Abstract
Once considered a hallmark of human uniqueness, brain asymmetry has emerged as a feature shared with several other species, including chimpanzees, one of our closest living relatives. Most notable has been the discovery of asymmetries in homologues of cortical language areas in apes, particularly in the planum temporale (PT), considered a central node of the human language network. Several lines of evidence indicate a role for genetic mechanisms in the emergence of PT asymmetry; however, the genetic determinants of cerebral asymmetries have remained elusive. Studies in humans suggest that there is heritability of brain asymmetries of the PT, but this has not been explored to any extent in chimpanzees. Furthermore, the potential influence of non-genetic factors has raised questions about the reproducibility of earlier observations of PT asymmetry reported in chimpanzees. As such, the present study was aimed at examining both the heritability of phenotypic asymmetries in PT morphology, as well as their reproducibility. Using magnetic resonance imaging, we evaluated morphological asymmetries of PT surface area (mm2) and mean depth (mm) in captive chimpanzees (n = 291) derived from two genetically isolated populations. Our results confirm that chimpanzees exhibit a significant population-level leftward asymmetry for PT surface area, as well as significant heritability in the surface area and mean depth of the PT. These results conclusively demonstrate the existence of a leftward bias in PT asymmetry in chimpanzees and suggest that genetic mechanisms play a key role in the emergence of anatomical asymmetry in this region.
Keywords: asymmetry, reproducibility, chimpanzee, planum temporale, heritability
1. Introduction
Ever since the seminal publication of Charles Darwin's Origin of Species [1], several lines of comparative evidence have demonstrated the close kinship shared between humans and chimpanzees. The genetic similarities of chimpanzees to humans [2] and the relative shortness of our evolutionary separation [3] indicate that many features of the modern human phenotype have evolutionary roots that pre-date our divergence from the last common ancestor [4]. In this regard, brain asymmetries, particularly those within language-associated areas, have been suggested as a key difference between humans and our nearest ancestors and living relatives [5–7]. While earlier studies seemed to reinforce the assertion of human uniqueness in terms of brain asymmetry [5,8–11], this assumption has been challenged by discoveries of population-level behavioural and neuroanatomical asymmetries in other species (e.g. [12,13]), including structural asymmetries in primate brains for homologues of areas implicated in human language and speech production among primates (e.g. [14–16]).
One key brain region is the planum temporale (PT) – the bank of cerebral cortex that lies posterior to Heschl's gyrus and considered an integral component of the language network [17–22]. The leftward asymmetry of the PT is the most pronounced and consistently reported asymmetry in the human brain [22,23], and has received considerable attention in relation to language dominance [24–26]. In particular, the surface area of the PT is on average larger in the left hemisphere, which is significant in that it overlaps with Wernicke's area, a key brain region involved in auditory and lexical processing which is associated with functional cerebral lateralization for language. In humans, deviations from normal PT asymmetry are associated with severe deficits in language comprehension and production [25,27–30]. In addition, comparisons of sulcal depth in regions surrounding the PT have also proven useful as markers of neurological dysfunction as well as species-specific morphology, prompting further exploration of PT asymmetry and its functional implications [31–33].
Population-level leftward asymmetry of the PT has been documented in olive baboons, suggesting the emergence of this leftward bias in primates by at least 30–40 Ma [34]. Chimpanzees are also known to have significant leftward asymmetries of the PT, both cytoarchitecturally [35] and morphologically [36–38], and there is some evidence that PT asymmetries are associated with handedness [39].
In humans, asymmetry in the PT as well as surrounding sulci emerges early in development [40–45], indicating a potential role for genetic mechanisms in the emergence of PT asymmetry. However, very few individual genes have so far been implicated in any aspect of lateralization of the human brain [46–49], and the genetic determinants of cerebral asymmetries are unknown and remain elusive [19,50,51]. Studies in humans suggest that there is heritability of brain asymmetries, notably within the PT [52–54], but this issue has not been explored in a wide range of nonhuman primates [55–58] or, to any extent, in our closest living relatives, the chimpanzees.
To this end, we examined the repeatability and heritability of asymmetries in the PT of common chimpanzees. Specifically, in vivo and postmortem magnetic resonance imaging (MRI) scans were obtained from two captive chimpanzee populations that are genetically isolated from each other (i.e. the two populations are geographically isolated from one another and there is no gene flow between the two groups), but for whom there are well-documented pedigrees dating back to the founder animals [59]. By measuring PT surface area (mm2) and the mean sulcal depth (mm) in these two populations, we had a unique opportunity to evaluate the consistency with which PT phenotypic asymmetries could be observed across a variety of non-genetic factors including MRI scanner magnet strength, sex, handedness and rearing history. For example, some have suggested that population-level behavioural asymmetries in nonhuman primates, including chimpanzees, may be influenced by their early handling by right-handed humans [60]. In rodents, there is good evidence that early handling can induce population-level behavioural asymmetries [61]. Within our sample, we had chimpanzee subjects with differing early social rearing experiences with human carers, and this allowed us to test this hypothesis as it relates to PT asymmetries. If early handling experiences by humans influence PT asymmetries, then we hypothesized that apes with more extensive caregiver contact would differ from chimpanzees with less history of human handling. Furthermore, through the use of heritability analyses we explored the proportion of variance in PT asymmetry in chimpanzees associated with genetic factors. We hypothesized that if population-level PT asymmetries are reproducible across chimpanzee populations and under genetic control, then significant leftward biases and heritability would be evident in the surface area and/or sulcal depth of the PT in both cohorts.
2. Material and methods
(a). Subjects
The PT was measured from in vivo (n = 229) and postmortem (n = 62) magnetic resonance images in a sample of 291 chimpanzees (Pan troglodytes) housed at two research facilities in North America. One sample included 155 individuals from the National Center for Chimpanzee Care (NCCC), which is part of the University of Texas MD Anderson Cancer Center. The remaining 136 chimpanzees were housed at the Yerkes National Primate Research Center (YNPRC) of Emory University. The entire sample ranged in age from 3 to 52 years at the time of MRI scanning (mean = 27.6, s.d. = 11.0) and included 165 females and 126 males, respectively. Within the entire sample, there were 135 mother-reared (MR), 92 nursery-reared (NR) and 64 wild-caught (WC) chimpanzees.
