Abstract
Background
Kratom (Mitragyna speciosa Korth.) has been used in Southeast Asia for hundreds of years to increase energy, for relaxation, and to diminish opioid withdrawal. Kratom use has recently spread to Western countries. Kratom could potentially be used for the treatment of opioid withdrawal and pain, but more insight is needed into its abuse potential. Therefore, we investigated the rewarding properties of the primary kratom alkaloid mitragynine and its active metabolite 7-hydroxymitragynine, and morphine as a reference drug in male and female rats. These compounds have agonist activity at mu-opioid receptors.
Methods
The compounds were tested in an intracranial self-stimulation (ICSS) procedure, which allows for the evaluation of the rewarding/aversive and sedative effects of drugs. Rewarding doses of drugs increase the brain reward thresholds, and aversive drug doses have the opposite effect.
Results
Mitragynine, 7-hydroxymitragynine, and morphine affected the brain reward thresholds. A high dose of 7-hydroxymitragynine (3.2 mg/kg) increased the brain reward thresholds, whereas an intermediate dose of morphine (10 mg/kg) decreased the reward thresholds. 7-Hydroxymitragynine and morphine affected the response latencies. Five mg/kg of morphine increased response latencies. 7-Hydroxymitragynine tended to increase the response latencies, but the post hoc analyses did not reveal a significant effect. There were no sex differences in the effects of mitragynine, 7-hydroxymitragynine, and morphine on the reward thresholds and the response latencies.
Conclusions
These initial findings indicate that mitragynine and 7-hydroxymitragynine are not rewarding in the ICSS procedure. The present results suggest that these kratom alkaloids do not have abuse potential.
Keywords: Reward, ICSS, kratom, mitragynine, 7-hydroxymitragynine, rats
1. Introduction
Mitragyna speciosa Korth., which is often referred to as kratom, has been used in Southeast Asia for centuries (Jansen and Prast, 1988). Kratom is a tropical tree that is native to countries such as Thailand, Myanmar, and Malaysia (Eisenman, 2014). Kratom use was first described in the early 1800s, and it has traditionally been used for a wide range of purposes (Jansen and Prast, 1988). In Thailand, workers used kratom to relieve fatigue and increase productivity when working outdoors. Kratom was also widely used for medical purposes and in religious ceremonies. Kratom can be consumed in many ways. Traditionally fresh Kratom leaves were chewed, or dried leaves were ground and eaten after being placed in warm water (Suwanlert, 1975). The leaves can also be smoked or used to brew tea (Singh et al., 2017).
Twenty-five alkaloids have been identified that may contribute to the physiological effects of kratom (Hassan et al., 2013). Mitragynine is the main alkaloid in kratom. The alkaloid 7-hydroxymitragynine is only a minor kratom constituent, but it is much more potent than mitragynine (Obeng et al., 2020). Mitragynine constitutes about 66 percent of the total alkaloid content and 7-hydroxymitragynine 2 percent (Hassan et al., 2013). 7-Hydroxymitragynine is also an active metabolite of mitragynine (Kruegel et al., 2019).
The kratom alkaloids act upon mu, delta, and kappa-opioid rereceptors. Mitragynine and 7-hydroxymitragynine act as low-efficacy agonists at mu-opioid receptors and as competitive antagonists at kappa and delta-opioid receptors (Kruegel and Grundmann, 2018). 7-Hydroxymitragynine has a tenfold higher potency at the mu-opioid receptor than mitragynine (EC50 values: 0.0345 ± 0.0045 and 0.339 ± 0.178 μM; G protein BRET assay)(Kruegel et al., 2016). In an in-vitro assay (guinea pig ileum preparation), 7-hydroxymitragynine had a 13 and 46-fold higher potency than morphine and mitragynine (Takayama et al., 2002). Mitragynine also inhibits radioligand binding at adenosine (A2A, 65.66 % inhibition), adrenergic (Alpha 2, 61.29 % inhibition), dopamine (D2, 54.22 % inhibition), and serotonin receptors (5-HT2C 58.77 % inhibition, 5-HT7 64.41 % inhibition)(Boyer et al., 2008). Furthermore, blockade of alpha2-adrenoceptors with idazoxan or inhibiting serotonin synthesis with p-chlorophenylalanine blocks the antinociceptive effects of mitragynine in mice (Matsumoto et al., 1996).
Kratom has complex behavioral effects in humans. At low doses, it induces stimulatory effects, and at high doses, it induces sedative, relaxing, and analgesic effects (Kruegel and Grundmann, 2018). Therefore, at low doses, kratom acts as a psychostimulant and at high doses like an opioid. In the US, kratom is used for pain relief, anxiety, depression, and opioid withdrawal (Garcia-Romeu et al., 2020). Chronic use of kratom can lead to dependence and withdrawal signs, which strongly resemble opioid withdrawal (Saingam et al., 2013; Singh et al., 2014). Kratom can also induce tolerance and dependence in rats (Harun et al., 2020).
