Abstract
Background
Nowadays, microbial infections have caused increasing economic losses in aquaculture industry and deteriorated worldwide environments. Many of these infections are caused by opportunistic pathogens through cell-density mediated quorum sensing (QS). The disruption of QS, known as quorum quenching (QQ), is an effective and promising way to prevent and control pathogens, driving it be the potential bio-control agents. In our previous studies, AHL lactonase AiiK was identified with many characteristics, and constitutive expression vector pELX1 was constructed to express heterologous proteins in Lactobacillus casei MCJΔ1 (L. casei MCJΔ1). In this study, recombinant strain pELCW-aiiK/L. casei MCJΔ1 (LcAiiK) and wild-type Aeromonas hydrophila (A. hydrophila) were co-cultured to test the QQ ability of LcAiiK against A. hydrophila.
Results
A cell wall-associated expression vector pELCW for L. casei MCJΔ1 was constructed. Localization assays revealed that the expressed AiiK was anchored at the surface layer of LcAiiK via vector pELCW-aiiK. LcAiiK (OD600 = 0.5) degraded 24.13 μM of C6-HSL at 2 h, 40.99 μM of C6-HSL at 12 h, and 46.63 μM of C6-HSL at 24 h. Over 50% LcAiiK cells maintained the pELCW-aiiK plasmid after 15 generations of cultivation without erythromycin. Furthermore, LcAiiK inhibited the swimming motility, extracellular proteolytic activity, haemolytic activity and biofilm formation of A. hydrophila AH-1 and AH-4.
Conclusion
The AHL lactonase AiiK is firstly and constitutively expressed at the surface layer of L. casei MCJΔ1. LcAiiK displayed considerable AHL lactonase activity and great QQ abilities against A. hydrophila AH-1 and AH-4 by attenuating their QS processes instead of killing them. Therefore, the LcAiiK can be exploited as an anti-pathogenic drug or a bio-control agent to control the AHL-mediated QS of pathogenic bacteria.
Keywords: Quorum sensing, Quorum quenching, AHL lactonase AiiK, Lactobacillus casei MCJΔ1, Aeromonas hydrophila
Introduction
Microbial infections have caused increasing economic losses in aquaculture industry and deteriorated worldwide environments year by year [1]. The acute hemorrhagic septicemia in fish and diarrhea in human caused by microbial infections are reported frequently [2–5]. These symptoms are mainly caused by a kind of gram-negative opportunistic pathogens [3–5]. Aeromonas hydrophila (A. hydrophila) is a representative gram-negative opportunistic pathogen, ubiquitous in fresh and estuarine water, and can infect fish, crabs, shrimps, and even humans [4, 6]. A. hydrophila can also cause various symptoms including tissue swelling, necrosis, and ulceration in fish [7]. The bacterial infections in fish and human depend on the cell-density mediated system termed quorum sensing (QS) [6].
QS, a cell-to-cell communication mechanism in bacteria, coordinates the expression of specialized structural gene sets via specific receptors-sensing signal molecules when bacteria present at high cell densities [8, 9]. The N-Acyl homoserine lactone (AHL), a common and important QS signal molecule in gram-negative bacteria, consists of a homoserine lactone and an acyl side chain of four or more carbon atoms. AHL-mediated QS traits encompass virulence factor production, swarming motility, biofilm maturation, and so on, which impart a significant advantage in survival of bacterial populations [10–12].
AHL-mediated QS triggers the expression of potential virulence and pathogenicity factors, including production of cytotoxic enterotoxin, exoprotease, lipase and hemolysin, shifting of swarming motility, and formation of biofilm in A. hydrophila [6, 13]. The biofilm formation of A. hydrophila stimulates strong resistance to multiple antibiotics [14]. Furthermore, virulence factors and swarming motility associated with QS make the diseases and infections caused by A. hydrophila hard to be cured [14]. However, finding new and effective antibiotics is harder, and thus therapies for controlling the QS-mediated pathogenicity without causing the emergence of resistance are promising alternatives [15, 16]. The disruption of QS, known as quorum quenching (QQ), is of great potential in alleviating the detrimental symptoms caused by QS-mediated pathogenic events, so it could be applied as a bio-control measure for pathogenic bacterial prevention and control [9, 17–20].
In recent years, four QQ approaches have been employed to inactivate the signal molecules and alleviate the symptoms of bacterial infections caused by QS: (1) using the purified QQ enzymes, (2) expressing QQ enzymes in pathogenic bacteria, (3) isolating and identifying new QQ strains, and 4) constructing recombinant QQ strains (Table 1). In the first case, AHL lactonases AidP [9], MomL [17], AiiK [18], RmmL [19], AiiAQSI-1 [20], and AiiAB546 [21] were expressed by Escherichia coli or Pichia pastoris, purified and used to ease the pathogenicity of different pathogens. The purified QQ enzymes functioned very well; however, the purification was complicated and the cost for purification was high. The purified AHL lactonases had poor resistance to environment when applied in a real situation. In the second case, AHL lactonases AiiA [22], AiiM [23], AttM [24], and HqiA [25] were expressed in different pathogens, and their pathogenicity decreased. This approach occurred only under ideal research conditions, because pathogens are difficult to be modified in true situation. In the third case, Bacillus licheniformis T-1 isolated from freshwater was found to have reduced the pathogenicity of A. hydrophila cb15 [7]. This was due to the presence of QQ enzyme gene in the isolated B. licheniformis T-1; however, it is very difficult to isolate a strain with great AHL lactonase activity because of lacking suitable and efficient screening methods. In the last case, Zhang et al. constructed a recombinant QQ strain BbMomL, and it significantly reduced the secretion of pathogenic factors and the pathogenicity of P. carotovorum subsp. carotovorum and Pseudomonas aeruginosa PAO1 [26]. After the recombinant QQ strain was constructed, it was applied directly against pathogens without further purification steps. Although most previous studies have focused on applying purified QQ enzymes or expressing QQ enzymes in pathogenic bacteria to inactivate the AHLs, little work has been done on constructing a recombinant QQ strain expressing the AHL lactonase on its surface to directly attenuate the symptoms caused by QS.
Table 1.
Four QQ approaches were used to alleviate the effects of QS against pathogenic bacteria
| Approaches of QQ | Enzyme type | Origin | Expression strains | Application and references | |
|---|---|---|---|---|---|
| Purified QQ enzymes | AidP | AHL lactonase | Planococcus sp. | E. coli BL21 | AidP attenuated the pathogenicity of P. carotovorum in Chinese cabbage [2] |
| MomL | AHL lactonase | M. olearia Th120 | E. coli BL21(DE3) | MomL attenuated virulence of P. aeruginosa in a Caenorhabditis elegans infection mode [17] | |
| AiiK | AHL lactonase | K. huakuii | E. coli BL21(DE3) | AiiK inhibited biofilm formation and attenuated extracellular proteolytic activity and pyocyanin production of P. aeruginosa PAO1 [18] | |
| RmmL | AHL lactonase | R. mobilis YJ3 | E. coli BL21(DE3) | RmmL reduced pyocyanin production of P. aeruginosa PAO1 and extracellular protease activity of V. anguillarum VIB72 [19] | |
| AiiAQSI-1 | AHL lactonase | Bacillus sp. QSI-1 | E. coli BL21(DE3) | AiiAQSI-1 inhibited swimming motility, extracellular protease, hemolysin factor, and biofilm formation of A. hydrophila YJ-1 [20] | |
| AiiAB546 | AHL lactonase | Bacillus sp. B546 | P. pastoris | AiiAB546 decreased mortality rate and delayed mortality time of fish when co-injection with A. hydrophila in common carp [21] | |
| Expressed QQ enzymes in pathogenic bacteria | AiiA | AHL lactonase | Bacillus sp. 240B1 | E. carotovora stain SCG1 | The introduction of aiiA gene in E. carotovora stain SCG1 decreased extracellular pectolytic activities and pathogenicity of E. carotovora [22] |
| AiiM | AHL lactonase | M. testaceum StLB037 | P. c. c. NBRC 3830 | The introduction of aiiM gene in P. carotovorum subsp. carotovorum NBRC 3830 attenuated soft rot symptoms on potato slices [23] | |
| AttM | AHL lactonase | A. tumefaciens c58 | S. scabies | The introduction of attM gene suppressed pathogenicity of S. scabies towards potato tuber [24] | |
| HqiA | AHL lactonase | Metagenomic library from soil | P. c. c. CECT 225T | The introduction of hqiA gene in plant pathogen P. carotovorum efficiently interfered swarming motility and maceration enzymes production [25] | |
| Isolated and identified new QQ strains | B. licheniformis T-1 | AHL lactonase | Freshwater culture pond sediment | No | B. licheniformis T-1 reduced pathogenicity of A. hydrophila cb15 in zebrafish coinjection [12] |
| Constructed recombinant QQ strains | BbMomL | AHL lactonase | M. olearia Th120 | B. brevis | BbMomL reduced secretion of pathogenic factors and pathogenicity of P. carotovorum subsp. carotovorum and P. aeruginosa PAO1 [26] |
| LcAiiK | AHL lactonase | K. huakuii | L. casei MCJΔ1 | LcAiiK attenuated swimming motility, virulence factor production, and biofilm formation of A. hydrophila AH-1 and AH-4 (This study) | |
In our previous studies, a constitutive expression vector pELX1 was constructed and used to intracellularly express heterologous proteins in Lactobacillus casei MCJΔ1 (L. casei MCJΔ1) [27]. AiiK, identified as an AHL lactonase from Kurthia huakuii LAM0618T (K. huakuii LAM0618T), showed characteristics of efficient degradation of AHLs, variable substrate spectrum, suitable thermostability, and great protease-resistance [18]. In the present study, in order to express the AiiK at surface layer of L. casei MCJΔ1, plasmid pELCW-aiiK was constructed and transformed into L. casei MCJΔ1. The recombinant strain pELCW-aiiK/L. casei MCJΔ1 (LcAiiK) was co-cultured separately with A. hydrophila AH-1 and AH-4 to test its QQ ability against A. hydrophila, an opportunistic pathogen isolated from dead grass carp. LcAiiK attenuated the production of virulence factors and inhibited the swimming activity and biofilm formation of A. hydrophila.
