Abstract
Introduction
The World Health Organization (WHO) Framework Convention on Tobacco control recognizes the need for tobacco product regulation. In line with that, the WHO Study Group on Tobacco Product Regulation (TobReg) proposed to regulate nine toxicants in mainstream cigarette smoke, including aldehydes, volatile organic compounds (VOCs), and carbon monoxide (CO). We analyzed their relations in 50 commercially available cigarette brands, using two different smoking regimes, and their dependence on sugar and humectant concentrations in tobacco filler.
Methods
We measured sugar and humectant in tobacco filler and aldehydes, VOCs, and tar, nicotine, and CO (TNCO) in mainstream smoke. The general statistics, correlations between emission yields, and correlations between contents and emissions yields were determined for these data.
Results
For aldehydes, several significant correlations were found with precursor ingredients in unburnt tobacco when smoked with the Intense regime, most prominently for formaldehyde with sucrose, glucose, total sugars, and glycerol. For VOCs, 2,5-dimethylfuran significantly correlates with several sugars under both International Standards Organization (ISO) and Intense smoking conditions. A correlation network visualization shows connectivity between a sugar cluster, an ISO cluster, and an Intense cluster, with Intense formaldehyde as a central highest connected hub.
Conclusions
Our multivariate analysis showed several strong connections between the compounds determined. The toxicants proposed by WHO, in particular, formaldehyde, can be used to monitor yields of other toxicants under Intense conditions. Emissions of formaldehyde, acetaldehyde, acrolein, and 2,5-dimethylfuran may decrease when sugar and humectants contents are lowered in tobacco filler.
Implications
Our findings suggest that the aldehydes and VOCs proposed by TobReg are a representative selection for smoke component market monitoring purposes. In particular, formaldehyde yields may be useful to monitor emissions of other toxicants under Intense conditions. Since the most and strongest correlations were observed with the Intense regime, policymakers are advised to prescribe this regime for regulatory purposes. Policymakers should also consider sugars and humectants contents as targets for future tobacco product regulations, with the additional advantage that consumer acceptance of cigarette smoke is proportional to their concentrations in the tobacco blend.
Introduction
Smoking remains the main cause of morbidity and mortality worldwide, even in countries with extensive tobacco control measures. Therefore, the World Health Organization (WHO) Framework Convention on Tobacco Control (FCTC) recognizes the need for tobacco product regulation in addition to preventing initiation, promoting cessation, and protecting the public from exposure to secondhand smoke.1 Articles 9 and 10 of the treaty advise parties to take measures that reduce the toxicity, addictiveness, and attractiveness of tobacco products.
Regarding toxicity, the WHO Study Group on Tobacco Product Regulation (TobReg) proposed to set product standards for cigarettes and a strategy to use them to mandate a reduction in the toxicant yields for cigarette smoke.2 Cigarette mainstream smoke consists of more than 8000 different compounds, including more than 100 toxicants.3–5 Nine toxicants were selected based on several criteria, including their toxicity indices, their potential to be lowered, and their representativeness for different chemical classes and different phases of smoke (gas and particulate), and toxicities that are associated with heart and lung disease, as well as cancer. Aldehydes (formaldehyde, acetaldehyde, and acrolein), volatile organic compounds (VOCs: benzene and 1,3-butadiene) and carbon monoxide (CO) are the components in the volatile phase that have been selected by WHO TobReg, while tobacco-specific nitrosamines (TSNAs: NNN and NNK) and polycyclic aromatic hydrocarbons (PAHs: benzo[a]pyrene, BaP) were selected from the particulate phase. Monitoring the yields of these compounds in cigarette mainstream smoke can be considered a baseline measurement and a starting point for the mandated lowering process.2,6
To generate cigarette mainstream smoke for regulatory purposes, TobReg recommended using the Canadian Intense smoking regime, rather than the US Federal Trade Commission (FTC)/International Standards Organization (ISO) testing regime.2 An important reason for this advice is that the ISO regime is less intense than human smoking behavior, especially in the case of cigarettes with a high degree of filter ventilation. As the main addictive component in cigarette smoke is nicotine, and smokers need a certain amount of nicotine to maintain their addiction, they adapt their smoking behavior to the nicotine quantities present in smoke.7 One of the main factors determining nicotine quantities is ventilation holes in the cigarette filter that dilute smoke. In response, smokers (partly) close the ventilation holes with their fingers and lips and smoke more intensely. The ventilation holes remain open in the ISO regime but are closed in the Intense regime.
Precursors of the volatile compounds could also be a regulatory target as lowering these may also result in lowered yields of the volatile phase monitor components. Two examples well known from the literature are sugars and humectants.
Unprocessed tobacco leaves contain many types of sugars, including glucose, fructose, and sucrose.8–10 Drying (curing) of the leaf can affect these contents; while air-cured tobacco contains virtually no sugars, flue-cured tobacco may contain concentrations up to 25% of its weight.11,12 Different types of sugars and sugar-containing ingredients, such as honey and fruit syrups, are added during manufacturing as binder, casing ingredient, flavor, formulation aid, or humectant.10,11,13 Combustion and pyrolysis processes of sugars, such as caramelization, produce many different chemical compounds, including aldehydes, furan derivatives, VOCs, organic acids, acrylamide, and PAHs.10,14–16 Sugars can also react with amines in tobacco to form Maillard products and aldehydes, ketones (eg, diacetyl), acids, acrylamide, pyrazines, and pyridines.8,10 Adding sugars to tobacco filler primarily enhances the yield of aldehydes and ketones, especially formaldehyde, acetaldehyde, acetone, acrolein, 2-furfural, and other furans.10
Several humectants, such as glycerol and propylene glycol, are commonly used as additives in manufactured cigarettes to maintain the moisture content of the tobacco filler.8,17 The humectant concentrations vary greatly among different cigarettes,18 with typical levels in tobacco filler of 1.1% for glycerol and 1.6% for propylene glycol.8,19 Both glycerol and propylene glycol are on the European Commission’s list of priority additives,20 which has been compiled based on frequency and amount of use and on their reported effects on toxicity, addictiveness or flavor properties.21 When pyrolyzed under conditions that approximate those of a burning cigarette, almost all glycerol present in tobacco is transferred to the pyrolysate, with trace formation of acrolein and glycolaldehyde.8,22,23 Similarly, 1,2-propylene glycol was transferred more than 85% intact, with the formation of small amounts of 1,3-propylene glycol, acetol (hydroxyacetone), acetic anhydride, and pyruvaldehyde.8
We measured the amount of aldehydes, VOCs, and CO in 50 commercially available cigarette brands, with two smoking regimes, ISO and WHO Intense (also known as Health Canadian Intense). As sugars and humectants are the main tobacco product contents and may be related to aldehyde and VOCs yields in cigarette smoke, we also measured these contents. Mutual correlations between analyte levels (in tobacco or smoke) were calculated. For a smaller subset of tobacco and smoke constituents (the latter based on TNCO and/or the WHO priority list), significant correlations (or their non-significance) were exported to make a network description file.
