Abstract
Background
Non-invasive mechanical ventilation (NIV) has become an alternative to an invasive artificial airway for the management of acute respiratory failure (ARF). NIV failure causes delayed intubation, which eventually has been associated with increased morbidity and mortality. This study aimed to develop the clinical scoring system of NIV failure in ARF patients.
Methods
This study was a diagnostic, retrospectively cross-sectional, and exploratory model at the Emergency Medicine Department in Ramathibodi Hospital between February 2017 and December 2017. We included all of the acute respiratory failure patients aged > 18 years and received non-invasive ventilation (NIV). Clinical factors associated with NIV failure were recorded. The predictive model and prediction score for NIV failure were developed by multivariable logistic regression analysis.
Result
A total of 329 acute respiratory failure patients have received NIV success (N = 237) and failure (N = 92). This study showed that NIV failure was associated with heart rate > 110 bpm, systolic BP < 110 mmHg, SpO2 < 90%, arterial pH < 7.30 and serum lactate. The clinical scores were classified into three groups: low, moderate, and high.
Conclusion
We suggested that the novel clinical scoring of the NIV failure in this study may use as a good predictor for NIV failure in the emergency room.
Keywords: NIV failure, Predictive score
Background
Acute respiratory failure (ARF) was a steady increase in the number of hospitalizations at an average annual rate of 11.3% in 2001 to 2009 with a decrease in inpatient mortality in the United States [1]. In Thailand, ARF was increased from 6.99 people per 100,000 in 2011 to 8.98 people per 100,000 in 2014 [2]. ARF characterized by the impaired respiratory system to exchange gases and to oxygenate the blood, resulting in hypoxia with or without hypercapnia [2]. Two main mechanisms of ARF include failure in pulmonary ventilation caused by neuromuscular diseases, chest wall deformities, obstructive pulmonary diseases, and failure in gas exchanges caused by adult acute respiratory distress syndrome, neonatal respiratory distress syndrome, acute cardiogenic pulmonary edema, severe status asthmaticus, pneumonia, airspace collapse (atelectasis) and pulmonary embolism [3]. The clinical signs and symptoms of patients with ARF refer to the two main manifestations of pulmonary diseases, including arterial hypercapnia and hypoxemia.
Noninvasive ventilation (NIV) refers to the delivery of mechanical ventilation without using an invasive artificial airway (endotracheal tube or tracheostomy tube) that markedly increases over the past two decades worldwide [4]. NIV has become an alternative to orotracheal intubation and invasive mechanical ventilation for the management of ARF, since it can decrease the length of stay in the ICU, reduce the number of possible complications, increase the quality of life, reduce risk of infection and improve the chance of survival, compared to conventional invasive ventilation [5–7]. The effectiveness of NIV varies according to the etiology of respiratory failure [8]. However, NIV failure causes delayed intubation and was associated with an increased risk of in-hospital death, ICU and hospital stay [9]. Thus, early prediction of NIV failure is important. However, the clinical scoring system is lacking. Therefore, this study aimed to develop the clinical scoring system of NIV failure in ARF patients at the Emergency Medicine Department of Ramathibodi Hospital, a Mahidol university-affiliated super tertiary care hospital in Bangkok, Thailand.
Method
This study was retrospectively cross-sectional study. Data was collected from Ramathibodi hospital database via Electronic Medical Record (EMR) by using NIV protocol record form between February 2017 and December 2017.
We included all acute respiratory failure patients aged > 18 years and received non-invasive ventilation (NIV) in the study period. We excluded the patients with denied intubation, used a tracheostomy tube, owned a personal non-invasive ventilator, and used non-invasive ventilation post-extubation.
The study variables were recorded for all eligible patients, including the baseline characteristic factor and potential clinical factors for NIV failure. Clinical factors included gender, age, vital signs at ED arrival (respiratory rate, heart rate, systolic blood pressure, oxygen saturation, body temperature), Glasgow coma scale, diagnosis, underlying disease, laboratory test, arterial blood gas, vasoactive agents and qSOFA score.
The outcomes were NIV success (did not receive intubation in this hospital admission) and the NIV failure (receive intubation in this hospital admission). Finally, we develop clinical scoring of failure on acute respiratory failure patients received NIV at the emergency department.
