Abstract
Background
Incorporating cognitive testing into routine clinical practice is a challenge in multiple sclerosis (MS), given the wide spectrum of both cognitive and physical impairments people can have and the time that testing requires. Shortened paper and verbal assessments predominate but still are not used routinely. Computer-based tests are becoming more widespread; however, changes in how a paper test is implemented can impact what exactly is being assessed in an individual. The Symbol Digit Modalities Test (SDMT) is one validated test that forms part of the cognitive batteries used in MS and has some computer-based versions. We developed a tablet-based SDMT variant that has the potential to be ultimately deployed to patients’ own devices.
Objective
This paper aims to develop, validate, and deploy a computer-based SDMT variant, the Cognition Reaction (CoRe) test, that can reliably replicate the characteristics of the paper-based SDMT.
Methods
We carried out analysis using Pearson and intraclass correlations, as well as a Bland-Altman comparison, to examine consistency between the SDMT and CoRe tests and for test-retest reliability. The SDMT and CoRe tests were evaluated for sensitivity to disability levels and age. A novel metric in CoRe was found: question answering velocity could be calculated. This was evaluated in relation to disability levels and age for people with MS and compared with a group of healthy control volunteers.
Results
SDMT and CoRe test scores were highly correlated and consistent with 1-month retest values. Lower scores were seen in patients with higher age and some effect was seen with increasing disability. There was no learning effect evident. Question answering velocity demonstrated a small increase in speed over the 90-second duration of the test in people with MS and healthy controls.
Conclusions
This study validates a computer-based alternative to the SDMT that can be used in clinics and beyond. It enables accurate recording of elements of cognition relevant in MS but offers additional metrics that may offer further value to clinicians and people with MS.
Keywords: cognition, multiple sclerosis, eHealth, electronic assessment, patient reported outcomes, neurology
Introduction
Background
Multiple sclerosis (MS) is an inflammatory demyelinating and degenerative disease of the central nervous system and the most common nontraumatic cause of disability in young adults worldwide [1]. The dominant phenotype is characterized by relapses (attacks) and remissions, known as relapsing-remitting MS (RRMS). In the majority of those affected with RRMS, the condition evolves, within 10 to 15 years, into secondary progressive MS (SPMS). About 15% of people with MS develop primary progressive MS (PPMS), characterized by progressive neurological dysfunction from onset [2].
Motor impairment forms the most overt impact of MS but cognitive impairment affects up to 40% of people with MS, rising to 80% in those with the progressive forms of the disease [3]. It has substantial impact on disability and can, when present in isolation, limit employment prospects [4]. However, in the early stages of MS, formal cognitive testing can show minimal changes in a wide variety of domains [5]. Later, as the disease advances, the picture becomes more coherent, with impairments in speed of information processing, attention, episodic memory, and executive function dominating. These impact independence and mood and can lead to social isolation [6].
Cognitive testing itself can be demanding on patients, causing difficulties for those with attentional disorders, fatigue, and physical limitations [7]. The time and attention required in a busy clinic environment makes test delivery in a routine context a challenge for both patient and assessors. To this end, in MS, a number of simplified tests of cognition have been developed for clinical use. These include the Brief International Cognitive Assessment for MS [8], the Brief Repeatable Battery of Neuropsychological Tests [9], and the Minimal Assessment of Cognitive Function in Multiple Sclerosis [10]. In most cases, these tests are still largely paper- or apparatus-based exercises completed in front of an assessor and take the form of a battery of tests that incorporate multiple testing methodologies.
One common element of the MS testing batteries is the Symbol Digit Modalities Test (SDMT) [11]. It assesses organic cerebral dysfunction and has a proven history as an effective outcome measure in a number of MS trials [10,11] and in other conditions [12]. The SDMT consists of matching symbols against digits within 90 seconds, the result being the total number of correct answers. Participants are given a practice number of attempts and then perform the timed assessment. The implementation of the test typically takes 5 minutes, including instruction and demonstration. The responses can be written or spoken out loud and recorded by the assessor [13].
A number of electronic variants of the SDMT have been developed [14,15], but as yet, they are not used routinely to assess cognitive impairment [16]. Their implementation varies from the original paper test, but the impact of these slight variations is as yet unclear, as impairment in individuals with MS can vary widely with different elements, such as fatigue, which can slow reactions, and physical issues such as ataxia or weakness, which can introduce further variability if a screen or keyboard needs to be manipulated. This is a further challenge if a test is to be administered without an assessor present. However, the computer-based approaches have the potential to offer additional information over the paper-based or oral approaches, as additional metrics can be quantified and these may enhance the information available from the test.
The United Kingdom Multiple Sclerosis Register (UKMSR) was established in 2011 as a means of capturing real-world evidence of living with MS in the United Kingdom. There are comprehensive data on 11,387 people with MS registered on the UKMSR via the internet and more than 13,000 consented clinically via a network of National Health Service (NHS) centers [17]. An online portal facilitates collection of longitudinal patient-reported outcomes (PROs) and real-world evidence of living with MS, but none of the instruments currently capture cognitive function. Given the need to understand in more depth the performance characteristics of electronic testing and the key role of cognitive impairment in MS, we developed an electronic variant of the SDMT that could be implemented rapidly and routinely at clinical centers to address this need. Ultimately, as an electronic register, if this type of testing is validated, then it could be also carried out in the patient’s home, which would also help patients who are unable to physically attend clinics.
Objectives
This paper aims to develop, validate, and deploy a computer-based SDMT variant, the Cognition Reaction (CoRe) test, that can reliably replicate the characteristics of the paper-based SDMT and assess its utility for deployment as a meaningful measure to assess cognition in an MS population.
Methods
Population
All participants gave informed consent, and the study has ethical approval from South West Central Bristol Ethics Committee (16/SW/0194). Participants were recruited from Morriston Hospital, Swansea Bay University Health Board and Charing Cross Hospital, Imperial College Healthcare NHS Trust. The people with MS that took part in the study were recruited at either progressive MS teaching days or as part of their routine visits to their respective hospitals. Demographic data and an Expanded Disability Status Score (EDSS) [18] were recorded at the time of testing. Healthy volunteers were recruited from Swansea University Medical School and Imperial College London to provide a control group of test scores with anonymized demographic data. Healthy volunteers were recruited from among the staff at the two clinical sites and included a mix of staff and PhD students from Swansea University. None of the healthy controls had MS and no one approached refused. All participants had completed at least full formal secondary education. There were no declared visual problems in the population.
