Abstract
In March 2020, a 74-year-old man affected by end-stage renal disease and on peritoneal dialysis was referred to an emergency room in Modena, Northern Italy, due to fever and respiratory symptoms. After ruling out COVID-19 infection, a diagnosis of chronic obstructive pulmonary disease exacerbation was confirmed and he was thus transferred to the nephrology division. Physical examination and blood tests revealed a positive fluid balance and insufficient correction of the uraemic syndrome, although peritoneal dialysis prescription was maximised. After discussion with the patient and his family, the staff decided to start hybrid dialysis, consisting of once-weekly in-hospital haemodialysis and home peritoneal dialysis for the remaining days. He was discharged at the end of the antibiotic course, after an internal jugular vein central venous catheter placement and the first haemodialysis session. This strategy allowed improvement of depuration parameters and avoidance of frequent access to the hospital, which is crucial in limiting exposure to SARS-CoV-2 in an endemic setting.
Keywords: dialysis, infectious diseases, TB and other respiratory infections
Background
A pandemic sustained by a novel coronavirus, SARS-CoV-2, rose between December 2019 and January 2020. Starting in Hubei Province in China, it soon spread worldwide.1 Italy has been one of the first non-Asian countries to be severely affected. Lockdown measures were quickly implemented, but the COVID-19 outbreak could not be avoided.2
In April 2020, Emilia Romagna was the second Italian region after Lombardia to have the most number of COVID-19 cases. According to the official report updated on 14 April 2020, the province of Modena (with a population of 708 346) accounted for 3180 (15.3%) of the 20 752 known regional cases.2 3
People affected by end-stage renal disease are among the most frail populations due to comorbidities and acquired immunodeficiency. Chronic kidney disease seems to be a significant risk factor for severe COVID-19 and therefore strategies to minimise exposure to SARS-CoV-2 are highly recommended.4 On the other hand, the need for renal replacement therapy (RRT) implies frequent access to hospital facilities for dialysis and outpatient follow-up, impeding domestic isolation.
Home dialysis (peritoneal and extracorporeal) could be the solution, but the necessary training for the patient and the caregiver, together with the technical issues (water and biological waste management, among others), makes the transition between RRTs5 difficult during an epidemic crisis.
Hybrid dialysis (HyD) is the combination of haemodialysis (HD) and peritoneal dialysis (PD).6–12 It is a promising yet underutilised strategy to optimise RRT efficiency and fluid balance and which could not only limit PD dropout rates but also reduce the number of hospital access, which is critical in a SARS-CoV-2 epidemic area.
We report a case of HyD implemented in a SARS-CoV-2 endemic area to avoid three times weekly in-hospital HD for a PD subject with inadequate uraemic syndrome correction.
The staff, the patient and the family’s concern was to ensure optimal RRT while limiting the risk of viral infection. HyD allowed to reach both goals, demonstrating better correction of uraemic syndrome while reducing in-hospital HD to just once weekly.
Case presentation
We present the case of a 74-year-old man living with his daughter (who incidentally is a nurse). He has a complex clinical history which includes nephroangiosclerotic end-stage renal disease (no renal biopsy was performed and diagnosis was based on clinical history) on continuous ambulatory peritoneal dialysis (CAPD) since February 2020 (Tenckhoff’s catheter placed on 28 January 2020), hypertension, chronic obstructive pulmonary disease (COPD) in active smoking, monoclonal gammopathy of uncertain significance and peripheral vasculopathy. His home prescription included diuretics (furosemide 75+75 mg, spironolactone 25 mg), bronchodilators (salmeterol/fluticasone), acetylsalicylic acid, vitamin D and calcium carbonate. He was referred to the emergency room on 15 March 2020 for fever (up to 38°C) lasting for 5 days and progressively worsening shortness of breath.
Investigations
On admission the patient’s arterial blood pressure was 120/70 mm Hg, heart rate was 88 beats per minute, respiratory frequency was 20 breaths per minute and body temperature was 37.8°C.
