Abstract
The spatial and temporal distribution of food resources can profoundly affect foraging decisions and prey selection, potentially resulting in shifts in diet in response to changes in resource availability. The masked palm civet (Paguma larvata) has long been regarded as a dietary generalist that feeds primarily on fruits and small mammals. Both types of food resources may vary spatially and temporally and the diet of P. larvata is expected to change in response to variation in the availability and distribution of these resources. To address the effects of such variation on foraging by masked palm civets, we studied a population of P. larvata inhabiting a highly heterogeneous habitat in central China consisting of primary forest, selectively logged forest, logged forest, broad-leaved and coniferous forest plantations, and cultivated farmland. Available food resources included wild fruits, cultivated fruits, leaves, plant cortexes, amphibians, reptiles, birds, small mammals, molluscs, and arthropods. The abundance of these food categories varied significantly among seasons and habitats and civets altered consumption of these categories according to their temporal and spatial availability. The diversity of items consumed also varied significantly among seasons and habitats. From June to October, wild fruits were the main food of civets in forest habitats, whereas cultivated fruits were the main food in farmland. In contrast, from November to May, civets in forested habitats consumed primarily rodents and birds. Concordant with these changes was a shift from foraging in primary forest (November-May) to foraging in logged forest and farmland (June-October) that appeared to be associated with the availability of fruits. These results demonstrate the ability of civets to change their diet, both spatially and temporally, in response to changing food resources. To better understand how foraging behavior of civets varies with resource availability, similar studies should be conducted in tropical environments characterized by year-round availability of fruit.
Keywords: China, diet, dietary shift, fruit availability, key food resource, Paguma larvata, trophic diversity
Foraging theory (MacArthur and Pianka 1966; Mitchell 1989; Stephens et al. 1986) predicts that use of a resource should be related to the fitness benefits that an animal receives from consuming that resource (Olsson et al. 2001). The most profitable foraging strategy for a predator may be to maximize the trade-off between energetic rewards and foraging costs, rather than to simply maximize energy gain (Stein 1977). Many factors can influence this trade-off, including the nutrient requirements (Delorme and Thomas 1999; Rode and Robbins 2000) and associated dietary preferences of the predator (Hanski et al. 1991; Sundell et al. 2000) as well as attributes of the prey such as size, vulnerability, and nutritional content (Hörnfeldt 1978; Sundell et al. 2003). Each of these factors may vary temporally and spatially, suggesting that the diets of predators also may vary over time and space.
The prey-switching and alternative prey hypotheses (Angelstam et al. 1984; Hörnfeldt 1978; Small et al. 1993; Thompson and Colgan 1990) suggest that choice of a prey item is influenced by its nature and abundance relative to other prey types. As a consequence, some predators switch between primary and alternative prey items as the availability of these food resources changes. This diet switching has been shown for the red fox (Vulpes vulpes—Ferrari and Weber 1995; Kjellander and Nordström 2003; Leckie et al. 1998), wolves (Canis lupus—Dale et al. 1994), coyotes (Canis latrans—Patterson et al. 1998; Prugh 2005), martens (Martes americana—Thompson and Colgan 1990), Tengmalm's owls (Aegolius funer eus—Hörnfeldt 1978), and long-eared owls (Asio otus—Hörnfeldt 1978). Although temporal switching of prey is well documented, spatial switching (e.g., foraging in different habitats) remains controversial (Prugh 2005). More generally, prey switching may be a consequence of the choice of prey types within a patch (the prey model) or of the choice of patches with different types of prey (the patch model—Norrdahl and Korpimäki 2000; Olsson et al. 2001).
To explore the effects of resource availability on foraging behavior, we examined spatial and temporal variation in the diets of masked palm civets (Paguma larvata) in a highly heterogeneous habitat in central China. P. larvata is known to live in a wide variety of habitats in both tropical and temperate zones (Heydon and Bulloh 1996; Narang 1996; Nowak 1999; Wang 1987). Although no systematic study of its diet has been conducted (Jiang et al. 2003; Lundrigan and Baker 2003), this species has been found to consume a wide variety of prey items and has long been regarded as a dietary generalist that relies primarily on fruits and small mammals but supplements its diet with birds, snakes, frogs, and invertebrates (Nowak 1999; Wang 1987; Wang and Fuller 2003). In the study area, fruits are seasonally available, whereas small mammals are available throughout the year (Song and Liu 1999). Given this temporal variability in resources, we hypothesized that civets would show seasonal dietary shifts between fruits and small mam mals. Because the spatial distribution of these resources varies across seasons, we also predicted that civets would exhibit spatial dietary shifts over time. In addition to providing a detailed characterization of the diet of P. larvata in a seasonal habitat, our study offers the 1st evidence that foraging by this species varies in response to resource availability. These findings have significant implications for understanding the ecology of both this species of predator and the diverse habitats in which it occurs.
Materials and Methods
Study area.—The study was carried out in Houhe National Nature Reserve (30°2′45″−8′40″N, 110°29′25″−40′45″E), which encompasses 10,340 ha along the middle reaches of the Yangtze River valley of China (Fig. 1). This reserve is in the transitional belt between the middle and northern subtropical zones and is characterized by 4 distinct seasons, including a cold winter and a hot, humid summer. Elevations range from 560 m to about 2,252 m above sea level. The mean annual temperature is 11.5°C and there are 211 frost-free days per year. The average annual precipitation is 1,814 mm.
Fig. 1.

Map of the study area in Houhe National Nature Reserve, southeastern China. Locations of the 6 habitat types, 20 transects (dashed lines), and 10 trapping grids (■) are shown. The solid lines denote streams. The location of the reserve within China is indicated in the inset.
The primary types of natural forest within the reserve are coniferous forest (including Pinus tabulaeformis, Pinus henryi, and Cryptomeria lanceolata), broad-leaved forest (including Sycopsis sinensis, Davidia involucrata, and Cercidiphyllum japonicum), and bamboo forest. Based on human activity, habitats within the reserve can be categorized as primary (unlogged) forest (PF), selectively logged forest (SLF), logged forest (LF), broad-leaved forest plantation (BFP), coniferous forest plantation (CFP), and farmland (FL). In all uncultivated habitats, the main fruiting plants are members of the families Rosaceae, Lardizabalaceae, Lauraceae, Actinidiaceae, Moraceae, and Cornaceae, which mature between August and November. Outside these months fruits are scarce (Song and Liu 1999; Wang et al. 1997), although some species (e.g., Elaeagnus henryi, Cerasus dielsiana, Fragaria orientalis, and Hove nia dulcis) mature during December through July.
Most logging in Houhe National Nature Reserve occurred before 1998. In LF, all commercially valuable trees with a diameter at breast height (dbh) of 10–20 cm were harvested for construction or firewood. In contrast, in SFL, approximately 1 tree (with a dbh > 20 cm) per 100 m2 was taken for use by local residents. The logging methods employed destroyed more than 50% of the trees with a dbh > 5 cm. After logging, a mosaic of vegetation types remained that was dominated by pioneer tree species, shrubs, vines, climbers, and herbs. BFPs and CFPs were established between 1998 and 2001. More than 99% of vegetation in CFP and more than 90% of vegetation in BFP consisted of introduced species, with the rest of the vegetation comprised of vines, climbers, and herbs.
Approximately 87 species of mammals have been reported from the study area. The potential prey of civets includes 2 species of hares, 11 species of insectivores, 5 species of squirrels, and 21 other species of rodents (Song and Liu 1999). In addition to the masked palm civet, 20 species of carnivores have been reported, including black bear (Ursus thibetanus), hog badger (Arctonyx collaris), yellow-throated marten (Martes fiavigula), Chinese ferret-badger (Melogale moschata), Siberian weasel (Mustela sibirica), yellow-bellied weasel (M. kathiah), and leopard cat (Prionailurus bengalensis). Particularly relevant to this study are reports of 2 other species of civets, the small Indian civet (Viverricula indica) and the large Indian civet (Viverra zibetha—Song and Liu 1999). However, more recent studies (Thomas et al. 2004, Y. Zhou et al., in litt.), however, failed to detect the latter 2 species, suggesting that they are either very uncommon or extinct in the study area. As a result, all foraging by civets can reasonably be attributed to P. larvata.