Methods
Materials
Standards of acetaldehyde, acrolein, formaldehyde, glycerol (99%), propylene glycol (99%), triethylene glycol (>99%), 1,3-butanediol (>99%), and fructose (99.9%), D-mannitol (99.9%) and benzene-d6 were purchased from Sigma-Aldrich (Darmstadt, Germany). A 54 VOC standard liquid mixture and standards of crotonaldehyde, butanal, and propanal were purchased from Accu standard Inc. (New Haven, CT). n-Heptadecane (for synthesis), glucose (99.9%), 2-propanol (Ph. Eur.), and sucrose (99.9%) were obtained from Merck (Darmstadt, Germany). Nicotine (>99%) was purchased from Fisher Scientific (Landsmeer, the Netherlands), acetonitrile (HPLC-grade, >99.9%), ethanol (>99.5%), phosphoric acid (85% solution in water), carbon disulphide (CS2), 2,4-dinitrophenylhydrazine (DNPH, >99.5%), and carboxen-572 (CX-572) were from Sigma-Aldrich, while methanol absolute was purchased from Biosolve (Valkenswaard, the Netherlands). Glass fiber filters (44-mm Cambridge filter pads) were purchased from Borgwaldt Gmbh (Germany).
Cigarettes were selected based on variation in added humectants, sugars, and ISO tar, nicotine, and carbon monoxide (TNCO) as apparent from manufacturer data on tobacco products and additives delivered to the Dutch regulator via the Electronic Model Tobacco Control (EMTOC) system and previous studies on sugar levels.24 Commercial cigarettes were sampled at points of sale (2017–2018). Additionally, three common research cigarettes (Kentucky research cigarettes 1R5F and 3R4F [University of Kentucky Tobacco and Health Research Institute, Lexington, KY] and Coresta Monitor 8 [Borgwaldt KC, Hamburg, Germany]) were included.
Sugar Analysis
For each brand, tobacco from all cigarettes in one box was pooled and homogenized. From this pooled sample, two samples were taken and analyzed for glucose, sucrose, and fructose. The sugar analysis was performed according to the method of Jansen et al.,24 including D-mannitol as internal standard on a high performance liquid chromatography (HPLC) system (Pro STAR, Varian, Middelburg, the Netherlands) equipped with an evaporative light scattering detector (ELSD; ZAM 3000, Schambeck SFD GmbH, Bad Honnef, Germany; 80°C, gas flow 1.7 mL/min) using an analytic column MetaCarb 67C (Varian Assoc., Middelburg, the Netherlands). Briefly, sugars were extracted from 1 g of tobacco with 40 mL of MilliQ water at room temperature. The analysis was run using an isocratic elution with MilliQ water, with a flow rate of 0.5 mL/min, column temperature of 85°C, with injection volume of 20 µL and a total runtime of 20 min.
Humectants and Nicotine Analysis
For each brand, tobacco from all cigarettes in one box was pooled and homogenized. From this pooled sample, three samples were taken and analyzed for glycerol, propylene glycol, and triethylene glycol. The analysis was performed according to TobLabNet method SOP06,25 with the following modifications: n-heptadecane was added to the samples as an internal standard for nicotine and nicotine was added to the calibration standard. These components are chromatographically well resolved from the components of interest and do, therefore, not interfere with the analysis.
Briefly, extraction of the components was performed with methanol, followed by measurement on a gas chromatography (GC) system (Shimadzu GC2010, Kyoto, Japan) equipped with a flame ionization detector, using n-heptadecane and 1,3 butanediol as internal standard. Separation of the components was performed using a DB-ALC1 30-m × 0.32-mm × 1.8-µm column (Agilent, Amstelveen, the Netherlands). The initial oven temperature was kept at 100ºC for 5 min, then raised with 40ºC/min to 250°C and held for 4 min. 1 µL of supernatant was injected in the 225°C injection port with a glass wool liner, split ratio 50:1 with a helium flow rate of 1.8 mL/min, and a total runtime of 13 min. We adopted the limit of reporting (LOR) in TobLabNet method SOP0625 as the limit of quantification (LOQ).
Mainstream Smoke Aldehyde and VOC Collection and Analysis
The cigarette smoke was generated on a semiautomated 20-channel, SM450 Cerulean linear smoking machine using the ISO26 and the WHO Intense27,28 smoking regime. Aldehydes and VOCs were sampled on a Cambridge filter pad combined with carboxen-572 cartridges.29,30 For the determination, single cigarettes were smoked in threefold.
Aldehyde determinations were conducted using TobLabNet method SOP08.30 The analysis was conducted on a Shimadzu HPLC system (Shimadzu) equipped with an SPD M20A photo-diode array detector. The mobile phase used was MilliQ water acetonitrile (ACN) using isocratic mode in a mixture of 1:1 (V/V) with a total run time of 40 min with a 1 mL/min flow rate.30 The column temperature was set at 30°C and injection volume of 10 µL was used according to the method.
VOCs were determined by TobLabNet SOP09.31 The determination was performed on a GC–mass spectrometry (GC–MS; Agilent 240 ion trap) using benzene-d6 as internal standard. An Inertcap aquatic-2, GL Sciences of 60-m × 0.25-mm × 1.4-µm was used. The initial oven temperature was kept at 40°C for 6 min., then raised with 6°C/min to 250°C, and held for 9 min. One microliter of the extracted sample was injected in the 200°C injection port with a glass wool liner, split ratio 10:1, a column flow of 1 mL/min, and a total runtime of 50 min. We adopted the LOR in TobLabNet method SOP0830 as the LOQ. To allow for the effect of differences in the amount of tobacco used and for comparison with the precursor concentrations measured in tobacco, tobacco filler weights were determined to convert smoke quantities to amounts per weight of tobacco.