Study size estimation
We collected the data of the acute respiratory failure patients received NIV between July and August 2017. There were 34 patients of NIV success (69.4%) and 15 patients of NIV failure (30.6%). The ratio of NIV success per NIV failure was 1: 2 STATA version 14.0 analysis software was used to calculate the sample size by employing a two-sample comparison of NIV success and NIV failure. The assumptions were as follows: alpha = 0.05 (two-sided test), power of sample size = 0.9, and the ratio of sample size = 1: 2 The sample size of 59 was obtained in NIV success population group and the sample size of 28 was obtained in NIV failure population group.
Statistical analysis
Data were analyzed using STATA version 14.0. All study variables were compared between the NIV success (did not receive intubation) and the NIV failure (receive intubation) groups by using exact probability test for categorical study variables, and T-test in continuous study variables. The predictive power of each variable was calculated using univariable logistic regression and presented as the area under the receiver operating characteristic (AuROC) curve with 95% confidence intervals (CIs). The potential predictors were categorized into three levels by multivariable logistic regression. Regression coefficients for each level of each clinical predictor were divided by the smallest coefficient of the model and rounded to the nearest 0 or 0.5, resulting in a scoring scheme. Discrimination of the prediction scores was presented as the AuROC curve, and 95% CIs for the clinical scoring of failure on acute respiratory failure patients received NIV. Calibration of the prediction was presented using the Hosmer-Lemeshow goodness-of-fit test. The number of reports and percentages of each group were presented with the positive likelihood ratio, 95% CIs, and p-value.
Results
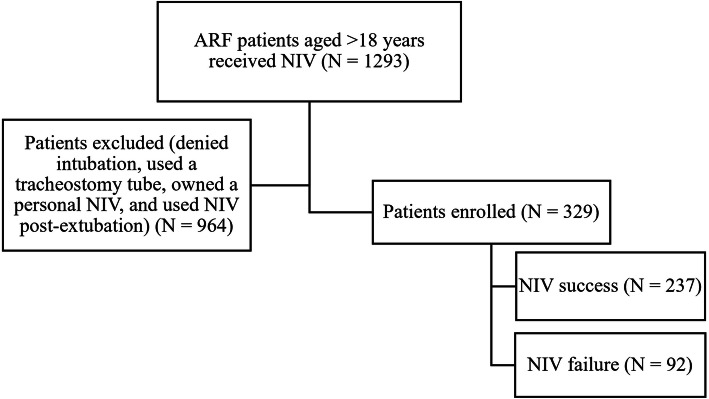
The study variables of NIV success and NIV failure on acute respiratory failure patients was collected between February 2017 and December 2017 at the Emergency Medicine Department of Ramathibodi Hospital, a university-affiliated super tertiary care hospital in Bangkok, Thailand (Fig. 1). A total of 329 acute respiratory failure patients were received NIV success (N = 237) and failure (N = 92). As illustrated in Table 1, the acute respiratory failure patients possessed five variable factors including heart rate > 110 bpm, systolic BP < 110 mmHg, SpO2 < 90%, arterial pH < 7.30 and serum lactate significantly demonstrated the failure for receiving NIV, with high discriminative performance (p = 0.005, AuROC = 0.582; p = 0.001, AuROC = 0.564; p = 0.001, AuROC = 0.601; p = 0.016, AuROC = 0.526 and p < 0.001, AuROC = 0.589, respectively).
Fig. 1.
Flow of patients through the study. NIV = Non-invasive mechanical ventilation
Table 1.
