Abstract
Introduction
In heart transplant recipients (HTRs), non-adherence (NA) to immunosuppressive (IS) medication and to recommended lifestyle behaviours are a common phenomenon and associated with higher risk of allograft rejection, organ loss and mortality. Risk factors for NA are highly diverse and still insufficiently researched. Precise measures of NA and an accurate understanding of its aetiology are of undisputable importance to detect patients at risk and intervene accordingly. The aim of this study is to assess the accuracy and concordance of different measures for NA as well as to determine potential risk factors.
Methods and analysis
This is a single-centre prospective observational trial. HTRs who are at least aged 18 are no less than 6 months post-transplant and receive tacrolimus (Prograf or Advagraf), cyclosporine (Sandimmun) or everolimus (Certican) as their prescribed IS medication are eligible for participation. We only include patients during the phase of medication implementation. At study enrolment, we assess depression, health-related quality of life, self-efficacy, social support, attachment, experiences and attitudes towards IS medication, emotional responses after transplantation, satisfaction with information about IS medication and perceptions and beliefs about medications. We further ask patients to rate their lifestyle behaviours concerning alcohol, smoking, diet, physical activity, sun protection and appointment keeping via questionnaires. Three different measurement methods for NA are applied at T0: self-reports, physician’s estimates and IS trough levels. NA is monitored prospectively using an electronic multicompartment pillbox (MEMS, VAICA) over a 3-month period. Meanwhile, participants receive phone calls every second week to obtain additional self-reports, resulting in a total of seven measurement points.
Ethics and dissemination
The study was approved by the Clinical Ethics Committee of the University Hospital Erlangen (Friedrich-Alexander-University, Erlangen-Nürnberg). Written informed consent is attained from all participants. The results of this study will be published in peer-reviewed journals and presented at conferences.
Trial registration number
DRKS00020496.
Keywords: cardiology, transplant medicine, statistics & research methods
Strengths and limitations of this study.
This is the first study assessing potential risk factors of immunosuppressive (IS) non-adherence (NA) and non-pharmacological NA by prospectively applying electronic monitoring in heart transplant recipients.
This study combines different measurement methods for NA, such as electronic monitoring, self-reports, physician’s estimates and IS trough-level variability.
One limitation of our study is a potential initial intervention effect induced by electronic monitoring; however, adherence behaviour is likely to stabilise after approximately 40 days.
This is a single-centre study with a moderate sample size.
Introduction
Adherence is defined as ‘the process by which patients take their medication as prescribed’.1 Especially in transplant recipients, regular and accurate intake of immunosuppressants is vital for organ survival.2 3 Immunosuppressive (IS) non-adherence (NA) rates in transplant recipients differ greatly depending on the respective organ, with a mean prevalence of approximately 22.6% of patients per year.4–10 For heart transplant recipients (HTRs), NA rates range between 4.6% and 39.2%,4–7 11 12 influenced by the choice of measurement methods, operational definitions and case finding methods. Due to a rising awareness of its detrimental impact on allograft rejection, organ loss and mortality,2 13 research on NA in HTRs has increased substantially in recent years. Already minor deviations from the medical regimen have been associated with hazardous effects on organ and patient survival.14 Various assessment tools for NA are currently available, comprising both indirect and direct measures. Direct measures include direct observation and blood assays, whereas for indirect measures pill counts, self-reports, collateral reports and electronic monitoring are used.15 16 The most frequently employed NA measure for HTRs is self-report.16 Despite their susceptibility to errors, such as memory bias and social desirability, self-reports are considered practical and inexpensive tools for NA assessment.15 17–20 Although electronic monitoring allows insights into patterns of adherence behaviour, its use can be quite expensive and labour intensive.15 17 Except for few studies,2 5 14 electronic monitoring is only very sparsely used in HTRs.16 Blood assays such as the trough-level variability are also frequently applied as an adherence measure, since they have increasingly been associated with rejection and mortality in HTRs.13 21 22 Collateral reports, however, are mostly considered unreliable measurement methods for NA.23 To increase sensitivity, some studies also use a combination of measurement methods to assess NA, which mostly results in very high NA rates.5 15 Few studies have examined the accuracy and concordance of these NA measures17 24–28; especially in HTRs, research is scarce. However, an accurate assessment of NA seems crucial in order to detect patients at risk and to intervene accordingly.
Besides the relevancy of immunosuppressant intake, certain lifestyle habits can also adversely affect organ survival as well as patient morbidity and mortality after transplantation.29–33 Nonetheless, a considerable amount of HTRs insufficiently adheres to certain lifestyle recommendations. Compared with other types of organs, HTRs display particularly high NA rates for physical exercise, with prevalences ranging between 33.7% and 49.1%.4 11 29 34 For following dietary recommendations, NA rates between 22.9% and 46% were observed, while those for keeping follow-up appointments varied between 5.7% and 37.3%.4 11 29 30 34 Alcohol use was found for approximately 4.9% to 27.8% of HTRs, while tobacco use was confirmed by 3.2% to 9.1%.4 11 29 34 Helmy et al29 further revealed that up to 39.9% of HTRs did not apply sun protection as recommended. Correlations between the various domains of lifestyle habits could not be detected,11 whereas IS NA was found to be interrelated with appointment keeping30 and smoking.12 Research on non-pharmacological NA is sparse and only few have examined the occurrence of and interrelations between various NA areas. However, research on solid organ transplant recipients that included HTRs found certain patient-related risk factors for unhealthy lifestyle behaviours. Post-transplant smoking was found to be more prevalent in men, but less in older patients and those with comorbid physical diseases such as diabetes and hypertension.33 Smoking in turn was found to be a risk factor for post-transplant at-risk drinking, while post-transplant alcohol use in general could be linked with male gender, being employed, and a history of psychiatric illness, among others.35 No significant risk factors were found for low physical exercise.36 Most of these studies are based on the results of kidney recipients, while research on heart recipients is rare. Especially studies connecting non-pharmacological NA with electronically monitored IS NA in HTRs are missing.
