Abstract
Specific features of visual objects innately draw approach responses in animals, and provide natural signals of potential reward. However, visual sampling behaviours and the detection of salient, rewarding stimuli are context and behavioural state-dependent and it remains unclear how visual perception and orienting responses change with specific expectations. To start to address this question, we employed a virtual stimulus orienting paradigm based on prey capture to quantify the conditional expression of visual stimulus-evoked innate approaches in freely moving mice. We found that specific combinations of stimulus features selectively evoked innate approach or freezing responses when stimuli were unexpected. We discovered that prey capture experience, and therefore the expectation of prey in the environment, selectively modified approach frequency, as well as altered those visual features that evoked approach. Thus, we found that mice exhibit robust and selective orienting responses to parameterized visual stimuli that can be robustly and specifically modified via natural experience. This work provides critical insight into how natural appetitive behaviours are driven by both specific features of visual motion and internal states that alter stimulus salience.
Keywords: behaviour, approach, mice, capture, natural, responses
1. Introduction
The ability to rapidly orient towards prey or away from predators from a distance is crucial for the survival of animals. Thus, visual systems are evolutionarily honed to selectively extract the sizes and speeds of object motion that appear in particular locations in the environment that are characteristic of natural predators and prey and transform that information into specific ethological behaviours across species [1–6]. Despite the use of such hardwired responses, it would be costly to release them in the wrong context. For example, even in the presence of an appetitive stimulus that should drive approach, animals should suppress this response when sated or in the presence of a competing, more salient threatening cue [7,8]. Innate approach responses, therefore, must be triggered by specific combinations of visual features that indicate reward, but they must be flexibly modulated by internal state, context, or experience on distinct time scales [9]. Furthermore, the ability to control visual orienting responses is impaired in several prevalent neurological diseases and neurodevelopmental disorders [10] such as trauma-induced visuospatial neglect [11], post-traumatic stress disorder (PTSD) [12,13], attention deficit and hyperactivity disorder (ADHD) [14], generalized anxiety disorder [15], schizophrenia [16] and autism [17]. Currently, the mechanisms underlying conserved visually guided approach responses in mammals and their context-dependent modulation remain unclear.
The mouse has emerged as a powerful model to determine the circuit mechanisms underlying context-dependent visual behaviour. To best exploit this model to understand approach behaviour, it is imperative to first determine which specific stimulus features mice naturally approach and explore and under which environmental conditions. Under ecologically relevant conditions, salient visual stimuli detected near the horizon may be appetitive. For example, small, moving objects in this location might indicate potential prey, as is often cited [1]. Conversely, similar cues could indicate social or predatory threat [18]. Here, we probed natural visual orienting responses in mice using parameterized virtual stimuli modelled after natural, live prey items such as crickets. Mice innately show a clear preference to approach objects that are a specific relative size and speed and located within specific regions of the monocular or binocular visual field along the azimuth. In addition, mice accurately intercept specific moving stimuli without the benefit of explicit experience with the stimulus, suggesting that the innate mechanisms for motion extrapolation in this model are rapidly tuned or require little tuning. Surprisingly, we find that mice frequently exhibit prolonged periods of immobility, or freezing, in response to moving visual stimuli in the lower visual field, and that this behavioural response constitutes the majority of responses evoked by moving stimuli in the lower visual field. These freezing responses precede about 50% of accurate and successful approaches towards novel moving objects, are triggered by distinct visual features relative to approach starts, and do not predict an increase in fleeing or avoidance behaviour. Therefore, the frequent freezing observed in the context explored here may relate better to its ability to improve motion perception and/or action preparation in response to potentially appetitive objects [19]. Most intriguingly, prey capture experience prior to exposure to novel moving stimuli robustly increased the ratio of approach to freezing by selectively altering those stimulus features that drive approach and their salience. That approaches are flexibly and selectively modulated by prey capture experience, suggests that distinct neural circuits encode the visual information that differentially drives each behaviour. Thus, we show which specific features of visual motion drive natural appetitive behaviour in the mouse and that it is reliably gated by specific internal states such as experience with prey.
2. Results
(a). Novel, artificially generated visual stimuli reliably elicit approach in C57BL/6 J mice
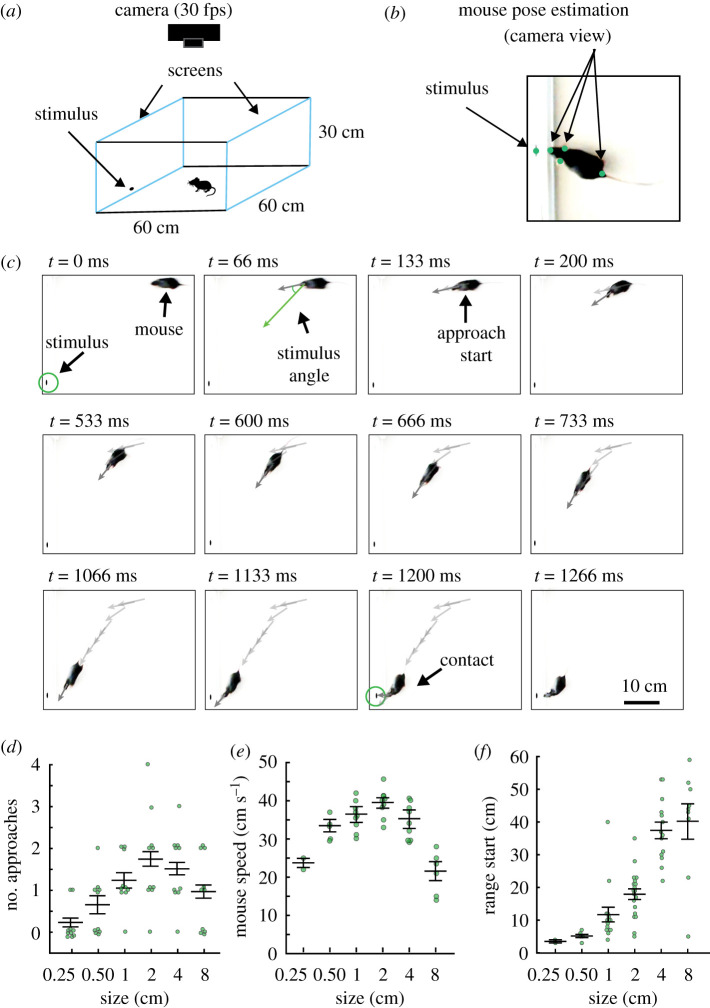