(b). MRI scanning
Both in vivo and postmortem MRI scan data were used in this study [62]. Seventy-seven chimpanzees were scanned using a 3.0 Tesla scanner (Siemens Trio, Siemens Medical Solutions USA, Malvern, Pennsylvania, USA) at YNPRC. T1-weighted images were collected using a three-dimensional gradient echo sequence (pulse repetition = 2300 ms, echo time = 4.4 ms, number of signals averaged = 3, matrix size = 320 × 320). Additionally, 139 NCCC and 13 YNPRC chimpanzees were scanned using a 1.5 Tesla Phillips machine (The Netherlands). T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, number of signals averaged = 8, and a 256 × 256 matrix). Postmortem T2-scans were obtained from 62 chimpanzees that had died from natural causes or were euthanized for humane reasons. For the postmortem scanning, either 4.7 or 7T magnets were used, and T2-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 22.0 s, echo time = 78.0 ms, number of signals averaged = 8–12, and a 256 × 192 matrix reconstructed to 256 × 256).
(c). Sulci extraction and measurement
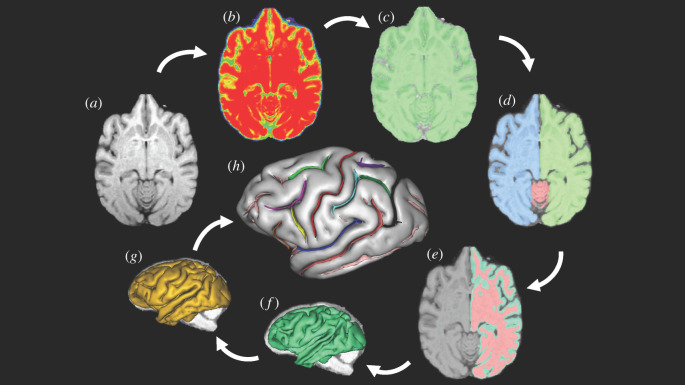
The sequence of post-image processing steps performed on the images is shown in figure 1a–h and have been described in detail elsewhere [62–64]. The pipeline of processing used to extract the sulci from the raw T1-weighted image derives from a pipeline initially dedicated to the human brain and freely distributed as a BrainVISA (BV) toolbox (http://brainvisa.info) [65]. The pipeline process of extracting the sulci from the cortex involved a number of steps [65] (figure 1a–h). The first step was to correct for spatial inhomogeneities in the signal intensity, providing a spatially smooth bias field with a stable distribution of tissue intensities (figure 1b). Next, the analysis of the signal histogram and mathematical morphology were performed using an automatic analysis of the voxel intensities for the entire brain to obtain a binary mask of the brain (figure 1c). The mask was then split into the left and right hemispheres and the cerebellum (figure 1d). A negative mould of the white matter was computed from the split-brain mask. The outside boundary of this mould results from a 5 mm morphological closing of the masked hemisphere, filling up the folds. The grey/white interface is the inside boundary that preserves deformations and assures the spherical topology of the mould (figure 1e). Finally, the mould was skeletonized to detect cortical folding, while topological constraints guaranteed the resulting surfaces would have no holes [65,66] (figure 1f,g). The folds making up the sylvian fissure in each hemisphere were selected manually (figure 1h) by the user, using a three-dimensional visualization. The sensitivity of the extraction of sulci can be influenced by factors such as the scanner magnet strength; thus, in all analyses, we used scanner magnet as a covariate in order to statistically control for this variable.
Figure 1.
An outline of the image processing pipeline as implemented in BrainVisa. (Online version in colour.)
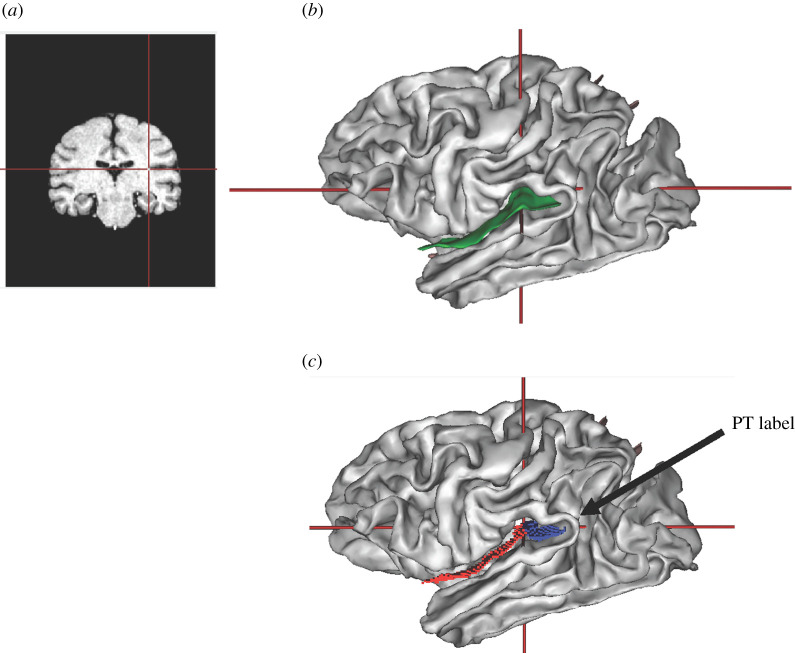
As noted above, the sylvian fissure was extracted during the pipeline procedure and manually labelled. To quantify the surface area (mm2) and mean depth of the PT (mm), we used the sulci editing function in BV. Specifically, the T1 scan and three-dimensional sulci display were opened simultaneously in the viewer window with the cursor visible in both windows (figure 2). On the T1 scan, the image was manipulated and rotated so that it was perfectly aligned in the x-, y- and z-axes, at the exact point at which the inferior limb of the insula was no longer visible in the anterior–posterior plane. When clicking with the mouse on this exact location, it would simultaneously display the anterior border of the PT in the sagittal plane. Using the scissors tool, we then section the sylvian fissure from its most medial to lateral point on the surface, which separated the sylvian fissure into that portion belonging to the PT and the remaining anterior region (figure 2). Using the labelling tool in BV, we then labelled the PT region and saved the image file for subsequent quantification of the PT surface area (mm2) and mean depth (mm) for each hemisphere.
