Abstract
Introduction
Effective August 2018, the U.S. Food and Drug Administration (FDA) required that nicotine addiction warnings be placed on ads for nicotine containing e-liquids. As per FDA comments, this provision pertains to visual ads communicated via social media, raising questions about compliance within the large e-liquid promotion community on Instagram.
Aims and Methods
This study examines use of warnings on promotional Instagram posts before and after provisions took effect on August 10, 2018. Netlytic was used to gather a sample of 500 promotional #eliquid and #ejuice posts from: May 2017, October 2017, March 2018, August 2018, and September 2018. The 1500 prewarning and 1000 postwarning posts were coded using content analysis. Changes in products and marketing strategies were also considered. Post volume was tracked monthly between May 2017 and February 2020.
Results
In the prewarning period, nicotine warning statements were absent on all posts. Following August 10, 2018, FDA compliant warnings were present on 13.6% of posts. Among US-based posts, 36.4% used the warnings, with warnings more common on posts made by e-liquid brands (52.3%) and posts promoting e-liquids with nicotine (40.0%). Promotional strategies and products did not significantly change. The share of posts made by US Instagram users decreased by 11%, although total post volume continued to grow.
Conclusions
Many e-liquid promotion posts on Instagram remained noncompliant with nicotine warnings after FDA provisions took effect. The large volume of international users also limited the impact of FDA-mandated warnings on the social media environment.
Implications
Further guidance and enforcement are needed to ensure that US e-liquid marketers on visual social media platforms adhere to current provisions, particularly for individual social media users who are sponsored by industry. The inherently global span of social media also indicates the importance of a shared approach to marketing regulations. Further work is needed to assess enforcement strategies viable for the social media environment.
Introduction
Marketing and promotion for electronic cigarettes (e-cigarettes) and the nicotine liquids used to refill them (e-liquids) have become widespread on social media.1,2 These posts often feature attractive visuals, including cartoons, and an emphasis on appealing nontobacco flavors.1,3,4 Despite evidence that exposure to these types of promotions on social media may increase youth vaping,5–8 this content remains largely unregulated in the United States. One of the few restrictions placed on social media marketing for vaping products is the mandated use of warning statements about nicotine addiction outlined in the U.S. Food and Drug Administration’s (FDA) 2016 Deeming Rule. Effective August 10, 2018, the FDA required visual advertising for all e-cigarette and e-liquid products containing nicotine to include the language “WARNING: This product contains nicotine. Nicotine is an addictive chemical.” As per FDA language in the Federal Register notice issuing the final Deeming Rule, warnings must also cover 20% of visual ads communicated via social media and smart phones.9 E-liquids that “do not contain tobacco or nicotine and are not made or derived from tobacco or nicotine do not meet the definition of covered tobacco product” and therefore do not need to carry an addiction warning.10
While initial evidence indicates that warnings on social media may have limited value for deterring vaping among those who already use nicotine,11,12 focus groups suggest that they may reduce interest among those who neither smoke nor vape.11 More broadly, the evidence on warning statements about vaping continues to be quite mixed with regard to their impact on the beliefs and behaviors of nicotine and nonnicotine users alike.13–18 For example, it is currently unclear if FDA-mandated nicotine addiction statements are more or less effective than the more detailed warnings voluntarily adopted by e-cigarette companies.16–18 There has also been some concern that warning statements may inadvertently lead smokers to continue use of combustible tobacco.19 Recent evidence suggests that e-cigarette warnings reduce interest in cigarette use among smokers.15 However, studies also indicate that many smokers continue smoking rather than switching to e-cigarettes due an unwillingness to “substitute one addiction for another,” 20 suggesting that addiction warnings may deter some harm reduction practices. While research on the ideal warning statement for e-cigarette products continues, questions about compliance with warning requirements continues as a separate and equally pressing concern.