Sample collection and identification.—The diet of the masked palm civet was studied by collecting feces and inspecting fresh foraging sites during July-November 2004 and April 2005-May 2006. Twenty transects measuring 2.1–3.0 km in length (X̄= 2.7 km ± 0.4 SE, range 2.1–3.0 km, total length = 54.3 km) and 4 m in width were established in the 6 forest types (X̄̄ = 5.8 transects per forest type ± 4.8 SE;Fig. 1). Each transect was systematically searched for feces every 2 weeks following the methods of Martinoli et al. (2001) and Joshi et al. (1995). During the 1st visit to each transect, all feces were removed to make sure that only fresh fecal samples were collected during later visits. The location and date of collection were recorded for each fecal sample. Samples were air-dried for a minimum of 4 weeks and then stored in air-tight bags until analysis.
Samples were identified as belonging to P. larvata based on the appearance (e.g., color, shape, and size), texture, and smell of feces; other evidence of civets (e.g., tracks, feeding signs, active dens, or daybeds) associated with feces; the opinion of local trappers (Wang 1999); and the presence of civet hairs in the fecal samples (ingested during grooming—Gatti et al. 2006; Juarez and Marinho 2002; Manfredi et al. 2004). Civet scats were readily distinguished from those of bears because of their smaller dimensions (scat diameter for civets = 5–20 mm versus > 40 mm for bears). The feces of mustelids were easily identified because of their characteristic odor and formation. The feces of masked palm civets were distinguished from those of leopard cats and the 2 other civets reported to occur in the study area by their characteristic shape and odor, as determined by comparison with a scat reference collection made from zoo specimens (Jâcomo et al. 2004). Although we used multiple ways to identify fecal samples, 237 of 2,149 feces collected during our study could not be assigned to species and were therefore excluded from dietary analyses. Collectively, these procedures suggest that the probability of misidentification of civet feces was low.
Diet determination and calculations of biomass.—Diet analyses were carried out according to the method of Kruuk and Parish (1981). Fecal samples were dissolved in distilled water and examined under a dissecting microscope. The number of individual prey items was estimated based on the number of paired or unique anatomical elements detected such as crania, mandibles, toothrows, wings, elytra, fruit cuticles, and seeds. Undigested remains of vertebrates were identified by comparison with reference collections of specimens from the study area (Marassi and Biancardi 2002). Fruit consumption was determined based on the remains of undigested seeds and fruit cuticles. A reference collection was used to identify seeds recovered from the samples (Genovesi et al. 1996). Identification of invertebrates was carried out according to Zhang et al. (2005).
The biomasses of the different foods consumed by P. larvata were determined from the fresh mass of each food item. For each species of fruit consumed, we estimated biomass by 1st estimating the number of individual fruits consumed. This was done by determining the mean number of seeds in fruits of each species collected directly from parent plants. These values were used to determine the number of fruits represented in each fecal sample, after which we estimated biomass of fruit consumed based on field measurements of masses of fruits (Silva et al. 2005). For unidentified seeds, we calculated biomass of fruit based on the mean masses of the identified fruits in the diet. The biomasses consumed from 2 prey items (ferret-badgers and pheasants) were assigned based on a feeding trial (Revilla and Palomares 2002) conducted with 3 civets captured from the study population. The design carefully followed the guidelines approved by the American Society of Mammalogists for the use of wild mammals in research (Gannon et al. 2007). For other identified vertebrate prey, we assumed that they were fully consumed by civets and biomass was estimated using mean body masses for these species. For unidentified animal prey, we assigned the mean mass of the different species identified from the same taxonomic group. Based on the results of feeding trials, the fresh biomasses of leaves and cortexes were assigned values of twice their dry mass in feces.
In addition to collecting fecal samples, we used the remains of recently consumed prey to characterize the diet of the study population. Fresh foraging sites were identified systematically by walking the 20 transects. We also searched for fresh foraging sites using radiotracking data collected as part of a concurrent study of civet behavior and by looking for civet paw prints and the fresh remains of prey whenever we walked through the study site. At the beginning of the study (May and June 2004), identification of paw prints and fresh food remains was achieved by comparison with a reference collection during feeding trials conducted with 3 civets captured from the study population. However, because civets usually swallow animals and some fruits whole, the calculation of biomass at fresh foraging sites was difficult. Thus, for these sites, we present only the number of individuals identified and the frequency of occurrence for the same or taxonomically similar food items.
For analyses of both fecal samples and foraging sites, dietary composition was expressed as the frequency of occurrence (FO = [number of the same species or taxonomic group × 100]/ [total number of feces or foraging sites sampled]) and as the percentage of fresh biomass intake (PB = [ingested biomass of the same species or taxonomic group × 100]/[total biomass consumed]—Reynolds and Aebischer 1991; Rosalino et al. 2005).
Estimating food availability.—In order to examine relationships between dietary variation and food availability, the abundances of the 2 main food items of civets—fruit and small mammals—were evaluated (Wang 1987; Wang and Fuller 2003). Based on a previous vegetation survey (Song and Liu 1999) and the experience of local trappers, 15 species of wild fruit plants used by civets were identified and surveyed in 2004; based on our 2004 analyses, an additional 6 species were surveyed in 2005. These species contributed >85% of the biomass of wild fruits in the diet of P. larvata. Availability of cultivated fruits (10 species surveyed in 2004 and 11 surveyed in 2005) also was evaluated. Distribution and fruit production of all species were surveyed along the 20 transects described above (Fig. 1); during the fruiting season, transects were surveyed every 2 weeks, at the same time as fecal samples were collected. Using the estimated biomass of fruits in each habitat, we calculated the availability (g/m2) of wild fruits, cultivated fruits, and all fruits for every month of the year.
Wang (1987) suggested that once a civet finds a tree with many mature fruits, it will repeatedly visit that foraging site. Therefore, we monitored 20 fruiting trees (10 Ilex macrocarpa, 5 Dendrobenthamia capitata, and 5 D. japonica) to determine the frequency of foraging visits to each tree and the relationship between the number of visits and the total fruit biomass of the tree. When a focal tree produced mature fruits, the total number of fruits on the tree and the number eaten (estimated from pericarp remains on the ground) were counted. Pericarp remains were identified by comparison with a reference collection made from feeding trials involving captive civets. If P. larvata had eaten fruits from a given tree, we removed all pericarp remains on the ground near that tree to ensure that only fresh pericarp remains were counted on the next survey. Trees were resurveyed every day until no fruits remained on the tree. When no fresh pericarp remains were found on the ground, we assumed that civets had stopped foraging at that tree. At this point, the final number of fruits remaining on the tree was counted. The total and final fruit biomass for each tree was calculated based on the mean mass of fruits of that species.
Small mammal populations in each habitat type were monitored by livetrapping following the protocol of Sun (2001). Trapping was conducted for 2 consecutive nights per month from April 2005 to May 2006. Ten grids (Fig. 1) were sampled, each consisting of an 8 × 8 array of trapping stations (n = 64 traps per grid); each row and column of traps was separated by 15 m, resulting in a total grid area of 1.4 ha (including a boundary strip of 7.5 m). We baited traps with cultivated peanut (Arachis hypogaea) during the evening and checked them the following morning. Each individual captured was marked, identified to species, and its body mass, sex, and reproductive condition were determined. The total biomass (g/m2) of each species in each habitat type was estimated from the biomass of individuals captured during each 2-day trapping session.
Statistical analyses.—Data are presented as means ± 1 SE unless otherwise stated; the specific statistical tests used are indicated in the text. SPSS 13.0 (SPSS 2003) was used for all statistical analyses. Because the data were not normally distributed, non-parametric tests were used to examine dietary variation of civets. Variation in the frequency of occurrence of each prey type in the feces of P. larvata was analyzed across months and habitats using Kruskal-Wallis tests. The level of significance of multiple comparisons was assessed using Bonferroni corrections.
Shannon's diversity index (H′) was used to examine changes in dietary diversity across habitats, months, and years. This index has been shown to be useful as an indicator of short-term dietary diversity (Revilla and Palomares 2002). For each fecal sample, H′ was calculated using the proportion of individuals of each species present in the sample. Variation in H′ was analyzed using a general linear model, with H′ as the dependent variable and month, year, and habitat as independent variables. To assess the influence of prey type on diversity, diversity values also were calculated using the proportion of ingested biomass represented by each prey type in each month. A multiple regression was performed with H′ as the dependent variable and the proportion of ingested biomass of each prey type (values subjected angular transformation) as independent variables.
To explore temporal variation in diet in greater detail, we grouped months of the year according to dietary composition using a hierarchical cluster analysis (using squared Euclidean distances). Multivariate analyses of variance (MANOVAs) were then performed using the grouping classifications obtained from the cluster analyses as an independent variable and the angular-transformed biomass values for each prey type as dependent variables. To determine the most important trophic resources, the significance of the contribution of each prey type to the final multivariate model was analyzed, after which the relationship between abundances of different prey types was examined using Spearman correlation coefficients.