Figure 2.
(a) Coronal view of T1 can and (b) lateral view of the three-dimensional brain with the Sylvian fissure outlined in green. Note that the cross bars in each image reflect the location of the point of closure of the inferior limb of the insula, which served as the anterior border to define the PT. (c) Lateral view of the three-dimensional brain showing the division of the Sylvian fissure into the anterior (red) and posterior (blue) regions after using the scissors to bifurcate the fold. (Online version in colour.)
(d). Heritability analyses
Consistent with our and others' previous work, to estimate heritability we used the software package SOLAR [67]. SOLAR uses a variance component approach to estimate the polygenic component of variance when considering the entire pedigree [64,68–71]. We used SOLAR to determine heritability in the average surface area and average depth for each sulcus by adding the left and right hemisphere values and dividing by two. For all heritability analyses, scanner strength (1.5T, 3T and 4.7T/7T), sex, age, rearing group and colony served as covariates in the analyses. To examine lateralization, an asymmetry quotient (AQ) was calculated using the equation |(right – left)/[(right + left)/2]|. Positive values indicate a right greater than left asymmetry and negative values indicate a left greater than right asymmetry. We also classified subjects as left-lateralized ( ≤ −0.025), right-lateralized (≥ 0.025) or having no bias (more than −0.0249 and less than 0.0249) using cutpoints based on their AQ values.
3. Results
(a). Descriptive data on planum temporale asymmetry
Consistent with previous reports, using one sample t-tests on the AQ values, we found significant population-level leftward asymmetries for PT surface area (t290 = −9.083, p < 0.001) and mean sulcal depth (t290 = −5.521, p < 0.001) in the total sample, and these results were significant when analysed separately within the three samples of chimpanzees that were scanned at different magnet strengths. Detailed results from these analyses are provided in the electronic supplementary material, table S1.
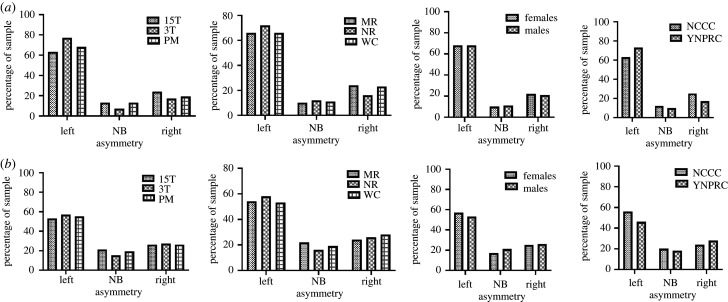
For descriptive purposes, we also report the percentage of chimpanzees that were classified as having a left, right or no bias (based on the AQ cut points) in PT surface area and mean depth asymmetry (figure 3a,b and electronic supplementary material, table S3). For each measure, the percentage of chimpanzees classified left, right and no bias in PT asymmetry is shown across scanner magnet strength, rearing history, sex and chimpanzee colony. These data corroborate the consistency in leftward biases within each scanner magnet cohort. Chi-square tests of independence revealed that significantly more chimpanzees were classified as left-lateralized compared to right and no bias for both PT surface area χ2(2, n = 291) = 159.28, p < 0.001 and mean sulcal depth χ2(2, n = 291) = 63.27, p < 0.001 and, like the mean AQ values, the distribution of lateralization was consistent across the brains scanned at different magnet strengths (figure 3).
Figure 3.
Per cent of chimpanzees classified as having a left, right or no bias in PT surface area (a) and mean depth (b) asymmetry. For each row, from left to right, the percentage of chimpanzees classified left, right and no bias in PT asymmetry is shown across scanner magnets and protocol, rearing history, sex and chimpanzee colony.
We also tested for consistency in PT asymmetries between chimpanzee populations, sexes and rearing groups using a multivariate analysis of covariance. The AQ values for the surface area and mean depth were the dependent measures, while sex (male, female), rearing group (MR, NR, WC) and colony (NCCC, YNPRC) were between-group factors. Scanner magnet and age were covariates. We found no overall significant main effects or interactions for this analysis (see electronic supplementary material, table S1). The AQ for the surface area and mean depth scores for the NCCC and YNPRC chimpanzees, was generally consistent across the two colonies, sexes and rearing groups (figure 3).
(b). Heritability analyses
Heritability in the left and right hemisphere PT surface areas and mean depths were determined for the entire sample. Scanner magnet strength, sex, age and colony were used as covariates. We found significant heritability in the mean hemisphere PT surface area (h2 = 0.22; p < 0.05) as well as the mean sulcal depth of the PT (h2 range = 0.42; p < 0.05). Detailed results from these analyses are provided in the electronic supplementary material, table S2. Additionally, we found a small, but significant, heritability for the AQ surface area (h2 = 0.13; p < 0.05), but not the mean depth AQ (h2 = 0.03; p > 0.05). We also estimated heritability within the NCCC and YNPRC colonies separately to examine consistency in heritability between the two populations. Within the NCCC population, significant heritability was found for both mean PT surface area and mean depth. By contrast, for the YNPRC population we failed to find significant heritability in mean PT surface area, although the mean depth, was significantly heritable. Thus, heritability in the mean depth of the PT was consistently significant between the NCCC and YNPRC chimpanzee populations. By contrast, heritability in surface area was not found to consistently be significant between the NCCC and YNPRC populations. Lastly, we performed genetic correlations between the left and right hemisphere surface area and mean depth values for the entire chimpanzee sample. A significant genetic correlation was found for mean depth (rhoG = 0.975, s.e. = 0.189 p < 0.001), but not for surface area, though the estimate approached conventional levels of statistical significance (rhoG = 0.755, s.e. = 0.212, p < 0.054).
