperature toward their Tp for 24 h generally does not alter Wolbachia titer, but in a few instances, reductions in host Tp seem to promote Wolbachia replication. Our results motivate future work on the causes and consequences of Wolbachia effects on Tp and other host behaviors.
RESULTS
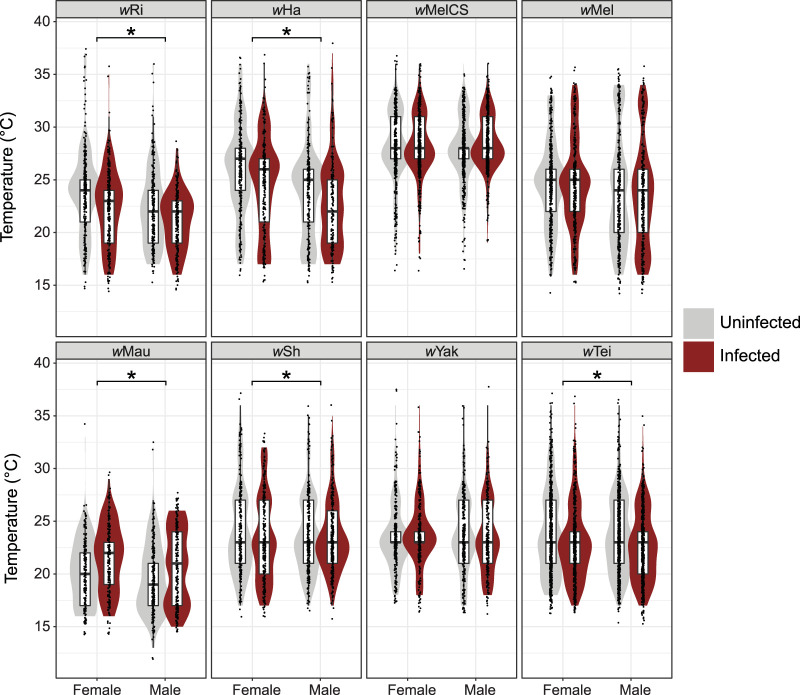
Wolbachia infections modify host temperature preference.
We used a thermal gradient apparatus to test whether eight different Wolbachia strains alter the temperature preference (Tp) of their Drosophila host species (see Fig. S1 and Table S1 in the supplemental material). For each strain, we measured the Tp of Wolbachia-infected hosts and uninfected flies of the same genotype. In total, we assayed the Tp of 10,401 flies in 347 replicates on the thermal gradient and analyzed our results using generalized linear mixed models (GLMMs) and a Poisson error structure (Table 1 and Fig. 1). Wolbachia infection status had a significant main effect on host Tp for five genotypes: wRi-infected D. simulans (χ2 = 6.158, P = 0.013), wHa-infected D. simulans (χ2 = 6.148, P = 0.013), wMau-infected D. mauritiana (χ2 = 7.540, P = 0.006), wSh-infected D. sechellia (χ2 = 4.531, P = 0.033), and wTei-infected D. teissieri (χ2 = 8.360, P = 0.004) (Table 1). These results were robust to whether the data were analyzed using GLMMs or linear mixed models (LLMs) (Table S2). Of the five Wolbachia strains with a significant effect on Tp, all host genotypes infected with A-group Wolbachia preferred a cooler temperature than uninfected flies (Fig. 2): wRi-infected D. simulans preferred a least-square (LS) mean temperature of 21.72°C ± 1.02°C (±standard error [SE]) compared to 23.12°C ± 1.02°C for uninfected flies, wHa-infected D. simulans preferred an LS mean of 23.56°C ± 1.01°C compared to the uninfected mean of 24.89°C ± 1.01°C, wSh-infected D. sechellia preferred an LS mean of 23.32°C ± 1.01°C compared to the uninfected mean of 23.98°C ± 1.01°C, and wTei-infected D. teissieri preferred an LS mean of 22.7°C ± 1.01°C compared to the uninfected mean of 23.7°C ± 1.01°C. In contrast, D. mauritana infected with B-group wMau preferred a warmer LS mean temperature of 21.15°C ± 1.01°C compared to the uninfected mean of 19.67°C ± 1.02°C.
TABLE 1.
Analysis of host Tp using generalized linear mixed models (GLMMs) and a Poisson error structurea
| Explanatory variable |
wRi |
wHa |
wMelCS |
wMel |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | χ2 | P value | Coefficient | χ2 | P value | Coefficient | χ2 | P value | Coefficient | χ2 | P value | |
| Infection status | 0.069 | 6.158 | 0.013* | 0.063 | 6.148 | 0.013* | −0.017 | 1.285 | 0.257 | −0.004 | 0.031 | 0.86 |
| Sex | −0.06 | 4.341 | 0.037* | −0.07 | 6.907 | 0.009* | −0.007 | 0.224 | 0.636 | −0.046 | 3.49 | 0.062 |
| Age | −0.003 | 0.016 | 0.898 | −0.001 | 0.02 | 0.887 | −0.019 | 11.426 | 0.001* | 0.012 | 2.251 | 0.134 |
| Run order | 0.001 | 0.013 | 0.909 | 0.009 | 1.002 | 0.317 | 0.011 | 4.914 | 0.027* | 0.005 | 0.366 | 0.545 |
| Infection-by-sex | −0.013 | 0.099 | 0.754 | −0.016 | 0.186 | 0.666 | 0.002 | 0.005 | 0.943 | 0.021 | 0.368 | 0.544 |
| Sample size | 1,015 | 857 | 1,727 | 1,341 | ||||||||
Statistically significant fixed effects at P < 0.05 are shown in bold text with asterisks.
FIG 1.
Box plots showing Tp for uninfected and infected flies of each genotype, separated by sex. An asterisk denotes a significant main effect of Wolbachia infection on Tp from the GLMMs (Table 1). Individual points are jittered to show overlap. We found a significant main effect of sex on Tp for wRi (χ2 = 4.341, P = 0.037) and wHa (χ2 = 6.907, P = 0.009).
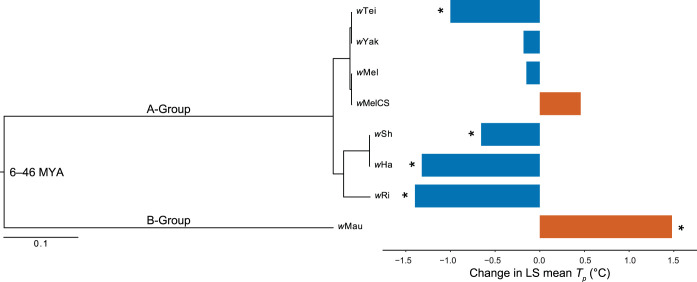
FIG 2.
Estimated Bayesian phylogram for A- and B-group Wolbachia strains examined in this study. The phylogram was estimated with 214 single-copy genes of identical length in all of the genomes, spanning 181,488 bp. All nodes have Bayesian posterior probabilities of 1. To the right, the change in least-square (LS) mean Tp between uninfected and infected flies is shown for each Wolbachia strain. LS means were generated from GLMMs (Table 1), and strains with a significant main effect on Tp are marked with an asterisk. The divergence time estimate (million years ago [MYA]) for A- and B-group Wolbachia is from Meany et al. (41).
The thermal gradient apparatus is composed of a 44 × 13× 1 cm aluminum plate and a 1-cm-high removable Plexiglas lid. The thermal gradient is subdivided into seven 10 × 6 cm sections (see Table S4 in the supplemental material). Download FIG S1, DOCX file, 0.4 MB (413.9KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genotype IDs for different Wolbachia-infected host species used in this study. Download Table S1, DOCX file, 0.01 MB (13KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Results and sample sizes from the LMM analyses of Tp data. Because the wHa, wMelCS, wMel, and wMau data were approximately normally distributed, we analyzed each data set using LMMs. Statistically significant fixed effects at P < 0.05 are shown in bold text with asterisks. Download Table S2, DOCX file, 0.01 MB (15.3KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In addition to Wolbachia infection status, we found other significant fixed effects on Tp. Sex had a significant main effect on Tp for both the wRi-infected D. simulans (χ2 = 4.341, P = 0.037) and wHa-infected D. simulans (χ2 = 6.907, P = 0.009) (Table 1). For both of these D. simulans genotypes, females preferred warmer temperatures than males, regardless of infection status (Fig. 1). For the wRi genotype, infected females preferred an LS mean temperature of 22.37°C ± 1.02°C compared to the uninfected female mean of 23.97°C ± 1.02°C. Infected males preferred an LS mean of 21.07°C ± 1.02°C compared to the uninfected male mean of 22.28°C ± 1.02. For the wHa genotype, infected females preferred an LS mean temperature of 24.41°C ± 1.02°C compared to the uninfected female mean of 25.98°C ± 1.02°C. Infected males preferred an LS mean of 22.75°C ± 1.02°C compared to the uninfected male mean of 23.83°C ± 1.02°C. The GLMMs also revealed a significant effect of fly age on Tp for wMelCS-infected D. melanogaster (χ2 = 11.426, P = 0.001), such that older flies tended to prefer cooler temperatures. Finally, we found that the run order each day had a significant effect on Tp for the wMelCS-D. melanogaster (χ2 = 4.914, P = 0.027) and the wMau-D. mauritiana genotypes (χ2 = 3.968, P = 0.046). In both instances, flies assayed earlier in the day tended to prefer cooler temperatures. This is consistent with prior findings that the Tp of D. melanogaster increases from morning to evening due to a circadian clock (64). In fact, a substrain of the Canton Special fly line (our wMelCS-D. melanogaster genotype) was specifically shown to have increasing Tp throughout the day (see Materials and Methods for a discussion on Canton Special substrains) (64). Circadian clock-dependent temperature preference rhythms help ectotherms maintain homeostasis throughout the day (65). We also detected a main effect of wMau on D. mauritiana Tp only after accounting for run order—wMau had only a marginal effect on Tp when we removed run order from the model (χ2 = 3.549, P = 0.06).