Patterns of temporal and spatial variability in the abundance of fruits and small mammals were analyzed with Kruskal-Wallis tests. Spearman correlation coefficients were used to estimate the relationship between food resources ingested by civets and their availability in different months or habitats. A general linear model, with the total fruit biomass as the dependent variable and tree species and foraging by civets as independent variables, was used to analyze differences in the total fruit biomass of trees at which civets fed versus trees at which no feeding occurred. The frequency of foraging at each fruiting tree and its relationship to total and final fruit biomass were analyzed using regression analyses. Variation in the number of fecal samples collected in different months and habitats were analyzed using a general linear model, with the number of fecal samples as the dependent variable and month and habitat as independent variables. To determine whether habitat switching occurred, Spearman correlation analyses were used to assess the relationship between the proportion of each food item ingested by civets and the habitats in which foraging occurred.
Results
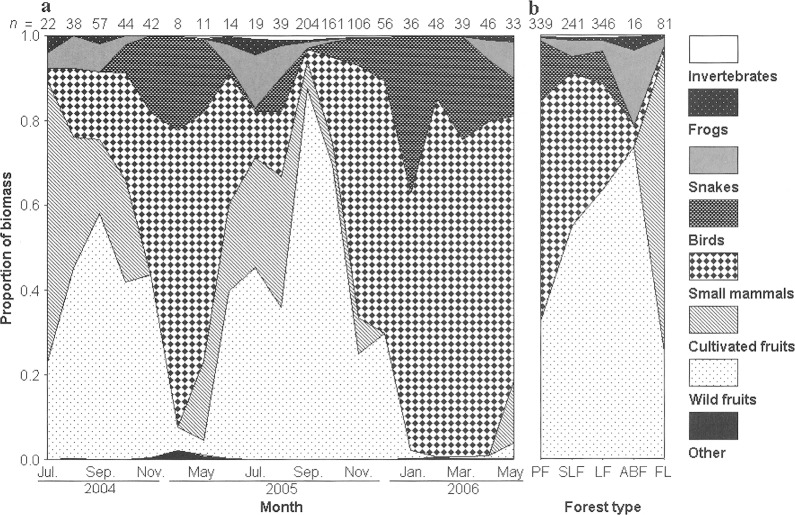
Diet composition and variation.—A total of 1,023 fecal samples was collected and 786 fresh foraging sites were detected. Of these, 203 fecal samples and 218 foraging sites were sampled between July and November 2004, whereas 820 fecal samples and 568 foraging sites were detected between April 2005 and May 2006. No civet feces and foraging sites were found in the coniferous plantation forest and so this habitat type was not included in subsequent analyses. The distribution of fecal samples and foraging sites differed among habitat types (χ2 = 110.53, d.f.= 4, P < 0.001), with the highest percentage in LF and the lowest percentage in BFP (Fig. 2).
Fig. 2.

Percentage of fecal samples (▲) and foraging sites (■) obtained from Paguma larvata in each habitat type in Houhe National Nature Reserve, central China. FL = cultivated farmland, BFP = broad-leaved forest plantation, LF = logged forest, SLF = selectively logged forest, and PF = primary forest.
A total of 2,235 food items was detected in feces; the mean ± 1 SD number of food items per fecal sample was 2.2 ± 1.3 (range 1–10). Sixty-seven species of wild fruits, 9 species of cultivated fruits, at least 50 species of vertebrates, and multiple invertebrates were found in civet scats (Appendix I). Animal prey included ferret-badgers (M. moschata), squirrels, rodents, shrews, birds, reptiles, amphibians, snails, crabs, and at least 4 different orders of insects. Wild fruits were the most common food items, accounting for more than 60% of the materials in fecal samples and 47% of the ingested biomass. This was followed by small mammals, which represented 29% and 30% of the materials in fecal samples and ingested biomass, respectively. Invertebrates accounted for about 26% of the materials in fecal samples and 0.5% of the ingested biomass.
As determined from fecal samples, the diet of civets was characterized by marked seasonal, annual, and habitat variation that affected all prey types (Fig. 3). Consumption of wild fruits varied significantly among years, months, and habitats (Fig. 3; Table 1). Consumption of small mammals, birds, and cortexes also varied among years and habitats; although there was significant monthly variation in the consumption of mammals and cortexes, consumption of birds did not vary significantly among months (Fig. 3; Table 1).
Fig. 3.
Proportion of biomass represented by each main prey type ingested by Paguma larvata across a) months and b) habitat types in Houhe National Nature Reserve, central China. The numbers shown above each panel represent the number of fecal samples analyzed for each time period or habitat. Habitat types as in Fig. 2.
Table 1.
Results of Kruskal-Wallis tests comparing the percent occurrence of different prey types in the diets of masked palm civets (Paguma larvata) from Houhe National Nature Reserve in central China, July 2004–May 2006. Diet composition was determined from analyses of fecal samples and remains found at fresh foraging sites. Sample sizes are given in Fig. 3.
| Years | Months | Habitats | ||||
|---|---|---|---|---|---|---|
| Food categories | χ2(d.f. = 2) | P | χ2 (d.f. = 11) | P | χ2(d.f. = 4) | P |
| Fecal sample analyses | ||||||
| Wild fruits | 13.98 | 0.001 | 26.39 | 0.006 | 25.67 | <0.001 |
| Cultivated fruits | 2.25 | 0.325 | 6.15 | 0.863 | 60.55 | <0.001 |
| Small mammals | 18.2 | <0.001 | 36.84 | <0.001 | 21.46 | <0.001 |
| Birds | 18.01 | <0.001 | 18.46 | 0.072 | 14.48 | 0.006 |
| Leaves | 5.13 | 0.077 | 15.73 | 0.152 | 13.11 | 0.011 |
| Cortexes | 6.78 | 0.034 | 19.98 | 0.046 | 11.25 | 0.024 |
| Snakes | 0.59 | 0.745 | 15.53 | 0.160 | 5.42 | 0.247 |
| Frogs | 0.4 | 0.819 | 12.97 | 0.295 | 2.63 | 0.622 |
| Mollusks | 10.61 | 0.005 | 11.97 | 0.366 | 4.02 | 0.403 |
| Arthropods | 22.51 | <0.001 | 9.1 | 0.613 | 2.47 | 0.650 |
| Foraging site analyses | ||||||
| Wild fruits | 20.563 | <0.001 | 56.593 | <0.001 | 18.221 | <0.001 |
| Cultivated fruits | 12.356 | 0.002 | 16.346 | 0.029 | ||
Fresh foraging sites were found almost exclusively (99% of 786 sites) in areas with fruiting trees, suggesting that wild and cultivated fruits are an important food source. Wild and cultivated fruits accounted for more than 84% and 15%, respectively, of the food items consumed at foraging sites (Appendix I). Because some wild fruits and animal prey leave no remains after being eaten, some prey types were difficult to detect at fresh foraging sites. Only 4 species of animal prey—Edward's Leopoldamys (Leopoldamys edwardsi), Temminck's tragopan (Tragopan temminckii), and 2 species of frog—were found to have been eaten, whereas 32 species of wild fruits, and all 9 species of cultivated fruits were consumed. Thus, only variability in consumption of wild and cultivated fruits was analyzed. Consumption of wild fruits varied significantly among years, months, and habitats (Table 1). Cultivated fruits only occurred in FL, and the consumption of this food category also varied among years and months (Table 1).
Diet diversity and key food resources.—Dietary diversity, measured as H′, differed significantly across years, months, and habitats (F = 2.576, d.f= 73, 949, P < 0.001; Fig. 4). The main effects of year, month, and habitat were each significant (F= 4.136, d.f. = 2, P = 0.016; F= 4.968, d.f. = 11, P < 0.001; and F= 14.034, d.f.= 4, P < 0.001, respectively), but no significant interaction effects were detected. Intra-sample diversity (e.g., H′ within years) was significantly lower in 2006 than in 2004 and 2005 (least square difference [LSD], F= 17.569, d.f.= 2, 1,020, P < 0.001; Fig. 4). In all habitats and years, diversity was higher between July and December (average H′ > 0.32 in all months) than between January and May (average H′ < 0.18 in all months), whereas June (H′ — 0.23) was not significantly different from any other month (LSD, F= 6.372, d.f. = 11, 1,011, P < 0.001). Diet diversity was significantly lower in FL than in other habitat types (LSD, F = 8.384, d.f. = 4, 1,018, P < 0.001); the remaining habitats did not differ significantly from each other (Fig. 4).
