Abstract
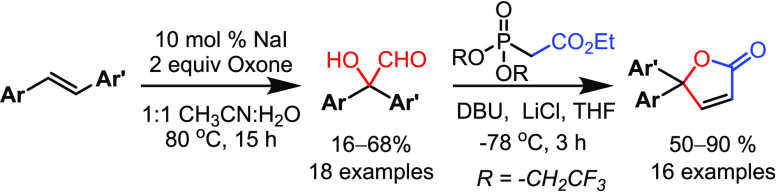
A one-pot oxone-mediated/iodine-catalyzed oxidative rearrangement of stilbenes leading to 2,2-diaryl-2-hydroxyacetaldehydes is described. Control experiments revealed that a 2,2-diarylacetaldehyde was initially formed that undergoes subsequent α-hydroxylation. The resulting α-hydroxyaldehydes have been subjected to a one-pot Still–Gennari olefination followed by cyclization, leading to 5,5-diaryl-γ-butenolides.
Introduction
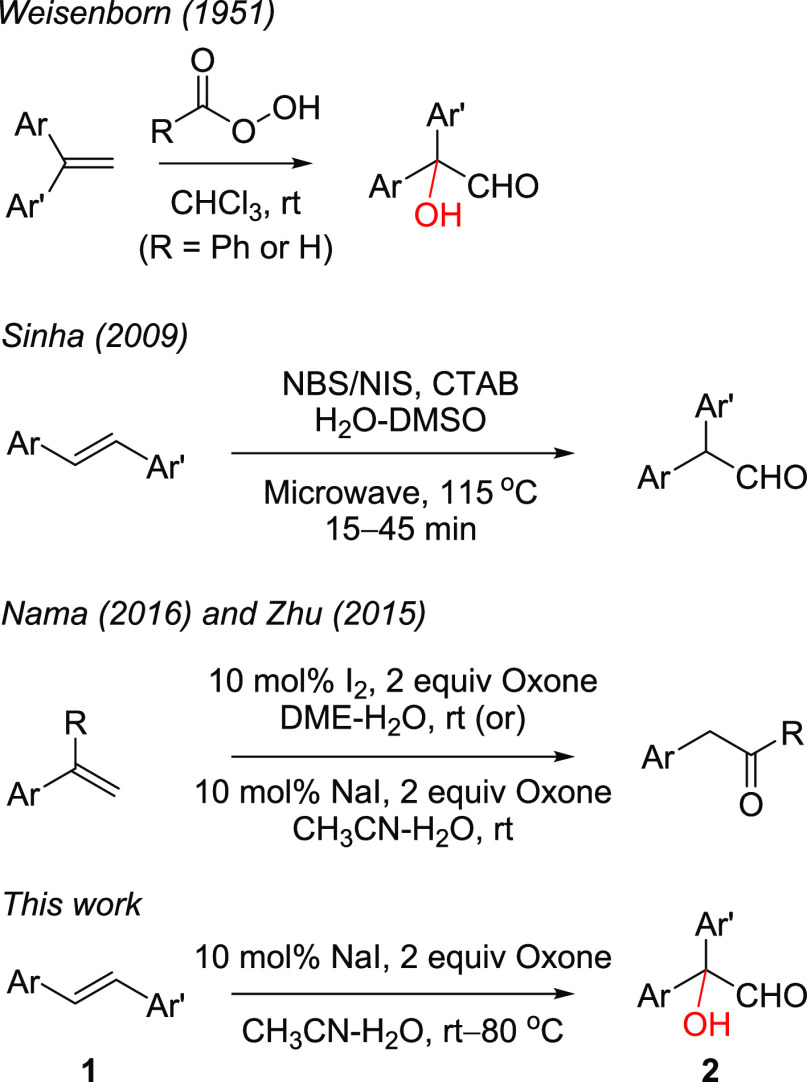
The oxidation/rearrangement/oligomerization of stilbenes is an important biochemical transformation that has inspired great innovation and the use of various oxidizing agents.1 The direct oxidation of stilbenes resulting in epoxides and/or vicinal diols is well established, and their acid/base-catalyzed rearrangements are well-studied reactions.2,3 There are occasions where the rearrangement of initially formed epoxides/diols has been noticed during the oxidation of stilbenes.4 In this context, the house rearrangement of stilbene oxides leading to diarylacetaldehyde is a well-studied reaction.2 In parallel, the Meinwald rearrangement of halohydrins or a one-pot halohydrin synthesis and its rearrangement have been established as an important alternative for the synthesis of diarylacetaldehyde without involving the epoxides.5 The diarylacetaldehydes are known to undergo decarbonylation, resulting in the corresponding benzophenones under oxidative and halogenation conditions.6 The possibility of combining all these events, i.e, olefin oxidation, pinacol rearrangement, and/or oxidative decarbonylation, is interesting, and this has been executed very recently for the direct synthesis of carbonyl compounds from the styrenes/stilbenes (Figure 1).7
Figure 1.
Selected oxidative rearrangement of stilbenes/aryl alkenes and the synthesis of α-hydroxy-2,2-diarylacetaldehyde 2.
In this manuscript, we document the direct conversion of stilbenes 1 to α-hydroxydiarylacetaldehydes 2. The possibility of obtaining α-hydroxydiarylacetaldehyde 2 from stilbenes was speculated considering a recent report from Fry and Merzel on the anodic oxidation of diphenylacetaldehyde leading to benzophenone, which indicated the occurrence of α-hydroxydiphenylacetaldehyde as an intermediate.8 Earlier, Weisenborn and Taub and Curtin and Bradley have documented the isolation of α-hydroxydiarylacetaldehydes during the epoxidation of the 1,1′-diaryethylenes.9 This option was employed with the idea of developing a general method for the synthesis of a 5,5-diaryl-γ-butenolide core,10 where the Still–Gennari olefination of α-hydroxydiarylacetaldehyde 2 and subsequent lactonization is a direct proposition.11
Results and Discussion
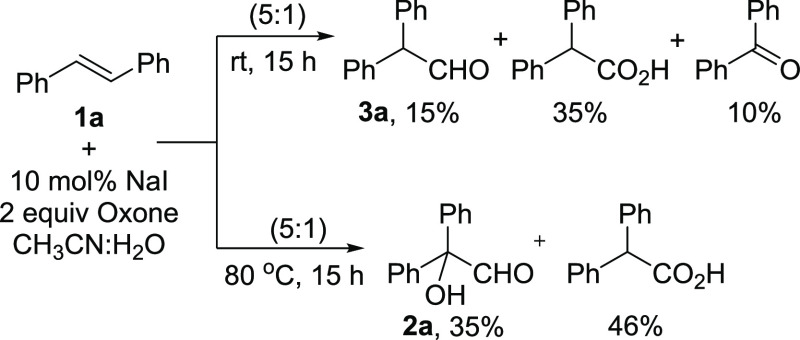
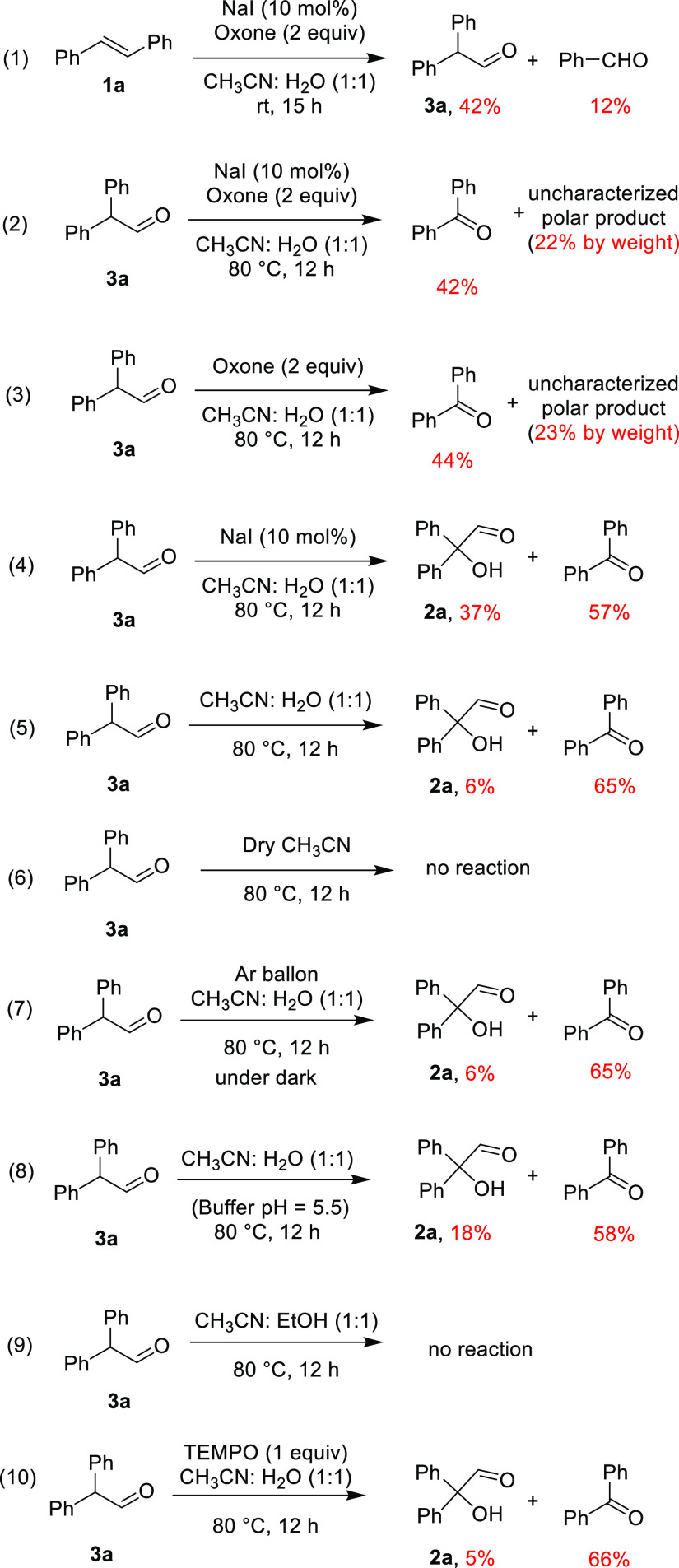
With this speculation, documented methods for the direct conversion of aryl alkenes to carbonyl compounds have been examined. Among these, the reports of Sinha et al.,5c Zhu and Zhao,5f and Nama et al.5g are interesting. Sinha and co-workers reported the conversion of stilbenes to diarylacetaldehydes employing NBS in the presence of a cationic surfactant under microwave irradiation.5c Nama et al. and Zhu and Zhao reported the iodine-catalyzed oxidative rearrangement of aryl alkenes to β-arylketones.5e−5g A close examination of these reports prompted the exploration of the NaI and oxone combination in our pursuit. Initial experiments employing simple stilbene as a substrate under Zhu and Zhao’s conditions (10 mol % NaI, 2 equiv of oxone in a 5:1 CH3CN/H2O mixture at rt) gave a mixture of diphenylacetaldehyde 3a (15%), diphenylacetic acid (35%), and benzophenone (10%) (Scheme 1). This prompted us to do parallel screening of reaction conditions by varying the reaction temperature and solvent ratio. Initial experiments with temperature variation revealed the formation of the requisite 2-hydroxy-2,2-diphenylacetaldehyde (2a). For example, when carried out at 80 °C with the same composition of the reaction contents, the required hydroxyacetaldehyde 2a was obtained in 35% yield along with 46% of diphenyl acetic acid. As shown in Table 1, changing the solvent composition also had some positive effect on the reaction outcome. When the reaction was conducted in a 1:1 mixture at rt, the formation of 3a in 42% yield along with the 12% benzaldehyde was observed.