The intracranial self-stimulation (ICSS) procedure has been widely used to study the acute rewarding effects of drugs and the aversive aspects of high doses of drugs (Barr et al., 2002; Der-Avakian and Markou, 2012). The ICSS procedure is widely used to determine if drugs have abuse potential (Negus and Miller, 2014). The rewarding effects of mitragynine and 7-hydroxymitragynine have not been evaluated in the ICSS procedure. In the ICSS procedure, rats are prepared with an electrode in the brain reward system, and the sensitivity to rewarding electrical stimuli is measured. Acute administration of low and intermediate doses of rewarding drugs increases the sensitivity to the rewarding electrical stimuli and decrease the brain reward thresholds (Esposito et al., 1978; Esposito et al., 1980; Igari et al., 2013). High and aversive doses of drugs and drug withdrawal decrease the sensitivity to rewarding electrical stimuli and increase the brain reward thresholds (Bruijnzeel et al., 2006; Cryan et al., 2003; Epping-Jordan et al., 1998). The ICSS methods can also be used to investigate the rewarding properties of opioids (Negus and Miller, 2014; Negus and Moerke, 2019). In the present study, we investigated the effects of mitragynine, 7-hydroxymitragynine, and morphine on brain reward function in male and female rats.
2. Materials and methods
2.1. Animals
Male and female Wistar rats (males 200–225 g, females 175–200 g, Charles River, Raleigh, NC) were used for this study. The rats were socially housed (2 per cage) with a rat of the same sex before the ICSS surgeries, and after the surgeries, they were singly housed. The rats were housed in a climate-controlled vivarium on a reversed 12 h light-dark cycle (light off at 7 AM). Food and water were available ad libitum in the home cage. The experimental protocols were approved by the University of Florida Institutional Animal Care and Use Committee.
2.2. Drugs
Salt and enantiomeric forms of the compounds used in the present study were as follows:(−)-7-hydroxymitragynine free base [synthesized from mitragynine as in (Obeng et al., 2020)], (−)-mitragynine hydrochloride [isolated as described in (Hiranita et al., 2019)], and (−)-morphine sulfate pentahydrate (National Institute on Drug Abuse, Drug Supply Program, Rockville, MD). Doses of the compounds used in the present study are expressed as the weight of the free base or salt forms listed above. All the compounds were dissolved in a vehicle consisting of sterile water (HyClone Water for injection, Fisher Scientific, Waltham, MA) containing 5% Tween 80 (polyoxyethylene sorbitan monooleate, Fisher Scientific) and 5% propylene glycol (Sigma-Aldrich Co., St. Louis, MO). The alkaloids mitragynine and 7-hydroxymitragynine were administered intraperitoneally (IP), and morphine was administered subcutaneously (sc). Each solution was filtered through a 0.2-μ pore size syringe filter (Millex-LG, 0.20 μm, SLLG025SS). The dose ranges of the studied compounds are based on our preliminary data and the literature (Hiranita et al., 2014; Hiranita et al., 2019; Hutchinson et al., 2012; Obeng et al., 2020).
2.3. Experimental design
The drugs were tested in drug naïve animals, and each drug was tested in a new group of animals. Mitragynine (males n=10 and females n=9; 3.2, 5.6, 10, 17.8, 32, and 56 mg/kg, IP) and 7-hydroxymitragynine (males n=12 and females n=11; 0.1, 0.32, 1, and 3.2 mg/kg, IP) were administered according to a Latin-square design. The low doses of morphine were also administered according to a Latin square design (male n=9 and females n=11; 1, 2.5, and 5 mg/kg, sc). The intermediate (10 mg/kg, sc) and a high dose of morphine (15 mg/kg, sc) were administered after the Latin square was completed). Mitragynine was administered 30 min before ICSS testing, 7-hydroxymitragynine was administered 15 min before ICSS testing, and morphine was administered 5-min before ICSS testing (Hiranita et al., 2019). There were three days between subsequent injections.
2.4. Electrode implantations and ICSS procedure
The rats were anesthetized with an isoflurane and oxygen vapor mixture and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). Electrodes were implanted in the medial forebrain bundle with the incisor bar set 5 mm above the interaural line (anterior-posterior −0.5 mm, medial-lateral ±1.7 mm, dorsal-ventral −8.3 mm from dura)(Qi et al., 2016). After the recovery period, the rats were trained on a modified discrete-trial ICSS procedure in operant conditioning chambers (Med Associates, Georgia, VT, USA). The operant conditioning chambers were housed in sound-attenuating chambers (Med Associates, Georgia, VT, USA). The operant conditioning chambers had a 5 cm wide metal response wheel that was centered on a sidewall, and a photobeam detector recorded every 90 degrees of rotation. Brain stimulation was delivered by constant current stimulators (Model 1200C, Stimtek, Acton, MA, USA). The rats were first trained to turn the wheel on a fixed ratio 1 (FR1) schedule of reinforcement. Each quarter-turn of the wheel resulted in the delivery of a 0.5-sec train of 0.1 ms cathodal square-wave pulses at a frequency of 100 Hz. After the acquisition of responding for stimulation on this FR1 schedule, defined as 100 reinforcements within 10 min, the rats were trained on a discrete-trial current-threshold procedure. The discrete-trial current-threshold procedure is a modification of a task developed by Kornetsky and Esposito, and previously described in detail (Bruijnzeel and Markou, 2003, 2004; Kornetsky and Esposito, 1979). Each trial began with the delivery of a noncontingent electrical stimulus, followed by a 7.5 s response window during which the animals could respond for a second identical stimulus. A response during this 7.5 s response window was labeled a positive response, while the lack of a response was labeled a negative response. During the 2 s period immediately after a positive response, additional responses had no scheduled consequences. The intertrial interval (ITI), which followed either a positive response or the end of the response window, had an average duration of 10 s (ranging from 7.5 s to 12.5 s). Responses that occurred during the ITI resulted in a 12.5 s delay of the onset of the next trial. During training on the discrete-trial procedure, the duration of the ITI and delay periods induced by time-out responses were increased until the animals performed consistently at standard test parameters. The training was completed when the animals responded correctly to more than 90% of the noncontingent electrical stimuli. It took 1 to 2 weeks of training for most rats to meet this response criterion. The rats were then tested on the current-threshold procedure in which stimulation intensities varied according to the classical psychophysical method of limits. Each test session consisted of four alternating series of descending and ascending current intensities starting with a descending sequence. Blocks of three trials were presented to the rats at a given stimulation intensity, and the intensity was altered systematically between blocks of trials by 5 μA steps. The initial stimulus intensity was set at 30 μA above the baseline current-threshold for each animal. Each test session typically lasted 30–40 min and provided two dependent variables for behavioral assessment (brain reward thresholds and response latencies). The brain reward threshold (μA) was defined as the midpoint between stimulation intensities that supported responding (positive responses on at least two of the three trials) and stimulation intensities that failed to support responding(positive responses on fewer than two of the three trials for two consecutive blocks of trials). The response latency (s) was defined as the time interval between the beginning of the noncontingent stimulus and a response. A decrease in reward thresholds is indicative of an increase in reward function (Kornetsky and Esposito, 1979). Drugs with sedative effects increase the response latencies, and stimulants decrease the response latency (Igari et al., 2013; Liebman, 1985).