4. Discussion
Our findings indicate that chimpanzees exhibit a robust and consistent pattern of population-level leftward asymmetry for the PT, which was evident across MRI scanner magnets, sexes, and colonies, and among chimpanzees that experienced different early social rearing experiences. Lastly, we found a small but significant heritability in the AQ scores for the PT mean depth but not the surface area. These findings should be interpreted with caution in light of the inconsistency in findings between the measures and the relative small effect size. Arguably, perhaps molecular biological methods might produce more compelling evidence for genetic factors influencing directional asymmetries than quantitative genetic approaches.
(a). Genetic factors
In human twin studies, PT morphological asymmetry has been shown to display heritability, an observation supported by human developmental studies [40,45,72], which have highlighted the early establishment of PT asymmetry in utero, suggesting genetic factors play a central role. More recent genome-wide analyses (GWAS) have confirmed the influence of genetic factors, with observations of significant heritability (14%) in PT asymmetry reported for the general population [73]. Although an earlier meta-analysis of PT asymmetry failed to detect any associations with gene loci [19], recent studies point towards significant associations between changes in loci of the BOK and DTYMK genes and PT asymmetry [73]. We believe there are three important aspects of the findings on heritability in the PT asymmetry values in the chimpanzee sample. First, the small, but significant, heritability we found in chimpanzee PT surface area approximates the 14% of heritability reported in a heterogenous sample of human subjects, suggesting similar contributions of genetic factors between the two species [73]. Second, Hopkins et al. [59] has previously found that overall tool use skill is significantly heritable in chimpanzees, and performance asymmetries in tool use skill are small, but significantly, heritable (h2 = 0.17), a value that is also comparable to the heritability estimate reported here for the PT. Third, the genetic correlation between the mean PT depth of the left and right hemispheres was significant and higher than for the surface area measures. The AQ values for surface area were significantly heritable, but this was not the case for the mean depth. It should be acknowledged that the higher genetic correlation between the two hemisphere values, the less likely it is that a specific gene may code for left–right asymmetry. Genetic correlations evaluate shared genetic variance between traits and higher values indicate that a common gene or sets of genes underlie the same phenotypes. Thus, if the left and right hemisphere genetic correlations are high, it suggests that the same gene(s) underlies their expression. If brain asymmetries reflect specific left or right hemisphere genetic regulation, then more lateralized brain regions would presumably have weaker interhemispheric correlations. This interpretation is supported by the results reported here, but whether this pattern could be expanded to additional brain regions remains unclear [58].
(b). Environmental factors
We found very little evidence that experiential, methodological or biological factors (i.e. sex) influenced PT directional asymmetries in either surface area or mean depth. Indeed, there is remarkable consistency in findings between these two chimpanzee populations, as well as between sexes, rearing groups and independent of the scanning procedure and magnet strength. With specific regard to rearing history, the findings reported here do not support any hypotheses suggesting that consistent, lateralized human handling in some way induces population-level asymmetries in chimpanzees. To be clear, we are not suggesting that early experiential factors have no influence on the development of brain asymmetries in chimpanzees; our results only suggest that early rearing either by conspecific mothers or in human nursery settings do not differentially influence the direction of PT asymmetries.
(c). Comparisons to other primates
Based on previous findings [37,39] and those reported here, chimpanzees show a population-level leftward asymmetry for the surface area, mean depth and grey matter volume of the PT [36,74]. Further, chimpanzees also show a leftward asymmetry in the cytoarchitectonic volume of BA22 or area Tpt [35]. Thus, leftward asymmetries in the PT are evident at multiple levels of analysis in chimpanzees. However, the evidence of population-level leftward asymmetries for the PT in other nonhuman primate species is less well established. For instance, there are few published data on PT asymmetries in other great apes, at either the morphological or cellular levels of analysis [75]. In more distantly related cercopithecid monkeys, baboons show a leftward asymmetry in the surface area of the PT [34] and, interestingly, there is some evidence that these asymmetries are present within the first few months of life similar to what is observed in humans [72]. By contrast, neither vervet nor rhesus monkeys show population-level asymmetries for the PT surface area and grey matter volume, when using traditional region-of-interest approaches [76–78]. In rhesus monkeys ranging between 1 and 19 month of age, Xia et al. [78] used a voxel-based approach to measure asymmetries in surface area and cortical thickness, and reported leftward asymmetries for the PT. Finally, there is one report of significant leftward asymmetries in the volume of BA22 in rhesus monkeys based on histologically defined boundaries [76].
There have been a number of comparative studies in apes and monkeys that have quantified the length of the Sylvian fissure as a proxy to estimating PT asymmetries by direct measures on the cortical surface [79] or from three-dimensional reconstructions of sulci from MRI scans or endocasts [56,58,80–82]. In general, the evidence suggests that both chimpanzees and various monkey species show a leftward bias in Sylvian fissure length, but to what extent that reflects asymmetries in PT surface area or volume remains unclear. Indeed, Cantalupo and colleagues [83] compared the measurement of PT asymmetries in relation to variation in different components of lateralization in Sylvian fissure length (i.e. anterior versus posterior sections) and found only small or non-significant associations. In our view, the methods and landmarks used in this study to define the PT could be readily adapted to other nonhuman primate brains and would facilitate a more comprehensive and fair assessment of lateralization in the posterior superior temporal gyrus across primate species.
