Abstract
Fusicoccin A (FC) is a fungal phytotoxin that stabilizes protein–protein interactions (PPIs) between 14-3-3 adapter proteins and their phosphoprotein interaction partners. Recently, FC has emerged as an important chemical probe of human 14-3-3 PPIs involved in cancer and neurobiology. These previous studies have established the structural requirements for FC-induced stabilization of 14-3-3·client phosphoprotein complexes; however, the effect of 14-3-3 isoforms on FC activity remains underexplored. This is a relevant question for the continued development of FC variants because there are seven isoforms of 14-3-3 in humans. Despite their sequence and structural similarities, a growing body of experimental evidence supports both tissue-specific expression of 14-3-3 isoforms and isoform-specific functions in vivo. Herein, we interrogate the isoform-specificity profile of FC in vitro using recombinant 14-3-3 isoforms and a library of fluorescein-labeled hexaphosphopeptides mimicking the C-terminal recognition domains of client proteins that are characterized targets of FC in vivo. Our results reveal modest isoform preferences for individual client phospholigands and demonstrate that FC differentially stabilizes PPIs involving 14-3-3σ. Together, these data support the feasibility of developing FC variants with enhanced isoform selectivity.
Introduction
14-3-3 proteins are a family of phospho-binding adapter molecules that form protein–protein interactions (PPIs) with hundreds of different client proteins.1,2 This large interactome is integrated with post-translational modifications to generate a dynamic signaling hub.3,4 At the molecular level, 14-3-3 PPIs are directed by phosphorylation of client proteins at serine or threonine residues within the consensus sequence RXXpZXP (X = any residue, pZ = phosphorylated S or T), although other recognition sequences exist.5 Once formed, this motif locates to an amphipathic groove on the surface of 14-3-3. The effect of 14-3-3 binding is dependent on the nature of the client and serves to finalize signal-induced events. Interactions with 14-3-3 proteins are known to modify protein trafficking or block interaction sites for effector proteins.6 In other settings, 14-3-3 proteins constrain binding partners into atypical conformations or serve as scaffolding to bring two clients proteins together.7,8 Despite this functional diversity, 14-3-3 PPIs have emerged as potential drug targets for cancer and neurological diseases.9,10 As such, the need for small-molecules to dissect the roles of individual 14-3-3 PPIs has become increasingly apparent.11
Fusicoccin A (FC) provides the best existing entry point to selective 14-3-3 PPI modulators (Figure 1).12 This phytotoxin is a well-characterized stabilizer of 14-3-3 PPIs in plants.13,14 More recently, FC has gained attention for its pro-apoptotic and neuroprotective properties in mammalian cell culture.15,16 Human 14-3-3 proteins are central in this pharmacology, as evidenced by studies connecting FC-induced stabilization of 14-3-3 PPIs in vitro to altered client function in vivo.17−23 These investigations have established that FC is incompatible with the prototypical RXXpZXP motif.22 Instead, FC selectively enhances contacts between 14-3-3 proteins and clients with a C-terminal recognition sequence (XpZBCOOH).24 The identity of the C-terminal residue within this atypical motif is critical for FC activity,25 and we and others have demonstrated that small residues (B = V, L, I, A, T or S) are required at this position.26,27
Figure 1.
Fusicoccin A (FC) is a phytotoxin produced by the fungus Fusicoccum amygdali. This cell-permeable diterpene glycoside stabilizes 14-3-3 PPIs utilizing a C-terminal recognition motif (X = any residue; pZ = phosphorylated S or T; B = V, L, I, A, T, or S) for molecular recognition.
While the scope of client proteins that can be targeted by FC is understood, the effect of different 14-3-3 isoforms on FC activity remains unclear. There are seven 14-3-3 isoforms in humans (α, β, ε, ζ, τ, η, and γ), each expressed by a single gene. Detailed structural analyses of these proteins show that the amino acid sequences and overall structures are highly conserved.1,6 This similarity is reflected in the ability of one isoform to compensate for loss of another.28 Nevertheless, as outlined in Table 1, there is convincing experimental evidence for both tissue-specific distribution of isoforms and isoform-specific functions.29−38 The extent to which these differences contribute to the pharmacology of FC is unknown. Therefore, as part of a program to develop FC variants with enhanced selectivity profiles, we analyzed the isoform-specificity profile of FC in vitro. To accomplish this objective, we focused on interactions between recombinant human 14-3-3 isoforms and a focused library of synthetic phosphopeptides mimicking the C-terminal recognition motifs of 14-3-3 client proteins that are known targets of FC in vivo. These experiments revealed small differences in FC activity across 14-3-3 isoforms, which were most notable for 14-3-3σ.
Table 1. Summary of Tissue Distribution and Isoform-Specific Functions of Human 14-3-3 Isoforms.