Previous work has shown that mice use visual cues to recognize prey [20–23]. In other species, specific combinations of visual features innately elicit approaches towards possible prey items [3,5,6]. However, the specific visual features of prey that draw innate approach responses in mice are unclear. We developed an assay based on natural prey capture behaviour but employing artificially generated visual stimuli displayed on a computer screen to elicit innate orienting responses in freely moving mice. We parametrically varied the size (figure 1) or speed (figure 2) of stimuli, black ellipses with a 2 : 1 aspect ratio of the major (horizontal) to minor axis, on a white background. First, a single cohort of mice was presented with a stationary, black ellipse on one of two computer screens comprising two of the four sides of an open field arena (figure 1a, blue outlines). The ellipse was centred on one of three possible locations along the azimuth of the target screen (centre or midway between centre and left adjacent or right adjacent wall). The bottom edge of the stimulus was maintained at 1 cm from the floor in elevation. Maintaining the 2 : 1 ratio, we varied the size of the stimulus along the horizontal axis from 0.25 to 8 cm, and quantified behaviour elicited within 60 s of the start of stimulus presentation. Stimuli were presented in a random order to each mouse. To derive behaviour measures, we tracked the stimulus as well as the nose, ears and tail base of the mice using DeepLabCut pose estimation software (figure 1b). We calculated the mouse's approach frequency, range (distance between stimulus and mouse head at approach start), locomotion speed, and stimulus angle, angle between the mouse's head and stimulus. Importantly, studies have shown that eye movements are coupled to head position in space and are aligned to head direction when performing natural visual behaviours such as prey capture and social investigation [24,25]. Thus, stimulus angle is a reliable measure of probable viewing angle and likely visual gaze which allowed us to estimate the visual stimulus features correlated with behavioural responses. We defined a successful approach as any time the mouse's nose came within 2 cm of the stimulus centre. Approaches were identified as in Hoy et al. [20]. Briefly, an approach start was defined as when mice decreased both their range and stimulus angle relative to the stimulus while moving an average speed of at least 15 cm s−1 starting from at least 5 cm away (figure 1c). Mice almost completely failed to approach stimuli less than or equal to 0.5 cm in size along the horizontal axis (figure 1d–f), and significantly slowed approach speeds to stimuli larger than 2 cm along the horizontal axis (figure 1e). By contrast, mice approached the stimulus with a 2 cm horizontal and 1 cm vertical axis most frequently, and with the highest locomotive speeds (figure 1d,e). Freezing, a period of immobility lasting at least 500 ms, was not significantly increased relative to habituation epochs with no stimuli shown (electronic supplementary material, data S1A) Thus, while mice approach a range of stimulus sizes, they preferred stimuli that were 2 cm in length along the horizontal axis (figure 1f). Given the nearly linear relationship between the distance where an approach started and stimulus size (figure 1f), we estimated the preferred angular stimulus size as the slope of a linear fit to the data. This yielded a preferred relative stimulus size of approximately 5 degrees (deg) of the visual angle. We were, therefore, able to determine that mice spontaneously orient towards and approach a preferred relative size of stimulus.
Figure 1.
Naive mice preferably approach specific sizes of stationary, two-dimensional visual stimuli. Naive mice are habituated to the arena and handlers, but have not been exposed to artificially generated stimuli or live crickets. (a) Schematic of arena with video recording performed overhead. Two sides of a white acrylic behaviour box consisted of computer monitors displaying blank white screens. For experiments with stationary stimuli, ellipses of different sizes were presented one at a time, in one of three different locations along the azimuth in a randomized order. (b) Example frame from a recorded behavioural video. Relative positional information (range and stimulus angle) between the mouse and stimulus are calculated from these pose estimation data (electronic supplementary material, video S1, employing moving stimuli). The positions of the stimulus, the mouse's nose, ears and tail base were all tracked and are shown as green circles. (c) A representative approach sequence towards a stationary ellipse, highlighting specific moments surrounding a successful approach: approach start and contact. Arrows indicate the stimulus angle across frames. (d) Mean approach frequency for each mouse exposed to six different objective sizes of stimuli. n = 10 mice. (e) Mean locomotor speed for mice that approached stimuli. n = 2, 5, 9, 9, 9 and 6 mice each stimulus, respectively. (f) Mean range of approach starts at each objective size of stimulus. n = 65, r = 0.73, adjusted r2 = 0.487. Error bars are ± standard error of the mean (SEM) in all panels. (Online version in colour.)
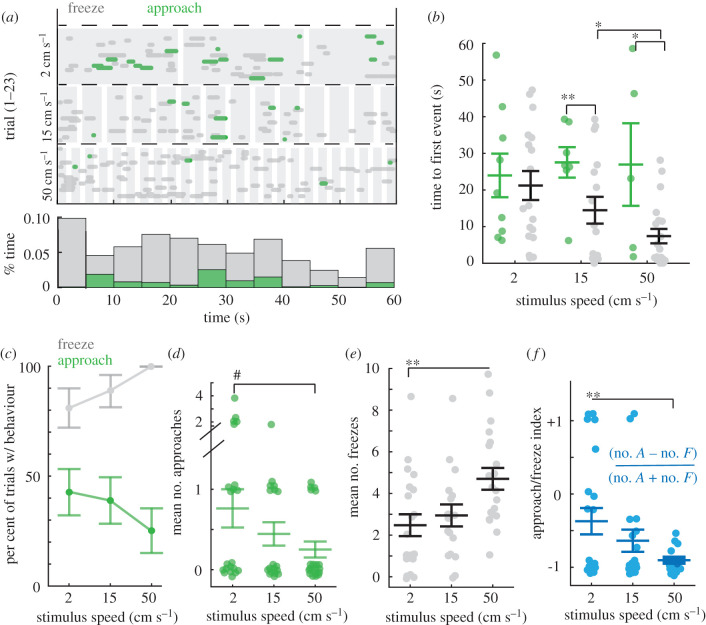
Figure 2.
Mice freeze or approach when presented with novel, small, moving stimuli. (a) Top, an ethogram representing when approach or freezing occurred relative to stimulus presentation (shaded in grey) of three different speeds of the stimulus. The responses observed in each trial (separate mouse) is denoted by a separate line in the ethogram. Green = approach, grey = freeze. Horizontal dashed line separates trial types based on stimulus speed, n = 23 mice. Bottom histogram, the proportion of time spent engaged in each type of response during each 5 s segment of the 60 s trial, collapsed across all speeds. (b) Mean time to the first orienting response of each type (green = approach or grey = freeze) for mice that display at least one type (two-way ANOVA, F = 9.1957, p < 0.001, Tukey's post hoc, * = p < 0.05 and ** = p < 0.01, n for each speed = 9, 7, 5 mice and 17, 16, 20 mice approach versus freeze, respectively). (c) Percentage of trials with at least one freeze (grey) or approach (green) by stimulus speed. Percentage of trials with freezes is significantly higher than that with approaches for each stimulus speed (Fisher's exact tests, p < 0.05, p < 0.01 and p < 0.0001, respectively). Error bars are standard deviation. (d) Number of approaches per mouse (one-way ANOVA, (F2 = 2.8418, p = 0.07, # = nearly significant). All conditions had significantly more approaches than a mean of 0 (Student's t-tests, p < 0.001, p < 0.01 and p < 0.05). (e) Number of freezes per mouse (one-way ANOVA, (F2 = 6.54, p < 0.01, Tukey's post hoc, ** = p < 0.01). Freezing evoked by all stimuli were highly significantly greater than a mean of 0 (Student's t-tests, p < 0.0001 in all cases). (f) Approach-to-freeze index by stimulus speed (one-way ANOVA, (F2 = 5.97, p < 0.01, Tukey's post hoc, ** = p < 0.01). Error bars are ± SEM unless noted otherwise. (Online version in colour.)
