Abstract
The fossil record of ‘lesser apes’ (i.e. hylobatids = gibbons and siamangs) is virtually non-existent before the latest Miocene of East Asia. However, molecular data strongly and consistently suggest that hylobatids should be present by approximately 20 Ma; thus, there are large temporal, geographical, and morphological gaps between early fossil apes in Africa and the earliest fossil hylobatids in China. Here, we describe a new approximately 12.5–13.8 Ma fossil ape from the Lower Siwaliks of Ramnagar, India, that fills in these long-standing gaps with implications for hylobatid origins. This ape represents the first new hominoid species discovered at Ramnagar in nearly a century, the first new Siwalik ape taxon in more than 30 years, and likely extends the hylobatid fossil record by approximately 5 Myr, providing a minimum age for hylobatid dispersal coeval to that of great apes. The presence of crown hylobatid molar features in the new species indicates an adaptive shift to a more frugivorous diet during the Middle Miocene, consistent with other proposed adaptations to frugivory (e.g. uricase gene silencing) during this time period as well.
Keywords: hylobatid, Asia, biogeography, lower Siwaliks, fossil, gibbon
1. Introduction
Hylobatid origins are shrouded in mystery. Despite being the most speciose group of living apes with a historically large distribution over East and Southeast Asia (figure 1) [1–5], the fossil record of hylobatids (=gibbons and siamangs or ‘lesser apes’) is woefully incomplete, with only a handful of teeth widely recognized as stem hylobatids before the Middle Pleistocene [6,7]. The paucity of fossil lesser apes is particularly vexing given that molecular data consistently estimate their divergence from other primates by at least 20 Ma [8–10], and their sister group, the great apes, are represented by a large and diverse fossil record in Asia by at least approximately 12.7 Ma [11]. Therefore, fossil hylobatids should be present in the African and/or Asian record well before the first widely recognized fossil taxon, Yuanmoupithecus, in the Late Miocene (approx. 7–9 Ma) of Yunnan, China [7,12]. Here, we report a new small-bodied ape specimen from the late Middle Miocene site of Ramnagar (figure 1), a classic locality in the Indian Lower Siwaliks correlating to the middle or lower half of the Chinji Formation on the Potwar Plateau, Pakistan [13–17]. Specimen VPL/RSP2 is a right lower third molar (M3) with strong morphological affinities to extant hylobatids, even stronger than Yuanmoupithecus, thereby extending the known time range of fossil hylobatids by approximately 5 Myr and providing an updated minimum age for their evolution and dispersal into Asia coeval to that of great apes. As this specimen is distinct from all other known fossil apes, we describe it as a new genus and species below and discuss other Asian Miocene specimens previously mentioned in the context of catarrhine evolution and hylobatid origins.
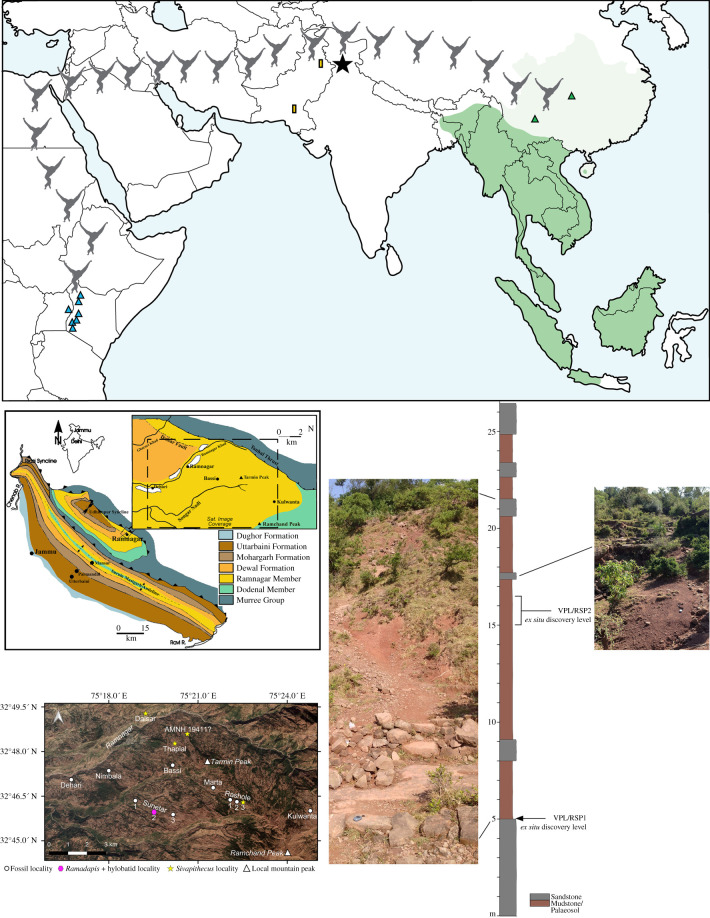
Figure 1.
Top: map illustrating the location of Kapi (black star) relative to modern (dark green) and historical (light green) populations of hylobatids and the approximate distribution of stem hominoid sites in East Africa (blue triangles). Green triangles mark the location of the hylobatid fossil taxa Bunopithecus and Yuanmoupithecus; yellow rectangles mark the location of the fossil catarrhine taxon Dionysopithecus sp. from Middle Miocene sites in Pakistan (see text). Bottom left: general geological map of the Siwalik Group surrounding Ramnagar with satellite imagery (GeoEye-1) of the Ramnagar region corresponding to the dashed insert of the geological map; bottom right: simplified stratigraphic section and photos of sequence at Sunetar 2 highlighting the ex situ discovery levels of primate specimens VPL/RSP1 (Ramadapis) and VPL/RSP2 (Kapi). Map by Free Vector Maps: http://freevectormaps.com.