Print ads for vaping products increasingly featured nicotine warnings once the FDA published its proposal for the Deeming Rule in 2014.21 However, print ads are dominated by a small number of major e-cigarette brands,21 while social media is home to large volumes of small independent brands and retailers,1 complicating enforcement and potentially making compliance less likely. Large volumes of users sponsored by e-liquid brands and retailers also raise questions about compliance across promoter types.1 While advertising warning statement provisions explicitly name only manufacturers, packagers, importers, distributors, and retailers of covered tobacco products, social media users who promote on behalf of these are also required to apply warning statements.22 The FDA and U.S. Federal Trade Commission (FTC) recently issued letters to several e-liquid manufacturers reminding them that “companies who use social media influencers to promote their products must comply with all applicable advertising requirements.” 23
This study examines use of mandated nicotine warnings on e-liquid promotion posts on Instagram before and after the FDA warning provisions for visual promotions took effect in 2018, as well as how usage varies by promoter type. Additionally, we consider if products promoted, post visuals, and promotional themes and claims changed as a result of the warning statement provisions. We also examine if the overall volume of e-liquid posts made on Instagram changed following the warning provision taking effect. We focus on Instagram because it is an image-driven platform, home to a large volume of content promoting vaping,1,24–26 and because it has a large young adult and adolescent user base.27 Prior research also suggests that exposure to e-cigarette messages on Instagram may have a greater impact on positive attitudes toward vaping than those on Facebook or YouTube.5 Compliance with warning provisions on Instagram represents an important priority for tobacco control.
Methods
Data Collection
The most recent 100 public posts tagged with #eliquid or #ejuice were collected every hour for 1-week periods using the online application Netlytic,28 which at the time had authorized third-party access to the Instagram API. This process was conducted at three 5-month intervals prior to the FDA’s warning provisions taking effect (May 2017, October 2017, March 2018) and in each of the 2 months following the effective date (August 2018, September 2018). For each sample period, duplicate posts and comments were removed based on URL matching and a random sample of 500 posts was chosen for analysis. This yielded 1500 prewarning posts and 1000 postwarning posts for analysis. Posts were manually screen captured and new posts were drawn from the sample to replace any dead links in order to maintain desired sample sizes. Additionally, the total number of Instagram posts with the hashtags #eliquid and #ejuice was recorded via Instagram’s hashtag search function on the first of each month from May 2017 through February 2020. The hashtags #vape, #ecig, and #vapelife, identified as common in prior literature,29 were also tracked to determine the overall volume of posting about e-cigarettes.
Codebook Development
The codebook was built on one developed for a comprehensive content analysis of marketing strategies for e-liquid on Instagram.1 The May and October 2017 samples were initially coded during this prior study, with the added time periods coded using a more streamlined codebook focused on warning statements, products, and promotional themes. The final codebook considered:
Instagram user type (based on user-profiles using a previously developed typology of shops, brands, other vape industry, and ambassadors/sponsored users1).
Post language and user location (based on location tags, store links, captions, and hashtag locations).
Descriptive metadata (likes, comments, followers, etc.).
Mentions of age restrictions for purchasing content.
Political statements and calls for advocacy about vaping.
Post visuals, promotional themes, and health claims identified in prior research.1
The nicotine level of promoted e-liquids (eg, 0 and 5 mg), as determined by examining the post captions and the labels of the e-liquid bottles depicted.
The flavors of e-liquids promoted in posts, as described in prior research,1 as well as promotion of cartridge-style e-liquids.
Presence of FDA-mandated nicotine warning statement language on post images.
Presence of other health related warnings in images or in captions.
Coding and Analysis
LL and MW coded posts across all time periods using a qualitative content analysis approach and the MAXQDA 2018 software.30 Posts made by casual users and vaping enthusiasts who posted about e-liquids or e-cigarettes but lacked any ties to industry in their user-profiles or posts were excluded from further analysis, as were promotional posts that did not mention or depict e-liquids or e-liquid brands in images, text, or hashtags. Data on prewarning period posts were aggregated to compare to posts made in the period immediately following the warning statement provisions taking effect. Coding reliability for the first two periods has already been established in prior work.1 To ensure continued reliability, LL and MW conducted additional rounds of double coding on 10% of the randomly selected posts from the March, August, and September 2018 samples. The estimated intercoder reliability coefficient (Cohen’s Kappa) for the all double-coded posts was on average .85, with 96% agreement between the two coders. In addition to qualitative analysis, we also conducted descriptive quantitative analysis using a t test for continuous variables and z test for binary variables to explore differences in posting characteristics between pre- and post-FDA mandate of warning statement provision. We also used Pearson chi-square test to assess the association between appearances of warning statements and types of users. Since we conducted a large number of statistical tests to explore differences between the pre- and postpolicy implementation, we used a Bonferroni-corrected p value of .001 as a threshold for statistical significance. All quantitative analyses were done with STATA 14 (StataCorp, 2015).