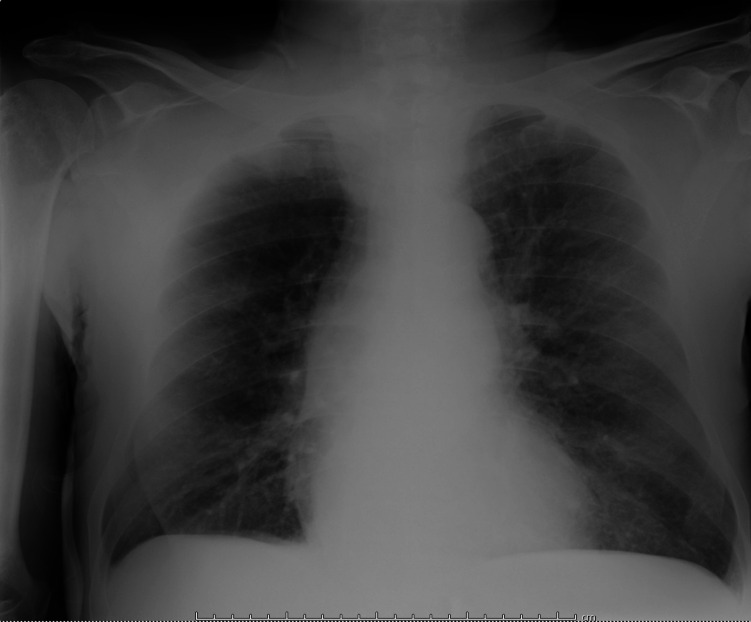
Laboratory results showed leucocytosis (17 570/μL, neutrophil count 83.2%) and high reactive C protein (7 mg/dL, normal value <0.7 mg/dL) and procalcitonin (1.4 ng/mL, normal value <0.5 ng/mL), with an interstitial pattern on chest X-ray (figure 1) and with desaturation (82% with fractional inspired oxygen (FiO2) 21%). Urea (153 mg/dL) and creatinine (6.32 mg/dL) were compatible with end-stage renal disease.
Figure 1.
Chest X-ray on admission showing an interstitial pattern.
Hypercapnic respiratory failure was described on blood gas analysis, with pH of 7.34, pO2 of 42 mm Hg (FiO2 21%, sO2 78%), pCO2 of 57 mm Hg and HCO3− of 30 mmol/L. Real-time reverse transcriptase PCR assay on a nasopharyngeal swab for SARS-CoV-2 was taken (negative on admission as well as at discharge). Urinary Streptococcus pneumoniae and Legionella pneumophila antigens were negative. Peritoneal cell count was 28/μL (no neutrophils), with negative cultures.
On echocardiogram a mild left ventricular hypertrophy was reported, with normal ejection fraction (65%) and continent valves (no sign of endocarditis), normal aortic caliper, and absence of pericardial effusion. Abdominal ultrasound was normal, except for the small-sized kidneys.
Differential diagnosis
During the SARS-CoV-2 outbreak, the differential diagnosis for respiratory failure always includes COVID-19. Chest X-ray described an interstitial pattern which could be COVID-19-related.1
High-resolution CT was not performed because our institutional policy during COVID-19 emergency is to reserve CT to severe respiratory failure, to avoid overload in the radiology department and to reduce the risk of SARS-CoV-2 exposure among potentially negative patients.
Neutrophilia, high C Reactive Protein (CRP) and Procalcitonin (PCT) are compatible with bacterial infection, while leucopenia with negative PCT is more frequently associated with viral pneumonia.1 Blood gas analysis in COVID-19 usually shows hypoxic and hypocapnic respiratory failure, secondary to compensatory tachypnoea.
To be sure to rule out bacterial superinfection in COVID-19, urine, blood and peritoneal solution cultures were performed, which were all negative, as well as three nasopharyngeal SARS-CoV-2 swabs.
Considering the patient’s blood cell count (neutrophilic leucocytosis), inflammatory markers (elevated CRP and PCT) and blood gas (hypoxic and hypercapnic respiratory failure), COPD exacerbation was the most probable diagnosis.
Treatment
Antibiotics (ceftriaxone 2 g, azithromycin 500 mg daily), intravenous steroids (methylprednisolone 20 mg two times per day) and low-flux (2 L/min) oxygen therapy were immediately started with suspicion for COPD exacerbation. Fluid overload (3.5 kg over his usual weight of 74.5 kg) was managed with high-dose diuretic therapy (furosemide 250+125+125 mg per os) and with optimisation of PD prescription.
At home the patient performed four manual hysotonic (1.36%) peritoneal dwells, 2 L for 6 hours each. During hospital stay, he was switched to automated PD, with a total of 20 L semihypertonic diurnal exchange combined with a nocturnal icodextrin 1 L dwell.
Outcome and follow-up
Blood cell count and respiratory parameters quickly normalised. After 5 days on admission, his pO2 increased to 57 mm Hg, pCO2 decreased to 47 mm Hg (FiO2 21%) and white cell count reached 7.910×109/L (neutrophil count 69.9%), with normal PCT and mildly elevated CRP (0.9 mg/dL). Steroids were withdrawn, and oxygen therapy could be suspended after 1 week from admission.