Note that this published work and the nomenclatural acts it contains have been registered in ZooBank. The Life Science Identifiers (LSIDs) for this publication are: urn:lsid:zoobank.org:act:2D7C942A-EC42-46FF-AF8F-A2341B5C87E5 and urn:lsid:zoobank.org:act:436CC8FF-118D-4F39-92D8-5C92B18005C4.
2. Systematic palaeontology
Order Primates Linnaeus, 1758
Suborder Anthropoidea Mivart, 1864
Infraorder Catarrhini Geoffroy St. Hilaire, 1812
Superfamily Hominoidea Gray, 1825
Family Hylobatidae Gray, 1870
Kapi ramnagarensis gen. et sp. nov.
(a). Etymology
Genus name from the Hindi word for a common anthropoid ape or monkey (kapi). Species name in reference to Ramnagar (Jammu and Kashmir), India, where the type specimen was found.
(b). Generic diagnosis
Kapi differs from Oligocene and Miocene catarrhine taxa such as propliopithecids, pliopithecids, and dendropithecids in the combination of the following lower molar features: transverse orientation of the mesial cusps with the metaconid even with or slightly mesial to the protoconid, reduced buccal cingulum, peripheral placement of the cusps creating reduced basal crown flare, small entoconid–hypoconulid pair, and a broad, open occlusal basin. Further differs from most pliopithecids in the mesiodistal orientation of the cristid obliqua (also found in some pliopithecines), the transverse orientation of the hypoconid and entoconid, the more central placement of the hypoconulid on M3, and the lack of any crests between the protoconid and hypoconid associated with the pliopithecine triangle. Differs from Oligocene–Miocene proconsulids in the combination of the reduced entoconid–hypoconulid pair, transversely aligned mesial and distal cusps, the more peripheral placement of the cusps on the tooth crown (leading to reduced crown flare), and the reduction of the buccal cingulum (although a reduced cingulum is also present in some proconsulids). Differs from most hominids and hylobatids in the retention of a reduced but moderately developed buccal cingulum and a relatively long, broad mesial fovea. Further differs from hominids in its relatively small size. Further differs from known hylobatid genera in its overall more ovoid and relatively narrower shape (except Symphalangus), distal tapering, and less inflated cusps (a more detailed diagnosis of Kapi can be found in the electronic supplementary material).
(c). Specific diagnosis
As for genus.
(d). Holotype
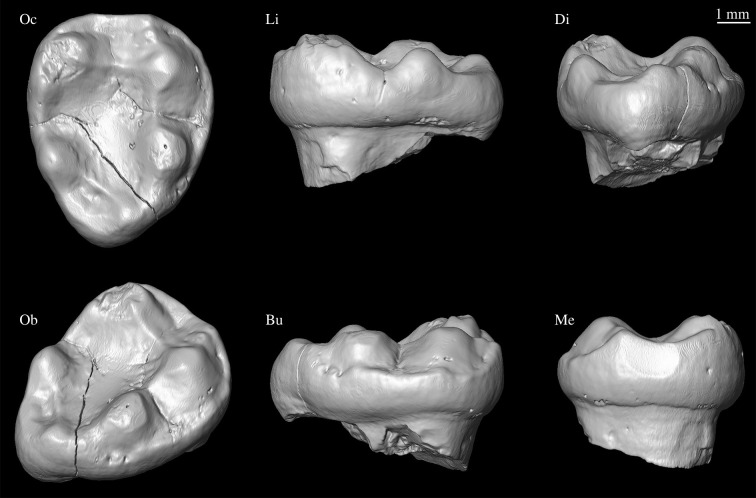
VPL/RSP2 (Vertebrate Paleontology Laboratory, Panjab University Department of Geology/Ramnagar Sunetar Primate 2); a complete and slightly worn right M3 crown (figure 2).
Figure 2.
VPL/RSP2 in various views. Clockwise from top left: Oc, Occlusal; Li, Lingual; Di, Distal; Me, Mesial; Bu, Buccal; Ob, Oblique. A three-dimensional surface rendering derived from µCT scans of the specimen is available at MorphoSource.org (media # M53248-96377; www.morphosource.org/Detail/MediaDetail/Show/media_id/53248).
(e). Hypodigm
The holotype is the only known specimen.
(f). Horizon
Lower Siwalik deposits; approximately 12.5–13.8 Ma (see electronic supplementary material, Geological background section).
(g). Localities/sites
Sunetar 2; approximately 4.5 km S/SE of Ramnagar, Jammu and Kashmir, India (figure 1).
(h). Description
VPL/RSP2 corresponds to a low-crowned, bunodont M3 from a catarrhine slightly smaller than Hoolock in molar size (figure 2; mesiodistal (MD) = 7.8 mm; buccolingual (BL) = 6.3 mm). It is mesiodistally longer than broad (breadth–length index of 0.79 calculated from photos; see electronic supplementary material for extended description), indicating proportions most similar to those of typical proconsulids, but considerably broader, on average, than those of pliopithecids, and slightly broader than those of modern Symphalangus, propliopithecids, as well as dendropithecids, although much overlap exists between individual specimens. It is relatively narrow compared to many modern gibbons, and slightly narrower than Yuanmoupithecus (0.81) and Bunopithecus (0.82).
The crown of VPL/RSP2 is ovoid in occlusal outline, tapering distally such that the distal moiety is narrower than the mesial moiety. There are five well-developed cusps, low and conical in shape, arranged around the periphery of the crown. The buccal wall of the crown displays a reduced, semi-continuous cingulum. The metaconid is the most voluminous and highest cusp, followed by the hypoconid and protoconid, which are subequal in elevation. The entoconid is similar in elevation to the hypoconid and protoconid, but relatively smaller in basal area. As is typical for apes, the hypoconulid is the smallest of the five cusps and located slightly towards the buccal side of the crown (figure 2).