Results
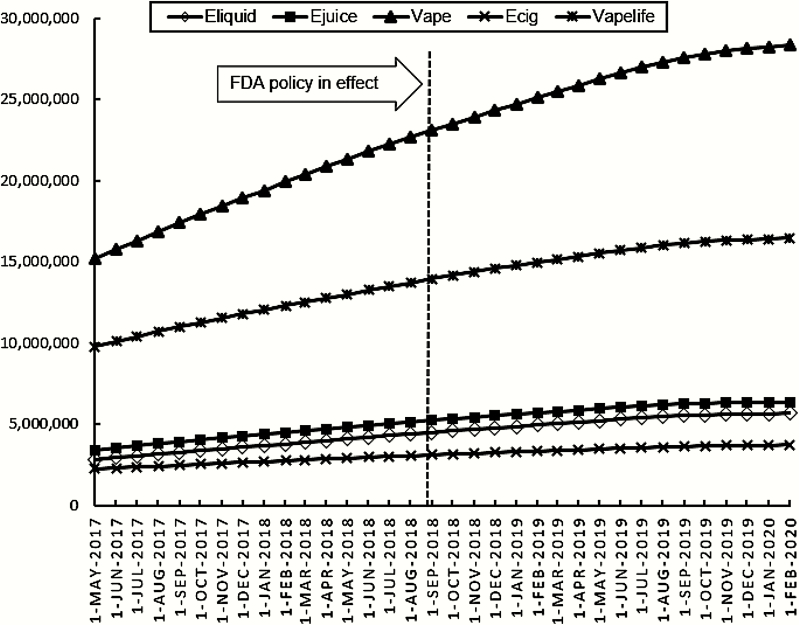
Of the 1500 prewarning posts, 61.7% (n = 926) were made by users with ties to the vaping industry that promoted e-liquid use or specific brands of e-liquid (Table 1). For the postwarning posts, this decreased to 55.2% (n = 552). All subsequent results refer to these promotional posts with some form of tie the vaping industry. In the prewarning period, the United States was the largest source of promotional e-liquid posts (38.0%) followed by Europe (20.8%). However, the proportion of posts from the United States significantly declined in the postwarning period (p < .001), dropping to 27.9% and being eclipsed by posts originating in Europe (29.2%). Posts from Central/South America and unspecified locations also increased. Despite this shift, there was no significant change in the number of posts made in English, with over 70% of posts fully in English. Further, the overall volume of posts using the hashtags #eliquid and #ejuice continued to rise consistently between May 2017 and February 2020 at the estimated rates of 90 977 #eliquid and 93 500 #ejuice posts per month (Figure 1).
Table 1.
Descriptive Statistics of Instagram E-liquid Marketing Posts Before and After FDA Nicotine Warning Statement Provisions Took Effect
| Mean (SD) or percent | ||||
|---|---|---|---|---|
| All posts | Pre-FDA warnings | Post-FDA warnings | p a | |
| N | 1478 | 926 | 552 | |
| Post metadata | ||||
| Views | 761.3 | 910.7 | 510.7 | .637 |
| (15 759.5) | (18 820.1) | (8428.8) | ||
| Likes | 151.9 | 139.3 | 173.0 | .376 |
| (706.6) | (608.2) | (846.6) | ||
| Comments | 5.9 | 6.5 | 5.0 | .736 |
| (79.4) | (99.5) | (16.0) | ||
| Posts | 1278.2 | 1282.2 | 1271.4 | .945 |
| (2928.0) | (2383.7) | (3666.3) | ||
| Followers | 11 285.5 | 9522.6 | 14 242.7 | .127 |
| (57 475.0) | (31 169.2) | (84 912.0) | ||
| Following | 1907.8 | 1874.2 | 1964.3 | .431 |
| (2127.7) | (2137.8) | (2111.4) | ||
| Geographical area | ||||
| United States | 34.2% | 38.0% | 27.9% | <.001 |
| Canada/Mexico | 5.0% | 5.0% | 5.1% | .929 |
| Central/South America | 2.3% | 1.2% | 4.2% | <.001 |
| Africa | 0.4% | 0.5% | 0.2% | .294 |
| Asia/Pacific | 17.2% | 19.1% | 14.0% | .011 |
| Europe | 24.0% | 20.8% | 29.2% | <.001 |
| Middle East | 3.1% | 2.7% | 3.8% | .237 |
| Not specified | 13.8% | 12.6% | 15.8% | .092 |
| Language | ||||
| English | 72.7% | 72.3% | 73.6% | .586 |
| Other (English comprehension) | 11.8% | 13.3% | 9.2% | .020 |
| Other (no English comprehension) | 15.5% | 14.5% | 17.2% | .159 |
| Media type | ||||
| Images | 94.5% | 94.0% | 95.5% | .215 |
| Video | 5.5% | 6.1% | 4.5% | .215 |
| User type | ||||
| Ambassador/sponsored/rep | 28.6% | 27.1% | 31.0% | .111 |
| Brand/manufacturer | 22.7% | 23.1% | 21.9% | .597 |
| Vape shop | 38.6% | 37.9% | 39.9% | .456 |
| Other vape industry | 10.2% | 11.9% | 7.3% | .004 |
| Warnings | ||||
| FDA compliant warning statement on image | 5.1% | 0.0% | 13.6% | <.001 |
| Other warning in image or caption | 11.6% | 5.5% | 21.7% | <.001 |
FDA = Food and Drug Administration.
