Abstract
The herbicidal activity of long-acting formulations of metribuzin and tribenuron methyl herbicides embedded in granules prepared from a mixture of degradable poly(3-hydroxybutyrate) and birch wood flour was studied in laboratory-grown weeds of various species and in wheat Triticum aestivum and barley Hordeum vulgare stands infested by weeds. The constructed formulations effectively suppressed all species of weeds studied. The biological effectiveness of herbicide formulations toward intact plants in wheat and barley stands infested with weeds was close to 100%, which was significantly higher than the effect of their free forms. The more effective suppression of weeds by embedded herbicides was beneficial for the growth of crops whose aboveground biomass was 8–13 to 20% greater than that of the crops in the treatments with free herbicides. Embedded metribuzin and tribenuron methyl exhibit sustained and pronounced herbicidal activity and are effective for pre-emergence soil application for crops infested with weeds of various species.
1. Introduction
Intensive farming involves increased use of various chemicals to control pests, weeds, and pathogens of crops. No more than 10% of pesticides reach their targets; most of these substances accumulate in biological objects and pollute soils and waterbodies, causing the death of beneficial organisms and upsetting the balance in natural ecosystems.1 Therefore, considerable research effort has been focused on the development and application of new pesticide formulations. Researchers seek to develop less toxic and more selective pesticides and reduce application rates. A very promising line of research aimed at reducing the risk of uncontrolled spread and accumulation of pesticides in the biosphere is the development of new-generation pesticides with targeted and controlled release of the active substance.
A number of research teams are engaged in creating new formulations, which will efficiently suppress the growth of undesirable plants and enhance crop productivity, causing the lowest possible harm to the ecosystem. In recent years, encapsulation of herbicides in the polymer matrix is gaining importance as controlled release of herbicides results in higher herbicidal activity than application of free forms of herbicides. For instance, slow-release formulations of alachlor and norflurazon herbicides were prepared by encapsulating them into ethylcellulose in order to reduce the risk of their uncontrolled spread and accumulation of toxicants in soil.2,3 The major issue in creating such long-acting systems with controlled release of pesticide is the material that is used as the basis for embedding active substances. Various materials of synthetic and natural origin are suitable for this application. However, the use of synthetic materials, which are not destroyed in the natural environment, can pose an environmental threat because of their accumulation in the environment as foreign compounds and even pollutants. Hence, employing biocompatible and biodegradable polymers is one of the top priorities in developing new slow-release formulations of herbicides. Biodegradable materials are degraded by natural soil microflora, enabling gradual release of pesticides, while ballast or polluting compounds are not introduced into the environment and do not accumulate. The use of such natural polymeric materials as cellulose, agarose, dextrans, carrageenan, starch, alginate, and albumin or gelatin for these purposes4 was not very successful. The disadvantage of these materials is their low mechanical strength and rather quick hydrolysis in aqueous media, which does not allow the development of true long-acting forms of pesticides.
Biodegradable polymeric materials that can be processed into durable products by various available methods include polyhydroxyalkanoates (PHAs). These microbial polymers are biodegraded slowly, and, therefore, they can function for a long time in soil. PHAs can be used in various applications,5−8 including the construction of long-acting pesticide formulations.9 As these polymers are decomposed via a truly biological degradation and do not undergo hydrolysis in liquid media, the products made from them may function, e.g., in soil, for months. The rates of release and delivery of the active substance can be varied within a wide range by controlling the degradation rate of the PHA matrix by using formulations that have different shapes and containing different amounts of pesticides. The available literature data on using PHAs to construct environmentally safe pesticide formulations are limited. However, poly(3-hydroxybutyrate) [P(3HB)], the best studied and most commonly used polymer of the PHA group, has been successfully used to construct slow-release pesticide formulations. In one of the first studies, a PHA matrix was used to deposit the Ronilan and Sumilex pesticides.10 The deposition of hexachlorocyclohexane and lindane into a PHA polymer matrix has been described, and the kinetics of polymer degradation and the release of pesticides into the soil have been studied.11,12 Similar results were presented in a study by Suave et al., which reported the encapsulation of malathion pesticide in P(3HB)/poly(ε-caprolactone) microspheres.13 The encapsulation of ametrine and atrazine pesticides in microspheres from a PHA copolymer [copolymer of 3-hydroxybutyrate with 3-hydroxyvalerate] has been described.14,15 Prudnikova et al. (2013) described the embedding of the Zellek Super herbicide into similar copolymer matrices shaped as films and granules, which provided a gradual and prolonged release of the pesticide.16 In another work, a copolymer of 3-hydroxybutyrate with 4-hydroxybutyrate was used to deposit selective trifluralin herbicide into polymer microparticles prepared by solvent evaporation from emulsion; the formulation suppressed laboratory-grown weed plant Echinochloa crus-galli more effectively than the free herbicide.17 One recent study reported the use of the P(3HB) polymer for the construction of long-acting formulations of the MET herbicide.18 Those formulations were studied in laboratory soil microecosystems, and slow degradation of the polymer matrix was achieved with a gradual release of the pesticide, which accumulated in the soil and suppressed two model weeds, Melilotus albus (M. albus) and Chenopodium album (C. album).
These few results indicate the potential of PHAs for the creation of long-acting formulations of pesticides. However, there are some limitations for the widespread use of PHAs for these purposes. These polymers are still very expensive, and their use today is justified for fabricating high-cost products such as implants for reconstructive surgery, organ and tissue engineering, and delivery of drugs and biologically active substances in technologies of targeted regeneration and therapy.19,20 PHA cost reduction is necessary to increase production outputs and expand the scope of applications, and this is currently a key task in the biotechnology of these polymers. For this, technologies for PHA biosynthesis are optimized, and new productive strains and cheap carbon raw materials, including waste, are used.5−7 Another approach is to use PHA blends with available and cheap materials. Such studies have begun in recent years. Although serious research has been carried out in Russia using P(3HB) as a matrix for loading herbicides, fungicides, and nitrogen fertilizers,9,11,12,16,18 no studies have been conducted on loading pesticides into the blended matrix rather than into the pure polymer until recently. In order to reduce the cost of PHA products for technical purposes, including construction of long-acting pesticide formulations, a series of PHA-based materials blended with natural materials (clay, peat, and wood flour) were developed and studied for the first time.21 In a work published in 2020, the authors prepared P(3HB) blends with natural materials, produced formulations in the shape of granules and pellets, studied their structure and properties, and investigated their degradation behavior in laboratory soil microecosystems.21 Another study reported embedding of fungicides into a matrix of P(3HB) mixed with natural fillers and the functioning of formulations in laboratory soil ecosystems with the gradual release of pesticides from the formulations into soil within 2–3 months, i.e., the duration of the growing season.22 These positive results provided the scientific background for the experiments on biological efficacy of long-acting formulations of pesticides against plant pathogens or weeds.
In the present work, the herbicidal activity of metribuzin and tribenuron methyl herbicides embedded in the matrix of degradable poly(3-hydroxybutyrate) blended with wood flour was studied in laboratory systems of weeds of various species and in weed-infested wheat and barley stands. Two herbicides with different modes of action were studied: metribuzin (MET) and tribenuron methyl (TBM).23 Metribuzin [4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-5(4H)-one] is a systemic selective herbicide of the class of 1,2,4-triazines, having a broad spectrum activity against some dicots and grass weeds. MET has a long-lasting effect, acting via both leaves and soil. The mode of action is based on inhibiting the Hill reaction (water photolysis) and photosynthetic electron transport between primary and secondary electron acceptors in Photosystem II. MET effectively protects soybean, maize, cereal, potato, and tomato crops from annual dicots and grass weeds. Tribenuron methyl [methyl ester of 2-(6-methyl-4-methoxy-1,3,5-triazin-2-yl(methyl) carbamoylsulfamoyl) benzoic acid] is a systemic selective herbicide of the sulfonylurea family. The mode of action is based on inhibiting acetolactate synthase, which takes part in the biosynthesis of branched-chain amino acids (valine, leucine, and isoleucine), causing a decrease in the levels of these amino acids in plant tissues followed by disruption of protein and nucleic acid synthesis. TBM effectively protects cereal crops from dicots and grass weeds.
2. Results
2.1. Suppression of Weeds by Free and Embedded Herbicides
In the first stage, the efficacy of the long-acting MET and TBM formulations was studied by estimating the effects of the free herbicides on various weed species that differ in their anatomical and physiological parameters and sensitivity to herbicides. These weed species commonly occur in agricultural crops.