Risk factors for IS NA on the other hand are well examined, although highly diverse and multifactorial in nature. They can reach from health system factors and behaviours of healthcare providers to factors on the individual patient level.37 When developing interventions, especially the identification of modifiable factors on the patient level is of primary interest. A broad literature search on studies comprising HTRs revealed a variety of patient-related factors such as depression,38 lower social support,4 6 11 39 lower quality of life,34 non-white ethnicity,4 13 negative feelings,40 41 attitudes to medication or treatment,41–43 higher frequency of medication intake,44 longer time since transplantation6 40 42 and younger age40 to be associated with NA. In renal transplant recipients, also anxiety,45 male sex,45 46 lower self-efficacy47–49 and avoidant attachment50 were found to be linked to increased NA. Although a considerable amount of research is devoted to the reasons and risk factors for IS NA, there is still a wide array of contradictory and heterogeneous results. Further, research examining risk factors exclusively in HTRs is sparse. Most studies examining risk factors for NA use self-reports, physicians’ estimates or trough levels. To our knowledge, no study has examined potential risk factors for NA in HTRs that were measured by applying electronic monitoring. What is more, studies on risk factors for NA to recommended lifestyle behaviours are missing. In order to develop adequate adherence interventions, assessing the accuracy of NA measures as well as determining factors that promote NA is of paramount importance. With this study, we cover relevant topics on adherence after heart transplantation and wish to fill substantial gaps in current research.
Study aims
The aim of our study is to investigate electronically monitored NA in HTRs over a period of 3 months during the phase of medication implementation.1 51 The following three research questions (RQs) will be addressed:
Measurement methods: Do different measurement methods of NA correlate with each other at the beginning of the study? Does electronically monitored NA coincide with self-reported NA during the course of the study?
Psychosocial predictors: Can certain psychosocial factors predict electronically monitored NA in HTRs?
Non-pharmacological adherence and lifestyle behaviours: To what extend do HTRs comply with lifestyle requirements? Does IS medication coincide with healthy lifestyle behaviours? What are potential risk factors for these behaviours?
Methods and analysis
Setting and recruitment
This work is part of the APT (Adherence and psychological health after transplantation) research project of the Department of Psychosomatic Medicine and Psychotherapy and takes place in cooperation with the Department of Cardiac Surgery and the Department of Nephrology and Hypertension in Erlangen. This substudy is conducted at the outpatient clinic of the Department of Cardiac Surgery of the University Hospital of Erlangen. During the course of 1 year, the sample is consecutively derived from HTRs undergoing their routine follow-up examination. Prior to their appointment, eligible patients are contacted via telephone and receive study information and questionnaires by mail, if interested.
Study population
Inclusion criteria for the study are patients who are at least 18 years old, have undergone heart transplantation at least 6 months ago and receive either tacrolimus (Advagraf or Prograf), cyclosporine (Sandimmun) or everolimus (Certican) as their prescribed IS medication. Patients with neurocognitive disabilities, who have no sufficient knowledge of the German language, and/or have severe mental disorders will be excluded from the study. Medication adherence was no eligibility criterion. We specifically focus on medication implementation and exclude cases of initiation.1 51 Of 100 eligible patients per year, we expect a responder rate of approximately 50%, resulting in a potential sample of 50 HTRs. We attain written informed consent from all participating patients (see online supplemental files 1 and 2). The study was approved by the Clinical Ethics Committee of the University Hospital Erlangen (Friedrich-Alexander-University, Erlangen-Nürnberg, FAU).
bmjopen-2020-038637supp001.pdf (657.5KB, pdf)
bmjopen-2020-038637supp002.pdf (139.4KB, pdf)
Study design and measurement points
This is a prospective clinical observational trial. At study enrolment, each patient is asked to fill in a questionnaire battery on potential psychosocial predictors of NA and recommended lifestyle behaviours. Further, self-reports, collateral reports and IS trough levels are assessed. Electronic monitoring will take place for 3 months. Meanwhile, each participant will receive phone calls in an interval of 2 weeks. Self-reports on potential incidents of NA referring to the last 2 weeks will be obtained. At the end of the study, patients will receive a feedback on their medication intake behaviour. The timeline of our study procedure is viewed in figure 1.
Figure 1.
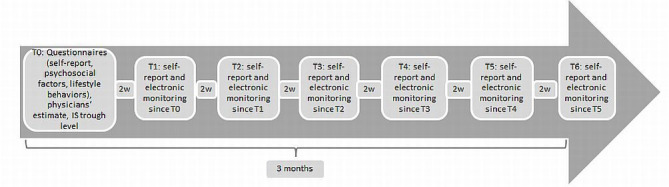
Timeline of study procedure. 2w=2 weeks.
Measurement methods
IS adherence measures
Collateral report
Adherence assessments are made after the patients’ appointments with their treating physician. The respective cardiologist is asked to rate the patients’ IS adherence both on a 5-point Likert Scale (1=very good, 5=very bad) and on a 10 cm (0%–100%) Visual Analogue Scale (VAS) according to their subjective estimate of the patients’ global adherence behaviour. Similar measures were used in the previous research.23 24
Self-report
As a means to assess self-reported NA, the patients equally receive a 10 cm (0%–100%) VAS (part of the Basel Assessment of Adherence to Immunosuppressive Mediation Scale (BAASIS20) Interview, in order to reach comparability with the collateral report. We further apply the BAASIS Questionnaire20, which consists of four items that relate to the medication taking behaviour of the last 4 weeks. It assesses four types of IS NA (dose taking, drug holiday, timing adherence >2 hour and dose reduction) that can be rated on a 6-point scale (0=never, 5=everyday). Patients who consent to at least one of these four items are classified as non-adherent. We added a further question on timing adherence covering an additional intake interval of ±30 min. During the course of the study, we also ask for the absolute frequency of IS intake deviations since the last phone call (recall period of approximately 2 weeks).