aStatistical significance tests were based on t test for mean difference and z test for percentage (proportional) difference.
Figure 1.
Volume of Instagram posts using selected hashtags between May 2017 and February 2020.
Use of Warning Statements on Post Visuals
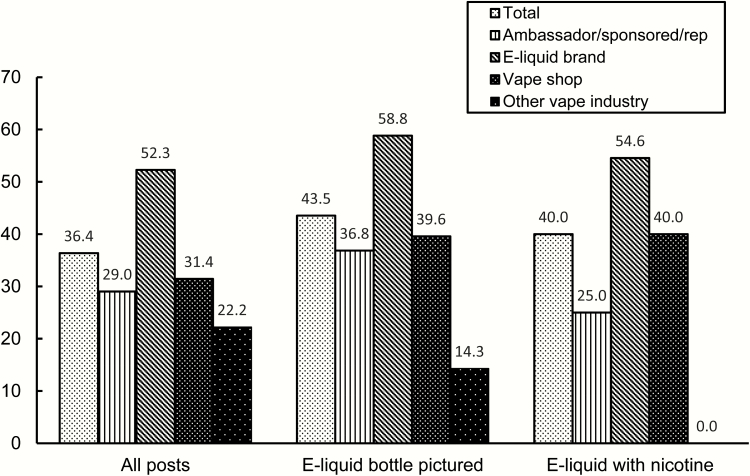
In the prewarnings period, no posts were made with FDA-mandated warning statements. In the postwarnings period, this increased to 13.6% for the overall sample of promotional posts. Use of other warnings, including nicotine warnings in captions and nicotine and hazard warnings on the bottles in post images, increased from 5.5% to 21.7%. Among promotional posts that were identified as originating with US Instagram users, warning statements were present on 36.4% of posts in the postwarning period (Figure 2). During this period, 70.1% of US posts depicted e-liquid bottles and 29.2% of posts disclosed that the e-liquid promoted contained nicotine (Table 2). Warning usage was higher on these post types, with 43.5% of posts depicting e-liquid bottles and 40.0% of posts promoting e-liquids with nicotine using the mandated warnings. Although there was no over-arching statistically significant difference across user types (Χ2 = 7.04, p = .07), users who indicated that they were sponsored or had an affiliation with an e-liquid brand or store tended to be less likely than actual brands to use warnings (Figure 2). The highest rates of compliance were found on posts made by e-liquid brands that depicted e-liquid bottles (58.8%) or promoted e-liquids that explicitly contained nicotine (54.6%).
Figure 2.
Appearance of nicotine addiction warning statements on Instagram post images from US-based accounts after FDA nicotine warning provisions took effect (%). FDA = Food and Drug Administration.
Table 2.
