Abstract
Background
To date, no report on coronavirus disease 2019 (COVID-19) pediatric patients in a large urban center with data on underlying comorbidities and coinfection for hospitalized cases has been published.
Methods
This was a case series of Chicago COVID-19 patients aged 0–17 years reported to the Chicago Department of Public Health (CDPH) from March 5 to April 8, 2020. Enhanced case investigation was performed. χ 2 and Wilcoxon 2-sample tests were used to compare characteristics among hospitalized and nonhospitalized cases.
Results
During March 5–April 8, 2020, 6369 laboratory-confirmed cases of COVID-19 were reported to CDPH; 64 (1.0%) were among children aged 0–17 years. Ten patients (16%) were hospitalized, and 7 (70%) required intensive care (median length of hospitalization, 4 days [range, 1–14 days]). Reported fever and dyspnea were significantly higher in hospitalized patients than in nonhospitalized patients (9/10 vs 28/54, P = .04 and 7/10 vs 10/54, P = .002, respectively). Hospitalized patients were significantly younger than nonhospitalized patients (median, 3.5 years vs 12 years; P = .03) and all either had an underlying comorbidity or coinfection. Among the 34 unique households with multiple laboratory-confirmed infections, the median number of laboratory-confirmed infections was 2 (range, 2–5), and 31 (91%) households had at least 1 COVID-19–infected adult. For 15 households with available data to assess transmission, 11 (73%) were adult-to-child, 2 (13%) child-to-child, and 2 (13%) child-to-adult.
Conclusions
Enhanced case investigation of hospitalized patients revealed that underlying comorbidities and coinfection might have contributed to severe disease. Given frequency of household transmission, healthcare providers should consider alternative dispositional planning for affected families of children living with comorbidities.
Keywords: coinfections, comorbidities, COVID-19, epidemiology, hospitalization
In the first US urban jurisdiction case series summarizing findings of enhanced case investigation of hospitalized pediatric COVID-19 patients, household transmission was frequent, and all hospitalized patients were found to have an underlying comorbidity or coinfection.
On March 12, 2020, the Chicago Department of Public Health (CDPH) received its first report of a pediatric patient with laboratory-confirmed coronavirus disease 2019 (COVID-19). In the month that followed, a total of 64 pediatric cases (children aged 0–17 years) were reported through April 8, 2020. Most affected children had mild or moderate illness and did not require hospitalization. However, some children with underlying comorbidities or those diagnosed with coinfections had severe manifestations requiring hospitalization. Symptomology of children with COVID-19 throughout the United States has been described [1]; however, to date no report on pediatric patients in a large urban center with data on underlying comorbidities and coinfection for the most severe cases has been published. This report characterizes the clinical and epidemiological characteristics of COVID-19 in Chicago children in the first month of known community transmission.
METHODS
Data were submitted on laboratory-confirmed COVID-19 cases into the Illinois’ National Electronic Disease Surveillance System (I-NEDSS) by electronic laboratory reporting or directly by providers as required by Illinois state administrative rules pertaining to control of communicable diseases. Additionally, results of COVID-19 laboratory tests were reported to I-NEDSS and de-duplicated by person to determine the percentage of Chicago residents who tested positive for COVID-19. Standard case investigation data were collected including demographic characteristics, signs and symptoms of illness, hospitalization (eg, date of admission, intensive care unit [ICU] requirement) and epidemiologic risk factors such as exposures to ill persons, travel, and comorbidities. Routine case investigation was completed by public health communicable disease investigators under medical director supervision. If routine case investigation was not completed for a patient, a physician investigator performed phone interviews with families to complete the investigation. If families could not be reached, medical records were requested and chart abstraction was completed. For hospitalized patients, enhanced data collection confirmed length of hospitalization, laboratory evidence of coinfection, and clarification of underlying diagnoses. Additional household members with a laboratory-confirmed infection were identified via interviews as well as provider and laboratory reports in I-NEDSS. Within a household, transmission was determined to be adult-to-child if the adult’s reported onset date was prior to the child’s; transmission from child-to-adult and child-to-child were similarly assessed. If onset dates were the same, or the household reported a common exposure, such as travel or attending an event having reports of COVID-19, household transmission was not assessed. The χ 2 and Wilcoxon 2-sample tests were performed to compare demographic characteristics and reported symptoms among hospitalized and nonhospitalized cases.