| isoform (gene) | tissue localization | isoform-specific roles in disease | references |
|---|---|---|---|
| σ (SFN) | lung, breast, uterus, ovary, blood, skin, liver, pancreas, cornea | •epigenetically suppressed in epithelial carcinomas | (9, 28, 31) |
| •functions as a tumor suppressor | |||
| β (YWHAB) | brain, lung, colon, gastric lining, liver, bladder, kidney | •overexpressed in squamous cell carcinoma | (28, 35) |
| ε (YWHAE) | brain (hippocampus), renal, liver, breast, gastric lining | •YWHAE deleted in Miller–Dieker syndrome | (10, 36) |
| •found in Lewy bodies from Parkinson’s patients | |||
| ζ (YWHAZ) | brain, breast, lung, colon, head and neck, oral, ovary, esophagus | •overexpressed in cancer and correlates with poor prognosis | (28, 32, 33) |
| •found in NTFs from Alzheimer’s patents; binds to Tau | |||
| τ (YWAHQ) | brain (frontal cortex), breast, lung, prostate | •overexpression protects from dopaminergic cell loss | (10, 34) |
| •diminished expression in Alzheimer’s patients | |||
| η (YWHAH) | brain (frontal cortex), liver, lung, prostate | •binding to α-synuclein disrupted in Parkinson’s disease | (10, 34) |
| •diminished expression in Alzheimer’s patients | |||
| γ (YWHAG) | brain, breast, liver, lung | •correlated with amyotrophic lateral sclerosis (ALS) | (10, 28, 37) |
| •overexpressed in lung cancer; p53 reduces 14-3-3γ mRNA |
Results and Discussion
Design
The ability of FC to differentially stabilize contacts between 14-3-3 proteins and C-terminal phospholigands has been reported.24 However, an explicit analysis of FC activity across human 14-3-3 isoforms has not been described. Thus, we set out to characterize the isoform-specificity profile of FC in vitro using recombinant 14-3-3 isoforms and established fluorescence polarization (FP) assays.22,26 An analysis of 14-3-3 sequences revealed 69–88% homology between isoforms, which each exhibit different subcellular localization patterns.39 Five isoforms are abundant in the cytosol (β, ε, ζ, σ, and τ). Conversely, 14-3-3γ is predominantly found in the nucleus and 14-3-3η is localized in mitochondria. Thus, we focused on the dominant cytosolic 14-3-3 isoforms because we expect FC to be most effective at targeting cytosolic PPIs in vivo.
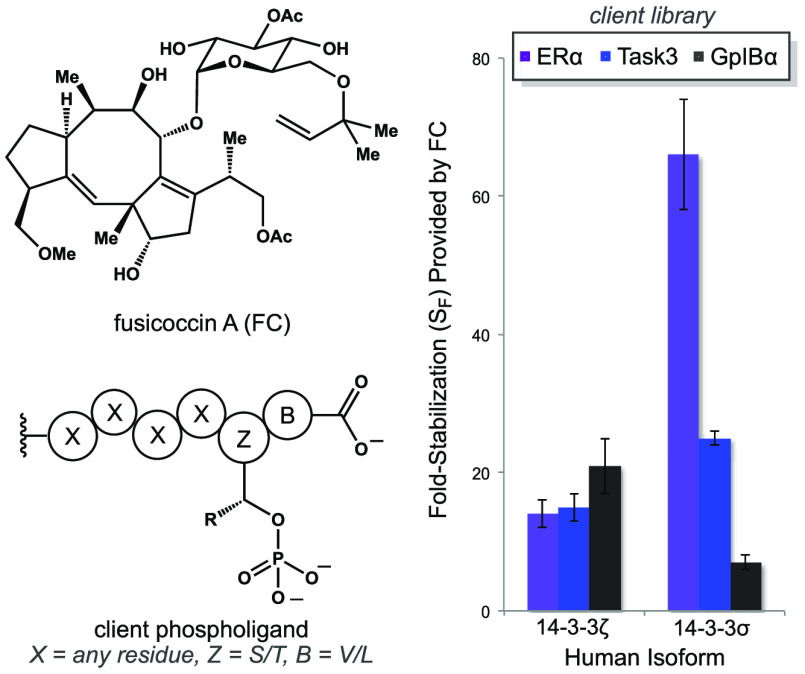
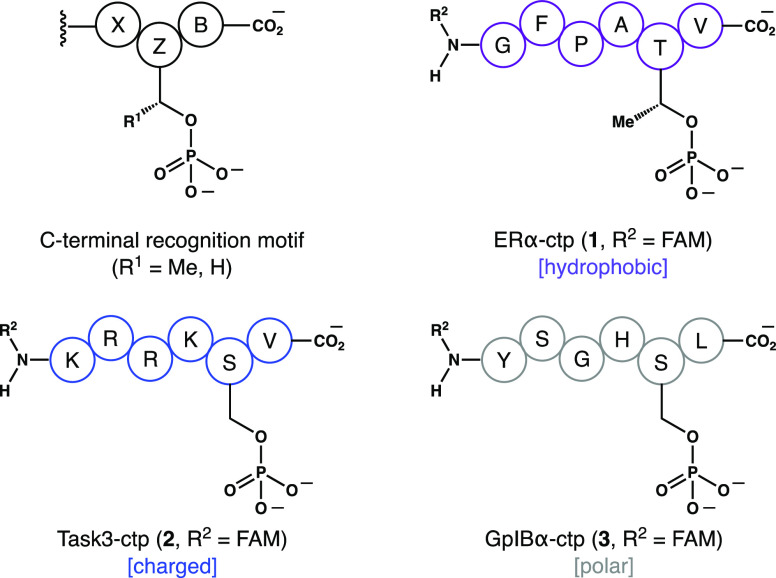
The interaction profile of FC is broad, and therefore, the choice of client protein targets was carefully considered. There are at least 119 proteins in humans with a potential C-terminal recognition motif.26 In addition, certain noncanonical 14-3-3 PPIs are compatible with FC.18,19 Thus, to address the question of 14-3-3 isoform specificity, we focused on clients that are well-characterized targets of FC in vivo. These include the transcription factor ERα,21 the potassium ion channel Task3,22 and the platelet adhesion receptor GpIBα.23 As shown in Figure 2, we used synthetic N-fluorescein-tagged, C-terminal hexaphosphopeptides (ctp) that recapitulate the functional 14-3-3 recognition domains of these clients. Importantly, this phospholigand library was also variable with regard to relative hydrophobicity, number of charged residues, identity of the phosphorylated side chain (pZ = S or T), and identity of the C-terminal residue (B = V or L).
Figure 2.
N-Fluorescein-labeled hexaphosphopeptides used as 14-3-3 client phospholigands in this study.