E-liquid Marketing in US-Based Promotional Instagram Posts Before and After FDA Nicotine Warning Statement Provisions Took Effect
| All posts | Pre-FDA warnings | Post-FDA warnings | p a | |
|---|---|---|---|---|
| N | 506 | 352 | 154 | |
| Age restriction | ||||
| Eighteen and over | 20.2% | 18.2% | 24.7% | .094 |
| Image content | ||||
| E-liquid bottle | 70.4% | 70.5% | 70.1% | .791 |
| Cartoons/illustrations | 54.0% | 54.8% | 52.0% | .550 |
| Brand mimicry | 3.4% | 3.1% | 3.9% | .658 |
| Person | 19.8% | 18.8% | 22.1% | .387 |
| Hand-check | 13.4% | 15.9% | 7.8% | .014 |
| Political statements | ||||
| Antiregulation | 6.1% | 6.3% | 5.8% | .861 |
| Other unclear/no statement | 93.9% | 93.8% | 94.2% | .861 |
| Flavor | ||||
| Tobacco | 2.6% | 2.3% | 3.3% | .524 |
| Menthol/mint | 9.3% | 7.1% | 14.3% | .010 |
| Other flavor(s) | 72.8% | 71.0% | 77.0% | .145 |
| Product type | ||||
| Cartridge | 2.8% | 3.1% | 2.0% | .458 |
| Nicotine | 22.1% | 20.5% | 29.2% | .031 |
| Nicotine free | 6.9% | 6.8% | 7.1% | .895 |
| Nicotine not specified | 72.3% | 74.4% | 67.5% | .110 |
| Themes | ||||
| Taste | 41.9% | 43.2% | 39.0% | .376 |
| Pleasurable effects | 14.8% | 16.2% | 11.7% | .189 |
| Community/social | 4.2% | 4.8% | 2.6% | .247 |
| Cute | 11.7% | 10.5% | 14.3% | .224 |
| Edgy/cool | 19.8% | 21.6% | 15.6% | .119 |
| Sex | 3.8% | 3.7% | 3.9% | .912 |
| Humor | 1.8% | 1.7% | 2.0% | .849 |
| Tricks | 5.7% | 5.7% | 5.8% | .942 |
| Claims | ||||
| Cessation | 5.7% | 5.1% | 7.1% | .366 |
| Modified risk | 3.0% | 3.4% | 2.0% | .373 |
| No smoke | 24.1% | 26.7% | 18.2% | .039 |
| Health benefits | 5.7% | 6.8% | 3.3% | .112 |
| Quality | 2.8% | 4.0% | 0.0% | .012 |
| Other claims | 5.1% | 5.7% | 3.9% | .403 |
| Any claim | 37.2% | 41.8% | 26.6% | .001 |
FDA = Food and Drug Administration.
aStatistical significance tests were based on z test for percentage (proportional) difference.
Changes to Promotional Themes, Political Activity, Products, and Health Claims
For US-based posts, there were no significant changes in the choice of promotional themes used or products promoted (Table 2). Although the promotion of menthol/mint products doubled from 7.1% of posts to 14.3% of posts (p = .01), no flavor experienced a statistically significant change. Posts promoting other (nontobacco) flavors remained most common at 77% of all postwarning period posts. There was a small insignificant increase in the promotion of e-liquids explicitly labeled or described as nicotine free (p = .90). Promotion of e-liquids noted as containing nicotine increased from 20.5% to 29.2% of posts, but without statistical significance (p < .05). Use of claims about the benefits of e-liquids decreased from 41.8% of posts to 26.6% of posts (p = .001), driven primarily by a decrease in claims implying that e-cigarettes are a smokeless product (eg, hashtags such as #smokefree).
Discussion
This content analysis of Instagram posts promoting e-liquids before and after the FDA’s nicotine warning statement provisions took effect in August 2018 found that Instagram users in the United States have begun to use the required warnings. However, overall compliance remains relatively poor, albeit with differences by both post and user type. Relative to posts by e-liquid brands, those by vape shops and by individual social media users who indicate ties to the e-cigarette industry appear to be a particular concern with regard to noncompliance. The lower usage of warnings by sponsored users suggests that there may be some confusion about if brand ambassadors and those who receive free promotional products are subject to the same regulations as more explicit e-liquid marketing. As per FDA and FTC complaints, individual users who are posting on behalf of brands or retailers must also use nicotine warnings.23 Although Instagram banned influencer posts that are sponsored by the e-cigarette industry on December 18, 2019,31 this is unlikely to have a significant impact on the large number of e-liquid brand ambassadors with relatively small numbers of followers whose posts are unlikely to use the official Instagram sponsored post feature.1
Based on the current analysis, there is little evidence that promotional posts have begun to feature the nicotine-free version of e-liquids in order to avoid using warning statements. There is also little indication that posters have begun to alter their promotional themes or image subjects since the FDA warning statement provisions took effect. However, both of these remain areas of potential concern that should be monitored. Prior research indicates that magazine ads for tobacco products became increasingly visually oriented and colorful as regulation became stricter (including mandated warning statements) and public concern about health effects grew.32 As warning compliance begins to increase, it is possible that promotional posts will shift their visuals and themes to offset the impact of warnings. Future research should monitor for increases in posts that use cute or edgy/cool imagery, particularly through cartoons, as well as posts that heavily stress appealing e-liquid flavors using bright colors and images of fruits/candy.1,33
