Abstract
In this investigation, we used a combination of field- and laboratory-based approaches to assess if influenza A viruses (IAVs) shed by ducks could remain viable for extended periods in surface water within three wetland complexes of North America. In a field experiment, replicate filtered surface water samples inoculated with duck swabs were tested for IAVs upon collection and again after an overwintering period of approximately 6–7 months. Numerous IAVs were molecularly detected and isolated from these samples, including replicates maintained at wetland field sites in Alaska and Minnesota for 181–229 days. In a parallel laboratory experiment, we attempted to culture IAVs from filtered surface water samples inoculated with duck swabs from Minnesota each month during September 2018–April 2019 and found monthly declines in viral viability. In an experimental challenge study, we found that IAVs maintained in filtered surface water within wetlands of Alaska and Minnesota for 214 and 226 days, respectively, were infectious in a mallard model. Collectively, our results support surface waters of northern wetlands as a biologically important medium in which IAVs may be both transmitted and maintained, potentially serving as an environmental reservoir for infectious IAVs during the overwintering period of migratory birds.
Keywords: avian, environment, influenza, persistence, reservoir, wetland
1. Introduction
Influenza A viruses (IAVs) maintained in wild bird hosts episodically spillover to domestic poultry where they may cause clinical disease and ultimately lead to economically costly outbreaks [1]. In rare instances, such viruses may spread to other domestic livestock [2,3] or companion animals [4–6], spill back into wild birds [7] and/or infect humans [8–9], sometimes resulting in fatal outcomes. On at least four occasions, avian-origin IAVs have co-infected mammalian hosts and reassorted with swine- and/or human-adapted influenza strains leading to pandemic viruses [10]. Thus, the maintenance of avian-origin IAVs in biotic reservoirs and the physical environment has important implications for economic interests and food security, as well as human and animal health.
The maintenance and spread of IAVs in avian hosts have been the focus of extensive global research and surveillance efforts [11]. However, far less research has been focused on the viability of viruses in the environment, particularly surface waters through which IAVs are presumably transmitted among wild birds, the natural biological reservoir. Wild bird-origin IAVs have repeatedly been detected from surface water [12–19] and sediment [20–22] collected from a broad range of freshwater and estuarine wetlands using a variety of virological and molecular methods. These findings are consistent with the premise that environmental transmission serves as an important mechanism of viral spread among wild bird hosts and may facilitate spillover to domestic animals. However, sporadic detections of IAVs from surface waters and sediment provide limited inference regarding how long viruses remain infectious in the physical environment, an important parameter regulating environmental transmission [23].
Most of the published literature on the duration of viral infectivity in water or sediments is based on temperature-controlled experiments conducted in laboratory settings [24–32] rather than field experiments carried out under natural conditions found in the environment [33]. In this investigation, we aimed to bridge the data gap between field observations and controlled laboratory investigations by developing and applying novel field-based and laboratory methods for refining inference on the duration of IAV infectivity in wetland complexes across a latitudinal gradient in North America. Specifically, we assessed if IAVs shed by ducks could remain viable for extended periods of time in surface water collected from the environment when maintained under naturally occurring temperatures. Furthermore, we incorporated a comparative laboratory experiment and an experimental challenge study to assess how field-based results relate to information obtained through laboratory models. Our combined field- and laboratory-based research approach builds upon a recently developed method for assessing the duration of IAV infectivity in the environment [33]. Here, we aimed to elucidate the potential for avian-origin viruses to remain infective in North American wetlands for extended periods, including from the time of departure of migratory birds from their northern staging areas in late summer/autumn until their subsequent return to such areas the following spring.
2. Materials and methods
We direct readers to electronic supplementary material, file S1 for complete and detailed materials and methods.
(a). Field experiment
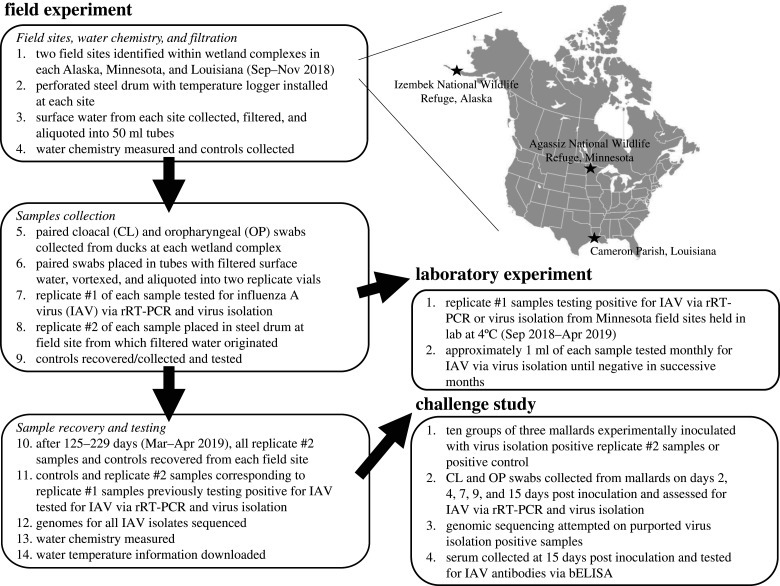
To assess if avian-origin IAVs remain viable in wetland surface waters for extended periods of time when maintained under naturally occurring temperatures, we first set up an experiment at two field sites within each of three wetland complexes of North America (figure 1): Izembek National Wildlife Refuge (NWR), Alaska (55.3° N, 162.8° W); Agassiz NWR, Minnesota (48.3–48.4° N, 96.0–96.1° W); and Cameron Parish, Louisiana (29.7–30.0° N, 92.8–93.2° W). Briefly, surface water from six field sites was collected, chemically characterized and filtered to 0.22 µm. Approximately 100–120 vials containing 40 ml of the filtered surface from each field site were inoculated with paired cloacal (CL) and oropharyngeal (OP) swab samples from hunter-harvested or live-captured ducks and divided into replicate samples (i.e. replicate #1 and replicate #2). Replicate #1 was tested for IAV upon collection by real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) [34] where a cycle threshold (Ct) value < 45 was considered ‘positive' and via virus isolation in embryonating chicken eggs (ECE) [35]. Prior to or concurrent with the testing of replicate #1, the replicate #2 sample was submerged within a steel perforated drum equipped with a temperature logger at the field site from which filtered surface water was originally obtained. Submersed replicate #2 vials, additional water chemistry samples, and appropriate controls were left to overwinter within steel drums at field sites until retrieval in spring 2019. After a period of 125–229 days, replicate #2 field samples were recovered, and those that corresponded to a replicate #1 sample that previously tested positive for IAV by rRT-PCR or virus isolation were tested using the same molecular and culture methods. Water chemistry samples and experimental controls were also recovered/collected and tested using methods reported in further detail within electronic supplementary material, file S1. RNA was extracted from all IAV positive field samples and genomically characterized using previously reported methods [36]. IAVs from virus isolation positive replicate #2 samples recovered in spring that shared greater than 99% nucleotide identity at all gene segments with viruses recovered from the paired replicate #1 sample were inferred to remain infectious throughout the duration of the field experiment.