Materials and methods
Bacterial strains and growth conditions
Strain K. huakuii LAM0618T was cultured in tryptic soy broth (TSB) at 30 °C with shaking. All E. coli strains were propagated in Luria–Bertani (LB) medium at 37 °C and 180 rpm. Strain L. casei MCJΔ1 was fostered in Man-Rogosa-Sharpe (MRS, tryptone 10.0 g/L, yeast extract 4.0 g/L, glucose 20.0 g/L, beef extract 8.0 g/L, NaAc·3H2O 8.3 g/L, Tween-80 1.0 mL/L, triammonium citrate 2.0 g/L, K2HPO4·3H2O 2.62 g/L, MgSO4·7H2O 0.41 g/L, MnSO4·H2O 0.056 g/L, pH 6.8) broth at 37 °C. Divalent metal ions-free MRS (DMIF-MRS, tryptone 10.0 g/L, yeast extract 4.0 g/L, glucose 20.0 g/L, beef extract 8.0 g/L, NaAc·3H2O 8.3 g/L, Tween-80 1.0 mL/L, triammonium citrate 2.0 g/L, K2HPO4·3H2O 2.62 g/L, pH 6.8) was used to test the effects of divalent metal ions on AHL lactonase activity of LcAiiK. A reporter strain Chromobacterium violaceum CV026 was grown in LB medium at 30 °C and 180 rpm. A. hydrophila AH-1 and A. hydrophila AH-4 (16S rDNA sequences showed in supplementary material), isolated from dead grass carp (Ctenopharyngodon idellus), were proliferated in nutrient broth (NB, peptone 10.0 g/L, beef extract powder 3.0 g/L, and NaCl 5.0 g/L) at 30 °C and 180 rpm. Antibiotics were added to the medium when required: ampicillin (100 μg/mL) for E. coli, kanamycin (50 μg/mL) for C. violaceum CV026, and erythromycin (50 μg/mL) for recombinant L. casei MCJΔ1. Strains and plasmids used in this study are listed in Table 2.
Table 2.
Bacterial strains and plasmids
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| Kurthia huakuii LAM0618T | Wild type | ACCC 06121 |
| Escherichia coli DH5α | λ−ф80dlacZΔM15 Δ (lacZYA-argF) U169 recA1 endA hsdR17 (r−K m−K) supE44 thi-1 gyrA relA1 | Tiangen |
| Lactobacillus casei MCJΔ1 | pMC11-cured strain | Chen et al. (2014) [27] |
| Chromobacterium violaceum CV026 | ATCC 31532 derivative, cviI::Tn5xylE Kmr, Smr | From Dr. Guishan Zhang |
| Aeromonas hydrophila AH-1 | Wild type | Isolated from dead grass carp |
| Aeromonas hydrophila AH-4 | Wild type | Isolated from dead grass carp |
| Plasmids | ||
| pELX1 | Expression vector, Ampr | Chen et al. (2014) [27] |
| pUC55-NlpC | pUC55 containing NlpC gene | Constructed by BGI |
| pELCW | Expression vector, Ampr | This study |
| pELCW-aiiK | pELCW containing aiiK gene | This study |
Construction of expression vectors pELCW and pELCW-aiiK
The expression vector pELCW was constructed based on pELX1 [27]. The NlpC gene encoding a cell wall-associated protein (accession: WP_022667204, surface layer protein) was synthesized and inserted into pUC55 (pUC55-NlpC was constructed by BGI company, Shanghai, China). Then the obtained plasmid pUC55-NlpC was used as a template to amplify the NlpC gene using FastPfu DNA polymerase (TransGen Biotech, Beijing, China) and NlpC-F-SOE and NlpC-R primers (PCR1, Table 3). The DNA sequence coding for His tag (in italics) and partial multiple cloning sites (MCS, in bold) were included in the NlpC-R primer. The NlpC gene, His-tag gene, and partial MCS were arranged in a row within the PCR1 product, and the PCR1 product was named as NlpC-His-MCS (NHM) gene. Meanwhile, the PslyA gene was amplified using pELX1 and FastPfu DNA polymerase with PslyA-F and PslyA-R-SOE primers (PCR2, Table 3). The splicing overlapping extension (SOE) PCR (amplification composition shown in Additional file 1: Table S1) [28] was used to fuse the PslyA gene and NHM gene using PslyA-F and NlpC-R primers (Table 3). The SOE-PCR product and pELX1 vector were digested with EcoRI and BglП at 37 °C for 4 h, and then purified by Cycle-Pure Kit (Omega Bio-Tek, USA) and Gel Purification Kit (TIANGEN, China), respectively. The digested products were linked using T4 DNA ligase (Thermo Scientific, USA) for constructing the expression plasmid pELCW.
Table 3.
Specific PCR used in this study
| Genes | Primers | Amplification parameters |
|---|---|---|
| NHM(PCR1) | NlpC-F-SOE: CAAGGAGGAAAAGACCACATGGTAGATGCAAAGAAAGTATTG | 95 °C for 5 min, 95 °C for 30 s, 57 °C for 30 s, and 72 °C for 60 s |
| NlpC-R: GGAAGATCTCCATGGCTCGAGATGATGATGATGATGGTGTAGTGAAGGACGAACAGC | ||
| PslpA(PCR2) | PslpA-F: CCGGAATTCAAGCGGTAGGTG | 95 °C for 5 min, 95 °C for 30 s, 57 °C for 30 s, and 72 °C for 40 s |
| PslpA-R-SOE: CAATACTTTCTTTGCATCTACCATGTGGTCTTTTCCTCCTTG | ||
| PslpA-NHM(SOE-PCR) | PslpA-F: CCGGAATTCAAGCGGTAGGTG | 95 °C for 5 min, 95 °C for 30 s, 56 °C for 30 s, and 72 °C for 100 s |
| NlpC-R: GGAAGATCTCCATGGCTCGAGATGATGATGATGATGGTGTAGTGAAGGACGAACAGC | ||
| aiiK(PCR3) | aiiKF: GGAAGATCTATGTGTCAAAATAAAAAGTTGTAC | 95 °C for 5 min, 95 °C for 30 s, 54 °C for 30 s, and 72 °C for 60 s |
| aiiKR: CGGGGTACCTTATTCGTAATACCCTTCCGTTGA | ||
| Sequencing | EcoRI-PSlpA-F: CCGGGAATTCAAGCGGTAGGTGAAATATTAC | 95 °C for 5 min, 95 °C for 30 s, 55.5 °C for 30 s, and 72 °C for 120 s |
| BamHI-T-R: GGCCGGATCCAGCTTGCGTTTGATTTTC |
His tag was marked in italics, and multiple cloning sites (MCS) were marked in bold
The genomic DNA of K. huakuii LAM0618T was utilized to amplify the aiiK gene using the aiiKF and aiiKR primers (PCR3, Table 3). The aiiK gene and pELCW vector were digested with BglП and KpnI at 37 °C for 4 h, and purified as mentioned earlier. Then ligation was performed by T4 DNA ligase to form pELCW-aiiK. Both pELCW and pELCW-aiiK were transformed into E. coli DH5α for storage, and then sequenced by company BGI (Shanghai, China) with the EcoRI-PSlyA-F and BamHI-T-R primers (Table 3).