The herbicidal activity of the embedded and free MET and TBM was studied in stands of two weeds: Amaranthus retroflexus (A. retroflexus) and Sinapis arvensis (S. arvensis), TBM in Sisymbrium loeselii (S. loeselii) and Elsholtzia ciliata (E. ciliata), and MET in Avena fatua L. (A. fatua) and Setaria macrocheata (S. macrocheata). The choice of herbicides for controlling weeds was based on different sensitivities of the weed species to herbicides and recommendations for the use of herbicides to control specific weeds.23
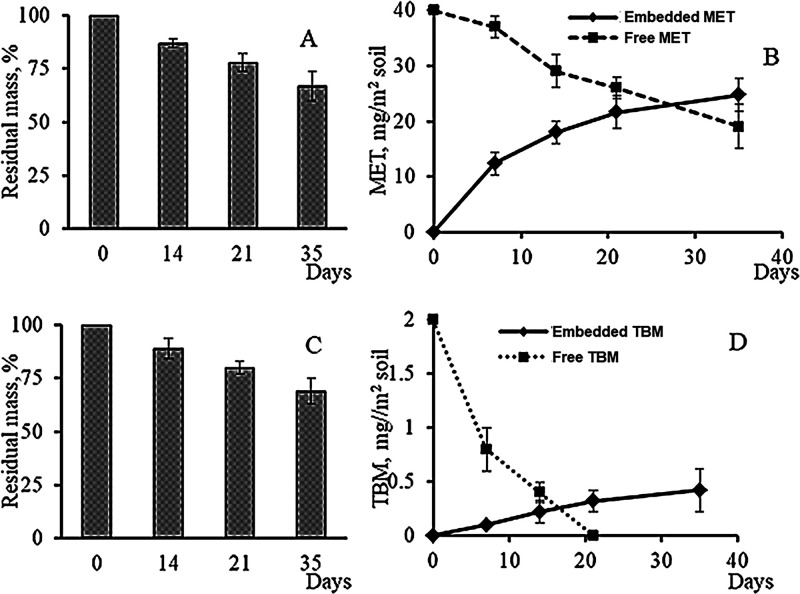
Both MET and TBM killed the weeds. In the treatments with embedded herbicides, the active ingredients were released from the polymer matrix as the granules were gradually degraded in soil. Figure 1 shows a typical pattern of herbicide accumulation in soil that occurred as the mass of the granules decreased because of biodegradation. Poly(3-hydroxybutyrate), unlike such degradable polymers as polylactide and polyglycolide, is not hydrolyzed in aqueous medium, and its degradation in soil is a true biological process, which is performed by soil microflora.7 Therefore, PHA degradation is an extended process, lasting for months or longer. This is the factor that makes these polymers promising materials for constructing long-acting formulations. The embedded MET and TBM exhibited comparable biodegradation behavior in soil (Figure 1A,C). The mass loss of granules was gradual, reaching 32–35% by the end of the experiment (35 d). Approximately 40–50% of the polymer contained in the granules (50%) was degraded during the experiment. It corresponded to the gradual release of the herbicides from the granules. Analysis of the curves showing herbicide concentrations in soil suggested that, by the end of the experiment (day 30–35), approximately 50–60% MET had been released to soil and its concentration reached 25–28 mg/m2 (Figure 1B). MET is highly soluble (1350 to 2040 mg/L, depending on the soil pH and type). MET release from the granules and the increase in MET concentration in soil occurred gradually with no burst release, including the processes soon after application of the herbicide when it was released not only because of the degradation of the polymer matrix but also because of dissolution of the herbicide on the surface of the granules and diffusion through pores. Solubility of TBM is much lower (2.04 mg/L), and its application rates are almost 20 times lower. This affected its release kinetics and soil concentration, which increased gradually, reaching approximately 0.4 mg/m2 (about 20% of the initial content of TBM in the granules) by the end of the experiment (Figure 1D). The curves representing MET and TBM concentrations in soil in the experiments with the free forms show their dramatic decrease. The TBM concentration had dropped by 50% by day 10 and by 100% by day 20 (Figure 1D). The reason for that was quick inactivation of TBM in soil. MET may remain active in soil for months. Its concentration in soil decreased rather gradually, reaching 50% of the initial concentration by the end of the experiment (Figure 1B). This could be associated with the high solubility of MET and removal of the herbicide from soil during watering events. Thus, embedded herbicides, in contrast to their free forms, are released gradually, persisting in soil and controlling weeds for extended periods of time.
Figure 1.
Mass loss kinetics of slow-release formulations of (A) metribuzin and (B) tribenuron methyl loaded into degradable matrices (polymer/wood flour) and concentrations of (C) metribuzin and (D) tribenuron methyl released from the formulations in soil at different time points.
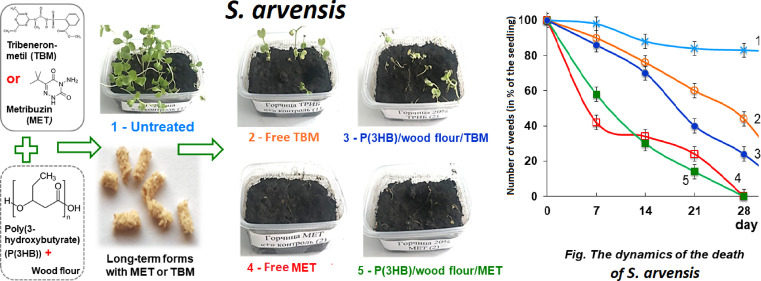
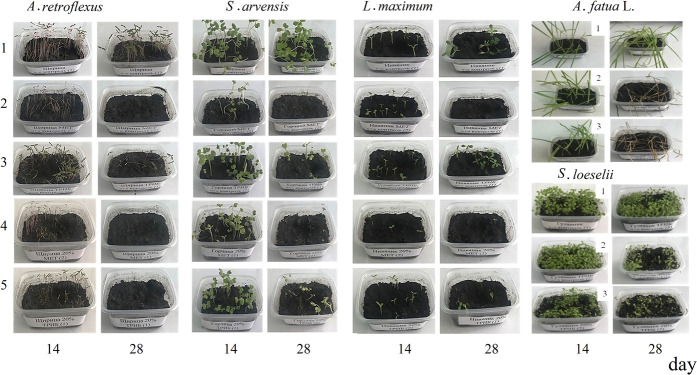
Differences were revealed between the herbicidal activities of MET and TBM, the activities of the embedded and unembedded herbicides, and sensitivity of the weeds to the two herbicides, which was estimated from the plant death rate. Figure 2 provides photographs of weeds affected by the free and embedded herbicides.
Figure 2.
Photographs of laboratory stands of weeds of various species affected by free and embedded herbicides. A. retroflexus and S. arvensis: 1 - intact plants without application of herbicides; free forms of herbicides: 2 - MET and 3 - TBM; embedded forms: 4 - P(3HB)/wood flour/MET and 5 - P(3HB)/wood flour/TBM; A. fatua L.: 1 - untreated, 2 - free form (MET), and 3 - P(3HB)/wood flour/MET; and S. loeselii: 1 - untreated, 2 - free form (TBM), and 3 - P(3HB)/wood flour/TBM.
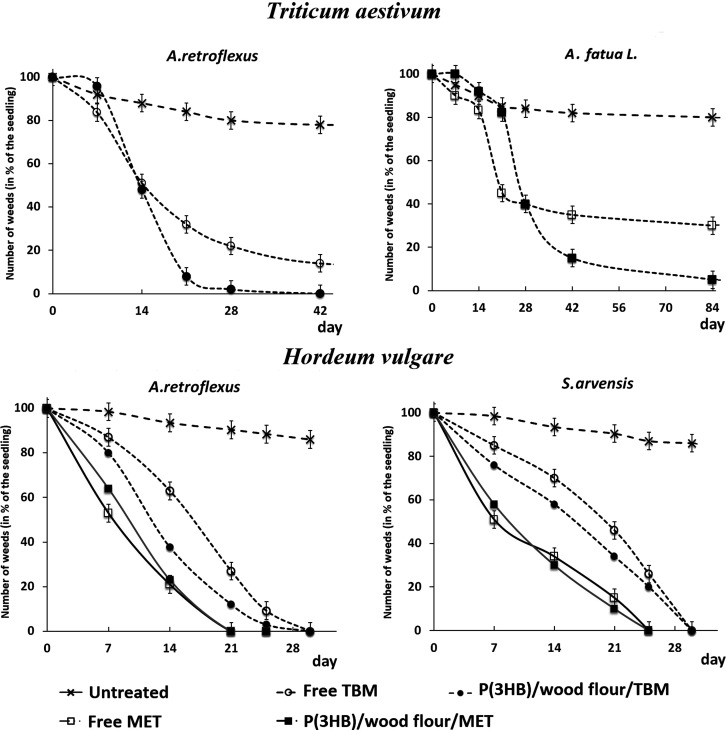
The herbicidal effects of the embedded herbicides and the differences revealed between MET and TBM and the forms of their use (free or embedded in polymer matrix) are illustrated by the photographs of weed plants (Figure 2) and the dynamics of weed death (Figure 3). In all experiments with MET, almost all weeds died by the end of the experiment, and their death occurred, as a rule, sooner than in the experiments with TBM whose activity was higher in the embedded form compared to the free form.