Wolbachia effects on Tp may exhibit phylogenetic signal.
Notably, hosts infected with A-group Wolbachia preferred cooler temperatures, whereas the one species infected with B-group Wolbachia preferred a warmer temperature. We conducted a phylogenomic analysis to test whether closely related Wolbachia strains exhibit similar effects on host Tp. We generated a Wolbachia phylogram and used the change in LS mean Tp of each host genotype to test for phylogenetic signal (Fig. 2). A Pagel’s λ value of 1 is consistent with a model of character evolution that entirely agrees with the phylogeny (i.e., Wolbachia effects on host Tp exhibit strong phylogenetic signal), whereas a λ value of 0 indicates that character evolution occurs independently of phylogenetic relationships (66, 67). Our maximum likelihood-fitted λ value was high (λ = 0.778 [0, 0.984]), but not significantly different from a model assuming no phylogenetic signal (likelihood ratio test, P = 0.203). Simulations suggest that a much larger number of Wolbachia strains are required to statistically distinguish λ ≈ 0.8 from zero (Fig. S2). A simulated N = 25 tree had a fitted λ with extremely large confidence intervals (λ = 0.886 [0, 1]), whereas the N = 50 tree had a λ estimate that does not overlap with zero (λ = 0.860 [0.376, 0.977]). Unfortunately, far fewer strains exist in laboratory culture, precluding such an analysis. Nevertheless, our finding that most A-group Wolbachia decreased host Tp and the one B-group strain increased host Tp hints that divergent Wolbachia may have contrasting effects on host behavior.
Distribution of maximum likelihood estimates of λ from 1,000 bootstrap replicates. The bootstrap analysis for our Wolbachia phylogram (Fig. 2) is shown to the left. To the right are simulated phylogenies with an increasing number of Wolbachia strains included (N = 25, 50, 100). For simulated trees, character evolution was simulated with our λ estimate of 0.778 using the “sim.bdtree” and “sim.char” functions in the geiger R package (147). For each graph, fitted λ values for the original phylogeny are shown above with a vertical dashed line. Note that fitted λ values for the simulated phylogenies differ slightly from λ = 0.778, because “sim.char” uses a Brownian-motion model to simulate character evolution along the phylogeny. Below each graph, the mean estimate of λ from the 1,000 replicates () is shown with associated 95% confidence intervals. The bootstrapping analyses generally show that small phylogenies (N = 8, 25) have a large number of near-zero λ values arising by random chance, which increases the uncertainty of parameter estimation. Indeed, small phylogenies are likely to generate near-zero λ values by chance, not necessarily because the phylogeny is unimportant for trait evolution (146). As the number of strains in our analysis increases (N = 50, 100), bootstrapped estimates of λ cluster around the true λ value fitted to the original phylogeny. Download FIG S2, DOCX file, 0.1 MB (76.7KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
24-h temperature shifts generally do not alter Wolbachia titer.
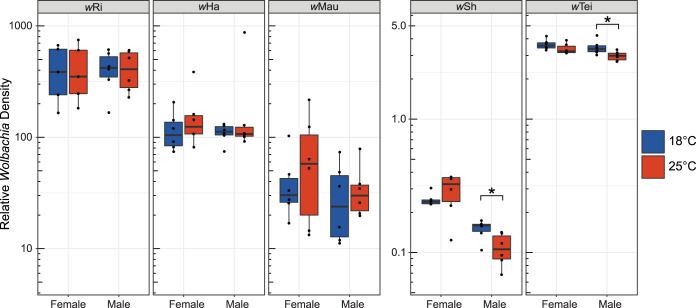
Truitt et al. speculated that the altered Tp of infected flies represents a host-induced behavior to reduce Wolbachia titer and ameliorate the negative effects of infection (47). According to this hypothesis, shifting species infected with A-group Wolbachia (wRi, wHa, wSh, and wTei) to a cool temperature should reduce Wolbachia titer in host bodies (i.e., behavioral chill), whereas shifting D. mauritiana infected with wMau to a warm temperature should reduce Wolbachia titer (i.e., behavioral fever). We tested whether shifting infected hosts toward their Tp increases or decreases Wolbachia titer (Fig. 3). We reared the five infected genotypes mentioned above at an intermediate temperature of 21.5°C and collected female and male virgins for temperature shift experiments. Adults were maintained as virgins, kept at 21.5°C until they were 3 days old, and then shifted to either a cold (18°C) or warm (25°C) incubator for 24 h, after which we measured Wolbachia titer.
FIG 3.
Boxplots of relative Wolbachia density from temperature shift experiments for the five Wolbachia strains showing main effects on host Tp (Table 1). Relative Wolbachia density is shown for virgin females and males shifted to cold (18°C) and warm (25°C) temperatures for 24 h. Graphs are separated into strains with high titer (wRi, wHa, and wMau) and low titer (wSh and wTei). Asterisks denote significant differences in titer between males shifted to 18°C and 25°C based on Wilcoxon rank sum tests at P < 0.05.
For wRi-infected D. simulans, Wolbachia titer did not differ between the 24-h cold and warm temperature treatments for females (W = 12, P = 1) or males (W = 19, P = 0.937). Similarly, for wHa-infected D. simulans, titer did not differ between the temperature treatments for females (W = 13, P = 0.485) or males (W = 18, P = 1). We also observed no significant difference in titer between temperature treatments for wMau-infected D. mauritiana females (W = 14, P = 0.589) or males (W = 14, P = 0.589). For wSh-infected D. sechellia, we detected no difference in Wolbachia titer between females from each temperature treatment (W = 13, P = 0.485); however, we found that males significantly differed in titer between cold and warm treatments (W = 32, P = 0.026). Male D. sechellia shifted to 18°C had a higher median relative Wolbachia density (0.16) than males shifted to 25°C (0.11). This pattern suggests that shifting infected males toward their Tp increases Wolbachia titer. We found a similar result for wTei-infected D. teissieri. While we detected no difference in Wolbachia titer between the treatments for females (W = 28, P = 0.132), males differed significantly in titer between the cold and warm treatments (W = 31, P = 0.041). As with D. sechellia, male D. teissieri shifted to 18°C had a higher median relative Wolbachia density (3.36) than males shifted to 25°C (2.98). Importantly, the wSh and wTei results suggest that males shifted to a colder temperature experience an increase in titer; however, these titer increases are not significant at a threshold of P = 0.005 after a Bonferroni correction for multiple tests.
DISCUSSION
Our analyses suggest that Wolbachia may generally influence host thermoregulatory behavior. Five of the eight Wolbachia strains we assayed had a significant effect on host Tp: wRi in D. simulans, wHa in D. simulans, wMau in D. mauritiana, wSh in D. sechellia, and wTei in D. teissieri. In contrast to past reports (47, 48), we found no evidence for wMelCS or wMel effects on D. melanogaster Tp, which we predict is due to host background effects (see below). Temperature is considered a major ecological factor limiting the distribution of Drosophila (55, 56, 68–72) and many other species (73–75). Body temperature is an important determinant of performance and fitness (50, 54, 76–82), and ectotherms depend on thermoregulatory behavior to maintain body temperature within a narrow range (49–53, 55). Given that Wolbachia have spread through most insect species and other ectotherms (2, 25, 26), our results motivate additional analyses of Wolbachia effects on Tp and thermoregulation of other host taxa.
Interestingly, Drosophila species infected with A-group Wolbachia generally preferred cooler temperatures, whereas D. mauritiana infected with divergent B-group wMau preferred warmer temperatures, suggesting divergent Wolbachia effects on host Tp. Our simulations indicate that an unreasonably large number of strains (N ~ 50) is required to test whether A- and B-group Wolbachia effects on Tp exhibit phylogenetic signal (see Fig. S2 in the supplemental material). Indeed, this number of infected species is not currently available to the research community. Nonetheless, our results specifically motivate analyses of whether other B-group Wolbachia increase Tp. The only other B-group strains that infect hosts in the D. melanogaster subgroup (wNo and wSn) almost always occur as coinfections with other Wolbachia (41). wNo co-occurs with wHa in D. simulans (83–86), and wSn co-occurs with wSh in D. sechellia (85, 87). D. simulans and D. sechellia genotypes singly infected with these B-group Wolbachia are currently unavailable. While phylogenetic relationships could be an important determinant of Wolbachia effects on host Tp, increases or decreases in Tp could also be idiosyncratic from one host genotype to the next.
Our phylogenetic analysis demonstrates that, in some instances, very closely related Wolbachia strains may have different effects on hosts. For example, wTei and wYak diverged only about 1,500 years ago and share very high sequence similarity (0.0039% third-position pairwise differences) (32), yet wTei altered the Tp of D. teissieri and wYak had no effect on D. yakuba (Fig. 2). Similarly, wHa and wSh have high sequence similarity according to our analysis (0.00008% third-position pairwise differences) and likely spread recently via introgression (41, 88), yet our mean estimates of titer for wHa in D. simulans (157.1) and wSh in D. sechellia (0.2) differ by nearly 3 orders of magnitude (Fig. 3). Host background effects may explain why closely related Wolbachia can have variable effects on their hosts. Our results from uninfected flies indicate that Tp varies among host genotypes within species. For D. simulans, the Tp of the Wolbachia-cleared wRi (mean = 23.13°C) and wHa (24.97°C) genotypes was significantly different (Wilcoxon test, W = 84398, P < 0.001). This was also true for the mean Tp of the uninfected wMelCS (27.9°C) and wMel (24.3°C) D. melanogaster genotypes (Wilcoxon test, W = 429288, P < 0.001). Prior work has similarly found that Tp of D. melanogaster varies in North America along a latitudinal cline (55). Indeed, host genomes seem to modify Wolbachia titer (89), maternal Wolbachia transmission (90), components of host fitness (91–93), and the strength of cytoplasmic incompatibility (94–96).