2.5. Statistics
The brain reward thresholds and response latencies were expressed as a percentage of pre-test day baselines. The ROUT method was used to identify outliers (Motulsky and Brown, 2006). The maximum false discovery rate (Q) was set to 0.1 percent. Three outliers were identified, and these rats were removed from the statistical analysis. The reward thresholds and response latencies were analyzed using a two-factor analysis of variance (ANOVA), with drug treatment as a within-subjects factor and sex as a between-subjects factor. A Bonferroni’s post hoc test was conducted to compare drug effects to vehicle effects (Figure 4). P values that were less than 0.05 were considered significant. Significant main effects and interactions are reported in the Results section. Data were analyzed with SPSS version 25 and GraphPad Prism version 7.
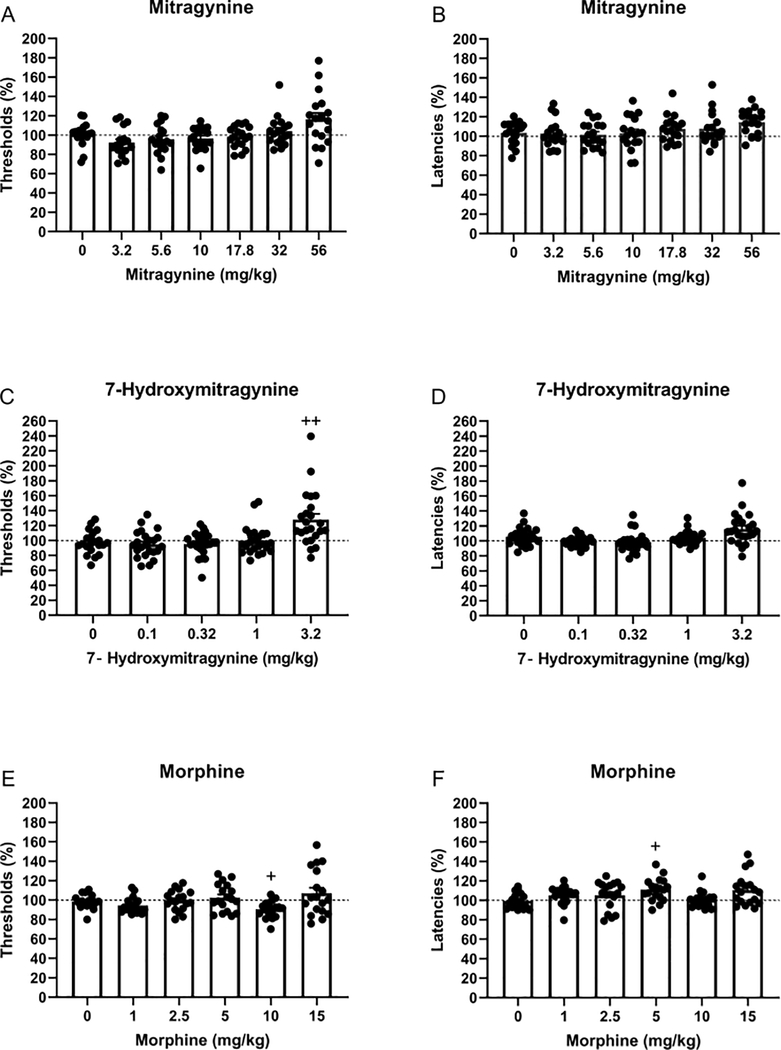
Figure 4. Effects of mitragynine, 7-hydroxymitragynine, and morphine on brain reward thresholds and response latencies.
The figures show the effect of mitragynine (A, B), 7-hydroxymitragynine (C, D), and morphine (E, F) on the reward thresholds and latencies for both males and females combined. Plus signs indicate an increase or decrease in the reward thresholds or response latencies compared to rats treated with vehicle. +, p<0.05. ++, p<0.01. A,B: N=18; C,D: N=23; E,F: N=18. Data are expressed as means ± SEM.