Figure 1.
Flow chart and inset providing an overview of wetland complexes, experimental work-flow and methods incorporated in this study. The map of the United States and Canada was used under CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0/).
(b). Laboratory experiment
To compare results from our field-based experiment to a laboratory-controlled approach (figure 1), we used the remaining volume of all replicate #1 samples from Agassiz NWR, Minnesota that tested positive for IAVs via virus isolation to assess viral viability through time. Briefly, replicate #1 samples were maintained in their original tube at 4°C. Approximately 1 ml of each sample was tested by virus isolation in ECE monthly through April 2019. Samples which were virus isolation negative for two successive months were considered to no longer contain viable IAV and not subjected to further testing.
(c). Challenge study
To further evaluate the infectivity of IAVs in field samples that were deployed at wetland field sites, we experimentally inoculated 10 groups of three mallards (Anas platyrhynchos) with virus isolation positive replicate #2 samples recovered in April 2019 or a positive inoculation control (figure 1). Briefly, 15-day-old mallard ducks housed in self-contained isolation units were inoculated by the intranasal, intracloacal, oral and ocular routes with 100, 100, 200 and 50 µl of inoculum, respectively. Ducks were observed daily for clinical signs of disease. CL and OP swabs were collected from each bird and placed in separate vials at 2, 4, 7, 9 and 15 days post-inoculation (dpi). At 15 dpi, ducks were bled to evaluate seroconversion, and then euthanized using pentobarbital. All CL and OP swabs were screened for IAVs using rRT-PCR [37] and samples with rRT-PCR Ct values (≤ 38) were subjected to virus isolation as previously described. Allantoic fluids causing hemagglutination after 4 days of incubation at 37°C were considered purportedly virus isolation positive. RNA was extracted from at least one isolate and at least one swab for every purported virus isolation positive mallard and subjected to full genome sequencing as reported above. IAVs from mallard samples sharing ≥ 99% nucleotide identity at all gene segments with viruses recovered from corresponding inoculum were inferred to be infectious (i.e. virus isolation positive) in our mallard model. Sera collected prior to inoculation (two weeks of age) and at 15 dpi were tested for antibodies to type A influenza with a blocking enzyme-linked immunosorbent assay (bELISA).
3. Results
(a). Field experiment
Of 686 samples of filtered environmental surface water inoculated with paired CL/OP swabs from ducks and initially screened, 80 and 51 samples tested positive for IAVs via rRT-PCR and virus isolation, respectively (table 1). Viral RNA was detected from samples collected from Minnesota (n = 65), Alaska (n = 11) and Louisiana (n = 4). Viable IAVs were also identified via virus isolation in samples collected from Minnesota (n = 40) and Alaska (n = 11), but not Louisiana (n = 0).
Table 1.
Summary of influenza A virus screening of filtered surface water samples inoculated with paired cloacal and oropharyngeal swab samples collected from ducks prior to and following placement in wetlands in Alaska, Minnesota and Louisiana for a period of 125–229 days.
| wetland complex and field site | filtered water/swab samples (n=) | replicate #1 samples rRT-PCR+a (n=) | replicate #1 samples VI+b (n=) | date replicate #2 samples were submerged | replicate #2 sample recovery date | duration (days) replicate #2 samples were submerged in wetlands | replicate #2 samples rRT-PCR+ (n =) | replicate #2 samples VI+ (n=) |
|---|---|---|---|---|---|---|---|---|
| Izembek NWR, Alaska Bluebill Lake |
120 | 6 | 7 | 2 Sep–4 Oct 2018 | 14 Apr 2019 | 192–224 | 1 | 1 |
| Izembek NWR, Alaska Red Salmon Lake |
120 | 5 | 4 | 12 Sep–15 Oct 2018 | 14 Apr 2019 | 181–214 | 1 | 1 |
| Agassiz NWR, Minnesota Farmes Pool |
109 | 38 | 20 | 11 Sep 2018 | 28 Apr 2019 | 229 | 2 | 4 |
| Agassiz NWR, Minnesota Tamarack/Northwest Pool |
99 | 27 | 20 | 14–21 Sep 2018 | 28 Apr 2019 | 219–226 | 4 | 4 |
| Cameron Parish, Louisiana Private rice field |
118 | 4 | 0 | 16 Nov 2018 | 22 Mar 2019 | 126 | not tested | not tested |
| Cameron Parish, Louisiana Rockefeller Wildlife Refuge |
120 | 0 | 0 | 17 Nov 2018 | 22 Mar 2019 | 125 | not tested | not tested |
arRT-PCR positive (+).
bVirus isolation (VI) positive (+).