Scheme 1. Selected Conditions for the Oxidative Rearrangement of Stilbene 1a.
Table 1. Optimizationa.
| entry | iodine source | solvent ratio | yieldb |
|---|---|---|---|
| 1 | NaI | 5:1 | 35c |
| 2 | NaI | 1:1 | 66 |
| 3 | I2 | 1:1 | 47 |
| 4 | KI | 1:1 | 48 |
| 5 | CuI | 1:1 | 42 |
| 6 | NIS | 1:1 | 19 |
| 7 | NH4I | 1:1 | 55 |
| 8 | NBS | 1:1 | 40d |
| 9 | NaIe | 1:1 | 23 |
| 10 | NaIf | 1:1 | 48 |
| 11 | I2 | dioxane/H2O | 46 |
| 12 | NaI | DMSO/H2O | NR |
| 13 | NaI | MeOH/H2O | 2 |
| 14 | NaI | DME/H2O | 5 |
| 15 | NaI | DMF/H2O | 30 |
| 16 | NaI | acetone/H2O | 1 + diketone |
| 17 | NaI + CTAB | 1:1 | 56 |
| 18 | NaI + TBAI | 1:1 | 48 |
| 19 | NaIg | 1:1 | 28 |
All reactions were carried out on an ∼0.5 mmol scale, 2 equiv of oxidant, and 0.1 equiv of iodine source; all reagent and substrate addition was done at room temperature (25 °C) and stirred for the next 15 h at 80 °C.
Isolated yields.
Diphenyl acetic acid as a major product.
Benzoin as a major product.
1 equiv of oxone.
2.5 equiv of oxone used.
Reaction was carried out under microwave irradiation at 100 °C.
The best results were obtained when the reaction was conducted at 80 °C in a 1:1 CH3CN/H2O mixture for 15 h. The starting stilbene was completely consumed, the required 2,2-diaryl-2-hydroxyacetaldehyde (66%) was obtained as the major product, and a mixture of benzaldehyde/benzophenone (10%) was also isolated. Under similar conditions when different iodine sources such as I2, KI, CuI, NIS, and NH4I were employed, the required 2,2-diaryl-2-hydroxyacetaldehyde was obtained in 19–55% yield along with benzaldehyde and/or benzophenone as the minor products (entries 3–7). Furthermore, when NBS was used as the halogen source, benzoin was obtained in 40% yield (entry 8). Decreasing or increasing the amount of oxone was not encouraging (entries 9 and 10). Further experiments were carried out to shift the reaction outcome completely toward the formation of the required product 2a by changing the solvent combination, using additives, and using microwave. The results are not satisfactory.
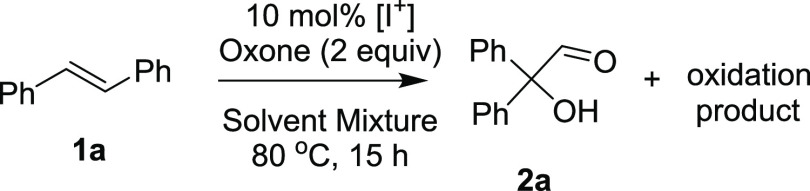
Having realized the possibility of synthesizing 2,2-diphenyl-2-hydroxyacetaldehyde directly from stilbene, next we looked at the course of the reaction. The next concern was the hydroxylation reaction and the role of the oxone or the byproducts resulting after the initial oxidation. In order to understand this, we conducted a set of control experiments employing freshly prepared diphenylacetaldehyde 3a with the exclusion of the reaction components one after the other. As shown in Scheme 2, when 3a was refluxed under the employed conditions in the presence of oxone and NaI in a mixture of 1:1 acetonitrile/water, benzophenone in 42% yield and an uncharacterized polar product in 22% yield were isolated. Next, a similar outcome was noticed when the reaction was carried out in the absence of NaI. Interestingly, when only oxone was replaced, the requisite hydroxyaldehyde 2a was isolated in 37% yield along with 57% benzophenone, indicating that NaI is playing a role in α-hydroxylation.
Scheme 2. Control Experiments.
Next, when diphenylacetaldehyde 3a was heated in a mixture of 1:1 acetonitrile/water in the absence of both oxone and NaI, the formation of hydroxyaldehyde 2a (6%) and benzophenone (65%) was noticed, and when water was also removed, the starting 3a was seen to be intact. These experiments revealed that the presence of water is essential. In addition, a separate experiment was carried out where the solution of 3a in a mixture of 1:1 acetonitrile/water was purged with argon for 15 min and heated under an argon atmosphere in the dark in a closed flask covered with silver foil. In this case too, complete conversion of the starting diphenylacetaldehyde was noticed and resulted in the isolation of 2a in 6% yield and benzophenone in 65% yield. Interestingly, when the reaction was carried out by adjusting the pH to 5.5 (measured for normal optimized conditions), the yield of hydroxyaldehyde was improved to 18%; however, benzophenone (58%) was the major product, indicating that the pH of the reaction medium plays a role in the stability of the intermediate hydroxyacetaldehyde. As a control, when we replaced water with ethanol as a proton source or in dry acetonitrile, there was no formation of 2a or benzophenone. Finally, to examine the possibility of a radical pathway, the reaction was carried out in the presence of TEMPO as a radical suppresser that resulted in the isolation 2a in 5% yield and benzophenone in 66% yield.
All these results indicate that the reaction is proceeding via oxidation of diphenylacetaldehyde to hydroxyaldehyde, that any external oxidant may not be required for α-hydroxylation,9,12 that the presence of water is important, and that it does not follow the radical pathway. This is in accordance with earlier reports where water was found to be the source of oxygen for oxidation of diphenylacetaldehyde to hydroxyaldehyde.8a The ready deformylation of the hydroxyaldehyde to the benzophenone under neutral conditions and the isolation of the hydroxyacetaldehyde in moderate yields when conducted in the presence of acidic buffer suggest that the deformylation is pH-sensitive. Also, though the role of NaI on the α-hydroxylation step is not clear, its presence appears to be important.
Coming to α-hydroxylation of 3a, as discussed previously, there are two proposals postulated. One is a radical pathway comprising of oxygen abstracting the α-hydrogen followed by recombination of the carbon centered and peroxide radicals, which would results in the α-hydroperoxido-diphenylacetaldehyde.7a The other one is an anodic oxidation pathway that involves the addition of water to a cationic radical generated after adding an electron to the enol of the diphenylacetaldehyde.8 The preliminary results in our case favor the second pathway; however, further studies are warranted to understand the course of oxidation and are currently in progress.
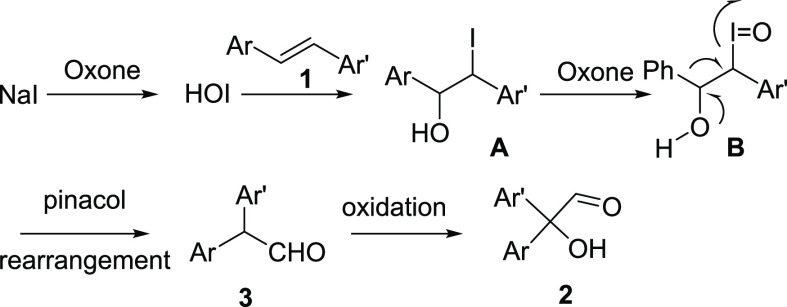
With this information in hand and with the help of earlier proposals on the iodine-mediated oxidative rearrangements,5 we propose the following tentative mechanism (Scheme 3). Initially, NaI is oxidized to HOI, which adds to the alkene in the presence of water to form the iodohydrin A. Further oxidation of iodine with oxone leads to the unstable hypervalent iodine intermediate B, which undergoes aryl ring migration to form the diphenylacetaldehyde 3, which subsequently undergoes oxidation, leading to the corresponding hydroxyaldehyde 2.
Scheme 3. Tentative Mechanism.
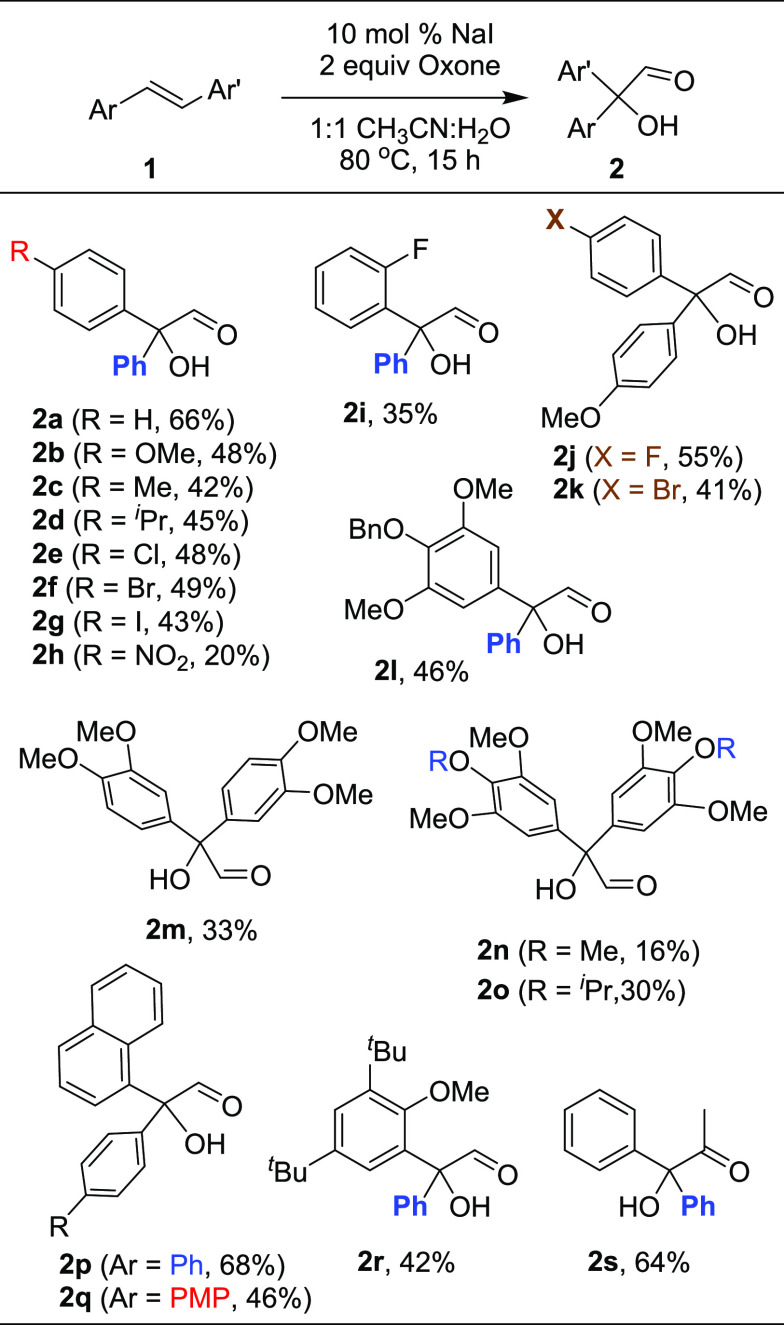
Next, the compatibility of various stilbenes under the current conditions has been examined. In general, substituted stilbenes having either electron-donating or electron-withdrawing groups on the benzene ring are found to be compatible (Scheme 4). However, the yields are moderate to good. In all cases, the corresponding cleaved aldehyde(s), in some cases, the corresponding acid13 and/or benzophenone, were obtained as minor products. Initially, stilbenes 1b–1h having substituents at the p-position (OMe, Me, iso-propyl, Cl, Br, I, and NO2, respectively) on one of the aryl rings have been employed. The reactions proceeded smoothly and provided the corresponding 2,2-diaryl-2-hydroxyacetaldehydes 2b–2h in moderate yields (20–49%). Further ortho-substituted stilbene 1i was employed for the same reaction condition and gave the corresponding ortho-substituted hydroxyacetaldehyde 2i (35%) yield. Similarly, the oxidative rearrangement of unsymmetrical stilbenes having different substituents on both aryl rings 1j and 1k gave the requisite hydroxyacetaldehydes 2j and 2k (41–55%) yield. Next, we examined a benzyloxy-protected stilbene 1l, which gave product 2l in 46% yield. However, an increase in the number of methoxy groups on the aryl ring of stilbenes 1m–1o resulted in the α-hydroxydiarylaldehydes 2m–2o in lower yields (16–33%). The scope of oxidation was further examined employing stilbenes 1p and 1q having a naphthalene ring on one side. Here too, the oxidative rearrangement was facile and provided the hydroxyaldehydes 2p and 2q in good yields. The stilbene 1r having a tert-butyl group on one side of the aryl ring was also compatible under these conditions and gave the corresponding oxidation product 2r (42%). Interestingly, the oxidative rearrangement of stilbene 1s having methyl substitution on alkene was also facile and here we observed that the phenyl group is more prone to such migration than the methyl group, providing the hydroxyketone 2s in 64% yield.