Construction of the recombinant strain of pELCW-aiiK/L. casei MCJΔ1
Competent cells of L. casei MCJΔ1 were prepared based on our previous study [27]. Electrotransformation was performed as follows: 200 ng of pELCW-aiiK or pELCW was mixed with the 80-μL competent cells. The mixture was transferred into a 2-mm electroporation cuvette (Bio-Rad, USA), and then incubated on ice for 10 min. Subsequently, the electroporation was carried out at 1500 V and 5 ms with an Eppendorf Multiporator (Eppendorf, Hamburg, Germany). After electroporation, the mixture was transferred to 920-μL pre-warmed MRSS (MRS with 0.3 M sucrose) broth, and then incubated at 37 °C for 3 h for recovery. At last, the mixture was plated on MRS agar with erythromycin and incubated at 37 °C for 48 h to screen for recombinant strains.
Detection of AHL lactonase activity of LcAiiK
Lactobacillus casei MCJΔ1 harboring pELCW-aiiK was inoculated into 100 mL of fresh MRS medium (containing erythromycin) at the ratio of 1%. After incubation at 37 °C for 20 h, the cells were harvested by centrifugation and washed twice with 10 mM phosphate buffer saline (PBS, pH 7.4). These washed cells were re-suspended in 10 mM PBS, and the suspension was subjected to detect the AHL lactonase activity. The reaction mixture (500 μL) containing LcAiiK cells (OD600 = 5.0) and 50 μM N-Hexanoyl-l-homoserine lactone (C6-HSL) in 10 mM PBS (pH 7.4) was incubated at 37 °C for 3 h. Then, sodium dodecyl sulfate (SDS) was added into the mixture to terminate the reaction. The unreacted C6-HSL was extracted based on our previous study [18]. For the negative control, LcAiiK cells were replaced by pELCW/L. casei MCJΔ1 (LcCW) cells with the other conditions being the same. For the positive control, 4 μg/μL of AiiK purified from E. coli BL21 (DE3) was used to replace LcAiiK cells. At last, the extracted C6-HSL was detected by using the violacein generation bioassay and quantified using the high performance liquid chromatography (HPLC).
In the violacein generation bioassay, 1 mL overnight culture of C. violaceum CV026 was mixed well with 24 mL molten LB agar (1.6%), and the mixture was poured onto the plates. After the agar solidification, a sterile filter paper disk with a diameter of 5.5 mm was placed on every plate. The extracted C6-HSL samples were dropped onto the filter paper discs, and the plates were incubated at 30 °C for 16 h to generate violacein.
In the HPLC analysis, the Agilent Eclipse Plus C18 (4.6 × 250 mm, 5 μm) column and Agilent Technologies 1200 series were employed. The extracted C6-HSL was separated at 22 °C with a constant flow rate of 0.7 mL/min in isocratic elution (acetonitrile/water = 31/69, v/v) and then detected at 210 nm.
Localization assays of AiiK in LcAiiK
The surface layer proteins are localized on the outer layer of the peptidoglycan, lysozyme can degrade the peptidoglycan of gram-positive bacterial cell wall, and release the surface layer proteins. To verify that the AiiK was expressed on the surface layer of LcAiiK cells, the localization of AiiK was carried out. LcAiiK cells (OD600 = 0.5) were treated with lysozyme (Amresco, China) (20 mg/mL) in 10 mM PBS (pH 7.4) at 37 °C for 1 h, 2 h, and 3 h. After incubation, the mixture was centrifuged at 12,000 rpm for 2 min to collect the supernatant for detecting AHL lactonase activity. The substrate C6-HSL (50 μM) was added into and incubated at 37 °C for 12 h. The unreacted C6-HSL was quantified using the method described above. For the positive control, the supernatant was replaced with LcAiiK cells (OD600 = 0.5). For the negative control, the supernatant was replaced with the same volume of lysozyme solution (lysozyme was dissolved in 10 mM PBS, pH 7.4).
Characteristics of LcAiiK
The characteristics of LcAiiK were determined by detecting its AHL lactonase activity. The reaction mixture (500 μL) contained LcAiiK cells (OD600 = 0.5) and C6-HSL (50 μM) in PBS (10 mM, pH 7.4). In C6-HSL degradation assay, the reaction mixture was incubated at 37 °C for 0, 1, 2, 4, 6, 8, 10, 12, 16, 20, and 24 h, respectively, and the residual C6-HSL was quantified by HPLC. The optimal cell density (OD600) of LcAiiK to degrade C6-HSL was determined using different optical densities (OD600 = 0.1, 0.5, 1.0, 2.0, and 3.0), and the reaction mixture was incubated at 37 °C for 12 h. The optimal reaction temperature was determined by incubating the reaction mixture at 25 °C, 30 °C, 35 °C, 37 °C, 40 °C, 45 °C, and 50 °C. The effects of divalent metal ions on LcAiiK in vivo were examined by cultivating LcAiiK in DMIF-MRS broth with addition one kind of divalent metal ions (1 mM Zn2+, 1 mM Mg2+, 1 mM Mn2+, 1 mM Co2+, and 1 mM Ni2+), the cultures were incubated at 37 °C for 20 h. After incubation, the cells were harvested and subjected to detect AHL lactonase activity. The effects of divalent metal ions on LcAiiK in vitro were examined by culturing LcAiiK in DMIF-MRS broth at 37 °C for 20 h. After incubation, the harvested cells and one kind of divalent metal ions (1 mM Zn2+, 1 mM Mg2+, 1 mM Mn2+, 1 mM Co2+, and 1 mM Ni2+) were added to detect the AHL lactonase activity.
Determination of plasmid stability
To calculate the plasmid stability of pELCW-aiiK in strain L. casei MCJΔ1, the LcAiiK was inoculated into MRS at ratio of 1% without erythromycin for continuous passage culture of 15 generations, and every generation was propagated for 12 h. At every generation, colony-forming units (cfu) were determined by MRS agar plates and selective MRS agar plates (50 μg/mL erythromycin). The plasmid stability of pELCW-aiiK was calculated as the ratio of cfu number on selective MRS agar versus that on MRS agar [27]. Therefore, the plasmid stability per generation was calculated by equation:
Herein, “L” is plasmid stability, “n” is continuous generations cultured, “” is the average cfu numbers on selective MRS agar, and “” is the average cfu numbers on MRS agar.
Effect of LcAiiK on swimming motility in A. hydrophila
The effect of LcAiiK on swimming motility in A. hydrophila was determined based on the method described by Jahid et al. with minor modifications [6]. Fifty microliter of co-culture mixture containing LcAiiK at various concentrations (OD600 = 0.1, 0.2, and 0.4) and A. hydrophila AH-1 or AH-4 (OD600 = 0.1) in 10 mM PBS (pH 7.4) was inoculated onto the center of NA (NB with 0.3% agar) plates, and then incubated at 25 °C for 24 h. After incubation, the diameter of strain lawn was measured. The LcCW cells was used as negative control, and 10 mM PBS was used as control check (CK).
Effect of LcAiiK on haemolytic activity in A. hydrophila
Blood agar plates were utilized to evaluate the effect of LcAiiK on haemolytic activity in A. hydrophila [29]. Eighty microliter of co-culture mixture, comprising LcAiiK (OD600 = 0.5) and A. hydrophila AH-1 or AH-4 (OD600 = 0.1) in 10 mM PBS (pH 7.4), was inoculated into the hole on blood agar plate and incubated at 30 °C for 24 h. The zone of complete haemolysis was measured to assess haemolytic activity of A. hydrophila. The negative control and CK were prepared as mentioned earlier.