3. Results
3.1. Experiment 1: Mitragynine and ICSS testing
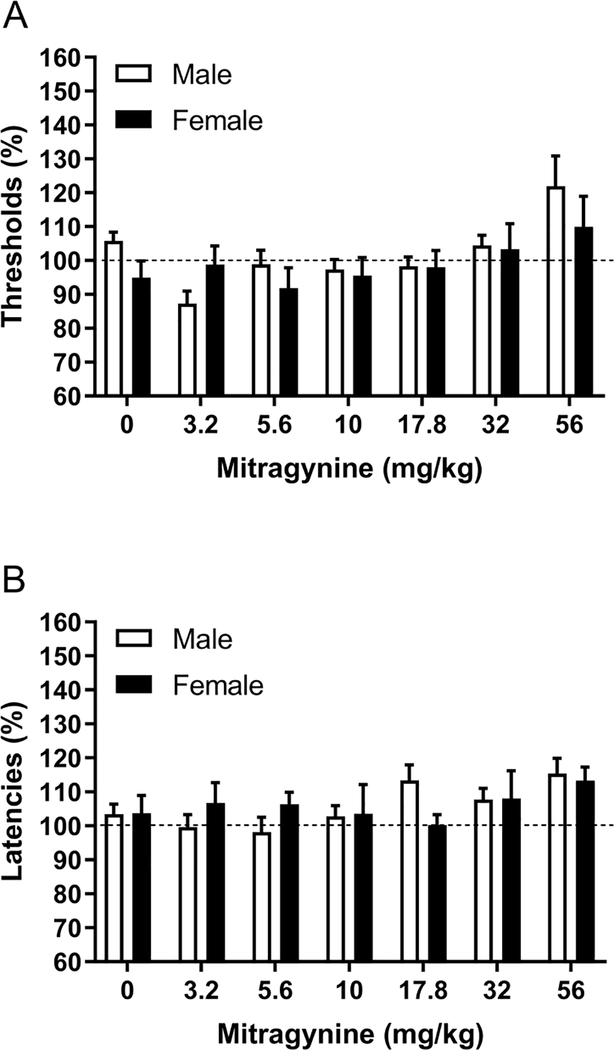
There was an effect of mitragynine on the brain reward thresholds (Dose F6,96=4.11, p<0.01; Figure 1A), and there was no sex difference in the effect of mitragynine on the reward thresholds (Sex F1,16=1.19, ns; Dose x Sex F6,102=0.54, ns). Mitragynine dose and sex did not affect the response latencies (Dose F6,96=1.61, ns; Sex F1,16=0.00, ns; Dose x Sex F6,96=1.09, ns; Figure 1B). There were no sex differences, and therefore the combined effects of mitragynine in the males and females are presented in figures 4A and B.
Figure 1. Effects of mitragynine on brain reward thresholds and response latencies in males and females.
The figures show the effect of mitragynine on the reward thresholds (A) and response latencies (B). Males, N=10; Females, N=8. Data are expressed as means ± SEM.
3.2. Experiment 2: 7-hydroxymitragynine and ICSS testing
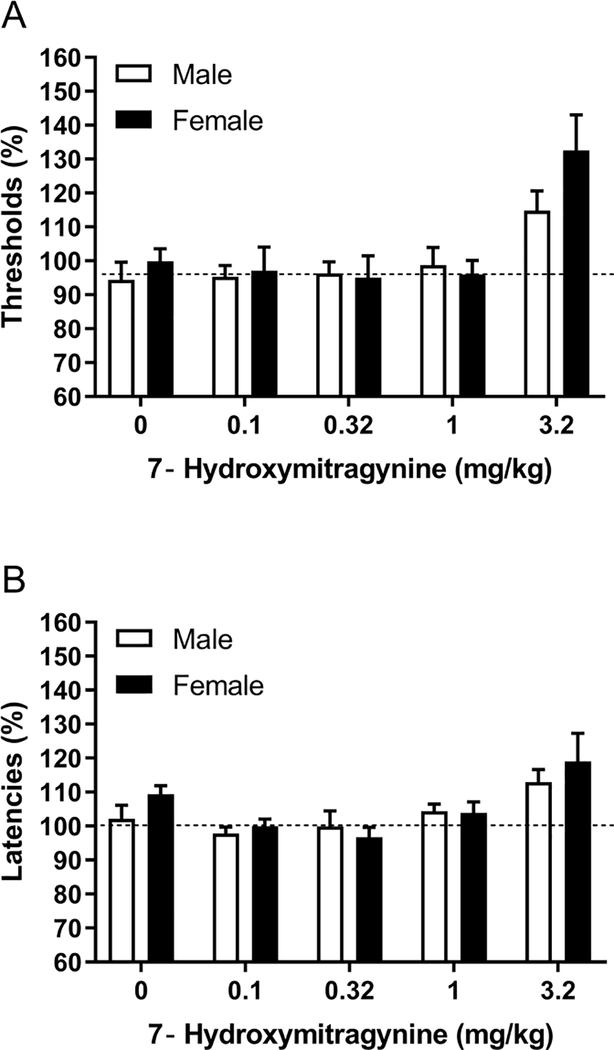
Low doses of 7-hydroxymitragynine did not affect the brain reward thresholds, and a high dose increased the brain reward thresholds (Dose F4,84=9.86, p<0.0001; Figure 2A). There was no sex difference in the effect of 7-hydroxymitragynine on the brain reward thresholds (Sex F1,21=2.55, ns; Dose x Sex F4,84=9.86, ns). A similar effect of 7-hydroxymitragynine was observed on the response latencies (Figure 2B). Low doses did not affect the response latencies, and a high dose slightly increased the response latencies (Dose F4,84=6.81, p<0.0001). There was no sex difference in the effect of 7-hydroxymitragynine on the response latencies (Sex F1,21=0.86, ns; Dose x Sex F4,84=0.63, ns). The combined effects of 7-hydroxymitragynine in males and females are presented in figures 4C and D. The post hoc analysis shows that 3.2 mg/kg of 7-hydroxymitragynine increased the reward thresholds.
