Abstract
Optical imaging in the shortwave infrared (SWIR, 1000–2000 nm) region of the electromagnetic spectrum provides high resolution imaging in complex systems. Here, we explore substituent placement on dimethylamino flavylium polymethine dyes, a class of SWIR fluorophores. We find that the position of substituent affects the λmax and fluorescence quantum yield. Quantum mechanical calculations suggest that steric clashes control the extent of π-conjugation. These insights provide a design principle for the development of novel fluorophores for enhanced SWIR imaging.
Graphical Abstract
Introduction
Fluorophores in the shortwave infrared (SWIR, 1000–2000 nm) region of the electromagnetic spectrum have recently garnered excitement as tools for biological imaging.1–3 The SWIR region is advantageous for optical imaging in complex systems because of the increased depth penetration of light through tissue, enhanced image resolution, and low energy photons as compared with the visible and near-infrared regions.4,5 Imaging in the SWIR originally necessitated single-wall carbon nanotubes (SWCNT)6–9, rare earth nanomaterials10–12 and quantum dots13–15 as contrast agents, which have biocompatibility or bioaccumulation concerns.11, 16–19 In contrast with these nanostructures, small molecule fluorophores have low toxicity and are readily cleared from the body.20 However, it is challenging to obtain fluorophores with absorption and emission above 1000 nm with fluorescence quantum yields (ΦF) greater than ~0.3%.21–23
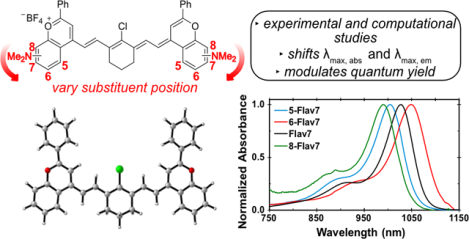
In 2017, we reported a bright flavylium heptamethine SWIR fluorophore, deemed Flav7 (1, Figure 1).24 Flav7 contains a dimethylamino substituent at the seven-position, which provided a λmax above 1000 nm. Recently, we have varied the electron-donating substituent at the seven-position and found that the λmax,abs can be modulated by ~80 nm and form a linear free energy relationship with σ meta Hammett constants.25 The identity of the substituent had minimal effect on the quantum yield of the series analyzed (ΦF = 0.42–0.62%). Finding that the seven-position represented the meta-position prompted our interest in placing the dimethylamino group at other positions on the flavylium heterocycle (Figure 1). Additional π-donation resulting from para and ortho substitution can lead to more significant electronic contributions from substituents, although the ortho position can be complicated by sterics.26 Herein, we report how dimethylamino substituents at putative ortho-, meta-, and para-positions on flavylium heptamethine fluorophores affect photophysical properties exploring experimental and quantum mechanical analyses.
Figure 1.
Systematic exploration of structural modifications of Flav7 (1). Previous work on derivatives at the seven-position, includeing methoxy and diphenylamine with a shift in λmax.25 Current work on exploring dimethylamino substitutions at different positions (five-, six-, and eight- on the heterocycle (3–5).
Results and Discussion
The dimethylamino flavylium heptamethine dyes were synthesized by leveraging chemistry developed to access Flav7 derivatives.25 The dimethylamino group was installed through a Buchwald Hartwig amination from either triflated or brominated flavones (6a-c) to provide dimethylamino substituents at the five-, six-, or eight-position (7a-c), respectively (Scheme 1). The addition of methyl-Grignard followed by quenching with tetrafluoroboric acid yielded flavylium hetereocycles (8a-c), which could then be treated with linker 9 and 2,4-di-tert-butyl-4-methylpyridine to provide 5-, 6-, and 8-Flav7 (3–5, respectively). These dyes are named with the first number representing the position of the dimethylamino substituent on the flavylium heterocycle, and Flav7 references the flavylium heptamethine.
Scheme 1.
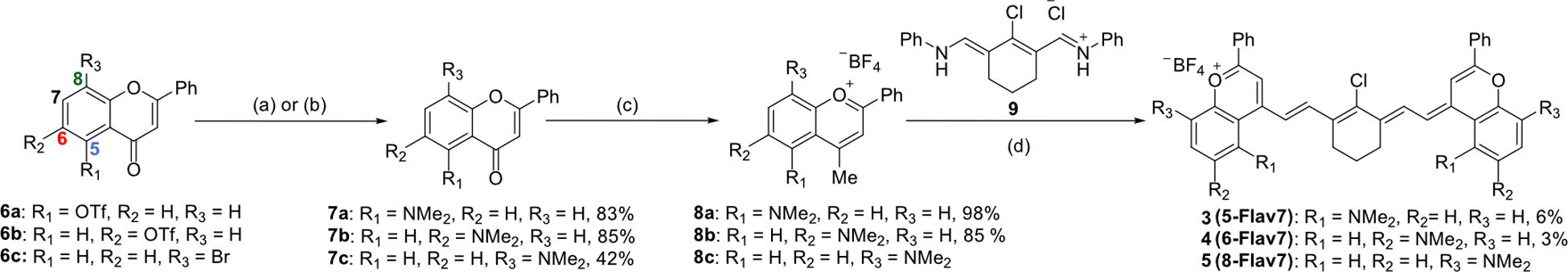
Synthetic scheme for dimethylamino flavylium heptamethines 3–5 with substituents at the five-, six-, and eight- positions. (a) RuPhos Pd G3 (0.10 eq), RuPhos (0.10 eq), Cs2Co3, HNMe2, toluene, 110 °C, 24 h. for 6a and 6b. (b) SPhos Pd G3 (0.10 eq), SPhos (0.10 eq) Cs2Co3, HNMe2, toluene, 110 °C, 24 h. for 6c. (c) MeMgBr (1.4M), THF, r.t. d) 2,6-di-tert-butyl-4methyl-pyridine, n-butanol/toluene or acetic anhydride, 100 °C, 15 min. Refer to SI for further experimental details.