RESULTS
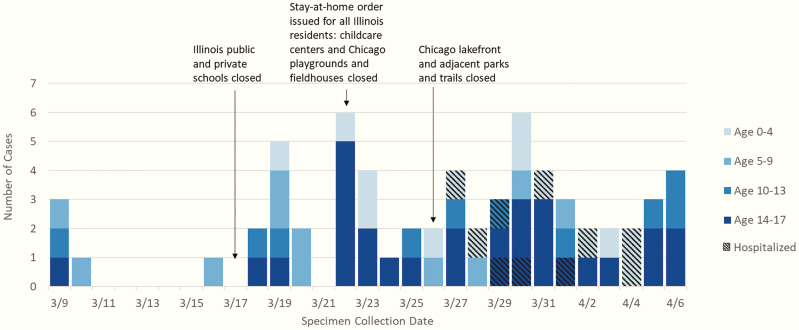
During March 5–April 8, 2020, 6369 laboratory-confirmed cases of COVID-19 were reported to CDPH; 64 (1.0%) were among children <18 years of age (Figure 1). Testing volume and positivity increased for both children and adults over the study period. The percentage of those tested who were positive ranged from 0% to 18.4% for children aged 0–17 years and 7.4% to 32.7% for adults aged ≥18 years. Overall pediatric positivity during this time was 110 of 1045 (10.5%). The median age of pediatric patients with COVID-19 during this time was 11 years (interquartile range, 7–16 years) (Table 1). Twenty-nine (45%) cases were among children aged 14–17 years and 15 (23%) among children aged 0–4 years; 8 (13%) were among infants 0–11 months of age. Males accounted for 56% of patients. Race and ethnicity of patients aligned with Chicago demographics overall [2]: 34% were black non-Hispanic, 39% white non-Hispanic, 25% Hispanic, and 6% Asian.
Figure 1.
Number of laboratory-confirmed coronavirus disease 2019 (COVID-19) cases among children aged ≤ 17 years (N = 64) by age, hospitalization status, and specimen collection date, Chicago, Illinois, March–April 2020.
Table 1.
Characteristics of Children Aged ≤ 17 Years With Laboratory-Confirmed COVID-19 (N = 64), Chicago, Illinois, March–April 2020
| Characteristic | No. (%) |
|---|---|
| Age, y, median (IQR) | 11 (7–16) |
| Age group, y | |
| 0–4 | 15 (23) |
| 5–9 | 11 (17) |
| 10–13 | 10 (16) |
| 14–17 | 29 (45) |
| Gender | |
| Female | 28 (44) |
| Male | 36 (56) |
| Race/ethnicity | |
| Hispanic | 16 (25) |
| Black, non-Hispanic | 20 (31) |
| White, non-Hispanic | 22 (34) |
| Asian, non-Hispanic | 3 (5) |
| Other, non-Hispanic | 2 (3) |
| Unknown | 1 (2) |
| Exposure/risk history | |
| Travel outside Illinoisa | 2 (7) |
| Laboratory-confirmed case in household member | 40 (63) |
| Underlying comorbidityb | 13 (26) |
| Symptoms | |
| Cough | 48 (75) |
| Nasal congestion/rhinorrhea/anosmia | 19 (30) |
| Sore throat | 16 (25) |
| Dyspneac | 17 (27) |
| Fever | 36 (56) |
| Headache | 18 (28) |
| Myalgia | 15 (23) |
| Chills | 5 (8) |
| Abdominal pain | 8 (13) |
| Diarrhea | 10 (16) |
| Nausea/vomiting | 4 (6) |
| Disease severity | |
| Hospitalized | 10 (16) |
| Hospitalized in ICUd | 7 (70) |
| Underlying comorbidityd,e | 7 (70) |
| Coinfectiond,f | 4 (40) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
aAmong those with supplementary data available (n = 27). Travel was reported to New York City (n = 2).
bAmong those with comorbidity data available (n = 50).
cIncludes shortness of breath, increased work of breathing, wheezing, chest pain.
dAmong those hospitalized (n = 10).
eSome patients had multiple underlying comorbidities, which included atopy (n = 1), cardiac or congenital heart disease (n = 2), chronic lung disease (n = 3), Trisomy 21 (n = 2), and immunocompromising condition (n = 2).
fCoinfections included adenovirus, Escherichia coli urinary tract infection, Mycoplasma pneumoniae, human rhinovirus/enterovirus, and rotavirus.