Scheme 4. Substrate Scope.
Having access to a good number of α-hydroxydiarylacetaldehydes, we proceeded further to synthesize γ,γ-diaryl-γ-butenolides employing the Still–Gennari Wittig olefination followed by cyclization (Scheme 5). Generally employed methods for their synthesis involve either adding the aryl Grignard reagents to lactones/esters or starting with the benzophenones. These methods require multiple steps.10 After screening a couple of conditions reported for cis-Wittig olefination,11 treatment of hydroxyaldehyde 2a with ethyl 2-[bis(2,2,2-trifluoroethoxy)phosphoryl]acetate in the presence of DBU and LiCl at −78 °C gave the required diaryl-γ-butenolide 4a in very good yields. Inspired with this initial success, all the earlier synthezised 2,2-diaryl-2-hydroxyacetaldehydes have been subjected for the cis-Wittig olefination followed by cyclization to obtain the corresponding lactones in moderate to good yields.
Scheme 5. Substrate Scope of γ,γ-Diaryl-γ-butenolide Synthesis.
It was notable that all the diarylhydroxyaldehydes with electron-donating groups as well as electron-withdrawing groups on the benzene ring gave similar yields. When the para-subtituted hydroxyaldehydes 2b–2h were subjected to this lactonization conditions, the corresponding lactones 4b–4h were obtained in good yields (50–78%). Further hydroxyaldehyde 2i having a substituent at the o-position has been employed for the same reaction condition and gave the corresponding o-substituted γ-butenolides 4i in 71%. Similarly, the unsymmetrically substituted hydroxyaldehydes 2j and 2k led to the synthesis of the lactones 4j and 4k in good yields (66% and 51%, respectively). The benzyl-protected hydroxyaldehyde 2l gave the lactone 4l in 65% yield. Furthermore, the highly substituted γ-butenolides (4m–4o) were successfully prepared in good yields (56–66%) using the corresponding hydroxyaldehydes 2m–2o. The olefination cum lactonization of the naphthalene-substituted hydroxyaldehydes 2p and 2q proceeded smoothly to provide the corresponding lactones 4p and 4q in excellent yields (76% and 89%, respectively).
Conclusions
In conclusion, an unprecedented NaI-catalyzed oxone-mediated oxidative rearrangement of stilbenes to 2,2-diaryl-2-hydroxyacetaldehydes has been documented. With the help of control experiments, a tentative mechanism postulating the possible oxygen transfer from the water to the initially formed diarylacetaldehyde for the final α-hydroxylation has been proposed. These diarylhydroxyacetaldehydes have been converted to 5,5-diaryl-γ-butenolides by employing a two-carbon cis-Wittig reaction and intramolecular lactonization. Currently, work in the direction of understanding the mechanism of α-hydroxylation is in progress.
Experimental Section
General Information
Commercial reagents were used without any purification. Column chromatography was carried out by using silica gels (60–120, 100–200, and 230–400 mesh). 1H and 13C NMR chemical shifts are reported relative to chloroform-d (δ = 7.25) or TMS, and coupling constants (J) are reported in hertz (Hz). The following abbreviations have been used to designate signal multiplicity: s = singlet, d = doublet, t = triplet, q = quartet, sxt = sextet, hept = septet, m = multiplet, and b = broad. High-resolution mass spectra (HRMS) were recorded on a Q Exactive Hybrid Quadrupole Orbitrap mass spectrometer, where the mass analyzer used for analysis is an Orbitrap. Melting points were recorded on a digital microscopy melting apparatus and uncorrected. All starting stilbenes were prepared according to well-known literature procedures.14
General Procedure for the Oxidation of Stilbenes 1
In general, all reactions were carried out employing 100 mg of stilbene 1.
At rt, a solution of stilbene 1 (100 mg, 0.55 mmol, 1 equiv) in a 1:1 CH3CN:H2O system (4 mL) was treated with sodium iodide (8.3 mg, 0.05 mmol, 10 mol %) and stirred for 5 min and then oxone (337 mg, 1.1 mmol, 2 equiv) was added slowly. The resulting suspension was heated at 80 °C for 15 h by which time the TLC showed the complete disappearance of the starting stilbene. The reaction mixture was concentrated under reduced pressure and partitioned between EtOAc (20 mL) and water (10 mL). The organic layer was separated, and the aqueous layer was extracted with EtOAc (2 × 20 mL). The combined organic layer was washed with Na2S2O8 (3 × 20 mL), brine (20 mL), dried (Na2SO4), and concentrated under reduced pressure. The resulting crude was purified by column chromatography to afford the hydroxyaldehyde 2 along with small amounts of side product aldehyde and sometimes ketone.
Representative Procedure for a 5 mmol Scale
At rt, to a solution of stilbene 1a (900 mg, 5 mmol) in 1:1 CH3CN:H2O (40 mL) was added sodium iodide (75 mg, 0.5 mmol) and then stirred for 5 min followed by addition of oxone (3072 mg, 10 mmol) slowly at 25 °C and the contents were heated at 80 °C for 15 h. Usual workup followed by purification by column chromatography afforded compound 2a (699 mg, 66% yield) as a white solid.
General Procedure for the Preparation of γ,γ-Disubstituted α,β-Unsaturated-γ-lactones 4
In general, all reactions were carried out employing 100 mg of hydroxyaldehyde 2.
To a suspension of anhydrous lithium chloride (2 equiv) in dry THF (5 mL) under a nitrogen atmosphere, ethyl [bis(2,2,2-trifluoroethoxy)phosphinyl]acetate (1.5 equiv) was added and stirred for 15 min at 25 °C. Then, the reaction mixture was cooled to 0 °C, treated with DBU (2 equiv), and stirred at the same temperature for an additional 30 min. Next, the reaction mixture was cooled to −78 °C and a solution of aldehyde 2 (1 equiv) in THF (5 mL) was added dropwise and continuously stirred for 3 h. Then, the reaction mixture was warmed to 0 °C, quenched by adding sat. NH4Cl, and concentrated on a rotary evaporator under reduced pressure. The crude was dissolved in ethyl acetate (20 mL), washed with H2O (10 mL) followed by brine, and concentrated. The crude was purified by column chromatography to procure the γ,γ-disubstituted α,β-unsaturated-γ-lactone product 4.
2-Hydroxy-2,2-diphenylacetaldehyde (2a)
Purified by column chromatography (5% EtOAc in petroleum ether, Rf = 0.5); yield: 78 mg (66%); colorless solid; mp: 55–57 °C (lit.15 52–55 °C); IR(neat) νmax 3477, 2925, 1723, 1491, 1448, 1336, 1176, 962, 794, 754, 699 cm–1; 1H NMR (400 MHz, CDCl3): δ 9.9 (s, 1H), 7.3 (s, 10H), 3.9 (br. s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 198.0 (d), 139.3 (s, 2C), 128.8 (d, 4C), 128.5 (d, 2C), 127.4 (d, 4C), 83.4 (s) ppm; HRMS (ESI): calcd. for C14H12O2Na: 235.0730 [M + Na]+; found 235.0729.
2-Hydroxy-2-(4-methoxyphenyl)-2-phenylacetaldehyde (2b)
Purified by column chromatography (5% EtOAc in petroleum ether, Rf = 0.5); yield: 55 mg (48%); colorless solid; mp: 72–74 °C (lit.9b 73–74 °C); IR(neat) νmax 3477, 2951, 1721, 1608, 1511, 1448, 1302, 1252, 1173, 102, 964, 831, 751, 701 cm–1; 1H NMR (400 MHz, CDCl3): δ 10.0 (s, 1H), 7.38–7.45 (m, 5H), 7.29 (d, J = 8.5 Hz, 2H), 6.95 (d, J = 9.2 Hz, 2H), 4.38 (s, 1H), 3.84 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 198.0 (d), 159.6 (s), 139.3 (s), 131.3 (s), 128.7 (d, 2C), 128.7 (d, 2C), 128.3 (d), 127.3 (d, 2C), 114.1 (d, 2C), 83.1 (s), 55.2 (q) ppm; HRMS (ESI) m/z: calcd. for C15H14O3Na: 265.0835 [M + Na]+; found: 265.0832.
2-Hydroxy-2-phenyl-2-(p-tolyl)acetaldehyde (2c)16
Purified by column chromatography (5% EtOAc in petroleum ether, Rf = 0.5); yield: 49 mg (42%); colorless syrup; IR(neat) νmax 3477, 2921, 1720, 1512, 1448, 1173, 1014, 815, 751, 699 cm–1; 1H NMR (400 MHz, CDCl3): δ 9.86 (s, 1H), 7.29 (s, 5H), 7.14 (d, J = 2.4 Hz, 4H), 4.29 (s, 1H), 2.27 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 198.2 (d), 139.5 (s), 138.4 (s), 136.5 (s), 129.6 (d, 2C), 128.8 (d, 2C), 128.5 (d), 127.5 (d, 2C), 127.4 (d, 2C), 83.4 (s), 21.2 (q) ppm; HRMS (ESI): calcd. for C15H14O2Na: 249.0886 [M + Na]+; found: 249.0883.