Mainstream Smoke TNCO Analysis
The mainstream cigarette smoke quantities of TNCO were determined according to the ISO26,32–35 or the WHO Intense smoking regime (TobLabNet method SOP0127 and SOP1028) using a semiautomated 20-channel, SM450 Cerulean linear smoking machine. Briefly, nicotine35 and water34 were analyzed using gas chromatograph-coupled with a dual detector (flame ionization detector (FID) and thermal conductivity detector (TCD)) using analytic columns CP-WAX51 (25 m × 0.25 mm × 0.2 µm) for nicotine and porabond Q (25 m × 0.32 mm × 5 µm) for water. The gas chromatograph was obtained from Shimadzu (Shimadzu GC2010, Den Bosch, the Netherlands). Tar was determined by subtracting the weight of nicotine and water from the weight of the particles collected on the Cambridge Filter Pad during smoking. CO was determined in the gas phase of the smoke according to ISO 845433 (ISO regime) or TobLabNet SOP1028 (Intense regime). Tobacco filler weights were determined to convert smoke yields to amounts per weight of tobacco.
Description Dataset for the Statistical Analysis
Starting from the various types of measurement, data for tobacco or smoke composition were converted to weight units (milligrams or micrograms) per gram tobacco. VOCs that exceeded the limit of detection in fewer than 10 samples for the combined ISO and WHO Intense data were rejected as having insufficient data points. The resulting data were collected as a table in Microsoft Excel and used for further statistical analysis and visualization in R version 3.5.1. This dataset comprised measurements on 56 compounds in 53 tobacco products. The 56 compounds comprised 6 tobacco constituents (4 sugars and 2 humectants), 25 smoke constituents under ISO (TNCO, 3 aldehydes, and 19 VOCs), and the same 25 smoke constituents under the Intense regime. Among the 53 tobacco products were 50 cigarettes sampled from the Dutch market and 3 common research cigarettes (1R5F, 3R4F, and CM8).
Statistical Analyses
For each of the compounds, we used the (average) values of each of the 53 individual cigarette brands to determine the following aggregated univariate statistics: the number of data points, the overall aggregated average, SD, minimum, and maximum value, and—where applicable—the ratio between ISO and WHO Intense smoke constituent yields.
Mutual correlations between analyte pairs were calculated as the Pearson correlation coefficient (and the corresponding p-value) over the (typically 53) individual cigarette brand values. p-values were adjusted for multiple testing using the Benjamini–Hochberg False Discovery Rate. Correlation coefficients (R values) were shown as a heat map using the gplots package. For a smaller subset of tobacco and smoke constituents (the latter based on TNCO and/or the WHO priority list), significant correlations were exported to make a network description file for visualization in Cytoscape (www.cytoscape.org).36
For smoke constituents that are included in the TNCO and/or on the WHO priority list and that showed a significant correlation with at least one of the sugar or humectant contents, a regression model was fitted using the yields of the smoke constituent as a function of total sugar, propylene glycol, and glycerol concentrations per brand. The intercept of the model served as an estimate of the smoke constituent yields in the absence of sugars and humectants; this value was used to determine the relative contribution of sugars and humectants to the total smoke emission levels.
Results
General Statistics
Starting from the combined data table, we first calculated various univariate descriptive statistics, the results of which are given in Table 1. The average content found for total sugars was 120 mg/g, propylene glycol 4.6 mg/g, and glycerol 7.8 mg/g. For smoke components, the yields found ranged over several orders of magnitude (Table 1). Ratios between average Intense and ISO varied from 0.08 to 41 but were mostly around 3, with a median value of 3.2.
Table 1.
Statistical summary of tobacco and smoke component levels
| Analyte | n | Average | SD | Min | Max | Ratio INT/ISO |
|---|---|---|---|---|---|---|
| Tobacco | ||||||
| Sucrose (mg/g) | 53 | 13 | 7 | 3 | 28 | |
| Glucose (mg/g) | 53 | 71 | 18 | 16 | 115 | |
| Fructose (mg/g) | 53 | 36 | 11 | 7 | 63 | |
| Sugars total (mg/g) | 53 | 120 | 30 | 30 | 190 | |
| Propylene glycol (mg/g) | 52 | 4.6 | 4.2 | 0 | 14.8 | |
| Glycerol (mg/g) | 52 | 7.8 | 6.3 | 1.1 | 23.7 | |