Of 50 (78%) patients with comorbidity data available, 13 (26%) had 1 or more preexisting conditions including 5 (10%) with chronic lung disease (ie, asthma or reactive airway disease), 3 (6%) with cardiac or congenital heart disease, 2 (4%) with Trisomy 21, 2 (4%) with immunocompromising conditions, 2 (4%) with atopy, and 1 (2%) with a history of prematurity.
Among all 64 patients, cough was the most commonly reported symptom in 48 (75%) patients, and fever was present in 36 (56%), with remaining symptoms presented in Table 1. Three (5%) patients denied any symptoms. Ten patients (16%) were hospitalized. The median length of hospitalization was 4 days (range, 1–14 days) for the 9 patients who had been discharged at the time of this report. Reported presence of fever and dyspnea were significantly higher in hospitalized patients compared to nonhospitalized patients (9/10 vs 28/54, P = .04 and 7/10 vs 10/54, P = .002, respectively). Hospitalized patients were also significantly younger than nonhospitalized patients (median, 3.5 years vs 12 years; P = .03). Of those hospitalized, all either had an underlying comorbidity or co-infection detected; 7 (70%) had an underlying comorbidity and 4 (40%) had coinfection. Three (30%) had a history of chronic lung disease, 2 (20%) had a history of cardiac or congenital heart disease, 2 (20%) had Trisomy 21, and 2 (20%) had immunodeficiency (1 with immune dysfunction related to a genetic disorder; 1 with recent myelosuppressive chemotherapy). All hospitalized patients were evaluated for viral and bacterial co-infection with multiplex respiratory pathogen panels. Four (40%) hospitalized patients had known coinfections: 1 with elevated Mycoplasma pneumoniae immunoglobulin M; 1 with positive nasopharyngeal adenovirus polymerase chain reaction (PCR); 1 with positive nasopharyngeal human rhinovirus/enterovirus PCR, positive nasopharyngeal adenovirus PCR, and Escherichia coli urinary tract infection; and 1 with positive rotavirus stool antigen (3 years after last recorded rotavirus vaccine administration date).
Of 10 hospitalized patients, 7 (70%) required ICU admission. Of these, 4 (57%) had at least 1 underlying comorbidity. Two (50%) had a history of cardiac or congenital heart disease, 1 (25%) had a history of chronic lung disease, 2 (50%) had Trisomy 21, and 1 (25%) had immunodeficiency. All 4 patients with coinfections required ICU admission. One patient who required intensive care had an underlying condition and coinfection. Infants (aged 0–11 months) represented 4 (40%) hospitalized children and 4 (57%) ICU patients. No patients met Centers for Disease Control and Prevention case definition for multisystem inflammatory syndrome in children (MIS-C) and at the time of manuscript submission, no COVID-19–related pediatric deaths were reported in this cohort.
Among 27 (42%) patients with known travel history, 2 (7%) reported travel to New York City within the 14 days prior to symptom onset. Forty (63%) had at least 1 additional household member with laboratory-confirmed COVID-19. Among the 34 unique households with multiple laboratory-confirmed infections, the median number of laboratory-confirmed infections was 2 (range, 2–5), and 31 (91%) households had at least 1 adult who also had a laboratory-confirmed infection. For 15 households for which all necessary data to determine transmission were available, 11 (73%) were adult-to-child, 2 (13%) were child-to-child, and 2 (13%) were child-to-adult. None of the 64 pediatric patients were associated with a recognized outbreak in a congregate setting.