The study variables of NIV success and NIV failure in acute respiratory failure patients
| Baseline characteristics | Success (N = 237) | Failure (N = 92) | p-value | AuROC (95% CI) | ||
|---|---|---|---|---|---|---|
| Gender, Female (N, %) | 130 | 54.85% | 39 | 42.39% | 0.049 | 0.438 (0.378–0.498) |
| Age (years) | 74.78 ± 12.78 | 73.37 ± 13.79 | 0.382 | – | ||
| Age > 75 | 150 | 63.29% | 53 | 57.61% | 0.377 | 0.472 (0.412–0.531) |
| Vital signs at ED arrival | ||||||
| Respiratory rate (bpm) | 29.46 ± 5.39 | 30.76 ± 6.88 | 0.070 | – | ||
| Respiratory rate > 30 | 96 | 40.51% | 52 | 56.52% | 0.010 | 0.580 (0.520–0.640) |
| Heart rate (bpm) | 97.82 ± 21.51 | 105.87 ± 26.67 | 0.005 | – | ||
| Heart rate > 110 | 64 | 27.00% | 40 | 43.48% | 0.005 | 0.582 (0.524–0.641) |
| Systolic BP (mmHg) | 156.68 ± 32.99 | 142.03 ± 39.27 | < 0.001 | – | ||
| SBP < 110 | 16 | 6.75% | 18 | 19.57 | 0.001 | 0.564 (0.520–0.608) |
| Oxygen saturation (%) | 92.31 ± 6.03 | 87.84 ± 11.21 | < 0.001 | |||
| SpO2 < 90 | 55 | 23.21% | 39 | 43.33% | 0.001 | 0.601 (0.543–0.659) |
| Body temperature (°C) | 37.13 ± 0.86 | 37.39 ± 0.90 | 0.015 | |||
| Body temp > 37.5 | 58 | 24.47 | 37 | 40.22 | 0.007 | 0.579 (0.521–0.636) |
| Glasgow coma scale | ||||||
| GCS 13–15 | 229 | 96.62% | 84 | 93.33% | 0.222 | 0.517 (0.488–0.545) |
| GCS 9–12 | 8 | 3.38% | 6 | 6.67% | ||
| Laboratory test | ||||||
| White blood cell (× 103/μL) | 9362.62 ± 4531.24 | 13,929.24 ± 15,127.59 | < 0.001 | – | ||
| WBC > 10,000 | 78 | 32.91% | 53 | 57.61% | < 0.001 | 0.624 (0.565–0.682) |
| Hemoglobin (g/dL) | 10.98 ± 2.16 | 11.51 ± 2.08 | 0.048 | – | ||
| HgB < 12 in female or HgB < 13 in male | 174 | 73.42% | 68 | 73.91% | 1.000 | 0.503 (0.449–0.556) |
| Hematocrit (%) | 33.94 ± 7.07 | 35.03 ± 7.96 | 0.227 | – | ||
| Hct < 36 in female or Hct < 40 in male | 161 | 67.93% | 55 | 59.78% | 0.196 | 0.459 (0.401–0.518) |
| Blood urea nitrogen (mg/dL)(Med, IQR) | 29.31 ± 21.82(22.00, 25.00) | 33.84 ± 26.85(24.50, 32.00) | 0.115 | – | ||
| BUN > 18 | 142 | 59.92% | 62 | 67.39% | 0.255 | 0.537 (0.480–0.595) |
| Creatinine (mg/dL) | 2.30 ± 2.77(1.28, 1.59) | 2.19 ± 2.48(1.21, 1.55) | 0.742 | – | ||
| Cr > 1.02 | 146 | 61.60% | 51 | 55.43% | 0.318 | 0.469 (0.409–0.529) |
| Sodium (mEq/L) | 136.45 ± 5.19 | 134.71 ± 6.71 | 0.013 | – | ||
| 136–145 | 147 | 62.23% | 46 | 50.00% | 0.090 | 0.558 (0.498–0.618) |
| < 136 | 86 | 36.29% | 45 | 48.91% | ||
| > 145 | 4 | 1.69% | 1 | 1.09% | ||
| Potassium (mEq/L) | 4.23 ± 0.66 | 4.33 ± 0.92 | 0.275 | – | ||
| 3.5–5.1 | 169 | 71.31% | 61 | 66.30% | 0.247 | 0.532 (0.474–0.591) |
| < 3.5 | 41 | 17.30% | 14 | 15.22% | ||