| Smoke ISO | ||||||
| Tar (mg/g) | 49 | 12 | 5 | 2 | 19 | 3.5 |
| Nicotine (mg/g) | 49 | 1.0 | 0.4 | 0.2 | 2.2 | 3.1 |
| CO (mg/g) | 49 | 13 | 5 | 3 | 20 | 3.3 |
| Formaldehyde (µg/g) | 53 | 20 | 13 | 0 | 58 | 3.4 |
| Acetaldehyde (µg/g) | 53 | 470 | 280 | 0 | 890 | 3.0 |
| Acrolein (µg/g) | 53 | 55 | 37 | 2 | 132 | 4.3 |
| 1,3-Butadiene (µg/g) | 53 | 50 | 21 | 8 | 92 | 3.0 |
| Benzene (µg/g) | 53 | 46 | 19 | 5 | 80 | 2.8 |
| 1,2,4-Trimethyl-benzene (µg/g) | 53 | 3.2 | 2.0 | 0 | 7.5 | 2.3 |
| 1,3,5-Trimethyl-benzene (µg/g) | 53 | 2.4 | 1.3 | 0 | 4.7 | 3.7 |
| Ethylbenzene (µg/g) | 53 | 12 | 6 | 0 | 21 | 3.4 |
| mp-Xylene (µg/g) | 53 | 10 | 5 | 0 | 18 | 3.2 |
| n-Propylbenzene (µg/g) | 53 | 1.7 | 1.1 | 0 | 3.8 | 3.8 |
| o-Xylene (µg/g) | 53 | 4.3 | 2.2 | 0 | 7.9 | 3.3 |
| Styrene (µg/g) | 53 | 11 | 6 | 0 | 25 | 4.1 |
| tert-Butylbenzene (µg/g) | 53 | 1.6 | 0.9 | 0 | 3.0 | 2.8 |
| Toluene (µg/g) | 53 | 92 | 39 | 10 | 157 | 2.8 |
| 2,5-Dimethylfuran (µg/g) | 53 | 9.0 | 3.7 | 1.4 | 16.6 | 2.6 |
| Isoprene (µg/g) | 53 | 93 | 39 | 19 | 196 | 3.1 |
| Phenol (µg/g) | 53 | 6.6 | 4.9 | 0 | 23.1 | 2.5 |
| Naphthalene (µg/g) | 53 | 0.02 | 0.10 | 0 | 0.62 | 41 |
| sec-Butylbenzene (µg/g) | 53 | 0.6 | 0.8 | 0 | 2.9 | 6.0 |
| cis-1,3-Dichloro-1-propene (µg/g) | 53 | 3.3 | 3.1 | 0 | 14.5 | 3.4 |
| trans-1,3-Dichloro-1-propene (µg/g) | 53 | 1.0 | 1.9 | 0 | 6.5 | 1.7 |
| Trichloromethane (µg/g) | 53 | 3.6 | 17.2 | 0 | 123.6 | 0.08 |
| Smoke Intense | ||||||
| Tar (mg/g) | 40 | 40 | 7 | 26 | 53 | |
| Nicotine (mg/g) | 40 | 3.1 | 0.5 | 2.1 | 4.3 | |
| CO (mg/g) | 40 | 42 | 10 | 22 | 71 | |
| Formaldehyde (µg/g) | 53 | 67 | 20 | 28 | 118 | |
| Acetaldehyde (µg/g) | 53 | 1400 | 200 | 900 | 2000 | |
| Acrolein (µg/g) | 53 | 230 | 40 | 150 | 330 | |
| 1,3-Butadiene (µg/g) | 53 | 150 | 30 | 90 | 250 | |
| Benzene (µg/g) | 53 | 130 | 20 | 80 | 170 | |
| 1,2,4-Trimethyl-benzene (µg/g) | 53 | 7.4 | 4.1 | 2.9 | 21.4 | |
| 1,3,5-Trimethyl-benzene (µg/g) | 53 | 9.0 | 1.6 | 5.7 | 12.1 | |
| Ethylbenzene (µg/g) | 53 | 40 | 6 | 26 | 55 | |
| mp-Xylene (µg/g) | 53 | 32 | 5 | 21 | 43 | |
| n-Propylbenzene (µg/g) | 53 | 6.3 | 0.9 | 4.3 | 8.6 | |
| o-Xylene (µg/g) | 53 | 14 | 2 | 10 | 19 | |
| Styrene (µg/g) | 53 | 45 | 8 | 30 | 59 | |
| tert-Butylbenzene (µg/g) | 53 | 4.6 | 1.3 | 2.0 | 7.6 | |
| Toluene (µg/g) | 53 | 260 | 40 | 170 | 360 | |
| 2,5-Dimethylfuran (µg/g) | 53 | 23 | 5 | 6 | 38 | |
| Isoprene (µg/g) | 53 | 290 | 60 | 120 | 430 | |
| Phenol (µg/g) | 53 | 16 | 10 | 4 | 55 | |
| Naphthalene (µg/g) | 53 | 0.8 | 0.6 | 0 | 1.9 | |
| sec-Butylbenzene (µg/g) | 53 | 3.8 | 2.2 | 0 | 8.9 | |
| cis-1,3-Dichloro-1-propene (µg/g) | 53 | 11 | 14 | 0 | 49 | |
| trans-1,3-Dichloro-1-propene (µg/g) | 53 | 1.7 | 4.4 | 0 | 16.2 | |
| Trichloromethane (µg/g) | 53 | 0.3 | 0.6 | 0 | 1.9 |
Correlations Between Smoke Components
We determined the correlations between the various compound levels and found that 518 out of 1540 possible associations were statistically significant. This corresponded to an absolute R-value >0.32 (p <.009; false discovery rate (FDR) 5%; Supplementary Table 1). Significant correlations were found more often in cases where analytes of the same class (aldehydes or VOCs) were determined under the same smoking regime (ISO or Intense; Supplementary Table 1), which points at a common aspect of their formation during the combustion process. More specifically, 212 significant correlations were found for cases where two compounds were measured under ISO, 128 for cases where both measurements were under Intense regime, 146 for an ISO–Intense combination. The other 32 significant correlations included at least one sugar or humectant determination.
Correlations Between Precursors and Smoke Components
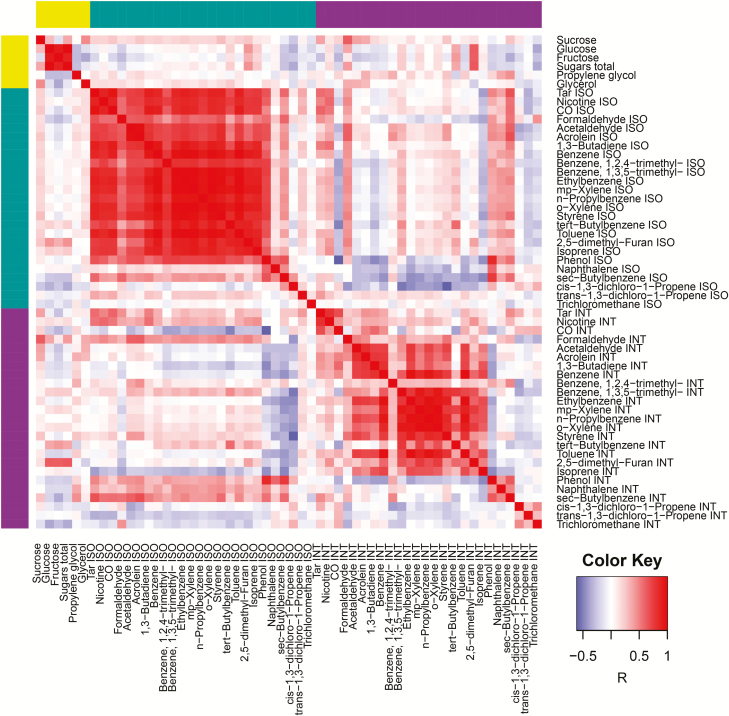
We found several significant correlations between aldehydes and precursor ingredients in unburnt tobacco (Figure 1) but only for the Intense smoking regime. These correlations were most often found for formaldehyde, which showed a significant correlation with sucrose, glucose, total sugars, and glycerol. For VOCs, significant correlations were found less frequently, but 2,5-dimethylfuran showed a correlation with several sugars under both ISO and Intense smoking conditions. Among the various sugars, sucrose showed the highest number of significant correlations, namely to tar, formaldehyde, acetaldehyde, and acrolein, all under Intense conditions.