(b). Stimulus speed biases the probability of approach versus freezing behaviour
Given that dynamic stimuli are highly salient to animals, we next measured the responses of freely moving mice to stimuli that moved along the azimuth with steady linear speeds. The stationary stimulus that evoked the most approaches was 2 × 1 cm, thus we varied the speed of this stimulus, ranging from 2 cm s−1 to 50 cm s−1 in order to determine whether a specific stimulus speed could increase spontaneous approaches. We found that introducing motion instead led to a significant increase in freezing frequency at all speeds of motion tested relative to stationary stimuli and habituation epochs (figure 2a; electronic supplementary material, data S1). From stimulus onset, mice froze more frequently and immediately relative to when they began approaches to the same stimuli (figure 2a,b; electronic supplementary material, data S1). Freezing frequency also significantly increased as the speed of the stimuli increase, while surprisingly, the number of approaches decreased as speed increased (figure 2c–e). Interestingly, freezing responses preceded approaches 48% of the time (figure 2a; electronic supplementary material, videos S4, S6 and S7) and the proportion of approaches preceded by freezing steadily increased as stimulus speed increased, 31%, 63% versus 80% at each speed increment, respectively (see ethogram in figure 2a). Thus, these two orienting responses were related, yet differentially biased by stimulus speed. Specifically, the number of approaches relative to freezes per subject, per speed significantly decreased by stimulus speed (electronic supplementary material, data S2A) and this relationship can be clearly observed in comparing the behaviour index, number of approaches minus the number of freezes divided by the total of both, by speed (figure 2f). Despite this shift, mice found each stimulus similarly behaviourally salient, as the percentage of trials where at least one approach or freezing event was observed was not significantly different as a function of stimulus speed (91%, 78% versus 87%, each speed increment, respectively, n = 23, Fisher's exact test, p > 0.05 for all pairwise comparisons, figure 2a). In other words, all stimulus speeds were similarly behaviourally salient as they evoked at least one type of visual response with high probability, between 78 and 91%. Finally, we observed no significant differences between the sexes in approach nor freeze frequency after correcting for multiple comparisons, Mann–Whitney U, p = 0.0478 and p = 0.102, freezes versus approaches and n = 15 and 8, females versus males, respectively (electronic supplementary material, data S2B).
(c). Behavioural choice depends on relative stimulus size and speed as well as stimulus-angle
We estimated the relative sizes and speeds of stimuli that best drove specific behaviour by calculating the angular size and speed of stimuli as they would appear at the retina preceding specific behaviours. We hypothesized that the probability of approach would be inversely proportional to the size of the stimulus (mice preferring relatively smaller stimuli) and proportional to increasing speeds of motion (enhancing salience). However, mice were most likely to approach relatively smaller objects moving at the slowest objective speed: stimuli less than 10 deg of the visual arc and moving less than 50 deg s−1 (figure 3d, green circles). On the other hand, relatively smaller stimuli, less than 6 deg in size and moving at a relative speed of greater than 50 deg s−1 preferentially drove freezing (figure 3a–d), although freezing was more prominent across all speeds experienced relative to approaches.
Figure 3.
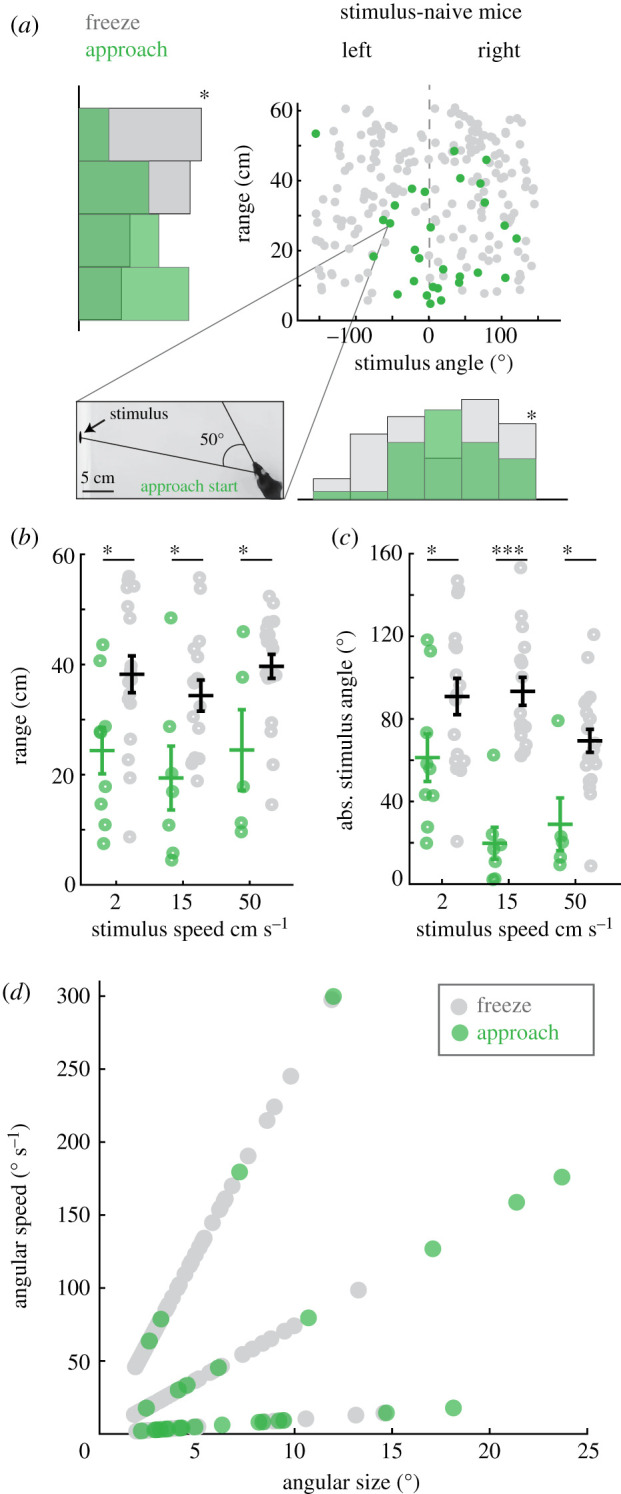
Freeze and approach orienting responses in naive mice are evoked by distinct combinations of stimulus size, speed, and location in the visual field. (a) Distribution of mouse ranges (cm) and stimulus angles (deg) relative to the mouse's head, where freezes occur (grey) or approaches start (green). * = p < 0.05, Kolmogorov-Smirnov (KS) test on range distributions, * = Ashman's D > 2 for distribution of stimulus angles at freeze starts. Plots show all individual behavioural events for all 23 mice, n = 29 and 199 approach starts and freezes, respectively. Inset, representative frame from a video where the highlighted data point is measured. The frame is annotated to show the stimulus angle relative to the mouse's head when that particular approach started. (b) Mean trial-averaged range at all three speeds. Each speed condition is represented as a separate category on the x-axis, approach and freeze data are also separated along the x-axis by a fixed amount and then jittered in the ×dimension to improve visualization of each distribution where approaches start (green circles) or freezing occurs (grey circles) (two-way ANOVA, (F1 = 19.99, p < 0.0001, Tukey's post hoc, * = p < 0.05, n = 9, 7, 5 mice and 17, 16, 20 mice for each stimulus speed, approaches or freezes, respectively). (c) Mean trial-averaged absolute stimulus angles relative to the mouse at approach starts or freezes (two-way ANOVA, (F2 = 3.17, p < 0.05, Tukey's post hoc, * = p < 0.05, *** = p < 0.0001). (d) Angular size versus angular speed of stimuli at approach start (green) or freeze (grey) reveals angular size and speed preferences for each behaviour. (Online version in colour.)