IS trough-level variability
IS levels are routinely checked at each follow-up examination at the outpatient clinic as well as at the patients’ respective resident doctors every 8–10 weeks. We will use the IS trough level that is measured at study enrolment as well as up to three antecedent measures,52 since reliability improves with an increased number of measurement points.23 53 For frame of reference, each IS level is positioned with respect to its respective target level that can be changed individually by the treating cardiologist depending on the clinical course and time since transplantation. The graded standard target levels for the different IS regimens can be viewed in the online supplemental file 3. We then will assess IS trough level variability (CV%: percent coefficient of variation53) that has been associated with graft rejection, mortality and NA.21 22 54 For the calculation of CV%, each IS level will be standardised by dividing it by its respective target level. For each standardised IS trough level, we will calculate means and SD. By dividing the SD by the mean and multiplying it by 100, we will gain the CV%.53 54 Higher CV%s are associated with a higher fluctuation of IS trough levels.53 The IS trough levels for the different IS regimens will be dealt with equally.
bmjopen-2020-038637supp003.pdf (68.3KB, pdf)
Electronic monitoring
Electronic monitoring of NA is taking place with a multicompartment pillbox (VAICA SimpleMed), which provides medication storage for 7 days with up to four doses per day. Medication extraction is automatically recorded and transferred to a web portal where the individual IS medication schedules of each patient are registered. Data transfer is done using cellular reception. For timing adherence, we set intervals at ±30 min and ±2 hours. Reminder functions (visual/acoustic signals) are disabled to keep a possible intervention effect at a minimum. In addition, participants are requested to keep a diary for incidences when medication extraction does not coincide with pill intake or the medication is taken from another source (pocket, take-away box etc). Irrespective of each patient’s dosing frequency and regimen, the percentage of (on time) taken immunosuppressants will be calculated per day, for each measurement point (T1–T6), as well as for the whole study course, attaining values between 0% and 100%. If the patient does not use the pill box for a certain period (due to hospital stay, vacation, bad reception), the respective days are registered as missing.
Psychosocial variables
For the assessment of patient-related psychosocial risk factors, a variety of instruments is applied (see table 1). The choice of potential determinants is limited to individual patient-related factors amenable to change and modifiable by interventions.
Table 1.
Psychosocial constructs and applied questionnaires
| Psychosocial construct | Instrument | Information |
| Depression | PHQ-974 75 | Self-report screening instrument of depression, nine Items, 4-point scale |
| Perceived social support | FSozU-776 | Self-report instrument on social support (practical support, emotional support, social integration), short form of F-SozU, seven items, 5-point scale |
| Perceived health-related quality of life | WHOQoL-BREF77 | Self-report instrument on perceived health-related quality of life (physical health, psychological health, social relationships, environment), short form of WHOQoL-100, 26 items, 5-point scale |
| Self-efficacy | SWE78 79 | Self-report questionnaire, 10 items, 4-point scale |
| Attachment | RSQ80 81 | Self-report questionnaire, 30 items, 5-point scale |
| Subjective experiences and attitudes towards immunosuppressive medication | MESI82 | Self-report questionnaire, seven items, 5-point scale |
| Emotional responses after organ transplantation | TxEQ83 84 | Self-report questionnaire on emotional responses after Tx (guilt, worry, disclosure, adherence, responsibility), 23 items, 5-point scale |
| Satisfaction with information about immunosuppressive medication | SIMS-D85 86 | Self-report questionnaire, 17 items, 5-point scale |
| Perceptions of and beliefs about medications | BMQ87 88 | Self-report questionnaire, 18 items, 5-point scale |
PHQ-9: Patient Health Questionnaire [Gesundheitsfragebogen für Patienten], FSozU-7: Fragebogen zur sozialen Unterstützung, WHOQoL-BREF: World Health Organization Quality of Life - Short Form, SWF: General Self Efficacy Scale [Skala zur allgemeinen Selbstwirksamkeitserwartung], RSQ: Relationship Scales Questionnaire, MESI: Medication Experience Scale for Immunosuppressants [Medikamenten-Erfahrungs-Skala für Immunsuppressiva], TxEQ: The Transplant Effects Questionnaire [Fragebogen zur psychischen Verarbeitung einer Organtransplantation], SIMS-D: The Satisfaction with Information about Medicines Scale, BMQ: The Beliefs about Medicines Questionnaire.
Non-pharmacological NA and lifestyle behaviour
To assess non-pharmacological NA to recommended lifestyle behaviours, we use self-report data on physical activity, diet, sun protection, smoking, appointment keeping and alcohol use. For better comparability, we apply similar measurement methods used in the previous research.29 How each component is measured is viewed in table 2.
Table 2.
Methods to assess non-pharmacological NA to recommended lifestyle behaviours
| Variable | Instrument | Information |
| Physical activity | Brief physical activity assessment tool89 | Two items, frequency of intense and moderate physical activity during an average week (adherent: sufficiently active, non-adherent: insufficiently active) |
| Smoking | Self-developed (based on measure used by Helmy et al29) | One item on current smoking status (adherent: never smoked/stopped before HTx, non-adherent: stopped after HTx, smokes sometimes/several times a week/daily) |
| Alcohol use | Self-developed (based on measure used by Helmy et al29) | Two items on current alcohol use (frequency of alcohol use per average week, usual quantity of alcohol intake), non-adherent:>1 drink/day (0.33 L) (women), >2 drinks/day (men) |
| Sun protection | According to measure developed by Helmy et al29 | Four items on current sun protection (using sun screen, wearing protective clothing, staying in the shade, being sensitive to the time of day), 5-point scale, adherent: always using ≥1 of protection methods, non-adherent: not always using at least 1 |
| Diet | According to measure developed by Helmy et al29 | One item on adherence to general dietary recommendations, four items on daily diet (sugar, low calorie, low saturated fats and low salt), 5-point scale, adherent: min. score 4–5 on all dietary recommendation, non-adherent: scores of 1–3 on any scale |
| Appointment keeping | Self-developed (based on measure used by Helmy et al29) | One item, frequency of unexcused absence at scheduled follow-up appointments since transplantation (adherent: 0, non-adherent:≥1) |
HTx, heart transplantation.