Fig. 4.

Temporal variation in diet diversity for Paguma larvata in Houhe National Nature Reserve, central China. Diversity (H′) was calculated from the proportion of total biomass represented by different prey types in fecal samples collected from this species. Data are partitioned according to habitat type; data from 2004 to 2006 were pooled for analysis. Samples sizes are provided in the text and in Fig. 3.
To determine the influence of prey type on monthly diversity, we included all 10 food categories in multiple regression analyses of the factors influencing H′. From this initial model, only wild fruits, cultivated fruits, small mammals, birds, and snakes were retained in the final model, in which H′ = 12.811 arcsine(wild fruits) + 13.049 arcsine(cultivated fruits) + 12.873 arcsine(small mammals) + 11.946 arcsine(birds) + 13.678 arcsine(snakes) − 10.748 (adjusted R2 = 0.867, F= 13.997, d.f. = 9, 9, P < 0.001).
The simplest classification obtained from the cluster analyses (2 temporal groups) was significant (MANOVA Pillai's trace = 0.87, F= 8.38, P < 0.001). The 2 temporal groups identified were June-October and November-May. Only 4 of the 10 prey types considered in these analyses were significantly clustered. In order of decreasing importance, these were wild fruits, cultivated fruits, small mammals, and birds (F = 87.948–23.362, d.f. — 1, 17, P < 0.001 in all cases). Among the independent variables considered in our model, significant negative correlations were found between the prevalence of wild fruits and small mammals (R = −0.761, n = 19, P < 0.001), the prevalence of wild fruits and birds (R = −0.794, n = 19, P< 0.001), the prevalence of cultivated fruits and small mammals (R= −0.635, n= 19, P= 0.003), and the prevalence of cultivated fruits and birds (R = −0.669, n= 19, P= 0.002). These results indicate that fruits characterize the diet of P. larvata between June and October, whereas small mammals and birds characterize the diet between November and May (Fig. 3).
Food availability, use, and dietary switching.—Abundance of fruit differed among months (χ2 = 48.72, d.f. = 11, P < 0.001) and habitats (χ2= 18.75, d.f. = 4, P = 0.001) but not among years (χ2 = 3.19, d.f. = 2, P — 0.074). Ingested biomass of fruits was correlated with availability of fruit (R — 0.633, n= 95, P < 0.001). Although no species of wild fruit were found in CFP and FL, similar results were obtained for the remaining habitats when only wild fruits were considered (χ2 = 36.33, d.f.= 11, P < 0.001; χ2 = 33.85, d.f. = 4, P < 0.001; and x2 = 1.92, df = 1, P = 0.166, for months, habitats, and years, respectively). Ingested biomass of wild fruits also was correlated with their availability (R = 0.651, n — 95, P < 0.001). Cultivated fruits were only present and consumed by civets in FL. Their availability was greater in 2004 than in 2005 (χ2 = 4.28, d.f. = 1, P = 0.039) and differed among months (χ2 = 16.62, d.f= 11, P= 0.020). Ingested biomass and availability of cultivated fruits also were correlated with each other (R= 0.535, n = 95, P = 0.018). Thirteen of 20 fruiting trees (7 of /. macrocarpa, and 3 each of Dendrobenthamia capitata and D. japonica) were used by P. larvata; the total fruit biomass of trees at which civets fed was greater than that of the remaining trees, although this difference was not significant (F — 2.739, d.f. = 5, 14, P= 0.063). The frequency of foraging at a given fruiting tree was positively correlated with the total fruit biomass for that tree (R = 0. 548, n= 13, P= 0.043). Regression analyses of foraging frequency on total fruit biomass indicated that trees should no longer be visited when the final fruit biomass drops below 4.5 kg per tree (R2 = 0. 341, F= 5.688, d.f.= 1, 11, P = 0.036).
Twenty-two mammals (including 3 species of squirrels, 13 other rodents, 5 species of insectivores, and the ferret-badger) were trapped. The most frequently captured mammal was Edward's Leopoldamys (L. edwardsi; 22% of captures), followed by the sulfur-bellied rat (Niviventer confucianus; 18% of captures); the least frequently captured mammal was the Asian red-cheeked squirrel (Dremomys rufigenis; 0.14% of captures). The abundance of small mammals did not differ significantly among months (χ2 = 8.145, d.f. = 11, P = 0.700) and years (χ2 = 1.960, d.f= 1, P = 0.162), but did differ significantly among habitats (χ2 = 52.79, d.f. = 4, P < 0.001), with rank based on abundance (highest to lowest) being PF > SLF > LF > FL > BFP. The biomass of small mammals ingested by P. larvata was negatively correlated with their abundance and fruit availability across months (R = —0.714, n= 14, P= 0.004, and R = −0.601, n= 19, P = 0.007, respectively) and positively correlated with their abundance across habitat types (R = 0.900, n = 5, P = 0.037). The highest consumption of mammals occurred when both small mammals and fruits were at their lowest abundance (Fig. 3), with abundance of fruits being ≤20 g/m2 (Fig. 5). When fruit consumption by civets was analyzed as a function of small mammal abundance, no significant effect was detected (R= 0.199, n = 70, P = 0.099).
Fig. 5.
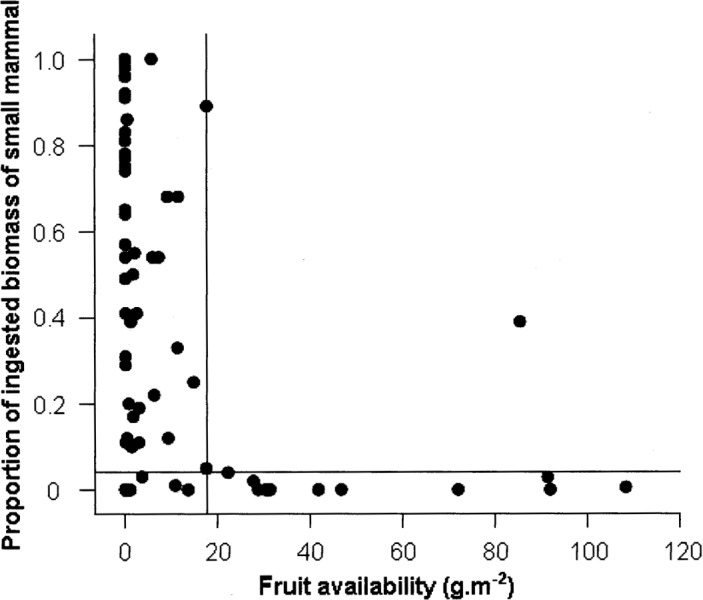
Relationship between small mammal biomass ingested by civets and fruit availability. Data for 2004 to 2006 were pooled for analysis. The vertical line indicates the point at which Paguma larvata switched from consuming primarily fruits to consuming primarily small mammals. The junction between the vertical and horizontal line indicates the threshold at which this species is expected to exhibit diet switching.
In response to temporal variation in food abundance, P. larvata switched its dietary habits from fruits to small mammals and birds in May-June and from small mammals and birds to fruits in November-December (Fig. 6a). With regard to spatial variation in food resources, the number of fecal samples collected differed significantly across months and habitats (F = 77.068, d.f= 48, 974, P < 0.001; Fig. 7), suggesting that the location of foraging varied. Significant negative correlations were found between the proportion of ingested biomass obtained in PF versus LF (R = −0.570, n= 19, p = 0.011) and between the proportion of ingested biomass obtained in PF versus FL (R= −0.641, n= 19, P= 0.003). These findings suggest that P. larvata shifts its foraging from PF to LF or FL habitat in spring when some fruits (F. orientalis, Cerasus, and Berchemia huana) are abundant in LF and cultivated fruits start to mature in FL. Conversely, the animals appear to switch foraging sites from LF or FL to PF in the fall, when wild fruits such as Diospyros lotus and H. dulcis are mature (Fig. 6b).
Fig. 6.