Structural Analysis
We began with a detailed analysis of the structural data for FC in complex with 14-3-3σ and the C-terminal motif of ERα (Protein Data Bank [PDB] ID: 4JDD, 2.1 Å resolution).21 The overall structures of 14-3-3 isoforms have been reviewed, and thus, only a summary of the relevant FC interactions are given here.5,6 The monomeric unit of 14-3-3σ comprised a bundle of nine α-helices (αA to αI). As shown in Figure 3A, both the ERα phosphopeptide (i.e., 1, R2 = H) and FC locate to the amphiphilic 14-3-3 phospho-binding groove (green), which comprised α-helices αC, αE, αG and αI. The phosphopeptide partially fills this channel and is held in place via contacts between the phosphothreonine and a triad of polar residues (i.e., R56, R129, and Y130) of 14-3-3σ that form a conserved phospho-binding pocket. Moving up the binding groove, hydrogen bonds (2.5–2.9 Å) formed between the phospholigand and K49 and N175 of 14-3-3σ are apparent. These 14-3-3σ/ERα contacts are expected to be conserved for all C-terminal phospholigand binding to 14-3-3σ. Importantly, FC locates to a hydrophobic surface created by the union of 14-3-3σ and the phospholigand. Binding of the natural product is supported by simultaneous hydrophobic contacts with 14-3-3σ and ERα.11 The C-terminal residue of ERα forms a critical hydrophobic contact with the 5-8-5 carbotricycle of FC.26 In contrast, interactions between FC and 14-3-3σ are limited to the convex periphery of the natural product. These include three hydrogen bonds: (1) K122 of 14-3-3σ and the C3 alkyl group on FC; (2) D215 of 14-3-3σ and the C8 alcohol of FC; and (C) a water-mediated hydrogen bond formed between N42 of 14-3-3σ and the C9 glycoside of FC.
Figure 3.

Structural and sequence analysis of the 14-3-3σ·ERα·FC ternary complex (PDB 4JDD). (a) Renderings of the ternary complex showing the 14-3-3 phospholigand-binding groove (green) and contacts between 14-3-3σ and both FC and ERα. (b) Sequence homology analysis of the phospholigand-binding groove across all human isoforms. Fully conserved residues are shown in blue.
Having identified the key contacts in the 14-3-3σ·ERα·FC ternary complex, we carried out a multiple sequence alignment (Figure S1) to assay sequence homology across human 14-3-3 isoforms. As shown in Figure 3B, we found that residues within the phospholigand-binding grove are almost identical between isoforms. This similarity in 14-3-3 contrasts with the diversity of C-terminal client protein sequences found to be compatible with FC.26 Nevertheless, the residues of 14-3-3σ contacting FC (including D122, D215, and N42) and the client phosphopeptide (including R56, R129, Y130, K49, and N175) are strictly conserved across all of the human isoforms.
Analyzing Dose–Response (EC50) Across 14-3-3 Isoforms
To evaluate the extent to which sequence homology within the 14-3-3 phospho-binding groove correlates with similarities in FC activity, we determined EC50 values for FC across the primary cytosolic 14-3-3 isoforms (i.e., β, ε, ζ, σ, and τ) using the suite of client phospholigands shown in Figure 2. As expected, each PPI in this series afforded a dose–response curve that allowed us to extract relative EC50 values for FC (Table 2).40 With both ERα-ctp (1) and Task3-ctp (2) as clients, the EC50 values for FC were similar across all isoforms, demonstrating a lack of isoform-specific association of FC with these binary protein complexes. In contrast, using the GpIBα phospholigand (3), we observed more significant isoform-specific differences in EC50 values for FC. The lowest EC50 value, 4.2 ± 1.1 μM, was obtained with 14-3-3σ. This measurement was consistent with EC50 values determined using 14-3-3σ and clients 1 (3.6 ± 1.1 μM) and 2 (3.2 ± 1.1 μM). Conversely, the highest EC50 value, 78 ± 1.1 μM, was obtained with 14-3-3ε. This approximately 18-fold difference lies well outside the standard deviation of our measurements and demonstrates that, for some clients, FC exhibits clear isoform-specific activity.
Table 2. Compiled EC50 Values (μM) for FC Across the Dominant Cytosolic Human 14-3-3 Isoformsa,b.
| 14-3-3 isoform | ERα-ctp (1) | Task3-ctp (2) | GpIBα-ctp (3) |
|---|---|---|---|
| β | 1.8 ± 1.1 | 3.0 ± 1.1 | 20 ± 1.2c |
| ε | 2.8 ± 1.1 | 1.3 ± 1.1 | 78 ± 1.3c |
| ζ | 3.5 ± 1.2 | 2.1 ± 1.1 | 7.6 ± 1.1 |
| σ | 3.6 ± 1.1 | 3.2 ± 1.1 | 4.2 ± 1.1 |
| τ | 2.8 ± 1.1 | 2.2 ± 1.1 | 38 ± 1.2c |
Reported EC50 values represent the average of two independent experiments.
The [FC] was varied from 48 nM to 100 μM; [phospholigand] = 100 nM; [14-3-3] = 600 nM.
Values represent the upper limit of the EC50.