Patient and public involvement
Patients and the public are not involved in the design, recruitment and conduction of this study.
Statistical analysis plan
Data will be analysed using SPSS V.21 for Microsoft Windows as well as the nlme-package55 and the lme4-package56 for R V.3.5.1. For descriptive statistics, we will depict frequencies, mean values, SDs and ranges. For group comparisons (non-responder-analyses), we will use independent T-tests, Mann-Whitney-U-tests and χ² tests. Cohen’s kappa and the Intraclass correlation coefficient (ICC) will be used to conduct analyses on the association of measurement methods and the relation between lifestyle behaviours and NA (RQs 1 and 3). For prospective analyses, we will perform multiple linear regressions with the percentage frequency of electronically monitored NA as the outcome variable (RQs 1 and 2). To assess changes in probability of adherence over time, two alternative linear mixed models (strictly linear and piecewise linear) on the percentage frequency of NA of each measurement point (T1–T6) will be conducted (RQ 1). In order to examine potential risk factors for non-pharmacological adherence, logistic regression analyses will be applied (RQ 3). If predictor count in regression analyses is restricted due to limited sample size, we will conduct preliminary analyses (eg, Spearman’s ρ, Pearson’s r, ICC) in order to insert only variables that significantly correlate with the outcome. Bonferroni-Holm corrections will be made for all statistical tests in order to adjust for the alpha-error of multiple testing. Results will be interpreted on a significance level of p<0.05.
Progress
Recruitment started in September 2019. Of 48 eligible patients, 45 could be contacted via telephone before their follow-up examination. Of those, 18 (40%) agreed to participate and are currently taking part in our study, whereas 27 declined participation. Reasons for refusal are lack of study interest, lack of time, sickness and inconvenience or impossibility to integrate the pillbox into daily routine. Sociodemographic and biomedical data of all patients already included in our study are depicted in table 3.
Table 3.
Sociodemographic and biomedical data of current participants
| Patients (N=18) | |
| Age (M, SD, range) | 56.93 (±15.59), 32–82 |
| Sex (n, %) | |
| Male | 18 (100) |
| Female | 0 (0) |
| Marital status (n, %) | |
| Single | 3 (16.67) |
| Married/in a relationship | 11 (61.11) |
| Separated/divorced | 1 (5.56) |
| No information | 3 (16.67) |
| Immunosuppressive medication (n, %) | |
| Advagraf (once daily) | 1 (5.6) |
| Prograf (two times daily) | 7 (38.89) |
| Sandimmun (two times daily) | 6 (33.3) |
| Certican (two times daily) | 2 (11.1) |
| Combination of Certican and Sandimmun | 1 (5.56) |
| Combination of Certican and Prograf | 1 (5.56) |
| Last transplantation (median, SD, range) | 2010 (±4.72), 2003–2017 |
Discussion
To our knowledge, this is the first study assessing potential risk factors of IS NA and non-pharmacological NA by prospectively applying electronic monitoring as the main NA measure. To date, no study has combined electronic monitoring, self-reports, physicians’ estimates and IS trough level variability to measure NA in HTRs. Although, measuring NA might induce an intervention effect resulting in temporarily improved adherence, adherence behaviour is likely to stabilise on its base level after approximately 35–40 days.57 58 Yet, by applying a combination of NA measures, especially electronic monitoring, more accurate statements about prevalences of NA and its potential risk factors in HTRs can be made. Precise measures and a detailed knowledge of potential determinants are crucial in order to develop adequate adherence interventions and reduce the fatal consequences of NA. In recent years, several well-designed interventions for the improvement of IS adherence have been published.59–63 Most of these interventions are tailored to meet the needs of renal transplant recipients. Interventions specifically for HTRs are sparse or non-existent, especially since research on prevalences and risk factors of NA are mostly focused on renal transplant recipients.4
Further, more research on lifestyle behaviours is required to fully understand its reasons as well as potential implications on rejection and organ survival in HTRs. The potentially harmful consequences of insufficient physical activity, inadequate sun protection, tobacco and alcohol use and unhealthy diet are already well established in healthy populations.64–68 But especially HTRs who are obliged to lifelong immunosuppression intake are at an even greater risk of developing chronic diseases and health issues when engaging in unhealthy lifestyle behaviours.29 69 70 In order to prevent comorbid diseases and health damages, the development of interventions specifically targeting lifestyle habits and its potential risk factors33 35 are substantial. Two recent studies on renal transplant recipients showed an improvement of sun-protective behaviour after an educational intervention.71–73 Interventions for the improvement of other lifestyle behaviours are lacking. For the development of adequate lifestyle interventions, the investigation of reasons and risk factors for these unhealthy behaviours is a prerequisite that we wish to meet with this study.
If patients with acute forms of severe depression, excessive substance abuse or other self-harming behaviours are detected during study recruitment, immediate interventions or transferrals to the respective outpatient clinics can be initiated.
With the combination of lifestyle habits, psychosocial factors and a bandwidth of IS NA measures, especially electronic monitoring, our study will provide a promising opportunity to shed further light onto highly clinically relevant topics. We expect that our findings will contribute to the refinement of NA measures as well as the development of comprehensive interventions for HTRs.
Ethics and dissemination
The study was approved by the Clinical Ethics Committee of the University Hospital Erlangen (Friedrich-Alexander-University, Erlangen-Nürnberg, FAU). Written informed consent is attained from all participants. Patient data are pseudonymised. Each patient receives a three-digit code under which personal and medical data are saved. In a password protected file, the patients’ name can be linked to the three-digit code to which only the study director has access to. At the end of the project, the file on patient allocation will be deleted. The results of this study will be published in peer-reviewed journals and presented at national and international conferences.