While the proportion of US-based posts decreased following the FDA warning statement provisions taking effect, this was offset by a growth of posts from other nations. Overall, the volume of #eliquid and #ejuice posts made each month continued to grow at the same rapid pace. As a global platform where Instagram users who search for these hashtags see all relevant content regardless of where the posts originate from, the large volume of non-US posts without warning statements waters down the effect of US warning mandates in the Instagram ecosystem. Further, many e-liquid companies ship internationally, making international posts relevant for US residents. These posts are enabled by nations that lack policies for warning statements on marketing34 and poor compliance by the e-cigarette industry in nations that prohibit online marketing for e-cigarette products. However, some progress does appear to have been made on the latter. The United Kingdom prohibits the promotion of e-cigarette products with nicotine on online media (in alignment with European Union rules against e-cigarette advertising on “information society services”) but allows the sharing of factual information on company/retailer websites.35 In December 2019, the U.K.’s Advertising Standards Authority ruled against several e-cigarette product companies and retailers that were marketing via their own Instagram pages, ruling that an Instagram page is not equivalent to a website because it can easily be viewed by people not “actively seeking out information about e-cigarettes” and that the inclusion of hashtags such as #vapelife render a post promotional rather than factual.36,37 Ultimately, the companies/retailers were told to cease posting promotional content on public Instagram pages unless steps are taken to ensure posts are “only be distributed to those following their account and would not be seen by other users.” 37
However, even with some progress to limit posts from the United Kingdom, the sheer volume of e-liquid posts complicates warning statement enforcement by US regulators. As of February 2020, there were over six million #eliquid posts on Instagram, many of them made by casual e-cigarette users and by retailers/brands located outside of the United States. One approach to addressing the challenges associated with warning statement enforcement on a platform with thousands of new posts generated daily would be to apply a deep learning approach to automate detection. This would require the ability to detect the warning statement in the image, as well as the ability to with some certainty predict if the post is promotional and if the posts originates from the United States. While international posters technically also need to comply with the FDA-mandated warnings for products advertised or distributed within the United States, further legal analysis is needed as to how this is interpreted in the context of social media posts originating from other nations. Posts that meet specified criteria, but lack warnings could then be flagged for manual review.
Specifically, deep learning—a form of artificial intelligence that automatically discovers patterns in data—allows for a fast and precise identification of themes featured in thousands of images.38 Proof of concept for how this might work for e-cigarette products has already begun, including a recent study that applied large-scale deep learning image classification to ~50 000 Instagram posts about e-cigarettes.38 By applying transfer learning and fine-tuning to create a customized image classifier capable of recognizing vaping images, a convolutional neural networks model was trained to identify one class in each image (eg, man, woman, mod, pod, e-juice, and other) with 0.90 accuracy.38–40 The model identified labels of thousands of images in less than a week, which offered a significant savings of time and cost. While the predictions were not 100% accurate, the method could be used to narrow down the number of posts that would need to be reviewed by enforcement agencies. Efforts are currently planned to use the same approach to evaluate compliance with FDA requirements for warning statements in a large sample of Instagram posts. This work will help determine the viability of detecting both post content and warning statement usage.
Our analysis was limited to posts from public Instagram accounts that remained active 2 weeks after they were originally posted. Therefore, the analysis does not reflect posts that were deleted within this time window. We were also unable to capture any e-liquid promotion posts that did not use either the e-liquid or e-juice hashtag. Identification of sponsored users was made based on mentions of specific stores or brands in user information or explicit statements about sponsorship at the post level. Due to the volume of e-liquid products on the market, we were not able to establish that the specific e-liquid product promoted in a post was sold by the vape shop or brand mentioned by the user. As sponsored users may also post products that they were not incentivized to post about, it is not possible to say with certainty that all posts made by sponsored users are truly sponsored posts. This may overstate the number of sponsored posts that we anticipated should use warning statements, as well as complicate enforcement efforts. Further research is needed to understand the relationships between sponsored users and brands/retailers and the extent to which they are aware of warning statement requirements. Further research is also needed to determine if and how semi-automated detection of violations can account for these types of nuances.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Funding
Research reported in this publication was supported by National Cancer Institute (NCI) and Food and Drug Administration (FDA) Center for Tobacco Products (CTP) under award number R03CA216528. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the Food and Drug Administration.
Declaration of Interests
None declared.