CoRe Test App
The Cognition Reaction (CoRe) test was inspired by the SDMT; however, there are some key differences. The CoRe test presents 9 different symbols displayed at the top of the screen, with corresponding numbers, 1 through 9, underneath. The symbols are randomized every time the app is launched, and the center of the screen displays 2 symbols, the one to be identified now and the next one. At the bottom of the screen, there are a number of buttons labelled 1 through 9 that participants tap to match the central symbol on the screen. Data recorded include the number of symbols accurately tapped, as for the SDMT, but in addition, CoRe automatically registers the time between responses and the number of incorrect responses. Further details of the app are presented in Multimedia Appendix 1 [19,20]. The app is entirely self-contained, with no requirement for internet access. The CoRe test app can be seen in Figure 1.
Figure 1.
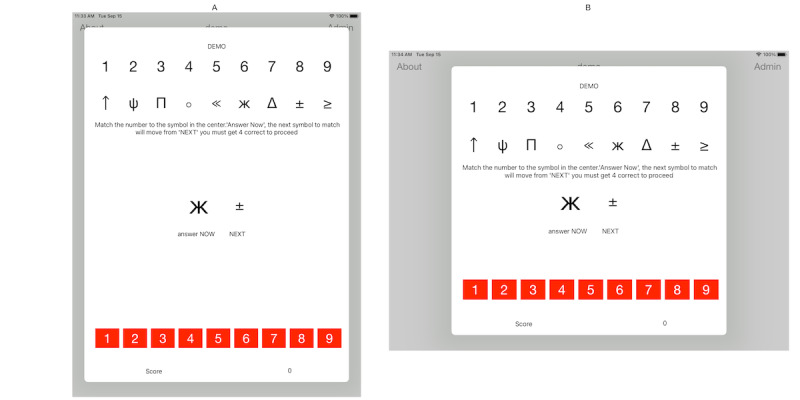
Cognitive Reaction test app shown running in portrait and landscape modes.
App Testing
For the MS population, participants first completed the paper SDMT using the traditional written method, requiring the paper test, a pen, and a stopwatch. Following this, participants were handed an iPad and given an introduction by a researcher from the UKMSR team, merely demonstrating the 2 orientations that the device could be placed in. The orientation that participants chose was not recorded as part of this assessment. They were then invited to follow the written directions on the app. They were first presented with a demonstration mode and encouraged to run through at least once. A score of 4, which was displayed on the screen, was required to progress to the main test. This could be repeated if desired. Once ready, participants hit “start” and were given 90 seconds to complete the test. A countdown timer was displayed on the screen of the iPad. Visual acuity was not formally assessed, and no participants claimed that they could not see the icons on the tablet screen. Test environments were controlled for noise and disturbance. Some participants were retested 1 month later in the same environment to determine the consistency of the results.
Statistical Analysis
Analysis was carried out using the Pandas library for Python (version 3.773) [21] and the R statistical language (version 3.6.0; R Foundation for Statistical Computing) [22]. Graphs and images were generated using Seaborn [23] and ggplot2 (version 3.0.0) [24]. Correlation was used to compare the validity of the paper and electronic versions of the tests and the test-retest reliability of the CoRe test. Pearson r was calculated for test scores from the CoRe test and the SDMT, with mean difference evaluated using a 2-tailed paired samples t test and differences in variances compared using a Pitman-Morgan test for paired samples. Intraclass correlation was also performed on the first and second CoRe and SDMT test results. A Bland-Altman analysis was used as an additional measure of equivalency. The sensitivity of the CoRe and SDMT scores to disability levels and age were measured using analysis of variance (ANOVA) statistics, with post hoc Tukey tests used to determine any significant differences between groups.
To utilize the additional data generated by the CoRe test, the question answering velocity (QAV) was quantified as a measure of cognitive function. This was defined as the total number of correct answers given at a time divided by total current time elapsed in the test (correct answers/seconds). Multivariate linear regression was performed to determine if any relationship existed between the QAV and the time period of the questionnaire. The CoRe test lasts a total of 90 seconds, and responses were divided into thirds to study the rates of change over the first, second, and third sections of responses for each patient. For analysis, EDSS scores were divided into 3 categories: low (EDSS of 0-2.5), medium (EDSS of 3-5.5), and high (EDSS of 6-10), as was age, with categories of 18-34 years, 35-54 years, and >55 years.
Results
Demographics
A total of 102 people with MS were recruited to the study (Table 1), of whom 30 returned within 1 month for a repeat test. All patients were over 18 years of age and had no significant comorbidities that would exclude them from being able to complete the paper or CoRe tests. No participants were excluded from the study, and none reported a relapse of MS at any point in the testing. Mean age of the people with MS cohort tested was younger than the overall MS Register population, with a slightly lower proportion of patients with PPMS and SPMS (Table 1). A total of 45 anonymous healthy controls were tested during the development of the app; apart from not completing an initial paper SDMT, conditions were similar to the MS cohort. Both healthy controls and people with MS had completed at least 12 years of education.
Table 1.