Supplementary Material
Acknowledgments
We wish to thank the staff of the Department of Cardiac Surgery at the University Hospital of Erlangen for enabling the organisation and conduction of this project. We especially would like to thank all patients who are taking part in our study.
Footnotes
Contributors: ML designed and drafted the manuscript and is currently conducting the study. YE supervises and supports the conceptualisation and conduction of the study. MW enabled the initiation of this project and supervises study coordination. MS supports patient recruitment. All authors revised the manuscript critically for important intellectual content, have given final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Vrijens B, De Geest S, Hughes DA, et al. . A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012;73:691–705. 10.1111/j.1365-2125.2012.04167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobbels F, De Geest S, van Cleemput J, et al. . Effect of late medication non-compliance on outcome after heart transplantation: a 5-year follow-up. J Heart Lung Transplant 2004;23:1245–51. 10.1016/j.healun.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 3.Denhaerynck K, Dobbels F, Cleemput I, et al. . Prevalence, consequences, and determinants of nonadherence in adult renal transplant patients: a literature review. Transpl Int 2005;18:1121–33. 10.1111/j.1432-2277.2005.00176.x [DOI] [PubMed] [Google Scholar]
- 4.Dew MA, DiMartini AF, De Vito Dabbs A, et al. . Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation 2007;83:858–73. 10.1097/01.tp.0000258599.65257.a6 [DOI] [PubMed] [Google Scholar]
- 5.De Bleser L, Dobbels F, Berben L, et al. . The spectrum of nonadherence with medication in heart, liver, and lung transplant patients assessed in various ways. Transpl Int 2011;24:882–91. 10.1111/j.1432-2277.2011.01296.x [DOI] [PubMed] [Google Scholar]
- 6.Dew MA, Dabbs AD, Myaskovsky L, et al. . Meta-Analysis of medical regimen adherence outcomes in pediatric solid organ transplantation. Transplantation 2009;88:736–46. 10.1097/TP.0b013e3181b2a0e0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Geest S, Burkhalter H, Bogert L, et al. . Describing the evolution of medication nonadherence from pretransplant until 3 years post-transplant and determining pretransplant medication nonadherence as risk factor for post-transplant nonadherence to immunosuppressives: the Swiss transplant cohort study. Transpl Int 2014;27:657–66. 10.1111/tri.12312 [DOI] [PubMed] [Google Scholar]
- 8.Villeneuve C, Rousseau A, Rerolle J-P, et al. . Adherence profiles in kidney transplant patients: causes and consequences. Patient Educ Couns 2020;103:189–98. 10.1016/j.pec.2019.08.002 [DOI] [PubMed] [Google Scholar]
- 9.Bertram A, Fuge J, Suhling H, et al. . Adherence is associated with a favorable outcome after lung transplantation. PLoS One 2019;14:e0226167. 10.1371/journal.pone.0226167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paterson TSE, Demian M, Shapiro RJ, et al. . Impact of Once- versus twice-daily tacrolimus dosing on medication adherence in stable renal transplant recipients: a Canadian single-center randomized controlled trial. Can J Kidney Health Dis 2019;6:205435811986799. 10.1177/2054358119867993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dew MA, Dimartini AF, De Vito Dabbs A, et al. . Adherence to the medical regimen during the first two years after lung transplantation. Transplantation 2008;85:193–202. 10.1097/TP.0b013e318160135f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denhaerynck K, Berben L, Dobbels F, et al. . Multilevel factors are associated with immunosuppressant nonadherence in heart transplant recipients: the International BRIGHT study. Am J Transplant 2018;18:1447–60. 10.1111/ajt.14611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliva M, Singh TP, Gauvreau K, et al. . Impact of medication non-adherence on survival after pediatric heart transplantation in the U.S.A. J Heart Lung Transplant 2013;32:881–8. 10.1016/j.healun.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 14.De Geest S, Abraham I, Moons P, et al. . Late acute rejection and subclinical noncompliance with cyclosporine therapy in heart transplant recipients. J Heart Lung Transplant 1998;17:854–63. [PubMed] [Google Scholar]
- 15.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97. 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 16.Senft Y, Kirsch M, Denhaerynck K, et al. . Practice patterns to improve pre and post-transplant medication adherence in heart transplant centres: a secondary data analysis of the International bright study. Eur J Cardiovasc Nurs 2017:1474515117747577. 10.1177/1474515117747577 [DOI] [PubMed] [Google Scholar]
- 17.Monnette A, Zhang Y, Shao H, et al. . Concordance of adherence measurement using self-reported adherence questionnaires and medication monitoring devices: an updated review. Pharmacoeconomics 2018;36:17–27. 10.1007/s40273-017-0570-9 [DOI] [PubMed] [Google Scholar]
- 18.J. Foster B, L.H. Pai A. Adherence in adolescent and young adult kidney transplant recipients. Open Urol Nephrol J 2014;7:133–43. 10.2174/1874303X01407010133 [DOI] [Google Scholar]
- 19.Nevins TE, Nickerson PW, Dew MA. Understanding medication nonadherence after kidney transplant. J Am Soc Nephrol 2017;28:2290–301. 10.1681/ASN.2017020216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobbels F, Berben L, De Geest S, et al. . The psychometric properties and practicability of self-report instruments to identify medication nonadherence in adult transplant patients: a systematic review. Transplantation 2010;90:205–19. 10.1097/TP.0b013e3181e346cd [DOI] [PubMed] [Google Scholar]