DISCUSSION
Studies on COVID-19 in children from China reported mild disease and variable clinical presentation [3, 4]. In a recent report on 2572 US pediatric patients with COVID-19, complete data on characteristics were limited due to the preliminary nature of data collection from local health departments [1]. Completion and reliability of characteristics such as symptoms (100%), preexisting underlying medical conditions (78%), and hospitalization status (100%) was improved in the Chicago cohort compared to 9.4%, 13%, and 33%, respectively, in the national cohort [1]. For 25 of 50 patients, underlying medical conditions were based solely upon I-NEDSS reporting data; however, all comorbidity data for hospitalized patients were confirmed with chart review and discussions with relevant staff at treating hospitals. While national pediatric clinical presentations were similar in the smaller Chicago cohort, the addition of information on comorbidities and coinfection in hospitalized children is instructive to focus public health efforts on shielding children at higher risk of complications from household transmission. Review of medical records and family interview revealed a higher ICU hospitalization rate (11%) compared to the upper estimate of national pediatric data (2%) [1]. The discrepancy might be related to incomplete reporting in the national dataset or lack of case investigation.
Upon review of testing volume across the city, infants represented 17.5% (183/1044) of all Chicago children who were known to be tested during this time period and 13% of all pediatric patients, similar to national data (15%) [1]. In the Chicago cohort, infants accounted for 40% of all hospitalizations and 57% of ICU hospitalizations, and many were found to have underlying comorbidities or coinfection, supporting a heightened risk perception for this group of infants or suggesting that risk perception plays a role in hospitalization. Data on pediatric coinfection with SARS-CoV-2 are scant in the literature. A recent research letter [5] revealed coinfection in 24 of 116 (21%) specimens positive for SARS-CoV-2; the youngest patient in the SARS-CoV-2 coinfection group was aged 9 years. While patients with coinfections did not differ significantly in age from those infected with SARS-CoV-2 only (mean age, 46.9 years vs 51.1 years, a 4.2-year difference [95% confidence interval, –4.8 to 13.2]), no specific pediatric data on coinfection and disease severity were presented.
Enhanced case investigation of all hospitalized revealed that, as with infants, underlying comorbidities and coinfection might have contributed to severe disease. Of concern was the finding that the majority of cases (63%) had multiple persons with confirmed COVID-19 living at home. Considering that school closures and shelter-in-place directives were instituted during this time period, multiple laboratory-confirmed infections within a household suggest that household transmission was more likely than community transmission for pediatric patients. Healthcare providers and public health systems should consider increased collaboration to support alternative dispositional planning for affected individuals living with children and adults with comorbidities. While the broad recommendation to shelter in place has slowed the spread of infection, shielding children with underlying cardiac and pulmonary comorbidities may be indicated. Additionally, guidance to reduce transmission in the home should continue to be reinforced with all persons who are confirmed or suspected to have COVID-19.
Collection of reliable past medical history, details on disease course, and epidemiologic exposures related to transmission will continue to inform public health policy regarding resuming pediatric congregate activities as containment strategies reemerge as primary prevention during the deceleration phase of the COVID-19 pandemic. To reduce burden on healthcare providers, public health systems should explore automatic electronic health record transfer of variables related to hospital course, currently in process through a recent CDPH public health order, to facilitate continued public health impact.
Notes
Acknowledgements. The authors acknowledge the patients described in this report; the health care providers who treated them and collaborated with public health; Dr. Tristan McPherson for his contributions to the CDPH COVID-19 response and to this report.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bialek S, Gierke R, Hughes M, et al. Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. US Census Bureau. QuickFacts: Chicago City, Illinois. Available at: http://www.census.gov/quickfacts/chicagocityillinois. Accessed April 9, 2020.
- 3. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics 2020; 145:e20200702. [DOI] [PubMed] [Google Scholar]
- 4. Lu X, Zhang L, Du H, et al. Chinese Pediatric Novel Coronavirus Study Team SARS-CoV-2 infection in children. N Engl J Med 2020; 382:1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim D, Quinn J, Pinsky B, et al. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA 2020; 323:2085–6. [DOI] [PMC free article] [PubMed] [Google Scholar]