We isolated pure 5-Flav7 (3) and 6-Flav7 (4). However, the eight-substituted flavylium (8c) readily reacted with oxygen to form a monomethine dye, characterized by an absorption peak at 722 nm (Figure S1).24 To minimize this, crude 8c was immediately taken to the next reaction. The resulting 8-Flav7 (5) proved difficult to purify due to its instability.27 Thus, we gained as much photophysical information as possible from the crude sample. The absorption coefficient (ε) was not determined due to insufficient purity.
We evaluated the photophysical properties of the newly synthesized dyes in comparison with Flav7 (1, Figure 2). The absorbance and emission spectra clearly show that the position of the dimethylamino affect the λmax,abs and λmax,em. Previously, we correlated the seven-position with the meta position through Hammett analysis.25 We expected that the six-position would correspond to the para-position and that substituent at this position would show pronounced effects due to enhanced π-donation. Indeed, we observed this result, as 6-Flav7 (4, Figure 2, red) is 20 nm bathochromically shifted from Flav7 (1, Figure 2, black).
Figure 2.
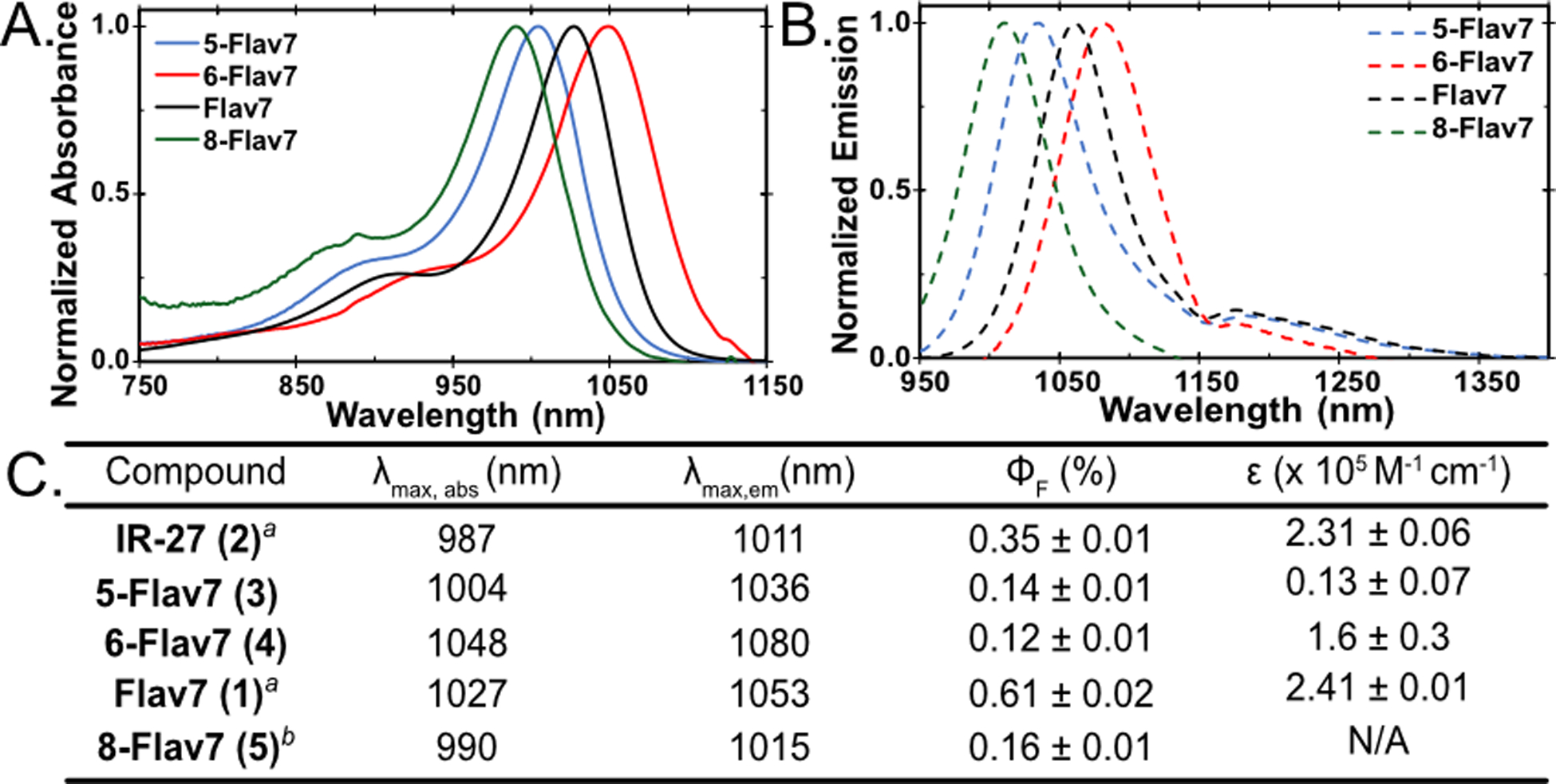
Normalized (A) absorbance and (B) emission of the flavylium polymethines discussed. (C) Photophysical data of unsubstituted (IR-27, 2) and dimethylamino substituted heptamethines (1, 3–5). All samples were taken in dichloromethane. aData was previously reported.25 bPhotophysical data was taken with crude sample.