| > 5.1 | 27 | 11.39% | 17 | 18,48% | ||
| Arterial blood gas | ||||||
| pH | 7.40 ± 0.04 | 7.40 ± 0.07 | 0.506 | – | ||
| Acidosis < 7.30 | 3 | 1.27% | 6 | 6.52% | 0.016 | 0.526 (0.500–0.552) |
| PaO2 (mmHg) | 121.70 ± 37.55 | 125.28 ± 59.73 | 0.517 | – | ||
| PaO2 < 60 | 13 | 5.49% | 7 | 7.61% | 0.451 | 0.511 (0.480–0.541) |
| PaCO2 (mmHg) | 39.44 ± 6.25 | 37.85 ± 11.28 | 0.106 | – | ||
| PaCO2 > 50 | 11 | 4.64% | 5 | 5.43% | 0.778 | 0.504 (0.477–0.531) |
| HCO3 (mEq/L) | 22.14 ± 4.14 | 20.53 ± 6.61 | 0.014 | – | ||
| > 22 | 105 | 44.49% | 30 | 33.33% | 0.078 | 0.444 (0.386–0.503) |
| Lactate (mmol/L) | 2.61 ± 1.03 | 3.50 ± 2.67 | < 0.001 | – | ||
| < 4 | 227 | 95.78 | 72 | 78.26 | < 0.001 | 0.589 (0.544–0.633) |
| 4–8 | 8 | 3.38 | 12 | 13.04 | ||
| > 8 | 2 | 0.84 | 8 | 8.70 | ||
| FiO2 | 0.26 ± 0.16 | 0.32 ± 0.23 | 0.017 | – | ||
| PaO2 / FiO2 (mmHg) | 531.67 ± 181.01 | 499.40 ± 229.79 | 0.181 | – | ||
| PaO2 / FiO2 < 300 | 33 | 13.92% | 20 | 21.74% | 0.095 | 0.539 (0.491–0.587) |
| Vasoactive agents (N, %) | 19 | 8.05% | 16 | 17.58% | 0.017 | 0.452 (0.409–0.495) |
| qSOFA score > 2 | 14 | 5.91% | 16 | 17.78% | 0.002 | 0.559 (0.517–0.602) |
| Diagnosis (N, %) | ||||||
| Volume overload | 32 | 13.50% | 4 | 4.35% | 0.017 | 0.546 (0.516–0.576) |
| Congestive heart failure | 115 | 48.52% | 29 | 31.52% | 0.006 | 0.585 (0.528–0.642) |
| Tracheobronchitis | 23 | 9.75% | 11 | 11.96% | 0.550 | 0.489 (0.451–0.527) |
| COPD | 55 | 23.31% | 23 | 25.00% | 0.774 | 0.492 (0.439–0.544) |
| Pneumonia | 63 | 26.58% | 43 | 46.74% | 0.001 | 0.399 (0.341–0.458) |
| Pleural effusion | 15 | 6.33% | 8 | 8.70% | 0.473 | 0.488 (0.455–0.521) |
| Pulmonary embolism | 4 | 1.69% | 3 | 3.26% | 0.404 | 0.492 (0.472–0.512) |
| ARDS | 0 | 0.00% | 5 | 5.43% | 0.002 | 0.473 (0.450–0.496) |
| Sepsis | 28 | 11.81% | 23 | 25.00% | 0.006 | 0.434 (0.385–0.483) |
| Septic shock | 1 | 0.42% | 16 | 17.39% | < 0.001 | 0.415 (0.376–0.454) |
| Anemia | 16 | 6.75% | 3 | 3.26% | 0.297 | 0.518 (0.493–0.542) |
| Influenza | 26 | 10.97% | 15 | 16.30% | 0.196 | 0.473 (0.430–0.516) |
| Neurological disease | 1 | 0.42% | 3 | 3.26% | 0.068 | 0.486 (0.467–0.505) |
| Acute kidney injury | 39 | 16.46% | 31 | 33.70% | 0.001 | 0.414 (0.360–0.468) |
| Revisit in 7 days (N, %) | 15 | 6.33% | 8 | 8.70% | 0.473 | 0.488 (0.455–0.521) |
The multivariable analysis showed item score of the significant predictors in the NIV failure including heart rate > 110 bpm (score = 0, 1), systolic BP < 110 mmHg (score = 0, 2), SpO2 < 90% (score = 0, 1), arterial pH < 7.30 (score = 0, 3) and serum lactate (score = 0, 2, 4) (Table 2).
Table 2.