Figure 1.
Correlation heatmap. Row and column colors: ochre, tobacco; teal, International Standards Organization (ISO); purple, Intense.
Correlation Network Analysis
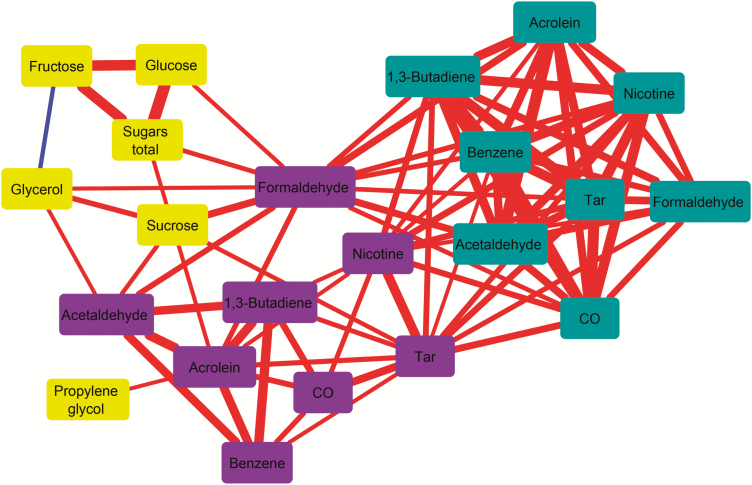
For further interpretation of the various mutual correlations between analyte relations, we visualized a subset of the data as a correlation network (Figure 2). For this, we used the significant correlations between tobacco components and/or TNCO and/or volatile smoke components listed by the WHO (CO, formaldehyde, acetaldehyde, acrolein, butadiene, and benzene) as network connections. This resulted in a network with 18 nodes and 50 edges (Figure 2). It can be seen from the network that smoke components within each regime are strongly connected, which results in the network having three subnetworks that correspond to sugars and glycerol, ISO smoke components, and Intense smoke components, respectively. There are several connections between the ISO and Intense subnetworks, mainly by means of Intense tar and Intense formaldehyde acting as connecting hubs. Additionally, the sugar subnetwork shows connections to the Intense subnetwork by means of sucrose and Intense formaldehyde acting as hubs. In agreement with these findings, Intense formaldehyde is the most connected compound in the network, with a total number of 13 connections.
Figure 2.
Correlation network for tar, nicotine, and carbon monoxide and World Health Organization Tobacco Product Regulation compounds. Node colors: ochre, tobacco; teal, International Standards Organization; purple, Intense. Line colors: red, positive correlation; blue, negative correlation. Line width indicates correlation strength and is proportional to the correlation coefficient.
Relative Contribution of Sugars and Humectants to Smoke Constituent Yields
The influence of sugar and humectant concentrations on the emission yields of smoke constituents is consistently significant under Intense conditions (Table 1; Figures 1 and 2). Moreover, most of the significant correlations were found for TNCO and/or WHO-prioritized compounds (Supplementary Table 1). To gain insight into the effects of product legislation guidelines, we estimated the relative amount for WHO-prioritized smoke constituents that can be attributed to sugars and humectants by means of regression modeling. The results are shown in Table 2. For constituents where a significant correlation was found, the relative contribution ranges from 2% (tar) to 85% (formaldehyde). For other components, relative contribution values are not significantly associated with any sugar or humectant (Table 2).
Table 2.
Relative contribution by sugars and humectants to smoke constituent yields
| Smoke constituent | Relative contribution |
|---|---|
| Tar | 2% |
| Nicotine | Not significant |
| CO | Not significant |
| Formaldehyde | 85% |
| Acetaldehyde | 6% |
| Acrolein | 17% |
| 1,3-Butadiene | Not significant |
| Benzene | Not significant |
Discussion
In a multivariate analysis of aldehydes, VOCs, and TNCO yields in cigarette mainstream smoke and sugars and humectant concentrations in tobacco filler, we found several strong connections. Overall, Intense formaldehyde is the most connected compound in the network, with a total number of 13 connections. This indicates that Intense formaldehyde as a single measurement would be significantly predictive for 13 other smoke or tobacco constituents as in shown Figure 2. However, it needs to be noted that formaldehyde’s predictive value may change as the products change over time. Manufacturing processes may change, for example, in response to regulation (type of tobacco, tobacco blends, inclusion of new additives, and removal of additives such as sugars and humectants) and, therefore, monitoring should not be restricted to a single component.
In general, the correlations between smoke components are higher for smoke generated with the ISO regime as compared to the WHO Intense regime, which is presumably due to the relative range (SD/average) being higher for the ISO regime as a result of additional variation due to filter ventilation. This additional source of variation can contribute to different smoke component yields in a similar way and, thus, lead to higher and more often significant correlation coefficients within the ISO subset. The ISO and Intense subnetworks are connected, mainly by means of Intense tar and Intense formaldehyde.
Moreover, we found several significant correlations between aldehydes and precursor ingredients in unburnt tobacco but only for the Intense smoking regime. With that regime, emission yields are around three times higher than with the ISO regime, leading more often to significant correlations.
These correlations were most often found for formaldehyde, which showed a significant correlation with sucrose, glucose, total sugars, and glycerol. For VOCs, significant correlations were found less frequently as expected, but 2,5-dimethylfuran showed a correlation with several sugars under both ISO and Intense smoking conditions. Among the various sugars, sucrose showed the highest number of significant correlations, namely to tar, formaldehyde, acetaldehyde, and acrolein, all under Intense conditions. Sucrose and Intense formaldehyde acted as hubs connecting the sugar subnetwork to the Intense subnetwork.