Interestingly, mice prominently displayed clear differences in behavioural choice depending on where stimuli were detected within the visual field which was estimated from measuring the stimulus angle, the angle between the mouse's head and stimulus. The stimulus angle when mice started to approach the preferred stimulus (2 cm s−1) was most often less than 60 deg (figure 3a,c, green circles and electronic supplementary material, data S3A–C). By contrast, freezes started more often when the stimulus angle was greater than 60 deg (figure 3a,c, grey circles and electronic supplementary material, data S3D–F). These findings have implications for which region of the visual field stimuli must appear in order to generate specific orienting behaviours as head position is coupled to eye position [25]. These biases are unlikely to be explained by preferences for occupying specific allocentric locations within the testing environment as stimuli were randomly presented on either screen and we observed no spatial occupancy bias within the arena in the absence of stimuli (16.7 ± 6.2%, 18.3 ± 4.9%, 18.3 ± 5.5%, 13.3 ± 4.4% per cent time in each quadrant, 1–4, respectively, during the baseline habituation period, n = 23, pairwise Mann Whitney U, p > 0.05). Stimuli thus appeared randomly to either the right or left of the mouse and at variable ranges. Our data, therefore, demonstrate that distinct visual features selectively drive orienting behaviours and that freezing is linked to approach responses, yet is dissociable.
(d). Prey capture experience selectively increases approach frequency to specific stimulus speeds
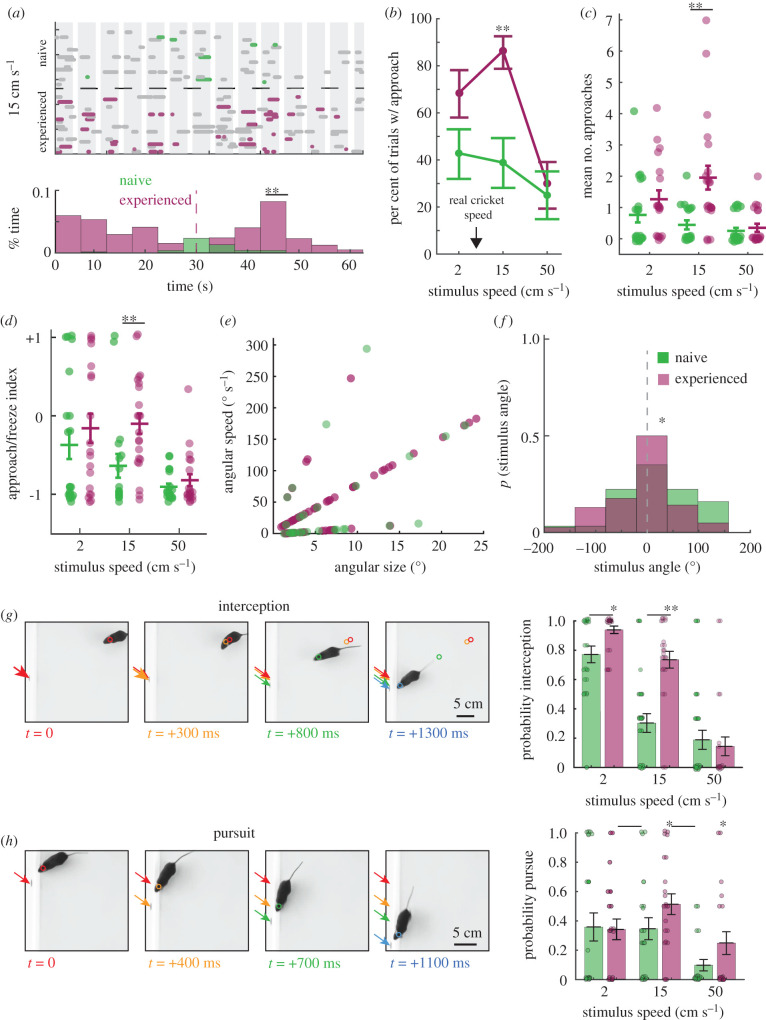
Results so far were from mice that had no experience hunting for insects. We hypothesized that innate orienting responses could be modified by prey capture experience as mice become more efficient at capturing live prey with experience [20]. To address this hypothesis, we quantified visually evoked orienting responses towards artificial stimuli in mice that had first captured live crickets (electronic supplementary material, Methods). We quantified speed-dependent approach and freezing frequencies in prey capture-experienced mice (electronic supplementary material, data S4) as well as the features of stimuli observed at the beginning of each type of orienting response (electronic supplementary material, data S5).
The significant differences found in these measures between naive and prey capture-experienced mice were specific to approach behaviour (figure 4; electronic supplementary material, data S3). Prey capture-experienced mice selectively increased their approaches towards the two slower objective speeds of 2 cm s−1 and 15 cm s−1 with the largest increases occurring in response to the 15 cm s−1 stimulus (electronic supplementary material, data S3 and S4 and figure 4a–d). These findings suggested that prey capture experience selectively altered approaches near the range of speeds of live prey motion, as well as altered the saliency of the features that evoke approach. The average speed of crawling crickets was 5 + 2.3 cm s−1 and was similar to the slowest objective speed of stimulus used here (figure 2d).
Figure 4.
Prey capture experience selectively alters approach frequency towards specific speeds and where stimuli are detected in the visual field. (a) Direct comparison of previously shown ethograms from the onset of the presentation of the 15 cm s−1 speed stimulus for each mouse (electronic supplementary material, data S4 and S5). Magenta = prey capture-experienced mice approaches, green = naive mice approaches. Grey = freezes by naive (top panel) or prey capture-experienced (bottom panel) mice. The overlaid histograms of these distributions (KS test, ** = p < 0.01, n = 8 versus 43 approach events from naive versus prey capture-experienced mice, respectively, from the 15 cm s−1 condition). (b) Per cent of trials where approaches were observed for three speeds of stimuli (Fisher's exact test, ** = p < 0.01, n = 23). Error bars are standard deviation. Black arrow highlights average measured crawling speed of crickets. (c) Mean number of approaches per mouse compared directly between naive (green) and prey capture-experienced (magenta) mice (Tukey's post hoc, ** = p < 0.01, n = 21, 18 and 20 for naive mice and n = 19, 22 and 20 for prey capture-experienced mice, for each speed, respectively). (d) Approach/freeze index as shown in figure 2f (Tukey's post hoc, ** = p < 0.01). (e) Angular size versus angular speed of stimuli at approach start for naive mice (green) or prey capture-experienced mice (magenta). (f) The distribution of all stimulus angles at approach start for all speeds for naive (green) versus prey capture-experienced (magenta) mice (KS test, * = p < 0.05, n = 29 and 73 approaches for naive versus prey capture-experienced mice, respectively). (g) Representative interception behaviour and mean trial-averaged interception probability across mice that displayed at least one approach start [21] when presented each speed of stimulus. (h) Representative pursuit behaviour, chasing stimulus after contact is made from initial approach, and mean trial-averaged pursuit probability across mice that displayed at least one approach start when presented with each speed of stimulus. Student's t-test, pairwise comparison between naive versus prey capture-experienced mice, * = p < 0.05, ** = p < 0.01, n = 21, 19 and 17, and 23, 23 and 17 mice at each speed, naive versus prey capture-experienced, respectively. Error bars are ± SEM. Coloured arrows or circles indicate the stimulus or mouse position, respectively, in sequential moments at the times indicated. (Online version in colour.)