Replicate #2 samples that had been submersed in wetlands at Agassiz NWR, Minnesota and Izembek NWR, Alaska and recovered after a period of 181–229 days yielded detections of viral RNA (n = 6 and 2, respectively) and/or viable IAVs (n = 8 and 2, respectively; table 1). Replicate #2 samples from Cameron Parish, Louisiana were not screened given the lack of viable IAVs identified through initial screening results. When considering only samples in which infectious IAVs were identified, the percentage of paired samples for which both the replicate #1 and replicate #2 sample were virus isolation positive was 20% for samples from Agassiz NWR, Minnesota (8/40) and 18% for samples from Izembek NWR, Alaska (2/11; table 1). Samples recovered in spring from which viable IAVs were isolated had been submersed in wetland field sites for 209–229 days. As calculated using 30.5 days per month, these time periods equate to 6.9–7.5 months. No infectious IAVs nor viral RNA were detected in any of the negative controls tested from either the initial or final collection timepoint.
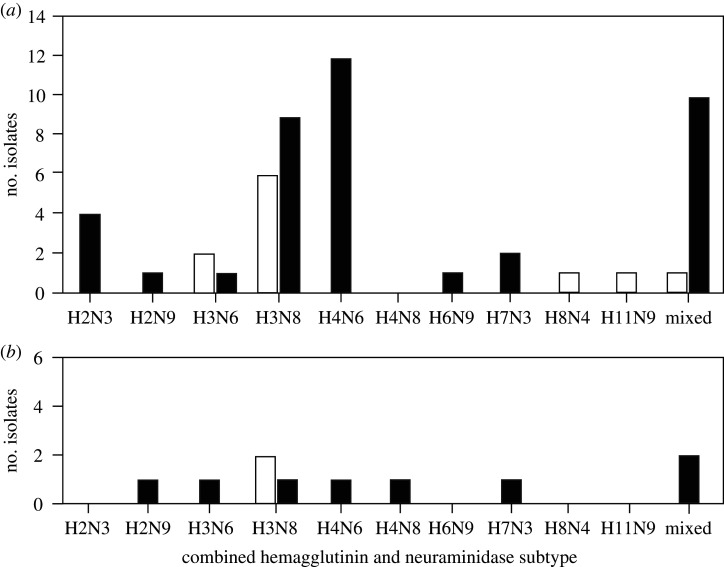
We obtained genomic information for all 51 viruses isolated from replicate #1 samples collected in Alaska and Minnesota during September–October 2018 and 10 viruses isolated from replicate #2 samples recovered from field sites at these locales in April 2019. Predominate combined hemagglutinin (HA) and neuraminidase (NA) subtypes for isolates recovered through the screening of replicate #1 samples collected in September–October 2018 included H3N8 (6/11 or 54% of isolates from Alaska and 9/40 or 23% of isolates from Minnesota) and H4N6 (12/40 or 30% of isolates from Minnesota) with mixed infections also being detected at both locations (1/11 or 9% of isolates from Alaska and 10/40 or 25% of isolates from Minnesota; figure 2). There were no predominate combined HA-NA subtypes among isolates recovered from replicate #2 samples from either location (figure 2). In two instances, a mixed infection was isolated upon screening of replicate #1 samples, whereas a pure culture of either H3N8 or H4N8 IAV was isolated upon testing of the corresponding replicate #2 sample. All isolates recovered from submerged replicate #2 samples shared greater than 99.6% nucleotide identity at each viral gene segment with the virus isolated from the corresponding replicate #1 sample. Genomic data were submitted to GenBank and can be accessed with accession numbers MN987946–MN988457 and MN998578–MN998579.
Figure 2.
Bar charts depicting combined subtypes of influenza A virus (IAV) isolates recovered from initial samples (replicate #1) of filtered surface water inoculated with paired cloacal and oropharyngeal swabs from ducks (a) and samples retrieved from field sites (replicate #2) after being maintained at ambient temperature in the field for a period of 209–229 days (b). Bars are shaded to represent subtype combinations for viruses isolated from samples from Alaska (white) and Minnesota (black). In two instances, a mixed infection was isolated upon initial screening of samples, whereas a pure culture of either H3N8 or H4N8 IAV was isolated upon testing of the corresponding duplicate sample.
Measures of pH for both unfiltered and filtered surface water samples from Alaska and Minnesota were near or slightly above neutral (range: 7.21–8.42) in August or September 2018 and lower (5.25–7.38) for corresponding paired samples collected (unfiltered water) or recovered (filtered water) in April 2019 (table 2). Measures of pH for unfiltered and filtered water samples from field sites in Louisiana were comparatively lower for both initial samples (6.10–6.60) and corresponding paired samples collected or recovered later (4.86–5.99; table 2). Specific conductance values were generally higher for unfiltered and filtered water samples collected in August–September 2018 at field sites in Alaska (84–96 µS cm−1) and Minnesota (527–615 µS cm−1) as compared to corresponding paired samples recovered or collected in April 2019 (74–87 µS cm−1 for Alaska sites; 242–549 µS cm−1 for Minnesota sites; table 2). Specific conductance values for surface water samples collected from Louisiana field sites exhibited a greater range of values than more northern field sites (75–1534 µS cm−1) and were not consistently lower upon sample collection/recovery in March 2019 (table 2). The mean temperature of surface water during the study period at field sites in Alaska and Minnesota in which IAVs remained viable was 4.2–4.9°C (−0.1–22.9°C), whereas the mean surface water temperature range for sites in Louisiana was 14.3–15.5°C (5.4–26.5°C; figure 3) [38].