Figure 2. Effects of 7-hydroxymitragynine on brain reward thresholds and response latencies in males and females.
The figures show the effect of 7-hydroxymitragynine on the reward thresholds (A) and response latencies (B). Males, N=12; Females, N=11. Data are expressed as means ± SEM.
3.3. Experiment 3: Morphine and ICSS testing
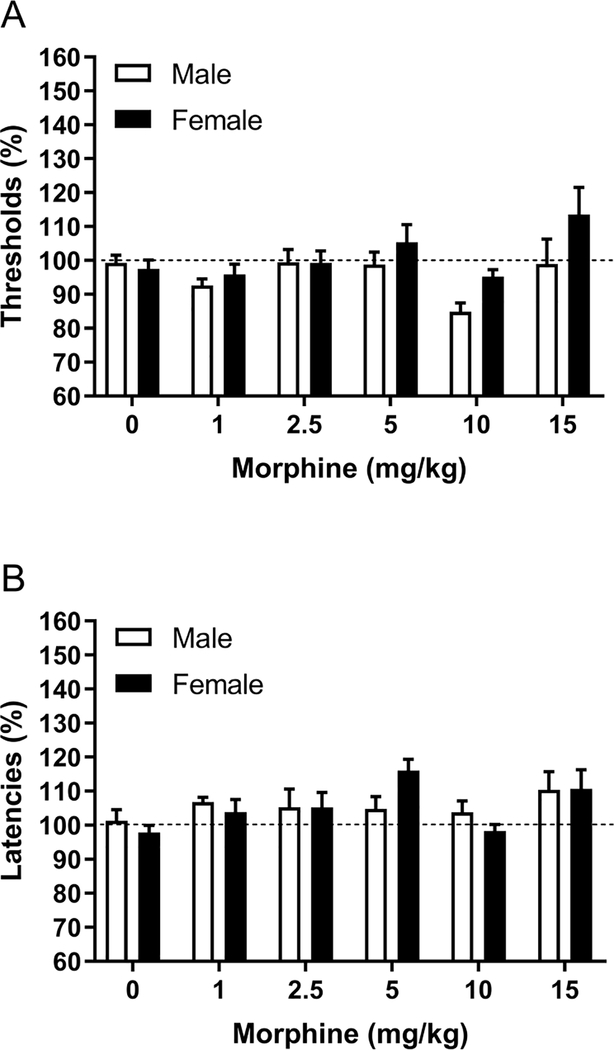
Treatment with morphine affected the brain reward thresholds (Dose F5,80=4.04, p<0.01; Figure 3A). The ANOVA analysis did not reveal a sex difference in the effect of morphine on the reward thresholds (Sex F1,16=2.62, ns; Dose x Sex F5,80=1.20, ns; Figure 3B). Morphine also affected the response latencies (Dose F5,80=3.11, p<0.05). Morphine had a similar effect on the response latencies of the male and the female rats (Sex F1,16=0.00, ns; Dose x Sex F5,80=1.32, ns). The combined effects of morphine in the males and females are depicted in figures 4E and F. The post hoc analysis shows that 10 mg/kg of morphine decreased the reward thresholds, and 5 mg/kg of morphine increased the latencies.
Figure 3. Effects of morphine on brain reward thresholds and response latencies in males and females.
The figures show the effect of morphine on the reward thresholds (A) and response latencies (B). Males, N=8; Females, N=10. Data are expressed as means ± SEM.
4. Discussion
In the present studies, we investigated the effects of mitragynine, 7-hydroxymitragynine, and morphine on brain reward function and response latencies in male and female rats. The ANOVA analyses indicated that mitragynine, 7-hydroxymitragynine, and morphine affected the brain reward thresholds and that there was no sex difference in the effects of these drugs on the reward thresholds. The post hoc analyses indicated that 10 mg/kg of morphine lowered the brain reward thresholds and 3.2 mg/kg of 7-hydroxymitragynine increased the brain reward thresholds. A high dose of mitragynine, 56 mg/kg, tended to increase the reward thresholds, but the post hoc analyses did not reveal a significant effect. The ANOVA analysis indicated that 7-hydroxymitragynine and morphine affected the response latencies. Five mg/kg of morphine increased response latencies. 7-Hydroxymitragynine also tended to increase the response latencies, but the post hoc analyses did not reveal any significant effects. There was no sex difference in the effects of 7-hydroxymitragynine and morphine on the response latencies. These preliminary findings indicate that morphine is rewarding and 7-hydroxymitragynine aversive.