Demographics of cohort and healthy controls undertaking the CoRe test. The UKMSR population is shown for comparison.
| Characteristic | Total UKMSRa | CoReb cohort (MSc) | CoRe cohort (healthy controls) | Cohort differenced, chi-square test (df) | Cohort differenced, t test (df) | P value | |
| Total participants, n | 11,387 | 102 | 45 | N/Ae | N/A | N/A | |
| Gender, n (% ) |
|
|
|
0.3 (1) | N/A | .57 | |
|
|
Female | 8387 (73.7) | 70 (68.6) | 28 (62.2) |
|
|
|
|
|
Male | 3000 (26.3) | 32 (31.4) | 17 (37.8) |
|
|
|
| MS type, n (%) |
|
|
|
N/A | N/A | N/A | |
|
|
RRMSf | 5988 (52.6) | 86 (83.2) | N/A |
|
|
|
|
|
PPMSg | 1492 (13.1) | 5 (5.6) | N/A |
|
|
|
|
|
SPMSh | 2945 (25.9) | 9 (9.3) | N/A |
|
|
|
|
|
Other | 962 (8.4) | 2 (1.9) | N/A |
|
|
|
| Age (years), mean (SD) | 53.6 (11.8) | 44.0 (11.0) | 38.1 (11.9) | N/A | 2.891 (145) | .004 | |
| Age at diagnosis (years), mean (SD) | 39.2 (10.3) | 34.6 (10.6) | N/A | N/A | N/A | N/A | |
| EDSSi, median (range) | 6 (0-9.5) | 3.5 (1-8) | N/A | N/A | N/A | N/A | |
aUKMSR: United Kingdom Multiple Sclerosis Register.
bCoRe: Cognitive Reaction.
cMS: multiple sclerosis.
dDifference between people with multiple sclerosis and healthy controls.
eN/A: not applicable.
fRRMS: relapsing-remitting multiple sclerosis.
gPPMS: primary progressive multiple sclerosis.
hSPMS: secondary progressive multiple sclerosis.
iEDSS: Expanded Disability Status Score.
CoRe Test in People With MS and Control Group: Comparison of Total Correct Responses
The first set of CoRe test scores for people with MS were compared with those of the healthy control group. Mean test results for people with MS were 39.0 (SD 13.3), while mean scores for the healthy control group were 56.1 (SD 15.9). An unpaired t test found that people with MS had significantly lower scores (t145=–6.769; P<.001), with no significant difference in variance between the groups (F101,44=0.701; P=.15).
CoRe Test and SDMT in People With MS: Comparison of Total Correct Responses
People with MS completed both the CoRe test and SDMT together on 2 occasions, 1 month apart. The first test response distributions for the CoRe test and SDMT were normally distributed (Shapiro-Wilk tests with P=.48 and P=.61, respectively) and were strongly correlated (Pearson r100=0.800; P<.001). First test participants scored a mean of 4.40 responses lower for the CoRe test compared with the SDMT, as seen in Table 2 (paired samples t101=5.390; P<.001), but there was no significant difference in the variance (Pitman-Morgan test: t100=–0.879; P=.38), with good agreement between tests (Figure 2). When the CoRe test and SDMT were repeated for a second time, the mean CoRe test score was not significantly lower than the SDMT (1.4 responses difference; t29=0.954; P=.35). Again, there was a strong correlation between the second CoRe test and second SDMT (Pearson r28=0.842; P<.001). Table 2 shows the baseline and retest responses for those who completed it.
Table 2.
Baseline and retest SDMT and CoRe test total responses at baseline and retest 1 month later.
| Test | Participants, n | Score, mean (SD), range | |
| Baseline |
|
|
|
|
|
SDMTa | 102 | 43.4 (12.6), 15-76 |
|
|
CoReb test | 102 | 39.0 (13.3), 11-73 |
| Retest |
|
|
|
|
|
SDMT | 30 | 41.9 (14.6), 14-76 |
|
|
CoRe test | 30 | 40.5 (13.9), 20-70 |
aSDMT: Symbol Digit Modalities Test.
bCoRe: Cognitive Reaction.
Figure 2.
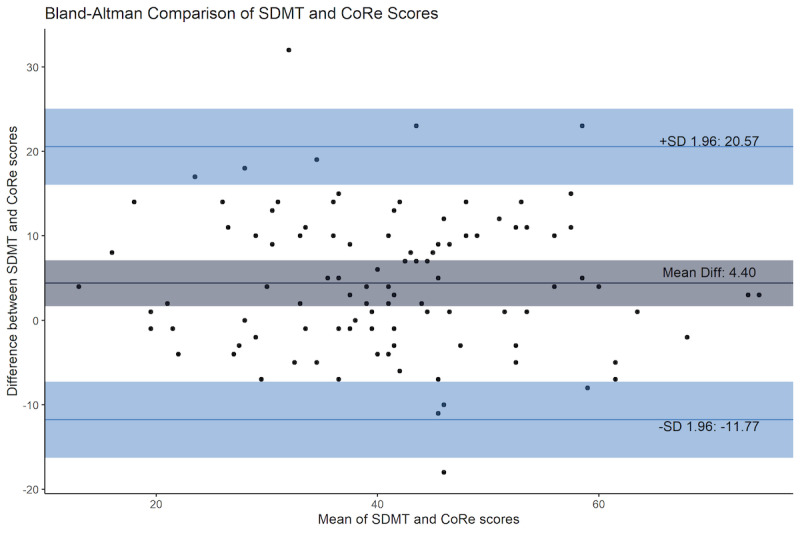
Bland-Altman comparison of first CoRe test with paper SDMT scores. CoRe: Cognitive Reaction; SDMT: Symbol Digit Modalities Test.
CoRe Test and SDMT Test-Retest Reliability
First and second CoRe test and SDMT scores were evaluated for test-retest reliability and scores at a 1-month interval. The CoRe tests were normally distributed and demonstrated consistency (Pearson correlation coefficient r=0.947; t28=15.60; P<.001). Differences in means were normal (Shapiro-Wilk test P=.81) and not significantly different (t29=–0.944; P=.35), with equal variances (Pitman-Morgan t28=1.784; P=.09). The intraclass correlation coefficient between the first and second CoRe tests was found to be 0.942 (95% CI 0.882-0.0972; F29,30=33.2; P<.001) (Figure 3).
Figure 3.