In conclusion, the present study provides important confirmatory data that the leftward asymmetries in the PT of chimpanzees is robust and is evident across two distinct genetically isolated populations. Further, leftward asymmetries in the PT were consistently found across two cohorts studied and were found to be independent of the (1) MRI magnet strength and scanning protocol, (2) the sex of the individual, and (3) early social rearing experiences. Surface area and mean depth of the PT were significantly heritable, and these patterns of results were largely consistent between the two chimpanzee populations. The collective findings suggest that asymmetries in the PT have a strong biological basis, and that this evolutionary foundation was probably evident in the last common ancestor of chimpanzees and humans, serving as a pre-adaptation for modern human language and speech [84]. The presence of PT asymmetries in the last common ancestor may have set the stage for the emergence of lateralization to the left hemisphere in language functions in modern humans.
Supplementary Material
Data accessibility
MR imaging data as used in this study may be accessed through the National Chimpanzee Brain Resource (NCBR) (https://www.chimpanzeebrain.org).
Authors' contributions
All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design; collection and analysis of data; statistical analysis and interpretation; drafting of the manuscript: M.A.S., C.C.S., S.J.S. and W.D.H. Obtained funding: C.C.S. and W.D.H. Preparation of figures/tables: M.A.S. and W.D.H. Critical revision of the manuscript for important intellectual content: all authors.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Science Foundation (grant nos. BCS-0515484, BCS-0549117, BCS- BCS-0824531, DGE-0801634), the National Institutes of Health (grant nos. NS42867) and the James S. McDonnell Foundation (grant no. 22002078). MRI data were provided by the National Chimpanzee Brain Resource (www.chimapanzeebrain.org) which was supported by NIH grant no. NS092988.
References
- 1.Darwin C. 1859. On the origin of species. London, UK: John Murray. [Google Scholar]
- 2.The Chimpanzee Sequencing and Analysis Consortium. 2005. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature 437, 69–87. ( 10.1038/nature04072) [DOI] [PubMed] [Google Scholar]
- 3.Bradley BJ. 2008. Reconstructing phylogenies and phenotypes: a molecular view of human evolution. J. Anat. 212, 337–353. ( 10.1111/j.1469-7580.2007.00840.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherwood CC, Subiaul F, Zawidzki TW. 2008. A natural history of the human mind: tracing evolutionary changes in brain and cognition. J. Anat. 212, 426–454. ( 10.1111/j.1469-7580.2008.00868.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corballis MC. 1992. The lopsided brain: evolution of the generative mind. New York, NY: Oxford University Press. [Google Scholar]
- 6.Crow TJ. 2009. A theory of the origin of cerebral asymmetry: epigenetic variation superimposed on a fixed right-shift laterality. 15, 289–303. ( 10.1080/13576500902734900) [DOI] [PubMed] [Google Scholar]
- 7.Holloway RL, De La Coste-Lareymondie MC. 1982. Brain endocast asymmetry in pongids and hominids: some preliminary findings on the paleontology of cerebral dominance. Am. J. Phys. Anthropol. 58, 101–110. ( 10.1002/ajpa.1330580111) [DOI] [PubMed] [Google Scholar]
- 8.Beaton AA. 1997. The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender and dyslexia: a review of the evidence. Brain Lang. 60, 255–322. ( 10.1006/brln.1997.1825) [DOI] [PubMed] [Google Scholar]
- 9.Ettlinger GF. 1988. Hand preference, ability and hemispheric specialization: how far are these factors related in the monkey? Cortex 24, 389–398. ( 10.1016/S0010-9452(88)80002-9) [DOI] [PubMed] [Google Scholar]
- 10.Li X, Crow TJ, Hopkins WD, Gong Q, Roberts N. 2018. Human torque is not present in chimpanzee brain. Neuroimage 165, 285–293. ( 10.1016/j.neuroimage.2017.10.017) [DOI] [PubMed] [Google Scholar]
- 11.Warren JM. 1980. Handedness and laterality in humans and other animals. Physiol. Psychol. 8, 351–359. ( 10.3758/BF03337470) [DOI] [Google Scholar]
- 12.Neubauer S, Gunz P, Scott NA, Hublinn JJ, Mitteroecker P. 2020. Evolution of brain lateralization: a shared hominid pattern of endocranial asymmetry is much more variable in humans than in great apes. Sci. Adv. 6, eaax9935 ( 10.1126/sciadv.aax99) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ocklenburg S, Gunturkun O. 2018. The lateralized brain: the neuroscience and evolution of hemispheric asymmetries. London, UK: Academic Press. [Google Scholar]
- 14.Annett M. 2002. Handedness and brain asymmetry: the right shift theory. Hove, UK: Psychology Press. [Google Scholar]
- 15.Bradshaw JL, Rogers LJ. 1993. The evolution of lateral asymmetries, language, tool use, and intellect. San Diego, CA: Academic Press. [Google Scholar]
- 16.Hopkins WD. 2013. Behavioral and brain asymmetries in chimpanzees: a case for continuity. Ann. N Y Acad. Sci. 1288, 27–35. ( 10.1111/nyas.12109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altarelli I, Leroy F, Monzalvo K, Fluss J, Billard C, Dehaene-Lambertz G, Galaburda AM, Ramus F. 2014. Planum temporale asymmetry in developmental dyslexia: revisiting an old question. Human Brain Mapping 35, 5717–5735. ( 10.1002/hbm.22579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckert MA, Leonard CM, Possing ET, Binder JR. 2006. Uncoupled leftward asymmetries for planum morphology and functional language processing. Brain Lang. 98, 102–111. ( 10.1016/j.bandl.2006.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guadalupe T, et al. 2015. Asymmetry within and around the human planum temporale is sexually dimorphic and influenced by genes involved in steroid hormone receptor activity. Cortex 62, 41–55. ( 10.1016/j.cortex.2014.07.015) [DOI] [PubMed] [Google Scholar]