We predict that host background effects also underlie our finding that Wolbachia does not influence D. melanogaster Tp, in contrast to past reports (47, 48). Arnold et al. (48) found a small, yet statistically significant, reduction in Tp of wMelCS-infected D. melanogaster (25.06°C versus 25.78°C for uninfected flies), and Truitt and colleagues (47) found that a wMelCS variant identical to our own (according to 720 genes totaling 733,923 bp) reduced D. melanogaster Tp by nearly 4°C. The effect size reported by Truitt et al. (47) is more than two and a half times greater than the largest effect we document here for any strain, and more than five times larger than the reduction in Tp observed by Arnold and colleagues (48). The wMelCS variant assayed in Truitt et al. (47) was introduced into the foreign DrosDel w1118 isogenic background using chromosome replacement (97), while Arnold et al. (48) used a standard Oregon RC line that was orginally established in the 1920s (8, 98, 99). Our wMelCS-infected genotype is a substrain of the Canton Special line that was also established in the 1920s (100, 101), and substrains of Canton Special can exhibit phenotypic variation due to founder effects and drift (102). It is also worth considering that experimental differences could contribute to differences among Tp studies; for example, differences in the apparatus used to measure Tp (47, 48), fly mating status (103, 104), or statistical approaches could influence Tp estimates. Our analyses accounted for diurnal variation in Tp and host immobilization in the cold (see Materials and Methods), whereas prior analyses did not (47, 48). Regardless, we expect that future analyses of reciprocally introgressed host and Wolbachia genotypes will reveal that host and Wolbachia genomes, and their interaction, contribute to the variation in Tp observed here.
Our temperature shift experiments indicate that changes to Tp of infected host genotypes generally do not alter Wolbachia titer, but in a few instances, reductions in Tp may increase Wolbachia replication within host bodies (Fig. 3). wSh-infected D. sechellia and wTei-infected D. teissieri preferred cooler temperatures than uninfected flies (Fig. 2), and infected males reared at 21.5°C tended to have higher Wolbachia titer when shifted to a cold 18°C treatment for 24 h, compared to a warm 25°C treatment (Fig. 3). Moghadam et al. (105) reported a similar effect of cold temperature on Wolbachia titer in male D. melanogaster, in which males developed at 13°C had higher microbial diversity and a higher relative abundance of Wolbachia than males developed at 23°C and 31°C (based on 16S rRNA sequencing). Our results are consistent with a hypothesis of parasite manipulation, in which Wolbachia alters host behavior to seek environmental conditions that promote Wolbachia growth (16, 18–20, 22, 23). Importantly, however, we found no temperature-associated increases in titer for wSh- and wTei-infected females or for any other Wolbachia strains we assessed. Future work should explore whether changes to male Tp and Wolbachia titer alter traits that determine Wolbachia infection spread through host populations. Increased Wolbachia titer in males is unlikely to affect rates of maternal Wolbachia transmission, but perhaps temperature-associated titer increases could alter the strength of cytoplasmic incompatibility caused by males infected with wSh or wTei (85, 87, 95, 106). Other studies have also reported male-biased effects on Wolbachia titer (42, 62, 107); for example, our own work demonstrated that maternal transmission of wYak to sons is more efficient than to daughters when D. yakuba mothers are reared in cold 20°C conditions (42).
Our findings do not provide support for the hypothesis proposed by Truitt et al. (47) that modifications to Tp represent an adaptive host response (e.g., behavioral chill) to reduce Wolbachia titer and mitigate the negative effects of infection (47). In particular, Truitt et al. (47) speculated that wMelCS is costly to the host because the strain has a higher titer and growth rate than wMel (97) and that wMelCS-infected D. melanogaster prefers colder temperatures to reduce Wolbachia titer and limit costly infections. The authors did not measure wMelCS titer or estimate host fitness components to test this hypothesis (47), although very recent work has demonstrated that wMelCS-infected D. melanogaster has reduced Wolbachia titer when raised at 18°C compared to 25°C (108). We found no effects of wMelCS or wMel on Tp of D. melanogaster and no evidence that decreases in Tp reduce Wolbachia titer for other infected systems (Fig. 3). Nonetheless, the observation that most Wolbachia-infected hosts have altered Tp motivates future analyses of host behaviors that might mitigate negative aspects of infection, especially because Wolbachia can have costly effects on hosts (37, 109–111). We found no association between changes to Tp and a decrease in adult Wolbachia titer, but perhaps infected females seek oviposition sites that reduce the efficiency of Wolbachia maternal transmission (51). Wolbachia maternal transmission is reduced in relatively cold temperatures in Drosophila (42) and hot temperatures in mosquitoes (60, 61). Future work should evaluate whether reductions in host Tp lead to reduced Wolbachia titer and maternal transmission downstream over the course of offspring development. For example, mosquito larvae have reduced wAlbB titer when reared at temperatures of <20°C (63). Temperature shifts longer than 24 h may also be required to generate reductions in titer, especially if infected hosts seek their Tp throughout their lifecycles.
Our results add to mounting literature showing that temperature is an important abiotic factor mediating interactions between Wolbachia and their hosts (112). Wolbachia titer seems to be especially sensitive to temperature (42, 58, 60, 61, 63, 113–116). Our 24-h temperature shift experiments suggest that Wolbachia titer can change over very short time periods due to environmental conditions. Lau et al. (63) similarly found that Wolbachia titer can change within a single host generation, such that cold temperatures (<20°C) reduce wAlbB titer in mosquitoes at the larval stage, but then titer rebounds in adulthood when fourth instar larvae are shifted to warmer conditions (>21°C) (63). Temperature-induced changes to Wolbachia titer are likely to have cascading effects, given that titer influences other host phenotypes (57). For example, exposure to heat stress is associated with correlated declines in Wolbachia titer and the severity of cytoplasmic incompatibility in wMel-transinfected mosquitoes (60, 61). In Drosophila hosts, temperature has been shown to modify the strength of cytoplasmic incompatibility (37, 58, 94, 117), maternal transmission (42, 110), and host fitness effects (118–120). Clearly, more work on how temperature influences Wolbachia-host interactions is needed.
Conclusion.
We show that A- and B-group Wolbachia bacteria induce changes to host Tp and that short shifts in temperature can increase titer in some Wolbachia-infected males. Behavioral changes like these are likely to have fundamental consequences for host physiology and thermoregulation. Wolbachia also modifies a range of other ecologically important host traits in Drosophila species, including reproduction (1, 2), virus blocking (8, 9, 121, 122), nutrient provisioning (123, 124), and activity levels (12, 17). Given that Tp and many other Drosophila traits vary clinally (55, 125), future studies should consider the role of Wolbachia in classic Drosophila clines (72). For example, wMel infection frequencies (120) and the Tp of D. melanogaster (55) both vary spatially in eastern North America.
Understanding the impact of Wolbachia on host performance and fitness is crucial for predicting evolutionary outcomes of Wolbachia-host interactions (39). The initial spread of Wolbachia through new host populations is driven by beneficial effects on host fitness that cause infections to deterministically spread from low initial frequencies (36–38). Yet, strong positive host effects have not been directly connected to spread in nature for any Wolbachia-infected host species (39, 41, 95, 126), although wRi recently evolved to confer a 10% fecundity advantage to D. simulans (111). Few data exist for other components of host fitness, but protection from viruses and nutrient provisioning remain candidates for potential host benefits (8, 9, 121–124, 126, 127). Basic research on how Wolbachia modifies different components of host fitness, like the effects on Tp reported here, represents a key step to uncovering how Wolbachia benefit hosts and spread to become a global pandemic.
MATERIALS AND METHODS
Fly lines.
We evaluated eight different Wolbachia strains infecting six different species in the D. melanogaster subgroup (see Table S1 in the supplemental material). For two of these host species, we tested multiple Wolbachia-infected genotypes: wRi- and wHa-infected D. simulans and wMelCS- and wMel-infected D. melanogaster. With the exception of the wMelCS D. melanogaster line (Canton S Berkeley), all our Wolbachia-infected genotypes were naturally sampled to form isofemale lines, such that single gravid females were collected from the field and placed individually in vials. wMelCS is found only at low frequency in global populations of D. melanogaster (99, 128, 129), because the strain has been largely replaced by a recent sweep of wMel in roughly the last 5,000 years (32, 99, 128, 129). wMelCS was originally identified in the common laboratory strain Canton Special (99–101), and a substrain (Canton S Berkeley) was kindly provided to us by Michael Turelli. All lines were maintained on standard cornmeal medium prior to experiments (Table S3).
Fly food recipe for cornmeal media. To the right, the nutritional content of the food is shown based on calculations from https://brodericklab.com/DDCC.php. Download Table S3, DOCX file, 0.01 MB (12.8KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We generated Wolbachia-uninfected genotypes by treating each infected line with 0.03% tetracycline for four generations. In the fourth generation, we used PCR to confirm that flies were cleared of Wolbachia. We amplified both the Wolbachia surface protein (wsp) and a second set of primers for the arthropod-specific 28S rDNA that served as a positive control (41, 95). We also used quantitative PCR (qPCR) on 10 females homogenized together as a more sensitive confirmation of Wolbachia removal (see qPCR details below). We then reconstituted the gut microbiome of the tetracycline-cleared flies by rearing them on food where infected males of the same genotype had fed and defecated for the prior 48 h. Tetracycline-cleared flies were given at least three more generations before we conducted experiments to avoid detrimental effects of the antibiotic treatment on mitochondrial function (130).
Host temperature preference assays.