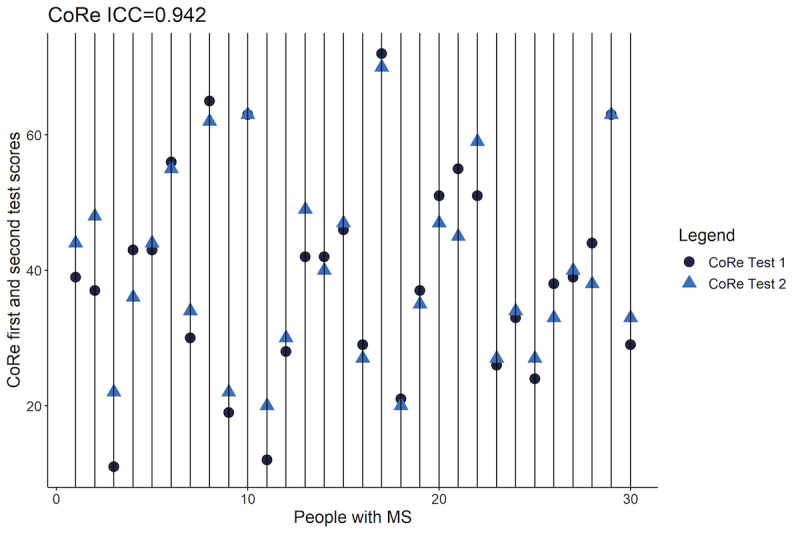
Intraclass correlation coefficients between the first and retested CoRe tests. CoRe: Cognitive Reaction; ICC: intraclass correlation coefficient; MS: multiple sclerosis.
Test-retest correlations were observed in the same people completing the SDMT at a 1-month interval. Scores were normally distributed and consistent (Pearson correlation r=0.936; t28=14.052; P<.001) and differences in means were normal (Shapiro-Wilk test P=.44) and not significantly different (t29=–0.919; P=.37), with equal variances (Pitman-Morgan t28=–0.743; P=.46). The intraclass correlation coefficient between the first and second SDMT tests was found to be 0.935 (95% CI 0.869-0.968; F29,30=29.6; P<.001).
CoRe Test Total Correct Response Score Is Impacted by Age and Disability in MS, Whereas SDMT Is Only Affected by Disability
We examined the impact of age, gender, and EDSS on the total correct responses (Figure 4). An ANOVA for SDMT scores with respect to age and EDSS found no significant impact of age (aged 18-34 years: mean 48.1, SD 15.5; aged 35-54 years: mean 43.2, SD 11.8; and 55+ years: mean 38.3, SD 9.0; F2=1.036; P=.36), but significance for EDSS (low EDSS: mean 49.8, SD 12.9; medium EDSS: mean 41.4, SD 12.3; high EDSS: mean 38.6, SD 9.8; F2=8.574; P<.001); post hoc Tukey tests showed higher scores in those in the lowest EDSS category compared with those in the highest EDSS category (P<.001) and compared with the medium EDSS category (P=.01). No significant difference was found between the low and medium EDSS categories.
Figure 4.
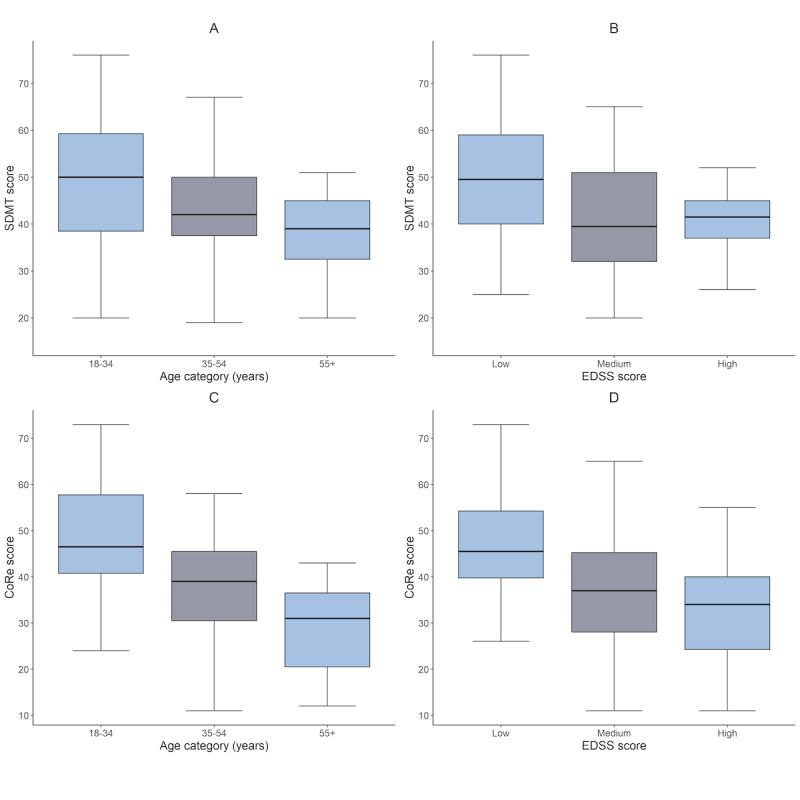
Mean SDMT and CoRe scores with age categories and EDSS scores. CoRe: Cognitive Reaction; EDSS: Expanded Disability Status Score; SDMT: Symbol Digit Modalities Test.
In contrast, an ANOVA for CoRe test scores showed a significant difference in the total responses with age (aged 18-34 years: mean 48.6, SD 13.5; aged 35-54 years: mean 38.3, SD 11.6; and >55 years: mean 28.9, SD 9.8; F2=8.633; P<.001) and EDSS (low EDSS: mean 47.4, SD 11.6; medium EDSS: mean 36.8, SD 12.7; high EDSS: mean 32.1, SD 10.7; F2=18.151; P<.001). Post hoc Tukey tests showed those in the age group of 18 to 34 years had significantly higher scores than those in the 34 to 54 years (P=.01) and 55+ years group (P=.001), with no difference between the medium and high age groups. The lowest EDSS category was associated with higher CoRe test scores than both other groups (P<.001), with no difference between the medium and high EDSS groups.
Gender was not found to be significant for either SDMT or CoRe test scores.
Speed of Reaction (Question Answering Velocity) Derived From the CoRe Test Increases Throughout the Test and Correlates With Age, Gender, and Disability
Due to the way data are acquired for the CoRe test, we were able to measure the speed of reaction to each question and calculate the QAV as correct answers over time elapsed (seconds) continuously throughout the assessment. There was a significant range of QAV over the time frame of the test in people with MS, as illustrated in Figure 5, which shows the two individuals with the lowest and highest scores in the CoRe test. Breaking down the total correct answers into 3 sections also allowed us to quantify the change in QAV over the course of the CoRe test. Multiple linear regression models with the variables age, gender, and EDSS in people with MS found that QAV increased in each third of the test in people with MS and healthy controls (Table 3).