We then reasoned that the five-position could serve as the other meta position and the eight-position would be the ortho position. We expected that the absorption and emission of 5-Flav7 (3) would be similar to Flav7 (1), and the same comparison could be drawn for 8-Flav7 (5) and 6-Flav7 (4). However, we observed that both 3 and 5 were hypsochromically shifted in comparison to Flav7 (1). 5-Flav7 (3, Figure 2, blue) has a λmax,abs of 1004 nm, a blue shift of 23 nm from Flav7 (1). 8-Flav7 (5, Figure 2, green) has a λmax,abs at 990 nm, nearly identical to parent dye IR-27 (2). These results were counterintuitive to predicted λmax,abs based on Hammett parameters and prompted a quantum mechanical study.
We performed a conformational search of 10,000 structures enforcing the all-trans configuration along the polymethine chain while searching conformational flexibility of the phenyl groups (Refer to SI). We optimized the ten lowest conformers of each molecule with the M06–2X28 density functional and the 6–31+G(d,p) basis set. We used the integral equation formalism polarizable continuum model (IEFPCM)29 for all calculations in the presence of dichloromethane. The computed range of free energies between the ten lowest energy conformers is 0.0–0.7 kcal mol–1 (Figure S2).
To evaluate the photophysical properties, we used the global minima for Flav7 dyes (1, 3–5) as well as unmodified IR-27 (2). The conjugated C-C bonds in the polymethine chains ranged from 1.39–1.41 Å. However, the substituents altered the planarity of the π-system along the polymethine chain and the flavylium heterocycle. We deconvoluted these effects with two angular parameters, α and β (Figure 3A,B). We define α as the angle between the plane of the polymethine and the plane of the flavylium, whereas β is the angle between the plane of the flavylium and the substituent. Analysis of the α angles shows little distortion between the polymethine plane and the flavylium heterocycles when the dimethylamino group is at the six-, seven-, or right- position (α = 5°–7°, Figure 3B). At the five-position, the NMe2 C-H bonds clash with the vinyl C-H bond of the polymethine chain, which results in a larger angle of 18°.
Figure 3.
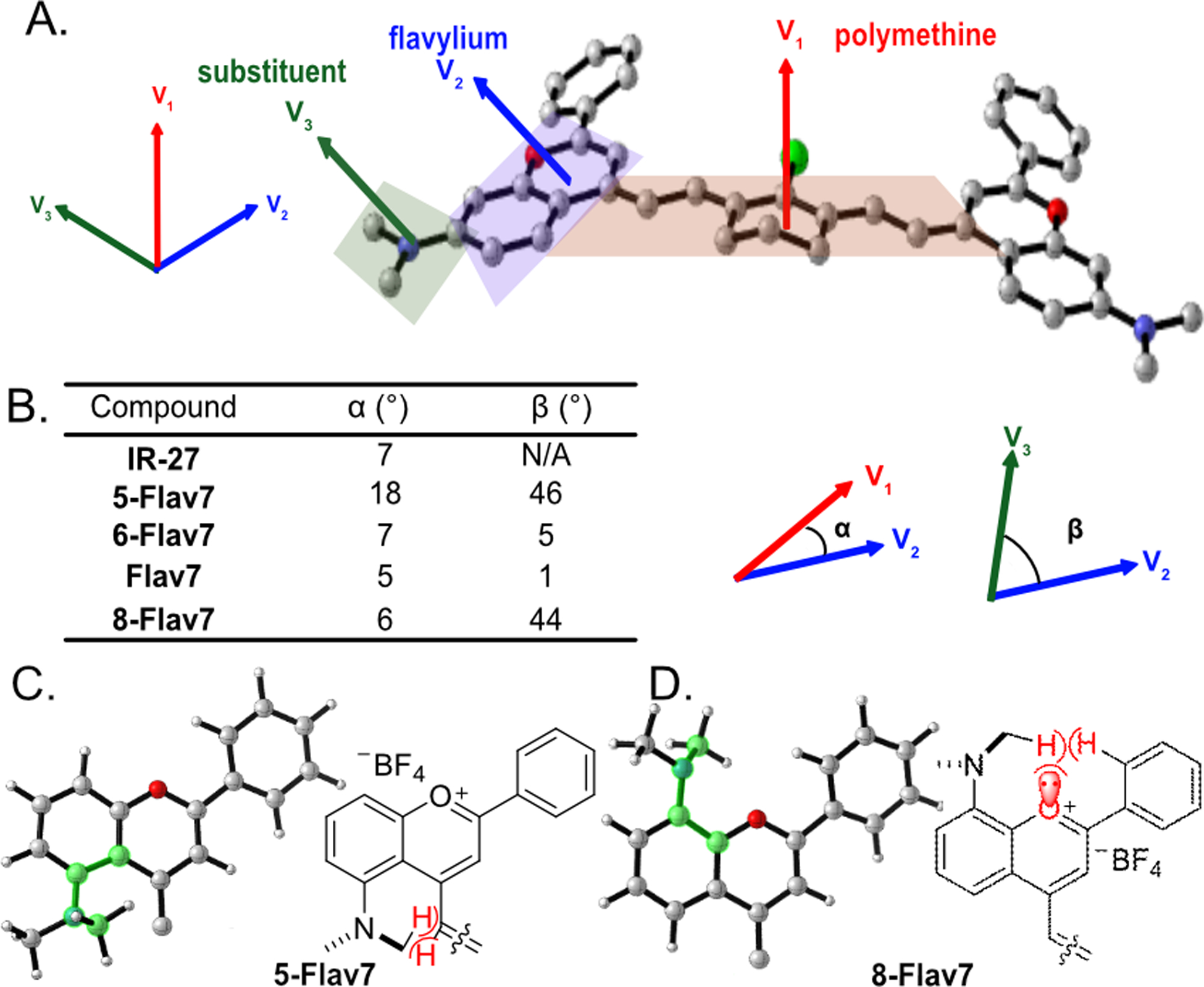