Trends in Affinities of 14-3-3·Phospholigand Complexes
Having established that FC exhibits isoform-specific activity, we investigated the extent to which the affinity of individual phospholigands varies across 14-3-3 isoforms in the absence of FC. Thus, phospholigands 1–3 were titrated with 14-3-3 isoforms to establish the intrinsic affinity (apparent Kd) of each protein/peptide complex. As shown in Table 3, the results of these experiments revealed clear differences in 14-3-3 isoform specificity for individual clients. Titrations with phospholigand 1 showed that 14-3-3β had the strongest affinity (apparent Kd = 0.72 ± 0.1 μM), whereas the same interaction using 14-3-3σ was comparatively weaker (apparent Kd = 6.6 ± 0.6 μM). This represents an approximately 9-fold preference between the two isoforms. On the other hand, with phospholigand 2, 14-3-3ζ gave the highest affinity (apparent Kd = 1.3 ± 0.1 μM), whereas 14-3-3ε showed the lowest affinity (apparent Kd = 6.0 ± 0.4 μM). Although this 4.6-fold difference in isoform preference is smaller than observed with phospholigand 1, it lies outside of the standard deviation of our measurements. Finally, for phospholigand 3, we observed that 14-3-3β exhibited the highest affinity (apparent Kd = 18 ± 3 μM), whereas 14-3-3σ showed the lowest affinity (apparent Kd = 47 ± 6 μM). It should be noted, however, that the standard deviation of data collected using 3 was larger than with other phospholigands, presumably because this peptide binds to 14-3-3 weakly compared to 1 and 2. Consistent with the titrations shown in Table 1, these data support isoform-dependent interactions for certain clients.
Table 3. Intrinsic Affinity (Apparent Kd, μM) of 14-3-3·Phospholigand Complexesa,b.
| 14-3-3 isoform | ERα-ctp (1) | Task3-ctp (2) | GpIBα-ctp (3) |
|---|---|---|---|
| β | 0.72 ± 0.1 | 2.4 ± 0.2 | 18 ± 3 |
| ε | 3.4 ± 0.2 | 6.0 ± 0.4 | 28 ± 5 |
| ζ | 1.5 ± 0.2 | 1.3 ± 0.1 | 20 ± 2 |
| σ | 6.6 ± 0.6 | 2.1 ± 0.1 | 47 ± 6 |
| τ | 1.1 ± 0.1 | 2.2 ± 0.2 | 20 ± 3 |
Reported Kd (μM) values represent the average of two independent experiments.
The [14-3-3] was varied from 40 nM to 160 μM; [phospholigand] = 100 nM.
Stabilization of 14-3-3·Phospholigand Interactions with FC
To examine whether differences in the intrinsic affinities outlined in Table 3 were enhanced by FC, we repeated our titrations of phospholigands 1–3 with 14-3-3 isoforms in the presence of 80 μM FC. At this concentration, FC stabilized all combinations of client phosphopeptides and 14-3-3 isoforms tested (Figure 4). However, we observed notable changes in isoform-specific trends relative to the titrations carried out in the absence of FC. For example, for phospholigand 1, 14-3-3β showed the highest FC-stabilized affinity (apparent Kd = 0.05 ± 0.001 μM), whereas 14-3-3τ exhibited the weakest interaction (apparent Kd = 0.13 ± 0.004 μM). In this case, the difference between the best and worst performing isoform (2.6-fold) is notably less than observed for the same titrations carried out in the absence of FC (9.1-fold). In contrast, for phospholigand 2, 14-3-3σ and 14-3-3ζ exhibited the highest affinities (apparent Kd = 0.08 ± 0.002 and 0.08 ± 0.005 μM, respectively), whereas 14-3-3ε gave the lowest affinity (apparent Kd = 0.16 ± 0.020 μM). Again, the magnitude of the isoform specificity was reduced (2.0-fold) in the presence of FC in this series. A similar trend was observed for phospholigand 3; 14-3-3β showed the highest affinity (apparent Kd = 0.87 ± 0.2 μM) and 14-3-3ε had the lowest affinity (apparent Kd = 1.7 ± 0.2 μM), resulting in a 2.0-fold isoform preference.
Figure 4.
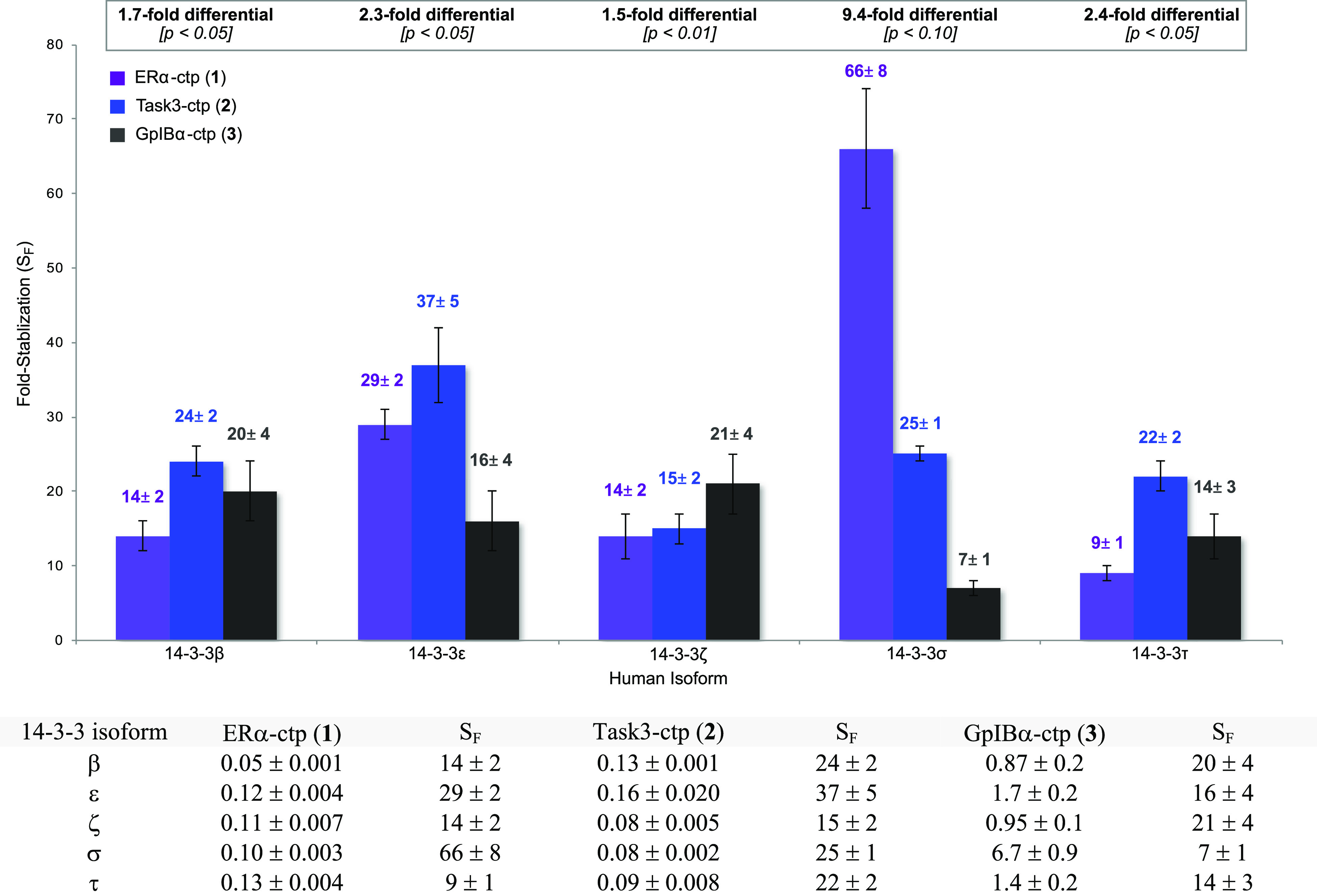
Stabilized affinity (apparent Kd, μM) and fold-stabilization (SF) of 14-3-3·phospholigand complexes in the presence of 80 μM FC. (a) Reported Kd (μM) values represent the average of two independent experiments carried out in the presence of FC. (b) Fold-stabilization (SF) was determined by dividing the intrinsic apparent Kd reported in Table 3 by the stabilized apparent Kd shown above. (c) The [14-3-3] was varied from 40 nM to 160 μM; [phospholigand] = 100 nM; [FC] = 80 μM.