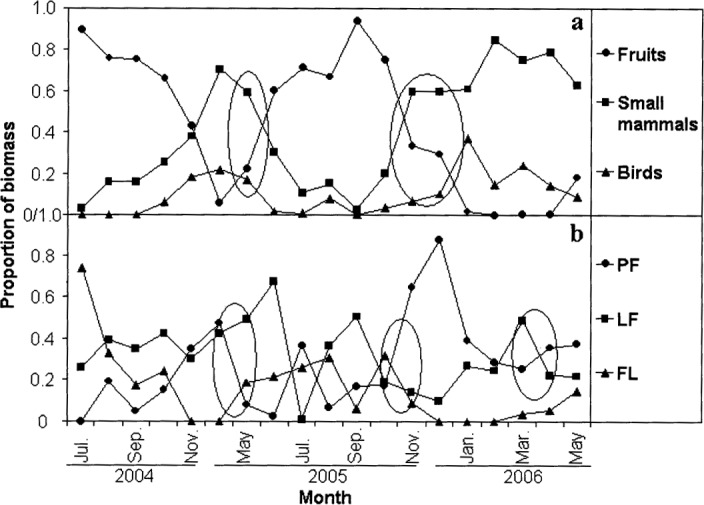
Changes in diet and foraging habitat for Paguma larvata in Houhe National Nature Reserve, central China, a) The proportions of prey biomass in fecal samples represented by fruits, small mammals, and birds, b) The proportions of prey biomass in fecal samples obtained from primary forest (PF), logged forest (LF), and farmland (FL). Ellipses denote apparent shifts in diet composition and foraging habitat.
Fig. 7.

Temporal and habitat variation in the number of fecal samples collected from Paguma larvata in Houhe National Nature Reserve, central China. Habitat tvnes as in Fig. 2.
Discussion
Diet composition and primary food items.—Although other studies have documented the diet of civets (Rabinowitz 1991; Wang and Fuller 2003), this is the 1st study to consider seasonal and habitat differences in the diet of the masked palm civet (P. larvata). Examination of our data showed that P. larvata from central China consumed a wide variety of food items and that the composition of the diet varied temporally and spatially. These findings add to our understanding of civet ecology by demonstrating significant dietary variation among seasons and habitats. In particular, these data provide one of the few demonstrations of spatial (habitat) dietary switching in a small carnivore.
Although no systematic study of the diet of civets has been carried out, fruits typically have been regarded as the principal food resource of these animals (Grassman 1998; Jiang et al. 2003; Lundrigan and Baker 2003; Nowak 1999; Wang 1987). In subtropical southeastern China, fruits are the most conspicuous food items ingested by P. larvata, with the Chinese abelia (Abelia chinensis) being consumed most frequently (Wang 1999). In Hong Kong, Corlett (1996) found seeds of 15 species of fruit in civet scats and Tsang and Corlett (2005) found that the large green fruits of Shaba holly (Ilex chapaensis) were consumed only by P. larvata. In tropical forests in Thailand, where ripe fruit is available throughout the year, civets ate fruits of many species (Grassman 1998; Kitamura et al. 2002; Rabinowitz 1991). Our study, which was conducted where both wild and cultivated fruits are available only seasonally, indicated that fruits were the primary food source for P. larvata during the period between June and October.
In contrast, outside of the fruiting season (i.e., in spring and winter) the diet of P. larvata consisted of a large proportion of small mammals, mainly ferret-badgers, squirrels, and rodents. These results agree with those of Wang (1999), who also found that small mammals (particularly rodents) were important food items for civets in southeastern China in the spring and winter, when fruits were scarce. In our study, small mammals were consumed by P. larvata primarily in PF, SLF, and LF, where rodents are relatively abundant and cultivated fruits are absent. The negative correlation between the consumption of small mammals and their seasonal availability, however, is contrary to our expectation that P. larvata, as a carnivore, would select small mammals.
A greater number of fecal samples was collected during the autumn than during the spring and summer. This may reflect differences in the preservation of samples (feces may disintegrate more quickly during the spring and summer when rainfall is greater—Juarez and Marinho 2002). At the same time, food intake may vary seasonally, because frugivorous mammals often increase feeding and accumulate fat before annual periods of food shortage (Zhao 1994). A greater number of fecal samples was collected in 2005 and 2006 than during 2004. This may be explained by an increase in civet density in 2005 and 2006. This increase may reflect a ban on hunting (including civets) instituted in 2003 when the severe acute respiratory syndrome virus (SARS-CoV) was believed to have jumped from a wild animal host to humans (Guan et al. 2003).
Food availability, use, and dietary switching.—Food abundance and distribution have been shown to affect the behavior (e.g., feeding, social, and spacing behavior) of a variety of mammals (Isbell et al. 1998; Lurz et al. 2000; Wilmshurst et al. 1999), including carnivores (Ferrari and Weber 1995; Joshi et al. 1995; Prange et al. 2004; Raymond et al. 1990). The dietary choices of small carnivores depend primarily on temporal and spatial variation in foraging costs, which are affected primarily by the availability of critical prey species (Alves-Costa et al. 2004; Erlinge 1981; Joshi et al. 1995; Raymond et al. 1990). Perhaps as a result, use of alternative food resources is characteristic of generalist carnivores (Martinoli et al. 2001). Specifically, as availability of the primary prey species decreases, the relative benefits of consuming alternative foods should increase (Agrawal and Klein 2000; Genovesi et al. 1996). Therefore, a decrease in availability of staple resources can cause a shift in diet toward alternative food items (e.g., Ferrari and Weber 1995; Prugh 2005; Thompson and Colgan 1990).
In our study area, fruits showed significant temporal and spatial variation, being more abundant in summer and autumn and in primary forests (Song and Liu 1999). Correspondingly, the consumption of fruits by civets was highest from June to October, and occurred mainly in forested areas. The highest consumption of small mammals occurred when both small mammals and fruits were at their lowest abundance. Birds (mainly pheasants) appeared in the diet in winter and spring, probably because in these seasons, the loss of leaves on the trees made them easier to capture (Song and Liu 1999; Wang et al. 1997). These results suggest that P. larvata alters its diet depending on the availability of fruit resources.
Optimal foraging theory (Sacks and Neale 2002; Schoener 1971; Stein 1977) predicts that the attributes (e.g., vulnerability) of a food resource also may influence foraging decisions by predators. Stickney (1991) found that if bird eggs were temporarily abundant and easily obtained, they become the primary prey item in the diet of foxes (Vulpes lagopus) in summer in spite of a high abundance of rodents. The alternative prey hypothesis (Angelstam et al. 1984; Hörnfeldt 1978; Thompson and Colgan 1990) asserts that predators switch prey types when numbers of their primary prey are low. For example, Bêty et al. (2002) found that predation by foxes on lemmings and snow goose eggs increased only when rodent populations declined. In this case, the alternative prey was relatively difficult to capture and thus the predator concentrated its foraging activity on this prey only when the abundance of its primary rodent prey was low (Norrdahl and Korpimäki 2000). Our results are consistent with this scenario. The abundance of mature fruits in the summer and autumn meant that fruits became the primary food source in these seasons despite a high abundance of rodents. Accordingly, civets switched their foraging habitat from PF to LF or FL. These findings suggest that although P. larvata is considered to be a generalist carnivore, the animals prefer fruits to rodents when the former are available, presumably because of the higher profitability of consuming fruit.
Our study took place in the subtropical zone, where fruits are abundant in summer and autumn but scarce during other seasons (Song and Liu 1999). Because of the absence of systematic studies of the diets of civets in tropical areas where fruits are available throughout the year, we cannot compare the foraging behavior of civets in areas where fruits are seasonally versus continually available. We predict that in tropical areas where fruits are available throughout the year, dietary switching will not occur and that fruits, with their higher profitability, will be the main food of P. larvata throughout the year. Complementary studies in tropical areas are called for in order to gain a more complete understanding of the effects of spatial and temporal variation in food resources on foraging behavior of civets.
Acknowledgements
This study was financially supported by the 6th Framework Programme of the European Commission (EPISARS, no. SP22-CT-2004–511063) to SZ; the Kadoorie Farm and Botanic Garden, Hong Kong Special Administrative Region, People's Republic of China to YZ; and Tran-Century Training Program Foundation for the Talents by the State Education Commission, People's Republic of China to XW. We thank the personnel of Houhe Nature Reserve, and Prof. H. Z. Wang, Dr. X. S. Liu, Dr. L. Zhang, Mr. J. Wang, and Dr. B. K. Bai for their assistance during the fieldwork. We thank Prof. M. Cao, Dr. Z. H. Tang, and Mrs. B. Liang for their help during the laboratory work. We thank Prof. J. Z. Song for assistance in identification of fruiting plants occurring in fecal samples. We thank Prof. J. C. Hu and Prof. Q. S. Wang for their advice and assistance in identification of vertebrates occurring in fecal samples. We thank Dr. T. Dahmer, Dr. Y. Kaneko, Dr. H. B. Wang, Dr. P. Myers, Dr. L. I. Grassman, Jr., and Dr. B. Dole for helping us with the literature. We also thank E. Lacey and 2 anonymous reviewers for constructive comments on the manuscript.