Relations Between Aldehydes in Mainstream Smoke
High correlations between individual aldehydes have been reported before but not for formaldehyde.37,38 We also found significant but rather lower correlations between formaldehyde and acetaldehyde (R = 0.49) and acrolein (R = 0.45) than between the two other aldehydes (R = 0.80). Formaldehyde is a small, volatile molecule and very reactive. In previous studies, this lower correlation was hypothesized37 to be partly due to the wide analytic variation and problems with trapping already reported by Uchiyama et al.29 Reilly et al. hypothesized that this may be due to part of the formaldehyde already being present in tobacco leaf, unlike the other aldehydes purely resulting from combustion.38 We think that this seems unlikely for such a highly volatile compound and find Reilly’s other suggestions more likely, which is that it could be that formaldehyde has more important precursors in tobacco leafs. In conclusion, while formaldehyde yields are significantly predictive for those of many precursor ingredients in the tobacco leaf, they are less typical as a representative of the aldehyde class.
Sugar and Humectants as Precursors of Volatile Toxicants in Mainstream Smoke
The finding that aldehydes, but not benzene or butadiene, are considerably and significantly correlated to sugars in tobacco is in line with pyrolysis data on sugars that show the formation of formaldehyde and, to a lesser extent, other aldehydes.10,39 Previous publications, mostly from the tobacco industry, concluded that adding sugars to tobacco increases many compounds, including phenol, furans, 2-butanone, isoprene, benzene, toluene, benzo[k]fluoranthene, organic acids, 2-furfural, acrolein, and, most prominently, formaldehyde.8,9,40–43 It was also concluded that an association between sugars and acetaldehyde does not exist,8,41–43 but reanalysis of some industry studies showed that added sugars determined 10%–50% of the acetaldehyde levels.44 Recent work of Cheah et al. also shows that the addition of sugars to Burley tobacco, which contains virtually no natural sugars, causes an increase of the aldehydes acetaldehyde, acrolein, crotonaldehyde, propionaldehyde, and butanal in the mainstream tobacco smoke.45 2,5-Dimethylfuran showed a correlation with several sugars under both ISO and Intense smoking conditions and is known to be formed upon thermal degradation of some sugars.10,46
Regarding humectants, a relation was found between glycerol and Intense formaldehyde and Intense acetaldehyde and propylene glycol and Intense acrolein. Literature data on pyrolysis of humectants did not show these relations but, in aerosol of electronic cigarettes, with carrier liquids consisting of glycerol and/or propylene glycol, formaldehyde, acetaldehyde, and acrolein were produced by the degradation of these e-liquids.47,48
Regulatory Implications
The strong correlations found in our study between the aldehydes, VOCs, and TNCO yields measured in cigarette mainstream smoke for both ISO and WHO Intense smoking conditions suggest that formaldehyde, acetaldehyde, acrolein, benzene, and butadiene are a representative selection for the aldehyde and VOCs classes as advised by TobReg.2 In general, the most and strongest correlations were observed with the Intense regime, which is an additional argument to include or exclusively use this regime for regulatory purposes. Another important argument is that the ISO regime is fundamentally flawed, and filter ventilation helps to mislead the public by the false perception that “low-delivery” cigarettes deliver reduced quantities of harmful constituents. However, the vast body of evidence shows that smokers “self-titrate” their respective nicotine doses via compensatory smoking (blocking vent holes with fingers or lips, take large puffs or more frequent puffs).7
Our finding that several significant correlations exist between aldehydes and the precursor ingredients sugars and glycerol in unburnt tobacco and, for the VOC, 2,5-dimethylfuran with several sugars (the latter also with ISO conditions) implies that the smoke yields of these compounds will potentially decrease when sugar and humectants content concentrations are lowered in tobacco filler. An additional argument for lowering is that consumer acceptance of cigarette mainstream smoke is proportional to the sugar content and the humectants content of the tobacco blend.8,49 Thus, policymakers are advised to consider these two classes of tobacco ingredients as targets for future tobacco products content regulations, being mindful that the ingredients are not substituted with alternatives that have an even larger health impact. As sugars can be both naturally present or added as tobacco ingredient, it is important to regulate total sugar contents and not just added sugar contents.
Strength, Limitations, and Future Research
The current study, a multivariate analysis of relations within and between several contents and emissions in a market sample of more than 50 cigarette brands, corroborates previous results regarding the relation between sugars in tobacco and aldehydes in smoke.45 Moreover, the current results indeed make clear that the relation even holds when sugar contents are not the only variable as in the previous study, where sugars were added to Burley tobacco that contains virtually no sugars. Commercial cigarettes, on the other hand, differ in many aspects regarding their physical design and the composition of the tobacco blend.50
There are some limitations to our study too. First, for Intense TNCO, our dataset contains 40 data points instead of 53 for the other compounds. Some cigarette brands with more than one product on the Dutch market may not have all of their products included in the dataset, and the same applies to the three common research cigarettes. However, we expect that this will still capture the product variation in the overall market. Second, it needs to be noted that the correlations we found between the content and emission yields are not necessarily proof of causation as the relation may also be due to a mediating factor, such as other product constituents or burning temperature. For instance, relative amounts listed in Table 2 indicate percentages attributed to sugar and humectants contents (up to 85%), but that is not necessarily through direct combustion product formation. Although in line with previous findings, the relation could also be caused by means of association with other product properties that play a role, such as burning temperature or filter ventilation. Studying the causality of these relations could be the subject of future work.
Regarding other future research topics, the finding that correlations between tobacco composition and smoke toxicant emissions are stronger under the Intense regime indicates that ISO-based findings and recommendations previously reported in the literature may need further scrutiny. Finally, future research should also study the particulate phase toxicants in cigarette smoke selected by TobReg (ie, TSNAs [NNN, and NNK] and PAHs [BaP]),2 their mutual relations, and their relations to precursors in unburned tobacco.
Conclusions
A multivariate analysis of toxicants in the volatile fraction of cigarette mainstream smoke, and their potential precursors sugars and humectants in tobacco filler, showed several connections between these compounds determined in a market sample of more than 50 brands. For both ISO and WHO Intense smoking conditions, we found strong correlations between the aldehydes, VOCs, and TNCO yields measured in cigarette mainstream smoke. Thus, for smoke component market monitoring purposes, the monitor compounds proposed by TobReg (resp. formaldehyde, acetaldehyde, acrolein, and benzene, and butadiene) are a representative selection for the aldehyde and VOCs classes. In particular, formaldehyde yields may be useful to monitor exposure levels to other toxicants under Intense conditions.