When combined with the intrinsic affinities in Table 3, the stabilized affinities in Figure 4 provide a measure of the extent to which FC enhances each 14-3-3/client combination. The resulting fold-stabilization (SF) for each PPI is summarized by the graph in Figure 4. Analysis of these data revealed several trends. Considering individual phospholigands across 14-3-3 isoforms, we observed that for 1, FC provides 7.3-fold greater stabilization with 14-3-3σ versus 14-3-3τ. Conversely, for both phospholigands 2 and 3, FC exhibits a differential SF of ≤3-fold between the highest and lowest affinity isoforms. Analysis of FC activity on individual isoforms across the phospholigand library shows that 14-3-3σ was distinct. Viewed in this way, FC provided a 9.4-fold greater degree of stabilization (ΔSF) for the 14-3-3σ·ERα-ctp PPI (66 ± 8) than for the 14-3-3σ·GplBα-ctp PPI (7 ± 1). In contrast, we observed smaller changes in FC-induced stabilization across remaining 14-3-3/phospholigand combinations (ΔSF <2.5-fold). These differences are small, and thus, unlikely to impact the functional behavior of FC in a substantial way. Moreover, the magnitude of the isoform preferences observed herein is too small to allow us to establish their structural and/or dynamic origin. Such mechanistic investigations await future examination involving full-length client proteins, rather than model peptides, which could attenuate isoform selectivity. However, these data confirm isoform-dependent effects on FC activity, and they are clearly more substantial for 14-3-3σ than other isoforms evaluated in this study. Notably, the hydrophobic ERα sequence (1) was the most effectively stabilized by FC in complex with 14-3-3σ. Consequently, these trends should be considered in the future for design and biological evaluation of 14-3-3 PPI stabilizers based on the FC scaffold.
Conclusions
In summary, we examined the 14-3-3 isoform-specificity profile of fusicoccin A (FC), a natural product stabilizer of 14-3-3 functions in vivo. A sequence analysis of human 14-3-3 isoforms, along with inspection of available crystallographic data, demonstrated that the residues contacting both FC and client phospholigands bound in the 14-3-3 binding groove are strictly conserved across the different isoforms. Based on this observation, it was anticipated that FC-induced stabilization of 14-3-3·client phospholigand complexes would be very similar across the 14-3-3 isoforms. Nevertheless, isoform preferences were observed using a series of phospholigands mimicking the 14-3-3 interaction motifs of characterized FC targets in vivo. These isoform-dependent interactions were most notable in the absence of FC; however, our data suggest that FC-induced stabilization of PPIs involving 14-3-3σ depend on the nature of the client. This outcome is intriguing given that 14-3-3σ mediates cell death pathways and is suppressed in several cancers.31,41 It is also notable that the biological role(s) of 14-3-3σ are distinct relative to the other 14-3-3 family members (Table 1).1 Although the observed isoform specificity of FC was modest, this selectivity might be more significant in vivo, where full-length client proteins can make additional contacts with 14-3-3 and FC. Consequently, isoform specificity might be exploited via the rational design of non-natural FC variants provided the salient structure–activity relationships responsible for isoform selectivity can be identified.
Experimental Section
Materials
FC was purchased from Enzo Life Sciences and used directly. N-Fluorescein-labeled hexaphosphopeptides that mimic the reported C-terminal recognition motifs of ERα (1), Task3 (2) and GpIBα (3) were obtained from Peptide 2.0 in >99% purity and used as received. pET-22b(+) expression vectors used for recombinant protein expression were obtained from Genscript. All other chemicals and consumables were purchased and used as received.
Proteins
Cloning, expression, and purification of human 14-3-3β (UniProt ID P31946), 14-3-3ε (UniProt ID Q04917), 14-3-3ζ (UniProt ID P63104), and 14-3-3τ (UniProt ID P27348) were performed as previously described for 14-3-3σ (UniProt ID P31947).26 Protein concentrations were determined spectrophotometrically. The purity of recombinant protein was analyzed by SDS-PAGE (Figure S2).