The protoconid has a short but well-developed preprotocristid and postprotocristid. The metaconid is slightly mesial to the protoconid and has a short and rounded premetacristid. The metaconid and entoconid are widely spaced by a long postmetacristid. The hypoconid has a short prehypocristid (cristid obliqua) that is parallel to the long axis of the crown. Both the postentocristid and the posthypoconulid cristid are low and ill-defined. The mesial fovea is broad and rectangular, delimited distally by a well-differentiated mesial transverse crest (hypometacristid and hypoprotocristid). The mesial marginal ridge is relatively sharp and well developed. The distal fovea is intermediate in size, but poorly defined.
The talonid basin is expansive and has a simple Y-shaped groove pattern with no secondary wrinkling. A well-developed postcristid and hypoentocristid link the hypoconulid and entoconid, forming the mesial-most boundary of the distal fovea, separating it from the talonid basin. The metaconid is damaged, but there may be traces of a small mesostylid or tubercle on the postmetacristid. A small tubercle is also present on the preprotocristid. There is no evidence of a pliopithecine triangle and no retention of the paraconid.
3. Morphometric and phylogenetic analyses
Two-dimensional morphometric analyses of M3 shape as well as a cladistic analysis of 272 craniodental and postcranial features in extant and fossil catarrhine taxa support Kapi as a stem hylobatid. We quantified M3 crown shape and cusp position as characterized by 14 homologous landmarks (following [18]; see electronic supplementary material, figure S1 and table S1) and conducted a phylogenetic analysis using parsimony inference on a modified version of a recent matrix (electronic supplementary material, datasets S1–S2) [19]. Our comparative morphometric sample includes 166 M3 specimens: five crown hylobatid genera (n = 79), three crown hominid genera (n = 56), two propliopithecid genera (n = 6), six pliopithecid genera (n = 9), four dendropithecid genera (n = 7), five proconsulid genera (n = 7), the stem hylobatid Yuanmoupithecus (n = 1), and Kapi (n = 1) (electronic supplementary material, table S2, figure S2, dataset S3). Landmark data were imported into MorphoJ [20] and Morphologika2 [21] and then subjected to a generalized least-square Procrustes superimposition to focus on size-adjusted shape variables. A Principal Components Analysis (PCA) was performed using Procrustes coordinates and wireframe models were created to visualize the extreme landmark configurations. Using Discriminant Function Analysis pairwise tests implemented in MorphoJ [20], we also created wireframes and deformation grids to observe shape deformations from the mean shape configuration (reference configuration) of each of our major taxonomic groups to the shape of VPL/RSP2 (M3) (target configuration; electronic supplementary material, figure S3). Following [18], hierarchical phenetic trees were obtained from Procrustes distances using a neighbour-joining (NJ) cluster analysis with propliopithecids assigned as the outgroup.
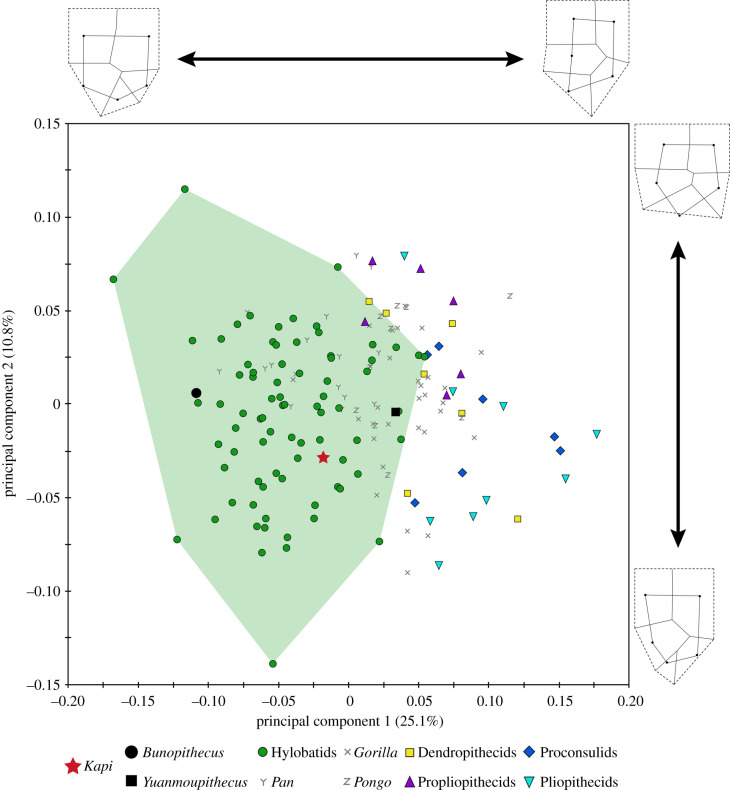
Although catarrhine M3s are variable in morphology and have been previously discounted in taxonomic identification [22], our multivariate results demonstrate that extant hylobatid M3s are distinct from stem catarrhines and stem hominoids, at least at the broad taxonomic levels analysed here (figure 3; electronic supplementary material, tables S3–S4; see also [3]; and [23–26] for other hominoids). These results are in line with recent research suggesting that, while morphologically variable within a taxon, anthropoid M3s evolve more quickly and are more distinctive between taxa, thereby making them more taxonomically informative than M1s and M2s in many cases [27]. VPL/RSP2 falls exclusively within crown hominoid space in the PCA plot, well within the crown hylobatid minimum convex polygon and closest to a number of crown hylobatid specimens on PC1 and PC2. While crown hylobatids do overlap in PC space with crown hominids (great apes), all crown hylobatids are easily differentiated from hominids on the basis of size, which is excluded from the shape analysis presented here (figure 3; see also electronic supplementary material, figure S2). Yuanmoupithecus plots within the small area of overlap between crown hylobatid, crown hominid, and stem catarrhine/hominoid taxa, but closest to crown hominoid specimens on PC1 and PC2. The Pleistocene gibbon Bunopithecus falls exclusively within crown hylobatid morphospace.