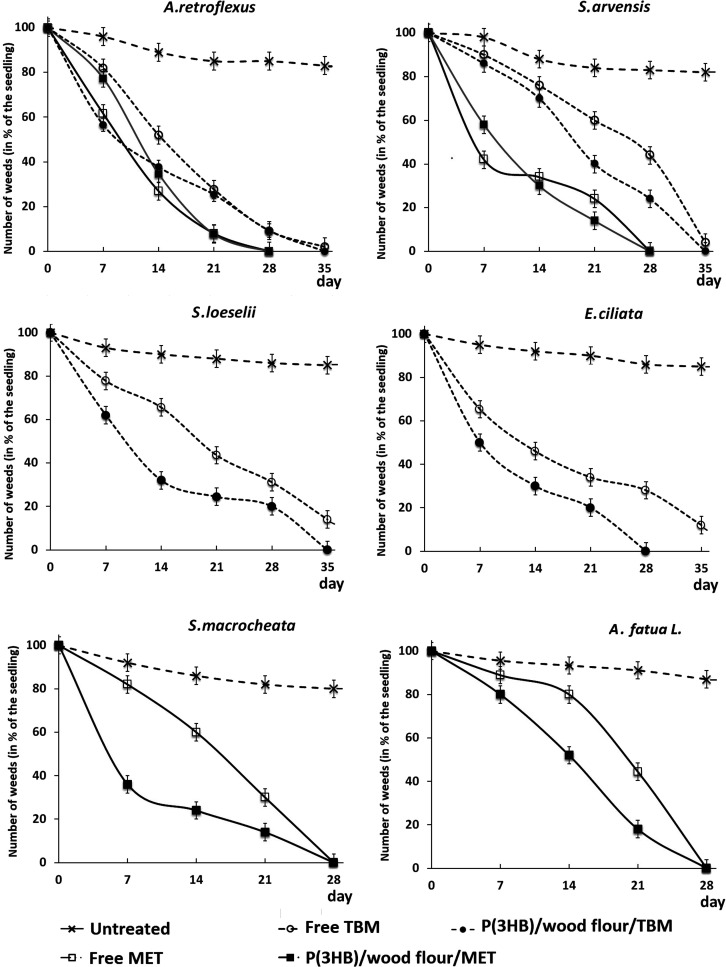
Figure 3.
Dynamics of the death of weeds of various species treated with embedded and free herbicides relative to intact plants (untreated).
The experiment with A. retroflexus and S. arvensis showed that the embedded metribuzin and tribenuron methyl had pronounced herbicidal activity against both weeds. In general, the effects of the embedded MET and TBM were not inferior to or even higher than the herbicidal activities of their free forms (Figure 3). Thus, in the experiment with A. retroflexus and S. arvensis, both herbicides suppressed the development of both weeds, and this was noted as early as day 7. The death of the majority of both weeds affected by MET was observed somewhat earlier than in the experiments with TBM, and this difference was more pronounced for S. arvensis. The number of live A. retroflexus and S. arvensis plants affected by free and embedded MET by day 14 did not exceed 30% and day 25 with 3–5%; at day 28, all plants had died. In the experiments with TBM, the curve of weed death was slightly different due to a slightly lower herbicidal activity; complete death of weeds at day 28 (as was the case with MET) was not observed. At that time point, up to 8% of A. retroflexus plants and up to 30 and 45% of S. arvensis plants remained alive for the embedded and free TBM, respectively. Only seven days later, almost complete death of A. retroflexus and S. arvensis was observed in the experiment with embedded TBM with up to 5–7% of the plants remaining alive in the case of free TBM.
The effects of the embedded and free TBM on two other weeds (E. ciliata and S. loeselii) were similar to the effects of this herbicide in the experiment with A. retroflexus and S. arvensis (Figure 3). In the stands of both weeds (E. ciliata and S. loeselii), the free and embedded TBM showed different herbicidal activities. The herbicidal effect of embedded TBM was already evident within 7 days; after 14 days, the death of 50% of plants of both species was noted. Within 21 days, the residual number of weeds in the treatment with the free form of TBM was about 35–38% and in the treatment with the embedded, approximately 20%, suggesting that the embedded TMB was more active compared to its free form. Within 28 days, all E. ciliata plants died in the treatment with the embedded TBM; in the treatment with the free form of this herbicide, approximately 20% of the weeds remained viable. The effect of TBM on S. loeselii was somewhat weaker; at day 28, approximately 35% of the plants did not die in the treatment with the free form of the herbicide and approximately 18% in the treatment with the embedded. Complete death of plants here was noted at day 35. With the free form of the herbicide, at day 35, approximately 10% of both weeds remained alive (Figure 3).
The efficacies of two forms of MET were studied in the stands of A. fatua L. and S. macrocheata (Figure 3). MET actively suppressed both weeds. In the case of S. macrocheata, complete plant death occurred seven days later. In both treatments, the embedded MET was more effective than its free form. Thus, the embedding of MET and TBM in the degradable matrix did not reduce the biological activity of these herbicides toward weeds; moreover, in some cases, it increased their herbicidal efficacy.
The biological effectiveness of herbicides at different time points of the experiment is provided in Table 1. The biological efficacy of the MET [P(3HB)/wood flour/MET] and TBM [P(3HB)/wood flour/TBM] formulations compared to their free form was calculated as relative to the number of weeds in the untreated laboratory system.
Table 1. Biological Efficacy of the P(3HB)/Wood Flour/MET and P(3HB)/Wood Flour/TBM Formulations Compared to the Efficacy of Free MET and TBMa.
| biological
efficacy Cispr (%) |
|||
|---|---|---|---|
| sample day | 14 | 21 | 28 |
| A. retroflexus | |||
| free MET | 66.7 ± 5.0 | 95.0 ± 4.9 | 100.0 |
| embedded MET | 57.1 ± 4.7 | 90.0 ± 5.7 | 97.5 ± 5.1 |
| free TBM | 44.8 ± 3.0 | 64.0 ± 4.2 | 88.0 ± 5.4 |
| embedded TBM | 53.3 ± 3.8 | 67.0 ± 4.4 | 92.1 ± 6.1 |
| S. arvensis | |||
| free MET | 60.5 ± 4.1 | 71.4 ± 4.8 | 100.0 |
| embedded MET | 65.1 ± 3.9 | 83.3 ± 5.3 | 100.0 |
| free TBM | 13.6 ± 1.9 | 28.1 ± 1.6 | 45.0 ± 3.6 |
| embedded TBM | 18.6 ± 2.0 | 52.4 ± 3.5 | 70.0 ± 5.1 |
| S. macrocheata | |||
| free MET | 30.2 ± 2.6 | 60.5 ± 4.0 | 100.0 |
| embedded MET | 70.0 ± 4.7 | 81.6 ± 5.1 | 100.0 |
| A. fatua L. | |||
| free MET | 14.3 ± 1.3 | 51.2 ± 3.2 | 100.0 |
| embedded MET | 43.3 ± 3.1 | 80.7 ± 5.1 | 100.0 |
| S. loeselii | |||
| free TBM | 27.3 ± 1.6 | 50.5 ± 3.3 | 60.6 ± 4.3 |
| embedded TBM | 64.9 ± 3.3 | 69.0 ± 5.0 | 74.6 ± 4.7 |
| E. ciliata | |||
| free TBM | 54.1 ± 4.0 | 57.5 ± 3.1 | 62.5 ± 3.9 |
| embedded TBM | 66.2 ± 4.7 | 75.0 ± 4.7 | 100.0 |
Cispr - corrected percent mortality that shows the decrease in the number of weeds caused by application of herbicides (% of the initial infestation or the control) corrected for the control.