Appendix
Appendix I.
Diet composition of masked palm civets (Paguma larvata) based on fecal analysis (FA) and examination of fresh foraging sites (EF) in Houhe National Nature Reserve in central China, July 2004–May 2006. Number of the same or taxonomically similar food items (n), percentage of occurrence (PO), and percentage of consumed biomass (PB) are given.
| Food item | FA (n = 1,023) | EF (n = 786) | |||
|---|---|---|---|---|---|
| n | PO | PB | n | PO | |
| Wild fruits | 615 | 60.117 | 47.835 | 662 | 84.224 |
| Actinidia chinensis | 147 | 14.370 | 2.528 | 62 | 7.888 |
| Actinidia callosa | 2 | 0.196 | 0.015 | 6 | 0.763 |
| Actinidia kolomikta | 46 | 4.497 | 0.138 | 22 | 2.799 |
| Actinidiaceae | 1 | 0.098 | 0.001 | 9 | 1.145 |
| Clematoclethra scandens | 30 | 2.933 | 0.052 | 4 | 0.509 |
| Holboellia grandiflora | 41 | 4.008 | 1.289 | 39 | 4.962 |
| Holboellia coriacea | 15 | 1.466 | 0.398 | 129 | 16.412 |
| Decaisnea fargesii | 9 | 0.880 | 0.209 | 4 | 0.509 |
| Sinofranchetia chinensis | 9 | 0.880 | 0.650 | 6 | 0.763 |
| Akebia trifoliata | 6 | 0.587 | 0.308 | 17 | 2.163 |
| Sorbus hemsleyi | 90 | 8.798 | 1.050 | 26 | 3.308 |
| Amygdalus persica | 4 | 0.391 | 0.080 | 0 | 0.000 |
| Prunus salicina | 10 | 0.978 | 0.590 | 26 | 3.308 |
| Cerasus dielsiana | 17 | 1.662 | 1.053 | 3 | 0.382 |
| Cerasus 1 | 3 | 0.293 | 0.067 | 0 | 0.000 |
| Cerasus 2 | 2 | 0.196 | 0.005 | 1 | 0.127 |
| Cerasus 3 | 13 | 1.271 | 0.103 | 0 | 0.000 |
| Cerasus 4 | 1 | 0.098 | 0.013 | 0 | 0.000 |
| Rubus parken | 1 | 0.098 | 0.182 | 0 | 0.000 |
| Fragaria orientalis | 4 | 0.391 | 0.482 | 0 | 0.000 |
| Pyracentha fortuneana | 71 | 6.940 | 0.016 | 0 | 0.000 |
| Rosa | 13 | 1.271 | 0.026 | 0 | 0.000 |
| Crataegus hupehensis | 24 | 2.346 | 0.669 | 1 | 0.127 |
| Cotoneaster | 1 | 0.098 | 0.001 | 0 | 0.000 |
| Rosaceae 1 | 1 | 0.098 | 0.170 | 0 | 0.000 |
| Rosaceae 2 | 1 | 0.098 | 0.135 | 0 | 0.000 |
| Rosaceae 3 | 10 | 0.978 | 0.132 | 0 | 0.000 |
| Rosaceae 4 | 6 | 0.587 | 0.250 | 0 | 0.000 |
| Rosaceae 5 | 10 | 0.978 | 0.090 | 0 | 0.000 |
| Ficus heteromorpha | 23 | 2.248 | 1.025 | 12 | 1.527 |
| Morus alba | 2 | 0.196 | 0.118 | 0 | 0.000 |
| Broussonetia kazinoki | 7 | 0.684 | 0.164 | 0 | 0.000 |
| Broussonetia papyrifera | 5 | 0.489 | 0.165 | 8 | 1.018 |
| Ilex macrocarpa | 140 | 13.685 | 13.665 | 49 | 4.962 |
| Ilex pedunculosa | 4 | 0.391 | 0.125 | 0 | 0.000 |
| Ilex pernyi | 7 | 0.684 | 0.313 | 0 | 0.000 |
| Ilex | 4 | 0.391 | 0.134 | 0 | 0.000 |
| Schisandra | 26 | 2.542 | 0.452 | 7 | 0.891 |
| Kadsura longipedunculata | 28 | 2.737 | 0.800 | 23 | 4.198 |
| Hovenia dulcis | 56 | 5.474 | 0.897 | 43 | 5.471 |
| Cells cerasifera | 58 | 5.670 | 1.421 | 27 | 3.435 |
| Dendrobenthamia japonica | 169 | 16.520 | 12.918 | 59 | 7.506 |
| Dendrobenthamia capitata | 21 | 2.053 | 0.719 | 36 | 4.580 |
| Kalopanax septemlobus | 31 | 3.030 | 0.656 | 2 | 0.254 |
| Diospyros lotus | 61 | 5.963 | 0.474 | 20 | 2.545 |
| Rubiaceae | 17 | 1.662 | 0.789 | 0 | 0.000 |
| Elaeagnus henryi | 8 | 0.782 | 0.051 | 2 | 0.254 |
| Trichosanthes kirilowii | 9 | 0.880 | 0.016 | 0 | 0.000 |
| Choerospondias axillaris | 15 | 1.466 | 0.935 | 5 | 0.636 |
| Sinomenium | 8 | 0.782 | 0.059 | 0 | 0.000 |
| Stachyurus chinensis | 2 | 0.196 | 0.172 | 2 | 0.254 |
| Linder a me gap hy lia | 3 | 0.293 | 0.135 | 0 | 0.000 |
| Smilax stans | 2 | 0.196 | 0.021 | 0 | 0.000 |
| Typhonium giganteum | 7 | 0.684 | 0.164 | 0 | 0.000 |
| Physalis alkekengi | 1 | 0.098 | 0.178 | 0 | 0.000 |
| Poncirus trigoliata | 1 | 0.098 | 0.047 | 1 | 0.127 |
| Quercus multinervis | 2 | 0.196 | 0.002 | 0 | 0.000 |
| Rubiaceae | 2 | 0.196 | 0.042 | 0 | 0.000 |
| Coriaria nepalensis | 1 | 0.098 | 0.012 | 0 | 0.000 |
| Berchemia huana | 2 | 0.196 | 0.008 | 1 | 0.127 |
| Unidentified fruit 1 | 1 | 0.098 | 0.000 | 0 | 0.000 |
| Unidentified fruit 2 | 3 | 0.293 | 0.012 | 1 | 0.127 |
| Unidentified fruit 3 | 1 | 0.098 | 0.316 | 0 | 0.000 |
| Unidentified fruit 4 | 2 | 0.196 | 0.017 | 1 | 0.127 |
| Unidentified fruit 5 | 5 | 0.489 | 0.106 | 1 | 0.127 |
| Unidentified fruit 6 | 1 | 0.098 | 0.002 | 0 | 0.000 |
| Other unidentified fruits | 19 | 1.857 | 0.004 | 7 | 0.891 |
| Cultivated fruits | 75 | 7.331 | 11.028 | 118 | 15.013 |
| Eriobotrya japonica | 6 | 0.587 | 1.148 | 7 | 0.891 |
| Amygdalus persicaa | 20 | 1.955 | 2.184 | 27 | 3.435 |
| Amygdalus persicaa | 25 | 2.444 | 3.381 | 22 | 2.799 |
| Amygdalus persicaa | 9 | 0.880 | 1.985 | 16 | 2.036 |
| Amygdalus persicaa | 7 | 0.684 | 0.725 | 7 | 0.891 |
| Pyrusb | 2 | 0.196 | 0.533 | 14 | 1.781 |
| Pyrusb | 2 | 0.196 | 0.510 | 5 | 0.636 |
| Pyrusb | 2 | 0.196 | 0.260 | 5 | 0.636 |
| Pyrus | 0 | 0.000 | 0.000 | 3 | 0.382 |
| Diospyros kakic | 2 | 0.196 | 0.302 | 10 | 1.272 |
| Diospyros kakfc | 0 | 0.000 | 0.000 | 2 | 0.254 |
| Leaves | 74 | 7.234 | 0.055 | 0 | 0.000 |
| Cortexes | 35 | 3.421 | 0.045 | 0 | 0.000 |
| Small mammals | 299 | 29.228 | 30.450 | 1 | 0.127 |
| Melogale moschata | 12 | 1.173 | 2.439 | 0 | 0.000 |
| Sciuridae | 43 | 4.203 | 4.551 | 0 | 0.000 |
| Leopoldamys edwardsi | 36 | 3.519 | 9.841 | 1 | 0.127 |
| Rattus fulvescens | 24 | 2.346 | 0.832 | 0 | 0.000 |
| Rattus niviventer | 18 | 1.760 | 0.615 | 0 | 0.000 |
| Apodemus | 14 | 1.369 | 0.202 | 0 | 0.000 |
| Insectivora | 6 | 0.587 | 0.089 | 0 | 0.000 |
| Unidentified small mammals | 154 | 15.054 | 11.882 | 0 | 0.000 |
| Birds | 72 | 7.038 | 7.052 | 3 | 0.382 |
| Tragopan temminckii | 15 | 1.466 | 3.558 | 3 | 0.382 |
| Unidentified pheasants | 11 | 1.075 | 2.609 | 0 | 0.000 |
| Pas serif ormes | 47 | 4.594 | 0.885 | 0 | 0.000 |
| Snakes | 26 | 2.542 | 2.463 | 0 | 0.000 |
| Zaocys dhumnades | 1 | 0.098 | 0.106 | 0 | 0.000 |
| Trimeresurus stejnegeri | 3 | 0.293 | 0.254 | 0 | 0.000 |
| Trimeresurus jerdonii | 1 | 0.098 | 0.098 | 0 | 0.000 |
| Elaphe taeniura | 2 | 0.196 | 0.175 | 0 | 0.000 |
| Unidentified snakes | 19 | 1.857 | 1.830 | 0 | 0.000 |
| Frogs | 13 | 1.271 | 0.579 | 2 | 0.254 |
| Paad | 8 | 0.782 | 0.397 | 1 | 0.127 |