Figure 3.
PCA resulting from two-dimensional morphometric analysis of overall M3 crown shape characterized by 14 homologous landmarks (see wireframes; cusps = black circles). Kapi plots comfortably within hylobatid space (=green polygon), and completely outside the sampled distribution of stem catarrhine and stem hominoid taxa. By contrast, Yuanmoupithecus plots within the small area of overlap between stem and crown catarrhine/hominoid taxa, including crown hylobatids. (Online version in colour.)
PC1 is most clearly driven by differences in the position of the hypoconulid relative to the protoconid and hypoconid (in a straight line buccally in pliopithecids, more central/slightly buccal in hylobatids), the position of the cusps/width of the occlusal basin relative to the outline of the crown (pliopithecids = internally placed cusps, narrow occlusal basins, increased flare, large cingulum; hylobatids = peripherally placed cusps, wide occlusal basins, reduced flare, reduced cingulum), and the alignment of buccal and lingual cusps (pliopithecids = buccal cusps more mesial than lingual cusps, hylobatids = buccal and lingual cusps aligned transversely). VPL/RSP2 exhibits a negative value on PC1 due to its transversely aligned and peripherally placed mesial and distal cusps, wide occlusal basin, reduced cingulum, low degree of flare and more centrally positioned hypoconulid. PC2 does not separate most taxa (except propliopithecids on the positive end), but appears related to crown elongation and distal tapering (negative values = more elongated and tapered, positive values = less elongated and tapered), along with similar features as seen on PC1 including the position of the cusps relative to the crown outline, the position of the hypoconulid, and the alignment of buccal and lingual cusps. VPL/RSP2 exhibits slightly negative values, consistent with its slight distal tapering. The NJ cluster analysis based on the morphometric data places Yuanmoupithecus and Kapi in a cluster with crown hominoids, with Yuanmoupithecus at the base of the cluster and Kapi as the sister to hylobatids. Dendropithecids, proconsulids, and pliopithecids are placed in a separate cluster as the sister group to Yuanmoupithecus + crown hominoids (figure 4).
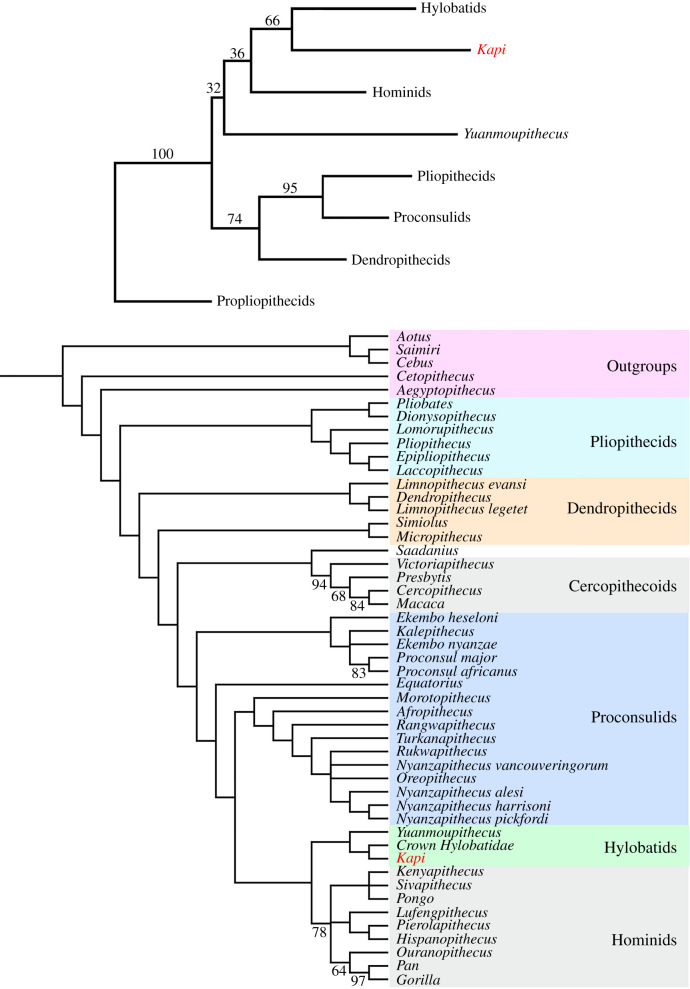
Figure 4.
Top: neighbour-joining cluster analysis derived from Procrustes distances of the same 14 landmark morphometric dataset as in figure 3. Numbers above branches indicate bootstrap values based on 10 000 replicates. Bottom: strict consensus of 18 most parsimonious trees (MPTs) resulting from phylogenetic analysis of stem catarrhine taxa including 272 characters (craniodental + postcranial), with outgroups constrained successively. Numbers below branches indicate any bootstrap values over 50%. Kapi is reconstructed as a stem hylobatid in both the cluster analysis and all MPTs. Tree length = 1455 steps. (Online version in colour.)
The resulting trees from our cladistic analysis are consistent with the morphometric analyses and recover both Kapi and Yuanmoupithecus as crown hominoids, and both fossil taxa are most parsimoniously reconstructed as stem hylobatids (figure 4; electronic supplementary material, datasets S1–S2 for character list and matrix). In all 18 most parsimonious trees (MPTs), Yuanmoupithecus is the sister taxon to a crown hylobatids + Kapi clade. Aside from the inclusion of Yuanmoupithecus and Kapi, the relationships among other catarrhines are broadly the same as those previously presented [19].