Effect of LcAiiK on extracellular proteolytic activity in A. hydrophila
The effect of LcAiiK on extracellular proteolytic activity in A. hydrophila was evaluated by conducting an extracellular proteolytic assay according to Bhakti et al. with modifications [30]. A. hydrophila AH-1 or AH-4 was inoculated into fresh NB with different concentrations of LcAiiK (OD600 = 0.1, 0.2, and 0.4), then the mixture was incubated at 30 °C and 180 rpm for 20 h. After incubation, the NB culture supernatant was used as the crude enzyme extract for detecting the extracellular proteolytic activity. The reaction mixture contained 250 μL of supernatant and 250 μL of 2% azocasein. After reacting at 30 °C for 3 h, 1.2 mL 10% trichloroacetic acid was added and centrifuged at 6000 g for 10 min. Then 1.2 mL of supernatant was mixed with 1.0 mL of 1 M NaOH, and the optical density was assessed at 440 nm. The negative control and CK were prepared as mentioned earlier.
Effect of LcAiiK on biofilm formation by A. hydrophila
The effect of LcAiiK on the biofilm formation by A. hydrophila was examined based on the method described by Dong et al. with some modifications [18]. About 200 μL co-culture mixture, containing LcAiiK with various concentrations (OD600 = 0.1, 0.2, and 0.4) and A. hydrophila AH-1 or AH-4 (OD600 = 0.1), was dispensed into a 96-well microtiter plate and statically incubated at 30 °C for 12 h. After incubation, planktonic cells from the plate were transferred out gently for dilution plate count of A. hydrophila AH-1 and AH-4. The biofilm cells were washed very gently with 10 mM PBS for three times, and then stained with 20 μL of 0.2% crystal violet at 25 °C for 15 min. The stained biofilm cells were washed very gently with distilled water for three times. Ethanol (100 μL, 95%) was added to extract crystal violet, and the absorbance at 590 nm was measured. The negative control and CK were prepared as mentioned earlier.
Statistical analysis
All data were processed using Excel (version 2019) as mean ± standard deviation (sd), and differences with P < 0.05 and P < 0.01 were deemed significant.
Results
Construction of recombinant strain LcAiiK
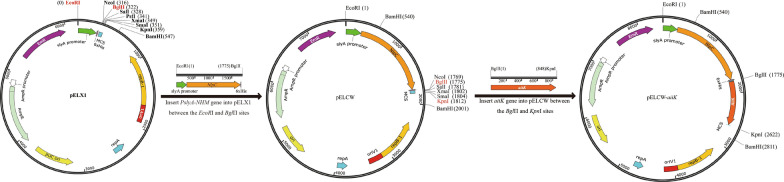
To construct the cell wall-associated expression vector pELCW, we inserted gene NlpC at the end of promoter PslyA and in the front of His-tag gene on plasmid pELX1. The NlpC gene was used as a guide peptide sequence to help target protein anchor at the surface layer. The SOE-PCR product (PslyA-NHM gene) (Additional file 1: Fig. S1) was inserted into pELX1 between the EcoRI and BglП sites (Fig. 1). We named the new plasmid pELCW, which was constructed from pELX1 with cell wall-associated expression function. Furthermore, the aiiK gene was inserted into pELCW between the BglП and KpnI sites (Fig. 1), generating the plasmid pELCW-aiiK (Fig. 1).
Fig. 1.
Flow chart of construction of the cell wall-associated expression vectors pELCW and pELCW-aiiK
Screening of the recombinant strain by colony PCR and plasmid sequencing indicated that the pELCW-aiiK/L. casei MCJΔ1 and pELCW/L. casei MCJΔ1 were constructed correctly. The recombinant strain pELCW-aiiK/L. casei MCJΔ1 was designated as LcAiiK (AiiK expressed by L. casei MCJΔ1) whereas the recombinant strain pELCW/L. casei MCJΔ1 was designated as LcCW.
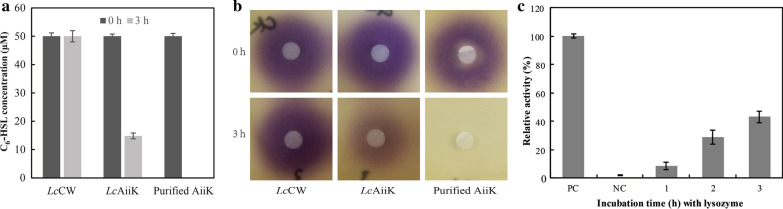
Detection of AHL lactonase activity of LcAiiK and localization assays of AiiK in LcAiiK
The results showed that LcAiiK (OD600 = 5.0) could degrade 35.18 μM C6-HSL at 37 °C within 3 h and LcCW didn’t exhibit any AHL lactonase activity to C6-HSL (Fig. 2a). The purified AiiK (4 μg/mL) from E .coli BL21 (DE3) degraded 50 μM C6-HSL at 37 °C within 3 h (Fig. 2a). Meanwhile, the same results were detected by violacein generation bioassay of C. violaceum CV026 (Fig. 2b). Here, LcAiiK cells (without any treatment) were used to directly degrade C6-HSL, and LcAiiK cells did degrade C6-HSL (Fig. 2a). From this, we can speculate that AiiK was expressed and located at the outermost layer of LcAiiK cells. Therefore, LcAiiK exhibited significant AHL lactonase activity, and the protein AiiK was expressed at the outermost layer of LcAiiK cells (Fig. 2a and b).
Fig. 2.
a AHL lactonase activity of LcAiiK detected by HPLC (LcCW cells were used as negative control, purified AiiK was used as positive control). b AHL lactonase activity of LcAiiK detected by violacein generation of C. violaceum CV026 (LcCW cells were used as negative control, purified AiiK was used as positive control). c Localization assays of AiiK in LcAiiK, PC represents positive control and NC represents negative control. Data are shown as mean ± SD, n = 3
Although the protein AiiK was expressed at the outermost layer of LcAiiK cells, it remained unclear whether AiiK was expressed at surface layer of LcAiiK. The localization assays of AiiK revealed that AHL lactonase activity of supernatant increased with incubation time compared with negative control (Fig. 2c). The lysozyme can degrade peptidoglycan of cell wall and release surface layer proteins. Thus, AHL lactonase activity of supernatant increased significantly within the treatment of lysozyme, which indicated that AiiK was expressed at the surface layer of LcAiiK.
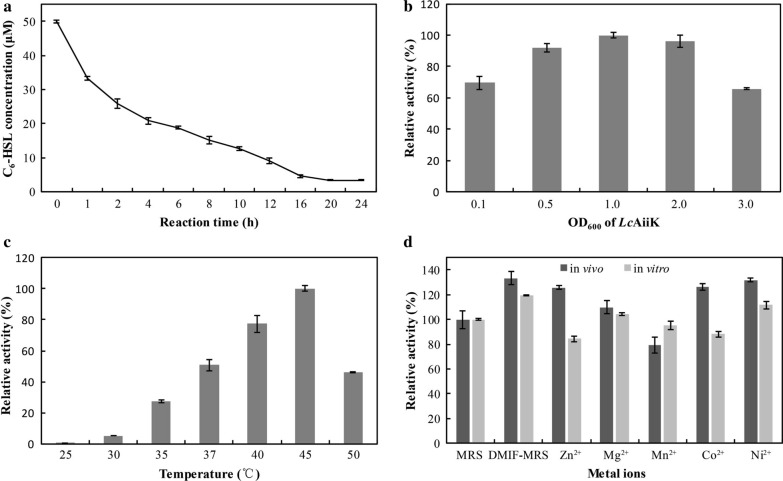
Characteristics of LcAiiK
Many previous studies have reported that C6-HSL is a vital signal molecule mediating QS processes such as motility, haemolytic activity, extracellular proteolytic activity, and biofilm formation in A. hydrophila [6, 26, 31–33]. Thus, C6-HSL was used as substrate to detect AHL lactonase activity of LcAiiK in our study. LcAiiK (OD600 = 0.5) degraded 24.13 μM of C6-HSL at 2 h, 40.99 μM of C6-HSL at 12 h, and 46.63 μM of C6-HSL at 24 h (Fig. 3a). The optimal OD600 of LcAiiK to degrade C6-HSL was determined at value of 1.0 (Fig. 3b). The optimal reaction temperature of LcAiiK to degrade C6-HSL was 45 °C (Fig. 3c). The in vivo experiments showed that Zn2+, Mg2+, Co2+, and Ni2+ increased AHL lactonase activity of LcAiiK, however, Mn2+ slightly decreased its activity (Fig. 3d). Moreover, the in vitro experiments revealed that Mg2+ and Ni2+ increased AHL lactonase activity of LcAiiK, however, Zn2+, Mn2+, and Co2+ decreased its activity (Fig. 3d). Interestingly, LcAiiK cultured in DMIF-MRS was found to exhibit higher AHL lactonase activity than that cultured in MRS from both in vivo and in vitro experiments (Fig. 3d). These characteristics provided the foundation and guiding significance for the practical use of LcAiiK.