In the first experiment, we investigated the effect of mitragynine on the ICSS brain reward thresholds and the response latencies. The ANOVA analyses indicated that mitragynine affects the reward thresholds and the response latencies. A low dose of mitragynine (3.2 mg/kg, post hoc non-significant) slightly lowered the reward thresholds, and a high dose slightly increased the reward thresholds (56 mg/kg, post hoc non-significant). It is interesting to note that the effect of mitragynine on the brain reward thresholds is similar to the effects of the alkaloid nicotine. An intermediate dose of nicotine lowers the reward thresholds (0.3 mg/kg, 14 percent decrease), and a high dose increases (0.6 mg/kg, 14 percent increase) the reward thresholds (Igari et al., 2013). A low dose of mitragynine (3.2 mg/kg) induced a 13 percent decrease in the brain reward thresholds in the male rats, which is a similar decrease in thresholds, as has been observed with 0.3 mg/kg of nicotine. Several other studies have investigated the rewarding properties of mitragynine. Two studies investigated the rewarding effects of 10 mg/kg of mitragynine in a conditioned place preference procedure. In both studies, mitragynine led to the development of place preference (Yusoff et al., 2017, 2018). Mitragynine-induced place preference was blocked by the opioid receptor antagonist naloxone and the GABAB receptor agonist baclofen (Yusoff et al., 2017, 2018). In another study, the reinforcing properties of mitragynine were investigated with an intravenous self-administration procedure. The rats were trained to self-administer methamphetamine and then switched to mitragynine, heroin, or saline (Yue et al., 2018). The rats that were switched to heroin had a higher level of operant responding than the rats that were switched to saline. However, the rats that were switched to mitragynine had a similar level of operant responding as the saline-control rats. Another self-administration study reported that rats do not acquire the self-administration of mitragynine (Hemby et al., 2019). Overall, these studies suggest that the noncontingent administration of mitragynine can be slightly rewarding under specific test conditions. Self-administration studies suggest that mitragynine has no reinforcing properties.
In the present study, we also investigated the rewarding effects of 7-hydroxymytragynine in the ICSS procedure. Low doses of 7-hydroxymytragynine did not affect the brain reward thresholds, but a high dose (3.2 mg/kg) elevated the brain reward thresholds. This suggests that a high dose of 7-hydroxymytragynine has aversive effects and impairs brain reward function. We are not aware of any other studies that investigated the rewarding effects of 7-hydroxymytragynine in the ICSS procedure or a place conditioning procedure in rats. However, a study with mice reported that 2 mg/kg induces place preference (Matsumoto et al., 2008). The effects of 7-hydroxymytragynine have been assessed in drug discrimination and self-administration procedures. One study conducted a substitution test with 7-hydroxymytragynine in rats trained to discriminate morphine (Harun et al., 2015). It was shown that 1 and 3 mg/kg of 7-hydroxymytragynine substituted for morphine. This suggests that relatively high doses of 7-hydroxymytragynine exert opioid-like effects. Rats also acquire the self-administration of 7-hydroxymitragynine (Hemby et al., 2019). It is somewhat surprising that rats self-administer 7-hydroxymytraginine, but that 7-hydroxymytragynine does not enhance brain reward function in the ICSS procedure. It is unlikely that the lack of rewarding effects was due to sedation because low doses of 7-hydroxymitragynine did not affect the response latencies. It might be possible that the ICSS procedure has a low sensitivity to determine the rewarding effects of drugs with opioid-like effects. Not all addictive drugs enhance responding in the ICSS procedure (Wise, 1996). Although potent stimulants mostly enhance ICSS, conflicting findings have been reported with ethanol and opioids (Wise, 1996). Therefore, we also investigated the effects of morphine in our ICSS procedure.
There was a significant effect of morphine on the brain reward thresholds. Low doses did not affect the brain reward thresholds. The 10 mg/kg dose of morphine-induced a significant decrease in brain reward thresholds in the rats. Stratmann and Craft used a similar discrete trial ICSS procedure to study the effects of morphine (0.56, 1, 1.8, 3.2, and 5.6 mg/kg) on ICSS thresholds in male and female Sprague—Dawley rats (Stratmann and Craft, 1997). The low doses did not affect the brain reward thresholds and the highest dose impaired responding in half the rats. This is in line with another study that reported that 3.2 mg/kg of morphine impairs ICSS responding in drug naïve male Sprague—Dawley rats (Miller et al., 2015). However, repeated administration of the same dose facilitated ICSS responding. In contrast, it has also been reported that morphine (0.5–10 mg/kg) enhances brain reward function in a discrete trial ICSS procedure in male Brown Norway/Fischer 344 F1 (F344/BNF1) hybrid rat (Jha et al., 2004). There is evidence that the sedative effects of morphine can impair performance in the ICSS procedure. Therefore, several studies investigated the effects of a high dose of morphine, 10 mg/kg, 3.5 h after its administration using a rate-frequency curve paradigm. These studies indicate that a high dose of morphine is rewarding 3.5 h after its administration in male Sprague-Dawley and Wistar rats (Katsidoni et al., 2014; O’Neill and Todtenkopf, 2010). Interestingly, we also found that the 10 mg/kg dose of morphine is rewarding, and we observed this effect 5-min after the administration of morphine. The 10 mg/kg dose of morphine did not affect the response latencies, which indicates that this dose did not impair responding in the ICSS test. This is in line with a study that showed that 10 mg/kg does not impair locomotor activity during the first hour of the open field test in male Holtzman rats (Babbini and Davis, 1972). These findings suggest that the rewarding effects of opioids can be studied with the ICSS procedure.
In conclusion, these studies indicate that mitragynine, 7-hydroxymitragynine, and morphine affect the brain reward system. A high doses of 7-hydroxymitragynine impaired reward function and an intermediate dose of morphine (10 mg/kg) enhanced reward function. These preliminary ICSS studies suggest that the kratom alkaloids are not rewarding, and high doses might be aversive.
Highlights.
Mitragynine and 7-hydroxymitragynine are not rewarding in the ICSS procedure.
A high dose of 7-hydroxymitragynine is aversive in the ICSS procedure.
There are no sex differences in the effects of mitragynine and 7-hydroxymitragynine.
Morphine is rewarding in the ICSS procedure.
Acknowledgments
Funding
This work was supported by NIH grants DA47855 and DA48353.
Footnotes
Conflict of interest statement:
The authors have no conflicts of interest to disclose.