On the other hand, forced dehydration with diuretics and a higher catabolic rate secondary to infection worsened his urea and creatinine (240 mg/dL and 7.09 mg/dL, respectively), despite maximal PD prescription, which could not ensure adequate depuration and ultrafiltration rates.
Transition to HD was discussed and agreed with the patient and his caregiver; a central venous catheter was placed in the right internal jugular vein.
HyD was proposed to reduce the number of hospital access for HD. He was very motivated to go on with home PD and accepted once-weekly HD sessions, combined with 6 days per week of CAPD (two hysotonic and two semihypertonic 2 L per 6-hour dwells).
The patient was discharged after the first HD session.
After 3 months of HyD he maintained adequate depuration parameters (urea 185 mg/dL, creatinine 6.93 mg/dL), without anaemia (haemoglobin 137 g/L), and showing well-controlled hyperparathyroidism (Ca 9 mg/dL, P 4.9 mg/dL). His compliance to water intake restriction remains non-optimal, with an average weekly weight gain of 2.5 kg, supporting the choice of HyD over PD alone.
As SARS-CoV-2 circulation in Italy is gradually reducing, the goal of avoiding COVID-19 infection was reached.
Future withdrawal of HD will be considered according to residual renal function (RRF), diuresis and dialysis efficiency.
Discussion
HyD, also known as bimodal, combination or complementary dialysis therapy,5 consists of a combination of one or two HD sessions per week with 5–7 days of PD. There are only few reports in the literature about this strategy, mainly from Japan,6 7 12 UK8 and Canada.9 HyD remains anecdotal probably because PD itself is still underutilised worldwide. However, in Japan HyD accounts for up to 5.5% of PD patients.7 HyD is not a routine RRT option in our centre and in Italy in general, as the lack of literature from our country on this subject testifies.
HyD can be used as a starting prescription to combine the advantages of continuous (PD) and intermittent (HD) RRT,7 8 11 12 but it is more often considered as a bridge from one technique to another10 11 in patients experiencing insufficient solute or water removal due to deteriorating RRF.
The main downside of HyD is the presence of both a peritoneal catheter and a vascular access (mostly a central venous catheter), amplifying the risk of access-related complications.6 8
In analogy to what has been reported in the literature, we think that HyD can be an effective strategy for patients dropping out from PD to HD during the SARS-CoV-2 outbreak, with the aim of reducing the number of hospital access for patients with end-stage renal disease.
In our report, HyD allowed our patient to stay home 6 days a week, reducing the risk of COVID-19 infection, while maintaining a good quality of life and improving his global performance status. HyD shares with PD the need of a caregiver who is able to perform home dialysis. A healthcare professional sibling, as in the case reported, is an essential resource, not only from a practical perspective but also to support the patient in the choice of a mixed technique, whose rationale is fairly more complex than standard RRTs.
Although in the case we described we needed Central Venous Catheter (CVC) placement (Arteriovenous Fistula (AVF) creation was temporarily suspended as general elective surgery), it has to be noted that it would have been any way inevitable when switching from PD to HD, which also implies surgical removal of the peritoneal catheter.
HyD could have been avoided if the patient had shown an adequate compliance to stay on PD alone, reducing hospital access to just once monthly.
The major limitation of this paper lies in its case report nature. We cannot be sure if general social distancing measures would have been sufficient to avoid SARS-CoV-2 infection in such a frail subject. The lack of trials comparing COVID-19 incidence in PD, HD and HyD subjects does not allow to draw definite conclusions, although we think that this report could be interesting for patients and nephrologists, increasing attention on an RRT strategy that is still heavily underutilised.
In conclusion HyD is a promising niche in the field of RRT whose use can be supported during the SARS-CoV-2 outbreak.
Patient’s perspective.
It wasn’t the first time I referred to the emergency room. I live in the mountains near Modena, so during winter my cough usually gets worse and I often need my doctor’s counseling. But this time I was really worried, firstly because now I’m on dialysis - I’ve been taught to report when I don’t breathe well - and secondly because of this new virus. I had cough and fever, it wasn’t really different from previous episodes. I immediately understood that doctors were concerned that I could have had contracted the coronavirus, but they reassured me day by day because the overall situation wasn’t so bad. It was a relief to know that the swab ended negative. It was a little painful to perform, but the hardest part was to be alone in a room for more than a week, visited by nurses and doctors only. I couldn’t see my daughter and I was craving for smoking - there was no chance to be allowed to go outside for a cigarette - so I started repeatedly asking if I could go home. The staff explained me that my lungs were getting better, but that drugs and peritoneal dialysis were insufficient to avoid water to fill my lungs, so they couldn’t dismiss me yet. That’s when we started talking about switching to hemodialysis. I already knew hemodialysis, and I chose peritoneal dialysis to stay at home, also because my daughter is a nurse and she could help me. The idea of coming to the hospital three times per week wasn’t appealing at all and I feared to feel sick after each session. The doctors explained me that we could try to start with a mixed scheme, performing just one hemodialysis at the hospital and keeping my usual home therapy in the other days of the weeks. I had to place anyhow a venous catheter, so I decided to give it a try. I was dismissed after the first hemodialysis, I was ok, just a little nauseated. At home I went on with my usual therapy and I was glad to be informed that the last swab performed was negative too. For the moment I have to go to the hospital on Mondays, but I’m really feeling better. They told me that if I can control my water intake I’ll have to possibility to get back to peritoneal dialysis alone. It’s not easy, but I’m doing my best!