Figure 5.
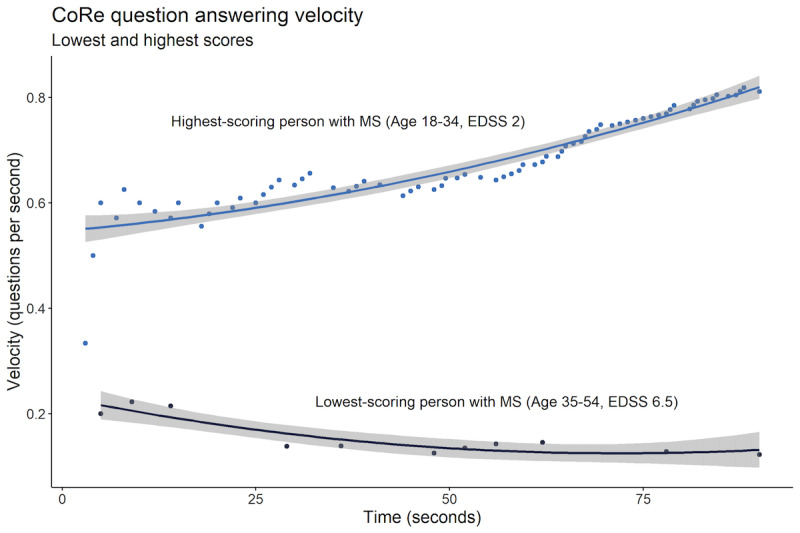
A polynomial regression of QAV for those people with MS with the lowest and highest scores in the cohort. CoRe: Cognitive Reaction; EDSS: Expanded Disability Status Score; MS: multiple sclerosis; QAV: question answering velocity.
Table 3.
Multivariate models in people with MS (R2=0.396; F5,3973=520.4; P<.001) and healthy controls (R2=0.323; F4,2521=300.1; P<.001) for QAV over the time frame of the Cognitive Reaction test, with additional covariates age and gender. EDSS scores are given for people with MS only.
| Variable | QAVa of people with MSb | QAV of healthy controls | |||
|
|
β coefficient (95% CI) | P value | β coefficient (95% CI) | P value | |
| Second section compared to first | .045 (0.037 to 0.053) | <.001 | .070 (0.056 to 0.085) | <.001 | |
| Third section compared to first | .071 (0.063 to 0.080) | <.001 | .110 (0.094 to 0.123) | <.001 | |
| Age | –.005 (–0.005 to –0.006) | <.001 | –.008 (–0.007 to –0.008) | <.001 | |
| Female gender | .049 (0.041 to 0.056) | <.001 | –.043 (–0.055 to –0.031) | <.001 | |
| EDSSc | –.017 (–0.015 to –0.019) | <.001 | N/Ad | N/A | |
aQAV: question answering velocity.
bMS: multiple sclerosis.
cEDSS: Expanded Disability Status Score.
dN/A: not applicable.
Both groups answered more quickly as the test progressed (the control group at an even faster rate than people with MS), with the second and third sections of their correct answers being completed in less time than the first. The gradient is similar in both populations (Figure 6). In both populations, increased age was associated with slowing of QAV by 0.007 to 0.008 questions per second for each year increase in age. For control participants, female gender was associated with a slowing of QAV by 0.034 questions per second, whereas in people with MS, female gender was associated with an increase in QAV of 0.049 questions per second. However, disability slowed QAV by 0.017 questions per second for every increase in EDSS by 1 point (Table 3).
Figure 6.
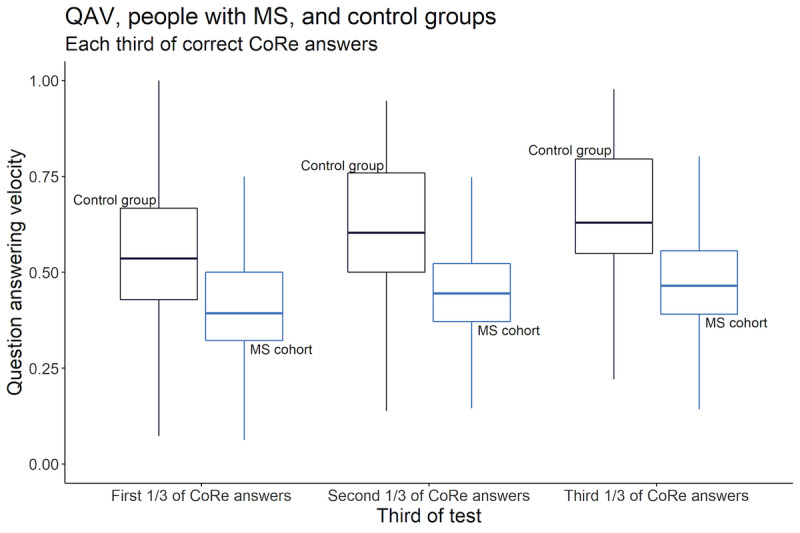
Comparison of increase in speed between each test third for healthy and MS populations. CoRe: Cognitive Reaction; MS: multiple sclerosis; QAV: question answering velocity.
We next directly compared the variables associated with CoRe test QAV and the CoRe test total response score. A regression using the variables age, gender, and EDSS score found that the CoRe test QAV was significantly impacted by all 3 factors, whereas the CoRe test score (total correct answers) found significant impacts only from EDSS and age (Table 4).
Table 4.
Impact of age, gender, and EDSS on total response score (R2=0.383; F3,98=20.3; P<.001) in people with MS cohort.
| Variable | CoRea test score | |
|
|
β coefficient (95% CI) | P value |
| EDSSb | –2.103 (–3.390 to –0.808) | .002 |
| Age | –.489 (–0.713 to –0.265) | <.001 |
| Female gender | 4.413 (–0.155 to 8.981) | .06 |
aCoRe: Cognitive Reaction.
bEDSS: Expanded Disability Status Score.