Fig. 3.
Characteristics of LcAiiK. a C6-HSL degradation curve of LcAiiK within 24 h. b Optimal OD600 of LcAiiK for degrading C6-HSL. c Optimal reaction temperature of LcAiiK. d Effect of divalent metal ions on AHL lactonase activity of LcAiiK in vivo and vitro [reaction was performed with LcAiiK cells (OD600 = 0.5) at 37 °C for 12 h]. Data are shown as mean ± SD, n = 3
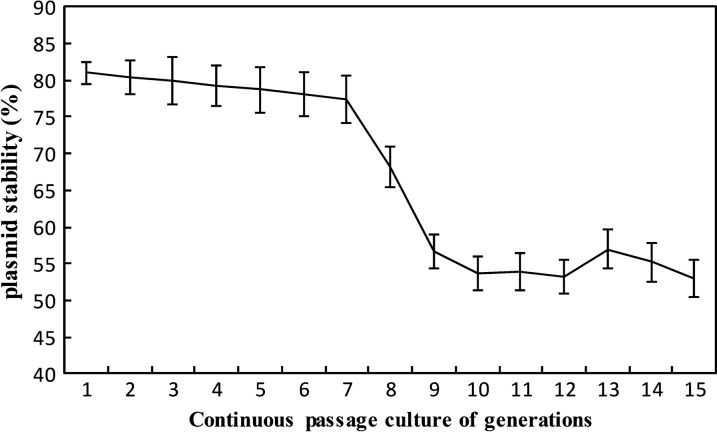
Determination of plasmid stability
The plasmid stability of pELCW-aiiK was measured by counting the colonies after continuous passage culture in MRS for 15 generations under the nonselective condition. The results revealed that plasmid stability of pELCW-aiiK decreased slightly from 81.00% to 77.38% during the first 7 generations, then tobogganed to 56.69% at generation 9, and finally remained stable from generation 10 to 15 with the value of 53.04% (Fig. 4). In short, over 50% of LcAiiK cells maintained plasmid pELCW-aiiK after 15 generations without erythrocin. From this result, we speculated that the LcAiiK might maintain the QQ ability for a period which was sufficient for LcAiiK cells to maintain the pELCW-aiiK plasmid while proliferating.
Fig. 4.
pELCW-aiiK plasmid stability of LcAiiK during continuous passage culture in MRS for 15 generations under the nonselective condition. Data are shown as mean ± SD, n = 3
LcAiiK’s application in QQ on A. hydrophila
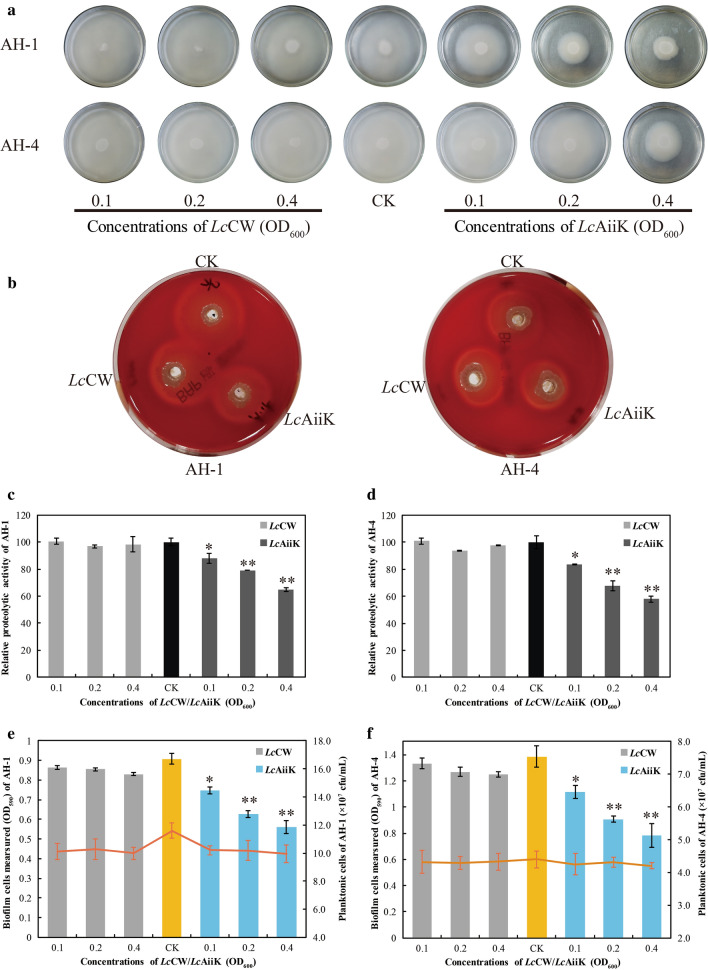
LcAiiK was found to quench the AHL-mediated QS processes of A. hydrophila AH-1 and AH-4 in this study (Fig. 5). LcAiiK significantly hampered the swimming motility of A. hydrophila AH-1 and AH-4, compared to CK and negative control (Fig. 5a). The inhibition ratio of swimming motility was dependent on the dose of LcAiiK (Fig. 5a). LcAiiK showed slight inhibition effect on haemolytic activity of A. hydrophila AH-1 and AH-4, compared to CK and negative control (Fig. 5b). LcAiiK (OD600 = 0.4) reduced extracellular proteolytic activity of A. hydrophila AH-1 and AH-4 by 35.29% (Fig. 5c) and 42.01% (Fig. 5d) after co-culture, respectively, and LcCW (negative control) did not exhibit inhibition effect on that of A. hydrophila AH-1 (Fig. 5c) and AH-4 (Fig. 5d). Moreover, the increased application of LcAiiK resulted in the obvious decrease in biofilm formation of A. hydrophila AH-1 (Fig. 5e) and AH-4 (Fig. 5f), compared to CK and negative control. Furthermore, plate counting results indicated that planktonic cells of A. hydrophila AH-1 and AH-4 remained stable at the value of 10.1 × 107 cfu/mL (Fig. 5e) and 4.3 × 107 cfu/mL (Fig. 5f) after co-culture, respectively. And this reflected that LcAiiK inhibited biofilm formation of A. hydrophila AH-1 and AH-4, but not kill them when co-culture. Therefore, these results demonstrated that LcAiiK obviously attenuated the swimming motility, haemolytic activity, extracellular proteolytic activity, and biofilm formation of A. hydrophila AH-1 and AH-4.
Fig. 5.
Effect of LcAiiK on the swimming motility, virulence factor production, and biofilm formation in A. hydrophila AH-1 and AH-4. a The swimming motility of A. hydrophila AH-1 and AH-4. b The haemolytic activity of A. hydrophila AH-1 and AH-4. c The extracellular proteolytic activity of A. hydrophila AH-1. d The extracellular proteolytic activity of A. hydrophila AH-4. e Biofilm formation (column chart) was detected by crystal violet staining, and planktonic cells (red line chart) were detected by plate counting of A. hydrophila AH-1. f Biofilm formation (column chart) was detected by crystal violet staining, and planktonic cells (red line chart) were detected by plate counting of A. hydrophila AH-4. Data are shown as mean ± SD, n = 3. A t test was performed for testing differences between groups, and the ** and * indicate P < 0.01 and P < 0.05, respectively
Discussion
QS is a population-dependent behavior in bacteria for communicating with each other, which orchestrates expression of multiple genes triggered by signal molecules when external environment changes [8, 10, 34–38]. AHLs as important signal molecules mediating many QS processes were identified in multiple gram-negative bacterial species, and most bacteria were common pathogens existing in various environments [26]. Many studies have revealed that AHL-mediated QS was closely related to the pathogenicity, virulence factor production, and biofilm formation in gram-negative pathogens [2, 3]. The acute hemorrhagic septicemia in fish and diarrhea even death in human caused by microbial infections were closely related to the AHL-mediated QS in various gram-negative pathogens, especially A. hydrophila, A. salmonicida, and P. aeruginosa [4, 5, 39–42]. However, the antibiotic therapy for these gram-negative pathogens will accelerate the emergence of drug-resistance. Thus, it is urgent to develop a promising strategy to inhibit or quench these processes of AHL-mediated QS [43–45]. Interfering or quenching QS, known as QQ, is becoming a prospective tactic for reducing the pathogenicity triggered by AHL-mediated QS [9, 17–20, 46, 47]. QQ enzymes are of great importance in inhibiting or attenuating pathogenicity. Therefore, the QQ enzymes can be widely applied as bio-control agents.