(A) Represented by Flav7; torsion angles are defined by α (V1 to V2, red to blue) and β (V3 to V2, green to blue) using the normal of the plane of the polymethine (V1), flavylium (V2) and substituent (V3). (B) Table of α and β angles for Flav7 dyes. (C,D) Heterocycle structures of (C) 5-Flav7 and (D) 8-Flav7 at the S0 state, optimized with M06–2X/6–31+G(d,p). The polymethine chain is omitted for clarity. The dihedral angles at the substituted positions of the flavylium heterocycles are highlighted in green to show the rotation of the NMe2 group in 5-Flav7 and 8-Flav7.
The NMe2 substituents also alter the geometries near the vicinity of the flavylium heterocycle. The torsion angle β in Flav7 (1) and 6-Flav7 (4) are nearly planar (5° and 1°, respectively). 5-Flav7 (3) and 8-Flav7 (5) have β angles of 46° and 44°, respectively, which indicate significant out-of-plane distortions. The NMe2 groups rotate to minimize closed-shell repulsion to the vinyl C-H bond in 5-Flav7 (3, Figure 3C) and to the oxygen lone pair and adjacent C-H bond of the phenyl group in 8-Flav7 (5, Figure 3D).
We computed the frontier molecular orbitals (FMOs) to illustrate the differences in the electronic structures of these fluorophores. Displayed in Figures 4A and Figure S3 are the highest occupied molecular orbitals and lowest unoccupied molecular orbitals (HOMOs and LUMOs). The FMOs show that the extent of π-conjugation in the flavylium heterocycles varies with respect to the substituent site. Flav7 (1) and 6-Flav7 (4) have dimethylamino substituents that are nearly coplanar with flavylium heterocycles, allowing for the NMe2 to maximally extended orbital overlap in the HOMOs and LUMOs. This results in reduced HOMO-LUMO gaps and longer λmax,abs. The 46° out-of-plane torsion in 5-Flav7 (3) results in decreased π-conjugation of the nitrogen lone pair to the chromophore. The substantial out-of-plane distortion of 8-Flav7 (5) nearly eliminates π-conjugation of the nitrogen lone pair, which leads to unperturbed λmax,abs relative to parent dye, IR-27 (2).
Figure 4.
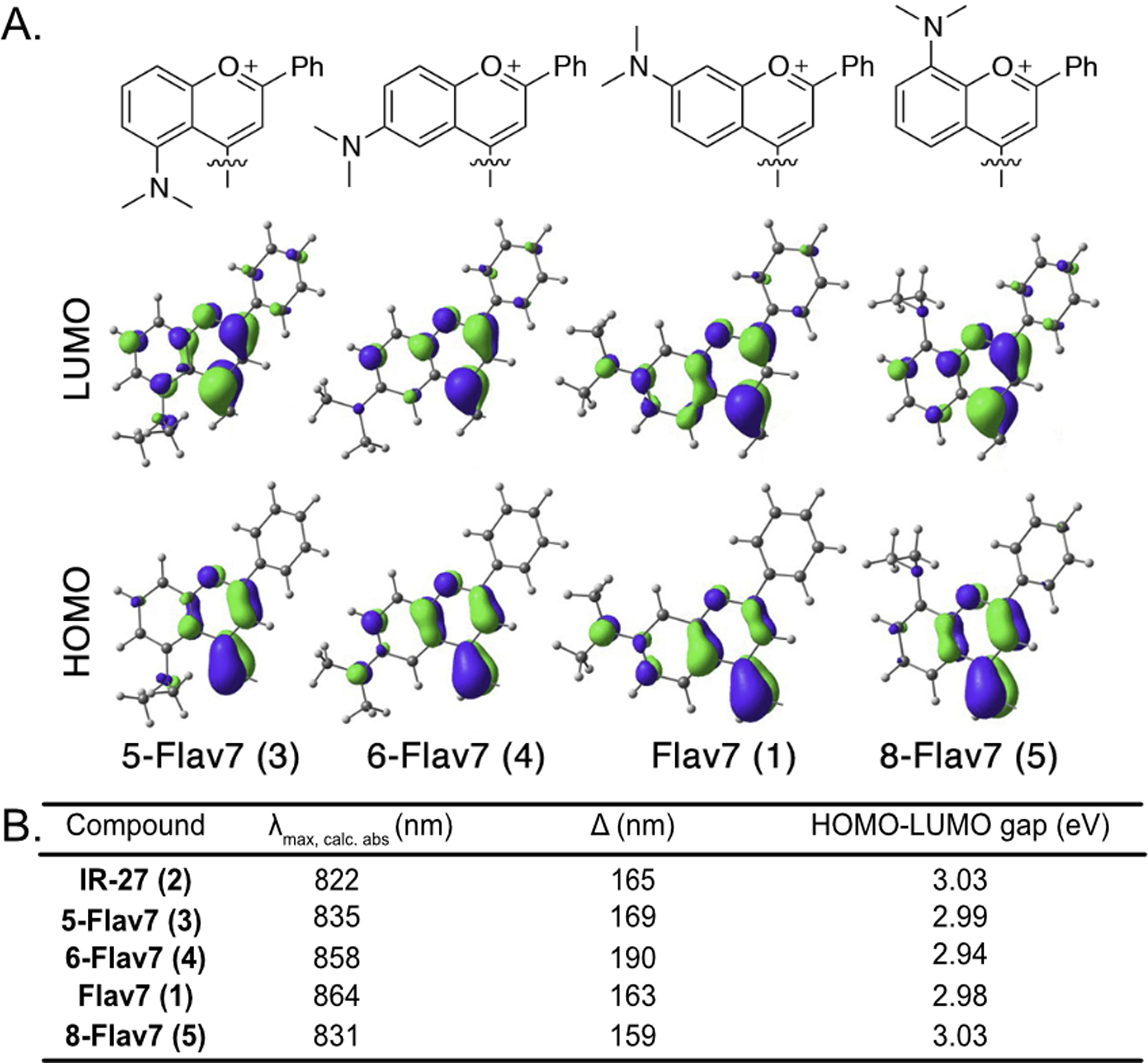
(A) HOMOs and LUMOs of Flav7 (1) and (5,6 and 8)-Flav7 (3–5) at M06–2X/6–31+G(d,p) level of theory. Polymethine chains were omitted for clarity. Explicit frontier molecular orbital information can be found in the SI. (B) Table of calculated λmax,calc,abs (nm) determined by theoretical HOMO-LUMO gap (eV). Δ is the difference between experimental and calculated λmax,abs.