- 20.Hickok G, Poeppel D. 2007. The cortical organization of speech processing. Nat. Rev. Neurosci. 8, 393–402. ( 10.1038/nrn2113) [DOI] [PubMed] [Google Scholar]
- 21.Pekkola J, Ojanen V, Autti T, Jaaskelainen TP, Mottonen R, Sams M. 2006. Attention to visual speech gestures enhances hemodynamic activity in the left planum temporale. Hum. Brain Mapp. 27, 471–477. ( 10.1002/hbm.20190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapleske J, Rossell SL, Woodruff PW, David AS. 1999. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res. Rev. 29, 26–49. ( 10.1016/S0165-0173(98)00047-2) [DOI] [PubMed] [Google Scholar]
- 23.Toga AW, Thompson M. 2003. Mapping brain asymmetry. Nature 4, 37–48. [DOI] [PubMed] [Google Scholar]
- 24.Galaburda AM, Corsiglia J, Rosen G, Sherman GF. 1987. Planum temporale asymmetry, reappraisal since Geschwind and Levitsky. Neuropsychologia 25, 853–868. ( 10.1016/0028-3932(87)90091-1) [DOI] [Google Scholar]
- 25.Geschwind N, Levitsky W. 1968. Human brain: left-right asymmetries in temporal speech region. Science 161, 837 186–837 187. ( 10.1126/science.161.3837.186) [DOI] [PubMed] [Google Scholar]
- 26.Josse G, Tzouio-Mazoyer N. 2004. Hemispheric specialization for language. Brain Res. Rev. 44, 1–12. ( 10.1016/j.brainresrev.2003.10.001) [DOI] [PubMed] [Google Scholar]
- 27.Borovsky A, Saygin AP, Bates E, Dronkers N. 2007. Lesion correlates of conversational speech production deficits. Neuropsychologia 45, 2525–2533. ( 10.1016/j.neuropsychologia.2007.03.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dronkers NF, Wilkins DP, Van Valin RD Jr, Redfern BB, Jaeger JJ. 2004. Lesion analysis of the brain areas involved in language comprehension. Cognition 92, 145–177. ( 10.1016/j.cognition.2003.11.002) [DOI] [PubMed] [Google Scholar]
- 29.Foundas AL, Bollich AM, Feldman J, Corey DM, Hurley M, Lemen LC, Heilman KM. 2004. Aberrant auditory processing and atypical planum temporale in developmental stuttering. Neurology 63, 1640–1646. ( 10.1212/01.wnl.0000142993.33158.2a) [DOI] [PubMed] [Google Scholar]
- 30.Wernicke C. 1874. Der aphasische symptomenkomplex. Breslau, Poland: Cohn, Weigert. [Google Scholar]
- 31.Ocklenburg S, Friedrich P, Fraenz C, Schlüter C, Beste C, Güntürkün O, Genç E. 2018. Neurite architecture of the planum temporale predicts neurophysiological processing of auditory speech. Sci. Adv. 4, eaar6830 ( 10.1126/sciadv.aar6830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Josse G, Kherif G, Flandin G, Seghier ML, Price CJ. 2009. Predicting language lateralization from gray matter. J. Neurosci. 29, 13 516–13 523. ( 10.1523/JNEUROSCI.1680-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Josse G, Mazoyer B, Crivello F, Tzourio-Mazoyer N. 2003. Left planum temporale: an anatomical marker of left hemispheric specialization for language comprehension. Cogn. Brain Res. 18, 1–14. ( 10.1016/j.cogbrainres.2003.08.007) [DOI] [PubMed] [Google Scholar]
- 34.Marie D, et al. 2018. Left brain asymmetry of the planum temporale in a nonhominid primate: redefining the origin of brain specialization for language. Cereb. Cortex 28, 1808–1815. ( 10.1093/cercor/bhx096) [DOI] [PubMed] [Google Scholar]
- 35.Spocter MA, Hopkins WD, Garrison AR, Stimpson CD, Erwin JM, Hof PR, Sherwood CS. 2010. Wernicke's area homolog in chimpanzees (Pan troglodytes): probabilstic mapping, asymmetry and comparison with humans. Proc. R. Soc. B 277, 2165–2174. ( 10.1098/rspb.2010.0011) [DOI] [Google Scholar]
- 36.Gannon PJ, Holloway RL, Broadfield DC, Braun AR. 1998. Asymmetry of chimpanzee Planum Temporale: Humanlike pattern of Wernicke's language area homolog. Science 279, 220–222. ( 10.1126/science.279.5348.220) [DOI] [PubMed] [Google Scholar]
- 37.Hopkins WD, Marino L, Rilling JK, MacGregor LA. 1998. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI). Neuroreport 9, 2913–2918. ( 10.1097/00001756-199808240-00043) [DOI] [PubMed] [Google Scholar]
- 38.Hopkins WD, Misiura M, Pope SM, Latash EM. 2015. Behavioral and brain asymmetries in primates: a preliminary evaluation of two evolutionary hypotheses. Yearb. Cogn. Neurosci. 1359, 65–83. ( 10.1111/nyas.12936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hopkins WD, Nir T. 2010. Planum temporale surface area and grey matter asymmetries in chimpanzees (Pan troglodytes): the effect of handedness and comparison within findings in humans. Behav. Brain Res. 208, 436–443. ( 10.1016/j.bbr.2009.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubois J, Hertz-Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene-Lambertz G. 2009. Structural asymmetries in the infant language and sensori-motor networks. Cereb. Cortex 19, 414–423. ( 10.1093/cercor/bhn097) [DOI] [PubMed] [Google Scholar]
- 41.Glasel H, Leroy F, Dubois J, Hertz-Pannier L, Mangin JF, Dehaene-Lambertz G. 2011. A robust cerebral asymmetry in the infant brain: a rightward superior temporal sulcus. Neuroimage 58, 716–723. ( 10.1016/j.neuroimage.2011.06.016) [DOI] [PubMed] [Google Scholar]