Table 2.
Summary of water chemistry measurements for surface water samples collected at field sites in Alaska, Minnesota and Louisiana.
| wetland complex—field site | samples type | date of initial sample collection | pH at initial measure | specific conductance (µS/cm) at initial measure | date of second sample collection/recoverya | pH at second measure/upon recovery | specific conductance (µS/cm) at second measure/upon recovery |
|---|---|---|---|---|---|---|---|
| Izembek NWR, Alaska Bluebill Lake |
filtered | 29 Aug 2018 | 7.58 | 84 | 14 Apr 2019 | 5.25 | 74 |
| Izembek NWR, Alaska Bluebill Lake |
unfiltered | 29 Aug 2018 | 7.21 | 84 | 14 Apr 2019 | 6.39 | 74 |
| Izembek NWR, Alaska Red Salmon Lake |
filtered | 4 Sep 2018 | 7.55 | 96 | 14 Apr 2019 | 5.67 | 87 |
| Izembek NWR, Alaska Red Salmon Lake |
unfiltered | 4 Sep 2018 | 7.39 | 94 | 14 Apr 2019 | 5.65 | 85 |
| Agassiz NWR, Minnesota Farmes Pool |
filtered | 9 Sep 2018 | 8.04 | 607 | 28 Apr 2019 | 6.54 | 549 |
| Agassiz NWR, Minnesota Farmes Pool |
unfiltered | 9 Sep 2018 | 7.75 | 615 | 28 Apr 2019 | 6.86 | 242 |
| Agassiz NWR, Minnesota Tamarack Pool |
filtered | 9 Sep 2018 | 8.42 | 527 | 28 Apr 2019 | 5.48 | 472 |
| Agassiz NWR, Minnesota Tamarack Pool |
unfiltered | 9 Sep 2018 | 8.09 | 534 | 28 Apr 2019 | 7.38 | 248 |
| Cameron Parish, Louisiana Private rice field |
filtered | 15 Nov 2018 | 6.10 | 279 | 23 Mar 2019 | 5.77 | 274 |
| Cameron Parish, Louisiana Private rice field |
unfiltered | 15 Nov 2018 | 6.26 | 304 | 23 Mar 2019 | 4.86 | 75 |
| Cameron Parish, Louisiana Rockefeller Wildlife Refuge |
filtered | 15 Nov 2018 | 6.60 | 1166 | 23 Mar 2019 | 5.99 | 1058 |
| Cameron Parish, Louisiana Rockefeller Wildlife Refuge |
unfiltered | 15 Nov 2018 | 6.14 | 1189 | 23 Mar 2019 | 4.95 | 1534 |
aFor unfiltered surface water samples, this date reflects the date of collection. For filtered water samples, this reflects the date that 250 ml sample bottles (filled on ‘date of initial sample collection') were recovered from perforated steel drums submerged within field sites.
Figure 3.
Mean daily surface water temperature for field sites at Izembek National Wildlife Refuge (black and grey lines), Alaska (AK); Cameron Parish (yellow and orange lines), Louisiana (LA); and Agassiz National Wildlife Refuge (purple and pink lines), Minnesota (MN) in which samples were held for a period of 125–229 days. (Online version in colour.)
(b). Laboratory experiment
During our laboratory experiment, we observed monotonic monthly declines in the number of samples testing positive for viable IAVs via virus isolation (figure 4). Of the 40 samples from which IAVs were isolated upon initial testing, five (13%) yielded infectious viruses approximately seven months later at the time of final re-testing in April 2019. Three of the five samples that remained infectious for the duration of the laboratory experiment corresponded to replicate #2 samples that remained infectious after being held at field sites within Agassiz NWR, Minnesota as part of our field experiment [38]. The other two IAVs that were consistently isolated throughout our laboratory experiment were not recovered from corresponding replicate #2 field samples [38].
Figure 4.
Number of paired swab samples in filtered environmental surface water from Farmes Pool (red) and Tamarack Pool (black) field sites at Agassiz National Wildlife Refuge, Minnesota yielding influenza A virus isolates in ovo each month September 2018–April 2019 when held at 4°C in the laboratory. Twenty samples were virus isolation positive from each field site upon initial screening in September 2018. (Online version in colour.)
(c). Challenge study
No clinical signs were observed among experimentally inoculated mallards, except for a single bird from the group inoculated with sample #48 maintained for 214 days within Bluebill Lake at Izembek NWR, Alaska. This bird was swabbed for IAV and euthanized 24 h post-experimental inoculation because it was weak, recumbent and not eating. Viral RNA was detected in CL and OP swab samples collected from all 10 groups of mallards experimentally inoculated with replicate #2 field samples maintained under ambient temperatures for a period of 209–229 days (figure 5) or the positive inoculation control. We detected purportedly virus isolation positive samples from seven groups of mallards [38]; however, we only confirmed replication of the IAV used as inoculum in two groups of birds through genomic sequencing (figure 5). More specifically, IAVs isolated from groups of mallards experimentally challenged with samples maintained at Izembek NWR, Alaska for 214 days and at Agassiz NWR, Minnesota for 226 days shared ≥99.8% nucleotide identity at all gene segments to viruses recovered from the corresponding inoculum (figure 5) [38].
Figure 5.