The biological efficacy of the embedded MET was 100% in the stands of the 4 weeds (A. retroflexus, S. arvensis, A. fatua L., and S. macrocheata) at day 28. This was comparable with the effect of MET in the free form toward all of the listed weeds. The biological effectiveness of the embedded TBM was slightly lower than the effectiveness of the embedded MET and reached 100% within 28 days only for E. ciliata, 92.1 and 74.6%, respectively, for A. retroflexus and S. loeselii, and 70% for S. arvensis. The herbicidal activity of the free form of TBM was inferior to the activity of the embedded herbicide; its biological effectiveness at day 28 was 88.0% only for A. retroflexus, 62.5% for E. ciliata, 60.6% for S. loeselii, and a little over 45% for S. arvensis. The slightly reduced herbicidal activity of TBM, which in some cases reached 100% at day 35, that is, somewhat later than MET, can be explained by the well-known fact that this herbicide is rapidly metabolized in the tissues of higher plants to forms non-toxic to plants, and its half-life in soil is much shorter than that of MET.23 Therefore, it is very important that embedding in the degradable matrix has been found to prolong and enhance its effect.
2.2. Biological Efficacy of Herbicides in Weed-Infested Wheat and Barley Stands
A series of long-duration experiments were performed with laboratory stands of weed-infested crops. The herbicidal activity of the embedded MET and TBM [P(3HB)/wood flour/MET and P(3HB)/wood flour/TBM] was compared with their free-form action relative to intact plants (untreated). MET or TBM free forms were applied to the soil at concentrations comparable with their concentrations in the treatments. This was achieved by varying the size and number of granules applied to the soil.
Figure 4 shows photographs of spring wheat crops infested with A. retroflexus and grown in laboratory soil microecosystems with free and embedded forms of TMB applied to the soil at different time points of the 35-day experiment.
Figure 4.

Photographs of laboratory crops of spring wheat infested with A. retroflexus weed: 1 - untreated plants, 2 - free TBM, and 3 - P(3HB)/wood flour/TBM formulations.
At day 14, the number of weeds decreased by a factor of almost 2, regardless of the form of TBM delivery, i.e., in laboratory soil microecosystems with free and embedded forms (Figure 5). Within 21 days, weeds affected by the embedded TBM declined to 10% whereas in the system with the free TBM, the remaining weeds reached approximately 37%. Within 28 days after sowing, approximately 3–5% of the weeds affected by the embedded TBM remained alive; later, all weeds died. Approximately 30% of the weeds affected by the free herbicide remained alive on day 28. At the end of the experiment (42 days), approximately 18% of A. retroflexus plants affected by the free TBM did not die.
Figure 5.
Dynamics of death of weeds in laboratory wheat and barley stands treated with free and embedded MET and TBM.
The biological efficacy of the free and embedded TBM estimated by the residual number of weeds in these groups was relatively negative on day 42 of the experiment: 80.3 and 100%, respectively. That is, the embedded TBM suppressed the A. retroflexus weed plants in the long-duration experiment more effectively than its free form (Table 2).
Table 2. Biological Efficacy Cispr (%) of Free and Embedded MET and TBM in Laboratory Experiments with Cereal Crop Seeds.
| weed-infested
wheat crops | |||||
|---|---|---|---|---|---|
| time
points of the experiment, day |
|||||
| herbicide form | 14 | 21 | 28 | 42 | 84 |
| A. retroflexus | |||||
| free TBM | 36.4 ± 2.6 | 58.3 ± 4.0 | 69.9 ± 4.2 | 80.3 ± 5.1 | |
| embedded TBM | 40.3 ± 2.8 | 89.6 ± 5.1 | 97.3 ± 2.9 | 99.5 ± 2.3 | |
| A. fatua L. | |||||
| free MET | 7.4 ± 0.7 | 47.1 ± 3.5 | 52.9 ± 2.7 | 58.8 ± 3.9 | 64.7 ± 3.8 |
| embedded MET | 8.6 ± 0.9 | 52.9 ± 3.4 | 82.4 ± 5.3 | 94.1 ± 4.9 | 100.0 |
| weed-infested barley crops | |||||
|---|---|---|---|---|---|
| time points of the experiment, day | |||||
| herbicide form | 7 | 14 | 21 | 28 | 35 |
| A. retroflexus | |||||
| free TBM | 13.2 ± 1.0 | 37.5 ± 2.8 | 73.2 ± 3.9 | 91.7 ± 4.9 | 100.0 |
| embedded TBM | 20.4 ± 1.2 | 52.6 ± 3.3 | 88.6 ± 5.0 | 97.1 ± 3.0 | 100.0 |
| free MET | 47.1 ± 3.1 | 79.4 ± 4.9 | 100.0 | ||
| embedded MET | 36.3 ± 2.7 | 77.1 ± 4.2 | 100.0 | ||
| S. arvensis | |||||
| free TBM | 15.1 ± 1.1 | 30.2 ± 2.1 | 54.3 ± 2.9 | 74.2 ± 4.2 | 100.0 |
| embedded TBM | 24.6 ± 1.8 | 42.0 ± 2.5 | 66.2 ± 3.5 | 80.1 ± 4.8 | 100.0 |
| free MET | 49.7 ± 3.0 | 66.4 ± 3.4 | 85.7 ± 4.3 | 100.0 | |
| embedded MET | 42.1 ± 2.7 | 70.7 ± 3.8 | 90.1 ± 5.6 | 100.0 | |
Thus, the embedding of TBM into the degradable blended matrix did not reduce its herbicidal activity; moreover, the experimental form of TBM was more effective toward A. retroflexus than the free form of this herbicide. In addition, the more effective suppression of the weeds by the embedded TBM produced a positive effect on the growth and development of wheat.
The experiment conducted to study the efficacy of the embedded MET was carried out under severer conditions. A. fatua L., which was used as the weed, forms a large biomass (comparable to wheat biomass). In addition, the number of weed seeds sown into soil was three times greater than the number of seeds of wheat (the A. fatua L.:wheat seed ratio was 20:6). A photograph of the laboratory wheat crops infested with A. fatua L. is shown in Figure 6; the dynamics of the death of weeds affected by the free form of MET and the experimental form (P(3HB)/wood flour/MET) are shown in Figure 5.
Figure 6.

Photographs of laboratory stands of wheat infested with A. fatua L. weed treated with various forms of MET: 1 - untreated plants, 2 - free MET, and 3 - P(3HB)/wood flour/MET formulations.
Despite the harsh experimental conditions, both free and embedded MET suppressed the development of A. fatua L., but the embedded MET was more effective. The death rate of the weed plants was the highest in 28 days when more than 50% of the plants died in the system with the free MET and more than 80% in the treatment with the embedded herbicide. Then, over a long period (up to 84 days), the number of weeds did not change in the system with the free MET. By contrast, in the treatment with the embedded MET, the number of weed plants did not exceed 10%, and by day 70, almost all weeds had died. In the laboratory systems with the free herbicide, the growing weeds reached approximately 40% and had remained almost at the same level until the end of the experiment (42 days).
The biological efficacy of the embedded MET was 100% toward the virulent weed A. fatua L.; with free MET, the effect was significantly lower (64.7%) (Table 2). Thus, the slow-acting MET formulation enabled a gradual and prolonged release of MET, significantly increasing the efficacy of this herbicide compared to a single application of the same amount of MET in the free form. More effective weed control by the embedded herbicide also had a positive effect on the growth and development of wheat: the height of the plants and the aboveground biomass were 18 and 22%, respectively, greater than the corresponding parameters in the wheat stand treated with the free MET.
Another experiment investigated the efficacy of the free and embedded MET and TBM in laboratory barley stands simultaneously infested with two weeds (A. retroflexus and S. arvensis). The experimental conditions and the comparative assessment of the effects of two different herbicides and their form of delivery to plants were the same as described above. The duration of the experiment was 35 days. Photographs of weed-infested barley and the dynamics of weed death are presented in Figures 5 and 7.
Figure 7.

Photographs of laboratory stands of barley infested with weeds A. retroflexus and S. arvensis and treated with various forms of MET and TBM: 1 - untreated plants, 2 - free MET, 3 - P(3HB)/wood flour/MET formulations, 4 - free TBM, and 5 - P(3HB)/wood flour/TBM formulations.
Thus, the results obtained in the present study suggest that the experimental long-acting formulations of MET and TBM embedded into poly(3-hydroxybutyrate) as a degradable blended polymer/wood flour matrix are effective for pre-emergence soil application in grain crops infested with weeds of various species.
2.3. Characteristics of Wheat and Barley Crops Infested with Weeds and Treated with Different Forms of Herbicides
The analysis of the experiments showed differences in the herbicidal activity of MET and TBM toward weeds. In addition, embedded herbicides were generally found to be more effective than free MET and TBM. The efficacy of the herbicides also varied depending on the weed species. Thus, the suppression of weeds that infested the laboratory wheat and barley crops was different in different experiments, and this could not but affect the growth and development of the crops (wheat and barley). Therefore, the effectiveness of the application of the experimental long-acting herbicide formulations was studied not only by measuring weed death rates in cereal crops but also by investigating the growth and development of wheat and barley plants competing with weeds.