To determine which specific relative visual perceptions were altered by experience, we again quantified the visual stimulus angles as well as angular size and speed of stimuli that were observed preceding and during each type of orienting response and compared the measures to naive mice responding to our stimuli (figure 4e,f; electronic supplementary material, data S3A–C). Mice were more likely to approach the 15 cm s−1 stimulus when it was a relatively larger angular size and moving at faster angular speeds (figure 4e) while also orienting to these stimuli earlier in the trials than naive mice, as indicated by a peak in approach frequency starting before 25 s (figure 4a). Additionally, we observed that mice adjusted their stimulus angle more rapidly at approach initiation (electronic supplementary material, data S3A, angular velocity of 107 ± 17 deg s−1 versus 156 ± 10 deg s−1, naive versus prey capture experienced, respectively, p < 0.05, Mann–Whitney U) and there was a significant difference in the distribution of stimulus angles preceding approach for prey capture experienced mice (figure 4f; electronic supplementary material, data S3A). Furthermore, we noted that stimulus angles were less variable overall around the centre of the stimulus angle distribution in the prey capture-experienced mice (centre of stimulus angle distributions = +10 deg for both distributions, but width at half max = 180 deg versus 60 degrees, naive versus prey capture-experienced mice, respectively), suggesting that stimuli were detected more equally between the predicted hemifields and proportionally more centrally (figure 4f ; electronic supplementary material, data S3A–C). Together, these data show that prey capture experience selectively altered the features of visual stimuli that evoke approach towards novel stimuli and enhanced the salience of specific visual features.
Finally, we sought to determine whether freezing responses were likely to reflect threat detection in the absence of a shelter. We, therefore, quantified the probability that mice responded to our stimuli with additional appetitive behaviour such as pursuit (following stimulus after approach), versus active avoidance behaviour such as increased thigmotaxis. If freezing indicated threat detection, an increase in thigmotaxis as stimulus speed increases was predicted. We also predicted that if our fastest moving stimuli were perceived as threatening, we should not observe significant approach and pursuit behaviours towards these stimuli. We found that both naive and prey capture-experienced mice significantly intercepted and pursued all speeds of our moving stimuli above a hypothetical mean of 0 (figure 4g,h; electronic supplementary material, videos S1, S2, S4 and S5). Mice also did not exhibit active avoidance behaviours such as fleeing to corners as scored by three independent observers (see all videos, https://dx.doi.org/10.5061/dryad.mw6m905v3), nor increased thigmotaxis after stimulus onset, relative to baseline conditions without stimuli (79 ± 5.6% thigmotaxis in baseline condition without stimuli, electronic supplementary material, data S6 and Hoy et al. [20,21,26]). Therefore, despite a high frequency of freezing to the presented stimuli in both naive and prey capture-experienced mice, these orienting responses are unlikely to indicate extreme anxiety or fear induced by our stimuli.
3. Discussion
We quantified the visual stimulus features that innately drive approach towards novel objects in mice and found that they are distinct from those that drive freezing and that they are selectively modified by prey capture experience. Specifically, naive mice prefer to approach relatively smaller and slower moving stimuli as compared to prey capture-experienced mice (figure 4e), but, both groups strongly preferred to approach stimuli located near their central visual field and moving nasally (posterior/temporal motion on the retina). By contrast, small, relatively faster-moving stimuli, mostly moving towards the periphery, reliably drove freezing in mice regardless of previous experience (figure 4a–f; electronic supplementary material, data S3). Thus, while it was possible that prey capture experience might reduce freezing responses to all stimuli in a non-selective manner, or, that smaller objects moving in the lower visual field would not induce freezing at all [27,28], freezing responses did not change significantly after prey capture experience and were consistently robust to small, fast-moving stimuli in the lower visual field (electronic supplementary material, data S3D–F, S4 and S5). Our data are most parsimonious with the idea that freezing in this context is enabling accurate perception of external motion [19,29] and augmenting the perception of objects [30] as opposed to reflecting a response to threat (figure 4g,h; electronic supplementary material, data S6). Indeed, freezing was specifically modulated by increasing stimulus motion (electronic supplementary material, data S1), preceded approximately 50% of approaches for naive mice (electronic supplementary material, videos S1 and S4) and mice often followed the trajectory of moving stimuli with saccadic head movements as stimuli moved towards their peripheral visual field (electronic supplementary material, videos S4, S6 and S7). However, future studies that apply dimensionality reduction methods to infer distinct stimulus-driven behavioural states will better address this issue [31–33].
We also note that mice keep linearly moving stimuli well-centred within their visual field during an approach regardless of the stimulus speed or experience (electronic supplementary material, data S3A–C and video S7). This observation indicates minimal motor lag and the ability to rapidly extrapolate motion information about moving targets innately [34]. Prey capture experience did not alter this basic behavioural response, yet, experienced mice did approach preferred stimuli more frequently and earlier within the trial period (figure 4; electronic supplementary material, data S3A–C). They also aligned the stimulus within the central visual field faster, i.e. displayed faster angular velocities as they centred stimuli in the visual field, from approach start (electronic supplementary material, data S3A). This observation indicates that a change in stimulus salience selectively modulates the activity of distinct circuitry dedicated to orienting towards moving targets and then keeping them centred within the visual field.
Our study suggests the probable behavioural relevance of several classes of previously identified direction-selective ganglion cell types (dsRGCs) in mice with size-selective responses and asymmetric retinal distribution [18,35–38]. A recent study precisely quantifying eye movement as coupled to head movement in mice during prey capture, showed that the position of the eyes, and therefore visual gaze, track the head position along the azimuth and in elevation [25]. This suggests that stimulus angle is a good approximation of the probable visual field position of the stimuli (figures 3 and 4; electronic supplementary material, data S3 and video S7, field of view 90 degrees). We show that distinct orienting responses are biased, although not exclusively controlled, by specific directions of motion along the nasal-temporal axis in the lower visual field. The role of genetically identifiable dsRGCs that preferentially encode temporal/posterior retinal motion, object motion towards the nose [39], or differ in their selectivity for motion along the nasal-temporal axis in the mouse retina [38] have been hypothesized to underlie salient object detection within ethological contexts and possibly underlie enhanced motion perception within the binocular visual field [18]. Here, we have provided the needed behavioural evidence to support tests of these remaining and intriguing hypotheses.