2-Hydroxy-2-(4-isopropylphenyl)-2-phenylacetaldehyde (2d)
Purified by column chromatography (5% EtOAc in petroleum ether, Rf = 0.5); yield: 51 mg (45%); colorless solid; mp: 50–52 °C; IR(neat) νmax 3482, 2951, 1720, 1509, 1450, 1338, 1175, 1014, 829, 793, 741, 699 cm–1; 1H NMR (400 MHz, CDCl3): δ 9.88 (s, 1H), 7.31 (s, 5H), 7.18 (s, 4H), 4.28 (s, 1H), 2.84 (quin, J = 6.9 Hz, 1H), 1.17 (d, J = 6.9 Hz, 6H) ppm; 13C NMR (100 MHz, CDCl3): δ 198.1 (s), 149.3 (s), 139.4 (s), 136.7 (s), 128.8 (d, 2C), 128.4 (d, 2C), 127.4 (d, 3C), 126.9 (d, 2C), 83.3 (s), 33.8 (d), 23.9 (q, 2C) ppm; HRMS (ESI): calcd. for C17H18O2Na+: 277.1199 [M + Na]+; found: 277.1196.
2-Chloro-2-(4-chlorophenyl)-2-phenylacetaldehyde (2e)17
Purified by column chromatography (5% EtOAc in petroleum ether, Rf = 0.5); yield: 55 mg (45%); yellow syrup; IR(neat) νmax 3466, 2922, 1720, 1490, 1400, 1092, 1011, 823, 823, 792, 758, 698 cm–1; 1H NMR (400 MHz, CDCl3): δ 9.96 (s, 1H), 7.28–7.55 (m, 9H), 4.41 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 197.5 (d), 139.0 (s), 137.7 (s), 134.6 (s), 129.0 (d, 2C), 128.9 (d, 2C), 128.8 (d), 128.7 (d, 2C), 127.3 (d, 2C), 83.0 (s) ppm; HRMS (ESI): calcd. for C14H10O2Cl:245.0373 [M – H]+; found: 245.0364.
2-(4-Bromophenyl)-2-hydroxy-2-phenylacetaldehyde (2f)
Purified by column chromatography (5% EtOAc in petroleum ether, Rf = 0.5); yield: 55 mg (49%); colorless syrup; IR(neat) νmax 3451, 2922, 1720, 1487, 1448,1395, 1153, 1072, 1006, 898, 817, 791, 756, 697, 653 cm–1; 1H NMR (500 MHz, CDCl3): δ 9.98 (s, 1H), 7.57 (d, J = 8.4 Hz, 2H), 7.40–7.46 (m, 3H), 7.35–7.37 (m, 2H), 7.30 (d, J = 8.4 Hz, 2H), 4.41 (s, 1H) ppm; 13C NMR (125 MHz, CDCl3): δ 197.5 (d), 138.9 (s), 138.3 (s), 132.0 (d, 2C), 129.1 (d, 2C), 129.0 (d), 128.7 (d, 2C), 127.3 (d, 2C), 122.8 (s), 83.0 (s) ppm; HRMS (ESI): calcd. for C14H10O2Br: 288.9871 [M – H]+; found: 288.9859.
2-Hydroxy-2-(4-iodophenyl)-2-phenylacetaldehyde (2g)
Purified by column chromatography (5% EtOAc in petroleum ether, Rf = 0.5); yield: 47 mg (43%); colorless syrup; IR(neat) νmax 3463, 2921, 1721, 1484, 1448, 1392, 1174, 1003, 817, 789, 755, 699 cm–1; 1H NMR (400 MHz, CDCl3): δ 9.95 (s, 1H), 7.75 (d, J = 7.93 Hz, 2H), 7.38–7.43 (m, 3H), 7.34 (d, J = 7.32 Hz, 2H), 7.14 (d, J = 8.55 Hz, 2H), 4.40 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 197.6 (d), 139.0 (s), 138.0 (d, 2C), 129.4 (d, 2C), 129.1 (d, 2C), 128.9 (d), 128.6 (s), 127.5 (d, 2C), 94.8 (s), 83.3 (s) ppm; HRMS (ESI): calcd. for C14H12O2I: 338.9876 [M + H]+; found: 338.9868.
2-Hydroxy-2-(4-nitrophenyl)-2-phenylacetaldehyde (2h)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 23 mg (20%); yellow syrup; IR(neat) νmax 3492, 2923, 1720, 1603, 1518, 1345, 1176, 852, 791, 751, 699 cm–1; 1H NMR (400 MHz, CDCl3): δ 10.01 (s, 1H), 8.26 (d, J = 8.39 Hz, 2H), 7.63 (d, J = 9.16 Hz, 2H), 7.35–7.50 (m, 3H), 7.30 (d, J = 6.87 Hz, 2H), 4.48 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 197.0 (d), 147.9 (s), 146.2 (s), 138.8 (s), 129.3 (d, 2C), 129.2 (d, 2C), 128.5 (d, 2C), 127.3 (d), 124.0 (d, 2C), 83.2 (s) ppm; HRMS (ESI): calcd. for C14H12O4N: 258.0761 [M + H]+; found: 258.0752.
2-(2-Fluorophenyl)-2-hydroxy-2-phenylacetaldehyde (2i)
Purified by column chromatography (5% EtOAc in petroleum ether, Rf = 0.5); yield: 41 mg (35%); colorless gum; IR(neat) νmax 3492, 2962, 1721, 1485, 1450, 1337, 1175, 962, 755, 699, 665 cm–1; 1H NMR (400 MHz, CDCl3): δ 10.06 (d, J = 5.49 Hz, 1H), 7.38–7.49 (m, 7H), 7.20–7.26 (m, 1H), 7.10–7.17 (m, 1H), 4.65 (br. s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 196.7 (dd, 4JC–F = 5.4 Hz), 159.8 (ds, 1JC–F = 246.6 Hz), 137.9 (s), 130.8 (dd, 3JC–F = 8.5 Hz), 129.9 (dd, 4JC–F = 3.9 Hz), 128.8 (d, 2C), 128.6 (d), 127.5 (ds, 2JC–F = 13.9 Hz), 126.9 (d, 2C), 124.8 (dd, 4JC–F = 3.1 Hz), 115.9 (dd, 2JC–F = 22.4 Hz), 82.1 (ds, 4JC–F = 3.1 Hz) ppm; HRMS (ESI): calcd. for C14H12O2F: 231.0816 [M + H]+; found: 231.0805.
2-(4-Fluorophenyl)-2-hydroxy-2-(4-methoxyphenyl)acetaldehyde (2j)
Purified by column chromatography (5% EtOAc in petroleum ether, Rf = 0.5); yield: 64 mg (55%); colorless syrup; IR(neat) νmax 3509, 2962, 1719, 1603, 1506, 1302, 1250, 1159, 1031, 828, 774.5 cm–1; 1H NMR (500 MHz, CDCl3): δ 9.91 (s, 1H), 7.38 (dd, J = 8.39, 5.34 Hz, 2H), 7.27 (d, J = 8.77 Hz, 2H), 7.11 (t, J = 8.58 Hz, 2H), 6.95 (d, J = 8.77 Hz, 2H), 4.43 (s, 1H), 3.83 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 197.6 (d), 162.6 (ds, 1JC–F = 248.0 Hz), 159.7 (s), 135.2 (s), 131.1 (s), 129.3 (dd, 2C, 3JC–F = 8.6 Hz), 128.6 (d, 2C), 115.6 (dd, 2C, 2JC–F = 22.0 Hz), 114.2 (d, 2C), 82.7 (s), 55.2 (q) ppm; HRMS (ESI): calcd. for C15H14O3: 261.0921 [M + H]+; found: 261.0914.
2-(4-Bromophenyl)-2-hydroxy-2-(4-methoxyphenyl)acetaldehyde (2k)
Purified by column chromatography (5% EtOAc in petroleum ether, Rf = 0.5); yield: 46 mg (41%); colorless syrup; IR(neat) νmax 3492, 2925, 1719, 1650, 1602, 1510, 1253, 1172, 1072, 1009, 828, 764 cm–1; 1H NMR (500 MHz, CDCl3): δ 9.81 (s, 1H), 7.46 (d, J = 8.59 Hz, 2H), 7.03–7.27 (m, 4H), 6.84 (d, J = 8.84 Hz, 2H), 4.27 (s, 1H), 3.74 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 197.4 (d), 159.9 (s), 138.4 (s), 131.9 (d, 2C), 131.0 (s), 129.1 (d, 2C), 128.73 (d, 2C), 122.8 (s), 114.4 (d, 2C), 82.8 (s), 55.4 (q) ppm; HRMS (ESI): calcd. for C15H14O3Br: 321.0121 [M + H]+; found: 321.0107.
2-(4-(Benzyloxy)-3,5-dimethoxyphenyl)-2-hydroxy-2-phenylacetaldehyde (2l)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 50 mg (46%); colorless solid; mp: 98–100 °C; IR(neat) νmax 3493, 2952, 1720, 1588, 1502, 1454, 1414, 1324, 1236, 1125, 981, 737, 699 cm–1; 1H NMR (400 MHz, CDCl3): δ 9.87 (s, 1H), 7.18–7.43 (m, 10H), 6.49 (s, 2H), 4.94 (s, 2H), 4.33 (s, 1H), 3.69 (s, 6H) ppm; 13C NMR (100 MHz, CDCl3): δ 197.6 (s), 153.8 (s, 2C), 139.2 (s), 137.7 (s), 137.2 (s), 134.5 (s) 128.9 (d, 2C), 128.6 (d), 128.4 (d, 2C), 128.2 (d, 2C), 127.9 (d), 127.5 (d, 2C), 104.8 (d, 2C), 83.4 (s), 75.0 (t), 56.2 (q, 2C) ppm; HRMS (ESI): calcd. for C23H22O5Na: 401.1359 [M + Na]+; found: 4011349.
2,2-Bis(3,4-dimethoxyphenyl)-2-hydroxyacetaldehyde (2m)
Purified by column chromatography (20% EtOAc in petroleum ether, Rf = 0.5); yield: 37 mg (33%); colorless solid; mp: 132–134 °C; IR(neat) νmax 3492, 2958, 1720, 1592, 1509, 1460, 1413, 1255, 1135, 1021, 864, 811, 759, 645 cm–1; 1H NMR (500 MHz, CDCl3): δ 9.89 (s, 1H), 6.92 (d, J = 1.3 Hz, 2H), 6.84–6.90 (m, 4H), 4.34 (br. s, 1H), 3.89 (s, 6H), 3.83 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 197.5 (d), 149.4 (s, 2C), 149.2 (s, 2C), 131.7 (s, 2C), 120.1 (d, 2C), 111.0 (d, 2C), 110.5 (d, 2C), 83.0 (s), 56.0 (q, 4C) ppm; HRMS (ESI): calcd. for C18H20O6Na: 335.1152 [M + Na]+; found: 335.1148.
2-Hydroxy-2,2-bis(3,4,5-trimethoxyphenyl)acetaldehyde (2n)
Purified by column chromatography (20% EtOAc in petroleum ether, Rf = 0.5); yield: 17 mg (16%); yellow syrup; IR(neat) νmax 3492, 2925, 1720, 1588, 1504, 1456, 1414, 1324, 1237, 1124, 1003 cm–1; 1H NMR (500 MHz, CDCl3): δ 9.90 (s, 1H), 6.59 (s, 4H), 4.39 (s, 1H), 3.87 (s, 6H), 3.83 (s, 12H) ppm; 13C NMR (125 MHz, CDCl3): δ 197.0 (d), 153.6 (s, 4C), 138.4 (s, 2C), 134.2 (s, 2C), 104.9 (d, 4C), 83.5 (s), 60.9 (s, 2C), 56.3 (s, 4C) ppm; HRMS (ESI): calcd. for C20H23O8: 391.1387 [M – H]+; found: 391.1384.