- 21.Flippin MS, Canter CE, Balzer DT. Increased morbidity and high variability of cyclosporine levels in pediatric heart transplant recipients. J Heart Lung Transplant 2000;19:343–9. 10.1016/S1053-2498(00)00061-9 [DOI] [PubMed] [Google Scholar]
- 22.Ringewald JM, Gidding SS, Crawford SE, et al. . Nonadherence is associated with late rejection in pediatric heart transplant recipients. J Pediatr 2001;139:75–8. 10.1067/mpd.2001.115067 [DOI] [PubMed] [Google Scholar]
- 23.Pabst S, Bertram A, Zimmermann T, et al. . Physician reported adherence to immunosuppressants in renal transplant patients: prevalence, agreement, and correlates. J Psychosom Res 2015;79:364–71. 10.1016/j.jpsychores.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 24.Lieb M, Hepp T, Schiffer M, et al. . Accuracy and concordance of measurement methods to assess non-adherence after renal transplantation - a prospective study. BMC Nephrol 2020;21:114. 10.1186/s12882-020-01781-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Alili M, Vrijens B, Demonceau J, et al. . A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol 2016;82:268–79. 10.1111/bcp.12942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denhaerynck K, Steiger J, Bock A, et al. . Prevalence and risk factors of non-adherence with immunosuppressive medication in kidney transplant patients. Am J Transplant 2007;7:108–16. 10.1111/j.1600-6143.2006.01611.x [DOI] [PubMed] [Google Scholar]
- 27.Butler JA, Peveler RC, Roderick P, et al. . Measuring compliance with drug regimens after renal transplantation: comparison of self-report and clinician rating with electronic monitoring. Transplantation 2004;77:786–9. 10.1097/01.TP.0000110412.20050.36 [DOI] [PubMed] [Google Scholar]
- 28.Schäfer-Keller P, Steiger J, Bock A, et al. . Diagnostic accuracy of measurement methods to assess non-adherence to immunosuppressive drugs in kidney transplant recipients. Am J Transplant 2008;8:616–26. 10.1111/j.1600-6143.2007.02127.x [DOI] [PubMed] [Google Scholar]
- 29.Helmy R, Duerinckx N, De Geest S, et al. . The International prevalence and variability of nonadherence to the nonpharmacologic treatment regimen after heart transplantation: findings from the cross-sectional BRIGHT study. Clin Transplant 2018;32:e13280. 10.1111/ctr.13280 [DOI] [PubMed] [Google Scholar]
- 30.De Geest S, Dobbels F, Martin S, et al. . Clinical risk associated with appointment noncompliance in heart transplant recipients. Prog Transplant 2000;10:162–8. 10.1177/152692480001000306 [DOI] [PubMed] [Google Scholar]
- 31.Weinrauch LA, Claggett B, Liu J, et al. . Smoking and outcomes in kidney transplant recipients: a post hoc survival analysis of the FAVORIT trial. Int J Nephrol Renovasc Dis 2018;11:155–64. 10.2147/IJNRD.S161001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Opelz G, Döhler B. Influence of current and previous smoking on cancer and mortality after kidney transplantation. Transplantation 2016;100:227–32. 10.1097/TP.0000000000000804 [DOI] [PubMed] [Google Scholar]
- 33.Duerinckx N, Burkhalter H, Engberg SJ, et al. . Correlates and outcomes of posttransplant smoking in solid organ transplant recipients: a systematic literature review and meta-analysis. Transplantation 2016;100:2252–63. 10.1097/TP.0000000000001335 [DOI] [PubMed] [Google Scholar]
- 34.Brocks Y, Zittermann A, Grisse D, et al. . Adherence of heart transplant recipients to prescribed medication and recommended lifestyle habits. Prog Transplant 2017;27:160–6. 10.1177/1526924817699959 [DOI] [PubMed] [Google Scholar]
- 35.Dobbels F, Denhaerynck K, Klem ML, et al. . Correlates and outcomes of alcohol use after single solid organ transplantation: a systematic review and meta-analysis. Transplant Rev 2019;33:17–28. 10.1016/j.trre.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 36.Berben L, Engberg SJ, Rossmeissl A, et al. . Correlates and outcomes of low physical activity posttransplant: a systematic review and meta-analysis. Transplantation 2019;103:679–88. 10.1097/TP.0000000000002543 [DOI] [PubMed] [Google Scholar]
- 37.World Health Organisation Adherence to long-term therapies: evidence for action, 2003. Available: https://www.who.int/chp/knowledge/publications/adherence_report/en/
- 38.Delibasic M, Mohamedali B, Dobrilovic N, et al. . Pre-Transplant depression as a predictor of adherence and morbidities after orthotopic heart transplantation. J Cardiothorac Surg 2017;12:62. 10.1186/s13019-017-0626-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobbels F, Vanhaecke J, Dupont L, et al. . Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation 2009;87:1497–504. 10.1097/TP.0b013e3181a440ae [DOI] [PubMed] [Google Scholar]
- 40.Shemesh Y, Peles-Bortz A, Peled Y, et al. . Feelings of indebtedness and guilt toward donor and immunosuppressive medication adherence among heart transplant (HTx) patients, as assessed in a cross-sectional study with the Basel assessment of adherence to immunosuppressive medications scale (BAASIS). Clin Transplant 2017;31:e13053 10.1111/ctr.13053 [DOI] [PubMed] [Google Scholar]
- 41.Kung M, Koschwanez HE, Painter L, et al. . Immunosuppressant nonadherence in heart, liver, and lung transplant patients: associations with medication beliefs and illness perceptions. Transplantation 2012;93:958–63. 10.1097/TP.0b013e31824b822d [DOI] [PubMed] [Google Scholar]
- 42.Cherubini P, Rumiati R, Bigoni M, et al. . Long-Term decrease in subjective perceived efficacy of immunosuppressive treatment after heart transplantation. J Heart Lung Transplant 2003;22:1376–80. 10.1016/S1053-2498(03)00023-8 [DOI] [PubMed] [Google Scholar]
- 43.Hugon A, Roustit M, Lehmann A, et al. . Influence of intention to adhere, beliefs and satisfaction about medicines on adherence in solid organ transplant recipients. Transplantation 2014;98:222–8. 10.1097/TP.0000000000000221 [DOI] [PubMed] [Google Scholar]