Measurements of the flag leaf height and air-dry biomass of aboveground plant parts served as indicators of the growth and development of weed-infested crops grown using free and embedded herbicides. The experiments lasted 84 days, and the plants went through all phases of development (germination, the third leaf emergence, tillering, booting, heading, and early grain maturation), which, according to the onset and duration of the phases, corresponded to the usual growth and development of the wheat and barley species used in this study in the conditions of the Siberian region (Russia). The crops of Triticum aestivum (T. aestivum) infested with A. fatua L. were treated with free and embedded metribuzin. The crops of Hordeum vulgare (H. vulgare) were infested with two weeds (A. retroflexus and S. arvensis) for which MET and TBM were used. The results of monitoring the development of wheat and barley crops are presented in Table 3.
Table 3. Growth and Development Parameters of Wheat and Barley Plants with Various Forms of Herbicidesa.
| flag
leaf height (cm) |
air-dry
biomass of aboveground plant parts (g/m2) |
|||
|---|---|---|---|---|
| treatments and controls | wheat | barley | wheat | barley |
| germination (day 7) | ||||
| untreated | 23.4 ± 0.1 | 17.8 ± 0.4 | 22.6 ± 1.8 | 38.0 ± 1.8 |
| free MET | 22.7 ± 0.2 | 17.3 ± 0.2 | 21.3 ± 2.0 | 36.9 ± 3.2 |
| free TBM | - | 16.9 ± 0.6 | - | 36.8 ± 2.7 |
| P(3HB)/wood flour/MET | 23.5 ± 0.1 | 17.0 ± 0.4 | 22.0 ± 1.0 | 38.0 ± 1.9 |
| P(3HB)/wood flour/TBM | - | 16.8 ± 0.7 | - | 39.0 ± 3.1 |
| tillering (day 28) | ||||
| untreated | 29.3 ± 2.3 | 30.9 ± 2.2 | 81.7 ± 3.9 | 92.1 ± 1.0 |
| free MET | 30.3 ± 3.0 | 31.6 ± 2.1 | 99.2 ± 4.3 | 93.8 ± 6.8 |
| free TBM | - | 33.0 ± 0.9 | - | 108.4 ± 8.2 |
| embedded MET | 31.2 ± 2.6 | 32.1 ± 3.1 | 95.7 ± 7.6 | 104.5 ± 9.5 |
| embedded TBM | - | 31.9 ± 2.0 | - | 105.3 ± 10.0 |
| booting (day 42) | ||||
| untreated | 31.6 ± 2.2 | 32.3 ± 1.8 | 120.5 ± 10.7 | 126.2 ± 9.7 |
| free MET | 38.0 ± 1.2 | 34.4 ± 2.7 | 147.5 ± 11.6 | 146.7 ± 10.9 |
| free TBM | - | 38.4 ± 3.6 | - | 153.1 ± 9.7 |
| embedded MET | 39.7 ± 3.1 | 39.0 ± 4.0 | 150.2 ± 10.3 | 169.2 ± 11.3 |
| embedded TBM | - | 40.0 ± 3.9 | - | 180.0 ± 13.4 |
| early grain maturation (day 84) | ||||
| untreated | 38.3 ± 2.5 | 40.4 ± 8.2 | 405.2 ± 5.7 | 521.7 ± 10.4 |
| free MET | 40.4 ± 10.4 | 42.2 ± 6.1 | 440.5 ± 10.3 | 530.9 ± 8.8 |
| free TBM | - | 43.0 ± 7.7 | - | 539.5 ± 11.3 |
| embedded MET | 44.2 ± 8.1 | 44.2 ± 9.4 | 460.1 ± 11.9 | 626.4 ± 10.1 |
| embedded TBM | - | 45.5 ± 7.8 | - | 630.1 ± 15.1 |
“-”means the absence of wheat grown by using TBM to control the weeds.
In the initial periods of grain growth (germination, the third leaf emergence, and tillering), despite the differences in the dynamics of weed death detected during these periods, no differences were found in the development rates of wheat and barley, regardless of which herbicide (MET or TBM) was applied and in what form (free or embedded). However, later (in the booting phase), the height of wheat and barley plants treated with the embedded MET and TBM significantly exceeded the height of untreated wheat and barley plants and was comparable to the height of the plants treated with the free herbicides. At the same time, there was a tendency toward an increase in the mass of aboveground biomass of both crops in the treatments with the embedded herbicides. This trend continued in the later phases, and the plant stems in the treatments exceeded the results in the crops treated with the free MET and TBM; at the same time, a more pronounced positive effect of the use of embedded herbicides was noted in barley crops. The positive effect of the application of embedded herbicides with a prolonged herbicidal effect persisted until the end of the experiment. Thus, in the early grain maturation (day 84) phase, the weight of the aboveground wheat biomass was 440.5 ± 10.3 and 460.1 ± 11.9 g/m2 with the free and embedded MET, respectively, which were 8.6 and 13.6% higher than the corresponding parameter in weed-infested wheat crops, respectively. A similar trend was recorded in barley crops that were infested simultaneously by two weeds and treated with two herbicides (MET and TBM) in two forms. In this experiment, the positive effect of embedded herbicides compared with their use in the free form was even more evident. In the treatments with the embedded MET and TBM, the weight of the barley aboveground biomass was 626.4 ± 10.1 and 630.1 ± 15.1 g/m2, respectively, which was 20% higher than in the systems treated with the free herbicides.
3. Discussion
The development and application of new-generation environmentally safe herbicide formulations is a priority for agrochemists, biotechnologists, and horticulturists. A current research focus is to develop less toxic and more selective pesticides and reduce application rates.
One of the challenges in developing slow-release pesticide formulations is maintaining the biological efficacy of the active ingredient loaded into the matrix. Therefore, it is necessary not only to develop the process of constructing slow-release herbicide formulations but also to study their biological activity in weed control relative to the herbicidal activity of the free active substance.
Among promising materials for creating long-acting formulations of herbicides suitable for soil use are degradable polymers of microbiological origin, polyhydroxyalkanoates (PHA) and the most common and best studied polymer of this class, a polymer of 3-hydroxybutyric acid, poly(3-hydroxybutyrate), P(3HB).
In the present work, the herbicidal activity of the MET and TBM herbicides embedded in the degradable poly(3-hydroxybutyrate) blended with wood flour was studied in laboratory stands of weeds of various species and weed-infested wheat and barley crops.
Experiments were conducted to study not only the herbicidal activity of the experimental pesticide formulations but also their degradation behavior in laboratory soil microecosystems and release kinetics of the embedded MET and TBM. Granular polymer forms were gradually degraded in soil, and the active ingredients were uniformly, without bursts, released and accumulated in soil. MET and TBM concentrations in soil were determined by the solubility of the herbicides and their application rates. In contrast to the free forms of MET and TBM whose concentrations in soil decreased considerably due to removal during watering events and inactivation (characteristic of TBM), the embedded herbicides were gradually released from the granules and persisted in soil, controlling the weeds over extended periods of time.
A comparative study of the effect of free and embedded MET and TBM was performed in individual stands of ubiquitous weeds of various species and in weed-infested stands of cereal crops (wheat and barley).
In the stands of six weed plants of various species, it was shown that the herbicidal activity of MET and TBM depends on the type of herbicide, its form of delivery to plants (embedded or free), and the weed species. The biological efficacy of the embedded MET was 100% toward almost all the weeds used in the present study in contrast to the embedded TBM. Moreover, it was established that the embedded TBM had more pronounced herbicidal activity than the free TBM; therefore, the possibility of enhancing its effect by embedding it in the degradable matrix is important. Thus, the activity of the herbicides tested in this study was not reduced by embedding them in the degradable matrix of P(3HB) blended with wood flour; moreover, the effect of the herbicides was even enhanced.