In this study, QQ enzyme AiiK was expressed at the surface layer of strain L. casei MCJΔ1. This is the first report that QQ enzyme was expressed in lactic acid bacteria Lactobacilli genus implemented by cell wall-associated constitutive expression vector pELCW-aiiK. Meanwhile, the cell wall-associated constitutive expression vector pELCW provides a genetic tool for DNA clone and a new perspective for heterologous gene expression at the surface layer of strain L. casei MCJΔ1. Many studies have reported that QQ enzymes were heterologously expressed by E. coli, but few works were done by utilizing other expression systems. So far, Chen et al. used the vector pPIC9 to express recombinant AiiAB546 in Pichia pastoris [21], and Zhang et al. applied B. brevis expression system to express MomL [26]. These two expression systems produced secreted proteins and the application of the proteins required prior purification steps. Herein, we utilized vector pELCW-aiiK to express the AiiK which anchored at the surface layer of LcAiiK, making LcAiiK cells can be applied directly without any processing steps.
Considering the characteristics of LcAiiK, our results revealed that LcAiiK maintained the same optimal reaction temperature with purified AiiK at 45 °C [18]. The effect of divalent metal ions on LcAiiK was slighter than that on purified AiiK [18]. Based on this finding, it could be speculated that LcAiiK is less affected by external environment (such as divalent metal ions) than purified AiiK. AHL lactonase activity of LcAiiK increased from 100.00% (day 1) to 192.10% (day 3), then dropped slowly to 155.87% (day 6) (Additional file 1: Fig. S2). However, the live LcAiiK cells were decreased quickly from 7.4 × 107 cfu/mL (day 1) to 7.8 × 106 cfu/mL (day 6) when LcAiiK was stored in 10 mM PBS at 4 °C (Additional file 1: Fig. S2). Based on this result, we speculated that the cell wall lysis released the AiiK from surface layer and increased AHL lactonase activity (Additional file 1: Fig. S2). LcAiiK could maintain 155.87% AHL lactonase activity after 6-day storage at 4 °C (Additional file 1: Fig. S2), while purified AiiK could only retain 20% AHL lactonase activity after 12-h storage at 37 °C [18]. This finding implied that LcAiiK endows higher stability compared to that of purified AiiK, which solves the drawback of instability of purified AiiK. The anchored AiiK at the surface layer of LcAiiK maintained good stability to sustainably degrade AHLs (Additional file 1: Fig. S2), which can be more useful in factual environment. Moreover, LcAiiK could retain over 50% plasmid stability of pELCW-aiiK within 15 generations, reflecting that plasmid pELCW-aiiK can exist in cells for a long time. Our previous study reported that AiiK could degrade multiple AHLs including C6-HSL, 3-Oxo-C6-HSL, C10-HSL, and C14-HSL [18]. Therefore, these characteristics of LcAiiK lay a solid foundation for its application in the field of QQ.
Herein, we verified the QQ ability of LcAiiK against A. hydrophila by co-culture. The reason why A. hydrophila was selected as the target strain was that this bacterium is an emerging gram-negative opportunistic pathogen that can cause various serious symptoms in fish, crabs, shrimps, and even humans [4, 6, 7]. Many studies have reported that the pathogenicity and human infections depend on the AHL-mediated QS in A. hydrophila [6, 13]. The main AHL signal molecules are C6-HSL and N-butanoyl-L-homoserine lactone (C4-HSL) in A. hydrophila [6, 13, 31–33]. Besides, the biofilm formation of A. hydrophila was highly associated with multiple antibiotics resistance, making the diseases or infections difficult to be cured [14]. In this study, the AiiK was expressed at the surface layer of LcAiiK with AHL lactonase activity (Fig. 2). LcAiiK exhibited an obvious QQ ability against A. hydrophila AH-1 and AH-4 by degrading their signal molecule C6-HSL (Figs. 5 and Fig. 6). Meanwhile, LcAiiK did not kill the planktonic cells of A. hydrophila AH-1 and AH-4 at co-culture condition, implying that LcAiiK did not accelerate the emergence of drug-resistance (Fig. 5e and f). Therefore, this might be a promising anti-pathogenic strategy to control pathogenic bacteria and to prevent antibiotic resistance. It was reported that recombinant strain BbMomL significantly reduced the secretion of pathogenic factors and the pathogenicity of P. carotovorum subsp. carotovorum and P. aeruginosa PAO1 [26]. Chen et al. expressed QQ enzyme AiiAB546 by pPIC9/P. pastoris expression system, and AiiAB546 decreased the mortality rate and delayed the mortality time of fish by co-injecting A. hydrophila and AiiAB546 into common carp [21]. In our study, the AiiK was expressed at the surface layer of LcAiiK. Therefore, LcAiiK cells harboring the AiiK protein on their cell walls were co-cultured directly with A. hydrophila AH-1 and AH-4 to quench their QS processes. This strategy is easy to apply as it only involves cultivation of LcAiiK cells without purification steps. Zhou et al. reported that Bacillus sp. QSI-1 significantly decreased haemolytic and protease activity of A. hydrophila YJ-1 [48], which was consistent with our present results.
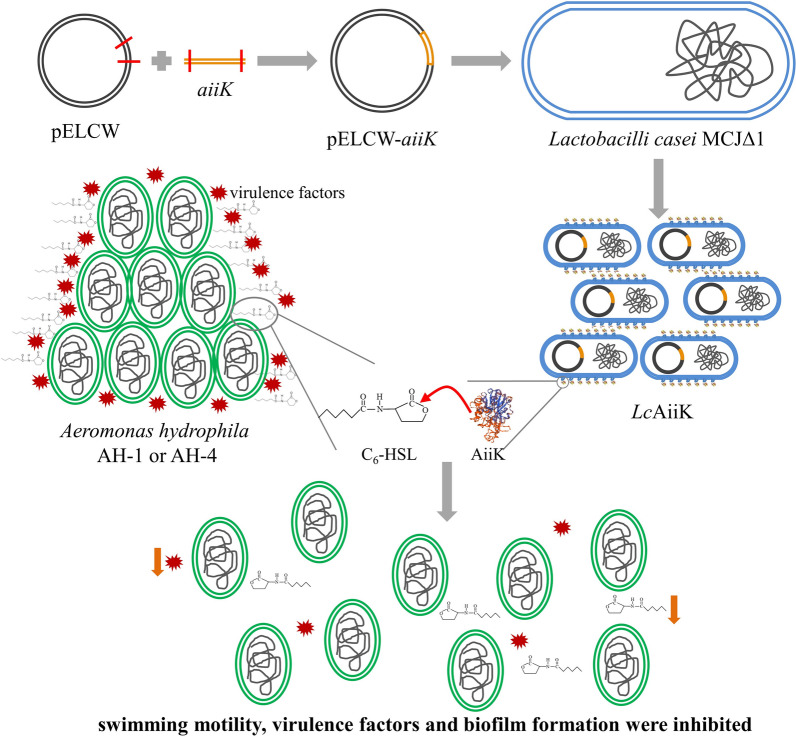
Fig. 6.