- 42.Habas PA, Scott JA, Roosta A, Rajagopalan V, Kim K, Rousseau F, Barkovich AJ, Glenn OA, Studholme C. 2012. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cereb. Cortex 22, 13–25. ( 10.1093/cercor/bhr053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill J, Dierker D, Neil J, Inder T, Knutsen A, Harwell J, Coalson T, Van Essen D. 2010. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J. Neurosci. 30, 2268–2276. ( 10.1523/JNEUROSCI.4682-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kasprian G, Langs G, Brugger PC, Bittner M, Weber M, Arantes M, Prayer D. 2011. The prenatal origin of hemispheric asymmetry: an in utero neuroimaging study. Cereb. Cortex 21, 1076–1083. ( 10.1093/cercor/bhq179) [DOI] [PubMed] [Google Scholar]
- 45.Li G, Nie J, Wang L, Shi F, Lyall AE, Lin W, Gilmore JH, Shen D. 2014. Mapping longitudinal hemispheric structural asymmetries of the human cerebral cortex from birth to 2 years of age. Cereb. Cortex 24, 1289–1300. ( 10.1093/cercor/bhs413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong XZ, et al. 2018. Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the ENIGMA Consortium. Proc. Natl Acad. Sci. USA 115, E5154–E5163. ( 10.1073/pnas.1718418115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ocklenburg S, Arning L, Gerding WM, Epplen JT, Gunturkun O, Beste C. 2013. FOXP2 variation modulates functional hemispheric asymmetries for speech perception. Brain Lang. 126, 279–284. ( 10.1016/j.bandl.2013.07.001) [DOI] [PubMed] [Google Scholar]
- 48.Scerri TS, Morris AP, Buckingham LL, Newbury DF, Miller LL, Monaco AP, Bishop DV, Paracchini S. 2011. DCDC2, KIAA0319 and CMIP are associated with reading-related traits. Biol. Psychiatry 70, 237–245. ( 10.1016/j.biopsych.2011.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun T, Walsh CA. 2006. Molecular approaches to brain asymmetry and handedness. Nat. Neurosci. Rev. 7, 655–662. ( 10.1038/nrn1930) [DOI] [PubMed] [Google Scholar]
- 50.de Kovel CGF, Francks C. 2019. The molecular genetics of hand preference revisited. Sci. Rep. 9, 5986 ( 10.1038/s41598-019-42515-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medland SE, et al. 2009. Genetic influences on handedness: Data from 25,732 Australian and Dutch twin families. Neuropsychologia 47, 33–337. ( 10.1016/j.neuropsychologia.2008.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geschwind DH, Miller BL, DeCarli C, Carmeli D. 2002. Heritability of lobar brain volumes in twins support genetic models of cerebral laterality and handedness. Proc. Natl Acad. Sci. USA 99, 3176–3181. ( 10.1073/pnas.052494999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jansen AG, Mous SE, White T, Posthuma D, Polderman TJC. 2015. What twin studies tell us about heritability of brain development, morphology and function. Neuropsychol. Rev. 25, 27–46. ( 10.1007/s11065-015-9278-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson P, et al. 2001. Genetic influences on brain structure. Nat. Neurosci. 4, 1253–1258. ( 10.1038/nn758) [DOI] [PubMed] [Google Scholar]
- 55.Atkinson EG, Rogers J, Mahaney MC, Cox LA, Cheverud JM. 2015. Cortical folding of the primate brain: an interdisciplinary examination of the genetic architecture, modularity, and evolvability of a significant neurological trait in pedigreed baboons (Genus Papio). Genetics 200, 651–666. ( 10.1534/genetics.114.173443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheverud JM, Falk D, Hildebolt C, Moore AJ, Helmkamp RC, Vannier J. 1990. Heritability and association of cortical petalia in rhesus monkeys (Macaca mulatta). Brain Behav. Evol. 35, 368–372. ( 10.1159/000115881) [DOI] [PubMed] [Google Scholar]
- 57.Fears SC, Scheibel K, Abaryan Z, Lee C, Jorgensen MJ, Fairbanks LA, Cantor RM, Freimer NB, Woods RP. 2011. Anatomic brain asymmetry in vervet monkeys. PLoS ONE 6, e28243 ( 10.1371/journal.pone.0028243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomez-Robles A, Hopkins WD, Schapiro SJ, Sherwood CC. 2016. The heritability of chimpanzee and human brain asymmetry. Proc. R. Soc. B 283, 1845 ( 10.1098/rspb.2016.1319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hopkins WD, Mareno MC, Schapiro SJ. 2019. Further evidence of left hemisphere dominance in motor skill by chimpanzees on a tool use task. J. Comp. Psychol. 133, 512–519. ( 10.1037/com0000183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGrew WC, Marchant LF. 1997. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in non-human primates. Yearb. Phys. Anthropol. 40, 201–232. () [DOI] [Google Scholar]
- 61.Denenberg VH, Yutzey DA. 1985. Hemispheric laterality, behavioral asymmetry, and the effects of early experience in rats. In Cerebral lateralization in nonhuman species (ed. Glick SD.), pp. 109–133. New York, NY: Academic Press. [Google Scholar]
- 62.Hopkins WD, et al. 2014. Evolution of the central sulcus morphology in primates. Brain Behav. Evol. 84, 19–30. ( 10.1159/000362431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bogart SL, Mangin JF, Schapiro SJ, Reamer L, Bennett AJ, Pierre PJ, Hopkins WD. 2012. Cortical sulci asymmetries in chimpanzees and macaques: a new look at an old idea. Neuroimage 61, 533–541. ( 10.1016/j.neuroimage.2012.03.082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hopkins WD, et al. 2017. Genetic factors and orofacial motor learning selectively influence variability in central sulcus morphology in chimpanzees (Pan troglodytes). J. Neurosci. 37, 5475–5483. ( 10.1523/JNEUROSCI.2641-16.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mangin JF, Riviere D, Cachia A, Duchesnay E, Cointepas Y, Papadopoulos-Orfanos D, Collins DL, Evans AC, Régis J. 2004. Object-based morphometry of the cerebral cortex. Medical Imaging 23, 968–982. ( 10.1109/TMI.2004.831204) [DOI] [PubMed] [Google Scholar]
- 66.Mangin JF. 2000. Entropy minimization for automatic correction of intensity nonuniformity. In Proc. MMBIA-2000. See https://ieeexplore.ieee.org/document/852374.