We assayed the temperature preference (Tp) of each genotype using a thermal gradient apparatus adapted from previous studies (131, 132). The rectangular thermal gradient comprised a 44 × 13 × 1 cm plate of aluminum with a removable Plexiglas lid (see Fig. S1 in the supplemental material). The Plexiglas lid enclosed a 1-cm-high space above the aluminum plate that allows flies to move around on the thermal gradient. We created an air-tight seal between the aluminum plate and the Plexiglas lid using double-sided tape and C-clamps. To keep flies on the temperature-controlled aluminum plate and off the lid, the Plexiglas was coated with Fluon (BioQuip Products), a slick barrier that prevents insects from obtaining a foothold (133, 134). A light-emitting diode (LED) light was placed above the apparatus to ensure that light was evenly distributed across the entire thermal gradient.
All Tp assays were conducted in a cold storage room with a constant temperature of 5°C. A hot plate set at 90°C was placed under one end of the aluminum plate to create a thermal gradient. All experiments began once the apparatus achieved thermal stability after approximately 0.5 h. The aluminum plate was subdivided into seven 10 × 6 cm sections (Fig. S1), and we recorded the temperature at the center of each section using a thermocouple (Digi-Sense Traceable) prior to the start of each experiment. The temperature decreased linearly along the gradient (R2 = 0.92), ranging from a mean of 34°C at the warmest end (section 1) to 17°C at the coldest end (section 7). Mean temperatures at the center point of each section across all experiments are reported in Table S4.
Mean temperature and standard error for each section of the custom-built thermal gradient apparatus, across all 347 experimental replicates in this study. Download Table S4, DOCX file, 0.01 MB (12.4KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The following protocol for our assay was adapted from previous experiments (47, 55, 131, 132). Trial runs revealed that a sample size of 50 to 60 flies allowed flies to distribute across the gradient without overcrowding in preferred temperature ranges, which is consistent with prior studies (47, 131). Flies were reared in a 25°C incubator under a 12-h light:12-h dark light cycle (Pericival model I-36LL) on a standard food diet (Table S3). For each genotype, we collected virgin flies as a batch and separated them into four treatment groups: uninfected females, infected females, uninfected males, and infected males. Flies of each treatment group were separated as virgins in groups of 60 in individual food vials and kept until they were 3 to 5 days old. We selected a single batch each day and ran all four treatment groups separately in a randomized order, such that all flies assayed on a given day were of the same batch and age. All experiments were run between 9 a.m. and 5 p.m. Before each run, we measured the temperature at the center of each section along the gradient and then transferred flies into the apparatus through a small hole located in the middle of the Plexiglas lid where the temperature averaged 22.7°C (Table S4). Flies were allowed to choose their preferred temperatures along the gradient for 30 min (47, 48, 131, 132). At the end of this period, we visually scored the numbers of flies in each section. For our records, we also used a camera mounted above the thermal gradient to take a picture of the distribution of flies in each section. A subset of flies located on the Plexiglas lid were removed from the analysis (132). After each run, the thermal gradient was cleaned with ethanol and allowed to dry. The total number of replicates run for each treatment group ranged from 6 to 21. The final number of flies recorded in each replicate varied due to variation in mortality and the number of flies located on the Plexiglas lid.
For each genotype, we analyzed the Tp data using generalized linear mixed models (GLMMs) and a Poisson error structure in R (135) with the “glmer” function in the lme4 package (136). We treated the Tp of each fly as the dependent variable and included infection status, sex, an infection-by-sex interaction, fly age (3, 4, or 5 days), and the run order of each replicate over the course of the day (1st, 2nd, 3rd, or 4th) as fixed effects. The replicate identifier (ID) of each run was included as a random effect. We then assessed the significance of fixed effects using an analysis of deviance with chi-squared tests. The Tp data for some genotypes more closely approximated a normal distribution (see Table S2), so we conducted an analogous set of tests using linear mixed models (LMMs) with the “lmer” function in the lme4 package. Here, we assessed significance of fixed effects using an analysis of variance (ANOVA) with Wald’s chi-squared tests. The LMMs produced qualitatively similar results to the GLMMs, so only results from the GLMMs are presented in the main text.
A preliminary analysis of the data revealed that flies seemed to form a bimodal distribution along the thermal gradient, with one cluster of flies located at the cold end of the gradient (section 7) where temperatures averaged about 17°C (Fig. S3). Given that 17°C generally falls below the average Tp of Drosophila species reported in previous experiments (47, 48, 55, 131), we hypothesized that flies were becoming immobilized in section 7 due to the cold temperature (51). A similar phenomenon has been identified for Caenorhabditis elegans in assays of Tp—the movement speed of C. elegans is dependent on temperature, which can leave worms “trapped” in cold sections of a thermal gradient (137). Thus, we removed the putatively immobilized flies in section 7 from each data set and reconducted our analyses. The analyses excluding section 7 are presented in the main text (Table 1); however, including section 7 did not alter our findings of Wolbachia effects on Tp (Table S5). We concluded that the data set excluding immobilized flies represents a more biologically accurate measure of Tp for each genotype.
Box plots showing temperature preference (Tp) for uninfected and infected flies of each genotype when the coldest section of the thermal gradient (section 7) is included. Individual points are jittered to show overlap. Download FIG S3, DOCX file, 0.5 MB (491.8KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Results and sample sizes from the GLMM and LMM analyses of Tp data, including the flies located in the coldest section of the thermal gradient apparatus (section 7). Statistically significant fixed effects at P < 0.05 are shown in bold text with asterisks. Download Table S5, DOCX file, 0.02 MB (18.6KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Wolbachia sequencing and phylogenomic analysis.
We conducted a phylogenomic analysis to characterize the evolutionary relationships among Wolbachia strains included in this study. Hosts infected with A-group Wolbachia (wRi, wHa, wSh, and wTei) preferred cooler temperatures, whereas D. mauritiana infected with B-group wMau preferred a warmer temperature. Therefore, we used a Wolbachia phylogram to test whether these Wolbachia effects on host Tp exhibit phylogenetic signal. We obtained Wolbachia sequences from publicly available genome assemblies, which included wRi (138), wHa (139), wMau (41), and wYak and wTei (32). We also obtained raw Illumina reads for a wSh-infected D. sechellia individual from a previously published data set (NCBI:SRA accession no. SRX3029362) (140). Importantly, two divergent Wolbachia strains may infect D. sechellia: A-group wSh and B-group wSn. In nature, wSh singly infects some individuals, but it also occurs as a coinfection with wSn (85). We confirmed that our D. sechellia genotype (PmuseumbananaI) is singly infected with wSh using qPCR primers described below, which can distinguish between A-group and B-group Wolbachia. Finally, we sequenced our wMelCS- and wMel-infected D. melanogaster genotypes (Canton S Berkeley and PC75, respectively) to compare the sequence similarity of our variants of these strains to those used in the prior assay of Tp by Truitt et al. (47, 97).
Tissue samples for genomic DNA were extracted using a DNeasy Blood & Tissue kit (Qiagen). DNA quantity was tested on a Nanodrop (Implen), and total DNA was quantified by Qubit fluorometric quantitation (Invitrogen). DNA was cleaned using Agencourt AMPure XP beads (Beckman Coulter, Inc.) following the manufacturer’s instructions, and eluted in 50 μl of 1× TE (Tris-EDTA) buffer for shearing. DNA was sheared using a Covaris E220 Focused Ultrasonicator (Covaris Inc.) to a target size of 400 bp. We prepared libraries using NEBNext Ultra II DNA Library Prep with Sample Purification beads (New England BioLabs). Final fragment sizes and concentrations were confirmed using a TapeStation 2200 system (Agilent). We indexed samples using NEBNext Multiplex Oligos for Illumina (Index Primers Set 3 and Index Primers Set 4), and 10 μl of each sample was shipped to Novogene (Sacramento, CA, USA) for sequencing using Illumina HiSeq 4000, generating paired-end 150 bp reads.
Reads were trimmed using Sickle version 1.33 (141) and assembled using ABySS version 2.0.2 (142). K values of 71, 81, and 91 were used, and scaffolds with the best nucleotide BLAST matches to known Wolbachia sequences with E values less than 10−10 were extracted as the draft Wolbachia assemblies. For each genotype, we chose the assembly with the highest N50 and the fewest scaffolds (Table S6). The wMelCS, wMel, and wSh genomes, along with the five previously published genomes were annotated using Prokka version 1.11, which identifies homologs to known bacterial genes (143). To avoid pseudogenes and paralogs, we only used genes present in a single copy with no alignment gaps in all of the genome sequences. Genes were identified as single copy if they uniquely matched a bacterial reference gene identified by Prokka. By requiring all homologs to have identical length in all of the Wolbachia genomes, we removed all loci with indels. A total of 214 genes totaling 181,488 bp met these criteria.
The scaffold count, N50, and total assembly size of each Wolbachia assembly. Download Table S6, DOCX file, 0.01 MB (12.6KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We also repeated this analysis to include the wMelCS and wMel genomes used in Truitt et al. (47). Here, we restricted our analysis to only wMelCS and wMel Wolbachia, with the goal of comparing sequence similarity between the variants used in this study to those from Truitt et al. (47). Given that many loci accumulate indels over time, the number of loci included in this analysis of wMel-like Wolbachia was relatively high, with a total of 720 genes totaling 733,923 bp that met our criteria. Based on these 720 genes, our wMelCS variant infecting the Canton S Berkeley genotype was identical to the wMelCS variant used in Truitt et al. (47). Our wMel variant infecting the PC75 genotype was also highly similar to wMel used in Truitt et al. (47), with only 0.000016% third-position pairwise differences (only 4 out of 244,641 third-codon positions).
We estimated a Bayesian phylogram of the 214 genes from the eight different Wolbachia strains using RevBayes 1.0.8 under the general tree reversible GTR + Γ model partitioned by codon position (144). Four independent runs were performed for each phylogenetic tree we estimated, and in each instance, all four runs converged on the same topology. All nodes were supported with Bayesian posterior probabilities of 1.