Our observation that prey capture experience altered the salience of visual features implicates specific downstream targets of retinal projections in mediating the behaviours we quantified. In the zebrafish, defined RGC types with specific stimulus selectivity have indeed been shown to underlie prey approach [40], while conditional, visual stimulus feature-driven decisions to approach or avoid and their conditional modulation are computed and encoded in the optic tectum [6,9], homologous to the superior colliculus. In the mouse, almost ninety per cent of RGCs project to the superior colliculus [41], which contains cells with response properties similar to those found in the retina [42–44] and is known to mediate visual attention [2,45] and orienting in ethological contexts in rodents [46–49]. In particular, the topographically mapped circuitry of the superficial superior colliculus that connects information from specific regions in the visual field to ipsilateral versus contralateral motor outputs that drive spatial orienting behaviour, is ideally organized to mediate the behaviours quantified here [50–52]. Although, from our findings, we hypothesize that even within the lower visual field or same regions of the visual field, distinct visual features may be directly coupled to either ipsilateral or contralateral motor outputs depending on direction of stimulus motion. Therefore, interesting near-future studies will seek to address whether circuit mechanisms similar to what has been observed in other species exist in the retino-collicular pathways of the mouse and whether they underlie the orienting response choices and their state-dependent modulation measured in this study [22,23,53].
4. Material and methods
We used C57BL/6 J, female and male mice between the ages of two to four months, 10 subjects were exposed to stationary stimuli that was varied in size and 23 subjects each comprised the naive and prey capture-experienced groups exposed to varying stimulus speed. Mice were group-housed, with regular access to water and food (Envigo, Teklad diet, 2919). The vivarium was maintained on a 12 h light/dark schedule, and all testing occurred within 3 h of the dark to light transition; 1–2 cm long crickets, Acheta domestica, from Fluker's Farm were used to give mice prey capture experience.
(a). Visual stimuli
Visual stimuli were generated with Matlab Psychophysics toolbox [54] and displayed on LCD monitors (60 Hz refresh rate, approx. 50 cd m−2 luminance) in a darkened room. The computer monitors replaced two sides of a rectangular behavioural arena that was 60 × 60 × 30 cm, length × width × height. We varied the major axis (horizontal) of the stationary stimulus from 0.25 to 8 cm and kept the aspect ratio between the major and minor axis at 2 : 1. We varied the speed of a 2 × 1 cm ellipse stimulus for separate cohorts of animals than were exposed to the stationary stimulus. Objective stimulus speed was varied over three steps between 2 cm s−1 to 50 cm s−1 and presented in a random order. Once stimuli traversed the screen, they reappeared from where they exited after 1 s and traversed in the opposite direction until the full 60 s trial was complete. Mice were exposed to relative speeds varying from 2 to approximately 300 deg s−1 at the retina. This is consistent with the range of speed selective responses that are encoded in the superior colliculus of mice [55–58].
(b). Behaviour
Prior to testing, mice were acclimated to handlers for 2 days, handled three times each day for 5 min each time. Mice were then acclimated to the arena for 4 days during which each mouse was placed in the arena three times a day for 5 min each. The day after this acclimation period, behavioural responses to a randomized presentation of either size-varying stationary stimuli or speed-varying motion stimuli of one size were recorded following a 3 min habituation session with no stimuli. For prey capture-experienced mice, each mouse was given a live cricket starting on their second day of habituation in the arena up to 4 days. All mice were returned to their home cages with standard food only.
(c). Data analysis
DeepLabCut [59] was used to digitize and extract coordinates of the mouse nose, ears, and tail base, as well as the centre point of the stimulus, throughout the duration of each trial. Tracked points with a ‘likelihood' value of less than 0.99 were rejected and dropped as inaccurate. The average percentage of dropped frames for the tracked points was 1.55, 0.05, 0.03, 1.89 and 1.51 per cent for the nose, ears, tail base and stimulus, respectively. Dropped frames were omitted from analysis and not interpolated. Tracks were entered into customized Matlab scripts to extract behavioural parameters [20,21] (electronic supplementary material, Methods). Freezes were identified as any time the nose and ear points moved less than 0.5 cm s−1 for a duration of 0.5–5 s. A successful approach was defined as any time the mouse's nose eventually came within 2 cm of the stimulus centre after moving towards the stimulus from a distance of at least 5 cm away with an average speed of at least 15 cm s−1 unless otherwise stated. Three human observers scored stimulus interception and pursuit (following a successful approach) behaviours.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Makayla Kesler-DeBarge and Rocio Olvera for scoring behaviour, running pose estimation routines and handling mice. We thank Drs Stephen Van Hooser, Cristopher Niell, Matt Smear, Angie Michaiel, Philip Parker and Thomas Kidd, for reading manuscript drafts. We are all grateful to our virtual reality core run by Dr Eelke Folmer.
Ethics
All experiments were conducted in accordance with protocols approved by the University of Nevada, Reno, Institutional Animal Care and Use Committee, in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Ten C57BL/6 J mice between the ages of two and four months were used for experiments in which a stationary stimulus was varied in size. Twenty-three C57BL/6 J mice between the ages of two and four months were used in each of two groups, naive and prey capture-experienced, for experiments in which stimulus speed was varied. Mice were group-housed, up to five animals per cage, with regular access to water and food (Envigo, Teklad diet, 2919) in an on-campus vivarium. The vivarium was maintained on a 12 h light/dark schedule, and all testing occurred within 3 h of the dark to light transition. The crickets used for prey capture were Acheta domestica, 1–2 cm in length, obtained from Fluker's Farm. They were group-housed in a cage and fed Fluker's Orange Cube Cricket Diet. Approval number: PHS assurance D16-00311, USDA Research Registration No. 88-R-0005, Animal protocol number: 2017-00716.
Data accessibility
Code, Raw video files and tracks generated from pose estimation data will be available via our laboratories website: http://www.hoylab.com/publications.html. Raw data are also available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.mw6m905v3 [60].
Authors' contributions
J.L.H. and N.M.P. designed research; J.L.H., N.M.P., R.I., K.M.A. and G.E.R. performed research; J.L.H., N.M.P. and G.E.R. analysed data; J.L.H., N.M.P. and K.M.A. edited the paper; J.L.H. and N.M.P. wrote the paper. H.L. rendered virtual reality videos of mouse view during behaviour.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by P20GM103650 (J.L.H. and the VR core) and partially supported by NSF grant for an REU site in Biomimetics and Soft Robotics (BioSoRo) with award number EEC grant no. 1852578NSF (J.L.H. and Dr Yantao Shen).