2-Hydroxy-2,2-bis(4-isopropoxy-3,5-dimethoxyphenyl)acetaldehyde (2o)
Purified by column chromatography (20% EtOAc in petroleum ether, Rf = 0.5); yield: 32 mg (30%); yellow syrup; IR(neat) νmax 3492, 2974, 1719, 1585, 1500, 1457, 1413, 1322, 1231, 1103, 929, 839, 733, cm–1; 1H NMR (400 MHz, CDCl3): δ 9.91 (s, 1H), 6.57 (s, 4H), 4.42 (s, 1H), 4.34–4.41 (hept, 2H), 3.78 (s, 12H), 1.31 (s, 12H) ppm; 13C NMR (100 MHz, CDCl3): δ 197.2 (d), 154.0 (s, 4C), 136.3 (s, 2C), 133.8 (s, 2C), 104.8 (d, 4C), 83.5 (s), 75.4 (d, 2C), 56.1 (q, 4C), 22.5 (q, 4C) ppm; HRMS (ESI): calcd. for C24H33O8: 449.2170 [M + H]+; found: 449.2166.
2-Hydroxy-2-(naphthalen-1-yl)-2-phenylacetaldehyde (2p)18
Purified by column chromatography (5% EtOAc in petroleum ether, Rf = 0.5); yield: 77 mg (68%); colorless solid; mp: 92–94 °C; IR(neat) νmax 3462, 2925, 1717, 1509, 1448, 1345, 1166, 1059, 947, 776, 745, 700 cm–1; 1H NMR (400 MHz, CDCl3): δ 10.20 (s, 1H), 7.78–8.04 (m, 3H), 7.14–7.65 (m, 9H), 4.70 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 198.5 (s), 139.3 (s), 136.6 (s), 135.2 (s), 131.0 (s), 130.0 (d), 129.1 (d, 2C), 128.9 (d), 128.5 (d), 127.0 (d, 2C), 126.7 (d), 126.2 (d), 125.9 (d), 125.4 (d), 124.6 (d), 84.1 (s) ppm; HRMS (ESI): calcd. for C18H14O2Na: 285.0886 [M + Na]+; found: 285.0881.
2-Hydroxy-2-(4-methoxyphenyl)-2-(naphthalen-1-yl)acetaldehyde (2q)
Purified by column chromatography (5% EtOAc in petroleum ether, Rf = 0.5); yield: 54 mg (46%); colorless solid; mp: 128–130 °C; IR(neat) νmax 3492, 2963, 1716, 1604, 1507, 1507, 1460, 1301, 1249, 1173, 1104, 1029, 951, 832, 802, 778, 647 cm–1; 1H NMR (400 MHz, CDCl3): δ 10.10 (d, J = 1.4 Hz, 1H), 7.94 (d, J = 8.7 Hz, 1H), 7.81–7.89 (m, 2H), 7.38–7.46 (m, 3H), 7.23–7.33 (m, 3H), 6.88 (d, J = 9.2 Hz, 2H), 4.60 (d, J = 1.4 Hz, 1H), 3.78 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 198.1 (s), 159.6 (s), 136.5 (s), 135.0 (s), 131.0 (s), 130.9 (s), 129.9 (d, 1C), 128.8 (d, 1C), 128.2 (d, 2C), 126.7 (d, 1C), 126.0 (d, 1C), 125.8 (d, 1C), 125.2 (d, 1C), 124.5 (d, 1C), 114.4 (d, 2C), 83.6 (s), 55.2 (s) ppm; HRMS (ESI): calcd. for C19H17O3: 293.1172 [M + H]+; found: 293.1159.
2-(3,5-Di-tert-butyl-2-methoxyphenyl)-2-hydroxy-2-phenylacetaldehyde (2r)
Purified by column chromatography (5% EtOAc in petroleum ether, Rf = 0.5); yield: 42 mg (42%); colorless solid; mp: 132–134 °C; IR(neat) νmax 3492, 2958, 1715, 1475, 1363, 1229, 1164, 1135, 998, 887, 799, 756, 700 cm–1; 1H NMR (400 MHz, CDCl3): δ 10.15 (s, 1H), 7.74–7.94 (m, 7H), 5.20 (s, 1H), 4.08 (s, 3H), 1.94 (s, 9H), 1.63 (s, 9H) ppm; 13C NMR (100 MHz, CDCl3): δ 193.7 (s), 155.2 (s), 146.7 (s), 141.9 (s), 139.4 (s), 135.7 (s), 128.3 (d, 2C), 127.9 (d, 1C), 127.2 (d, 2C), 127.0 (d, 1C), 125.9 (d, 1C), 82.6 (s), 63.4 (q, 1C), 35.6 (s), 34.5 (s), 31.7 (q, 3C), 31.2 (q, 3C) ppm; HRMS (ESI): calcd. for C23H31O3: 355.2268 [M + H]+; found: 355.2262.
1-Hydroxy-1,1-diphenylpropan-2-one (2s)
Purified by column chromatography (5% EtOAc in petroleum ether, Rf = 0.5); yield: 75 mg (64%); colorless solid; mp: 64–66 °C (lit.17 64–66 °C); IR(neat) νmax 3462, 2925, 1706, 1492, 1447, 1335, 1157, 1055, 754, 696 cm–1; cm–1; 1H NMR (400 MHz, CDCl3): δ 7.28 (s, 10H), 4.76 (s, 1H), 2.17 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 208.7 (s), 141.4 (s, 2C), 128.5 (d, 4C), 128.3 (d, 4C), 128.1 (d, 2C), 85.8 (s), 26.3 (q) ppm; HRMS (ESI): calcd. for C15H14O2Na: 249.0886 [M + Na]+; found: 249.0884.
5,5-Diphenylfuran-2(5H)-one (4a)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 69 mg (62%); colorless solid; mp: 134–136 °C (lit.10e 133–134 °C); IR(neat) νmax 2925, 1760, 1449, 1211, 1095, 985, 921, 818, 759, 697 cm–1; 1H NMR (400 MHz, CDCl3): δ 7.96 (d, J = 5.49 Hz, 1H), 7.30–7.43 (m, 10H), 6.20 (d, J = 5.49 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 172.1 (s), 158.8 (d), 139.2 (s, 2C), 128.7 (d, 4C), 128.6 (d, 2C), 126.6 (d, 4C), 119.7 (d), 92.2 (s) ppm; HRMS (ESI): calcd. for C16H13O2: 237.0910 [M + H]+; found: 237.0908.
5-(4-Methoxyphenyl)-5-phenylfuran-2(5H)-one (4b)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 79 mg (72%); colorless solid; mp: 118–120 °C (lit.19 118.6–119.6 °C); IR(neat) νmax 2951, 1762, 1609, 1512, 1254, 1179, 1092, 1032, 918, 832, 758, 699 cm–1; 1H NMR (400 MHz, CDCl3): δ 7.91 (d, J = 5.49 Hz, 1H), 7.30–7.40 (m, 5H), 7.22 (d, J = 9.16 Hz, 2H), 6.88 (d, J = 9.16 Hz, 2H), 6.18 (d, J = 5.49 Hz, 1H), 3.81 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 172.2 (s), 159.8 (s), 159.0 (d), 139.4 (s), 131.3 (s), 128.7 (d, 2C), 128.6 (d), 128.1 (d, 2C), 126.5 (d, 2C), 119.5 (d), 114.0 (d, 2C), 92.2 (s), 55.3 (q) ppm; HRMS (ESI): calcd. for C17H15O3: 267.1016 [M + H]+; found: 267.1012.
5-Phenyl-5-(p-tolyl)furan-2(5H)-one (4c)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 86 mg (78%); colorless solid; mp: 70–72 °C; IR(neat) νmax 2921, 1764, 1211, 1092, 919, 820, 757, 699 cm–1; 1H NMR (400 MHz, CDCl3): δ 7.93 (d, J = 5.49 Hz, 1H), 7.35 (d, J = 6.71 Hz, 5H), 7.16–7.22 (m, 4H), 6.18 (d, J = 5.49 Hz, 1H), 2.36 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 172.2 (s), 158.9 (d), 139.4 (s), 138.6 (s), 136.3 (s), 129.4 (d, 2C), 128.7 (d, 2C), 128.6 (d, 2C), 126.8 (d), 126.5 (d, 2C), 119.6 (d), 92.2 (s), 21.1 (s) ppm; HRMS (ESI): calcd. for C17H15O2: 251.1067 [M + H]+; found: 251.1064.
5-(4-Isopropylphenyl)-5-phenylfuran-2(5H)-one (4d)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 77 mg (70%); colorless solid; mp: 88–90 °C; IR(neat) νmax 2951, 1766, 1092, 919, 818, 699 cm–1; 1H NMR (400 MHz, CDCl3): δ 7.94 (d, J = 5.5 Hz, 1H), 7.33–7.38 (m, 5H), 7.23 (s, 4H), 6.19 (d, J = 5.5 Hz, 1H), 2.91 (hept, J = 6.9 Hz, 1H), 1.25 (d, J = 6.9 Hz, 6H) ppm; 13C NMR (100 MHz, CDCl3): δ 172.2 (s), 158.9 (d), 149.5 (s), 139.4 (s), 136.5 (s), 128.7 (d, 2C), 128.5 (d), 126.8 (d, 2C), 126.6 (d, 2C), 126.5 (d, 2C), 119.5 (d), 92.3 (s), 33.8 (d), 23.8 (q, 2C) ppm; HRMS (ESI): calcd. for C19H19O2: 279.1380 [M + H]+; found: 279.1378.
5-(4-Chlorophenyl)-5-phenylfuran-2(5H)-one (4e)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 55 mg (50%); colorless gum; IR(neat) νmax 2950, 1761, 1491, 1208, 1092, 1055, 985, 915, 815, 761, 697 cm–1; 1H NMR (400 MHz, CDCl3): δ 7.92 (d, J = 5.5 Hz, 1H), 7.26–7.40 (m, 9H), 6.23 (d, J = 5.5 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 171.7 (s), 158.3 (d), 138.8 (s), 137.8 (s), 134.8 (s), 128.9 (d, 2C), 128.9 (d), 128.9 (d, 2C), 128.0 (d, 2C), 126.5 (d, 2C), 120.0 (d), 91.6 (s) ppm; HRMS (ESI): calcd. for C16H12O2Cl: 271.0520 [M + H]+; found: 271.0520.
5-(4-Bromophenyl)-5-phenylfuran-2(5H)-one (4f)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 77 mg (71%); colorless solid; mp: 72–74 °C; IR(neat) νmax 2925, 1767, 1488, 1209, 1091, 918, 821, 760, 699 cm–1; 1H NMR (500 MHz, CDCl3): δ 7.91 (d, J = 5.3 Hz, 1H), 7.50 (d, J = 8.8 Hz, 2H), 7.34–7.40 (m, 3H), 7.29–7.32 (m, 2H), 7.20 (m, J = 8.8 Hz, 2H), 6.21 (d, J = 5.7 Hz, 1H) ppm; 13C NMR (125 MHz, CDCl3): δ 171.7 (s), 158.2 (d), 138.7 (s), 138.4 (s), 131.9 (d, 2C), 128.9 (d), 128.8 (d, 2C), 128.3 (d, 2C), 126.5 (d, 2C), 122.9 (s), 120.0 (d), 91.6 (s) ppm; HRMS (ESI): calcd. for C16H12O2Br: 315.0015 [M + H]+; found: 315.0009.