Schematic diagram of QQ mechanism of LcAiiK. The expression of AiiK at the surface layer of LcAiiK maintained AHL lactonase activity. LcAiiK can degrade C6-HSL produced by A. hydrophila and interrupt or inhibit the production of virulence factors of A. hydrophila
Interestingly, LcAiiK was found to exhibit higher AHL lactonase activity when it was cultured in DMIF-MRS than in MRS (Fig. 3d). Correspondingly, we speculated that the AHL lactonase activity of LcAiiK could be improved by optimizing the culture formula (such as divalent metal ions), and this speculation deserves further study. As for safety issue, L. casei is one kind of lactic acid bacteria which is commonly considered as an environment friendly probiotic. Furthermore, many studies have demonstrated that the L. casei is an important probiotic [49–52]. L. casei inhibited the growth of Streptococcus mutans in caries prevention [49], decreased the relative abundance of intestinal Escherichia-Shigella in suckling rabbit [50], and attenuated the biofilm development of Candida albicans [51]. The health-promoting feature of L. casei was documented by Hill et al., reporting the potentials of L. casei in the treatment or prevention of a variety of diseases [52]. Therefore, the probiotic capabilities of L. casei made LcAiiK safe to be applied. These probiotic capabilities also expanded the application range of LcAiiK, which suggested that LcAiiK could not only be used as probiotic, but also be exploited as an anti-pathogenic drug or a bio-control agent against the AHL-mediated QS pathogenic bacteria.
Conclusions
AHL lactonase AiiK is firstly expressed at the surface layer of L. casei MCJΔ1 via a cell wall-associated constitutive expression vector pELCW-aiiK. LcAiiK exhibited considerable AHL lactonase activity and displayed obvious QQ ability against A. hydrophila AH-1 and AH-4 by attenuating their swimming motility, virulence factor production, and biofilm formation instead of killing them. Therefore, the LcAiiK can be developed as an anti-pathogenic agent to control AHL-mediated QS pathogenic bacteria and prevent the emergence of antibiotic resistance.
Supplementary information
Additional file 1. Text 1. 16S rDNA sequence of A. hydrophila AH-1. Text 2. 16S rDNA sequence of A. hydrophila AH-4. Table S1. Amplification composition and condition of SOE-PCR. Fig. S1. Agarose gel electrophoresis of SOE-PCR product PslyA-NHM gene. Fig. S2. AHL lactonase activity and live cells of LcAiiK after storage at 4 °C.
Acknowledgements
Not applicable.
Authors’ contributions
SMZ, NP, and YXL conceived and supervised the study. WWD designed the experiments, performed the main experiments, acquired and analysed the data, and drafted the manuscript. YYC, ZLX, BF, QTC, YXC and ZYR performed partial experiments and contributed to the manuscript. All authors reviewed the manuscript and provided meaningful intellectual contributions to the study. All authors read and approved the final manuscript.
Funding
This study was financially supported by Grant 2662018JC016 of the Fundamental Research Funds for the Central Universities.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional file.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12934-020-01448-4.
References
- 1.Defoirdt T. Implications of ecological Niche differentiation in marine bacteria for microbial management in aquaculture to prevent bacterial disease. PLoS Pathog. 2016;12:e1005843. doi: 10.1371/journal.ppat.1005843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swift S, Karlyshev AV, Fish L, Durant EL, Winson MK, Chhabra SR, Williams P, Macintyre S, Stewart GSAB. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida identification of the LuxRI homologs AhyRI and AsaRI and their cognate N-acylhomoserine lactone signal molecul. J Bacteriol. 1997;179:5271–5281. doi: 10.1128/JB.179.17.5271-5281.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch MJ, Swift S, Kirke DF, Keevil CW, Dodd CER, Williams P. The regulation of biofilm development by quorum sensing in Aeromonas hydrophila. Environ Microbiol. 2002;4:18–28. doi: 10.1046/j.1462-2920.2002.00264.x. [DOI] [PubMed] [Google Scholar]
- 4.Vivas J, Carracedo B, Riano J, Razquin BE, Lopez-Fierro P, Acosta F, Naharro G, Villena AJ. Behavior of an Aeromonas hydrophila aroA live vaccine in water microcosms. Appl Environ Microbiol. 2004;70:2702–2708. doi: 10.1128/AEM.70.5.2702-2708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agger WA, Mccormick JD, Gurwith MJ. Clinical and microbiological features of Aeromonas hydrophila-associated diarrhea. J Cli Microbiol. 1985;21:909–913. doi: 10.1128/JCM.21.6.909-913.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jahid IK, Mizan MF, Ha AJ, Ha SD. Effect of salinity and incubation time of planktonic cells on biofilm formation, motility, exoprotease production, and quorum sensing of Aeromonas hydrophila. Food Microbiol. 2015;49:142–151. doi: 10.1016/j.fm.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Chen B, Peng M, Tong W, Zhang Q, Song Z. The Quorum Quenching Bacterium Bacillus licheniformis T-1 Protects Zebrafish against Aeromonas hydrophila Infection. Probiotics Antimicrob Proteins. 2019 doi: 10.1007/s12602-018-9495-7. [DOI] [PubMed] [Google Scholar]
- 8.Eickhoff MJ, Bassler BL. SnapShot: bacterial Quorum Sensing. Cell. 2018;174(1328–1328):e1321. doi: 10.1016/j.cell.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 9.See-Too WS, Convey P, Pearce DA, Chan KG. Characterization of a novel N-acylhomoserine lactonase, AidP, from Antarctic Planococcus sp. Microb Cell Fact. 2018;17:179. doi: 10.1186/s12934-018-1024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fetzner S. Quorum quenching enzymes. J Biotechnol. 2015;201:2–14. doi: 10.1016/j.jbiotec.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Chhabra SR, Harty C, Hooi DSW, Daykin M, Williams P, Telford G, Pritchard DI, Bycroft BW. Synthetic analogues of the bacterial signal (Quorum Sensing) molecule N-(3-Oxododecanoyl)-l-homoserine lactone as immune modulators. J Med Chem. 2003;46:97–104. doi: 10.1021/jm020909n. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson S, Williams P. Quorum sensing and social networking in the microbial world. J R Soc Interface. 2009;6:959–978. doi: 10.1098/rsif.2009.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sha J, Pillai L, Fadl AA, Galindo CL, Erova TE, Chopra AK. The type III secretion system and cytotoxic enterotoxin alter the virulence of Aeromonas hydrophila. Infect Immun. 2005;73:6446–6457. doi: 10.1128/IAI.73.10.6446-6457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Defoirdt T, Thanh LD, Van Delsen B, De Schryver P, Sorgeloos P, Boon N, Bossier P. N-acylhomoserine lactone-degrading Bacillus strains isolated from aquaculture animals. Aquaculture. 2011;311:258–260. doi: 10.1016/j.aquaculture.2010.11.046. [DOI] [Google Scholar]
- 15.Chen F, Gao Y, Chen X, Yu Z, Li X. Quorum quenching enzymes and their application in degrading signal molecules to block quorum sensing-dependent infection. Int J Mol Sci. 2013;14:17477–17500. doi: 10.3390/ijms140917477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickey SW, Cheung GYC, Otto M. Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat Rev Drug Discov. 2017;16:457–471. doi: 10.1038/nrd.2017.23. [DOI] [PubMed] [Google Scholar]