Fluorescence Polarization (FP) Assays
FP measurements were performed using a filter-based microplate reader (BioTek Synergy H1MF) with a fluorescein filter set (λex = 485/20 nm, λem = 535/25 nm) and an integration time of 50 ms in black, flat-bottom 96 well plates (FLUOTRAC, medium binding). The measured polarization values were converted to anisotropy (A = 2P/3-P) and processed using GraphPad Prism.
For the determination of EC50 values, a solution of 100 nM fluorescein-labeled phospholigand and 600 nM 14-3-3 was titrated with FC in buffer A containing 10 mM HEPES (pH 6.5), 150 mM NaCl, 0.1% (v/v) Tween 20, and 0.1% BSA. To obtain relative EC50 values,40 the anisotropy signal (mA) was corrected for FC contribution in the absence of 14-3-3 (control) and plotted against logarithmic FC concentration.42 The reported EC50 values were obtained by fitting the data into a four-parameter logistic curve, abbreviated 4PL, using the equation Y = bottom + (top – bottom)/(1 + 10∧((log EC50 – X)*HS)). In this model, the EC50 value is the concentration that provokes a response half-way between the basal (bottom) response and the maximal (top) response. Y is the anisotropy signal, X is the concentration of FC on log scale, and HS is the Hill slope, which was constrained to a constant value of 1.
For the determination of apparent Kd values, a solution of 100 nM fluorescein-labeled phospholigand was titrated with 14-3-3 in buffer A in the presence and absence of 80 μM FC.43,44 The anisotropy signal was corrected for background signal at zero 14-3-3 concentration prior to fitting the data. The reported Kd values were determined, as previously described,45 by plotting fraction bound (q) as a function of 14-3-3 protein concentration. The value q was calculated from the equation q = (Y – Y0)/(ΔY), where Y is the fluorescence anisotropy signal at each 14-3-3 concentration, Y0 is the initial fluorescence anisotropy signal in the absence of 14-3-3, and ΔY is the change in fluorescence anisotropy signal. For titrations in the absence of FC and titrations performed with low-affinity ligand 3 in the presence of FC, data were fitted to the hyperbolic equation q = P0/(Kd + P0), where P0 is the total 14-3-3 concentration, using the assumption that [P0] ≈ [P]free, which is applicable for weak binding. For titrations performed with ligands 1 and 2 in the presence of FC, the hyperbolic assumption is invalid because the concentration of the constant species is comparable to the Kd value. Therefore, these data were fit to the quadratic equation q = [(P0 + L0 + Kd) – ((P0 + L0 + Kd)2 – 4P0L0)0.5]/(2L0), where P0 is the total 14-3-3 concentration and L0 is the total ligand concentration. We developed a python script to solve this equation, which is provided in the SI.
Data Analysis
Reported values for EC50 and Kd represent the average of two independent experiments, where individual measurements were collected in triplicate and averaged. The reported error represents standard deviation. Fold-stabilization (SF) was calculated by dividing the apparent Kd in the absence of FC by the Kd of a replicate experiment in the presence of 80 μM FC. The error was propagated using the standard equation for the division of measured quantities.
Acknowledgments
This investigation was funded by the National Institutes of Health (R01-GM125926 to J.H.F.; R01-GM133843 and R01-GM115388 to B.G.M.) and the Pfeiffer Professorship for Cancer Research (B.G.M.). We acknowledge B. Stefanovic (FSU) for access to his microplate reader.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01454.
SDS gel image of recombinant human 14-3-3 isoforms, processed FP data, and sequence alignment for 14-3-3 isoforms (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Pennington K. L.; Chan T. Y.; Torres M. P.; Andersen J. L. The dynamic and stress-adaptive signaling hub of 14-3-3: emerging mechanisms of regulation and context-dependent protein-protein interactions. Oncogene 2018, 37, 5587–5604. 10.1038/s41388-018-0348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.; Crowther S.; Stafford M. J.; Campbell D. G.; Toth R.; MacKintosh C. Bioinformatic and experimental survey of 14-3-3 binding sites. Biochem. J. 2010, 427, 69–78. 10.1042/BJ20091834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.; Thornton J. M. Principles of protein-protein interactions. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 13–20. 10.1073/pnas.93.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seet B. T.; Dikic I.; Zhou M. M.; Pawson T. Reading protein modifications with interaction domains. Nat. Rev. Mol. Cell Biol. 2006, 7, 473–483. 10.1038/nrm1960. [DOI] [PubMed] [Google Scholar]
- Yang X.; Lee W. H.; Sobott F.; Papgrigoriou E.; Robinson C. V.; Grossmann J. G.; Sundström M.; Doyle D. A.; Elkins J. M. Structural basis for protein-protein interactions in the 14-3-3 protein family. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 17237–17242. 10.1073/pnas.0605779103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardino A. K.; Smerdon S. J.; Yaffe M. B. Structural determinants of 14-3-3 binding specificities and regulation of subcellular localization of 14-3-3-ligand complexes: a comparison of the X-ray structures of all human 14-3-3 isoforms. Semin. Cancer Biol. 2006, 16, 173–182. 10.1016/j.semcancer.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Yaffe M. B. How do 14-3-3 proteins work? Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett. 2002, 513, 53–57. 10.1016/S0014-5793(01)03288-4. [DOI] [PubMed] [Google Scholar]