- 44.Doesch AO, Mueller S, Akyol C, et al. . Increased adherence eight months after switch from twice daily calcineurin inhibitor based treatment to once daily modified released tacrolimus in heart transplantation. Drug Des Devel Ther 2013;7:1253–8. 10.2147/DDDT.S52820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belaiche S, Décaudin B, Dharancy S, et al. . Factors relevant to medication non-adherence in kidney transplant: a systematic review. Int J Clin Pharm 2017;39:582–93. 10.1007/s11096-017-0436-4 [DOI] [PubMed] [Google Scholar]
- 46.Prihodova L, Nagyova I, Rosenberger J, et al. . Adherence in patients in the first year after kidney transplantation and its impact on graft loss and mortality: a cross-sectional and prospective study. J Adv Nurs 2014;70:2871–83. 10.1111/jan.12447 [DOI] [PubMed] [Google Scholar]
- 47.Calia R, Lai C, Aceto P, et al. . Emotional self-efficacy and alexithymia may affect compliance, renal function and quality of life in kidney transplant recipients: results from a preliminary cross-sectional study. Physiol Behav 2015;142:152–4. 10.1016/j.physbeh.2015.02.018 [DOI] [PubMed] [Google Scholar]
- 48.Russell CL, Ashbaugh C, Peace L, et al. . Time-in-a-bottle (TIAB): a longitudinal, correlational study of patterns, potential predictors, and outcomes of immunosuppressive medication adherence in adult kidney transplant recipients. Clin Transplant 2013;27:E580–90. 10.1111/ctr.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva AN, Moratelli L, Tavares PL, et al. . Self-Efficacy beliefs, locus of control, religiosity and non-adherence to immunosuppressive medications in kidney transplant patients. Nephrology 2016;21:938–43. 10.1111/nep.12695 [DOI] [PubMed] [Google Scholar]
- 50.Calia R, Lai C, Aceto P, et al. . Attachment style predict compliance, quality of life and renal function in adult patients after kidney transplant: preliminary results. Ren Fail 2015;37:678–80. 10.3109/0886022X.2015.1010989 [DOI] [PubMed] [Google Scholar]
- 51.De Geest S, Zullig LL, Dunbar-Jacob J, et al. . ESPACOMP medication adherence reporting guideline (EMERGE). Ann Intern Med 2018;169:30–5. 10.7326/M18-0543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheel J, Reber S, Stoessel L, et al. . Patient-Reported non-adherence and immunosuppressant Trough levels are associated with rejection after renal transplantation. BMC Nephrol 2017;18:107. 10.1186/s12882-017-0517-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsiau M, Fernandez HE, Gjertson D, et al. . Monitoring nonadherence and acute rejection with variation in blood immunosuppressant levels in pediatric renal transplantation. Transplantation 2011;92:918–22. 10.1097/TP.0b013e31822dc34f [DOI] [PubMed] [Google Scholar]
- 54.Gueta I, Markovits N, Yarden-Bilavsky H, et al. . High tacrolimus Trough level variability is associated with rejections after heart transplant. Am J Transplant 2018;18:2571–8. 10.1111/ajt.15016 [DOI] [PubMed] [Google Scholar]
- 55.Pinheiro J, Bates D, DebRoy S, et al. . Nlme: linear and nonlinear mixed effect models. R Package Version 2014;3:1–117. [Google Scholar]
- 56.Bates D, Mächler M, Bolker B, et al. . Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw 2015;67:1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 57.Ekberg H, Tedesco-Silva H, Demirbas A, et al. . Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007;357:2562–75. 10.1056/NEJMoa067411 [DOI] [PubMed] [Google Scholar]
- 58.Denhaerynck K, Schäfer-Keller P, Young J, et al. . Examining assumptions regarding valid electronic monitoring of medication therapy: development of a validation framework and its application on a European sample of kidney transplant patients. BMC Med Res Methodol 2008;8:5. 10.1186/1471-2288-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foster BJ, Pai ALH, Zelikovsky N, et al. . A randomized trial of a multicomponent intervention to promote medication adherence: the teen adherence in kidney transplant effectiveness of intervention trial (TAKE-IT). Am J Kidney Dis 2018;72:30–41. 10.1053/j.ajkd.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Panyi N, Erim Y. [Manualized psychotherapy for the optimization of immunosuppressant adherence following kidney transplantation: Results of a feasibility study]. Z Psychosom Med Psychother 2017;63:189–201. 10.13109/zptm.2017.63.2.189 [DOI] [PubMed] [Google Scholar]
- 61.Reese PP, Bloom RD, Trofe-Clark J, et al. . Automated reminders and physician notification to promote immunosuppression adherence among kidney transplant recipients: a randomized trial. Am J Kidney Dis 2017;69:400–9. 10.1053/j.ajkd.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 62.Russell C, Conn V, Ashbaugh C, et al. . Taking immunosuppressive medications effectively (TIMELink): a pilot randomized controlled trial in adult kidney transplant recipients. Clin Transplant 2011;25:864–70. 10.1111/j.1399-0012.2010.01358.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russell CL, Moore S, Hathaway D, et al. . MAGIC study: aims, design and methods using SystemCHANGE™ to improve immunosuppressive medication adherence in adult kidney transplant recipients. BMC Nephrol 2016;17:84. 10.1186/s12882-016-0285-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shield KD, Parry C, Rehm J. Chronic diseases and conditions related to alcohol use. Alcohol Res 2013;35:155–73. [PMC free article] [PubMed] [Google Scholar]
- 65.Hernandez SL, Banks HE, Bailey AE, et al. . Relationships among chewing tobacco, cigarette smoking, and chronic health conditions in males 18-44 years of age. J Prim Prev 2017;38:505–14. 10.1007/s10935-017-0485-4 [DOI] [PubMed] [Google Scholar]