Author disclosures:
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babbini M, Davis W, 1972. Time-dose relationships for locomotor activity effects of morphine after acute or repeated treatment. British journal of pharmacology 46(2), 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Markou A, Phillips AG, 2002. A 'crash' course on psychostimulant withdrawal as a model of depression. Trends Pharmacol.Sci. 23, 475–482. [DOI] [PubMed] [Google Scholar]
- Boyer EW, Babu KM, Adkins JE, McCurdy CR, Halpern JH, 2008. Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth). Addiction 103(6), 1048–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Lewis B, Bajpai LK, Morey TE, Dennis DM, Gold M, 2006. Severe deficit in brain reward function associated with fentanyl withdrawal in rats. Biol.Psychiatry 59(5), 477–480. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A, 2003. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse 50(1), 20–28. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A, 2004. Adaptations in cholinergic transmission in the ventral tegmental area associated with the affective signs of nicotine withdrawal in rats. Neuropharmacology 47(4), 572–579. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A, 2003. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol.Psychiatry 54, 49–58. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A, 2012. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 35(1), 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman SW, 2014. The botany of Mitragyna speciosa (Korth.) Havil. And related species. Kratom and other Mitragynines: The chemistry and pharmacology of opioids from a non-opium source, 57–76.
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A, 1998. Dramatic decreases in brain reward function during nicotine withdrawal. Nature 393(6680), 76–79. [DOI] [PubMed] [Google Scholar]
- Esposito RU, Motola AH, Kornetsky C, 1978. Cocaine: Acute effects of reinforcement thresholds for self-stimulation behavior to the medial forebrain bundle. Pharmacology Biochemistry and Behavior 8(4), 437–439. [DOI] [PubMed] [Google Scholar]
- Esposito RU, Perry W, Kornetsky C, 1980. Effects of d-amphetamine and naloxone on brain stimulation reward. Psychopharmacology 69(2), 187–191. [DOI] [PubMed] [Google Scholar]
- Garcia-Romeu A, Cox DJ, Smith KE, Dunn KE, Griffiths RR, 2020. Kratom (Mitragyna speciosa): User demographics, use patterns, and implications for the opioid epidemic. Drug and alcohol dependence 208, 107849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harun N, Hassan Z, Navaratnam V, Mansor SM, Shoaib M, 2015. Discriminative stimulus properties of mitragynine (kratom) in rats. Psychopharmacology 232(13), 2227–2238. [DOI] [PubMed] [Google Scholar]
- Harun N, Johari IS, Mansor SM, Shoaib M, 2020. Assessing physiological dependence and withdrawal potential of mitragynine using schedule-controlled behaviour in rats. Psychopharmacology 237(3), 855–867. [DOI] [PubMed] [Google Scholar]
- Hassan Z, Muzaimi M, Navaratnam V, Yusoff NH, Suhaimi FW, Vadivelu R, Vicknasingam BK, Amato D, von Hörsten S, Ismail NI, 2013. From kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse, and addiction. Neuroscience & Biobehavioral Reviews 37(2), 138–151. [DOI] [PubMed] [Google Scholar]
- Hemby SE, McIntosh S, Leon F, Cutler SJ, McCurdy CR, 2019. Abuse liability and therapeutic potential of the Mitragyna speciosa (kratom) alkaloids mitragynine and 7-hydroxymitragynine. Addiction biology 24(5), 874–885. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Kohut SJ, Soto PL, Tanda G, Kopajtic TA, Katz JL, 2014. Preclinical efficacy of N-substituted benztropine analogs as antagonists of methamphetamine self-administration in rats. Journal of Pharmacology and Experimental Therapeutics 348(1), 174–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Leon F, Felix JS, Restrepo LF, Reeves ME, Pennington AE, Obeng S, Avery BA, McCurdy CR, McMahon LR, 2019. The effects of mitragynine and morphine on schedule-controlled responding and antinociception in rats. Psychopharmacology 236(9), 2725–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt A, Hiranita T, Wang X, Lewis S, Thomas J, Van Steeg K, Kopajtic T, Loram L, Sfregola C, 2012. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. Journal of Neuroscience 32(33), 11187–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igari M, Alexander JC, Ji Y, Qi X, Papke RL, Bruijnzeel AW, 2013. Varenicline and cytisine diminish the dysphoric-like state associated with spontaneous nicotine withdrawal in rats. Neuropsychopharmacology 39, 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen KL, Prast CJ, 1988. Ethnopharmacology of kratom and the Mitragyna alkaloids. Journal of Ethnopharmacology 23(1), 115–119. [DOI] [PubMed] [Google Scholar]
- Jha SH, Knapp CM, Kornetsky C, 2004. Effects of morphine on brain-stimulation reward thresholds in young and aged rats. Pharmacology Biochemistry and Behavior 79(3), 483–490. [DOI] [PubMed] [Google Scholar]
- Katsidoni V, Alexiou P, Fotiadou M, Pelecanou M, Sagnou M, Panagis G, 2014. Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit morphine's rewarding effect in rats. Psychopharmacology 231(23), 4467–4478. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, 1979. Euphorigenic drugs: effects on the reward pathways of the brain. Fed.Proc. 38(11), 2473–2476. [PubMed] [Google Scholar]