- Ottmann C.; Marco S.; Jaspert N.; Marcon C.; Schauer N.; Weyand M.; Vandermeeren C.; Duby G.; Boutry M. l.; Wittinghofer A.; Rigaud J.; Oecking C. Structure of a 14-3-3 coordinated hexamer of the plant plasma membrane H+-ATPase by combining X-ray crystallography and electron cryomicroscopy. Mol. Cell 2007, 25, 427–440. 10.1016/j.molcel.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Hermeking H. The 14-3-3 cancer connection. Nat. Rev. Cancer 2003, 3, 931–943. 10.1038/nrc1230. [DOI] [PubMed] [Google Scholar]
- Foote M.; Zhou Y. 14-3-3 proteins in neurological disorders. Int. J. Biochem. Mol. Biol. 2012, 3, 152–164. [PMC free article] [PubMed] [Google Scholar]
- Stevens L. M.; Sijbesma E.; Botta M.; MacKintosh C.; Obsil T.; Landrieu I.; Cau Y.; Wilson A. J.; Karawajczyk A.; Eickhoff J.; Davis J.; Hann M.; O’Mahony G.; Doveston R. G.; Brunsveld L.; Ottmann C. Modulators of 14-3-3 protein-protein interactions. J. Med. Chem. 2018, 61, 3755–3778. 10.1021/acs.jmedchem.7b00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N. C.; Graniti A. Fusicoccin: a fungal toxin that opens stomata. Nature 1969, 223, 1070–1071. 10.1038/2231070a0. [DOI] [Google Scholar]
- Fullone M. R.; Visconti S.; Marra M.; Fogliano V.; Aducci P. Fusicoccin effect on the in vitro interaction between plant 14-3-3 and plasma membrane H+ATPase. J. Biol. Chem. 1998, 273, 7698–7702. 10.1074/jbc.273.13.7698. [DOI] [PubMed] [Google Scholar]
- Würtele M.; Jelich-Ottmann C.; Wittinghofer A.; Oecking C. Structural view of a fungal phytotoxin acting on a 14-3-3 regulatory complex. EMBO J. 2003, 22, 987–994. 10.1093/emboj/cdg104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deVries-van Leeuwen I. J.; Kortekaas-Thijssen C.; Mandouckou J. A. N.; Kas S.; Evidente A.; de Boer A. H. Fusicoccin A selectively induces apoptosis in tumor cells after interferon-alpha priming. Cancer Lett. 2010, 293, 198–206. 10.1016/j.canlet.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Kaplan A.; Morquette B.; Kroner A.; Leong S.; Madwar C.; Sanz R.; Banerjee S. L.; Antel J.; Bisson N.; David S.; Fournier A. E. Small-molecule stabilization of 14-3-3 protein-protein interactions stimulates axon regeneration. Neuron 2017, 93, 1082–1093. 10.1016/j.neuron.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Andrei S. A.; de Vink P.; Sijbesma E.; Han L.; Brunsveld L.; Kato N.; Ottmann C.; Higuchi Y. Rationally designed semisynthetic natural product analogs for stabilization of 14-3-3 protein-protein interactions. Angew. Chem., Int. Ed. 2018, 57, 13470–13474. 10.1002/anie.201806584. [DOI] [PubMed] [Google Scholar]
- Stevers L. M.; Lam C. V.; Leysen S. F.; Meijer F. A.; van Scheppingen D. S.; de Vries R. M.; Carlile G. W.; Milroy L. G.; Thomas D. Y.; Brunsveld L.; Ottmann C. Characterization and small-molecule stabilization of the multisite tandem binding between 14-3-3 and the R domain of CFTR. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, E1152–E1161. 10.1073/pnas.1516631113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doveston R. G.; Kuusk A.; Andrei S. A.; Leysen S.; Cao Q.; Castaldi M. P.; Hendricks A.; Brunsveld L.; Chen H.; Boyd H.; Ottmann C. Small-molecule stabilization of the p53–14-3-3 protein-protein interaction. FEBS Lett. 2017, 591, 2449–2457. 10.1002/1873-3468.12723. [DOI] [PubMed] [Google Scholar]
- Scwarczynska M.; Molzan M.; Ottmann C. Activation of NF-kB signaling by fusicoccin-induced dimerization. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, E377–E386. 10.1073/pnas.1212990110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries-van Leeuwen I. J.; da Costa Pereira D.; Flach K. D.; Piersma S. R.; Haase C.; Bier D.; Yalcin X.; Michalides R.; Feenstra K. A.; Jimenez C. R.; de Greef T. F. A.; Brunsveld L.; Ottmann C.; Zwart W.; de Boer A. H. Interaction of 14-3-3 proteins with the estrogen receptor alpha F domain provides a drug target interface. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 8894–8899. 10.1073/pnas.1220809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders C.; Higuchi Y.; Koschinsky K.; Bartel M.; Schumacher B.; Thiel P.; Nitta H.; Preisig-Muller R.; Schlichthorl G.; Renigunta V.; Ohkanda J.; Daut J.; Kato N.; Ottmann C. A semisynthetic fusicoccane stabilizes a protein-protein interaction and enhances expression of K+ channels at the cell surface. Chem. Biol. 2013, 20, 583–593. 10.1016/j.chembiol.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Camoni L.; di Lucente C.; Visconti S.; Aducci P. The phytotoxin fusicoccin promotes platelet aggregation via 14-3-3-glycoprotein Ib-IX-V interaction. Biochem. J. 2011, 436, 429–436. 10.1042/BJ20102037. [DOI] [PubMed] [Google Scholar]
- Paiardini A.; Aducci P.; Cervoni L.; Cutruzzola F.; Di Lucente C.; Janson G.; Pascarella S.; Rinaldo S.; Visconti S.; Camoni L. The phytotoxin fusicoccin differentially regulates 14-3-3 proteins association to mode III targets. IUBMB Life 2014, 66, 52–62. 10.1002/iub.1239. [DOI] [PubMed] [Google Scholar]