Significant predictors and item score of the NIV failure in acute respiratory failure patients
| Predictors | Category | OR | 95% CI | p-value | Coefficienta | Score |
|---|---|---|---|---|---|---|
| Heart rate > 110 bpm | No | 1.00 | reference | – | – | 0 |
| Yes | 1.84 | 1.05–3.22 | 0.033 | 0.61 | 1 | |
| Systolic BP < 110 mmHg | No | 1.00 | reference | – | – | 0 |
| Yes | 3.04 | 1.39–6.62 | 0.005 | 1.11 | 2 | |
| SpO2 < 90% | No | 1.00 | reference | – | – | 0 |
| Yes | 2.44 | 1.40–4.26 | 0.002 | 0.89 | 1 | |
| Arterial pH < 7.30 | No | 1.00 | reference | – | – | 0 |
| Yes | 4.94 | 1.01–24.01 | 0.048 | 1.60 | 3 | |
| Serum lactate (mmol/L) | < 4 | 1.00 | reference | – | – | 0 |
| 4–8 | 3.09 | 1.13–8.47 | 0.029 | 1.13 | 2 | |
| > 8 | 12.22 | 2.38–62.68 | 0.003 | 2.50 | 4 |
aCoefficients from multivariable continuation ratio logistic regression
OR odds ratio; CI confidence interval; bpm beat per minute; BP blood pressure; SpO2 Pulse oxygen saturation
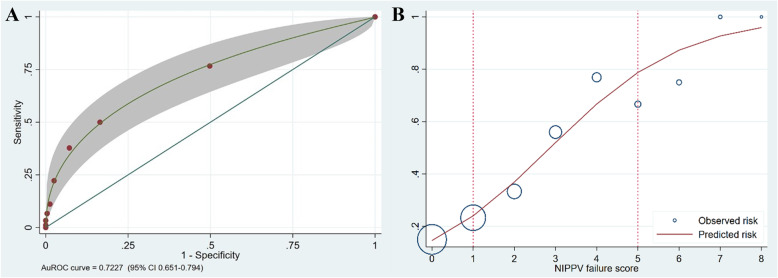
As shown in Fig. 2, this study exhibited that the AuROC curve was 72. 27% (95% CI: 0.651–0.794) for the ability of the clinical score to predict the failure of NIV, and the increased score-predicted risk correlated to the observed risk of the failure of NIV in acute respiratory failure patients. The clinical scoring of the NIV failure in acute respiratory failure patients was classified into three groups: low, score 0–1; moderate, score 2–4; and high, score > 5. The positive likelihood ratio in the high group was 8.78 (Table 3). The patient should undergo intubation or definite airway instead of NIV.
Fig. 2.
The AuROC and 95% Confidence Interval of the predictive power of the clinical scoring (a) and Observed risk (circles) vs score-predicted risk (solid line) (b) of the NIV failure in acute respiratory failure patients
Table 3.
Distribution of NIV failure vs NIV success into low, moderate and high probability categories, likelihood ratio of positive (LHR+) and 95% confidence interval (CI)
| Probability categories | Score | Failure N = 92 |
Success N = 237 |
LHR+ | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Low | 0–1 | 45 | 50.00 | 198 | 83.54 | 0.60 | 0.48–0.74 | < 0.001 |
| Moderate | 2–4 | 35 | 38.89 | 36 | 15.19 | 1.70 | 1.15–2.50 | 0.006 |
| High | > 5 | 10 | 11.11 | 3 | 1.27 | 8.78 | 2.47–31.17 | < 0.001 |
| Mean ± SD | 0.77 ± 1.04 | 2.09 ± 1.93 | < 0.001 | |||||
Discussion
Non-invasive mechanical ventilation (NIV) is an alternative therapy to avoid the life-threatening risks of invasive mechanical ventilation. It uses ventilatory support via the patient’s upper airway using a mask or similar device [3, 10]. Absolute contraindications of NIV contain cardiorespiratory arrest, extreme psychomotor agitation, severe hemodynamic instability, non-hypercapnic coma, and multiple organ failure [3]. NIV is a widely used and effective treatment for acute respiratory failure (ARF), particularly an acute exacerbation of chronic obstructive pulmonary disease (COPD) and cardiogenic pulmonary edema since the 1980s [11]. Previous studies demonstrated high success and low mortality rates of NIV in patients [12]. However, NIV failure may delay intubation, which may increase mortality and health care costs. Various clinical scoring strategies of NIV failure were assessed for early prediction. Five variables, including heart rate, acidosis, consciousness, oxygenation, and respiratory rate (HACOR) scores showed good predictive power for NIV failure in COPD patients, particularly for the prediction of early NIV failure (< 48 h) [13].