The obtained results are compared with the few publications that present the results of similar studies of herbicides in stands of higher plants of the target and non-target species. The most actively studied herbicide is MET. The review of the literature on the efficacy of MET use showed that, as a rule, the data characterize the biological effects of various concentrations of this herbicide on weeds, while there are very few articles reporting comparative studies of the efficacy of free and embedded MET. MET was used in maize fields to test its efficacy against the Portulaca oleracea, A. retroflexus, and Echinochloa colonum weeds, and the biomass of the weeds was reduced by 97.7, 96.9, and 97.2%, respectively.24 In that case, the effect of various doses of the herbicide was detected depending on the stage of growth of weeds. These results were consistent with the data reported by Medd et al. (2001), suggesting that herbicide efficacy varied depending on the weed species.25 This finding is in good agreement with the conclusion made by Riethmuller-Haage et al. (2006).26 It was supposed that the MET dose necessary to control weeds varied depending on the leaf area and number of leaves and the effect of herbicides became weaker as the plants grew.26 A few studies addressed the relationship between herbicidal activity and the form of herbicide delivery. Experiments with polycaprolactone nanocapsules with the atrazine herbicide whose mode of action is similar to that of MET, inhibition of plant photosynthesis, showed higher efficacy of encapsulated atrazine compared to the free herbicide used for post-emergence treatment of Amaranthus viridis and Bidens pilosa.27
Considerably fewer studies have been published on the effects of TBM in weed stands compared to the extensively studied MET. The latest literature data suggest that the effect of TBM, like the effect of MET, varies depending on the target weed species and the time and rate of herbicide application. Gherekhloo et al. (2018) described the dose-dependent effect of TBM on different generations of S. arvensis and showed that, as plants became more resistant, the standard dose of TBM (15 g/ha) needed to be increased by 2.2–16.8 times.28 Similar results were obtained in another study of the TBM effect on S. arvensis.30 TBM was found to be less effective in weed control than triazine and chlorotriazine herbicides.31 However, the ability of embedded or free sulfonylurea herbicides to inhibit growth and development of various weeds was confirmed in a number of studies, including studies performed with such weeds as Amaranthus tuberculatus, Amaranthus palmeri, and Amaranthus spp.(32,33)
One of the main tasks in constructing long-acting formulations of herbicides is the preservation of their biological effectiveness once they are embedded in the degradable matrix. Therefore, it is necessary to study their biological activity towards weed compared with their initial herbicidal activity. The most effective approach is to perform experiments with higher plants grown in laboratory and/or conduct field trials.
In the present work, pioneering studies of the efficacy of experimental herbicide formulations in suppressing weeds grown simultaneously in laboratory wheat and barley stands were carried out. A series of experiments with grain crops infested with one of the weeds (A. retroflexus or A. fatua L.) or two at the same time (A. retroflexus + S. arvensis) showed the high efficacy of the embedded MET and TBM, which was significantly higher than the effect of these herbicides in the free form. The results are consistent with the positive effect of the use of similar herbicides embedded into a matrix of P(3HB) alone.34 The formulations were constructed as films and microgranules, which were tested against the weeds such as white sweet clover M. albus and lamb’s quarters C. album in the presence of wheat T. aestivum, cv. Altaiskaya 70 as the subject crop for investigation. The experimental MET and TBM formulations showed pronounced herbicidal activity against the weed species used in the study. The efficacy of the embedded herbicides in inhibiting weed growth was comparable and sometimes higher than that of the commercial formulations. The amount of the biomass of the wheat treated with the experimental herbicide formulations was significantly greater than that of the wheat treated with commercial formulations.
Very few data have been reported in the literature on long-duration experiments performed to study the effectiveness of long-acting formulations of herbicides in higher plant stands grown simultaneously with weeds. At the same time, the use of the MET herbicide (the most extensively studied herbicide) for the suppression of weeds in crop stands has been described as beneficial.
Field trials of MET, which is effective against many broad-leaved weeds and herbs, confirmed its efficacy in traditional application against A. retroflexus and C. album in potato crops for pre-emergence tillage without a negative impact on the potato yield.35 In the work of Tagour et al. (2017), the effect of MET on the growth of different weeds that infest cereal crops was studied to show that the biomass of weeds remaining after treatment did not exceed 5–8%.24 MET is an effective herbicide against various broadleaf weeds and grasses, and traditional pre-emergence soil applications of this herbicide in potato fields proved that it effectively controlled A. retroflexus and C. album without reducing the potato yield.34 The effect of various concentrations of MET embedded in polyvinyl chloride, carboxymethyl cellulose (CMC), and carboxymethyl cellulose-kaolinite (CMC-KAO) composite was studied in the field experiment; the MET formulations were found to be effective in the weed-infested wheat stand.4,35
Application of the embedded MET and TBM had a positive effect on the growth of crops, compared with their free forms. More effective suppression of weeds by embedded herbicides enhanced the growth of crops whose aboveground biomass increased from 8–13% in the experiments with the free herbicides to 20% of the crops.
The experimental long-acting formulations of MET and TBM embedded in a degradable matrix of P(3HB) and wood flour had a long and pronounced herbicidal effect, effectively suppressing the development of weeds in wheat and barley stands and beneficial for the growth of grain crops. The experimental herbicide formulations effectively suppress weeds of various species and are suitable for pre-emergence applications.
4. Materials and Methods
4.1. Herbicides
Two herbicides with different modes of action were studied: metribuzin (MET) and tribenuron methyl (TBM) (China).
4.2. Materials for Embedding Herbicides
P(3HB) polymer samples were synthesized using a Cupriavidus necator (C. necator) B-10646 strain and proprietary technology.7 Cultivation of C. necator B-10646 cells was carried out in a 30 L fermenter (Bioengineering AG, Switzerland) under strictly aseptic conditions. The carbon substrate was fructose (E.U., purity: 99%) at a concentration of 5–10 g/L. A two-stage process was used. In the first stage, cells were grown under nitrogen deficiency (the amount of nitrogen supplied in this stage was 60 mg/g cell biomass synthesized, i.e., 50% of the cell’s physiological requirements); the cells were cultured in a complete mineral medium and with a fructose flux regulated in accordance with the requirements of the cells. In the second stage, cells were cultured in a nitrogen-free medium; the other parameters were the same as in the first stage. The temperature of the culture medium was 30 ± 0.5 °C, and the pH was 7.0 ± 0.1. The concentration of dissolved oxygen was maintained at DO 30%.
The polymer was extracted from bacterial cells with chloroform, and the extracts were precipitated using hexane. The extracted polymers were re-dissolved and precipitated again 3–4 times to prepare homogeneous specimens. The polymer had the following properties: a degree of crystallinity of 75%, melting point of 176 °C, thermal decomposition temperature of 287 °C, molecular weight (Mw) of 590 kDa, and polydispersity index of 3.
A natural material, wood flour, was used as a filler. It was produced by grinding wood of birch (Betula pendula Roth) using an MD250-85 woodworking machine (“Stanko Premyer”, Russia). Then, it was dried at 60 °C for 120 h until it reached constant weight, and a 0.5 mm mesh was used to separate the flour size fraction; its degree of crystallinity was 26% with an onset of thermal decomposition at 220 °C.
4.3. Experimental Herbicide Formulations
The polymer and wood flour were pulverized by an impact and shearing action in an ultracentrifugal mill ZM 200 (Retsch, Germany). To achieve high fineness of polymer grinding, the material and the mill housing with the grinding tools were preliminarily cooled at −80 °C for approximately 30 min in an Innova U101 freezer (New Brunswick Scientific, U.S.A.). Grinding was performed with a 2 mm holes sieve at a rotor speed of 18,000 rpm. The fractional composition of the polymer and filler powders was determined using a vibratory sieve shaker AS 200 control (Retsch, Germany). Then, polymer powder was mixed with the filler flour powder in a benchtop planetary mixer SpeedMixer DAC 250 SP (Hauschild Eng., Germany); the blend period was 1 min with the speed 1000 rpm.
Blends of powdered P(3HB) and natural material (wood flour) were mixed with herbicide powder. Herbicide granules were prepared using polymer paste wetted with ethanol and mixed with birch wood flour and the herbicide in a screw granulator (Fimar, Italy). The formulations contained the following percentages of the components: P(3HB)/wood flour/herbicide 50/30/20 (wt %). The granules were 3 mm in diameter and 4 to 6 mm long. The manufacturing scheme and a photograph of the granules are shown in Figure 8.
Figure 8.
Manufacturing scheme and photographs of granules.
4.4. Higher Plants
The following plant species were used as weeds: A. retroflexus, S. arvensis, S. loeselii, E. ciliata, A. fatua L., and S. macrocheata.
Wheat T. aestivum and barley H. vulgare were used as test crops. The short-cycle spring wheat variety “Novosibirskaya 15” was bred at the Siberian Research Institute of Plant Cultivation and Breeding (Russia); the variety was registered in the National Registry of Plant Varieties and approved for use in the Ural, West Siberian, and East Siberian Regions in 2013. The barley variety “Biom” was bred at the Siberian Research Institute of Plant Cultivation and Breeding as well.