4. Discussion
Based on the available evidence of lower molar anatomy, Kapi ramnagarensis represents the first new hominoid species discovered at Ramnagar in nearly 100 years. While caution is necessary given that only a single molar is documented, the analyses presented here demonstrate that Kapi is more similar to extant hylobatids in its known morphology than the widely accepted stem hylobatid Yuanmoupithecus. Thus, if one considers Yuanmoupithecus a stem hylobatid, Kapi is equally if not more likely to be one as well, making it the earliest known hylobatid in the fossil record (figures 3 and 4). The phylogenetic placement of these two taxa within hominoids, however, is admittedly difficult to assess in the absence of additional material. Based on shared similarities with extant hylobatids in the premolars and anterior dentition, Yuanmoupithecus perhaps represents a slightly different combination of dental morphologies in early hylobatid evolution compared to Kapi. Further specimens of both taxa are necessary to confidently resolve the polarity of stem hylobatid dental features.
The discovery of Kapi in approximately 12.5–13.8 Ma Lower Siwalik deposits helps to fill temporal, morphological, and biogeographic gaps in hominoid evolution. While much of hylobatid evolution remains unknown, it is now probable that they dispersed to Asia from Africa by the end of the Middle Miocene, possibly at the same time as great apes such as Sivapithecus just after the Middle Miocene Climatic Optimum [28,29]. Judging by the affinities of both Kapi and Yuanmoupithecus in our analyses, it seems most likely that hylobatids evolved from an African taxon dentally similar to dendropithecids or proconsulids, the two advanced catarrhine groups outside of crown hominoids with specimens closely approaching hylobatids in the multivariate and phylogenetic analyses. Therefore, it is entirely possible that early stem hylobatids are currently represented by some of the fossil material in the extensive East African Early Miocene record, but cannot yet be distinguished based on the lack of clear hylobatid dental synapomorphies among these fragmentary taxa.
In many ways, Kapi represents a logical intermediate or mosaic dental morphology between Early Miocene dendropithecids/proconsulids and extant hylobatids; it clearly displays the bunodonty, peripherally placed cusps, expanded basin and transversely aligned cusps as seen in living hylobatids, but also retains primitive features such as a (reduced) buccal cingulum, a relatively long mesial fovea, and possibly a vestigial mesostylid not typically observed in living gibbons and siamangs. It also does not display the expanded cusp areas typical of living hylobatids. Features of extant hylobatid molars, particularly the bunodonty and expansion of the occlusal basin relative to stem catarrhine taxa (figure 3; electronic supplementary material, figures S2–S3), indicate an adaptive shift to a dedicated diet of frugivory. The presence of these characters in Kapi suggest that this shift had begun by the end of the Middle Miocene, consistent with the silencing of the uricase gene (another proposed adaptation to frugivory) in hylobatids during this time period as well [30].
While other Eurasian fossils have been advanced as possible hylobatids in the past, none have held up to closer scrutiny and an improved understanding of the catarrhine fossil record. Pliopithecids (i.e. pliopithecoids), a well-represented group of catarrhines found in the Early to Late Miocene of Asia (e.g. Dionysopithecus, Platodontopithecus, Pliopithecus, and Laccopithecus), resemble hylobatids in certain cranial features, including a relatively short face, projecting inferior orbital rims, and a broad interorbital distance. However, they also lack a key synapomorphy found in all crown catarrhines, namely a completely ossified tubular ectotympanic (ear tube). In addition, they generally possess a unique combination of primitive features (e.g. very broad upper molars and an entepicondylar foramen in the distal humerus) along with autapomorphic lower molar anatomy (including the pliopithecine triangle), leading most experts to conclude that they are, in fact, late-occurring stem catarrhine taxa (figure 4) [6,7,19,31–33].
A worn M3 from the Middle Siwalik locality of Haritalyangar, India, was initially referred to as a possible hylobatid ancestor and ultimately placed in its own genus, Krishnapithecus [34,35]. However, Krishnapithecus has recently been demonstrated to be a late-occurring pliopithecid, with lower molars displaying a distinctive pliopithecine triangle among other pliopithecid features [36]. Notably, our cladistic analysis reconstructs the recently described and debated Pliobates from the Middle/Late Miocene boundary of Spain as a pliopithecid taxon as well (see also [19]).
One other taxon from South Asia, Dionysopithecus sp., represented by a handful of isolated teeth from the Lower Siwalik Kamlial Formation and Manchar Formation in Pakistan (approx. 16–17 Ma), has been discussed as a possible dendropithecid, proconsulid, pliopithecid, and even stem hylobatid [7,37–39]. Thus, the affinities of H-GSP 8114/609, the sole lower molar (M1) assigned to Dionysopithecus sp., were re-examined given its proximity in time and space to the M3 from Ramnagar as well as its possible status as a stem hylobatid or advanced stem catarrhine/hominoid (dendropithecid or proconsulid) in Asia. Although roughly similar in size, GSP 8114/609 is morphologically distinct from Kapi in its much higher crown, better developed cingulum, stronger occlusal crests, more restricted occlusal basin, and more centrally located cusps, clearly representing a different taxon. We conducted a separate morphometric analysis on GSP 8114/609 and a large sample of catarrhine M1s (electronic supplementary material, figures S4-S5, tables S4–S6, dataset S4). There is more overlap among all groups on the first two components of the M1 PCA, and GSP 8114/609 falls completely outside hylobatid multivariate space. Instead, it falls within an area of overlap between dendropithecids, pliopithecids, and crown hominids. Thus, while the taxonomic placement of the Manchar/Kamlial specimens is still unclear, they seem unlikely to belong to a fossil hylobatid.