References
- 1.Dean P, Redgrave P, Westby GW. 1989. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 12, 137–147. ( 10.1016/0166-2236(89)90052-0) [DOI] [PubMed] [Google Scholar]
- 2.Knudsen EI. 2020. Evolution of neural processing for visual perception in vertebrates. J. Comp. Neurol. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingle DJ. 1971. Prey-catching behavior of anurans toward moving and stationary objects. Vision Res. 11(Suppl. 3), 447–456. ( 10.1016/0042-6989(71)90057-5) [DOI] [PubMed] [Google Scholar]
- 4.Ewert J-P. 1980. Neurobiological basis for the recognition and localization of environmental signals: how does a toad brain recognize prey and enemy? In Neuroethology, pp. 69–128. Berlin, Germany: Springer-Verlag. [Google Scholar]
- 5.Bianco IH, Kampff AR, Engert F. 2011. Prey capture behavior evoked by simple visual stimuli in larval zebrafish. Front. Syst. Neurosci. 5, 101 ( 10.3389/fnsys.2011.00101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker AJ, Baier H. 2015. Sensorimotor decision making in the zebrafish tectum. Curr. Biol. 25, 2804–2814. ( 10.1016/j.cub.2015.09.055) [DOI] [PubMed] [Google Scholar]
- 7.Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. ( 10.1139/z90-092) [DOI] [Google Scholar]
- 8.Burnett CJ, Li C, Webber E, Tsaousidou E, Xue SY, Brüning JC, Krashes MJ. 2016. Hunger-driven motivational state competition. Neuron 92, 187–201. ( 10.1016/j.neuron.2016.08.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filosa A, Barker AJ, Dal Maschio M, Baier H. 2016. Feeding state modulates behavioral choice and processing of prey stimuli in the zebrafish tectum. Neuron 90, 596–608. ( 10.1016/j.neuron.2016.03.014) [DOI] [PubMed] [Google Scholar]
- 10.Rothbart MK, Posner MI, Rosicky J. 1994. Orienting in normal and pathological development. Dev. Psychopathol. 6, 635–652. ( 10.1017/S0954579400004715) [DOI] [Google Scholar]
- 11.Corbetta M, Kincade MJ, Shulman GL. 2002. Two neural systems for visual orienting and the pathophysiology of unilateral spatial neglect. In The cognitive and neural bases of spatial neglect (eds Karnath H-O, Milner AD, Vallar G), pp. 258–273. New York, NY: Oxford University Press. [Google Scholar]
- 12.Mueller-Pfeiffer C, et al. 2013. Atypical visual processing in posttraumatic stress disorder. NeuroImage: Clin. 3, 531–538. ( 10.1016/j.nicl.2013.08.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Block SR, King AP, Sripada RK, Weissman DH, Welsh R, Liberzon I. 2017. Behavioral and neural correlates of disrupted orienting attention in posttraumatic stress disorder. Cogn. Affect. Behav. Neurosci. 17, 422–436. ( 10.3758/s13415-016-0488-2) [DOI] [PubMed] [Google Scholar]
- 14.Wood C, Maruff P, Levy F, Farrow M, Hay D. 1999. Covert orienting of visual spatial attention in attention deficit hyperactivity disorder: does comorbidity make a difference? Arch. Clin. Neuropsychol. 14, 179–189. [PubMed] [Google Scholar]
- 15.Yiend J, et al. 2015. Mechanisms of selective attention in generalized anxiety disorder. Clin. Psychol. Sci. 3, 758–771. ( 10.1177/2167702614545216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold JM, Randolph C, Coppola R, Carpenter CJ, Goldberg TE, Weinberger DR. 1992. Visual orienting in schizophrenia. Schizophr. Res. 7, 203–209. ( 10.1016/0920-9964(92)90013-U) [DOI] [PubMed] [Google Scholar]
- 17.Landry O, Parker A. 2013. A meta-analysis of visual orienting in autism. Front. Hum. Neurosci. 7, 833 ( 10.3389/fnhum.2013.00833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Danaf RN, Huberman AD. 2019. Sub-topographic maps for regionally enhanced analysis of visual space in the mouse retina. J. Comp. Neurol. 527, 259–269. ( 10.1002/cne.24457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roelofs K. 2017. Freeze for action: neurobiological mechanisms in animal and human freezing. Phil. Trans. R. Soc. B 372, 20160206 ( 10.1098/rstb.2016.0206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoy JL, Yavorska I, Wehr M, Niell CM. 2016. Vision drives accurate approach behavior during prey capture in laboratory mice. Curr. Biol. 26, 3046–3052. ( 10.1016/j.cub.2016.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoy JL, Bishop HI, Niell CM. 2019. Defined cell types in superior colliculus make distinct contributions to prey capture behavior in the mouse. Curr. Biol. 29, 4130–4138. ( 10.1016/j.cub.2019.10.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shang C, et al. 2019. A subcortical excitatory circuit for sensory-triggered predatory hunting in mice. Nat. Neurosci. 22, 909–920. ( 10.1038/s41593-019-0405-4) [DOI] [PubMed] [Google Scholar]
- 23.Zhao Z-D, et al. 2019. Zona incerta GABAergic neurons integrate prey-related sensory signals and induce an appetitive drive to promote hunting. Nat. Neurosci. 22, 921–932. ( 10.1038/s41593-019-0404-5) [DOI] [PubMed] [Google Scholar]
- 24.Meyer AF, O'Keefe J, Poort J.Curr. Biol. In press. Two distinct types of eye-head coupling in freely moving mice. [DOI] [PMC free article] [PubMed]
- 25.Michaiel AM, Abe ETT, Niell CM. In press. Dynamics of gaze control during prey capture in freely moving mice eLife. [DOI] [PMC free article] [PubMed]
- 26.Blanchard RJ, Flannelly KJ, Blanchard DC. 1986. Defensive behavior of laboratory and wild Rattus norvegicus. J. Comp. Psychol. 100, 101–107. ( 10.1037/0735-7036.100.2.101) [DOI] [PubMed] [Google Scholar]
- 27.Yilmaz M, Meister M. 2013. Rapid innate defensive responses of mice to looming visual stimuli. Curr. Biol. 23, 2011–2015. ( 10.1016/j.cub.2013.08.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Franceschi G, Vivattanasarn T, Saleem AB, Solomon SG.. 2016. Vision guides selection of freeze or flight defense strategies in mice. Curr. Biol. 26, 2150–2154. ( 10.1016/j.cub.2016.06.006) [DOI] [PubMed] [Google Scholar]
- 29.Roseberry T, Kreitzer A. 2017. Neural circuitry for behavioural arrest. Phil. Trans. R. Soc. B 372, 20160197 ( 10.1098/rstb.2016.0197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lojowska M, Gladwin TE, Hermans EJ, Roelofs K. 2015. Freezing promotes perception of coarse visual features. J. Exp. Psychol. Gen. 144, 1080–1088. ( 10.1037/xge0000117) [DOI] [PubMed] [Google Scholar]
- 31.Berman GJ, Bialek W, Shaevitz JW. 2016. Predictability and hierarchy in Drosophila behavior. Proc. Natl Acad. Sci. USA 113, 11 943–11 948. ( 10.1073/pnas.1607601113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Datta SR. 2019. Q&A: Understanding the composition of behavior. BMC Biol. 17, Article number: 44 ( 10.1186/s12915-019-0663-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Datta SR, Anderson DJ, Branson K, Perona P, Leifer A. 2019. Computational neuroethology: a call to action. Neuron 104, 11–24. ( 10.1016/j.neuron.2019.09.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borghuis BG, Leonardo A. 2015. The role of motion extrapolation in amphibian prey capture. J. Neurosci. 35, 15 430–15 441. ( 10.1523/JNEUROSCI.3189-15.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farrow K, Masland RH. 2011. Physiological clustering of visual channels in the mouse retina. J. Neurophysiol. 105, 1516–1530. ( 10.1152/jn.00331.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yonehara K, Roska B. 2013. Motion detection: neuronal circuit meets theory. Cell 154, 1188–1189. ( 10.1016/j.cell.2013.08.027) [DOI] [PubMed] [Google Scholar]