Discussion
Summary of Findings
This study aims to validate an electronic variant of the SDMT, comparing the CoRe test with the established paper-based SDMT within an MS cohort in 2 independent UK centers, examining its overall reliability and suitability. In addition, we quantified an additional metric that can be extracted from the electronic implementation. The total response scores for the CoRe test were on average lower than the SDMT but showed good correlation with the paper test, though there are clear differences in responses across age groups. Having the understanding that the CoRe performs similarly across these deviations allows it to be compared with the paper-based test, though it is not a like-for-like match. However, the consistency of the test and its utility remain clear. The CoRe test showed consistent responses over time and demonstrated similar test-retest properties to the SDMT, as with other electronic implementations [14]. These findings suggest that the CoRe test is an appropriate alternative to measure of cognitive ability as assessed by the SDMT.
We confirmed that a reduction in correct responses for both the SDMT and CoRe test correlates with increasing disability, but in addition, a reduction in correct responses correlates with increasing age in people with MS. Using the advantages of an electronic implementation, we were able to measure the QAV and found that both people with MS and healthy controls increase their QAV throughout the test and also that in both groups, an increased QAV correlates with younger age and male gender. This implies these correlations are not associated with MS-specific cognitive decline. However, increased QAV is also associated with lower disability, only present in those with MS. In our testing, increasing age showed a reduction in correct responses over the test. This finding corresponds with other SDMT testing in populations [25], and there is evidence for older participants performing poorly over the duration of the test, with studies showing decreased reaction times (about 0.5 ms/year) [26] in simple reaction-style tests in older people. There is also the effect of older people’s familiarity with tablet computers [27] that could have some impact on this. This will be investigated in future testing.
There are a number of computer-based variants of the SDMT, one of the first being the computerized Symbol Digit Modalities Test (c-SDMT) [14], which showed excellent sensitivity in 119 people with MS versus 38 healthy controls, with people with MS performing significantly worse than the healthy controls. Use of the c-SDMT has not become widespread, most likely due to the technology platform that it was developed on and the stringent test description (Windows PC, 19-inch screen with participant at 15 inches from the screen), making deployment challenging. A more recent implementation of a computer-based SDMT is the processing speed test (PST) [15], which was also tested against a healthy control population and forms one element of the Floodlight assessment tool [28]. The PST showed similar results as we have demonstrated and has shown high reliability when reproduced within Floodlight on patients’ own devices. Small differences in implementation of the same paper-based test can impact what is being tested and need to be understood. The CoRe implementation requires the screen to be touched, which adds a visuospatial element to the assessment, and this will have an impact in some people with MS. It also presents 2 symbols in random order as opposed to a standard sheet of symbols; this change means that there is less likely to be a learning effect on retesting. A key issue with computer-based implementation is the impact of rapid hardware and software development, which results in a need to develop applications that can adapt to a changing environment. Another issue is the variety of devices, such as desktops, laptops, and smartphones, that are currently in use, especially if the test is to be performed without an assessor present. CoRe has been developed to run at multiple screen sizes and on different devices, with an interface—two symbols seen at a time—that is suited to a small screen. This will have to be tested separately.
Prior studies, and our results, show that data produced by electronic tests are consistent, repeatable, and have utility to clinicians, informing on a vital aspect of patient care [29]. The scores on both paper SDMT and the CoRe test fall with increasing disability. The CoRe test is more sensitive than the SDMT to age, with the SDMT being only affected in populations older than 55 years [27]. The electronic CoRe test allows greater analysis of this effect, demonstrating slower mean response times in higher ages and disability groups. There is some evidence that there may be a gender difference in cognitive tests [30], with males and females performing differently at various ages in different test types. Notably, this is seen with visual reaction times, and this would be consistent with the implementation of the test presented here. The fact that this extra variable of reaction time (QAV) can be measured as part of the CoRe test could have clinical or research utility in the future. Having additional quantifiable clinical measurement information via a simple-to-implement and rapid test could hopefully have some relevance to everyday clinical practice, research, and medication trials. Benedict et al [13] state that the current definition of “NEDA” (no evidence of disease activity) is predicated on largely physical outcome measures, but cognition is so fundamental to socialization, employment, and quality of life beyond pure health care that a prolonged measurement of cognitive aspects could add a compelling dimension to our understanding of disease activity.
Limitations
We identified some limitations with this study. First, there were few people with MS with progressive disease and advanced disability, and we did not have complete directly measured cognitive assessments. In addition, the population that took 1-month follow-up tests was limited, and we have only tested this on a single type of device here. The 1-month period chosen for retest represents the hospital visit pattern for some patients on a particular disease-modifying therapy. Differing retest periods should be tested in the future. Although testing was performed in the presence of a researcher, they had little or no input on the actual test itself—though this has been shown to not have effect on these types of tests [31]. We also did not consider the orientation of the device as having any effect. This could also be incorporated into future testing on other devices.
Given that the CoRe test is consistent and repeatable, we intend to test the app on other devices, including laptops and a variety of smartphones. This will facilitate completion of the test away from the clinic and will enable us to integrate the CoRe test into the range of PROs captured by the UKMSR. Additionally, this will allow us to carry out testing among participants with higher disability and more progressive disease at different intervals to ensure that the test maintains its reliability and repeatability. We recognize that the CoRe is not an exact replacement for SDMT. It is an entirely new test [32], but it is comparable and measurable compared with the SDMT.
Conclusion
The CoRe implementation of the SDMT test is reliable and correlates with the paper-based SDMT, while also offering the additional metric of patient reaction time (QAV). This will allow clinicians and researchers to capture important additional metrics in people with MS, and potentially in other diseases, quickly and reliably on existing tablet hardware.
Acknowledgments
We would like to thank all the participants of the UK MS Register but especially those people who directly took part in this research. Additionally, we thank the healthy participants that took part in the development of CoRe, and all the hard-working researchers and clinicians that take such a keen interest in the development of novel measures.