References
- 1. Laestadius LI Wahl MM, Pokhrel P, Cho YI. From Apple to Werewolf: a content analysis of marketing for e-liquids on Instagram. Addict Behav. 2019;91:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCausland K, Maycock B, Leaver T, Jancey J. The messages presented in electronic cigarette-related social media promotions and discussion: scoping review. J Med Internet Res. 2019;21(2):e11953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allem J-P, Cruz TB, Unger JB, Toruno R, Herrera J, Kirkpatrick MG. Return of cartoon to market e-cigarette-related products. Tob Control. 2019:28(5):555–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jackler RK, Ramamurthi D. Unicorns cartoons: marketing sweet and creamy e-juice to youth. Tob Control. 2017;26(4):471–475. [DOI] [PubMed] [Google Scholar]
- 5. Cho H, Li W, Shen L, Cannon J. Mechanisms of social media effects on attitudes toward e-cigarette use: motivations, mediators, and moderators in a National Survey of Adolescents. J Med Internet Res. 2019;21(6):e14303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pokhrel P, Fagan P, Herzog TA, et al. Social media e-cigarette exposure and e-cigarette expectancies and use among young adults. Addict Behav. 2018;78:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sawdey MD, Hancock L, Messner M, Prom-Wormley EC. Assessing the association between e-cigarette use and exposure to social media in college students: a cross-sectional study. Subst Use Misuse. 2017;217(14):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alpert JM, Chen H, Adams K-A. E-cigarettes and social media: attitudes and perceptions of young adults to social media messages. Addict Res Theory. 2019:1–10. doi:10.1080/16066359.2019.1663835 [Google Scholar]
- 9. Deeming Tobacco Products to be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act. Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. 2016. 81 FR 28973. [PubMed] [Google Scholar]
- 10. U.S. Food and Drug Administration. Zero-Nicotine: Self-certification and Alternative Required Warning Statement. 2018. https://www.fda.gov/tobacco-products/labeling-and-warning-statements-tobacco-products/covered-tobacco-products-and-roll-your-own-cigarette-tobacco-labeling-and-warning-statement#zeronicotine. Accessed April 7, 2020.
- 11. Laestadius LI, Penndorf KE, Seidl M, Cho YI. Assessing the appeal of Instagram electronic cigarette refill liquid promotions and warnings among young adults: mixed methods focus group study. J Med Internet Res. 2019;21(11):e15441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guillory J, Kim AE, Fiacco L, Cress M, Pepper J, Nonnemaker J. An experimental study of nicotine warning statements in e-cigarette tweets. Nicotine Tob Res. 2019;376(4):342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wackowski OA, Sontag JM, Hammond D, et al. The impact of e-cigarette warnings, warning themes and inclusion of relative harm statements on young adults’ e-cigarette perceptions and use intentions. Int J Environ Res Public Health. 2019;16(2):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berry C, Burton S, Howlett E. Are cigarette smokers’, e-cigarette users’, and dual users’ health-risk beliefs and responses to advertising influenced by addiction warnings and product type? Nicotine Tob Res. 2017;19(10):1185–1191. [DOI] [PubMed] [Google Scholar]
- 15. Brewer NT, Jeong M, Hall MG, et al. Impact of e-cigarette health warnings on motivation to vape and smoke. Tob Control. 2019;28(e1):e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shang C, Huang J, Chaloupka FJ, Emery SL. The impact of flavour, device type and warning messages on youth preferences for electronic nicotine delivery systems: evidence from an online discrete choice experiment. Tob Control. 2018;27(e2):e152–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee HY, Lin HC, Seo DC, Lohrmann DK. The effect of e-cigarette warning labels on college students’ perception of e-cigarettes and intention to use e-cigarettes. Addict Behav. 2018;76:106–112. [DOI] [PubMed] [Google Scholar]
- 18. Mays D, Smith C, Johnson AC, Tercyak KP, Niaura RS. An experimental study of the effects of electronic cigarette warnings on young adult nonsmokers’ perceptions and behavioral intentions. Tob Induc Dis. 2016;14(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wackowski OA, Hammond D, O’Connor RJ, Strasser AA, Delnevo CD. Considerations and future research directions for e-cigarette warnings—findings from expert interviews. Int J Environ Res Public Health. 2017;14(7):781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weaver SR, Heath JW, Ashley DL, Huang J, Pechacek TF, Eriksen MP. What are the reasons that smokers reject ENDS? A national probability survey of U.S. adult smokers, 2017–2018. Drug Alcohol Depen. 2020;211:107855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shang C, Chaloupka FJ. The trend of voluntary warnings in electronic nicotine delivery system magazine advertisements. Int J Environ Res Public Health. 2017;14(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Required Warning Statement Regarding Addictiveness of Nicotine, 21 CFR §1143.3. 2020. [Google Scholar]
- 23. US Food and Drug Administration. FDA, FTC Take Action to Protect Kids by Citing Four Firms That Make, Sell Flavored e-Liquids for Violations Related to Online Posts by Social Media Influencers on Their Behalf. 2019. https://www.fda.gov/news-events/press-announcements/fda-ftc-take-action-protect-kids-citing-four-firms-make-sell-flavored-e-liquids-violations-related. Accessed February 27, 2020.