The predicted λmax,abs values of dyes were calculated using configuration interactions of singles with corrections to doubles method (CIS(D))30,31 and the cc-pVDZ basis sets. The computed HOMO–LUMO gaps match the experimentally observed trend (Figure 4B). 6-Flav7 (4) has the smallest gap (2.94 eV), which correlates with the longest λmax,abs, whereas 8-Flav7 (5) has the largest gap (3.03 eV) and the shortest experimentally determined λmax,abs. The predicted λmax,abs is systematically blue-shifted 159–190 nm relative to experimental, likely due to contributions from double excitation that are unaccounted for with single-reference quantum mechanical methods.32,33
Our analysis of the λmax show that the position of substituents on the flavylium ring can affect λmax, comparable to the magnitude observed by varying the electronics at the seven-position. However, the major limitation of small molecule fluorophores in the SWIR is their low ΦF.21 At 0.61%, Flav7 (1) has a respectable ΦF for polymethine SWIR fluorophores. It is of interest to gain an understanding of how structural modifications impact ΦF to develop brighter probes. Previously, we found that functional groups at the seven-position showed little change in quantum yield of fluorescence.25 However, here we see that substituent placement can greatly alters the ΦF (Figure 2).
We measured ΦF values for 5-Flav7, 6-Flav7, and 8-Flav7 (3–5). All three were significantly less fluorescent than Flav7 (1), with ΦF ranging from 0.12–0.16%. We were particularly interested in the large difference between Flav7 (1) and 6-Flav7 (4), which are conformationally similar. Flavylium heterocycles are structurally similar to coumarin heterocycles, and large differences in ΦF between six- and seven-position substituted coumarin fluorophores has previously been observed.27 The low ΦF in 6-aminocoumarins (10) compared to 7-aminocoumarins (11) was attributed to a significant contribution of a twisted intermolecular charge transfer (TICT) state in which the amine donor twists out of plane by ~90° upon photoexcitation, forming a non-emissive species.34,35 We hypothesized a similar phenomenon could contribute to the loss of fluorescence in 6-Flav7 (4), as compared with the parent Flav7 (1).