Finally, a proximal humerus (GSP 28062) from site Y499 in the Chinji Formation (approx. 12.05 Ma) [40–42] is the only other small-bodied catarrhine specimen currently known from the Siwaliks close to the likely time range represented at Ramnagar. Interestingly, this specimen displays none of the specializations for extreme mobility present in living hylobatids and great apes, and instead retains a primitive catarrhine morphotype similar to pliopithecids and dendropithecids [42]. While an association with an as yet undiscovered Chinji-level pliopithecid or dendropithecid is perhaps most likely, if this proximal humerus is attributable to a stem hylobatid (e.g. Kapi), it would suggest that the suspensory features exhibited by extant hylobatids evolved independently from great apes and within the last around 12.5–13.8 Myr from a more primitive catarrhine morphotype. Such an association would also suggest that the common ancestor of crown apes was not a highly suspensory animal, a hypothesis that we consider likely (see also [43–45]), but that stands in contrast to the consensus view of ape evolution for much of the past century [46–49]. Additional early catarrhine and stem hylobatid fossils such as Kapi, particularly with associated postcrania, are necessary to resolve these competing views and gain a clearer insight into the first approximately 6–8 Myr of hylobatid evolution.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Terry Harrison provided access to Yuanmoupithecus and comments that greatly improved the manuscript. Kai He, Hira Naseer, and Nastassia Chittumuri assisted with photography and wireframe representation of the molar specimens. Two anonymous reviewers and an associate editor provided constructive comments that improved the manuscript. We thank Judy Galkin (AMNH), Eric Delson (AMNH), Terry Harrison (NYU), Hannah Taboada (NYU), Eileen Westwig (AMNH), Eleanor Hoeger (AMNH), Bill Kimbel (ASU), Julie Lawrence (ASU), Frieder Mayer (ZMB), Darrin Lunde (NMNH), Mark Omura (Harvard), Jessica Cundiff (Harvard), Larry Flynn (Harvard), and David Pilbeam (Harvard) for access to original specimens and casts in their care. We also thank Morgan Hill and the Microscopy and Imaging Facility at the AMNH for access and assistance with µCT scanning VPL/RSP2. Luci Betti-Nash prepared the map in figure 1.
Data accessibility
Original raw µCT image scan data and derived three-dimensional surface rendering of VPL/RSP2 are available on MorphoSource.org (www.morphosource.org/Detail/MediaDetail/Show/media_id/53248; https://doi.org/10.17602/M2/M96377). All data used in the morphometric analyses are provided as MorphoJ input files and the matrix used in the cladistic analysis is provided as a text file in the electronic supplementary material associated with this article.
Authors' contributions
C.C.G., A.O., and K.D.P. designed the study. C.C.G., C.J.C., B.A.P., N.P.S., and R.P. did field research. C.C.G., A.O., K.D.P., B.A.P., J.G.F., N.P.S., and R.P. collected and analysed comparative data on primate dental morphology. B.A.P. performed µCT imaging analyses. C.J.C. and R.P. studied the geological context and provided the geological background. C.C.G., K.D.P., B.A.P., N.P.S., and R.P. identified fauna at Ramnagar. A.O., C.C.G., and K.D.P. performed morphometric analyses. K.D.P. and C.C.G. performed the phylogenetic analyses. All authors wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Leakey Foundation, the PSC-CUNY faculty award programme, Hunter College, the AAPA professional development programme, the University of Southern California, the Institute of Human Origins (ASU), and the National Science Foundation (BCS Award nos. 1945736, 1945618). In addition, R.P. and N.P.S. were supported by MoES/P.O. (Geosci)/46/2015 and SERB-HRR/2018/000063.
References
- 1.Gao YT, Wen HR, He YH. 1981. The change of historical distribution of Chinese gibbons (Hylobates). Zool. Res. 2, 1–8. [Google Scholar]
- 2.Gu Y. 1989. Preliminary research on the fossil gibbons of the Chinese Pleistocene and recent. Hum. Evol. 4, 509–514. ( 10.1007/BF02436298) [DOI] [Google Scholar]
- 3.Ortiz A, Pilbrow V, Villamil C, Korsgaard J, Bailey SE, Harrison T. 2015. The taxonomic and phylogenetic affinities of Bunopithecus sericus, a fossil hylobatid from the Pleistocene of China. PLoS ONE 10, e0131206 ( 10.1371/journal.pone.0131206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turvey ST, Bruun K, Ortiz A, Hansford J, Hu S, Ding Y, Zhang T, Chatterjee HJ. 2018. New genus of extinct Holocene gibbon associated with humans in Imperial China. Science 360, 1346–1349. ( 10.1126/science.aao4903) [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y-Q, Jin C-Z, Wang Y, Ortiz A, He K, Harrison T. 2018. Fossil gibbons (Mammalia, Hylobatidae) from the Pleistocene of Chongzuo, Guangxi Zhuang Autonomous Region, China. Vert. PalAs. 56, 248–263. ( 10.19615/j.cnki.1000-3118.180403) [DOI] [Google Scholar]