| Pelophylax nigromaculata | 1 | 0.098 | 0.027 | 1 | 0.127 |
| Unidentified frogs | 4 | 0.391 | 0.154 | 0 | 0.000 |
| Molluscs | 31 | 3.030 | 0.066 | 0.000 | 0.000 |
| Arthropods | 240 | 23.460 | 0.426 | 0.000 | 0.000 |
| Coleoptera | 217 | 21.212 | 0.376 | 0.000 | 0.000 |
| Hymenoptera | 25 | 2.444 | 0.003 | 0.000 | 0.000 |
| Hemiptera | 3 | 0.293 | 0.004 | 0.000 | 0.000 |
| Homoptera | 4 | 0.391 | 0.006 | 0.000 | 0.000 |
| Unidentified insects | 8 | 0.782 | 0.006 | 0.000 | 0.000 |
| Crustacea | 2 | 0.196 | 0.031 | 0.000 | 0.000 |
Four plantings of peaches.
Four plantings of pears.
Two plantings of persimmons.
Includes Boulenger's spiny-frog (Paa boulengeri) and giant spiny-frog (Paa spinosa).
Literature Cited
- Agrawal A. A., Klein C. N. 2000. What omnivores eat: direct effects of induced plant resistance in herbivores and indirect consequences for diet selection by omnivores. Journal of Animal Ecology 69:525–535. [Google Scholar]
- Alves-Costa C. P., Fonseca G A. B., Christöfar C. 2004. Variation in the diet of the brown-nosed coati (Nasua nasua) in southeastern Brazil. Journal of Mammalogy 85:478–482. [Google Scholar]
- Angelstam P., Lindström E., Widén P. 1984. Role of predation in short-term population fluctuations of some birds and mammals in Fennoscandia. Oecologia 62:199–208. [DOI] [PubMed] [Google Scholar]
- Bêty J., Gauthier G., Korpimäki E., Giroux J. F. 2002. Shared predators and indirect trophic interactions: lemming cycles and arctic-nesting geese. Journal of Animal Ecology 71:88–98. [Google Scholar]
- Corlett R. T. 1996. Characteristics of vertebrate-dispersed fruits in Hong Kong. Journal of Tropical Ecology 12:819–833. [Google Scholar]
- Dale B. W., Adams L. G, Bowyer R. T. 1994. Functional response of wolves preying on barren-ground caribou in a multiple-prey ecosystem. Journal of Animal Ecology 63:644–652. [Google Scholar]
- Delorme M., Thomas D. W. 1999. Comparative analysis of the digestive efficiency and nitrogen and energy requirements of the phyllostomid fruit-bat (Artibeus jamaicensis) and the pteropodid fruit-bat (Rousettus aegyptiacus). Journal of Comparative Physiology, B. Biochemical, Systemic, and Environmental Physiology 169:123–132. [DOI] [PubMed] [Google Scholar]
- Erlinge S. 1981. Food preference, optimal diet and reproductive output in stoats Mustela erminea in Sweden. Oikos 36:303–315. [Google Scholar]
- Ferrari N., Weber J. M. 1995. Influence of the abundance of food resources on feeding habits of the red fox, Vulpes vulpes, in western Switzerland. Journal of Zoology (London) 236:117–129. [Google Scholar]
- Gannon W. L., Sikes R. S., the Animal Care, Use Committee of the American Society of Mammalogists 2007. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy 88:809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti A., Bianchi R., Rosa C. R. X., Mendes S. L. 2006. Diet of two sympatric carnivores, Cerdocyon thous and Procyon cancrivorus, in a restinga area of Espirito Santo State, Brazil. Journal of Tropical Ecology 22:227–230. [Google Scholar]
- Genovesi P., Secchi M., Boitani L. 1996. Diet of stone martens: an example of ecological flexibility. Journal of Zoology (London) 238:545–555. [Google Scholar]
- Grassman L. I., Jr. 1998. Movements and fruit selection of two Paradoxurinae species in a dry evergreen forest in southern Thailand. Small Carnivore Conservation 19:25–29. [Google Scholar]
- Guan Y., et al. 2003. Isolation and characterization of viruses related to the SARS Coronavirus from animals in southern China. Science 302:276–278. [DOI] [PubMed] [Google Scholar]
- Hanski L, Henttonen H., Hansson L. 1991. Specialist predators, generalist predators, and the microtine rodent cycle. Journal of Animal Ecology 60:353–367. [Google Scholar]
- Heydon M. J., Bulloh P. 1996. The impact of selective logging on sympatric civet species in Borneo. Oryx 30:31–36. [Google Scholar]
- Hörnfeldt B. 1978. Synchronous population fluctuations in voles, small game, owls, and tularemia in northern Sweden. Oecologia 32:141–152. [DOI] [PubMed] [Google Scholar]
- Isbell L. A., Pruetz J. D., Young T. P. 1998. Movements of vervets (Cercopithecus aethiops) and patas monkeys (Erythrocebus patas) as estimators of food resource size, density, and distribution. Behavioral Ecology and Sociobiology 42:123–133. [Google Scholar]
- Jâcomo A. T. A., Silveira L., Diniz-Filho J. A. F. 2004. Niche separation between the maned wolf (Chrysocyon brachyurus), crabeating fox (Dusicyon thous) and the hoary fox (Dusicyon vetulus) in central Brazil. Journal of Zoology (London) 262:99–106. [Google Scholar]
- Jiang Z. G, Li C. W., Zeng Y. 2003. Status of the research on masked palm civets. Chinese Journal of Zoology 38:120–122 (in Chinese with English summary). [Google Scholar]
- Joshi A. R., Smith J. L. D., Cuthbert F. J. 1995. Influence of food distribution and predation pressure on spacing behavior in palm civets. Journal of Mammalogy 76:1205–1212. [Google Scholar]
- Juarez K. M., Marinho J. 2002. Diet, habitat use, and home ranges of sympatric canids in central Brazil. Journal of Mammalogy 83:925–933. [Google Scholar]
- Kitamura S., et al. 2002. Interactions between fleshy fruits and frugivores in a tropical seasonal forest in Thailand. Oecologia 133:559–572. [DOI] [PubMed] [Google Scholar]
- Kjellander P., Nordström J. 2003. Cyclic voles, prey switching in red fox, and roe deer dynamics—a test of the alternative prey hypothesis. Oikos 101:338–344. [Google Scholar]
- Kruuk H., Parish T. 1981. Feeding specialization of the European badger (Meles meles) in Scotland. Journal of Animal Ecology 50:773–788. [Google Scholar]
- Leckie F. M., Thirgood S. J., Redpath S. M. 1998. Variation in the diet of red foxes on Scottish moorland in relation to prey abundance. Ecography 21:599–604. [Google Scholar]
- Lundrigan B., Baker S. 2003. Paguma larvata. http://animaldiversity.ummz.umich.edu/site/accounts/information/paguma_larvata.html. Accessed 2 August 2005.