To gain insight into whether TICT was contributing to observed differences in fluorescence 1 and 4, we synthesized a flavylium heptamethine that contained an azetidine at the six-position. Azetidines have been shown to minimize TICT states by preventing the substituent from twisting out of plane.36 Azet-6-Flav7 (12, Figure 5A) was synthesized following a similar procedure to 6-Flav7 (Scheme S1). We found the ΦF to be 0.21%, 1.75 times higher than the dimethylamino derivative. In contrast, azetidine at the 7-position (13) resulted in a slight decrease in ΦF compared with Flav7 (1) (0.51% vs. 0.61%, Figure 5C).25 We calculated fold change of in ΦF for each of the pairs of six- and seven-substituted fluorophores (4 vs 1, 12 vs 13, 10 vs 11; Figure 5D) and found that the NMe2 variants of both flavylium heptamethine and coumarin dyes had similar changes, whereas the azetidine functionalized Flav7 dyes displayed a reduced change. These data support the notion that TICT could be playing a larger role at the six-position, contributing to the observed difference in ΦF between Flav7 (1) and 6-Flav74 vs (4).
Figure 5.
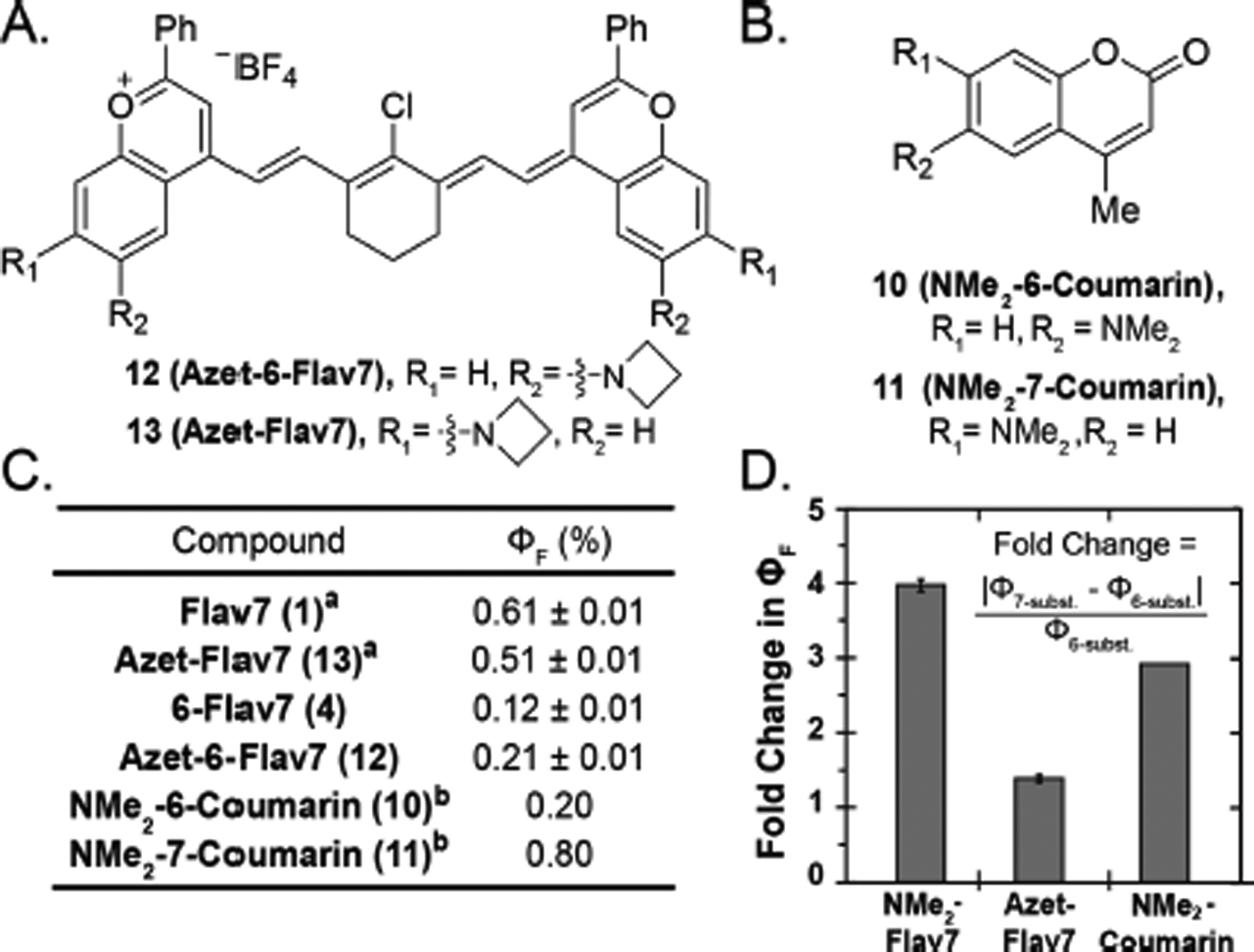
(A) Structures of azetidine-substituted flavylium heptamethines (12, 13). (B) Structure of 6- and 7-aminocoumarins (10, 11). (C) ΦF of flavylium heptamethine and coumarin dyes in dichloromethane and decanol, respectively. aPreviously reported by our group.25 bPreviously reported.34 (D) Fold change of ΦF between six- and seven-substituted flavylium heptamethine or coumarin fluorophore.