Summary of experimental challenge study to assess the infectivity of influenza A viruses (IAVs) in mallards using inoculum comprised filtered surface water and paired cloacal and oropharyngeal swabs from ducks and maintained at ambient temperature in wetlands at Izembek National Wildlife Refuge, Alaska (AK) or Agassiz National Wildlife Refuge, Minnesota (MN) for a period of 209–229 days (d). Duck silhouettes indicate the number of mallards inoculated and have been colour-coded to denote the number of birds that were inferred to be negative for influenza A infection (white); positive for viral RNA via rRT-PCR only (yellow); positive for viral RNA and for which a bELISA provided evidence for seroconversion (orange); or positive for influenza A infection using all criteria (red) assessed in the challenge study (i.e. rRT-PCR, bELISA and isolation of IAV nearly identical to virus in inoculum as confirmed via genomic sequencing). The inverted duck silhouette indicates the euthanasia of a single mallard 24 h post-inoculation. Sample numbers, cycle threshold values (Ct) and field sites corresponding to inoculum are indicated to the right of virus icons. Maps of AK and MN courtesy of FreeVectorMaps.com (http://freevectormaps.com). The duck silhouette was downloaded from PhyloPic (http://phylopic.org/) and used under public domain (image by Sharon Wegner-Larsen). (Online version in colour.)
All experimentally challenged mallards were seronegative for IAV antibodies prior to inoculation with virus isolation positive replicate #2 samples recovered in April 2019. We detected evidence of seroconversion in four groups of mallards using sera collected 15 dpi: one group of birds experimentally challenged with a sample maintained at Izembek NWR, Alaska; two groups of birds inoculated with samples maintained at Agassiz NWR, Minnesota (figure 5); and the fourth group of mallards inoculated with the positive control virus [38]. We note that only two mallards were used to assess seropositivity resulting from experimental challenge with sample #48 given the unanticipated loss of one bird 24 h following experimental inoculation. Based on all criteria assessed, including (i) positive detection of viral RNA via rRT-PCR, (ii) isolation of viable IAV in ovo that was confirmed as sharing greater than 99% nucleotide identity with IAV in the inoculum and (iii) evidence of seroconversion following experimental infection, we found evidence that one sample from each Alaska and Minnesota appeared to be infectious in a mallard model after being held at ambient temperature in the field for seven months (figure 5).
4. Discussion
We evaluated the duration of infectivity of avian-origin IAVs in three wetland complexes across a latitudinal gradient in North America and found that viruses may remain infectious for more than seven months in surface waters collected from Alaska and Minnesota field sites when maintained under naturally occurring temperatures. Physical conditions at northern wetlands in which IAVs remained infectious included cold temperatures (approaching 0°C), near-neutral pH, and relatively low to moderate specific conductance. These results are generally consistent with previous laboratory investigations [24,26,27,30] and prior evaluation using a field-based research approach [33] which found similar physical and chemical properties to be associated with extended periods of viral infectivity in distilled or filtered surface water. We note that a consistent decline in pH was observed among all filtered and non-filtered water samples collected from Alaska, Minnesota and Louisiana wetlands between our initial sampling efforts in late summer and autumn 2018 and subsequent sampling/recovery efforts in spring 2019. In the overwintering water bottles, this was probably caused by microbial oxidation of organic matter by bacteria passing through our filtration step [39], which produced carbon dioxide and thus acted to decrease the pH of the samples. At field sites, the specific factors resulting in a reduction in surface water pH over the winter season could not be easily identified and may have included (i) increased partial pressure of carbon dioxide due to heightened ecosystem respiration relative to primary production, (ii) the accumulation of weakly ionizable organic acids and/or (iii) the influx of acidic meltwaters in the spring [40–42]. Future investigations focused on pH variability in both sample vials and the environment over time may provide new insights regarding how this water quality parameter acts to limit viral viability in wetland surface waters.
When comparing the results of our field experiment in Minnesota to those obtained using a laboratory approach, we found results to be generally concordant. More specifically, in our laboratory experiment, a comparable (although slightly lower) percentage of IAVs remained infectious for the duration of the approximately seven-month study period as compared to results obtained from Minnesota in our field experiment. Though most of the IAVs that remained infectious throughout the duration of the laboratory experiment also remained infectious in the field experiment in Minnesota, we also repeatedly isolated two viruses in our laboratory investigation throughout the approximately seven-month study period that did not remain infectious after being held for a comparable time period in the field. This discrepancy may be due to experimental artefacts (e.g. unequal mixing of virus between our split field samples), differences among viruses with regard to the ambient temperature range at which they are able to remain viable or potential isolation issues associated with the formation of viral aggregates through time. Additional comparative studies using parallel field and laboratory approaches may be useful for resolving this uncertainty.
Results from the challenge study provided strong evidence that IAVs in two field samples, one from each Izembek NWR, Alaska and Agassiz NWR, Minnesota, remained infectious in an in vivo model following a period of approximately seven months during which samples were deployed in wetlands under ambient water temperatures. Results from the challenge study for IAVs in other field samples are less clear, on account of negative virus isolation results, the lack of antibody detection through serologic testing, or insufficient genomic evidence to support in vivo replication of the IAV used as inoculum. Differences observed between assessments of infectivity in mallards versus in ovo testing of field samples may arise from disparities in sensitivity of the tests, viral variability and the freezing and thawing of samples for rRT-PCR and virus isolation as part of the challenge study which could have affected the quality of the RNA or lowered the virus titers in samples. Regardless, the finding that some field samples from Alaska and Minnesota remained infectious to mallards after more than seven months in wetlands supports the premise that northern surface waters represent a biologically important medium in which IAVs are both transmitted and maintained.