Due to the lack of the best clinical scoring, this study assessed the NIV failure patients’ novel clinical scoring in acute respiratory failure patients at the emergency department. This study showed that NIV failure was associated with heart rate > 110 bpm, systolic BP < 110 mmHg, SpO2 < 90%, arterial pH < 7.30 and serum lactate. The clinical scores were classified into three groups: low, moderate, and high. We suggested that the low group increased the chance of successful NIV in ARF.
In this study, serum lactate levels (> 8 mmol/L) were the most relevant variables for predicting NIV failure, with a maximal score of 4 points. Blood lactate was considered a diagnostic hallmark of tissue hypoxia, respiratory muscle fatigue, and COPD severity [14]. Patients with a high lactate level are strongly correlated with increased mortality in various conditions [15–17]. Arterial pH (< 7.30) was the second most relevant variable, with a maximal score of 3 points, followed by systolic BP (< 110 mmHg) with a maximal score of 2 points. The pH level, an indicator of hypercapnia severity, has been documented as an essential predictor to assess NIV success [18].
Previous studies have been clearly reported that a lower baseline pH is a risk factor for NIV failure in certain conditions, especially in COPD patients [19, 20]. COPD patients with mild to moderate acidosis showed that NIV improved patient outcomes exclusively, the baseline pH was ≥7.30 [21]. In a certain study, systolic BP < 90 mmHg is considered a relative contraindication to NIV [22]. Heart rate (> 110 bpm) and SpO2 (< 90%) were less relevant, with a maximal score of 1 point. Generally, a heart rate < 110 bpm has been suggested as an indicator to withdrawn NIV in ARF [10]. SpO2, an arterial oxygen saturation measured by pulse oximetry, targets should be 88–92% in hypercapnic ARF patients treated with NIV. A previous study demonstrated that no single variable could predict NIV failure well. On the other hand, a combination of several variables may increase predictive accuracy [13].
There are limitations to this study. First, this study was retrospective data collection and conducted in a single center. The clinical parameters are affected by confounder variables, overlapping that can incorporate normal parameters. Some parameters are poor predictors—the predictor variables in other studies not statistically significant in this study. We now need to validate our results externally to establish our risk score’s actual value for management decisions.
Conclusion
Using combination of 5 variables including heart rate > 110 bpm, systolic BP < 110 mmHg, SpO2 < 90%, arterial pH < 7.30 and serum lactate. The clinical scores were classified into three groups: low, moderate, and high.
Acknowledgements
We thank Angela Morben, DVM, ELS, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
Abbreviations
- NIV
Non-invasive mechanical ventilation
- ARF
Acute respiratory failure
- AuROC
Area under the receiver operating characteristic curve
- CIs
Confidence intervals
- COPD
Chronic obstructive pulmonary disease
Authors’ contributions
WL and TT designed this study and protocol development. TT and CJ were responsible for data collection. CY and CJ were responsible for data analysis. WL and TT wrote the manuscript. TT and PN provided the final approval for this version to be published. CY and WL agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets analysed during the current study are not publicly available due to privacy issues but are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
This study was approved by the Faculty of Medicine, Committee on Human Rights Related to Research Involving Human Subjects, of Mahidol University’s Ramathibodi Hospital (COA. NO. MURA2018/608). The need for informed consent was waived by the ethics committee due to retrospective design.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stefan MS, Shieh M-S, Pekow PS, et al. Epidemiology and outcomes of acute respiratory failure in the United States, 2001 to 2009: a national survey. J Hosp Med. 2013;8(2):76–82. doi: 10.1002/jhm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niyomrat W, Masingboon K, Kunsongkeit W. Relationships between comfort and pain, anxiety, and social support in acute respiratory failure patients with non-invasive ventilator support. Thai Pharmaceutical Health Sci J. 2018;13(4):179–186. [Google Scholar]
- 3.Forte P, Mazzone M, Portale G, et al. Approach to respiratory failure in emergency department. Eur Rev Med Pharmacol Sci. 2006;10(3):135–151. [PubMed] [Google Scholar]