Using Intense smoking conditions, we found several significant correlations between aldehydes and the precursor ingredients sugars and glycerol in unburnt tobacco and, for the VOC, 2,5-dimethylfuran with several sugars (the latter also with ISO conditions). Moreover, most of the significant correlations between contents and emissions were found for WHO-prioritized compounds. This implies that emissions of formaldehyde, acetaldehyde, and acrolein, the toxicants advised by WHO for mandated lowering, as well as 2,5-dimethylfuran, will potentially decrease when sugar and humectants contents are lowered in tobacco filler.
Funding
This work was supported by the Netherlands Food and Consumer Product Safety Authority (project number 9.7.1).
Declaration of Interest
None declared.
Supplementary Material
Acknowledgments
The authors wish to thank Peter van Lierop, Walter Romagnolo, and Pieter Dingemanse for their technical assistance. Wouter Visser and Nuan Ping Cheah are acknowledged for critically reviewing the manuscript.
References
- 1. WHO Framework Convention on Tobacco Control. Partial guidelines for implementation of articles 9 and 10—regulation of the contents of tobacco products and regulation of tobacco product disclosures 2012. https://www.who.int/fctc/guidelines/Guideliness_Articles_9_10_rev_240613.pdf. Accessed November 7, 2019.
- 2. Burns DM, Dybing E, Gray N, et al. Mandated lowering of toxicants in cigarette smoke: a description of the World Health Organization TobReg proposal. Tob Control. 2008;17(2):132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health. 2011;8(2):613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodgman A, Perfetti TA.. The Chemical Components of Tobacco and Tobacco Smoke. New York, NY: CRC Press, Taylor and Francis Group; 2013. [Google Scholar]
- 5. Cai B, Li Z, Wang R, et al. Emission level of seven mainstream smoke toxicants from cigarette with variable tobacco leaf constituents. Regul Toxicol Pharmacol. 2019;103:181–188. [DOI] [PubMed] [Google Scholar]
- 6. WHO Framework Convention on Tobacco Control. Decision FCTC/COP7(14) Further development of the partial guidelines for implementation of Articles 9 and 10 of the WHO FCTC (regulation of the contents of tobacco products and Regulation of tobacco product disclosures) Tobacco Regulatory Science. 2016;4(2):61–72(12). https://www.who.int/fctc/cop/cop7/FCTC_COP7(14)_EN.pdf. Accessed November 7, 2019. [Google Scholar]
- 7. Talhout R, Richter PA, Stepanov I, Watson CV, Watson CH. Cigarette design features: effects on emission levels, user perception, and behavior. Tob Regul Sci. 2018;4(1):592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klus H, Scherer G, Müller L. Influence of additives on cigarette related health risks. Beitr Tabakforsch Int. 2012;25(3):412. [Google Scholar]
- 9. Roemer E, Schorp MK, Piadé JJ, Seeman JI, Leyden DE, Haussmann HJ. Scientific assessment of the use of sugars as cigarette tobacco ingredients: a review of published and other publicly available studies. Crit Rev Toxicol. 2012;42(3):244–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Talhout R, Opperhuizen A, van Amsterdam JG. Sugars as tobacco ingredient: effects on mainstream smoke composition. Food Chem Toxicol. 2006;44(11):1789–1798. [DOI] [PubMed] [Google Scholar]
- 11. Leffingwell JC. Leaf chemistry: basic chemical constituents of tobacco leaf and differences among tobacco types. In: Davis DL, Nielsen MT, eds. Tobacco: Production, Chemistry and Technology. Oxford, UK: Blackwell Science; 1999:265–284. [Google Scholar]
- 12. Cahours X, Verron T, Purkis S. Effect of sugar content on acetaldehyde yield in cigarette smoke. Beitr Tabakforsch Int. 2014;25(2):381. [Google Scholar]
- 13. Seeman JI, Laffoon SW, Kassman AJ. Evaluation of relationships between mainstream smoke acetaldehyde and “tar” and carbon monoxide yields in tobacco smoke and reducing sugars in tobacco blends of U.S. commercial cigarettes. Inhal Toxicol. 2003;15(4):373–395. [DOI] [PubMed] [Google Scholar]
- 14. Fagerson IS. Thermal degradation of carbohydrates; a review. J Agric Food Chem. 1969;17(4):747–750. [Google Scholar]
- 15. Mattonai M, Tamburini D, Colombini MP, Ribechini E. Timing in analytical pyrolysis: Py(HMDS)-GC/MS of glucose and cellulose using online micro reaction sampler. Anal Chem. 2016;88(18):9318–9325. [DOI] [PubMed] [Google Scholar]
- 16. Mattonai M, Ribechini E. A comparison of fast and reactive pyrolysis with insitu derivatisation of fructose, inulin and Jerusalem artichoke (Helianthus tuberosus). Anal Chim Acta. 2018;1017:66–74. [DOI] [PubMed] [Google Scholar]
- 17. Hoffmann D, Hoffmann I. The changing cigarette, 1950-1995. J Toxicol Environ Health. 1997;50(4):307–364. [DOI] [PubMed] [Google Scholar]
- 18. Rainey CL, Shifflett JR, Goodpaster JV, Bezabeh DZ. Quantitative analysis of humectants in tobacco products using gas chromatography (GC) with simultaneous mass spectrometry (MSD) and flame ionization detection (FID). Beitr Tabakforsch Int. 2014;25(6):576. [Google Scholar]
- 19. SCENIHR. Opinion on Additives Used in Tobacco Products (Opinion 1). Luxembourg: European Commission, Scientific Committee on Emerging and Newly Identified Health Risks; 2016. https://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_051.pdf. Accessed November 7, 2019. [Google Scholar]
- 20. SCHEER. Opinion on Additives Used in Tobacco Products (Opinion 2). Luxembourg: European Commission, Scientific Committee on Health, Environmental and Emerging Risks; 2016. https://ec.europa.eu/health/sites/health/files/scientific_committees/scheer/docs/scheer_o_001.pdf. Accessed November 7, 2019. [Google Scholar]
- 21. Hoet P, Rydzynski K, Vermeire T, et al. Recommendations to the European Commission implementing a priority list of additives that should have more stringent reporting requirements: The opinion of the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). Tob Control. 2018;27(2):225–228. [DOI] [PubMed] [Google Scholar]
- 22. Baker RR, Bishop LJ. The pyrolysis of tobacco ingredients. J Anal Appl Pyrolysis. 2004;71(1):223–311. [Google Scholar]
- 23. Purkis SW, Mueller C, Intorp M. The fate of ingredients in and impact on cigarette smoke. Food Chem Toxicol. 2011;49(12):3238–3248. [DOI] [PubMed] [Google Scholar]
- 24. Jansen E, Cremers J, Borst S, Talhout R. Simple determination of sugars in cigarettes. J Anal Bioanal Tech. 2014;5(6):1–3. [Google Scholar]
- 25. WHO TobLabNet. SOP 6—Standard operating procedure for determination of humectants in cigarette tobacco filler 2016. https://www. who.int/tobacco/publications/prod_regulation/sop-humectants- cigarette-tobacco-filler/en/. Accessed November 7, 2019.