4.5. Cultivation and Evaluation of Parameters of Weeds Affected by Herbicides
Herbicide formulations were investigated in laboratory crop stands. Field soil was placed into 500 cm3 plastic containers (400 g of soil/container). The soil was collected at the field of Krasnoyarsk State Agrarian University in the vicinity of Krasnoyarsk (Russia). This was meadow-chernozem soil whose soil profile was similar to the profile of chernozem soils with a thick humus-rich layer and loose granular structure. The soil was neutral with low hydrolytic activity, high contents of nitrogen, labile phosphorus, and exchangeable potassium. Properties of soil: pH 7.2; humus, 10.7%; hydrolytic acidity, 0.75 mmol/100 g; cation exchange capacity, 71.8 mmol/100 g; nitrate nitrogen, 16.0 mg/kg; exchangeable potassium, calcium, and magnesium, 110.7, 27.2, and 4.3 mg/kg. The soil was collected from the plot that had not been treated with pesticides, including metribuzin and tribenuron methyl. Thus, the plants grown in the experimental systems could not have developed resistance to these herbicides, and the effect of the herbicides was their true biological effect.
Plant seeds were introduced into the soil simultaneously with the embedded herbicides to a depth of 1.5–2.0 cm; the granules were packed into bags made of fine-meshed gauze (the number of granules per container was varied, which made it possible to set different concentrations of the herbicides in the formulations and their subsequent accumulations in the soil). In the laboratory system with the free forms, the soil was treated with the herbicides in similar concentrations according to the recommended application rates;23 the application rates of MET and TBM were 400 g/ha and 20 g/ha, respectively. The necessary concentrations of MET or TBM embedded in the matrix were achieved by varying the amounts of the granules buried in the soil simultaneously with seeds. All procedures were performed in triplicate.
Plants were grown in climate chambers (Fitotron-LiA-2, Russia). The temperature, lighting, and soil moisture content were controlled in the six-step mode: “night–early morning–late morning–early afternoon–late afternoon–evening”. The temperature was varied between 10 °C by night and 18 °C by day in the first seven weeks of the experiment and between 14 °C by night and 22 °C by day in the following five weeks. Lighting was varied between 0 and 300 μmol/m2/s in 100 μmol/m2/s increments. The lowest soil moisture content was 50%.
4.6. Degradation of Herbicide Formulations in Soil
Biodegradation of the experimental herbicide formulations was studied as follows. Three samples of each type were collected periodically to investigate biodegradation of the polymeric matrix and herbicide release. Mass loss was measured to estimate degradation. Three specimens of each type were periodically taken out of the soil, rinsed with distilled water, dried to a constant weight, and weighed on the analytical balance of accuracy class 4 (Metler, Toledo). The mass (X) was determined as follows:
where Y1 and Y2 denote the average mass of the samples (mg) before and after testing, respectively.
4.7. Herbicide Release Systems
Herbicides were extracted from soil with chloroform; then, chloroform was removed, and herbicide concentrations in soil were determined by chromatography. A 20 g soil sample was extracted with 100 mL of chloroform while agitating it on a shaker for 24 h at a temperature of 25 °C. A volume of 50 mL of the resulting extract was concentrated using a rotary evaporator, and the residue was re-dissolved in 2 mL of acetonitrile.
Herbicide concentrations were analyzed using high-performance liquid chromatography with an Agilent 1200 Infinity chromatographic system (Agilent Technologies, U.S.A.) equipped with a gradient pump, autosampler, a column thermostat, and a diode matrix detector. An Eclipse XDB-C18 column was used. Chromatography was performed in the gradient of water acidified with 0.1% acetic acid and acetonitrile with the acetonitrile concentration gradually increasing from 45 to 60%. Chromatography lasted 12 min. Detection was done at the wavelengths located at the absorption maxima of the herbicides: at 298 nm for metribuzin and at 220 nm for tribenuron methyl. The reference values were recorded at a wavelength of 360 nm. The range of the concentrations detected was between 1 and 500 μg/mL or higher. Calibration curves were plotted by using high-purity compounds: state standard reference samples (GSO) of metribuzin (GSO 7713-99) and tribenuron methyl (GSO 8628-2004). Calibration solutions of the active ingredients of concentrations of 0.1, 1, 10, 100, 500, and 1000 μg/mL were used.
4.8. Evaluation of the Efficacy of the Experimental MET and TBM Formulations
The physiological effects of herbicides on weeds and crops were studied and the efficacies of the free and embedded pesticides were compared in the laboratory using a wide range of target and non-target plants in experiments of different complexities and durations (28 to 84 days).
Indicators of the condition of the crops were observed by visual inspection and periodic digital photo documentation. The parameters determined in the weed systems and weed-infested crop stands were the weed plant density (the number of plants per 1 m2) and mass and the time when the majority of weed plants began to die.
The biological efficacy of the embedded herbicides was estimated using the corrected percent mortality, Cispr, derived from a modified Abbot’s formula, which shows the decrease in the number of weeds caused by application of herbicides (% of the initial infestation or the control) corrected for the control.
The growth and development of crops were evaluated by periodic measurements of the height of the plants and the aboveground biomass in different phases of crop development.
4.9. Statistics
Statistical analysis of the results was performed by conventional methods using the standard software package of Microsoft Excel, STATISTICA 8. Arithmetic means and standard deviations were found. The statistical significance of results was determined using a Student’s t-test (significance level: P ≤ 0.05). Statistical analysis of surface properties of the samples was performed by using embedded methods of DSA-4 software.
Acknowledgments
This study was financially supported by Project “Agropreparations of the New Generation: A Strategy of Construction and Realization” (agreement no. 074-02-2018-328) in accordance with Resolution No. 220 of the Government of the Russian Federation of April 9, 2010 “on measures designed to attract leading scientists to the Russian institutions of higher learning”.
The authors declare no competing financial interest.
References
- Hansen L. J.; Schwacke L. H.; Mitchum G. B.; Hohn A. A.; Wells R. S.; Zolman E. S.; Fair P. A. Geographic variation in polychorinated biphenyl and organochlorine pesticide concentrations in the blubber of bottlenose dolphins from the US Atlantic coast. Sci. Total Environ. 2004, 319, 147–172. 10.1016/S0048-9697(03)00371-1. [DOI] [PubMed] [Google Scholar]
- Fernández-Urrusuno R.; Gines J. M.; Morillo E. Development of controlled release formulations of alachlor in ethylcellulose. J. Microencapsulation 2000, 17, 331–342. 10.1080/026520400288300. [DOI] [PubMed] [Google Scholar]
- Sopeña F.; Cabrera A.; Maqueda C.; Morillo E. Controlled release of the herbicide norflurazon into water from ethylcellulose formulations. J. Agric. Food Chem. 2005, 53, 3540–3547. 10.1021/jf048007d. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Bhanjana G.; Sharma A.; Sidhu M. C.; Dilbaghi N. Synthesis, characterization and on field evaluation of pesticide loaded sodium alginate nanoparticles. Carbohydr. Polym. 2014, 101, 1061–1067. 10.1016/j.carbpol.2013.10.025. [DOI] [PubMed] [Google Scholar]