- 4.Scala R, Pisani L. Noninvasive ventilation in acute respiratory failure: which recipe for success? Eur Respir Rev. 2018;27(149):180029. doi: 10.1183/16000617.0029-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrer M, Esquinas A, Arancibia F, et al. Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med. 2003;168(1):70–76. doi: 10.1164/rccm.200209-1074OC. [DOI] [PubMed] [Google Scholar]
- 6.Antonelli M, Conti G, Rocco M, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998;339:429–435. doi: 10.1056/NEJM199808133390703. [DOI] [PubMed] [Google Scholar]
- 7.Mehta S, Hill NS. Noninvasive Ventilation. Am J Respir Crit Care Med. 2001;163(2):540–577. doi: 10.1164/ajrccm.163.2.9906116. [DOI] [PubMed] [Google Scholar]
- 8.Martín-González F, González-Robledo J, Sánchez-Hernández F, et al. Effectiveness and predictors of failure of noninvasive mechanical ventilation in acute respiratory failure. Med Int. 2016;40(1):9–17. doi: 10.1016/j.medin.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Corrêa TD, Sanches PR, de Morais LC, et al. Performance of noninvasive ventilation in acute respiratory failure in critically ill patients: a prospective, observational, cohort study. BMC Pulmonary Medicine. 2015;15(1):144. doi: 10.1186/s12890-015-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyatt J, Bellis F. British Thoracic Society guidelines on non-invasive ventilation. Emerg Med J. 2002;19(5):435. 10.1136/emj.19.5.435. [DOI] [PMC free article] [PubMed]
- 11.Wang T, Zhang L, Luo K, et al. Noninvasive versus invasive mechanical ventilation for immunocompromised patients with acute respiratory failure: a systematic review and meta-analysis. BMC Pulmonary Med. 2016;16(1):129. doi: 10.1186/s12890-016-0289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timenetsky KT, Aquino SH, Saghabi C, et al. High success and low mortality rates with non-invasive ventilation in influenza A H1N1 patients in a tertiary hospital. BMC Res Notes. 2011;4:375. doi: 10.1186/1756-0500-4-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan J, Wang S, Liu P, et al. Early prediction of noninvasive ventilation failure in COPD patients: derivation, internal validation, and external validation of a simple risk score. Ann Intensive Care. 2019;9(1):108. doi: 10.1186/s13613-019-0585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiavo A, Renis M, Polverino M, Iannuzzi A, Polverino F. Acid-base balance, serum electrolytes and need for non-invasive ventilation in patients with hypercapnic acute exacerbation of chronic obstructive pulmonary disease admitted to an internal medicine ward. Multidiscip Respir Med. 2016;11:23. doi: 10.1186/s40248-016-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schollin-Borg M, Nordin P, Zetterström H, et al. Blood Lactate Is a Useful Indicator for the Medical Emergency Team. Crit Care Res Pract. 2016;2016:5765202. doi: 10.1155/2016/5765202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen LW, Mackenhauer J, Roberts JC, et al. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127–1140. doi: 10.1016/j.mayocp.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filho RR, Rocha LL, Corrêa TD, et al. Blood lactate levels cutoff and mortality prediction in sepsis-time for a reappraisal? A retrospective cohort study. Shock. 2016;46(5):480–485. doi: 10.1097/SHK.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozyilmaz E, Ugurlu AO, Nava S. Timing of noninvasive ventilation failure: causes, risk factors, and potential remedies. BMC Pulmonary Med. 2014;14:19. doi: 10.1186/1471-2466-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Confalonieri M, Garuti G, Cattaruzza MS, et al. A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J. 2005;25(2):348–355. doi: 10.1183/09031936.05.00085304. [DOI] [PubMed] [Google Scholar]
- 20.Conti G, Antonelli M, Navalesi P, et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med. 2002;28(12):1701–1707. doi: 10.1007/s00134-002-1478-0. [DOI] [PubMed] [Google Scholar]
- 21.Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000;355(9219):1931–1935. doi: 10.1016/S0140-6736(00)02323-0. [DOI] [PubMed] [Google Scholar]
- 22.Ergan B, Nasiłowski J, Winck JC. How should we monitor patients with acute respiratory failure treated with noninvasive ventilation? Eur Respir Rev. 2018;27:148. doi: 10.1183/16000617.0101-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are not publicly available due to privacy issues but are available from the corresponding author upon reasonable request.