The approximately seven-month period during which viruses remained infectious at wetland field sites in Alaska and Minnesota is ecologically pertinent as this time span corresponds to the timing of the departure of migratory birds from northern staging areas in late summer/autumn and their subsequent return to such areas the following spring after overwintering at more southern latitudes. It is therefore plausible that surface waters of northern wetlands may serve as an environmental reservoir for infectious IAVs during the overwintering period of migratory waterfowl. This may explain prior observations of nearly identical genome constellations identified from wild bird samples collected in successive years at locations in Alaska and in the Czech Republic [43–45]. For example, migratory birds may have deposited IAVs in wetlands in Alaska during autumn staging and then migrants returning to their northern breeding grounds could have become infected with viruses maintained in surface waters while staging at these same areas the following spring.
Given the potential of IAVs to remain infectious in suitable northern wetlands, we encourage ambient environmental conditions to be considered in the design of future surveillance efforts for wild bird-origin IAVs, particularly those efforts targeting specific viruses determined to be important biological threats (e.g. highly pathogenic goose/Guangdong lineage viruses). For example, if surveillance activities detect economically costly or potentially pandemic IAVs in wild birds inhabiting wetland complexes with abundant cold, near-neutral surface water, it is plausible that such biological threats may also persist in the environment for extended periods of time, well beyond detection in infected hosts. Consideration should therefore be given to the development and implementation of surveillance methods that can be used to efficiently detect infectious IAVs in the environment. We also encourage the exploration of potential tools or management actions that may be used to reduce IAV infectivity in virally contaminated wetlands during outbreak scenarios should such biological threats be identified in the environment. Potentially effective approaches include the temporary draining of managed wetlands or the utilization of short-term changes in water temperature or pH. Although we appreciate that such actions may be logistically complicated, expensive, and/or associated with ecological consequences, these costs may be warranted in certain high-risk scenarios.
The lack of viable IAVs detected in samples collected in Cameron Parish, Louisiana during November 2018 precluded us from assessing the duration of infectivity for viruses at our most southernly field sites. However, if laboratory models are indeed congruent with results from field-based approaches, as appears to be the case based upon results from field sites in Alaska and Minnesota, we would expect the duration of infectivity to be much reduced at the Louisiana field sites we investigated based upon the observed lower pH of surface waters and higher average daily temperatures. Additional efforts to apply field-based approaches at a diversity of wetlands, including mid- and low-latitude field sites, would be useful to confirm or refute this hypothesis.
Supplementary Material
Acknowledgements
We appreciate field and laboratory support and intellectual input provided by Lindsay Carlson, Deborah Carter, Brady Fettig, Alinde Fojtik, Andy Greenawalt, Mason Hill, Nathan LaShomb, Brett Leach, Nikolai Lee, Scott Lee, Aliya McCarthy, Stacy Moskal, Hannah Munro and David Swayne. We thank Emily Weiser for her assistance with figure 3. We are grateful for logistical support provided by US Fish and Wildlife Service staff at Agassiz and Izembek national wildlife refuges. We appreciate reviews provided by John Pearce, Laura Hubbard and two anonymous reviewers on prior versions of this product. The contents of this product are the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH) and the US Department of Agriculture (USDA). This report was reviewed and approved by the US Geological Survey under the Fundamental Science Practices policy (http://www.usgs.gov/fsp/). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Ethics
All procedures involving live animals were reviewed and approved by Institutional Animal Care and Use Committees at the University of Georgia (approval A2016 05-020-Y3-A6) and the USDA U.S. National Poultry Research Center (IACUC USNPRC-2021-021).
Data accessibility
All data that support the findings of this publication have been made publicly available by the USGS (https://doi.org/10.5066/P98N5GKC) and the National Center for Biotechnology Information GenBank (accession numbers: MN987946–MN988457 and MN998578–MN998579).
Authors' contributions
A.M.R., A.B.R., J.Z.D., R.L.P. and D.E.S. conceived of the study. A.M.R., A.B.R., J.Z.D., J.T.A., S.D.L.C., A.S.L., P.L., D.J.P., G.J.R., E.S., M.P.-J., R.L.P. and D.E.S. designed the study. All authors participated in laboratory or fieldwork. A.M.R., A.B.R., R.L.P., S.Y., M.P.-J. and D.E.S. carried out analyses. A.M.R., A.B.R., R.L.P. and D.E.S. wrote the initial draft manuscript. All authors read and revised the manuscript, gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships which could have appeared to influence this work.
Funding
The research described in this product was funded by the USGS Alaska Regional Director's Office; the USGS Wildlife Program of the Ecosystems Mission area; the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract HHSN272201400006C; USDA Agricultural Research Service project 6040-32000-066-00D; and the Memorial University of Newfoundland Seed, Bridge and Multidisciplinary Fund #20190120. J.W. was supported by funding from the Natural Sciences and Engineering Research Council of Canada (Canadian Lake Pulse Network, NETGP 479720) and the Memorial University of Newfoundland School of Graduate Studies.