Conclusions
In conclusion, we have demonstrated that dimethylamino substituent placement significantly influences the photophysical properties of flavylium heptamethine dyes. Substituent steric effects impact the degree of conjugation and alter the λmax,abs. We have used density functional theory calculations to understand the origin of these unique photophysical properties. The seven-position appears to be advantageous for obtaining a high quantum yield and maintaining SWIR absorption maxima; however, further red-shifted dyes can be obtained by substituting the six-position. The insights garnered herein can contribute to the design of novel fluorophores to be utilized for high resolution SWIR in vivo imaging.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by grants from the Sloan Foundation (FG-2018-10855 to E.M.S.), NIH (1R01EB027172-01 to E.M.S.), and NSF (NSF-1940307 to S.A.L.; CHE-1905242 to E.M.S.). Instrumentation was funded through the NSF MRI (CHE-1048804) and NIH (1S10OD016387-01). S.A.L, and J.L. acknowledge the Massachusetts Green High-Performance Computing Center (MGHPCC) for computing resources and the staff of Research Computing for support. We thank Dr. J. Cox (Northeastern), P. Neal (Northeastern), I. Lim (UCLA) and E. Cosco (UCLA) for helpful discussions.
Footnotes
Supporting Information
Experimental procedures, supplemental Figures S1–3, full spec-troscopic data and computational analysis for compounds are included in the supporting information.
REFERENCES
- (1).Schnermann MJ Chemical Biology: Organic Dyes for Deep Bioimaging. Nature. 2017, 551, 176–177. [DOI] [PubMed] [Google Scholar]
- (2).Thimsen E; Sadtler B; Berezin MY Shortwave-Infrared (SWIR) Emitters for Biological Imaging: A Review of Challenges and Opportunities. Nanophotonics 2017, 6 (5), 1043–1054. [Google Scholar]
- (3).Weissleder R A Clearer Vision for in Vivo Imaging. Nat. Biotechnol 2001, 19 (4), 316–317. [DOI] [PubMed] [Google Scholar]
- (4).Smith AM; Mancini MC; Nie S Bioimaging: Second Window for in Vivo Imaging. Nat. Nanotechnol 2009, 4, 710–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Carr JA; Franke D; Caram JR; Perkinson CF; Saif M; Askoxylakis V; Datta M; Fukumura D; Jain RK; Bawendi MG; Bruns OT Shortwave Infrared Fluorescence Imaging with the Clinically Approved Near-Infrared Dye Indocyanine Green. Proc. Natl. Acad. Sci 2018, 115 (17), 4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hong G; Lee JC; Robinson JT; Raaz U; Xie L; Huang NF; Cooke JP; Dai H Multifunctional in Vivo Vascular Imaging Using Near-Infrared II Fluorescence. Nat. Med 2012, 18 (12), 1841–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Welsher K; Liu Z; Sherlock SP; Robinson JT; Chen Z; Daranciang D; Dai H A Route to Brightly Fluorescent Carbon Nanotubes for Near-Infrared Imaging in Mice. Nat. Nanotechnol 2009, 4 (11), 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Diao S; Blackburn JL; Hong G; Antaris AL; Chang J; Wu JZ; Zhang B; Cheng K; Kuo CJ; Dai H Fluorescence Imaging In Vivo at Wavelengths beyond 1500 Nm. Angew. Chemie Int. Ed 2015, 54 (49), 14758–14762. [DOI] [PubMed] [Google Scholar]
- (9).Robinson JT; Hong G; Liang Y; Zhang B; Yaghi OK; Dai H In Vivo Fluorescence Imaging in the Second Near-Infrared Window with Long Circulating Carbon Nanotubes Capable of Ultrahigh Tumor Uptake. J. Am. Chem. Soc 2012, 134 (25), 10664–10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Naczynski DJ; Tan MC; Zevon M; Wall B; Kohl J; Kulesa A; Chen S; Roth CM; Riman RE; Moghe PV Rare-Earth-Doped Biological Composites as in Vivo Shortwave Infrared Reporters. Nat. Commun 2013, 4, 2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Naczynski DJ; Tan MC; Riman RE; Moghe PV Rare Earth Nanoprobes for Functional Biomolecular Imaging and Theranostics. J. Mater. Chem. B 2014, 2, 2958–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Naczynski DJ; Sun C; Türkcan S; Jenkins C; Koh AL; Ikeda D; Pratx G; Xing L X-Ray-Induced Shortwave Infrared Biomedical Imaging Using Rare-Earth Nanoprobes. Nano Lett. 2015, 15 (1), 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Bruns OT; Bischof TS; Harris DK; Franke D; Shi Y; Riedemann L; Bartelt A; Jaworski FB; Carr JA; Rowlands CJ; et al. Next-Generation in Vivo Optical Imaging with Short-Wave Infrared Quantum Dots. Nat. Biomed. Eng 2017, 1 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Dong B; Li C; Chen G; Zhang Y; Zhang Y; Deng M; Wang Q Facile Synthesis of Highly Photoluminescent Ag2Se Quantum Dots as a New Fluorescent Probe in the Second Near-Infrared Window for in Vivo Imaging. Chem. Mater 2013, 25 (12), 2503–2509. [Google Scholar]
- (15).Hong G; Robinson JT; Zhang Y; Diao S; Antaris AL; Wang Q; Dai H In Vivo Fluorescence Imaging with Ag 2 S Quantum Dots in the Second Near-Infrared Region. Angew. Chemie Int. Ed 2012, 51 (39), 9818–9821. [DOI] [PubMed] [Google Scholar]