- 26. ISO4387:2000. Routine Analytical Cigarette-Smoking Machine—Definitions and Standard Conditions. Geneva, Switzerland: : International Organization for Standardization; 2011; Amd1:2008: 1–25. [Google Scholar]
- 27. WHO TobLabNet. SOP 1—standard operating procedure for intense smoking of cigarettes 2012. https://www.who.int/tobacco/publications/prod_regulation/sop_smoking_cigarettes_1/en/. Accessed November 7, 2019.
- 28. WHO TobLabNet. SOP 10—standard operating procedure for determination of nicotine and carbon monoxide in mainstream cigarette smoke under intense smoking conditions 2016. https://www.who.int/tobacco/publications/prod_regulation/sop_smoking_cigarettes_1/en/. Accessed November 7, 2019.
- 29. Uchiyama S, Tomizawa T, Inaba Y, Kunugita N. Simultaneous determination of volatile organic compounds and carbonyls in mainstream cigarette smoke using a sorbent cartridge followed by two-step elution. J Chromatogr A. 2013;1314:31–37. [DOI] [PubMed] [Google Scholar]
- 30. WHO TobLabNet. SOP 8—standard operating procedure for determination of aldehydes in mainstream cigarette smoke under ISO and intense smoking conditions 2018. https://www.who.int/tobacco/publications/prod_regulation/standard-operation-validation-08/en/. Accessed November 7, 2019.
- 31. WHO TobLabNet. SOP 9—standard operating procedure for determination of volatile organics in mainstream cigarette smoke under ISO and intense smoking conditions 2018. https://www.who.int/tobacco/publications/prod_regulation/standard-operation-validation-09/en/. Accessed November 7, 2019.
- 32. ISO3308:2012. Cigarettes—Determination of Total and Nicotine-Free Dry Particulate Matter Using a Routine Analytical Smoking Machine. Geneva, Switzerland: International Organization for Standardization; 2012. [Google Scholar]
- 33. ISO8454:2007. Cigarettes—Determination of Carbon Monoxide in the Vapour Phase of Cigarette Smoke—NDIR Method. Geneva, Switzerland: : International Organization for Standardization; 2010;1–7. [Google Scholar]
- 34. ISO10362-1:1999. Cigarettes—Determination of Water in Smoke Condensates—Part 1: Gas-Chromatographic Method. Geneva, Switzerland: : International Organization for Standardization; 2010;1–7. [Google Scholar]
- 35. ISO10315:2013. Cigarettes—Determination of Nicotine in Smoke Condensates—Gas-Chromatographic Method. Geneva, Switzerland: : International Organization for Standardization; 2013;1–7. [Google Scholar]
- 36. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pauwels CGGM, Klerx WNM, Pennings JLA, et al. Cigarette filter ventilation and smoking protocol influence aldehyde smoke yields. Chem Res Toxicol. 2018;31(6):462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reilly SM, Goel R, Trushin N, et al. Brand variation in oxidant production in mainstream cigarette smoke: carbonyls and free radicals. Food Chem Toxicol. 2017;106(Pt A):147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baker RR, Coburn S, Liu C. The pyrolytic formation of formaldehyde from sugars and tobacco. J Anal Appl Pyrolysis. 2006;77(1):12–21. [Google Scholar]
- 40. Coggins CR, Wagner KA, Werley MS, Oldham MJ. A comprehensive evaluation of the toxicology of cigarette ingredients: carbohydrates and natural products. Inhal Toxicol. 2011;23(Suppl 1):13–40. [DOI] [PubMed] [Google Scholar]
- 41. Baker RR. Sugars, carbonyls and smoke. Food Chem Toxicol. 2007;45(9):1783–1786. [DOI] [PubMed] [Google Scholar]
- 42. Baker RR. The generation of formaldehyde in cigarettes–overview and recent experiments. Food Chem Toxicol. 2006;44(11):1799–1822. [DOI] [PubMed] [Google Scholar]
- 43. Hahn J, Schaub J. Influence of tobacco additives on the chemical composition of mainstream smoke. Beitr Tabakforsch Int. 2014;24(3):100. [Google Scholar]
- 44. O’Connor RJ, Hurley PJ. Existing technologies to reduce specific toxicant emissions in cigarette smoke. Tob Control. 2008;17(Suppl 1):i39–i48. [DOI] [PubMed] [Google Scholar]
- 45. Cheah NP, Borst SE, Hendrickx LPA, et al. Effect of adding sugar to Burley tobacco on the emission of aldehydes in mainstream tobacco smoke. Tob Regul Sci. 2017. [Google Scholar]
- 46. Román-Leshkov Y, Barrett CJ, Liu ZY, Dumesic JA. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature. 2007;447(7147):982–985. [DOI] [PubMed] [Google Scholar]
- 47. Sleiman M, Logue JM, Montesinos VN, et al. Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environ Sci Technol. 2016;50(17):9644–9651. [DOI] [PubMed] [Google Scholar]
- 48. Jensen RP, Strongin RM, Peyton DH. Solvent chemistry in the electronic cigarette reaction vessel. Sci Rep. 2017;7:42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rodgman A. Some studies of the effects of additives on cigarette mainstream smoke properties. II. Casing materials and humectants. Beitr Tabakforsch Int. 2002;20(4):279. [Google Scholar]
- 50. Agnew-Heard KA, Lancaster VA, Bravo R, Watson C, Walters MJ, Holman MR. Multivariate statistical analysis of cigarette design feature influence on ISO TNCO yields. Chem Res Toxicol. 2016;29(6):1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.