We used the resulting phylogram to test whether Wolbachia effects on host Tp exhibit phylogenetic signal. For each genotype, we extracted the least-square (LS) mean Tp for infected and uninfected flies from the GLMMs and then used the change in LS mean Tp as a continuous character to calculate the maximum likelihood value of Pagel’s lambda (λ) (67). We used a likelihood ratio test to compare our fitted value of λ to a model assuming no phylogenetic signal (λ = 0) using the “phylosig” function in the R package phytools (145). We also employed a Monte Carlo-based method to generate 95% confidence intervals surrounding our λ estimate using 1,000 bootstrap replicates in the R package pmc (146). To evaluate whether larger phylogenies increase the accuracy of λ estimation, we simulated trees with an increasing number of Wolbachia strains (N = 25, 50, and 100) and our λ estimate of 0.778 using the “sim.bdtree” and “sim.char” functions in the geiger R package (147). We then reestimated confidence intervals surrounding λ using the larger simulated trees. See Fig. S2 for an extended description of the simulations.
Host temperature shift experiments.
We tested whether shifting infected hosts toward their Tp increases or decreases Wolbachia titer. We reared the five infected host genotypes with altered Tp at an intermediate temperature of 21.5°C. We separated female and male virgins, kept them at 21.5°C until they were 3 days old, and then shifted them to either a cold (18°C) or warm (25°C) incubator for 24 h. Flies were separated by sex and maintained in groups of 40 in individual food vials throughout the course of the experiment. Following 24 h of the cold/warm temperature treatment, flies were frozen in a −80°C freezer for subsequent analysis of Wolbachia titer.
We used qPCR to compare Wolbachia titer in flies shifted to 18°C versus 25°C. Flies from each temperature treatment were homogenized together in groups of 10. The final samples included six biological replicates for each sex and temperature treatment. DNA was extracted using a DNeasy Blood & Tissue kit (Qiagen). Preliminary analyses indicated that our extractions contained DNA quantities that are well within the recommended range for PowerUp SYBR green Master Mix (Thermo Fisher Scientific) used in our qPCRs. We used a Stratagene Mx3000P (Agilent Technologies) to amplify Drosophila- and Wolbachia-specific loci. In order to quantify the titers of the five different Wolbachia strains, we utilized multiple combinations of Drosophila and Wolbachia qPCR primers (Table S7). Efficiency curves were generated to confirm that each primer pair had adequate efficiency. All qPCRs were amplified using the following cycling conditions: 50°C for 2 min, 95°C for 2 min, and then 40 cycles, with one cycle consisting of 95°C for 15 s, 58°C for 15 s, and 72°C for 1 min. We used the average cycle threshold (Ct) value of three technical replicates for each sample. We estimated relative Wolbachia density as , where ΔCt = Cthost – CtWolbachia (148). We then used a Wilcoxon rank sum test to assess differences in titer between flies shifted to 18°C and 25°C.
qPCR primers used to measure relative Wolbachia density in temperature shift experiments. Download Table S7, DOCX file, 0.01 MB (13.6KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data availability.
Genome assemblies are deposited on GenBank (BioProject accession no. PRJNA658309). All other data are available on Dryad (https://doi.org/10.5061/dryad.j9kd51c8r).
ACKNOWLEDGMENTS
We thank Tim Wheeler for assistance in the lab and Will Conner for help with bioinformatic analyses. Isaac Humble helped construct the thermal gradient apparatus. Dave Begun, Michael Turelli, and Daniel Matute kindly provided the flies used in this study. The Cooper lab group, Michael May, and Gregg Thomas provided valuable feedback that improved the quality of the manuscript. We thank the Genomics Core and the Environmental Control for Organismal Research (ECOR) Laboratories at the University of Montana for their support. An invited editor and two anonymous reviewers provided comments that improved the manuscript.
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under award number R35GM124701 to B.S.C.
Footnotes
Citation Hague MTJ, Caldwell CN, Cooper BS. 2020. Pervasive effects of Wolbachia on host temperature preference. mBio 11:e01768-20. https://doi.org/10.1128/mBio.01768-20.
Contributor Information
Robert L. Unckless, University of Kansas.
Vaughn S. Cooper, University of Pittsburgh.
REFERENCES
- 1.Hoffmann AA, Turelli M. 1997. Cytoplasmic incompatibility in insects, p 42–80. In O’Neill SL, Hoffmann AA, Werren JH (ed), Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 2.Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 3.Baumann P. 2005. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol 59:155–189. doi: 10.1146/annurev.micro.59.030804.121041. [DOI] [PubMed] [Google Scholar]
- 4.Moran NA, McCutcheon JP, Nakabachi A. 2008. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 5.Douglas AE. 2009. The microbial dimension in insect nutritional ecology. Funct Ecol 23:38–47. doi: 10.1111/j.1365-2435.2008.01442.x. [DOI] [Google Scholar]
- 6.Brumin M, Kontsedalov S, Ghanim M. 2011. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Sci 18:57–66. doi: 10.1111/j.1744-7917.2010.01396.x. [DOI] [Google Scholar]
- 7.Mueller UG, Mikheyev AS, Hong E, Sen R, Warren DL, Solomon SE, Ishak HD, Cooper M, Miller JL, Shaffer KA, Juenger TE. 2011. Evolution of cold-tolerant fungal symbionts permits winter fungiculture by leafcutter ants at the northern frontier of a tropical ant-fungus symbiosis. Proc Natl Acad Sci U S A 108:4053–4056. doi: 10.1073/pnas.1015806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedges LM, Brownlie JC, O’Neill SL, Johnson KN. 2008. Wolbachia and virus protection in insects. Science 322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 9.Teixeira L, Ferreira Á, Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6:e1000002. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldhaar H. 2011. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol Entomol 36:533–543. doi: 10.1111/j.1365-2311.2011.01318.x. [DOI] [Google Scholar]
- 11.Goodacre SL, Martin OY. 2012. Modification of insect and arachnid behaviours by vertically transmitted endosymbionts: infections as drivers of behavioural change and evolutionary novelty. Insects 3:246–261. doi: 10.3390/insects3010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bi J, Wang Y-F. 2020. The effect of the endosymbiont Wolbachia on the behavior of insect hosts. Insect Sci 27:846–858. doi: 10.1111/1744-7917.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosokawa T, Fukatsu T. 2020. Relevance of microbial symbiosis to insect behavior. Curr Opin Insect Sci 39:91–100. doi: 10.1016/j.cois.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Hentschel U, Steinert M, Hacker J. 2000. Common molecular mechanisms of symbiosis and pathogenesis. Trends Microbiol 8:226–231. doi: 10.1016/S0966-842X(00)01758-3. [DOI] [PubMed] [Google Scholar]
- 15.Lefevre T, Thomas F. 2008. Behind the scene, something else is pulling the strings: emphasizing parasitic manipulation in vector-borne diseases. Infect Genet Evol 8:504–519. doi: 10.1016/j.meegid.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Poulin R. 2010. Parasite manipulation of host behavior: an update and frequently asked questions, p 151–186. In Macedo R. (ed), Advances in the study of behavior, vol 42. Elsevier, New York, NY. [Google Scholar]
- 17.van Houte S, Ros VI, van Oers MM. 2013. Walking with insects: molecular mechanisms behind parasitic manipulation of host behaviour. Mol Ecol 22:3458–3475. doi: 10.1111/mec.12307. [DOI] [PubMed] [Google Scholar]
- 18.Heil M. 2016. Host manipulation by parasites: cases, patterns, and remaining doubts. Front Ecol Evol 4:80. [Google Scholar]
- 19.Vale PF, Siva-Jothy A, Morrill A, Forbes MR. 2018. The influence of parasites, p 273–291. In Cordoba-Aguilar A, Gonzalez-Tokman D, Gonzalez-Santoyo I (ed), Insect behavior: from mechanisms to ecological and evolutionary consequences. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 20.Weinersmith KL. 2019. What’s gotten into you? A review of recent research on parasitoid manipulation of host behavior. Curr Opin Insect Sci 33:37–42. doi: 10.1016/j.cois.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Hart BL. 1988. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12:123–137. doi: 10.1016/S0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 22.De Roode JC, Lefèvre T. 2012. Behavioral immunity in insects. Insects 3:789–820. doi: 10.3390/insects3030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis VA. 2014. Infection-avoidance behaviour in humans and other animals. Trends Immunol 35:457–464. doi: 10.1016/j.it.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Fedorka KM, Kutch IC, Collins L, Musto E. 2016. Cold temperature preference in bacterially infected Drosophila melanogaster improves survival but is remarkably suboptimal. J Insect Physiol 93-94:36–41. doi: 10.1016/j.jinsphys.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Zug R, Hammerstein P. 2012. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One 7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ. 2015. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc Biol Sci 282:20150249. doi: 10.1098/rspb.2015.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Neill SL, Giordano R, Colbert AM, Karr TL, Robertson HM. 1992. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci U S A 89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raychoudhury R, Baldo L, Oliveira DC, Werren JH. 2009. Modes of acquisition of Wolbachia: horizontal transfer, hybrid introgression, and codivergence in the Nasonia species complex. Evolution 63:165–183. doi: 10.1111/j.1558-5646.2008.00533.x. [DOI] [PubMed] [Google Scholar]
- 29.Gerth M, Bleidorn C. 2016. Comparative genomics provides a timeframe for Wolbachia evolution and exposes a recent biotin synthesis operon transfer. Nat Microbiol 2:16241. [DOI] [PubMed] [Google Scholar]