- Ohkanda J.; Kusumoto A.; Punzalan L.; Masuda R.; Wang C.; Parvatkar P.; Akase D.; Aida M.; Uesugi M.; Higuchi Y.; Kato N. Structural effects of fusicoccin upon upregulation of 14-3-3 phospholigand interaction and cytotoxic activity. Chem. - Eur. J. 2018, 24, 16066–16071. 10.1002/chem.201804428. [DOI] [PubMed] [Google Scholar]
- Sengupta A.; Liriano J.; Miller B. G.; Frederich J. H; Analysis of interactions stabilized by fusicoccin A reveals an expanded suite of potential 14-3-3 binding partners. ACS Chem. Biol. 2020, 15, 305–310. 10.1021/acschembio.9b00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter M.; de Vink P.; Neves J. F.; Srdanovic S.; Higuchi Y.; Kato N.; Wilson A.; Landrieu I.; Brunsveld L.; Ottmann C. Selectivity via cooperativity: Preferential stabilization of the p65/14-3-3 interaction with semisynthetic natural products. J. Am. Chem. Soc. 2020, 142, 11772–11783. 10.1021/jacs.0c02151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwaelder S. M.; Darbousset R.; Cranmer S. L.; Ramshaw H. S.; Orive S. L.; Sturgeon S.; Yan Y.; Yao Y.; Krycer J. R.; Woodcock J.; Maclean J.; Pitson S.; Zheng X.; Henstridge D. C.; van der Wal D.; Gardiner E. E.; Berndt M. C.; Andrews R. K.; James D. E.; Lopez A. F.; Jackson S. P. 14-3-3ζ regulates the mitochondrial respiratory reserve linked to platelet phosphatidylserine exposure and procoagulant function. Nat. Commun. 2016, 7, 12862 10.1038/ncomms12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X.; Cui L.; Zeng Y.; Song W.; Gaur U.; Yang M. 14-3-3 proteins are on the crossroads of cancer, aging, and age-related neurodegenerative disease. Int. J. Mol. Sci. 2019, 20, 3518–3539. 10.3390/ijms20143518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W.; Liu X.; Qiao D.; Martinez J. D. Isoform-specific expression of 14-3-3 proteins in human lung cancer tissues. Int. J. Cancer 2005, 113, 359–363. 10.1002/ijc.20492. [DOI] [PubMed] [Google Scholar]
- Chandra S.; Fornai F.; Kwon H. B.; Yazdani U.; Atasoy D.; Liu X.; Hammer R. E.; Battaglia G.; German D. C.; Castillo P. E.; Sudhof T. C. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 14966–14971. 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. A.; Hermeking H.; Legauer C.; Kinzler K. W.; Vogelstein B. 14-3-3σ is required to prevent mitotic catastrophe after DNA damage. Nature 1999, 401, 616–620. 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- Neal C. L.; Xu J.; Li P.; Mori S.; Yang J.; Neal N. N.; Zhou X.; Wyszomierski S. L.; Yu D. Overexpression of 14-3-3ζ in cancer cells activates PI3K via binding the p85 regulatory subunit. Oncogene 2012, 31, 897–906. 10.1038/onc.2011.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Cao W.; Zhou J.; Zhang W.; Zhang X.; Lin W.; Fei Z.; Lin H.; Wang B. 14-3-3ζ positive expression is associated with a poor prognosis in patients with glioblastoma. Neurosurgery 2011, 68, 932–938. 10.1227/NEU.0b013e3182098c30. [DOI] [PubMed] [Google Scholar]
- Gu Q.; Cuevas E.; Raymick J.; Kanungo J.; Sarkar S. Downregulation of 14-3-3 proteins in Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 32–40. 10.1007/s12035-019-01754-y. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Nesland J. M.; Sou Z.; Trope C. G.; Holm R. The prognostic value of 14-3-3 isoforms in squamous cell carcinoma cases: 14-3-3β and ε are independent prognostic factors for these tumors. PLoS One 2011, 6, e24843 10.1371/journal.pone.0024843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S.; Chiba T.; Sakata E.; Kato K.; Mizuno Y.; Hattori N.; Tanaka K. 14-3-3η is a novel regulator of parkin ubiquitin ligase. EMBO J. 2006, 25, 211–221. 10.1038/sj.emboj.7600774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalice A.; Parisi P.; Nicita F.; Pizzardi G.; Del Balszo F.; Iannetti P. Neuronal migration disorders: clinical, neuroradiological and genetic aspects. Acta Paediatr. 2009, 98, 421–433. 10.1111/j.1651-2227.2008.01160.x. [DOI] [PubMed] [Google Scholar]
- Abdrabou A.; Brandwein D.; Wang Z. Differential subcellular distribution and translocation of seven 14-3-3 isoforms in response to EGF and during the cell cycle. Int. J. Mol. Sci. 2020, 21, e318 10.3390/ijms21010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebaugh J. L. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 2011, 10, 128–134. 10.1002/pst.426. [DOI] [PubMed] [Google Scholar]
- Li Z.; Liu J. Y.; Zhang J. T. 14-3-3 Sigma, the double-edged sword of human cancers. Am. J. Transl. Res. 2009, 1, 326–340. [PMC free article] [PubMed] [Google Scholar]
- The upper limit of FC concentration in buffer A was estimated to be 380 μM.
- Low FP signal (mP) was observed with lower concentration of FAM-phosphopeptides. The same 100 nM concentration was used in previous studies. See, references (22) and (26).
- A 14-3-3 protein concentration gradient of 40 nM to 160 μM was optimal. The FP signal (mP) at low concentrations of 14-3-3 was poor and often variable. Conversely, aggregation of 14-3-3 was observed at concentrations exceeding ∼200 μM in buffer A.
- Johnson K. A.Kinetic Analysis for the New Enzymology, 1st ed.; KinTek Corporation: Austin, TX, 2019; pp 65–85. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.