- 6.Fleagle JG. 2013. Primate adaptation and evolution, 3rd edn San Diego, CA: Academic Press. [Google Scholar]
- 7.Harrison T. 2016. The fossil record and evolutionary history of hylobatids. In Evolution of gibbons and siamang: phylogeny, morphology, and cognition (eds Reichard UH, Hirohisa H, Barelli C), pp. 91–110. New York, NY: Springer. [Google Scholar]
- 8.Perelman P, et al. 2011. A molecular phylogeny of living primates. PLoS Genet. 7, e1001342 ( 10.1371/journal.pgen1001342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finstermeier K, Zinner D, Brameier M, Meyer M, Kreuz E, Hofreiter M, Roos C. 2013. A mitogenomic phylogeny of living primates. PLoS ONE 8, e69504 ( 10.1371/journal.pone0069504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pozzi L, Hodgson J, Burrell AS, Sterner KN, Raaum RL, Disotell TR. 2014. Primate phylogenetic relationships and divergence dates inferred from complete mitochondrial genomes. Mol. Phylogenet. Evol. 75, 165–183. ( 10.1016/j.ympev.2014.02.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley J. 2005. Twenty-five years contemplating Sivapithecus taxonomy. In Interpreting the past: essays on human, primate and mammal evolution in honor of David Pilbeam (eds Lieberman DE, Smith RJ, Kelley J), pp. 123–143. Boston, MA: Brill Academic Publishers. [Google Scholar]
- 12.Pan Y. 2006. Primates Linnaeus 1758. In Lufengpithecus hudiensis site (eds Qi G, Dong W), pp. 131–148, 320–322 Beijing, China: Science Press. [Google Scholar]
- 13.Sehgal RK, Patnaik R. 2012. New muroid rodent and Sivapithecus dental remains from the Lower Siwalik deposits of Ramnagar (J and K, India): Age implication. Quatern. Int. 269, 69–73. ( 10.1016/j.quaint.2011.01.043) [DOI] [Google Scholar]
- 14.Gilbert CC, Patel BA, Singh NP, Campisano CJ, Fleagle JG, Rust KL, Patnaik R. 2017. New sivaladapid primate from Lower Siwalik deposits surrounding Ramnagar (Jammu and Kashmir State), India. J. Hum. Evol. 102, 21–41. ( 10.1016/j.jhevol.2016.10.001) [DOI] [PubMed] [Google Scholar]
- 15.Gilbert CC, Sehgal RK, Pugh KD, Campisano CJ, May E, Patel BA, Singh NP, Patnaik R. 2019. New Sivapithecus specimen from Ramnagar (Jammu and Kashmir), India and a taxonomic revision of Ramnagar hominoids. J. Hum. Evol. 135, 102665 ( 10.1016/j.jhevol.2019.102665) [DOI] [Google Scholar]
- 16.Parmar V, Magotra R, Norboo R, Prasad GVR. 2017. Rodent-based age appraisal of the Lower Siwalik Subgroup of Kalaunta, Ramnagar, Jammu, India. Aust. J. Palaeontol. 41, 124–133. ( 10.1080/03115518.2016.1196435) [DOI] [Google Scholar]
- 17.Parmar V, Prasad GVR, Norboo R. 2018. Middle Miocene small mammals from the Siwalik Group of Northwestern India. J. Asian Earth Sci. 162, 84–92. ( 10.1016/j.jseaes.2017.11.023) [DOI] [Google Scholar]
- 18.Gómez-Robles A, Olejniczak AJ, Martinon-Tórres M, Prado-Simón L, Bermúdez de Castro JM. 2011. Evolutionary novelties and losses in geometric morphometrics: a practical approach through hominin molar morphology. Evolution 65, 1772–1790. ( 10.1111/j.1558-5646.2011.01244.x) [DOI] [PubMed] [Google Scholar]
- 19.Nengo I. et al 2017. New infant cranium from the African Miocene sheds light on ape evolution. Nature 548, 169–174. ( 10.1038/nature23456) [DOI] [PubMed] [Google Scholar]
- 20.Klingenberg CP. 2011. MorphoJ: an integrated software package for geometric morphometries. Mol. Ecol. Resour. 11, 353–357. ( 10.1111/j.1755-0998.2010.02924.x) [DOI] [PubMed] [Google Scholar]
- 21.O'Higgins P, Jones N. 2006. Tools for Statistical Shape Analysis. Hull York Medical School. See http://sites.google.com/site/hymsfme/resources (accessed 5 June 2018).
- 22.Gamarra B, Nova Delgado M, Romero A, Galbany J, Perez-Perez A. 2016. Phylogenetic signal in molar dental shape of extant and fossil catarrhine primates. J. Hum. Evol. 94, 13–27. ( 10.1016/j.jhevol.2016.01.005) [DOI] [PubMed] [Google Scholar]
- 23.Ortiz A, et al. 2019. Morphometric analysis of fossil hylobatid molars from the Pleistocene of southern China. Anthropol. Sci. 127, 109–121. ( 10.1537/ase.190331) [DOI] [Google Scholar]
- 24.Uchida A. 1996. Craniodental variation among the great apes. Cambridge, MA: Peabody Museum of Archaeology and Ethnology. [Google Scholar]
- 25.Pilbrow VC. 2006. Population systematics of chimpanzees using molar morphometrics. J. Hum. Evol. 51, 646– 662. ( 10.1016/j.jhevol.2006.07.008) [DOI] [PubMed] [Google Scholar]
- 26.Pilbrow VC. 2010. Dental and phylogeographic patterns of variation in gorillas. J. Hum. Evol. 59, 16–34. ( 10.1016/j.jhevol.2010.01.009) [DOI] [PubMed] [Google Scholar]
- 27.Mongle C. 2019. Modeling hominin variability: the alpha taxonomy of Australopithecus africanus. PhD thesis, pp. 194 Stony Brook University, Stony Brook, NY. [Google Scholar]
- 28.Barry JC, Morgan ME, Flynn LJ, Pilbeam D, Jacobs LL, Lindsay EH, Raza SM, Solounias N. 1995. Patterns of faunal turnover and diversity in Neogene Siwaliks of Northern Pakistan. Palaeogeogr. Palaeocl. 115, 209–226. ( 10.1016/0031-0182(94)00112-L) [DOI] [Google Scholar]