- 30.Conner WR, Blaxter ML, Anfora G, Ometto L, Rota-Stabelli O, Turelli M. 2017. Genome comparisons indicate recent transfer of wRi-like Wolbachia between sister species Drosophila suzukii and D. subpulchrella. Ecol Evol 7:9391–9404. doi: 10.1002/ece3.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turelli M, Cooper BS, Richardson KM, Ginsberg PS, Peckenpaugh B, Antelope CX, Kim KJ, May MR, Abrieux A, Wilson DA, Bronski MJ, Moore BR, Gao J-J, Eisen MB, Chiu JC, Conner WR, Hoffmann AA. 2018. Rapid global spread of wRi-like Wolbachia across multiple Drosophila. Curr Biol 28:963–971.e8. doi: 10.1016/j.cub.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper BS, Vanderpool D, Conner WR, Matute DR, Turelli M. 2019. Wolbachia acquisition by Drosophila yakuba-clade hosts and transfer of incompatibility loci between distantly related Wolbachia. Genetics 212:1399–1419. doi: 10.1534/genetics.119.302349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dobson SL, Bourtzis K, Braig HR, Jones BF, Zhou W, Rousset F, O’Neill SL. 1999. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem Mol Biol 29:153–160. doi: 10.1016/S0965-1748(98)00119-2. [DOI] [PubMed] [Google Scholar]
- 34.Albertson R, Casper-Lindley C, Cao J, Tram U, Sullivan W. 2009. Symmetric and asymmetric mitotic segregation patterns influence Wolbachia distribution in host somatic tissue. J Cell Sci 122:4570–4583. doi: 10.1242/jcs.054981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pietri JE, DeBruhl H, Sullivan W. 2016. The rich somatic life of Wolbachia. MicrobiologyOpen 5:923–936. doi: 10.1002/mbo3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caspari E, Watson G. 1959. On the evolutionary importance of cytoplasmic sterility in mosquitoes. Evolution 13:568–570. doi: 10.1111/j.1558-5646.1959.tb03045.x. [DOI] [Google Scholar]
- 37.Hoffmann AA, Turelli M, Harshman LG. 1990. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics 126:933–948. https://www.genetics.org/content/126/4/933.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barton N, Turelli M. 2011. Spatial waves of advance with bistable dynamics: cytoplasmic and genetic analogues of Allee effects. Am Nat 178:E48–E75. doi: 10.1086/661246. [DOI] [PubMed] [Google Scholar]
- 39.Ross PA, Turelli M, Hoffmann AA. 2019. Evolutionary ecology of Wolbachia releases for disease control. Annu Rev Genet 53:93–116. doi: 10.1146/annurev-genet-112618-043609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kriesner P, Hoffmann AA. 2018. Rapid spread of a Wolbachia infection that does not affect host reproduction in Drosophila simulans cage populations. Evolution 72:1475–1487. doi: 10.1111/evo.13506. [DOI] [PubMed] [Google Scholar]
- 41.Meany MK, Conner WR, Richter SV, Bailey JA, Turelli M, Cooper BS. 2019. Loss of cytoplasmic incompatibility and minimal fecundity effects explain relatively low Wolbachia frequencies in Drosophila mauritiana. Evolution 73:1278–1295. doi: 10.1111/evo.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hague MT, Mavengere H, Matute DR, Cooper BS. 2020. Environmental and genetic contributions to imperfect wMel-like Wolbachia transmission and frequency variation. Genetics 215:1117–1132. doi: 10.1534/genetics.120.303330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell JA, Moran NA. 2006. Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc Biol Sci 273:603–610. doi: 10.1098/rspb.2005.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunbar HE, Wilson AC, Ferguson NR, Moran NA. 2007. Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS Biol 5:e96. doi: 10.1371/journal.pbio.0050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wernegreen JJ. 2012. Mutualism meltdown in insects: bacteria constrain thermal adaptation. Curr Opin Microbiol 15:255–262. doi: 10.1016/j.mib.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang B, Leonard SP, Li Y, Moran NA. 2019. Obligate bacterial endosymbionts limit thermal tolerance of insect host species. Proc Natl Acad Sci U S A 116:24712–24718. doi: 10.1073/pnas.1915307116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Truitt AM, Kapun M, Kaur R, Miller WJ. 2019. Wolbachia modifies thermal preference in Drosophila melanogaster. Environ Microbiol 21:3259–3268. doi: 10.1111/1462-2920.14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arnold PA, Levin SC, Stevanovic AL, Johnson KN. 2019. Drosophila melanogaster infected with Wolbachia strain wMelCS prefer cooler temperatures. Ecol Entomol 44:287–290. doi: 10.1111/een.12696. [DOI] [Google Scholar]
- 49.Angilletta MJ, Steury TD, Sears MW. 2004. Temperature, growth rate, and body size in ectotherms: fitting pieces of a life-history puzzle. Integr Comp Biol 44:498–509. doi: 10.1093/icb/44.6.498. [DOI] [PubMed] [Google Scholar]
- 50.Martin TL, Huey RB. 2008. Why “suboptimal” is optimal: Jensen’s inequality and ectotherm thermal preferences. Am Nat 171:E102–E118. doi: 10.1086/527502. [DOI] [PubMed] [Google Scholar]
- 51.Dillon ME, Wang G, Garrity PA, Huey RB. 2009. Thermal preference in Drosophila. J Therm Biol 34:109–119. doi: 10.1016/j.jtherbio.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garrity PA, Goodman MB, Samuel AD, Sengupta P. 2010. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev 24:2365–2382. doi: 10.1101/gad.1953710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffmann AA, Sgro CM. 2011. Climate change and evolutionary adaptation. Nature 470:479–485. doi: 10.1038/nature09670. [DOI] [PubMed] [Google Scholar]
- 54.Condon C, Cooper BS, Yeaman S, Angilletta MJ Jr.. 2014. Temporal variation favors the evolution of generalists in experimental populations of Drosophila melanogaster. Evolution 68:720–728. doi: 10.1111/evo.12296. [DOI] [PubMed] [Google Scholar]
- 55.Rajpurohit S, Schmidt PS. 2016. Measuring thermal behavior in smaller insects: a case study in Drosophila melanogaster demonstrates effects of sex, geographic origin, and rearing temperature on adult behavior. Fly (Austin) 10:149–161. doi: 10.1080/19336934.2016.1194145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffmann A. 2010. Physiological climatic limits in Drosophila: patterns and implications. J Exp Biol 213:870–880. doi: 10.1242/jeb.037630. [DOI] [PubMed] [Google Scholar]
- 57.López-Madrigal S, Duarte EH. 2019. Titer regulation in arthropod-Wolbachia symbioses. FEMS Microbiol Lett 366:fnz232. doi: 10.1093/femsle/fnz232. [DOI] [PubMed] [Google Scholar]
- 58.Clancy DJ, Hoffmann AA. 1998. Environmental effects on cytoplasmic incompatibility and bacterial load in Wolbachia-infected Drosophila simulans. Entomol Exp Applic 86:13–24. doi: 10.1046/j.1570-7458.1998.00261.x. [DOI] [Google Scholar]
- 59.Ulrich JN, Beier JC, Devine GJ, Hugo LE. 2016. Heat sensitivity of wMel Wolbachia during Aedes aegypti development. PLoS Negl Trop Dis 10:e0004873. doi: 10.1371/journal.pntd.0004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM, Hoffmann AA. 2017. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog 13:e1006006. doi: 10.1371/journal.ppat.1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ross PA, Ritchie SA, Axford JK, Hoffmann AA. 2019. Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Negl Trop Dis 13:e0007357. doi: 10.1371/journal.pntd.0007357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foo IJ-H, Hoffmann AA, Ross PA. 2019. Cross-generational effects of heat stress on fitness and Wolbachia density in Aedes aegypti mosquitoes. Trop Med Infect Dis 4:13. doi: 10.3390/tropicalmed4010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lau M-J, Ross PA, Endersby-Harshman NM, Hoffmann AA. 2020. Impacts of low temperatures on Wolbachia (Rickettsiales: Rickettsiaceae)-infected Aedes aegypti (Diptera: Culicidae). J Med Entomol. doi: 10.1093/jme/tjaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaneko H, Head LM, Ling J, Tang X, Liu Y, Hardin PE, Emery P, Hamada FN. 2012. Circadian rhythm of temperature preference and its neural control in Drosophila. Curr Biol 22:1851–1857. doi: 10.1016/j.cub.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Refinetti R, Menaker M. 1992. The circadian rhythm of body temperature. Physiol Behav 51:613–637. doi: 10.1016/0031-9384(92)90188-8. [DOI] [PubMed] [Google Scholar]
- 66.Freckleton RP, Harvey PH, Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat 160:712–726. doi: 10.1086/343873. [DOI] [PubMed] [Google Scholar]
- 67.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- 68.Hoffmann AA, Anderson A, Hallas R. 2002. Opposing clines for high and low temperature resistance in Drosophila melanogaster. Ecol Lett 5:614–618. doi: 10.1046/j.1461-0248.2002.00367.x. [DOI] [Google Scholar]
- 69.Umina PA, Weeks AR, Kearney MR, McKechnie SW, Hoffmann AA. 2005. A rapid shift in a classic clinal pattern in Drosophila reflecting climate change. Science 308:691–693. doi: 10.1126/science.1109523. [DOI] [PubMed] [Google Scholar]
- 70.Kellermann V, van Heerwaarden B, Sgrò CM, Hoffmann AA. 2009. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 325:1244–1246. doi: 10.1126/science.1175443. [DOI] [PubMed] [Google Scholar]
- 71.Kellermann V, Loeschcke V, Hoffmann AA, Kristensen TN, Fløjgaard C, David JR, Svenning J-C, Overgaard J. 2012. Phylogenetic constraints in key functional traits behind species’ climate niches: patterns of desiccation and cold resistance across 95 Drosophila species. Evolution 66:3377–3389. doi: 10.1111/j.1558-5646.2012.01685.x. [DOI] [PubMed] [Google Scholar]
- 72.Adrion JR, Hahn MW, Cooper BS. 2015. Revisiting classic clines in Drosophila melanogaster in the age of genomics. Trends Genet 31:434–444. doi: 10.1016/j.tig.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crisp MD, Arroyo MT, Cook LG, Gandolfo MA, Jordan GJ, McGlone MS, Weston PH, Westoby M, Wilf P, Linder HP. 2009. Phylogenetic biome conservatism on a global scale. Nature 458:754–756. doi: 10.1038/nature07764. [DOI] [PubMed] [Google Scholar]
- 74.Tittensor DP, Mora C, Jetz W, Lotze HK, Ricard D, Berghe EV, Worm B. 2010. Global patterns and predictors of marine biodiversity across taxa. Nature 466:1098–1101. doi: 10.1038/nature09329. [DOI] [PubMed] [Google Scholar]