- (16).Yang ST; Wang X; Jia G; Gu Y; Wang T; Nie H; Ge C; Wang H; Liu Y Long-Term Accumulation and Low Toxicity of Single-Walled Carbon Nanotubes in Intravenously Exposed Mice. Toxicol. Lett 2008, 181 (3), 182–189. [DOI] [PubMed] [Google Scholar]
- (17).Liu Z; Davis C; Cai W; He L; Chen X; Dai H Circulation and Long-Term Fate of Functionalized, Biocompatible Single-Walled Carbon Nanotubes in Mice Probed by Raman Spectroscopy. Proc. Natl. Acad. Sci. U. S. A 2008, 105 (5), 1410–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Choi HS; Liu W; Misra P; Tanaka E; Zimmer JP; Bawendi MG; Frangioni JV Renal Clearance of Quantum Dots. Nat. Biotechnol/ 2007, 25 (10), 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Fitzpatrick JAJ; Andreko SK; Ernst LA; Waggoner AS; Ballou B; Bruchez MP Long-Term Persistence and Spectral Blue Shifting of Quantum Dots in Vivo. Nano. Lett 2009, 9 (7), 2736–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Lavis LD; Raines RT Bright Ideas for Chemical Biology. ACS Chem. Biol 2008, 3 (3), 142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Casalboni M; De Matteis F; Prosposito P; Quatela A; Sarcinelli F Fluorescence Efficiency of Four Infrared Polymethine Dyes. Chem. Phys. Lett 2003, 373 (3–4), 372–378. [Google Scholar]
- (22).Wang S; Fan Y; Li D; Sun C; Lei Z; Lu L; Wang T; Zhang F Anti-Quenching NIR-II Molecular Fluorophores for in Vivo High-Contrast Imaging and PH Sensing. Nat. Commun 2019, 10 (1), 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Hong G; Zou Y; Antaris AL; Diao S; Wu D; Cheng K; Zhang X; Chen C; Liu B; He Y; et al. Ultrafast Fluorescence Imaging in Vivo with Conjugated Polymer Fluorophores in the Second Near-Infrared Window. Nat. Commun 2014, 5 (1), 1–9. [DOI] [PubMed] [Google Scholar]
- (24).Cosco ED; Caram JR; Bruns OT; Franke D; Day RA; Farr EP; Bawendi MG; Sletten EM Flavylium Polymethine Fluorophores for Near- and Shortwave Infrared Imaging. Angew. Chemie Int. Ed 2017, 56 (42), 13126–13129. [DOI] [PubMed] [Google Scholar]
- (25).Cosco ED; Spearman AL; Ramakrishnan S; Lingg JGP; Saccomano M; Pengshung M; Arus BA; Wong KCY; Glasl S; Ntziachristos V; Warmer M; McLaughlin RR; Bruns OT; Sletten EM Shortwave infrared polymethine fluorophores matched to excitation lasers enable noninvasive, multicolor in vivo imaging in real time. Nat. Chem 2020, in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hansch C; Leo A; Taft RW A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. 1991, 91, 165–195. [Google Scholar]
- (27).Purification of 8-Flav7 was attempted via silica column chromatography, HPLC, recrystallization, and washing with solvents (toluene, tetrahydrofuran, diethyl ether, ethyl acetate). No Title.
- (28).Zhao Y; Truhlar DG The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc 2008, 120 (1–3), 215–241. [Google Scholar]
- (29).Scalmani G; Frisch MJ Continuous Surface Charge Polarizable Continuum Models of Solvation. I. General Formalism. J. Chem. Phys 2010, 132 (11), 114110. [DOI] [PubMed] [Google Scholar]
- (30).Head-Gordon M; Rico RJ; Oumi M; Lee TJ A Doubles Correction to Electronic Excited States from Configuration Interaction in the Space of Single Substitutions. Chem. Phys. Lett 1994, 219 (1–2), 21–29. [Google Scholar]
- (31).Head-Gordon M; Maurice D; Oumi M A Perturbative Correction to Restricted Open Shell Configuration Interaction with Single Substitutions for Excited States of Radicals. Chem. Phys. Lett 1995, 246 (1–2), 114–121. [Google Scholar]
- (32).Zhou P Why the Lowest Electronic Excitations of Rhodamines Are Overestimated by Time-Dependent Density Functional Theory. Int. J. Quantum Chem 2018, 118 (23), e25780. [Google Scholar]
- (33).CIS(D) leads to smaller deviations relative to TD-DFT methods because of the built-in corrections to the doubly excited state. No Title.
- (34).Rettig W; Klock A Intramolecular Fluorescence Quenching in Aminocoumarines. Identification of an Excited State with Full Charge Separation. Can. J. Chem 1985, 63, 1649. [Google Scholar]
- (35).An azetidine substituted at the seven-position of coumarin has been previously synthesized and has shown some TICT character in water. Grimm JB; English BP; Chen J; Slaughter JP; Zhang Z; Revyakin A; Patel R; Macklin JJ; Normanno D; Singer RH; et al. A General Method to Improve Fluorophores for Live-Cell and Single-Molecule Microscopy. Nat. Methods 2015, 12 (3), 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Liu X; Qiao Q; Tian W; Liu W; Chen J; Lang MJ; Xu Z Aziridinyl Fluorophores Demonstrate Bright Fluorescence and Superior Photostability by Effectively Inhibiting Twisted Intramolecular Charge Transfer. J. Am. Chem. Soc 2016, 138, 6963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