References
- 1.Swayne DE. 2008. Epidemiology of avian influenza in agricultural and other man-made systems. In Avian influenza (ed. Swayne DE.), pp. 59–85. Ames, IA: Blackwell Publishing. [Google Scholar]
- 2.Cong YL, Pu J, Liu QF, Wang S, Zhang GZ, Zhang XL, Fan WX, Brown EG, Liu JH. 2007. Antigenic and genetic characterization of H9N2 swine influenza viruses in China. J. Gen. Virol. 88, 2035–2041. ( 10.1099/vir.0.82783-0) [DOI] [PubMed] [Google Scholar]
- 3.Nidom CA, et al. 2010. Influenza A (H5N1) viruses from pigs, Indonesia. Emerg. Infect. Dis. 16, 1515–1523. ( 10.3201/eid1610.100508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Meemak N, Pariyothorn N, Payungporn S, Theamboonlers A, Poovorawan Y. 2006. Avian influenza H5N1 in naturally infected domestic cat. Emerg. Infect. Dis. 12, 681 ( 10.3201/eid1204.051396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Songserm T, et al. 2006. Fatal avian influenza A H5N1 in a dog. Emerg. Infect. Dis. 12, 1744–1747. ( 10.3201/eid1211.060542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou H, et al. 2015. Serological evidence of avian influenza virus and canine influenza virus infections among stray cats in live poultry markets, China. Vet. Microbiol. 175, 369–373. ( 10.1016/j.vetmic.2014.12.018) [DOI] [PubMed] [Google Scholar]
- 7.Lycett SJ, et al. 2016. Role for migratory wild birds in the global spread of avian influenza H5N8. Science 354, 6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peiris JS, et al. 2004. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363, 617–619. ( 10.1016/S0140-6736(04)15595-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao R, et al. 2013. Human infection with a novel avian-origin influenza A (H7N9) virus. New England J. Med. 368, 1888–1897. ( 10.1056/NEJMoa1304459) [DOI] [PubMed] [Google Scholar]
- 10.Guan Y, Vijaykrishna D, Bahl J, Zhu H, Wang J, Smith GJ. 2010. The emergence of pandemic influenza viruses. Protein & Cell 1, 9–13. ( 10.1007/s13238-010-0008-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen B, Munster VJ, Wallensten A, Waldenström J, Osterhaus AD, Fouchier RA. 2006. Global patterns of influenza A virus in wild birds. Science 312, 384–388. ( 10.1126/science.1122438) [DOI] [PubMed] [Google Scholar]
- 12.Hinshaw VS, Webster RG, Turner B. 1979. Water-borne transmission of influenza A viruses? Intervirology 11, 66–68. ( 10.1159/000149014) [DOI] [PubMed] [Google Scholar]
- 13.Markwell DD, Shortridge KF. 1982. Possible waterborne transmission and maintenance of influenza viruses in domestic ducks. Appl. Environ. Microbiol. 43, 110–115. ( 10.1128/AEM.43.1.110-115.1982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sivanandan V, Halvorson DA, Laudert E, Senne DA, Kumar MC. 1991. Isolation of H13N2 influenza A virus from turkeys and surface water. Avian Dis. 35, 974–977. ( 10.2307/1591638) [DOI] [PubMed] [Google Scholar]
- 15.Ito T, Okazaki K, Kawaoka Y, Takada A, Webster RG, Kida H. 1995. Perpetuation of influenza A viruses in Alaskan waterfowl reservoirs. Arch. Virol. 140, 1163–1172. ( 10.1007/BF01322743) [DOI] [PubMed] [Google Scholar]
- 16.Lebarbenchon C, Yang M, Keeler SP, Ramakrishnan MA, Brown JD, Stallknecht DE, Sreevatsan S. 2011. Viral replication, persistence in water and genetic characterization of two influenza A viruses isolated from surface lake water. PLoS ONE 6, e26566 ( 10.1371/journal.pone.0026566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hénaux V, Samuel MD, Dusek RJ, Fleskes JP, Ip HS. 2012. Presence of avian influenza viruses in waterfowl and wetlands during summer 2010 in California: are resident birds a potential reservoir? PLoS ONE 7, e31471 ( 10.1371/journal.pone.0031471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okuya K, Kawabata T, Nagano K, Tsukiyama-Kohara K, Kusumoto I, Takase K, Ozawa M. 2015. Isolation and characterization of influenza A viruses from environmental water at an overwintering site of migratory birds in Japan. Arch. Virol. 160, 3037–3052. ( 10.1007/s00705-015-2610-0) [DOI] [PubMed] [Google Scholar]
- 19.Lickfett TM, Clark E, Gehring TM, Alm EW. 2018. Detection of influenza A viruses at migratory bird stopover sites in Michigan, USA. Inf. Ecol. Epidemiol. 8, 1474709 ( 10.1080/20008686.2018.1474709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang AS, Kelly A, Runstadler JA. 2008. Prevalence and diversity of avian influenza viruses in environmental reservoirs. J. Gen. Virol. 89, 509–519. ( 10.1099/vir.0.83369-0) [DOI] [PubMed] [Google Scholar]
- 21.Poulson RL, et al. 2017. Influenza A virus: sampling of the unique shorebird habitat at Delaware Bay, USA . R. Soc. Open Sci. 4, 171420 ( 10.1098/rsos.171420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Himsworth CG, Duan J, Prystajecky N, Coombe M, Baticados W, Jassem AN, Tang P, Sanders E, Hsiao W. 2020. Targeted resequencing of wetland sediment as a tool for avian influenza virus surveillance. J. Wildl Dis. 56, 397–408. ( 10.7589/2019-05-135) [DOI] [PubMed] [Google Scholar]
- 23.Rohani P, Breban R, Stallknecht DE, Drake JM.. 2009. Environmental transmission of low pathogenicity avian influenza viruses and its implications for pathogen invasion. Proc. Natl Acad. Sci. USA 106, 10 365–10 369. ( 10.1073/pnas.0809026106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stallknecht DE, Kearney MT, Shane SM, Zwank PJ. 1990. Effects of pH, temperature, and salinity on persistence of avian influenza viruses in water. Avian Dis. 34, 412–418. ( 10.2307/1591429) [DOI] [PubMed] [Google Scholar]