Group Collaborators:
Dr Judy Archer, Mid Yorkshire Hospitals NHS Trust
Dr Carlo Canepa, James Paget University Hospitals NHS Trust
Dr Viquar Chamoun, Buckingham Healthcare NHS Trust
Professor Jeremy Chataway, University College London Hospitals NHS Trust
Dr Abhijit Chaudhubl, Barking Havering and Redbridge Hospitals NHS Trust
Professor Alasdair Coles, Cambridge University Hospitals NHS Trust
Dr Matt Craner, Oxford University Hospitals NHS Trust
Professor H Emsley, Lancashire Teaching Hospitals NHS Trust
Dr Nikos Evangelou, Nottingham University Hospitals NHS Trust
Dr Leonora Finisku, Brighton and Sussex Hospitals NHS Trust
Dr Helen Ford, Leeds Teaching Hospitals NHS Trust
Ms Annmieke Fox, Poole Hospital NHS Trust
Ms Julie Foxton, Royal Berkshire NHS Trust
Dr Andrew Gale, Luton and Dunstable Hospital NHS Trust
Dr Ian Galea, University Hospital Southampton NHS Trust
Dr Andrew Graham, Ipswich Hospital NHS Trust
Dr Joe Guadango, Newcastle Upon Tyne Hospitals NHS Trust
Dr Dreedharan Harikrishnan, East Kent Hospitals University NHS Trust
Dr Tim Harrower, Royal Devon and Exeter NHS Trust
Dr Jeremy Hobart, Plymouth Hospitals NHS Trust
Dr Chris Kipps, Hampshire Hospitals NHS Trust
Ms Jo Kitley, Portsmouth Hospitals NHS Trust
Dr Monica Marta, Southend University Hospital NHS Trust
Dr Gavin McDonnell, Belfast Health and Social Care Trust
Dr Brendan McLean, Royal Cornwall Hospitals NHS Trust
Ms Charlotte Owen, Shrewsbury and Telford Hospital NHS Trust
Dr Laura Petzold, Maidstone and Tunbridge Wells NHS Trust
Dr David Rog, Salford Royal Hospital NHS Trust
Dr Klaus Schmierer, Barts Health NHS Trust
Professor Basil Sharrack, Sheffield Teaching Hospitals NHS Trust
Dr Zbignew Slowinski, Airedale NHS Trust
Dr Agne Straukiene, Torbay and South Devon NHS Trust
Dr Sigurlaug Sveinsbornsdottir, Basildon and Thurrock University Hospitals NHS Trust
Dr Stewart Webb, NHS Greater Glasgow and Clyde
Dr Heather Wilson, Royal Free London NHS Trust
Abbreviations
- ANOVA
analysis of variance
- CoRe
Cognitive Reaction
- c-SDMT
computerized Symbol Digit Modalities Test
- EDSS
Expanded Disability Status Score
- MS
multiple sclerosis
- NHS
National Health Service
- PPMS
primary progressive multiple sclerosis
- PRO
patient-reported outcome
- PST
processing speed test
- QAV
question answering velocity
- RRMS
relapsing-remitting multiple sclerosis
- SDMT
Symbol Digit Modalities Test
- SPMS
secondary progressive multiple sclerosis
- UKMSR
United Kingdom Multiple Sclerosis Register
Appendix
CoRe test Application Details.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges. The Lancet. 2017 Apr;389(10076):1336–1346. doi: 10.1016/s0140-6736(16)30959-x. [DOI] [PubMed] [Google Scholar]
- 2.Leray E, Yaouanq J, Le Page E, Coustans M, Laplaud D, Oger J, Edan G. Evidence for a two-stage disability progression in multiple sclerosis. Brain. 2010 Jul;133(Pt 7):1900–13. doi: 10.1093/brain/awq076. http://europepmc.org/abstract/MED/20423930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991 May;41(5):685–91. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- 4.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008 Dec;7(12):1139–51. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 5.de Gobbi Porto FH, Spíndola L, de Oliveira MO, Figuerêdo do Vale PH, Orsini M, Nitrini R, Dozzi Brucki SM. A score based on screening tests to differentiate mild cognitive impairment from subjective memory complaints. Neurol Int. 2013;5(3):e16. doi: 10.4081/ni.2013.e16. http://europepmc.org/abstract/MED/24147213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobelt G, Langdon D, Jönsson L. The effect of self-assessed fatigue and subjective cognitive impairment on work capacity: The case of multiple sclerosis. Mult Scler. 2019 Apr;25(5):740–749. doi: 10.1177/1352458518769837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumowski JF, Benedict R, Enzinger C, Filippi M, Geurts JJ, Hamalainen P, Hulst H, Inglese M, Leavitt VM, Rocca MA, Rosti-Otajarvi EM, Rao S. Cognition in multiple sclerosis. Neurology. 2018 Jan 17;90(6):278–288. doi: 10.1212/wnl.0000000000004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langdon D, Amato M, Boringa J, Brochet B, Foley F, Fredrikson S, Hämäläinen P, Hartung H, Krupp L, Penner I, Reder A, Benedict R. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) Mult Scler. 2012 Jun;18(6):891–8. doi: 10.1177/1352458511431076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bever C, Grattan L, Panitch H, Johnson K. The Brief Repeatable Battery of Neuropsychological Tests for Multiple Sclerosis: a preliminary serial study. Mult Scler. 1995 Nov;1(3):165–9. doi: 10.1177/135245859500100306. [DOI] [PubMed] [Google Scholar]
- 10.Benedict RHB, Cookfair D, Gavett R, Gunther M, Munschauer F, Garg N, Weinstock-Guttman B. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS) J Int Neuropsychol Soc. 2006 Jul;12(4):549–58. doi: 10.1017/s1355617706060723. [DOI] [PubMed] [Google Scholar]
- 11.Sheridan LK, Fitzgerald HE, Adams KM, Nigg JT, Martel MM, Puttler LI, Wong MM, Zucker RA. Normative Symbol Digit Modalities Test performance in a community-based sample. Arch Clin Neuropsychol. 2006 Jan;21(1):23–8. doi: 10.1016/j.acn.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Sachdev P, Anstey K, Parslow R, Wen W, Maller J, Kumar R, Christensen H, Jorm A. Pulmonary function, cognitive impairment and brain atrophy in a middle-aged community sample. Dement Geriatr Cogn Disord. 2006;21(5-6):300–8. doi: 10.1159/000091438. [DOI] [PubMed] [Google Scholar]