- Chen G. Q. New challenges and opportunities for industrial biotechnology. Microb. Cell Fact. 2012, 11, 111. 10.1186/1475-2859-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller M.; Maršálek L.; de Sousa Dias M. M.; Braunegg G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. 10.1016/j.nbt.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Volova T.G.; Shishatskaya E.I.; Sinskey A.J.. Degradable polymers: Production, properties, applications; Nova Science Pub. Inc.: New York, 2013, 389 p. [Google Scholar]
- Volova T.G.; Vinnik Yu.S.; Shishatskaya E.I.; Markelova N.M.; Zaikov G.E.. Natural-based polymers for biomedical applications; CRC Press: Toronto, 2017, 460 p. [Google Scholar]
- Volova T.G.; Shishatskaya E.I.; Prudnikova S.V.; Zhila N.O.; Boyandin A.N.. New generation formulations of agrochemicals: Current trends and future priorities; 4th ed.; Appl. Acad. Press: Toronto, 2020, 286 p. [Google Scholar]
- Savenkova L.; Gercberga Z.; Muter O.; Nikolaeva V.; Dzene A.; Tupureina V. PHB-based films as matrices for pesticides. Process Biochem. 2002, 37, 719–722. 10.1016/S0032-9592(01)00263-1. [DOI] [Google Scholar]
- Volova T. G.; Voinova O. N.; Kalacheva G. S.; Grodnitskaya I. D. The prospects of the use of resorbable polyesters for designing safe pesticides. Dokl. Biol. Sci. 2008, 419, 100–103. 10.1134/S0012496608020099. [DOI] [PubMed] [Google Scholar]
- Voinova O. N.; Kalacheva G. S.; Grodnitskaya I. D.; Volova T. G. Microbial polymers as a degradable carrier for pesticide delivery. Appl. Biochem. Microbiol. 2009, 45, 384–388. 10.1134/S0003683809040061. [DOI] [PubMed] [Google Scholar]
- Suave J.; Dall’Agnol E. C.; Pezzin A. P. T.; Meier M. M.; Silva D. A. K. Biodegradable microspheres of poly(3-hydroxybutyrate)/poly(e-caprolactone) loaded with malathion pesticide: Preparation, characterization, and in vitro controlled release testing. J. Appl. Polym. Sci. 2010, 117, 3419–3427. 10.1002/app.32082. [DOI] [Google Scholar]
- Lobo F. A.; de Aguirre C. L.; Silva M. S.; Grillo R.; de Melo N. F. S.; de Oliveira L. K.; de Morais L. C.; Campos V.; Rosa A. H.; Fraceto L. F. Poly(hydroxybutyrate-co-hydroxyvalerate) microspheres loaded with atrazine herbicide: Screening of conditions or preparation, physicchemical characterization, and in vitro release studies. Polym. Bull. 2011, 67, 479–495. 10.1007/s00289-011-0447-6. [DOI] [Google Scholar]
- Grillo R.; Pereira A. E. S.; Melo N. F. S.; Porto R. M.; Feitosa L. O.; Tonello P. S.; Filho N. L. D.; Rosa A. H.; Lima R.; Fraceto L. F. Controlled release system or ametryn using polymer microspheres: Preparation, characterization and release kinetics in water. J. Hazard. Mater. 2011, 186, 1645–1651. 10.1016/j.jhazmat.2010.12.044. [DOI] [PubMed] [Google Scholar]
- Prudnikova S. V.; Boyandin A. N.; Kalacheva G. S.; Sinskey A. J. Degradable polyhydroxyalkanoates as herbicide carriers. J. Polym. Environ. 2013, 21, 675–682. 10.1007/s10924-012-0561-z. [DOI] [Google Scholar]
- Cao L.; Liu Y.; Xu C.; Zhou Z.; Zhao P.; Niu S.; Huang Q. Biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate) microcapsules for controlled release of trifluralin with improved photostability and herbicidal activity. Mater. Sci. Eng. C 2019, 102, 134–141. 10.1016/j.msec.2019.04.050. [DOI] [PubMed] [Google Scholar]
- Volova T.; Zhila N.; Kiselev E.; Prudnikova S.; Vinogradova O.; Nikolaeva E.; Shumilova A.; Shershneva A.; Shishatskaya E. Poly(3-hydroxybutyrate)/metribuzin formulations: Characterization,controlled release properties, herbicidal activity, and effect on soil microorganisms. Environ. Sci. Pollut. Res. 2016, 23, 23936–23950. 10.1007/s11356-016-7636-7. [DOI] [PubMed] [Google Scholar]
- Kourmentza C.; Plácido J.; Venetsaneas N.; Burniol-Figols A.; Varrone C.; Gavala H. N.; Reis M. A. M. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. 10.3390/bioengineering4020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrahi R.; Fathi Z.; Özgür M.; Seydibeyoǧlu M. O.; Doustkhah E.; Khataee A. Polyhydroxyalkanoates (PHA): From production to nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619. 10.1016/j.ijbiomac.2019.12.181. [DOI] [PubMed] [Google Scholar]
- Thomas S.; Shumilova A. A.; Kiselev E. G.; Baranovsky S. V.; Vasiliev A. D.; Nemtsev I. V.; Kuzmin A. P.; Sukovatyi A. G.; Avinash R. P.; Volova T. G. Thermal, mechanical and biodegradation studies of biofiller based poly-3-hydroxybutyrate biocomposites. Int. J. Biol. Macromol. 2020, 155, 1373–1384. 10.1016/j.ijbiomac.2019.11.112. [DOI] [PubMed] [Google Scholar]
- Volova T.; Prudnikova S.; Boyandin A.; Zhila N.; Kiselev E.; Shumilova A.; Baranovskiy S.; Demidenko A.; Shishatskaya E.; Thomas S. Constructing slow-release fungicide formulations based on poly(3-hydroxybutyrate) and natural materials as a degradable matrix. J. Agric. Food Chem. 2019, 67, 9220–9231. 10.1021/acs.jafc.9b01634. [DOI] [PubMed] [Google Scholar]
- Rakitsky V.N.Handbook of Pesticides (Toxicological-Hygienic Characterization); 4th. ed.; Agrorus Publishers: Moscow, 2011. [Google Scholar]
- Tagour R. M. H.; Mosaad I. S. M. Effect of the foliar enrichment and herbicides on maize and associated weeds irrigated with drainage water. Ann. Agric. Sci. 2017, 62, 183–192. 10.1016/j.aoas.2017.11.004. [DOI] [Google Scholar]
- Medd R. W.; Van De Ven R. J.; Pickering D. I.; Nordblom T. Determination of environment-specific dose response relationships for clodinafop-propargyl on Avena spp. Weed Res. 2001, 41, 351–368. 10.1046/j.1365-3180.2001.00243.x. [DOI] [Google Scholar]
- Riethmuller-Haage I.; Bastiaans L.; Kropff M. J.; Harbinson J.; Kempenaar C. Can photosynthesis-related parameters be used to establish the activity of acetolactate synthase inhibiting herbicides on weeds?. Weed Sci. 2006, 54, 974–982. 10.1614/WS-06-010.1. [DOI] [Google Scholar]
- Pereira A. E. S.; Grillo R.; Melo N. F. S.; Rosa A. H.; Fraceto L. F. Application of poly(epsilon-caprolactone) nanoparticles containing atrazine herbicide as an alternative technique to control weeds and reduce damage to the environment. J. Hazard. Mater. 2014, 268, 207–215. 10.1016/j.jhazmat.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Gherekhloo J.; Hatami Z. M.; Alcántara-de la Cruz R.; Sadeghipour H. R.; De Prado R. Continuous use of tribenuron-methyl selected for cross-resistance to acetolactate synthase-inhibiting herbicides in wild mustard (Sinapis arvensis). Weed Sci. 2018, 66, 424–432. 10.1017/wsc.2018.23. [DOI] [Google Scholar]
- Kumar S.; Bhanjana G.; Sharma A.; Dilbaghi N.; Sidhu M. C.; Kim K. H. Development of nanoformulation approaches for the control of weeds. Sci. Total Environ. 2017, 586, 1272–1278. 10.1016/j.scitotenv.2017.02.138. [DOI] [PubMed] [Google Scholar]
- Qi Y.; Li J.; Fu G.; Zhao C.; Guan X.; Yan B.; Ren M. Effects of sublethal herbicides on offspring germination and seedling growth: Redroot pigweed (Amaranthus retroflexus) vs. velvetleaf (Abutilon theophrasti). Sci. Total Environ. 2018, 645, 543–549. 10.1016/j.scitotenv.2018.07.171. [DOI] [PubMed] [Google Scholar]
- Vieira B. C.; Luck J. D.; Amundsen K. L.; Gaines T. A.; Werle R.; Kruger G. R. Response of Amaranthus spp. following exposure to sublethal herbicide rates via spray particle drift. PLoS One 2019, 14, e0220014 10.1371/journal.pone.0220014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhila N.; Murueva A.; Shershneva E.; Shishatskaya E.; Volova T. Herbicidal activity of slow-release herbicide formulations in wheat stands infested by weeds. J. Environ. Sci. Health, Part B. 2017, 52, 729–735. 10.1080/03601234.2017.1356668. [DOI] [PubMed] [Google Scholar]
- Alebrahim M. T.; Majda R.; Rashed Mohassel M. H.; Wilcockson S.; Baghestani M. A.; Ghorban R.; Kudsk P. Evaluating the efficacy of pre- and post-emergence herbicides for controlling Amaranthus retroflexus L. and Chenopodium album L. in potato. Crop Prot. 2012, 42, 345–350. 10.1016/j.cropro.2012.06.004. [DOI] [Google Scholar]
- Kumar J.; Nisar K.; Shakil N. A.; Sharma R. Residue and bio-efficacy evaluation of controlled release formulations of metribuzin against weeds in wheat. Bull. Environ. Contam. Toxicol. 2010, 85, 357–361. 10.1007/s00128-010-0091-0. [DOI] [PubMed] [Google Scholar]