- 66.Lim SS, Vos T, Flaxman AD, et al. . A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2224–60. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leskinen T, Stenholm S, Aalto V, et al. . Physical activity level as a predictor of healthy and chronic disease-free life expectancy between ages 50 and 75. Age Ageing 2018;47:423–9. 10.1093/ageing/afy016 [DOI] [PubMed] [Google Scholar]
- 68.Green AC, Williams GM, Logan V, et al. . Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011;29:257–63. 10.1200/JCO.2010.28.7078 [DOI] [PubMed] [Google Scholar]
- 69.Naldi L, Fortina AB, Lovati S, et al. . Risk of nonmelanoma skin cancer in Italian organ transplant recipients. A registry-based study. Transplantation 2000;70:1479–84. 10.1097/00007890-200011270-00015 [DOI] [PubMed] [Google Scholar]
- 70.Söderlund C, Rådegran G. Immunosuppressive therapies after heart transplantation--The balance between under- and over-immunosuppression. Transplant Rev 2015;29:181–9. 10.1016/j.trre.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 71.Wu SZ, Jiang P, DeCaro JE, et al. . A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol 2016;75:1238–44. 10.1016/j.jaad.2016.06.031 [DOI] [PubMed] [Google Scholar]
- 72.Robinson JK, Guevara Y, Gaber R, et al. . Efficacy of a sun protection workbook for kidney transplant recipients: a randomized controlled trial of a culturally sensitive educational intervention. Am J Transplant 2014;14:2821–9. 10.1111/ajt.12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robinson JK, Friedewald JJ, Desai A, et al. . A randomized controlled trial of a mobile medical APP for kidney transplant recipients: effect on use of sun protection. Transplant Direct 2016;2. 10.1097/TXD.0000000000000561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Löwe B, Spitzer R, Zipfel S, et al. . Gesundheitsfragebogen für Patienten (PHQ-D). Komplettversion und Kurzform. Testmappe MIT manual, Fragebögen, Schablonen. Pfizer: Karlsruhe, 2002. [Google Scholar]
- 75.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fydrich T, Geyer M, Hessel A, et al. . Fragebogen zur Sozialen Unterstützung (F-SozU): Normierung an einer repräsentativen Stichprobe. Diagnostica 1999;45:212–6. 10.1026//0012-1924.45.4.212 [DOI] [Google Scholar]
- 77.Angermeyer C, Kilian R, Matschinger H. Deutschsprachige version Der who Instrumente Zur Erfassung von Lebensqualität WHOQOL-100 und WHOQOL-BREFM. Z Med Psychol 2002;11:44–8. [Google Scholar]
- 78.Schwarzer R, Jerusalem M. Generalized self-efficacy scale : Weinman J, Wright S, Johston M, Measures in health psychology: A user’s portfolio Causal and control beliefs. Windsor, England: Nfer-Nelson, 1995: p. 35–7. [Google Scholar]
- 79.Schwarzer R, Jerusalem M. Skalen Zur Erfassung von Lehrer- und Schülermerkmalen: Dokumentation Der psychometrischen Verfahren Im Rahmen Der Wissenschaftlichen Begleitung des Modellversuchs Selbstwirksame Schulen. Berlin: Freie Universität Berlin, 1999. [Google Scholar]
- 80.Steffanowski M, Oppl J, Meyerberg J, et al. . Psychometrische Überprüfung einer deutschsprachigen Version des Relationship Scales Questionnaire (RSQ) In: Bassler M, ed Störungsspezifische Therapieansätze - Konzepte und Ergebnisse. Gießen, 2001. [Google Scholar]
- 81.Griffin DW, Bartholomew K. The metaphysics of measurement: The case of adult attachment : Bartholomew K, Perlman D, Attachment processes in adulthood advances in personal relationships. 5 Jessica Kingsley, 1994: p. 17. [Google Scholar]
- 82.Goetzmann L, Klaghofer R, Spindler A, et al. . [The "Medication Experience Scale for Immunosuppressants" (MESI): initial results for a new screening instrument in transplant medicine]. Psychother Psychosom Med Psychol 2006;56:49–55. 10.1055/s-2005-867060 [DOI] [PubMed] [Google Scholar]
- 83.Klaghofer R, Sarac N, Schwegler K, et al. . Fragebogen zur psychischen Verabeitung einer Organtransplantation: Deutsche Validierung des Transplant Effects Questionnaire (TxEQ) [Questionnaire on emotional response after organ transplantation: German validation of the Transplant Effect Questionnaire (TxEQ-D)]. Z Psychosom Med Psychother 2008;54:174–88. [DOI] [PubMed] [Google Scholar]
- 84.Ziegelmann JP, Griva K, Hankins M, et al. . The Transplant Effects Questionnaire (TxEQ): The development of a questionnaire for assessing the multidimensional outcome of organ transplantation - example of end stage renal disease (ESRD). Br J Health Psychol 2002;7:393–408. 10.1348/135910702320645381 [DOI] [PubMed] [Google Scholar]
- 85.Mahler C, Jank S, Hermann K, et al. . Psychometric properties of a German version of the "Satisfaction with Information about Medicines Scale" (SIMS-D). Value Health 2009;12:1176–9. 10.1111/j.1524-4733.2009.00575.x [DOI] [PubMed] [Google Scholar]
- 86.Horne R, Hankins M, Jenkins R. The satisfaction with information about medicines scale (SIMS): a new measurement tool for audit and research. Qual Health Care 2001;10:135–40. 10.1136/qhc.0100135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999;14:1–24. 10.1080/08870449908407311 [DOI] [Google Scholar]
- 88.Mahler C, Hermann K, Horne R, et al. . Patients' beliefs about medicines in a primary care setting in Germany. J Eval Clin Pract 2012;18:409–13. 10.1111/j.1365-2753.2010.01589.x [DOI] [PubMed] [Google Scholar]
- 89.Marshall AL, Smith BJ, Bauman AE, et al. . Reliability and validity of a brief physical activity assessment for use by family doctors. Br J Sports Med 2005;39:294–7. discussion -7. 10.1136/bjsm.2004.013771 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038637supp001.pdf (657.5KB, pdf)
bmjopen-2020-038637supp002.pdf (139.4KB, pdf)
bmjopen-2020-038637supp003.pdf (68.3KB, pdf)