5-(4-Iodophenyl)-5-phenylfuran-2(5H)-one (4g)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 74 mg (69%); colorless solid; mp: 80–82 °C, IR(neat) νmax 2951, 1758, 1484, 1393, 1202, 1090, 985, 816, 759, 697 cm–1; 1H NMR (500 MHz, CDCl3): δ 7.91 (d, J = 5.3 Hz, 1H), 7.70 (d, J = 8.4 Hz, 2H), 7.35–7.39 (m, 3H), 7.28–7.31 (m, 2H), 7.07 (d, J = 8.4 Hz, 2H), 6.21 (d, J = 5.7 Hz, 1H) ppm; 13C NMR (125 MHz, CDCl3): δ 171.7 (s), 158.2 (d), 139.1 (s), 138.8 (s), 137.9 (d, 2C), 128.9 (d), 128.9 (d, 2C), 128.4 (d, 2C), 126.5 (d, 2C), 120.0 (d), 94.67 (s), 91.74 (s) ppm; HRMS (ESI): calcd. for C16H12O2I: 362.9876 [M + H]+; found: 362.9879.
5-(4-Nitrophenyl)-5-phenylfuran-2(5H)-one (4h)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 79 mg (72%); colorless gum; IR(neat) νmax 2920, 1759, 1519, 1347, 1207, 1091, 915, 851, 815, 760, 697 cm–1; 1H NMR (500 MHz, CDCl3): δ 8.24 (d, J = 9.2 Hz, 2H), 7.97 (d, J = 5.3 Hz, 1H), 7.53–7.57 (d, J = 8.9 Hz, 2H), 7.38–7.44 (m, 3H), 7.28–7.33 (m, 2H), 6.29 (d, J = 5.3 Hz, 1H) ppm; 13C NMR (125 MHz, CDCl3): δ 171.1 (s), 157.4 (d), 147.9 (s), 146.2 (s), 138.2 (s), 129.3 (d), 129.1 (d, 2C), 127.5 (d, 2C), 126.5 (d, 2C), 124.0 (d, 2C), 120.7 (d), 91.2 (s) ppm; HRMS (ESI): calcd. for C16H12O4N: 282.0761 [M + H]+; found: 282.0750.
5-(2-Fluorophenyl)-5-phenylfuran-2(5H)-one (4i)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 78 mg (71%); colorless solid; mp: 120–122 °C; IR(neat) νmax 2924, 1761, 1486, 1451, 1219, 1087, 984, 915, 820, 758, 696 cm–1; 1H NMR (400 MHz, CDCl3): δ 8.18 (dd, J = 5.6, 3.38 Hz, 1H), 7.64–7.71 (m, 1H), 7.33–7.46 (m, 4H), 7.15–7.33 (m, 3H), 7.08 (dd, J = 11.3, 8.3 Hz, 1H), 6.23 (d, J = 5.3 Hz, 1 H) ppm; 13C NMR (100 MHz, CDCl3): δ 171.7 (s), 159.3 (ds, 1JC-F = 247 Hz), 157.6 (dd, 3JC-F = 6.3 Hz), 138.3 (s), 130.7 (dd, 3JC-F = 8.5 Hz), 129.0 (d), 128.8 (d, 2C), 127.6 (dd, 4JC-F = 3.1 Hz), 126.8 (ds, 3JC-F = 12.3 Hz), 126.8 (d, 2C), 124.8 (dd, 4JC-F = 3.1 Hz), 120.1 (d), 116.2 (dd, 2JC-F = 22.4 Hz), 90.0 (s) ppm; HRMS (ESI): calcd. for C16H12O2F: 255.0816 [M + H]+; found: 255.0815.
5-(4-Fluorophenyl)-5-(4-methoxyphenyl)furan-2(5H)-one (4j)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 72 mg (66%); colorless solid; mp: 96–98 °C; IR(neat) νmax 2933, 1764, 1606, 1510, 1304, 1232, 1181, 1086, 1032, 964, 918, 832 cm–1; 1H NMR (400 MHz, CDCl3): δ 7.89 (d, J = 6.0 Hz, 1H), 7.30 (dd, J = 9.0, 5.26 Hz, 2H), 7.21 (m, J = 8.3 Hz, 2H), 7.04–7.09 (m, 2H), 6.90 (m, J = 9.0 Hz, 2 H), 6.20 (d, J = 5.3 Hz, 1H), 3.83 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 172.0 (s), 163.4 (ds, 1JC-F = 248.9 Hz), 159.9 (s), 158.7 (d), 135.3 (s), 131.0 (s), 128.5 (dd, 3JC-F = 8.5 Hz, 2C), 128.0 (d, 2C), 119.6 (d), 115.7 (dd, 2JC-F = 21.6, 2C), 114.1 (d, 2C), 91.64 (s), 55.33 (q) ppm; HRMS (ESI): calcd. for C17H14O3F: 285.0921 [M + H]+; found:285.0918.
5-(4-Bromophenyl)-5-(4-methoxyphenyl)furan-2(5H)-one (4k)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 55 mg (51%); colorless solid; mp: 90–92 °C; IR(neat) νmax 2930, 1762, 1607, 1511, 1305, 1253, 1179, 1085, 1031, 982, 919, 823 cm–1; 1H NMR (500 MHz, CDCl3): δ 7.86 (d, J = 5.7 Hz, 1H), 7.50 (d, J = 8.4 Hz, 2H), 7.14–7.21 (m, 4H), 6.88 (d, J = 9.2 Hz, 2H), 6.19 (d, J = 5.7 Hz, 1H), 3.81 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 171.9 (s), 159.9 (s), 158.4 (d), 138.5 (s), 131.8 (d, 2C), 130.7 (s), 128.2 (d, 2C), 128.1 (d, 2C), 122.8 (s), 119.8 (d), 114.1 (d, 2C), 91.6 (s), 55.3 (s) ppm; HRMS (ESI): calcd. for C17H14O3: 345.0121 [M + H]+; found: 345.0107.
5-(4-(Benzyloxy)-3,5-dimethoxyphenyl)-5-phenylfuran-2(5H)-one (4l)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 69 mg (65%); colorless solid; mp: 124–126 °C; IR(neat) νmax 2921, 1760, 1589, 1503, 1453, 1414, 1328, 1214, 1126, 970, 920, 835, 734, 699 cm–1; 1H NMR (500 MHz, CDCl3): δ 7.91 (d, J = 5.7 Hz, 1H), 7.48 (d, J = 7.3 Hz, 2H), 7.27–7.41 (m, 8H), 6.51 (s, 2H), 6.20 (d, J = 5.7 Hz, 1H), 5.01 (s, 2H), 3.77 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 173.0 (s), 158.7 (d), 153.7 (s, 2C), 139.1 (s), 137.7 (s), 137.3 (s), 134.7 (s), 128.8 (d), 128.7 (d, 2C), 128.4 (d, 2C), 128.2 (d, 2C), 127.9 (d), 126.6 (d, 2C), 119.7 (d), 104.3 (d, 2C), 90.2 (s), 75.0 (t), 56.3 (q, 2C) ppm; HRMS (ESI): calcd. for C25H23O5: 403.1540 [M + H]+; found: 403.1538.
5,5-Bis(3,4-dimethoxyphenyl)furan-2(5H)-one (4m)
Purified by column chromatography (30% EtOAc in petroleum ether, Rf = 0.5); yield: 60 mg (56%); yellow solid; mp: 113–115 °C; IR(neat) νmax 2931, 1762, 1515, 1463, 1414, 1263, 1143, 1025, 922, 840, 765 cm–1; 1H NMR (400 MHz, CDCl3): δ 7.88 (d, J = 5.5 Hz, 1H), 6.82 (s, 6H), 6.17 (d, J = 5.5 Hz, 1H), 3.88 (s, 6H), 3.82 (s, 6H) ppm; 13C NMR (100 MHz, CDCl3): δ 172.3 (s), 159.0 (d), 149.3 (s, 2C), 149.1 (s, 2C), 131.6 (s, 2C), 119.2 (d, 2C), 110.7 (d, 4C), 109.8 (d), 92.1 (s), 56.0 (q, 4C) ppm; HRMS (ESI): calcd. for C20H21O6: 357.1333 [M + H]+; found: 357.1328.
5,5-Bis(3,4,5-trimethoxyphenyl)furan-2(5H)-one (4n)
Purified by column chromatography (30% EtOAc in petroleum ether, Rf = 0.5); yield: 64 mg (60%); yellow solid; mp: 166–168 °C; IR(neat) νmax 2922, 1766, 1590, 1507, 1459, 1416, 1329, 1242, 1128, 1004, 830 cm–1; 1H NMR (400 MHz, CDCl3): δ 7.88 (d, J = 5.5 Hz, 1H), 6.51 (s, 4H), 6.20 (d, J = 5.5 Hz, 1H), 3.82 (s, 12H), 3.86 (s, 6H) ppm; 13C NMR (100 MHz, CDCl3): δ 172.0 (s), 158.6 (d, 2C), 153.3 (s, 4C), 138.4 (s, 2C), 134.4 (s), 119.6 (d), 104.1 (d, 4C), 92.1 (s), 60.9 (q, 3C), 56.3 (q, 3C) ppm; HRMS (ESI): calcd. for C22H25O8: 417.1544 [M + H]+; found: 417.1539.
5,5-Bis(4-isopropoxy-3,5-dimethoxyphenyl)furan-2(5H)-one (4o)
Purified by column chromatography (30% EtOAc in petroleum ether, Rf = 0.5); yield: 69 mg (66%); colorless solid; mp: 160–162 °C; IR(neat) νmax 2925, 1729, 1587, 1501, 1459, 1415, 1325, 1236, 1125, 926, 766 cm–1; 1H NMR (400 MHz, CDCl3): δ 7.88 (d, J = 6.1 Hz, 1H), 6.49 (s, 4H), 6.20 (d, J = 5.5 Hz, 1H), 4.37 (hept, J = 12.2, 6.10 Hz, 2H), 3.77 (s, 12H), 1.29 (d, J = 6.1 Hz, 12H) ppm; 13C NMR (100 MHz, CDCl3): δ 172.2 (s), 158.7 (d), 153.9 (s, 4C), 136.5 (s, 2C), 133.9 (s, 2C), 119.4 (d), 104.2 (d, 4C), 92.3 (s), 75.4 (d, 2C), 56.2 (q, 4C), 22.43 (q, 4C) ppm; HRMS (ESI): calcd. for C26H33O8: 473.2170 [M + H]+; found: 473.2166.
5-(Naphthalen-1-yl)-5-phenylfuran-2(5H)-one (4p)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 97 mg (89%); colorless solid; mp: 184–186 °C; IR(neat) νmax 2925, 1763, 1199, 1089, 969, 924, 778, 699 cm–1; 1H NMR (400 MHz, CDCl3): δ 8.15 (d, J = 5.5 Hz, 1H), 7.94 (d, J = 7.9 Hz, 1H), 7.88 (d, J = 8.5 Hz, 2H), 7.68 (d, J = 7.3 Hz, 1H), 7.49–7.55 (m, 1H), 7.44 (t, J = 7.6 Hz, 1H), 7.24–7.38 (m, 6H), 6.23 (d, J = 5.5 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 172.1 (s), 159.5 (d), 139.3 (s), 134.7 (s), 134.6 (s), 130.5 (d), 130.3 (s), 129.0 (d, 2C), 128.8 (d), 128.6 (d), 126.4 (d), 126.2 (d), 125.8 (d), 125.7 (d, 2C), 125.2 (d), 124.6 (d), 119.1 (d), 92.9 (s) ppm; HRMS (ESI): calcd. for C20H14O2Na: 309.0886 [M + H]+; found: 309.0873.