- 13.Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R, Multiple Sclerosis Outcome Assessments Consortium Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler. 2017 Apr;23(5):721–733. doi: 10.1177/1352458517690821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akbar N, Honarmand K, Kou N, Feinstein A. Validity of a computerized version of the symbol digit modalities test in multiple sclerosis. J Neurol. 2011 Mar;258(3):373–9. doi: 10.1007/s00415-010-5760-8. [DOI] [PubMed] [Google Scholar]
- 15.Rao SM, Losinski G, Mourany L, Schindler D, Mamone B, Reece C, Kemeny D, Narayanan S, Miller DM, Bethoux F, Bermel RA, Rudick R, Alberts J. Processing speed test: Validation of a self-administered, iPad-based tool for screening cognitive dysfunction in a clinic setting. Mult Scler. 2017 Dec;23(14):1929–1937. doi: 10.1177/1352458516688955. [DOI] [PubMed] [Google Scholar]
- 16.Klein OA, das Nair R, Ablewhite J, Drummond A. Assessment and management of cognitive problems in people with multiple sclerosis: A National Survey of Clinical Practice. Int J Clin Pract. 2018 Dec 03;73(3):e13300. doi: 10.1111/ijcp.13300. [DOI] [PubMed] [Google Scholar]
- 17.Middleton R, Rodgers W, Chataway J, Schmierer K, Rog D, Galea I, Akbari A, Tuite-Dalton K, Lockhart-Jones H, Griffiths D, Noble D, Jones K, Al-Din A, Craner M, Evangelou N, Harman P, Harrower T, Hobart J, Husseyin H, Kasti M, Kipps C, McDonnell G, Owen C, Pearson O, Rashid W, Wilson H, Ford D. Validating the portal population of the United Kingdom Multiple Sclerosis Register. Mult Scler Relat Disord. 2018 Aug;24:3–10. doi: 10.1016/j.msard.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983 Nov;33(11):1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 19.Kremenek T. Swift 4.0 Released! Swift Blog. 2017. Sep, [2019-03-05]. https://swift.org/blog/swift-4-0-released/
- 20.Apple Inc. Cupertino, CA: Apple Inc; 2017. [2019-03-05]. Xcode 10. https://developer.apple.com/documentation/xcode-release-notes/xcode-10-release-notes. [Google Scholar]
- 21.McKinney W. Data Structures for Statistical Computing in Python. Proceedings of the 9th Python in Science Conference; June 28-July 3, 2010; Austin, TX. 2010. pp. 51–56. [DOI] [Google Scholar]
- 22.R Core Team . R Foundation for Statistical Computing. Vienna, Austria: 2013. [2019-03-05]. R: A language and environment for statistical computing. http://www.R-project.org/ [Google Scholar]
- 23.Waskom M, et al . mwaskom/seaborn. Zenodo; 2018. Jul, [2019-03-05]. seaborn: v0.9.0. https://seaborn.pydata.org/ [Google Scholar]
- 24.Wickham H. ggplot2. New York, NY: Springer-Verlag; 2016. [2019-03-05]. ggplot2: Elegant Graphics for Data Analysis. https://ggplot2-book.org/ [Google Scholar]
- 25.Kiely KM, Butterworth P, Watson N, Wooden M. The Symbol Digit Modalities Test: Normative data from a large nationally representative sample of Australians. Arch Clin Neuropsychol. 2014 Dec;29(8):767–75. doi: 10.1093/arclin/acu055. [DOI] [PubMed] [Google Scholar]
- 26.Woods DL, Wyma JM, Yund EW, Herron TJ, Reed B. Factors influencing the latency of simple reaction time. Front Hum Neurosci. 2015;9:131. doi: 10.3389/fnhum.2015.00131. doi: 10.3389/fnhum.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaportzis E, Clausen MG, Gow AJ. Older Adults Perceptions of Technology and Barriers to Interacting with Tablet Computers: A Focus Group Study. Front Psychol. 2017 Oct 04;8:1687. doi: 10.3389/fpsyg.2017.01687. doi: 10.3389/fpsyg.2017.01687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Midaglia L, Mulero P, Montalban X, Graves J, Hauser SL, Julian L, Baker M, Schadrack J, Gossens C, Scotland A, Lipsmeier F, van Beek J, Bernasconi C, Belachew S, Lindemann M. Adherence and Satisfaction of Smartphone- and Smartwatch-Based Remote Active Testing and Passive Monitoring in People With Multiple Sclerosis: Nonrandomized Interventional Feasibility Study. J Med Internet Res. 2019 Aug 30;21(8):e14863. doi: 10.2196/14863. https://www.jmir.org/2019/8/e14863/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benedict RH, Wahlig E, Bakshi R, Fishman I, Munschauer F, Zivadinov R, Weinstock-Guttman B. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005 Apr 15;231(1-2):29–34. doi: 10.1016/j.jns.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Jorm AF, Anstey KJ, Christensen H, Rodgers B. Gender differences in cognitive abilities: The mediating role of health state and health habits. Intelligence. 2004 Jan;32(1):7–23. doi: 10.1016/j.intell.2003.08.001. [DOI] [Google Scholar]
- 31.Wojcik CM, Rao SM, Schembri AJ, Drake AS, Maruff P, Schindler D, Alberts J, Yasin F, Pol J, Weinstock-Guttman B, Benedict RH. Necessity of technicians for computerized neuropsychological assessment devices in multiple sclerosis. Mult Scler. 2020 Jan;26(1):109–113. doi: 10.1177/1352458518813287. [DOI] [PubMed] [Google Scholar]
- 32.Bauer RM, Iverson GL, Cernich AN, Binder LM, Ruff RM, Naugle RI. Computerized neuropsychological assessment devices: joint position paper of the American Academy of Clinical Neuropsychology and the National Academy of Neuropsychology. Arch Clin Neuropsychol. 2012 May;27(3):362–73. doi: 10.1093/arclin/acs027. http://europepmc.org/abstract/MED/22382386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CoRe test Application Details.