- 17.Tang K, Su Y, Brackman G, Cui F, Zhang Y, Shi X, Coenye T, Zhang XH. MomL, a novel marine-derived N-acyl homoserine lactonase from Muricauda olearia. Appl Environ Microbiol. 2015;81:774–782. doi: 10.1128/AEM.02805-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong W, Zhu J, Guo X, Kong D, Zhang Q, Zhou Y, Liu X, Zhao S, Ruan Z. Characterization of AiiK, an AHL lactonase, from Kurthia huakui LAM0618(T) and its application in quorum quenching on Pseudomonas aeruginosa PAO1. Sci Rep. 2018;8:6013. doi: 10.1038/s41598-018-24507-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai X, Yu M, Shan H, Tian X, Zheng Y, Xue C, Zhang XH. Characterization of a Novel N-Acylhomoserine Lactonase RmmL from Ruegeria mobilis YJ3. Mar Drugs. 2018;16:370. doi: 10.3390/md16100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B, Zhuang XY, Guo LY, Mclean RJC, Chu Wh. Recombinant N-acyl homoserine lactone-lactonase AiiAQSI-1 attenuates Aeromonas hydrophila virulence dactors, biofilm formation and reduces mortality in Crucian Carp. Mar Drugs. 2019;17:499. doi: 10.3390/md17090499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen RD, Zhou ZG, Cao YN, Bai YG, Yao B. High yield expression of an AHL-lactonase from Bacillus sp. B546 in Pichia pastoris and its application to reduce Aeromonas hydrophila mortality in aquaculture. Microb Cell Fact. 2010;9:39. doi: 10.1186/1475-2859-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong YH, Xu JL, Li XZ, Zhang LH. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc Natl Acad Sci. 2000;97:3526–3531. doi: 10.1073/pnas.97.7.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang WZ, Morohoshi T, Ikenoya M, Someya N, Ikeda T. AiiM, a novel class of N-acylhomoserine lactonase from the leaf-associated bacterium Microbacterium testaceum. Appl Environ Microbiol. 2010;76:2524–2530. doi: 10.1128/AEM.02738-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin L, Xu X, Zheng Y, Zhang C. Effects of AttM lactonase on the pathogenicity of Streptomyces scabies. Lett Appl Microbiol. 2018;67:270–277. doi: 10.1111/lam.13019. [DOI] [PubMed] [Google Scholar]
- 25.Torres M, Uroz S, Salto R, Fauchery L, Quesada E, Llamas I. HqiA, a novel quorum-quenching enzyme which expands the AHL lactonase family. Sci Rep. 2017;7:943. doi: 10.1038/s41598-017-01176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Wang J, Feng T, Du R, Tian X, Wang Y, Zhang XH. Heterologous expression of the marine-derived quorum quenching enzyme MomL can expand the antibacterial spectrum of Bacillus brevis. Mar Drugs. 2019;17:128. doi: 10.3390/md17020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, Lin J, Ma C, Zhao S, She Q, Liang Y. Characterization of pMC11, a plasmid with dual origins of replication isolated from Lactobacillus casei MCJ and construction of shuttle vectors with each replicon. Appl Microbiol Biotechnol. 2014;98:5977–5989. doi: 10.1007/s00253-014-5649-z. [DOI] [PubMed] [Google Scholar]
- 28.Heckman KL, Pease LR. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc. 2007;2:924–932. doi: 10.1038/nprot.2007.132. [DOI] [PubMed] [Google Scholar]
- 29.Chu W, Zhou S, Zhu W, Zhuang X. Quorum quenching bacteria Bacillus sp. QSI-1 protect zebrafish (Danio rerio) from Aeromonas hydrophila infection. Sci Rep. 2014;4:5446. doi: 10.1038/srep05446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel B, Kumari S, Banerjee R, Samanta M, Das S. Disruption of the quorum sensing regulated pathogenic traits of the biofilm-forming fish pathogen Aeromonas hydrophila by tannic acid, a potent quorum quencher. Biofouling. 2017;33:580–590. doi: 10.1080/08927014.2017.1336619. [DOI] [PubMed] [Google Scholar]
- 31.Khajanchi BK, Sha J, Kozlova EV, Erova TE, Suarez G, Sierra JC, Popov VL, Horneman AJ, Chopra AK. N-acylhomoserine lactones involved in quorum sensing control the type VI secretion system, biofilm formation, protease production, and in vivo virulence in a clinical isolate of Aeromonas hydrophila. Microbiology. 2009;155:3518–3531. doi: 10.1099/mic.0.031575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jahid IK, Lee NY, Kim A, Ha SD. Influence of glucose concentrations on biofilm formation, motility, exoprotease production, and quorum sensing in Aeromonas hydrophila. J Food Prot. 2013;76:239–247. doi: 10.4315/0362-028X.JFP-12-321. [DOI] [PubMed] [Google Scholar]
- 33.Zhou S, Zhang A, Yin H, Chu W. Bacillus sp. QSI-1 modulate quorum sensing signals reduce Aeromonas hydrophila level and alter gut microbial community structure in fish. Front Cell Infect Microbiol. 2016;6:184. doi: 10.3389/fcimb.2016.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 35.Rakoff-Nahoum S, Foster KR, Comstock LE. The evolution of cooperation within the gut microbiota. Nature. 2016;533:255–259. doi: 10.1038/nature17626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pader V, Hakim S, Painter KL, Wigneshweraraj S, Clarke TB, Edwards AM. Staphylococcus aureus inactivates daptomycin by releasing membrane phospholipids. Nat Microbiol. 2016;2:16194. doi: 10.1038/nmicrobiol.2016.194. [DOI] [PubMed] [Google Scholar]
- 37.Bandyopadhaya A, Tsurumi A, Maura D, Jeffrey KL, Rahme LG. A quorum-sensing signal promotes host tolerance training through HDAC1-mediated epigenetic reprogramming. Nat Microbiol. 2016;1:16174. doi: 10.1038/nmicrobiol.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JH, Lee J. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev. 2010;34:426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 39.Bjarnsholt T, Jensen PO, Burmolle M, Hentzer M, Haagensen JA, Hougen HP, Calum H, Madsen KG, Moser C, Molin S, Hoiby N, Givskov M. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology. 2005;151:373–383. doi: 10.1099/mic.0.27463-0. [DOI] [PubMed] [Google Scholar]
- 40.Dou Y, Song F, Guo F, Zhou Z, Zhu C, Xiang J, Huan J. Acinetobacter baumannii quorum-sensing signalling molecule induces the expression of drug-resistance genes. Mol Med Rep. 2017;15:4061–4068. doi: 10.3892/mmr.2017.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch G, Nadal-Jimenez P, Reis CR, Muntendam R, Bokhove M, Melillo E, Dijkstra BW, Cool RH, Quax WJ. Reducing virulence of the human pathogen Burkholderia by altering the substrate specificity of the quorum-quenching acylase PvdQ. Proc Natl Acad Sci. 2014;111(4):1568–1573. doi: 10.1073/pnas.1311263111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sokol PA, Sajjan U, Visser MB, Gingues S, Forstner J, Kooi C. The CepIR quorum-sensing system contributes to the virulence of Burkholderia cenocepacia respiratory infections. Microbiology. 2003;149:3649–3658. doi: 10.1099/mic.0.26540-0. [DOI] [PubMed] [Google Scholar]
- 43.Harms A, Maisonneuve E, Gerdes K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science. 2016;354:aaf4268. doi: 10.1126/science.aaf4268. [DOI] [PubMed] [Google Scholar]
- 44.Alekshun MN, Levy SB. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128:1037–1050. doi: 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Defoirdt T, Sorgeloos P, Bossier P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol. 2011;14:251–258. doi: 10.1016/j.mib.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol. 2007;3:541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- 47.Gupta K, Daroch P, Harjai K, Chhibber S. Parallels among natural and synthetically modified quorum-quenching strategies as convoy to future therapy. Microbiology. 2019 doi: 10.1099/mic.0.000826. [DOI] [PubMed] [Google Scholar]
- 48.Zhou S, Yu Z, Chu W. Effect of quorum-quenching bacterium Bacillus sp QSI-1 on protein profiles and extracellular enzymatic activities of Aeromonas hydrophila YJ-1. BMC Microbiol. 2019;19:135. doi: 10.1186/s12866-019-1515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin X, Chen X, Tu Y, Wang S, Chen H. Effect of Probiotic Lactobacilli on the Growth of Streptococcus mutans and multispecies biofilms isolated from children with active caries. Med Sci Monit. 2017;23:4175–4181. doi: 10.12659/MSM.902237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen XM, Cui HX, Chen B, Xu XR. Orally administered Lactobacillus casei exhibited several probiotic properties in artificially suckling rabbits. Asian-Australas J Anim Sci. 2019 doi: 10.5713/ajas.18.0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsubara VH, Wang Y, Bandara HM, Mayer MP, Samaranayake LP. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl Microbiol Biotechnol. 2016;100:6415–6426. doi: 10.1007/s00253-016-7527-3. [DOI] [PubMed] [Google Scholar]
- 52.Hill D, Sugrue I, Tobin C, Hill C, Stanton C, Ross RP. The Lactobacillus casei group: history and health related applications. Front Microbiol. 2018;9:2107. doi: 10.3389/fmicb.2018.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Text 1. 16S rDNA sequence of A. hydrophila AH-1. Text 2. 16S rDNA sequence of A. hydrophila AH-4. Table S1. Amplification composition and condition of SOE-PCR. Fig. S1. Agarose gel electrophoresis of SOE-PCR product PslyA-NHM gene. Fig. S2. AHL lactonase activity and live cells of LcAiiK after storage at 4 °C.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Additional file.