- 37.Jacoby J, Schwartz GW. 2017. Three small-receptive-field ganglion cells in the mouse retina are distinctly tuned to size, speed, and object motion. J. Neurosci. 37, 610–625. ( 10.1523/JNEUROSCI.2804-16.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bleckert A, Schwartz GW, Turner MH, Rieke F, Wong ROL. 2014. Visual space is represented by nonmatching topographies of distinct mouse retinal ganglion cell types. Curr. Biol. 24, 310–315. ( 10.1016/j.cub.2013.12.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. 2009. Genetic identification of an on-off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron 62, 327–334. ( 10.1016/j.neuron.2009.04.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semmelhack JL, Donovan JC, Thiele TR, Kuehn E, Laurell E, Baier H. 2014. A dedicated visual pathway for prey detection in larval zebrafish. Elife 3, e04878 ( 10.7554/eLife.04878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellis EM, Gauvain G, Sivyer B, Murphy GJ. 2016. Shared and distinct retinal input to the mouse superior colliculus and dorsal lateral geniculate nucleus. J. Neurophysiol. 116, 602–610. ( 10.1152/jn.00227.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reinhard K, Li C, Do Q, Burke EG, Heynderickx S, Farrow K. 2019. A projection specific logic to sampling visual inputs in mouse superior colliculus. Elife 8, e50697 ( 10.7554/eLife.50697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliveira AF, Yonehara K. 2018. The mouse superior colliculus as a model system for investigating cell type-based mechanisms of visual motor transformation. Front. Neural Cir. 12, 59 ( 10.3389/fncir.2018.00059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito S, Feldheim DA. 2018. The mouse superior colliculus: an emerging model for studying circuit formation and function. Front. Neural Cir. 12, 10 ( 10.3389/fncir.2018.00010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krauzlis RJ, Lovejoy LP, Zénon A. 2013. Superior colliculus and visual spatial attention. Annu. Rev. Neurosci. 36, 165–182. ( 10.1146/annurev-neuro-062012-170249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dean P, Redgrave P. 1984. The superior colliculus and visual neglect in rat and hamster. I. Behavioural evidence. Brain Res. 320, 129–141. ( 10.1016/0165-0173(84)90002-X) [DOI] [PubMed] [Google Scholar]
- 47.Dean P, Redgrave P. 1984. The superior colliculus and visual neglect in rat and hamster. II. Possible mechanisms. Brain Res. 320, 143–153. ( 10.1016/0165-0173(84)90003-1) [DOI] [PubMed] [Google Scholar]
- 48.Overton P, Dean P, Redgrave P. 1985. Detection of visual stimuli in far periphery by rats: possible role of superior colliculus. Exp. Brain Res. 59, 559–569. ( 10.1007/BF00261347) [DOI] [PubMed] [Google Scholar]
- 49.Westby GW, Keay KA, Redgrave P, Dean P, Bannister M. 1990. Output pathways from the rat superior colliculus mediating approach and avoidance have different sensory properties. Exp. Brain Res. 81, 626–638. ( 10.1007/BF02423513) [DOI] [PubMed] [Google Scholar]
- 50.Comoli E, Das Neves Favaro P, Vautrelle N, Leriche M, Overton PG, Redgrave P. 2012. Segregated anatomical input to sub-regions of the rodent superior colliculus associated with approach and defense. Front. Neuroanat. 6, 9 ( 10.3389/fnana.2012.00009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Redgrave P, Mitchell IJ, Dean P. 1987. Further evidence for segregated output channels from superior colliculus in rat: ipsilateral tecto-pontine and tecto-cuneiform projections have different cells of origin. Brain Res. 413, 170–174. ( 10.1016/0006-8993(87)90165-X) [DOI] [PubMed] [Google Scholar]
- 52.Redgrave P, Mitchell IJ, Dean P. 1987. Descending projections from the superior colliculus in rat: a study using orthograde transport of wheatgerm-agglutinin conjugated horseradish peroxidase. Exp. Brain Res. 68, 147–167. ( 10.1007/BF00255241) [DOI] [PubMed] [Google Scholar]
- 53.Procacci N, Hoy JL. 2019. Hungry for motion: the senses propel predation. Nat. Neurosci. 22, 843–845. ( 10.1038/s41593-019-0412-5) [DOI] [PubMed] [Google Scholar]
- 54.Brainard DH. 1997. The psychophysics toolbox. Spat. Vis. 10, 433–436. ( 10.1163/156856897X00357) [DOI] [PubMed] [Google Scholar]
- 55.Gale SD, Murphy GJ. 2018. Distinct cell types in the superficial superior colliculus project to the dorsal lateral geniculate and lateral posterior thalamic nuclei. J. Neurophysiol. 120, 1286–1292. ( 10.1152/jn.00248.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gale SD, Murphy GJ. 2014. Distinct representation and distribution of visual information by specific cell types in mouse superficial superior colliculus. J. Neurosci. 34, 13 458–13 471. ( 10.1523/JNEUROSCI.2768-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inayat S, Barchini J, Chen H, Feng L, Liu X, Cang J. 2015. Neurons in the most superficial lamina of the mouse superior colliculus are highly selective for stimulus direction. J. Neurosci. 35, 7992–8003. ( 10.1523/JNEUROSCI.0173-15.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L, Sarnaik R, Rangarajan K, Liu X, Cang J. 2010. Visual receptive field properties of neurons in the superficial superior colliculus of the mouse. J. Neurosci. 30, 16 573–16 584. ( 10.1523/JNEUROSCI.3305-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, Bethge M. 2018. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289. ( 10.1038/s41593-018-0209-y) [DOI] [PubMed] [Google Scholar]
- 60.Procacci NM, Allen KM, Robb GE, Ijekah R, Lynam H, Hoy JL. 2020. Data from: Context-dependent modulation of natural approach behaviour in mice. Dryad Digital Repository. ( 10.5061/dryad.mw6m905v3) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Procacci NM, Allen KM, Robb GE, Ijekah R, Lynam H, Hoy JL. 2020. Data from: Context-dependent modulation of natural approach behaviour in mice. Dryad Digital Repository. ( 10.5061/dryad.mw6m905v3) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Code, Raw video files and tracks generated from pose estimation data will be available via our laboratories website: http://www.hoylab.com/publications.html. Raw data are also available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.mw6m905v3 [60].