- Lurz P. W. W., Garson P. J., Wauters L. A. 2000. Effects of temporal and spatial variations in food supply on the space and habitat use of red squirrels, Sciurus vulgaris L. Journal of Zoology (London) 251:167–178. [Google Scholar]
- MacArthur R. H., Pianka E. R. 1966. On optimal use of a patchy environment. American Naturalist 100:603–609. [Google Scholar]
- Manfredi C, Lucherini M., Canepuccia A., Casanave E. B. 2004. Geographical variation in the diet of Geoffroy's cat (Oncifelis geoffroyi) in Pampas grassland of Argentina. Journal of Mammalogy 85:1111–1115. [Google Scholar]
- Marassi M., Biancardi C. M. 2002. Diet of the Eurasian badger (Meies meles) in an area of the Italian Prealps. Hystrix 13:19–28. [Google Scholar]
- Martinoli A., Preatoni D. G., Chiarenzi B., Wauters L. A., Tosi G. 2001. Diet of stoats (Mustela erminea) in an alpine habitat: the importance of fruit consumption in summer. Acta Oecologica 22:45–53. [Google Scholar]
- Mitchell W. A. 1989. Informational constraints on optimally foraging hummingbirds. Oikos 55:145–154. [Google Scholar]
- Narang M. L. 1996. Some notes on Himalayan palm civet, Paguma larvata (Hamilton-Smith) (Carnivora: Viverridae). Journal of the Bombay Natural History Society 93:80–81. [Google Scholar]
- Norrdahl K., Korpimäki E. 2000. Do predators limit the abundance of alternative prey? Experiments with vole-eating avian and mammalian predators. Oikos 91:528–540. [Google Scholar]
- Nowak R. M. 1999. Walker's mammals of the world. 6th ed. Johns Hopkins University Press, Baltimore, Maryland. [Google Scholar]
- Olsson O., Wiktander U., Malmqvist A., Nilsson S. G. 2001. Variability of patch type preferences in relation to resource availability and breeding success in a bird. Oecologia 127: 435–443. [DOI] [PubMed] [Google Scholar]
- Patterson B. R., Benjamin L. K., Messier F. 1998. Prey switching and feeding habits of eastern coyotes in relation to snowshoe hare and white-tailed deer densities. Canadian Journal of Zoology 76:1885–1897. [Google Scholar]
- Prange S., Gehrt S. D., Wiggers P. E. 2004. Influences of anthropogenic resources on raccoon (Procyon lotor) movements and spatial distribution. Journal of Mammalogy 85:483–490. [Google Scholar]
- Prugh L. R. 2005. Coyote prey selection and community stability during a decline in food supply. Oikos 110:253–264. [Google Scholar]
- Rabinowitz A. R. 1991. Behaviour and movements of sympatric civet species in Huai Kha Khaeng Wildlife Sanctuary, Thailand. Journal of Zoology (London) 7:37–47. [Google Scholar]
- Raymond M., Robitaille J. F., Lauzon P., Vaudry R. 1990. Prey-dependent profitability of foraging behaviour of male and female ermine, Mustela erminea. Oikos 58:323–328. [Google Scholar]
- Revilla E., Palomares F. 2002. Does local feeding specialization exist in Eurasian badgers? Canadian Journal of Zoology 80:83–93. [Google Scholar]
- Reynolds J. C, Aebischer N. J. 1991. Comparison and quantification of carnivore diet by faecal analysis: a critique, with recommendations, based on a study of the fox Vulpes vulpes. Mammal Review 21:97–122. [Google Scholar]
- Rode D. K., Robbins C. T. 2000. Why bears consume mixed diets during fruit abundance. Canadian Journal of Zoology 78:1640–1645. [Google Scholar]
- Rosalino L. M., Loureiro F., Macdonald D. W., Santos-Reis M. 2005. Dietary shifts of the badger (Meles meles) in Mediterranean woodlands: an opportunistic forager with seasonal specialisms. Mammalian Biology 70:12–23. [Google Scholar]
- Sacks B. N., Neale J. C. C. 2002. Foraging strategy of a generalist predator toward a special prey: coyote predation on sheep. Ecological Applications 12:299–306. [Google Scholar]
- Schoener T. W. 1971. Theory of feeding strategies. Annual Review of Ecology and Systematics 2:369–404. [Google Scholar]
- Silva S. L, Bozinovic F., Jaksic F. M. 2005. Frugivory and seed dispersal by foxes in relation to mammalian prey abundance in a semiarid ecosystem. Journal of Austral Ecology 30:749–756. [Google Scholar]
- Small R. J., Marcström V., Willebrand T. 1993. Synchronous and nonsynchronous population fluctuations of some predators and their prey in central Sweden. Ecography 16:360–364. [Google Scholar]
- Song C. S., Liu S. X. 1999. Scientific survey of Houhe Nature Reserve Hubei. China Forestry Publishing House, Beijing, China: (in Chinese). [Google Scholar]
- SPSS 2003. SPSS for Windows release 12.01. SPSS, Chicago, Illinois. [Google Scholar]
- Stein R. A. 1977. Selective predation, optimal foraging, and the predator-prey interaction between fish and crayfish. Ecology 58:1237–1253. [Google Scholar]
- Stephens D. W., Lynch J. F., Sorensen A. E., Gordon C. 1986. Preference and profitability: theory and experiment. American Naturalist 127:533–553. [Google Scholar]
- Stickney A. 1991. Seasonal patterns of prey availability and the foraging behaviour of arctic foxes (Alopex lagopus) in a waterfowl nesting area. Canadian Journal of Ecology 69:2853–2859. [Google Scholar]
- Sun R. Y. 2001. Principles of animal ecology. Beijing Normal University Press, Beijing, China: (in Chinese). [Google Scholar]
- Sundell J., Eccard J. A., Tiilikainen R., Ylönen H. 2003. Predation rate, prey preference and predator switching: experiments on voles and weasels. Oikos 101:615–623. [Google Scholar]
- Sundell J., Norrdahl K., Korpimäki E., Hanski I. 2000. Functional response of the least weasel (Mustela nivalis nivalis). Oikos 90:501–508. [Google Scholar]
- Thomas D., Tian S., Gui X. 2004 Camera-trapping to determine the status of south China tiger in Hupingshan National Nature Reserve, Hunan Province. Pp. 241–242 in Proceedings of the XlXth International Congress of Zoology (China Zoological Society , ed.). China Zoological Society, Beijing, China. [Google Scholar]
- Thompson I. D., Colgan P. W. 1990. Prey choice by marten during a decline in prey abundance. Oecologia 83:443–451. [DOI] [PubMed] [Google Scholar]
- Tsang A. C. W., Corlett R. T. 2005. Reproductive biology of the Ilex species (Aquifoliaceae) in Hong Kong, China. Canadian Journal of Botany 83:1645–1654. [Google Scholar]
- Wang H. B. 1999. Wildlife conservation in rural southeastern China: wildlife harvest and the ecology of sympatric carnivores. Ph.D. dissertation, University of Massachusetts, Amherst. [Google Scholar]
- Wang H., Fuller T. K. 2003. Food habits of four sympatric carnivores in southeastern China. Mammalia 67:513–519. [Google Scholar]
- Wang W. X., Fu Y. S., Yang Y., Cheng J., Li X. Y. 1997. Floral characteristics of the Houhe Nature Reserve in the southwest Huibei. Journal of Wuhan Botanical Research 15:353–362 (in Chinese with English summary). [Google Scholar]
- Wang Y. X. 1987. Paguma larvata. Pp. 282–293 in Fauna Sinica: Mammalia. Vol. 8. Carnivora (Gao Y. T., ed.). Science Press, Beijing, China: (in Chinese). [Google Scholar]
- Wilmshurst J. F., Fryxell J. M., Farm B. P., Sinclair A. R. E., Hensche C. P. 1999. Spatial distribution of Serengeti wildebeest in relation to resources. Canadian Journal of Zoology 77:1223–1232. [Google Scholar]
- Zhang L., Jones G., Parsons S., Liang B., Zhang S. 2005. The diet of flat-headed bats Tylonycteris pachypus and T. robustula in Guangxi, South China. Journal of Mammalogy 86:61–66. [Google Scholar]
- Zhao Q. K. 1994. Seasonal changes in body weight of Macaca thibetana at Mt. Emei, China. American Journal of Primatology 32:223–226. [DOI] [PubMed] [Google Scholar]