- Kruegel AC, Gassaway MM, Kapoor A, Váradi A.s., Majumdar S, Filizola M, Javitch JA, Sames D, 2016. Synthetic and receptor signaling explorations of the Mitragyna alkaloids: mitragynine as an atypical molecular framework for opioid receptor modulators. Journal of the American Chemical Society 138(21), 6754–6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel AC, Grundmann O, 2018. The medicinal chemistry and neuropharmacology of kratom: A preliminary discussion of a promising medicinal plant and analysis of its potential for abuse. Neuropharmacology 134, 108–120. [DOI] [PubMed] [Google Scholar]
- Kruegel AC, Uprety R, Grinnell SG, Langreck C, Pekarskaya EA, Le Rouzic V, Ansonoff M, Gassaway MM, Pintar JE, Pasternak GW, 2019. 7-Hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS central science 5(6), 992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman JM, 1985. Anxiety, anxiolytics and brain stimulation reinforcement. Neurosci.Biobehav.Rev. 9(1), 75–86. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Mizowaki M, Suchitra T, Murakami Y, Takayama H, Sakai S.-i., Aimi N, Watanabe H, 1996. Central antinociceptive effects of mitragynine in mice: contribution of descending noradrenergic and serotonergic systems. European journal of pharmacology 317(1), 75–81. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Takayama H, Narita M, Nakamura A, Suzuki M, Suzuki T, Murayama T, Wongseripipatana S, Misawa K, Kitajima M, 2008. MGM-9 [(E)-methyl 2-(3-ethyl-7a, 12a-(epoxyethanoxy)-9-fluoro-1, 2, 3, 4, 6, 7, 12, 12b-octahydro-8-methoxyindolo [2, 3-a] quinolizin-2-yl)-3-methoxyacrylate], a derivative of the indole alkaloid mitragynine: A novel dual-acting β-and κ-opioid agonist with potent antinociceptive and weak rewarding effects in mice. Neuropharmacology 55(2), 154–165. [DOI] [PubMed] [Google Scholar]
- Miller LL, Altarifi AA, Negus SS, 2015. Effects of repeated morphine on intracranial self-stimulation in male rats in the absence or presence of a noxious pain stimulus. Experimental and clinical psychopharmacology 23(5), 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky HJ, Brown RE, 2006. Detecting outliers when fitting data with nonlinear regression—a new method based on robust nonlinear regression and the false discovery rate. BMC bioinformatics 7(1), 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Miller LL, 2014. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacological reviews 66(3), 869–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Moerke MJ, 2019. Determinants of opioid abuse potential: Insights using intracranial self-stimulation. Peptides 112, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill KS, Todtenkopf MS, 2010. Using a rate-frequency curve method to assess the rewarding properties of morphine in the intracranial self-stimulation paradigm in rats. Journal of neuroscience methods 189(1), 75–79. [DOI] [PubMed] [Google Scholar]
- Obeng S, Kamble SH, Reeves ME, Restrepo LF, Patel A, Behnke M, Chear NJ-Y, Ramanathan S, Sharma A, Leon F, 2020. Investigation of the Adrenergic and Opioid Binding Affinities, Metabolic Stability, Plasma Protein Binding Properties, and Functional Effects of Selected Indole-Based Kratom Alkaloids. Journal of medicinal chemistry 63(1), 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Guzhva L, Yang Z, Febo M, Shan Z, Wang KK, Bruijnzeel AW, 2016. Overexpression of CRF in the BNST diminishes dysphoria but not anxiety-like behavior in nicotine withdrawing rats. Eur Neuropsychopharmacol 26, 1378–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saingam D, Assanangkornchai S, Geater AF, Balthip Q, 2013. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. International Journal of Drug Policy 24(4), 351–358. [DOI] [PubMed] [Google Scholar]
- Singh D, Müller CP, Vicknasingam BK, 2014. Kratom (Mitragyna speciosa) dependence, withdrawal symptoms and craving in regular users. Drug and alcohol dependence 139, 132–137. [DOI] [PubMed] [Google Scholar]
- Singh D, Narayanan S, Vicknasingam B, Corazza O, Santacroce R, Roman-Urrestarazu A, 2017. Changing trends in the use of kratom (Mitragyna speciosa) in Southeast Asia. Human Psychopharmacology: Clinical and Experimental 32(3), e2582. [DOI] [PubMed] [Google Scholar]
- Stratmann JA, Craft RM, 1997. Intracranial self-stimulation in female and male rats: no sex differences using a rate-independent procedure. Drug Alcohol Depend 46(1–2), 31–40. [DOI] [PubMed] [Google Scholar]
- Suwanlert S, 1975. A study of kratom eaters in Thailand. Bull Narc 27(3), 21–27. [PubMed] [Google Scholar]
- Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D, Koyama F, Matsumoto K, Moriyama T, Yamamoto LT, 2002. Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: discovery of opioid agonists structurally different from other opioid ligands. Journal of medicinal chemistry 45(9), 1949–1956. [DOI] [PubMed] [Google Scholar]
- Wise RA, 1996. Addictive drugs and brain stimulation reward. Annu.Rev.Neurosci. 19, 319–340. [DOI] [PubMed] [Google Scholar]
- Yue K, Kopajtic TA, Katz JL, 2018. Abuse liability of mitragynine assessed with a self-administration procedure in rats. Psychopharmacology 235(10), 2823–2829. [DOI] [PubMed] [Google Scholar]
- Yusoff NH, Mansor SM, Müller CP, Hassan Z, 2017. Opioid receptors mediate the acquisition, but not the expression of mitragynine-induced conditioned place preference in rats. Behavioural brain research 332, 1–6. [DOI] [PubMed] [Google Scholar]
- Yusoff NH, Mansor SM, Müller CP, Hassan Z, 2018. Baclofen blocks the acquisition and expression of mitragynine-induced conditioned place preference in rats. Behavioural brain research 345, 65–71. [DOI] [PubMed] [Google Scholar]