- 75.Quintero I, Wiens JJ. 2013. Rates of projected climate change dramatically exceed past rates of climatic niche evolution among vertebrate species. Ecol Lett 16:1095–1103. doi: 10.1111/ele.12144. [DOI] [PubMed] [Google Scholar]
- 76.Siddiqui W, Barlow C. 1972. Population growth of Drosophila melanogaster (Diptera: Drosophilidae) at constant and alternating temperatures. Ann Entomol Soc Am 65:993–1001. doi: 10.1093/aesa/65.5.993. [DOI] [Google Scholar]
- 77.Huey RB, Berrigan D. 2001. Temperature, demography, and ectotherm fitness. Am Nat 158:204–210. doi: 10.1086/321314. [DOI] [PubMed] [Google Scholar]
- 78.Chown SL, Nicolson S. 2004. Insect physiological ecology: mechanisms and patterns. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 79.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 80.Cooper BS, Hammad LA, Fisher NP, Karty JA, Montooth KL. 2012. In a variable thermal environment selection favors greater plasticity of cell membranes in Drosophila melanogaster. Evolution 66:1976–1984. doi: 10.1111/j.1558-5646.2011.01566.x. [DOI] [PubMed] [Google Scholar]
- 81.Huey RB, Kearney MR, Krockenberger A, Holtum JA, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Philos Trans R Soc Lond B Biol Sci 367:1665–1679. doi: 10.1098/rstb.2012.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoekstra LA, Siddiq MA, Montooth KL. 2013. Pleiotropic effects of a mitochondrial-nuclear incompatibility depend upon the accelerating effect of temperature in Drosophila. Genetics 195:1129–1139. doi: 10.1534/genetics.113.154914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Neill SL, Karr TL. 1990. Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature 348:178–180. doi: 10.1038/348178a0. [DOI] [PubMed] [Google Scholar]
- 84.Mercot H, Llorente B, Jacques M, Atlan A, Montchamp-Moreau C. 1995. Variability within the Seychelles cytoplasmic incompatibility system in Drosophila simulans. Genetics 141:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rousset F, Solignac M. 1995. Evolution of single and double Wolbachia symbioses during speciation in the Drosophila simulans complex. Proc Natl Acad Sci U S A 92:6389–6393. doi: 10.1073/pnas.92.14.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.James A, Dean M, McMahon M, Ballard J. 2002. Dynamics of double and single Wolbachia infections in Drosophila simulans from New Caledonia. Heredity (Edinb) 88:182–189. doi: 10.1038/sj.hdy.6800025. [DOI] [PubMed] [Google Scholar]
- 87.Giordano R, O’Neill SL, Robertson HM. 1995. Wolbachia infections and the expression of cytoplasmic incompatibility in Drosophila sechellia and D. mauritiana. Genetics 140:1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ballard JWO. 2000. Comparative genomics of mitochondrial DNA in members of the Drosophila melanogaster subgroup. J Mol Evol 51:48–63. doi: 10.1007/s002390010066. [DOI] [PubMed] [Google Scholar]
- 89.Funkhouser-Jones LJ, van Opstal EJ, Sharma A, Bordenstein SR. 2018. The maternal effect gene Wds controls Wolbachia titer in Nasonia. Curr Biol 28:1692–1702. doi: 10.1016/j.cub.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Serbus LR, Sullivan W. 2007. A cellular basis for Wolbachia recruitment to the host germline. PLoS Pathog 3:e190. doi: 10.1371/journal.ppat.0030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fry A, Palmer M, Rand D. 2004. Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity (Edinb) 93:379–389. doi: 10.1038/sj.hdy.6800514. [DOI] [PubMed] [Google Scholar]
- 92.Dean MD. 2006. A Wolbachia-associated fitness benefit depends on genetic background in Drosophila simulans. Proc Biol Sci 273:1415–1420. doi: 10.1098/rspb.2005.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gruntenko NE, Karpova EK, Adonyeva NV, Andreenkova OV, Burdina EV, Ilinsky YY, Bykov RA, Menshanov PN, Rauschenbach IY. 2019. Drosophila female fertility and juvenile hormone metabolism depends on the type of Wolbachia infection. J Exp Biol 222:jeb195347. doi: 10.1242/jeb.195347. [DOI] [PubMed] [Google Scholar]
- 94.Reynolds KT, Hoffmann AA. 2002. Male age, host effects and the weak expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genet Res 80:79–87. doi: 10.1017/S0016672302005827. [DOI] [PubMed] [Google Scholar]
- 95.Cooper BS, Ginsberg PS, Turelli M, Matute DR. 2017. Wolbachia in the Drosophila yakuba complex: pervasive frequency variation and weak cytoplasmic incompatibility, but no apparent effect on reproductive isolation. Genetics 205:333–351. doi: 10.1534/genetics.116.196238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cattel J, Nikolouli K, Andrieux T, Martinez J, Jiggins F, Charlat S, Vavre F, Lejon D, Gibert P, Mouton L. 2018. Back and forth Wolbachia transfers reveal efficient strains to control spotted wing Drosophila populations. J Appl Ecol 55:2408–2418. doi: 10.1111/1365-2664.13101. [DOI] [Google Scholar]
- 97.Chrostek E, Marialva MSP, Esteves SS, Weinert LA, Martinez J, Jiggins FM, Teixeira L. 2013. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet 9:e1003896. doi: 10.1371/journal.pgen.1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hartl D, Jungen H. 1979. Estimation of average fitness of populations of Drosophila melanogaster and the evolution of fitness in experimental populations. Evolution 33:371–380. doi: 10.1111/j.1558-5646.1979.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 99.Riegler M, Sidhu M, Miller WJ, O’Neill SL. 2005. Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr Biol 15:1428–1433. doi: 10.1016/j.cub.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 100.Stern C. 1943. Genic action as studied by means of the effects of different doses and combinations of alleles. Genetics 28:441–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stern C, Schaeffer EW. 1943. On primary attributes of alleles in Drosophila melanogaster. Proc Natl Acad Sci U S A 29:351–361. doi: 10.1073/pnas.29.11.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Colomb J, Brembs B. 2014. Sub-strains of Drosophila Canton-S differ markedly in their locomotor behavior. F1000Res 3:176. doi: 10.12688/f1000research.4263.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Isaac RE, Li C, Leedale AE, Shirras AD. 2010. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc Biol Sci 277:65–70. doi: 10.1098/rspb.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. 2011. Insect seminal fluid proteins: identification and function. Annu Rev Entomol 56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moghadam NN, Thorshauge PM, Kristensen TN, de Jonge N, Bahrndorff S, Kjeldal H, Nielsen JL. 2018. Strong responses of Drosophila melanogaster microbiota to developmental temperature. Fly (Austin) 12:1–12. doi: 10.1080/19336934.2017.1394558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Charlat S, Nirgianaki A, Bourtzis K, Merçot H. 2002. Evolution of Wolbachia-induced cytoplasmic incompatibility in Drosophila simulans and D. sechellia. Evolution 56:1735–1742.2.0.CO;2]. doi: 10.1111/j.0014-3820.2002.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 107.Correa CC, Ballard JWO. 2012. Wolbachia gonadal density in female and male Drosophila vary with laboratory adaptation and respond differently to physiological and environmental challenges. J Invertebr Pathol 111:197–204. doi: 10.1016/j.jip.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 108.Chrostek E, Martins N, Marialva MS, Teixeira L. 2020. Wolbachia-conferred antiviral protection is determined by developmental temperature. bioRxiv doi: 10.1101/2020.06.24.169169. [DOI] [PMC free article] [PubMed]
- 109.Stouthamer R, Luck R. 1993. Influence of microbe-associated parthenogenesis on the fecundity of Trichogramma deion and T. pretiosum. Entomol Exp Applic 67:183–192. doi: 10.1111/j.1570-7458.1993.tb01667.x. [DOI] [Google Scholar]
- 110.Turelli M, Hoffmann AA. 1995. Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics 140:1319–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA. 2007. From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol 5:e114. doi: 10.1371/journal.pbio.0050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Charlesworth J, Weinert LA, Araujo E Jr, Welch JJ. 2019. Wolbachia, Cardinium and climate: an analysis of global data. Biol Lett 15:20190273. doi: 10.1098/rsbl.2019.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mouton L, Henri H, Bouletreau M, Vavre F. 2006. Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology 132:49–56. doi: 10.1017/S0031182005008723. [DOI] [PubMed] [Google Scholar]
- 114.Mouton L, Henri H, Charif D, Boulétreau M, Vavre F. 2007. Interaction between host genotype and environmental conditions affects bacterial density in Wolbachia symbiosis. Biol Lett 3:210–213. doi: 10.1098/rsbl.2006.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bordenstein SR, Bordenstein SR. 2011. Temperature affects the tripartite interactions between bacteriophage WO, Wolbachia, and cytoplasmic incompatibility. PLoS One 6:e29106. doi: 10.1371/journal.pone.0029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sumi T, Miura K, Miyatake T. 2017. Wolbachia density changes seasonally amongst populations of the pale grass blue butterfly, Zizeeria maha (Lepidoptera: Lycaenidae). PLoS One 12:e0175373. doi: 10.1371/journal.pone.0175373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoffmann AA, Turelli M, Simmons GM. 1986. Unidirectional incompatibility between populations of Drosophila simulans. Evolution 40:692–701. doi: 10.1111/j.1558-5646.1986.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 118.Olsen K, Reynolds KT, Hoffmann AA. 2001. A field cage test of the effects of the endosymbiont Wolbachia on Drosophila melanogaster. Heredity (Edinb) 86:731–737. doi: 10.1046/j.1365-2540.2001.00892.x. [DOI] [PubMed] [Google Scholar]