- 29.Gilbert CC, Pugh KD, Fleagle JG. In press Dispersal of Miocene Hominoids (and Pliopithecoids) from Africa to Eurasia in Light of Changing Tectonics and Climate. In Biological consequences of plate tectonics: new perspectives on post-Gondwana break-up- a tribute to Ashok Sahni (eds Prasad GVR, Patnaik R). New York, NY: Springer. [Google Scholar]
- 30.Andrews P, Johnson RJ, 2015. Gibbons and hominoid ancestry. In Taxonomic tapestries: the threads of evolutionary, behavioural and conservation research (eds Behie AM, Oxenham MF), pp. 51–73. Acton: ANU Press. [Google Scholar]
- 31.Andrews P, Harrison T, Delson E, Bernor RL, Martin L. 1996. Distribution and biochronology of European and southwest Asian Miocene catarrhines. In The evolution of Western Eurasian neogene mammal faunas (eds Bernor RL, Fahlbusch V, Mittmann H-W), pp. 168–207. New York, NY: Columbia University Press. [Google Scholar]
- 32.Begun DR. 2002. The Pliopithecoidea. In The primate fossil record (ed. Hartwig WC.), pp. 221–240. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 33.Harrison T. 2013. Catarrhine origins. In A companion to paleoanthropology (ed. Begun DR.), pp. 376–396. New York, NY: Wiley-Blackwell. [Google Scholar]
- 34.Chopra SRK, Kaul S. 1979. A new species of Pliopithecus from the Indian Sivaliks. J. Hum. Evol. 8, 475–477. ( 10.1016/0047-2484(79)90085-X) [DOI] [Google Scholar]
- 35.Ginsburg L, Mein P. 1980. Crouzelia rhodanica, nouvelle espèce de Primate catarhinien, et essai sur la position systématique des Pliopithecidae. Bulletin du Museum d'Historie Naturelle de Paris 4, 57–85. [Google Scholar]
- 36.Sankhyan AR, Kelley J, Harrison T. 2017. A highly derived pliopithecid from the Late Miocene of Haritalyangar, India. J. Hum. Evol. 105, 1–12. ( 10.1016/j.jhevol.2017.01.010) [DOI] [PubMed] [Google Scholar]
- 37.Barry JC, Jacobs LL, Kelley J. 1986. An early Middle Miocene catarrhine from Pakistan with comments on the dispersal of catarrhines into Eurasia. J. Hum. Evol. 15, 501–508. ( 10.1016/S0047-2484(86)80030-6) [DOI] [Google Scholar]
- 38.Bernor R, Flynn L, Harrison T, Hussain ST, Kelley J. 1988. Dionysopithecus from southern Pakistan and the biochronology and biogeography of early Eurasian catarrhines. J. Hum. Evol. 17, 339–358. ( 10.1016/0047-2484(88)90075-9) [DOI] [Google Scholar]
- 39.Harrison T, Gu Y. 1999. Taxonomy and phylogenetic relationships of early Miocene catarrhines from Sihong, China. J. Hum. Evol. 37, 225–277. ( 10.1006/jhev.1999.0310) [DOI] [PubMed] [Google Scholar]
- 40.Kappelman J, et al. 1991. The earliest occurrence of Sivapithecus from the middle Miocene Chinji Formation of Pakistan. J. Hum. Evol. 21, 61–73. ( 10.1016/0047-2484(91)90036-U) [DOI] [Google Scholar]
- 41.Aziz HA, Krijgsman W, Hilgen FJ, Wilson DS, Calvo JP. 2003. An astronomical polarity timescale for the late middle Miocene based on cyclic continental sequences. J. Geophys. Res. 108, 2159 ( 10.1029/2002JB001818) [DOI] [Google Scholar]
- 42.Rose MD. 1989. New postcranial specimens of catarrhines from the Middle Miocene Chinji Formation, Pakistan: descriptions and a discussion of proximal humeral functional morphology in anthropoids. J. Hum. Evol. 18, 131–162. ( 10.1016/0047-2484(89)90067-5) [DOI] [Google Scholar]
- 43.Larson SG. 1998. Parallel evolution in the hominoid trunk and forelimb. Evol. Anthropol. 6, 87–99. () [DOI] [Google Scholar]
- 44.Moyà-Solà S, Köhler M, Alba DM, Casanovas-Vilar I, Galindo J. 2004. Pierolapithecus catalaunicus, a new Middle Miocene great ape from Spain. Science 306, 1339–1344. ( 10.1126/science.1103094) [DOI] [PubMed] [Google Scholar]
- 45.Pugh KD. 2020. Phylogenetic relationships of Middle-Late Miocene Hominoids: Implications for understanding great ape and human evolution. PhD thesis, City University of New York. [Google Scholar]
- 46.Keith A. 1923. Man's posture: its evolution and disorders. Brit. Med. J. 1, 451–454, 499–502, 545–548, 587–590, 624–626, 669–672 ( 10.1136/bmj.1.3246.451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schultz AH. 1930. The skeleton of the trunk and limbs of higher primates. Hum. Biol. 11, 303–438. [Google Scholar]
- 48.Gebo DL. 1996. Climbing, brachiation, and terrestrial quadrupedalism: historical precursors of hominid bipedalism. Am. J. Phys. Anthropol. 101, 55–92. () [DOI] [PubMed] [Google Scholar]
- 49.Pilbeam D. 1996. Genetic and morphological records of the Hominoidea and hominid origins: a synthesis. Mol. Phylogenet. Evol. 5, 155–168. ( 10.1006/mpev.1996.0010) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original raw µCT image scan data and derived three-dimensional surface rendering of VPL/RSP2 are available on MorphoSource.org (www.morphosource.org/Detail/MediaDetail/Show/media_id/53248; https://doi.org/10.17602/M2/M96377). All data used in the morphometric analyses are provided as MorphoJ input files and the matrix used in the cladistic analysis is provided as a text file in the electronic supplementary material associated with this article.