- 67.Almasy L, Blangero J. 1998. Multipoint quantitative-trait linkage analysis in general pedigrees. Am. J. Hum. Genet. 62, 1198–1211. ( 10.1086/301844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fears SC, et al. 2009. Identifying heritable brain phenotypes in an extended pedigree of vervet monkeys. J. Neurosci. 29, 2867–2875. ( 10.1523/JNEUROSCI.5153-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hopkins WD, Latzman RD, Mareno MC, Schapiro SJ, Gómez-Robles A, Sherwood CC. 2018. Heritability of gray matter structural covariation and tool use skills in chimpanzees (Pan troglodytes): a source-based morphometry and quantitative genetic analysis. Cereb. Cortex 29, 3702–3711. ( 10.1093/cercor/bhy250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kochunov PV, et al. 2010. Genetics of primary cerebral gyrification: heritability of length, depth and area of primary sulci in an extended pedigree of Papio baboons. Neuroimage 53, 1126–1134. ( 10.1016/j.neuroimage.2009.12.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rogers J, Kochunov PV, Lancaster JL, Sheeledy W, Glahn D, Blangero J, Fox PT. 2007. Heritability of brain volume, surface area and shape: an MRI study in an extended pedigree of baboons. Hum. Brain Mapp. 28, 576–583. ( 10.1002/hbm.20407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chi JG, Dooling EC, Gilles FH. 1977. Gyral development of the human brain. Ann. Neurol. 1, 86–93. ( 10.1002/ana.410010109) [DOI] [PubMed] [Google Scholar]
- 73.Carrion-Castillo A, Pepe A, Kong XZ, Fisher SE, Mazoyer B, Tzourio-Mazoyer N, Crivello F, Francks C. et al. 2020. Genetic effects on planum temporale asymmetry and their limited relevance to neurodevelopmental disorders, intelligence or educational attainment. Cortex 124, 137–153. ( 10.1016/j.cortex.2019.11.006) [DOI] [PubMed] [Google Scholar]
- 74.Zilles K, Dabringhaus A, Geyer S, Amunts K, Qü M, Schleicher A, Gilissen E, Schlaug G, Steinmetz H. 1996. Structural asymmetries in the human forebrain and the forebrain of non-human primates and rats. Neurosci. Biobehav. Rev. 20, 593–605. ( 10.1016/0149-7634(95)00072-0) [DOI] [PubMed] [Google Scholar]
- 75.Hopkins WD, Lyn H, Cantalupo C. 2009. Volumetric and lateralized differences in selected brain regions of chimpanzees (Pan troglodytes) and bonobos (Pan paniscus). Am. J. Primatol. 71, 988–997. ( 10.1002/ajp.20741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gannon PJ, Kheck N, Hof PR. 2008. Leftward interhemispheric asymmetry of macaque monkey temporal lobe language area homolog is evident at the cytoarchitectural, but not gross anatomic level. Brain Research 1199, 62–73. ( 10.1016/j.brainres.2007.12.041) [DOI] [PubMed] [Google Scholar]
- 77.Lyn H, Pierre P, Bennett AJ, Fears S, Woods R, Hopkins WD. 2011. Planum temporale grey matter asymmetries in chimpanzees (Pan troglodytes), vervet (Chlorocebus aethiops sabaeus), rhesus (Macaca mulatta) and bonnet (Macaca radiata) monkeys. Neuropsychologia 49, 2004–2012. ( 10.1016/j.neuropsychologia.2011.03.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xia J, Wang F, Wu Z, Wang L, Zhang C, Shen D, Li G. 2020. Mapping hemispheric asymmetries of the macaque cerebral cortex during early brain development. Hum. Brain Mapp. 41, 95–106. ( 10.1002/hbm.24789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yeni-Komshian G, Benson D. 1976. Anatomical study of cerebral asymmetry in the temporal lobe of humans, chimpanzees and monkeys. Science 192, 387–389. ( 10.1126/science.816005) [DOI] [PubMed] [Google Scholar]
- 80.Hou L, Xiang L, Crow TJ, Leroy F, Rivière D, Mangin JF, Roberts N. 2019. Measurement of Sylvian Fissure asymmetry and occipital bending in humans and Pan troglodytes. Neuroimage 184, 855–870. ( 10.1016/j.neuroimage.2018.08.045) [DOI] [PubMed] [Google Scholar]
- 81.Atkinson EG, Rogers J, Cheverud JM. 2016. Evolutionary and developmental implications of asymmetric brain folding in a large primate pedigree. Evolution 70, 707–715. ( 10.1111/evo.12867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hopkins WD, Pilcher DL, MacGregor L. 2000. Sylvian fissure length asymmetries in primates revisited: a comparative MRI study. Brain Behav. Evol. 56, 293–299. ( 10.1159/000047213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cantalupo C, Pilcher D, Hopkins WD. 2003. Are planum temporale and sylvian fissure asymmetries directly related? A MRI study in great apes. Neuropsychologia 41, 1975–1981. ( 10.1016/S0028-3932(02)00288-9) [DOI] [PubMed] [Google Scholar]
- 84.Hopkins WD, Russell JL, Cantalupo C. 2007. Neuroanatomical correlates of handedness for tolld use in chimpanzees (Pan troglodytes): implications for theories on the evolution of language. Psychol. Sci. 18, 971–977. ( 10.1111/j.1467-9280.2007.02011.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
MR imaging data as used in this study may be accessed through the National Chimpanzee Brain Resource (NCBR) (https://www.chimpanzeebrain.org).