Learning points.
Chronic kidney disease seems to be a relevant risk factor for severe COVID-19 and therefore strategies to minimise exposure to SARS-CoV-2 are recommended for people on renal replacement therapy.
Hybrid dialysis, which combines home peritoneal dialysis and in-hospital haemodialysis, can be a valid resource during the SARS-CoV-2 outbreak, not only to optimise depuration and fluid balance, but also to limit the need for hospital access.
The presence of a reliable caregiver (a nurse sibling in this case) is essential to support patients in their therapeutic choice and to safely perform home dialysis.
Acknowledgments
We thank Professor Gianni Cappelli for the support during the COVID-19 crisis. We thank all colleagues involved in this unexpected challenge for their precious collaboration.
Footnotes
Contributors: GM made substantial contribution to the concept design of the work, or acquisition, analysis or interpretation of data, and drafted the article. GA, FF and RM revised it critically for important intellectual content and approved the version to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Xie M, Chen Q. Insight into 2019 novel coronavirus - An updated interim review and lessons from SARS-CoV and MERS-CoV. Int J Infect Dis 2020;94:119–24. 10.1016/j.ijid.2020.03.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riccardo F, Andrianou X, Bella A, et al. Epidemia COVID-19, Aggiornamento nazionale: 16 aprile 2020. Roma (Italy): Istituto Superiore di Sanit (ISS), Task force COVID-19 del Dipartimento Malattie Infettive E Servizio di Informatica 2020:17.
- 3.Coronavirus R. Aggiornamento al 14 aprile 2020. Bologna (Italy):Assessorato regionale politiche per la Salute. Elaborazione Agenzia informazione e comunicazione 2020:11p. [Google Scholar]
- 4.Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol 2020;52:1193–4. 10.1007/s11255-020-02451-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holvoet E, Verhaeghe S, Davies S, et al. Patients’ experiences of transitioning between different renal replacement therapy modalities: A qualitative study. Perit Dial Int 2020;896860819896219. [DOI] [PubMed] [Google Scholar]
- 6.Fukui H, Hara S, Hashimoto Y, et al. Review of combination of peritoneal dialysis and hemodialysis as a modality of treatment for end-stage renal disease. Ther Apher Dial 2004;8:56–61. 10.1111/j.1526-0968.2004.00107.x [DOI] [PubMed] [Google Scholar]
- 7.Kawanishi H, Hashimoto Y, Nakamoto H, et al. Combination therapy with peritoneal dialysis and hemodialysis. Perit Dial Int 2006;26:150–4. 10.1177/089686080602600205 [DOI] [PubMed] [Google Scholar]
- 8.McIntyre CW. Bimodal dialysis: an integrated approach to renal replacement therapy. Perit Dial Int 2004;24:547–53. 10.1177/089686080402400614 [DOI] [PubMed] [Google Scholar]
- 9.McCormick BB, Chan CT, ORN Home Dialysis Research Group . Striving to achieve an integrated home dialysis system: a report from the Ontario renal network home dialysis attrition Task force. Clin J Am Soc Nephrol 2018;13:468–70. 10.2215/CJN.06900617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sriperumbuduri S, Biyani M, Brown PA, et al. Retrospective study of patients on hybrid dialysis: single-center data from North America. Perit Dial Int 2020;40:224–6. 10.1177/0896860819887284 [DOI] [PubMed] [Google Scholar]
- 11.Grubbs V, Moss AH, Cohen LM, et al. A palliative approach to dialysis care: a patient-centered transition to the end of life. Clin J Am Soc Nephrol 2014;9:2203–9. 10.2215/CJN.00650114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka M, Mise N, Nakajima H, et al. Effects of combination therapy with peritoneal dialysis and hemodialysis on left ventricular hypertrophy. Perit Dial Int 2011;31:598–600. 10.3747/pdi.2010.00273 [DOI] [PubMed] [Google Scholar]