- 24. Czaplicki L, Kostygina G, Kim Y, et al. Characterising JUUL-related posts on Instagram [published online ahead of print July 2, 2019]. Tob Control. doi:10.1136/tobaccocontrol-2018-054824 [DOI] [PubMed] [Google Scholar]
- 25. Majmundar A, Kirkpatrick M, Cruz TB, Unger JB, Allem J-P. Characterising KandyPens-related posts to Instagram: implications for nicotine and cannabis use [published online ahead of print May 30, 2019]. Tob Control. doi:10.1136/tobaccocontrol-2019-055006 [DOI] [PubMed] [Google Scholar]
- 26. Chu KH, Allem JP, Cruz TB, Unger JB. Vaping on Instagram: cloud chasing, hand checks and product placement. Tob Control. 2016;26(5):575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perrin A, Anderson M. Share of U.S. Adults Using Social Media, Including Facebook, Is Mostly Unchanged Since 2018 2019. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018/. Accessed February 29, 2020.
- 28. Gruzd A. Netlytic: Software for Automated Text and Social Network Analysis. 2018. https://netlytic.org/home/ [Google Scholar]
- 29. Laestadius LI, Wahl MM, Cho YI. #Vapelife: an exploratory study of electronic cigarette use and promotion on Instagram. Subst Use Misuse. 2016;51(12):1669–1673. [DOI] [PubMed] [Google Scholar]
- 30. Schreier M. Qualitative Content Analysis in Practice. London: SAGE; 2012. [Google Scholar]
- 31. Cavale S. Instagram Bans Influencers from Promoting Vaping Products. Reuters; 2019. https://www.reuters.com/article/us-instagram-vaping/instagram-bans-influencers-from-promoting-vaping-products-idUSKBN1YN15B. Accessed February 27, 2020. [Google Scholar]
- 32. King KW, Reid LN, Moon YS, Ringold DJ. Changes in the visual imagery of cigarette ads, 1954–1986. J Public Policy Mark. 1991;10(1):63–80. [Google Scholar]
- 33. Kirkpatrick MG, Cruz TB, Unger JB, Herrera J, Schiff S, Allem J-P. Cartoon-based e-cigarette marketing. Associations with susceptibility to use and perceived expectations of use. Drug Alcohol Depend. 2019;201:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johns Hopkins Institute for Global Tobacco Control. Country Laws Regulating E-cigarettes: A Policy Scan. https://www.globaltobaccocontrol.org/e-cigarette_policyscan. Last updated February 12, 2020; Accessed February 28, 2020.
- 35. Committee on Advertising Practices. Electronic Cigarette Advertising Prohibitions—Advertising Guidance. 2017. https://www.asa.org.uk/asset/97E623E4-3A64-4215-81A5C4BD6D82D1E0.A1727AC1-C340-4B08-9820666C89AE18CB/. Accessed February 27, 2020.
- 36. Advertising Standards Authority. ASA Ruling on British American Tobacco UK Ltd. 2019. https://www.asa.org.uk/rulings/british-american-tobacco-uk-ltd-G19-1018310.html. Accessed February 27, 2020.
- 37. Advertising Standards Authority. ASA Ruling on Ama Vape Lab Ltd. 2019. https://www.asa.org.uk/rulings/ama-vape-lab-ltd-A19-563349.html. Accessed February 27, 2020.
- 38. Vassey J, Metayer C, Kennedy CJ, Whitehead TP. #Vape: measuring e-cigarette influence on Instagram with deep learning and text analysis. Front Commun. 2020;4:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deshpande A. A Beginner’s Guide to Understanding Convolutional Neural Networks 2016. https://adeshpande3.github.io/A-Beginner%27s-Guide-To-Understanding-Convolutional-Neural-Networks/. Accessed February 27, 2020.
- 40. TensorFlow. Transfer Learning with a Pretrained ConvNet 2017. https://www.tensorflow.org/tutorials/images/transfer_learning. Accessed February 27, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.