- 25.Zarkov IS. 2006. Survival of avian influenza viruses in filtered and natural surface waters of different physical and chemical parameters. Revue de Médecine Vétérinaire 157, 471. [Google Scholar]
- 26.Brown JD, Swayne DE, Cooper RJ, Burns RE, Stallknecht DE. 2007. Persistence of H5 and H7 avian influenza viruses in water. Avian Dis. 51, 285–289. ( 10.1637/7636-042806R.1) [DOI] [PubMed] [Google Scholar]
- 27.Brown JD, Goekjian G, Poulson R, Valeika S, Stallknecht DE. 2009. Avian influenza virus in water: infectivity is dependent on pH, salinity and temperature. Vet. Microbiol. 136, 20–26. ( 10.1016/j.vetmic.2008.10.027) [DOI] [PubMed] [Google Scholar]
- 28.Domanska-Blicharz K, Minta Z, Smietanka K, Marché S, Van Den Berg T.. 2010. H5N1 high pathogenicity avian influenza virus survival in different types of water. Avian Dis. 54, 734–737. ( 10.1637/8786-040109-ResNote.1) [DOI] [PubMed] [Google Scholar]
- 29.Nazir J, Haumacher R, Ike AC, Marschang RE. 2011. Persistence of avian influenza viruses in lake sediment, duck feces, and duck meat. Appl. Environ. Microbiol. 77, 4981–4985. ( 10.1128/AEM.00415-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keeler SP, Dalton MS, Cressler AM, Berghaus RD, Stallknecht DE. 2014. Abiotic factors affecting the persistence of avian influenza virus in surface waters of waterfowl habitats. Appl. Environ. Microbiol. 80, 2910–2917. ( 10.1128/AEM.03790-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Li Y, Chen J, Chen Q, Chen Z. 2014. Perpetuation of H5N1 and H9N2 avian influenza viruses in natural water bodies. J. Gen. Virol. 95, 1430–1435. ( 10.1099/vir.0.063438-0) [DOI] [PubMed] [Google Scholar]
- 32.Dalziel AE, Delean S, Heinrich S, Cassey P. 2016. Persistence of low pathogenic influenza A virus in water: a systematic review and quantitative meta-analysis. PLoS ONE 11, e0161929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeves AB, Ramey AM, Koch JC, Poulson RL, Stallknecht DE. 2020. Field-based method for assessing duration of infectivity for influenza A viruses in the environment. J. Virol. Methods 277, 113818 ( 10.1016/j.jviromet.2020.113818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. 2002. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 40, 3256–3260. ( 10.1128/JCM.40.9.3256-3260.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stallknecht DE, Shane SM, Zwank PJ, Senne DA, Kearney MT. 1990. Avian influenza viruses from migratory and resident ducks of coastal Louisiana. Avian Dis. 34, 398–405. ( 10.2307/1591427) [DOI] [PubMed] [Google Scholar]
- 36.Reeves AB, Hall JS, Poulson RL, Donnelly T, Stallknecht DE, Ramey AM. 2018. Influenza A virus recovery, diversity, and intercontinental exchange: a multi-year assessment of wild bird sampling at Izembek National Wildlife Refuge, Alaska. PLoS ONE 13, e0195327 ( 10.1371/journal.pone.0195327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spackman E. 2014. Avian influenza virus detection and quantitation by real-time RT-PCR. In Animal influenza virus (ed. Spackman E.), pp. 105–118. New York, NY: Humana Press. [DOI] [PubMed] [Google Scholar]
- 38.Reeves AB, et al. 2020. Temporal viral viability data from avian influenza A viruses maintained in North American wetlands under experimental and environmental conditions. US Geol. Surv. data release. ( 10.5066/P98N5GKC) [DOI] [Google Scholar]
- 39.Hahn MW. 2004. Broad diversity of viable bacteria in ‘sterile’ (0.2 μm) filtered water. Res. Microbiol. 155, 688–691. ( 10.1016/j.resmic.2004.05.003) [DOI] [PubMed] [Google Scholar]
- 40.Kratz TK, Cook RB, Bowser CJ, Brezonik PL. 1987. Winter and spring pH depressions in northern Wisconsin lakes caused by increases in pCO2. Can. J. Fish. Aquat. Sci. 44, 1082–1088 ( 10.1139/f87-129) [DOI] [Google Scholar]
- 41.Baehr MM and Degrandpre MD. 2002. Under-ice CO2 and 02 variability in a freshwater lake. Biogeochemistry 61, 95–113. ( 10.1023/A:1020265315833) [DOI] [Google Scholar]
- 42.Finlay K, Vogt RJ, Bogard MJ, Wissels B, Tutolo BM, Simpson GL, Leavitt PR. 2015. Decrease in CO2 efflux from northern hardwater lakes with increasing atmospheric warming. Nature 519, 215–218. ( 10.1038/nature14172) [DOI] [PubMed] [Google Scholar]
- 43.Reeves AB, Pearce JM, Ramey AM, Meixell BW, Runstadler JA. 2011. Interspecies transmission and limited persistence of low pathogenic avian influenza genomes among Alaska dabbling ducks . Infect. Genet. Evol. 11:2004–2010. ( 10.1016/j.meegid.2011.09.011) [DOI] [PubMed] [Google Scholar]
- 44.Nagy A, Černíková L, Jiřincová H, Havlíčková M, Horníčková J. 2014. Local-scale diversity and between-year ‘frozen evolution’ of avian influenza A viruses in nature. PLoS ONE 9, e103053 ( 10.1371/journal.pone.0103053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill NJ, Ma EJ, Meixell BW, Lindberg MS, Boyce WM, Runstadler JA. 2016. Transmission of influenza reflects seasonality of wild birds across the annual cycle. Ecol. Lett. 19, 915–925. ( 10.1111/ele.12629) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of this publication have been made publicly available by the USGS (https://doi.org/10.5066/P98N5GKC) and the National Center for Biotechnology Information GenBank (accession numbers: MN987946–MN988457 and MN998578–MN998579).