- 119.Versace E, Nolte V, Pandey RV, Tobler R, Schlötterer C. 2014. Experimental evolution reveals habitat-specific fitness dynamics among Wolbachia clades in Drosophila melanogaster. Mol Ecol 23:802–814. doi: 10.1111/mec.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kriesner P, Conner WR, Weeks AR, Turelli M, Hoffmann AA. 2016. Persistence of a Wolbachia infection frequency cline in Drosophila melanogaster and the possible role of reproductive dormancy. Evolution 70:979–997. doi: 10.1111/evo.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Osborne SE, Leong YS, O’Neill SL, Johnson KN. 2009. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog 5:e1000656. doi: 10.1371/journal.ppat.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Martinez J, Longdon B, Bauer S, Chan Y-S, Miller WJ, Bourtzis K, Teixeira L, Jiggins FM. 2014. Symbionts commonly provide broad spectrum resistance to viruses in insects: a comparative analysis of Wolbachia strains. PLoS Pathog 10:e1004369. doi: 10.1371/journal.ppat.1004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brownlie JC, Cass BN, Riegler M, Witsenburg JJ, Iturbe-Ormaetxe I, McGraw EA, O’Neill SL. 2009. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog 5:e1000368. doi: 10.1371/journal.ppat.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Newton ILG, Rice DW. 2019. The Jekyll and Hyde symbiont: could Wolbachia be a nutritional mutualist? J Bacteriol 202:e00589-19. doi: 10.1128/JB.00589-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hoffmann AA, Weeks AR. 2007. Climatic selection on genes and traits after a 100 year-old invasion: a critical look at the temperate-tropical clines in Drosophila melanogaster from eastern Australia. Genetica 129:133–147. doi: 10.1007/s10709-006-9010-z. [DOI] [PubMed] [Google Scholar]
- 126.Shi M, White VL, Schlub T, Eden J-S, Hoffmann AA, Holmes EC. 2018. No detectable effect of Wolbachia wMel on the prevalence and abundance of the RNA virome of Drosophila melanogaster. Proc Biol Soc 285:20181165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nikoh N, Hosokawa T, Moriyama M, Oshima K, Hattori M, Fukatsu T. 2014. Evolutionary origin of insect-Wolbachia nutritional mutualism. Proc Natl Acad Sci U S A 111:10257–10262. doi: 10.1073/pnas.1409284111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nunes MDS, Nolte V, Schlötterer C. 2008. Nonrandom Wolbachia infection status of Drosophila melanogaster strains with different mtDNA haplotypes. Mol Biol Evol 25:2493–2498. doi: 10.1093/molbev/msn199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Richardson MF, Weinert LA, Welch JJ, Linheiro RS, Magwire MM, Jiggins FM, Bergman CM. 2012. Population genomics of the Wolbachia endosymbiont in Drosophila melanogaster. PLoS Genet 8:e1003129. doi: 10.1371/journal.pgen.1003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ballard J, Melvin R. 2007. Tetracycline treatment influences mitochondrial metabolism and mtDNA density two generations after treatment in Drosophila. Insect Mol Biol 16:799–802. doi: 10.1111/j.1365-2583.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- 131.Matute DR, Novak CJ, Coyne JA. 2009. Temperature-based extrinsic reproductive isolation in two species of Drosophila. Evolution 63:595–612. doi: 10.1111/j.1558-5646.2008.00588.x. [DOI] [PubMed] [Google Scholar]
- 132.Goda T, Leslie JR, Hamada FN. 2014. Design and analysis of temperature preference behavior and its circadian rhythm in Drosophila. J Vis Exp 83:e51097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dierick HA. 2007. A method for quantifying aggression in male Drosophila melanogaster. Nat Protoc 2:2712–2718. doi: 10.1038/nprot.2007.404. [DOI] [PubMed] [Google Scholar]
- 134.Dankert H, Wang L, Hoopfer ED, Anderson DJ, Perona P. 2009. Automated monitoring and analysis of social behavior in Drosophila. Nat Methods 6:297–303. doi: 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.R Core Team. 2018. R: a language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 136.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Soft 67:1–48. [Google Scholar]
- 137.Anderson JL, Albergotti L, Proulx S, Peden C, Huey RB, Phillips PC. 2007. Thermal preference of Caenorhabditis elegans: a null model and empirical tests. J Exp Biol 210:3107–3116. doi: 10.1242/jeb.007351. [DOI] [PubMed] [Google Scholar]
- 138.Klasson L, Westberg J, Sapountzis P, Näslund K, Lutnaes Y, Darby AC, Veneti Z, Chen L, Braig HR, Garrett R, Bourtzis K, Andersson SGE. 2009. The mosaic genome structure of the Wolbachia wRi strain infecting Drosophila simulans. Proc Natl Acad Sci U S A 106:5725–5730. doi: 10.1073/pnas.0810753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ellegaard KM, Klasson L, Näslund K, Bourtzis K, Andersson SG. 2013. Comparative genomics of Wolbachia and the bacterial species concept. PLoS Genet 9:e1003381. doi: 10.1371/journal.pgen.1003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schrider DR, Ayroles J, Matute DR, Kern AD. 2018. Supervised machine learning reveals introgressed loci in the genomes of Drosophila simulans and D. sechellia. PLoS Genet 14:e1007341. doi: 10.1371/journal.pgen.1007341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Joshi N, Fass J. 2011. Sickle: a sliding-window, adaptive, quality-based trimming tool for FastQ files.
- 142.Jackman SD, Vandervalk BP, Mohamadi H, Chu J, Yeo S, Hammond SA, Jahesh G, Khan H, Coombe L, Warren RL, Birol I. 2017. ABySS 2.0: resource-efficient assembly of large genomes using a Bloom filter. Genome Res 27:768–777. doi: 10.1101/gr.214346.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 144.Höhna S, Landis MJ, Heath TA, Boussau B, Lartillot N, Moore BR, Huelsenbeck JP, Ronquist F. 2016. RevBayes: Bayesian phylogenetic inference using graphical models and an interactive model-specification language. Syst Biol 65:726–736. doi: 10.1093/sysbio/syw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- 146.Boettiger C, Coop G, Ralph P. 2012. Is your phylogeny informative? Measuring the power of comparative methods. Evolution 66:2240–2251. doi: 10.1111/j.1558-5646.2011.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24:129–131. doi: 10.1093/bioinformatics/btm538. [DOI] [PubMed] [Google Scholar]
- 148.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The thermal gradient apparatus is composed of a 44 × 13× 1 cm aluminum plate and a 1-cm-high removable Plexiglas lid. The thermal gradient is subdivided into seven 10 × 6 cm sections (see Table S4 in the supplemental material). Download FIG S1, DOCX file, 0.4 MB (413.9KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genotype IDs for different Wolbachia-infected host species used in this study. Download Table S1, DOCX file, 0.01 MB (13KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Results and sample sizes from the LMM analyses of Tp data. Because the wHa, wMelCS, wMel, and wMau data were approximately normally distributed, we analyzed each data set using LMMs. Statistically significant fixed effects at P < 0.05 are shown in bold text with asterisks. Download Table S2, DOCX file, 0.01 MB (15.3KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Distribution of maximum likelihood estimates of λ from 1,000 bootstrap replicates. The bootstrap analysis for our Wolbachia phylogram (Fig. 2) is shown to the left. To the right are simulated phylogenies with an increasing number of Wolbachia strains included (N = 25, 50, 100). For simulated trees, character evolution was simulated with our λ estimate of 0.778 using the “sim.bdtree” and “sim.char” functions in the geiger R package (147). For each graph, fitted λ values for the original phylogeny are shown above with a vertical dashed line. Note that fitted λ values for the simulated phylogenies differ slightly from λ = 0.778, because “sim.char” uses a Brownian-motion model to simulate character evolution along the phylogeny. Below each graph, the mean estimate of λ from the 1,000 replicates () is shown with associated 95% confidence intervals. The bootstrapping analyses generally show that small phylogenies (N = 8, 25) have a large number of near-zero λ values arising by random chance, which increases the uncertainty of parameter estimation. Indeed, small phylogenies are likely to generate near-zero λ values by chance, not necessarily because the phylogeny is unimportant for trait evolution (146). As the number of strains in our analysis increases (N = 50, 100), bootstrapped estimates of λ cluster around the true λ value fitted to the original phylogeny. Download FIG S2, DOCX file, 0.1 MB (76.7KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Fly food recipe for cornmeal media. To the right, the nutritional content of the food is shown based on calculations from https://brodericklab.com/DDCC.php. Download Table S3, DOCX file, 0.01 MB (12.8KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mean temperature and standard error for each section of the custom-built thermal gradient apparatus, across all 347 experimental replicates in this study. Download Table S4, DOCX file, 0.01 MB (12.4KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Box plots showing temperature preference (Tp) for uninfected and infected flies of each genotype when the coldest section of the thermal gradient (section 7) is included. Individual points are jittered to show overlap. Download FIG S3, DOCX file, 0.5 MB (491.8KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Results and sample sizes from the GLMM and LMM analyses of Tp data, including the flies located in the coldest section of the thermal gradient apparatus (section 7). Statistically significant fixed effects at P < 0.05 are shown in bold text with asterisks. Download Table S5, DOCX file, 0.02 MB (18.6KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The scaffold count, N50, and total assembly size of each Wolbachia assembly. Download Table S6, DOCX file, 0.01 MB (12.6KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
qPCR primers used to measure relative Wolbachia density in temperature shift experiments. Download Table S7, DOCX file, 0.01 MB (13.6KB, docx) .
Copyright © 2020 Hague et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
Genome assemblies are deposited on GenBank (BioProject accession no. PRJNA658309). All other data are available on Dryad (https://doi.org/10.5061/dryad.j9kd51c8r).