5-(4-Methoxyphenyl)-5-(naphthalen-1-yl)furan-2(5H)-one (4q)
Purified by column chromatography (10% EtOAc in petroleum ether, Rf = 0.5); yield: 82 mg (76%); colorless solid; mp: 100–102 °C; IR(neat) νmax 2921, 1763, 1607, 1511, 1253, 1178, 1087, 1032, 919, 832, 777 cm–1; 1H NMR (400 MHz, CDCl3): δ 8.14 (d, J = 6.0 Hz, 1H), 7.80–7.98 (m, 3H), 7.70 (d, J = 7.5 Hz, 1H), 7.40–7.54 (m, 2H), 7.25–7.37 (m, 1H), 7.16 (d, J = 8.3 Hz, 2H), 6.83 (d, J = 9.0 Hz, 2H), 6.21 (d, J = 5.3 Hz, 1H), 3.78 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 172.2 (s), 159.8 (s), 159.4 (d), 134.8 (s), 134.6 (s), 131.1 (s), 130.5 (s), 130.2 (d), 128.8 (d), 127.3 (d, 2C), 126.4 (d), 126.2 (d), 125.8 (d), 125.0 (d), 124.7 (d), 119.1 (d), 114.3 (d, 2C), 92.9 (s), 55.3 (q) ppm; HRMS (ESI): calcd. for C21H17O3: 317.1172 [M + H]+; found: 317.1169.
Acknowledgments
We thank CSIR, New Delhi, for funding and for the award of a Senior Research Fellowship to R.G.K.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03328.
1H and 13C NMR spectra of all new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Shen T.; Wang X. N.; Lou H. X. Natural stilbenes: an overview. Nat. Prod. Rep. 2009, 26, 916–935. 10.1039/b905960a. [DOI] [PubMed] [Google Scholar]; b Chong J.; Poutaraud A.; Hugueney P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. 10.1016/j.plantsci.2009.05.012. [DOI] [Google Scholar]; c Jørgensen K. B. Photochemical Oxidative Cyclisation of Stilbenes and Stilbenoids-The Mallory-Reaction. Molecules 2010, 15, 4334–4358. 10.3390/molecules15064334. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Keylor M. H.; Matsuura B. S.; Stephenson C. R. J. Chemistry and Biology of Resveratrol-Derived Natural Products. Chem. Rev. 2015, 115, 8976–9027. 10.1021/cr500689b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a House H. O. The Acid-catalyzed Rearrangement of the Stilbene Oxides. J. Am. Chem. Soc. 1955, 77, 3070–3075. 10.1021/ja01616a041. [DOI] [Google Scholar]; b Cope A. C.; Trumbull P. A.; Trumbull E. R. Base-catalyzed Rearrangement of Epoxides. J. Am. Chem. Soc. 1958, 80, 2844–2849. 10.1021/ja01544a063. [DOI] [Google Scholar]; c Meinwald J.; Labana S. S.; Chadha M. S. Peracid Reactions. III.1 The Oxidation of Bicyclo[2.2.1]heptadiene2. J. Am. Chem. Soc. 1963, 85, 582–585. 10.1021/ja00888a022. [DOI] [Google Scholar]; d Crandall J. K.; Apparu M. Base-promoted isomerizations of epoxides. Org. React. 2004, 29, 345. 10.1002/0471264180.or029.03. [DOI] [Google Scholar]; e Shi H.; Du C.; Zhang X.; Xie F.; Wang X.; Cui S.; Peng X.; Cheng M.; Lin B.; Liu Y. Lewis Acid Assisted Electrophilic Fluorine-Catalyzed Pinacol Rearrangement of Hydrobenzoin Substrates: One-Pot Synthesis of (±)-Latifine and (±)-Cherylline. J. Org. Chem. 2018, 83, 1312–1319. 10.1021/acs.joc.7b02587. [DOI] [PubMed] [Google Scholar]; f Robinson M. W. C.; Davies A. M.; Buckle R.; Mabbett I.; Taylor S. H.; Graham A. E. Epoxide ring-opening and Meinwald rearrangement reactions of epoxides catalyzed by mesoporous aluminosilicates†. Org. Biomol. Chem. 2009, 7, 2559–2564. 10.1039/B900719A. [DOI] [PubMed] [Google Scholar]; g Robinson M. W. C.; Pillinger K. S.; Mabbett I.; Timms D. A.; Graham A. E. Copper(II) tetrafluroborate-promoted Meinwald rearrangement reactions of epoxides. Tetrahedron 2010, 66, 8377–8382. 10.1016/j.tet.2010.08.078. [DOI] [Google Scholar]; h Roy S.; Banerjee R.; Nangia A.; Kruger G. J. Conformational, Concomitant Polymorphs of 4,4-Diphenyl-2,5-cyclohexadienone: Conformation and Lattice Energy Compensation in the Kinetic and Thermodynamic Forms. Chem. - Eur. J. 2006, 12, 3777–3788. 10.1002/chem.200501417. [DOI] [PubMed] [Google Scholar]; i Cavdar H.; Saracoglu N. Ring opening of epoxides with NaHSO4: isolation of β-hydroxy sulfate esters and an effective synthesis for trans-diols. Tetrahedron 2009, 65, 985–989. 10.1016/j.tet.2008.11.092. [DOI] [Google Scholar]
- a Zincke T. Mittheilungen aus dem chemischen Institut zu Marburg. Ber. Dtsch. Chem. Ges. 1876, 9, 1761–1775. 10.1002/cber.187600902219. [DOI] [Google Scholar]; b Alloum A. B.; Labiad B.; Villemin D. Application of microwave heating techniques for dry organic reactions. J. Chem. Soc., Chem. Commun. 1989, 7, 386–387. 10.1039/C39890000386. [DOI] [Google Scholar]
- a Srinivasan K.; Michaud P.; Kochi J. K. Epoxidation of olefins with cationic (salen)manganese(III) complexes. The modulation of catalytic activity substituents. J. Am. Chem. Soc. 1986, 108, 2309–2320. 10.1021/ja00269a029. [DOI] [PubMed] [Google Scholar]; b Hanquet G.; Lusinchi X.; Milliet P. Transfert d’oxygene sur la double liaison ethylenique a partir d’un sel d’oxaziridinium. Tetrahedron Lett. 1988, 29, 3941–3944. 10.1016/S0040-4039(00)80388-3. [DOI] [Google Scholar]; c Kim T.; Mirafzal G. A.; Liu J.; Bauld N. L. Is hole transfer involved in metalloporphyrin-catalyzed epoxidation?. J. Am. Chem. Soc. 1993, 115, 7653–7664. 10.1021/ja00070a009. [DOI] [Google Scholar]; b Mesbahi E.; Safari N.; Gheidi M. Investigation of axial ligand effects on catalytic activity of manganese porphyrin, evidence for the importance of hydrogen bonding in cytochrome-P450 model reactions. J. Porphyrins Phthalocyanines 2014, 18, 354–365. 10.1142/S1088424614500102. [DOI] [Google Scholar]
- a Kikuchi H.; Kogure K.; Toyoda M. A Facile Preparation of 2-Arylpropionaldehyde from 1-Aryl-1-propene. Chem. Lett. 1984, 341–344. 10.1246/cl.1984.341. [DOI] [Google Scholar]; b Andrews L. E.; Bonnett R.; Appelman E. H. Substitution and addition reactions of organic substrates with hypofluorous acid. Tetrahedron 1985, 41, 781–784. 10.1016/S0040-4020(01)96457-9. [DOI] [Google Scholar]; c Sharma A.; Sharma N.; Kumar R.; Sharma U. K.; Sinha A. K. Water-promoted cascade synthesis of α-arylaldehydes from arylalkenes using N-halosuccinimides: an avenue for asymmetric oxidation using Cinchona organocatalysis†. Chem. Commun. 2009, 35, 5299–5301. 10.1039/B908717F. [DOI] [PubMed] [Google Scholar]; d Wang Z.; Li M.; Zhang W.; Jia J.; Wang F.; Xue S. CF3CO2ZnEt-mediated highly regioselective rearrangement of bromohydrins to aldehydes. Tetrahedron Lett. 2011, 52, 5968–5971. 10.1016/j.tetlet.2011.08.134. [DOI] [Google Scholar]; e Swamy P.; Reddy M. M.; Naresh M.; Kumar M. A.; Srujana K.; Durgaiah C.; Narender N. Hypoiodite-Catalyzed Regioselective Oxidation of Alkenes: An Expeditious Access to Aldehydes in Aqueous Micellar Media. Adv. Synth. Catal. 2015, 357, 1125–1130. 10.1002/adsc.201400986. [DOI] [Google Scholar]; f Zhu M.; Zhao Y. A convenient catalytic oxidative 1,2-shift of arylalkenes for preparation of α-aryl ketones mediated by NaI. Chin. Chem. Lett. 2015, 26, 248–250. 10.1016/j.cclet.2014.11.006. [DOI] [Google Scholar]; g Kodumuri S.; Peraka S.; Mameda N.; Chevella D.; Banothu R.; Nama N. Metal-free, catalytic regioselective oxidative conversion of vinylarenes: a mild approach to phenylacetic acid derivatives. RSC Adv. 2016, 6, 6719–6723. 10.1039/C5RA25296B. [DOI] [Google Scholar]; h Yi W.; Wang P.-F.; Lu M.; Liu Q.-Q.; Bai X.; Chen K.-D.; Zhang J.-W.; Liu G.-Q. Environmentally Friendly Protocol for the Oxidative Iodofunctionalization of Olefins in a Green Solvent. ACS Sustainable Chem. Eng. 2019, 7, 16777–16785. 10.1021/acssuschemeng.9b04298. [DOI] [Google Scholar]
- a Danilov S.; Venus-Danilova E. Isomerization of disubstituted aldehydes to ketones. Ber. Dtsch. Chem. Ges. (A B Ser.) 1926, 59, 1032–1043. [Google Scholar]; b Frimer A. A.; Gilinsky-Sharon P.; Aljadeff G.; Gottlieb H. E.; Hameiri-Buch J.; Marks V.; Philosof R.; Rosental Z. Superoxide anion radical (O2.bul.-)-mediated base-catalyzed autoxidation of enones. J. Org. Chem. 1989, 54, 4853–4866. 10.1021/jo00281a030. [DOI] [Google Scholar]; d Welch J. T.; Seper K. W. Peroxouranium oxide promoted oxidation of olefins with alkyl hydroperoxides. Synth. Commun. 1984, 14, 933–937. 10.1080/00397918408063763. [DOI] [Google Scholar]; e Althaus M.; Togni A.; Mezzetti A. Asymmetric oxidative α-fluorination of 2-alkylphenylacetaldehydes with AgHF2 and ruthenium/PNNP catalysts. J. Fluorine Chem. 2009, 130, 702–707. 10.1016/j.jfluchem.2009.05.008. [DOI] [Google Scholar]; f Havare N.; Plattner D. A. Oxidative cleavage of α-aryl aldehydes using iodosylbenzene. Org. Lett. 2012, 14, 5078–5081. 10.1021/ol301675v. [DOI] [PubMed] [Google Scholar]; g Hu G.; Ramakumar K.; Brenner-Moyer S. E. Metal- and O2-Free Oxidative C–C Bond Cleavage of Aromatic Aldehydes. J. Org. Chem. 2017, 82, 6972–6977. 10.1021/acs.joc.7b00784. [DOI] [PubMed] [Google Scholar]; h Shipilovskikh S. A.; Rubtsov A. E.; Malkov A. V. Oxidative Dehomologation of Aldehydes with Oxygen as a Terminal Oxidant. Org. Lett. 2017, 19, 6760–6762. 10.1021/acs.orglett.7b03512. [DOI] [PubMed] [Google Scholar]; i Tian X.; Ren Y.-L.; Cheng X.; Lu W. Aerobic Oxidative C(CO)–C Bond Cleavage under Catalyst-Free and Additive-Free Conditions. ChemistrySelect 2019, 4, 11496–11499. 10.1002/slct.201903197. [DOI] [Google Scholar]
- a Pettit G. R.; Lippert J. W.; Herald D. L. A Pinacol Rearrangement/Oxidation Synthetic Route to Hydroxyphenstatin. J. Org. Chem. 2000, 65, 7438–7444. 10.1021/jo000705j. [DOI] [PubMed] [Google Scholar]; b Sharma N.; Sharma A.; Kumar R.; Shard A.; Sinha A. K. One-Pot Two-Step Oxidative Cleavage of 1,2-Arylalkenes to Aryl Ketones Instead of Arylaldehydes in an Aqueous Medium: A Complementary Approach to Ozonolysis. Eur. J. Org. Chem. 2010, 6025–6032. 10.1002/ejoc.201000672. [DOI] [Google Scholar]; c Zeng X.; Xu D.; Miao C.; Xia C.; Sun W. Tetraethylammonium iodide catalyzed synthesis of diaryl ketones via the merger of cleavage of C–C double bonds and recombination of aromatic groups. RSC Adv. 2014, 4, 46494–46497. 10.1039/C4RA08764J. [DOI] [Google Scholar]; d Fraile J. M.; García N.; Mayoral J. A.; Santomauro F. G.; Guidotti M. Multifunctional Catalysis Promoted by Solvent Effects: Ti-MCM41 for a One-Pot, Four-Step, Epoxidation-Rearrangement-Oxidation-Decarboxylation Reaction Sequence on Stilbenes and Styrenes. ACS Catal. 2015, 5, 3552–3561. 10.1021/cs501671a. [DOI] [Google Scholar]
- a Merzel R. L.; Fry A. J. Competing Reaction Pathways in the Anodic Oxidation of Diphenylacetaldehyde: Differentiation by 18O Isotopic Labeling. J. Electrochem. Soc. 2012, 159, G117–G122. 10.1149/2.039210jes. [DOI] [Google Scholar]; b Ogibin Y. N.; Ilovaisky A. I.; Nikishin G. I. Rearrangement of trans-stilbene into diphenylacetaldehyde acetals induced by direct anodic oxidation. Russ. Chem. Bull. 1997, 46, 2089–2092. 10.1007/BF02495257. [DOI] [Google Scholar]
- Possible α-hydroxylation of intermediate diaryl acetaldehydes with peroxides has been postulated earlier by:; a Weisenborn F. L.; Taub D. The Reaction of Perbenzoic Acid with Certain Olefins. J. Am. Chem. Soc. 1952, 74, 1329–1330. 10.1021/ja01125a057. [DOI] [Google Scholar]; b Curtin D. Y.; Bradley A. Anomalous Oxidations of 1-p-Anisyl-1-phenylethylene with Performic and Perbenzoic Acid. J. Am. Chem. Soc. 1954, 76, 5777–5779. 10.1021/ja01651a046. [DOI] [Google Scholar]
- Some selected methods for synthesis of 5,5-diary-γ-butenolide core see:; a Grandguillot J. C.; Rouessac F. A new, convenient synthesis of 5,5-dialkyl-2(5H)-furanones. Synthesis 1979, 607–609. 10.1055/s-1979-28778. [DOI] [Google Scholar]; b Lehmann J.; Gossen A. Lactone, 14. Mitt.†: Synthese lactonverbrückter 1,1–Diarylethylamine. Arch. Pharm. 1987, 320, 1059–1064. 10.1002/ardp.198700009. [DOI] [Google Scholar]; c Kothiyal D. P.; Chamoli R. P. Synthesis of some new butenolactones. Ind. J. Chem., Sec. B. 1990, 21, 166–167. [Google Scholar]; d Kindo T.; Kodoi K.; Mitsudo T.-A.; Watanabe Y. A new route to 2(5H)-furanones via ruthenium-catalysed oxidative cyclocarbonylation of allylic alcohols. J. Chem. Soc., Chem. Commun. 1994, 755–756. 10.1039/c39940000755. [DOI] [Google Scholar]; e Tnay Y. L.; Chiba S. Copper-Catalyzed Aerobic C-C Bond Cleavage of Lactols with N-Hydroxy Phthalimide for Synthesis of Lactones. Chem. – Asian J. 2015, 10, 873–877. 10.1002/asia.201403196. [DOI] [PubMed] [Google Scholar]
- a Franci X.; Martina S. L. X.; McGrady J. E.; Webb M. R.; Donald C.; Taylor R. J. K. A comparison of the Still–Gennari and Ando HWE-methodologies with α,β-unsaturated aldehydes; unexpected results with stannyl substituted systems. Tetrahedron Lett. 2003, 44, 7735–7740. 10.1016/j.tetlet.2003.08.095. [DOI] [Google Scholar]; b Devalankar D. A.; Chouthaiwale P. V.; Sudalai A. Organocatalytic sequential α-aminoxylation and cis-Wittig olefination of aldehydes: synthesis of enantiopure γ-butenolides. Tetrahedron: Asymmetry 2012, 23, 240–244. 10.1016/j.tetasy.2012.02.004. [DOI] [Google Scholar]
- Liang Y.-F.; Jiao N. Highly Efficient C-H Hydroxylation of Carbonyl Compounds with Oxygen under Mild Conditions. Angew. Chem., Int. Ed. 2014, 53, 548–552. 10.1002/anie.201308698. [DOI] [PubMed] [Google Scholar]
- Parida K. N.; Moorthy J. N. Oxidation cascade with oxone: cleavage of olefins to carboxylic acids. Tetrahedron 2014, 70, 2280–2285. 10.1016/j.tet.2014.01.042. [DOI] [Google Scholar]
- a Sarabia F. J.; Ferreira E. M. Radical Cation Cyclopropanations via Chromium Photooxidative Catalysis. Org. Lett. 2017, 19, 2865–2868. 10.1021/acs.orglett.7b01095. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Baker R.; Sims R. J. A convenient modification of the wadsworth-Emmons method of olefin formation using a crown ether. Synthesis 1981, 2, 117. [Google Scholar]; c Shen X.; Liu P.; Liu Y.; Dai B. Synthesis of 1,2-Diarylethylenes by Pd-Catalyzed One-Pot Reaction of Benzyl Halides, Tosylhydrazide, and Aryl Aldehydes. Lett. Org. Chem. 2018, 15, 709–715. 10.2174/1570178615666171222163927. [DOI] [Google Scholar]; d Wen X.-M.; Tao L.; Xiang Y.-Z.; Fang Y.-G.; Yu X.-Q. A facile synthesis of trans-alkenes in micellar media. ARKIVOC 2005, 2005, 169–174. 10.3998/ark.5550190.0006.d15. [DOI] [Google Scholar]; e Roy X.; Schenck C. L.; Ahn S.; Lalancette R. A.; Venkataraman L.; Nuckolls C.; Steigerwald M. L. Quantum Soldering of Individual Quantum Dots. Angew. Chem., Int. Ed. 2012, 51, 12473–12476. 10.1002/anie.201206301. [DOI] [PubMed] [Google Scholar]; f Zhang J.; Peng Y.; Leng W.; Gao Y.; Xu F.; Chai J. Nitrogen ligands in two-dimensional covalent organic frameworks for metal catalysis. Chin. J. Catal. 2016, 37, 468–475. 10.1016/S1872-2067(15)61050-6. [DOI] [Google Scholar]; g Rosocha G.; Batey R. A. Synthesis of 2-bromo-1-aryl-1H-indenes via a Ag(I) promoted domino 2π-electrocyclic ring-opening/4π-electrocyclization reaction of 1,2-diaryl substituted gem-dibromocyclopropanes. Tetrahedron 2013, 69, 8758–8768. 10.1016/j.tet.2013.07.086. [DOI] [Google Scholar]; h Heynekamp J. J.; Weber W. M.; Hunsaker L. A.; Gonzales A. M.; Orlando R. A.; Deck L. M.; Vander Jagt D. L. Substituted trans-Stilbenes, Including Analogues of the Natural Product Resveratrol, Inhibit the Human Tumor Necrosis Factor Alpha-Induced Activation of Transcription Factor Nuclear Factor Kappa B. J. Med. Chem. 2006, 49, 7182–7189. 10.1021/jm060630x. [DOI] [PubMed] [Google Scholar]; i Yamashita M.; Hirano K.; Satoh T.; Miura M. Synthesis of Substituted Stilbenes via Direct Decarboxylative Coupling of Cinnamic Acids with Arylboronic Acids under Palladium Catalysis. Chem. Lett. 2010, 39, 68–69. 10.1246/cl.2010.68. [DOI] [PubMed] [Google Scholar]; j Azzena U.; Dettori G.; Idini M. V.; Pisano L.; Sechi G. Regioselective reductive demethoxylation of 3,4,5-trimethoxystilbenes. Tetrahedron 2003, 59, 7961–7966. 10.1016/j.tet.2003.08.009. [DOI] [Google Scholar]; k Niwa T.; Nakada M. A Non-Heme Iron(III) Complex with Porphyrin-like Properties That Catalyzes Asymmetric Epoxidation. J. Am. Chem. Soc. 2012, 134, 13538–13541. 10.1021/ja304219s. [DOI] [PubMed] [Google Scholar]; l Nishinaga A.; Iwasaki H.; Shimizu T.; Toyoda Y.; Matsuura T. Oxygenation of tert-Butylphenols with an Unsaturated Side Chain. J. Org. Chem. 1986, 51, 2257–2266. 10.1021/jo00362a018. [DOI] [Google Scholar]
- Zhang X.; Staples R. J.; Rheingoldb A. L.; Wulff W. D. Catalytic Asymmetric α-Iminol Rearrangement: New Chiral Platforms. J. Am. Chem. Soc. 2014, 136, 13971–13974. 10.1021/ja5065685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaiyama T.; Sakito Y.; Asami M.. Optically active or racemic aminal derivatives, process for preparing same and for converting same to alpha-hydroxyaldehydes. European Patent Office, EP0011417B1, 1983.
- Schlegel M.; Schneider C. Lewis acid-catalyzed Friedel–Crafts reactions toward highly versatile, α-quaternary oxime ethers. Chem. Commun. 2018, 54, 11124–11127. 10.1039/C8CC06823B. [DOI] [PubMed] [Google Scholar]
- Hou Z.; Wakatsuki Y. Product Class 12: Organometallic Complexes of Scandium, Yttrium, and the Lanthanides. Sci. Synth. 2003, 2, 849–942. [Google Scholar]
- Fujimoto N.; Nishino H.; Kurosawa K. Reaction of Olefins with Malonic Acid Derivatives in the Presence of Manganese(III) Acetate. Bull. Chem. Soc. Jpn. 1986, 59, 3161–3168. 10.1246/bcsj.59.3161. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.