Abstract
Antiviral countermeasures are needed to reduce the morbidity associated with Chikungunya virus (CHIKV) infection. This arbovirus reemerged in 2004 and causes periodic outbreaks in various areas throughout the world. While infection is rarely lethal, the majority of people infected with the virus develop a hallmark arthralgia as well as other disease manifestations. The virus is classified within three phylogenetic groups, namely, West African, East/Central/South African (ECSA), and Asian. Six strains of CHIKV covering the three phylogenetic groups were studied for their replication in cell culture, their ability to cause disease in susceptible mouse strains and susceptibility to antiviral treatment. Differential replication kinetics were observed for various CHIKV isolates in cell culture, which coincided with a decreased sensitivity to antiviral treatment as compared with ECSA and Asian clade viruses. This was confirmed in mouse infection studies with severe disease observed in mice infected with West African clade viruses, mild disease phenotype after infection with Asian clade viruses and an intermediate disease severity associated with ECSA virus infection. We also tested a broadly active antiviral, Favipiravir (T-705), which activity was inversely proportional to disease severity. These data suggest that some clades of CHIKV may cause more severe disease and may be more difficult to treat.
Introduction
Chikungunya virus (CHIKV) is an alphavirus that is transmitted by mosquitos in tropical and semi-tropical areas throughout the world. Originally discovered in Tanzania in 1952, this virus has since spread across the world and causes significant morbidity during regular outbreaks of disease. The viruses have been traditionally classified under three genotypes, namely the West African (WA), East-Central-South African (ECSA) and Asian. Two sub-lineages have emerged; one within the ECSA called the Indian Ocean (IOL) and one within the Asian clade for American viruses referred to as Asian/American (Langsjoen et al., 2018). More recent genetic analysis presents a revised phylogeny to take into account the spread of the virus and includes the epidemic clades ECSA-IOL, ECSA-MASA (Middle Africa/South American), AUL-Am (Asian Urban + American) and WA (Schneider et al., 2019).
Various mutations have been identified that are adaptive to specific mosquito vectors (Maljkovic Berry et al., 2019; Tsetsarkin et al., 2007; Tsetsarkin and Weaver, 2011), allowing the virus to increase its range and spread more efficiently in different areas. Since this reemergence in 2006 on the island of La Reunion, cases of chikungunya have been reported in various African, Asian, European and American countries (WHO Disease outbreak news). All three clades of CHIKV have been documented to cause outbreaks of disease over the past two decades (Maljkovic Berry et al., 2019; Sow et al., 2018; White et al., 2018). There also appears to be differences in pathogenesis in clinical disease depending on the strain of CHIKV a patient is infected with (Dupuis-Maguiraga et al., 2012). For example, 93% of patients experience cephalalgia headache after infection during an Indian outbreak, while on 43% experienced this symptom during an outbreak in Singapore.
Disease manifestations typically include fever and intense joint pain (arthralgia), but may also include rash, muscle pain, headache, and fatigue (Couderc and Lecuit, 2009). Less common consequences of CHIKV infection include cardiovascular disorders, neurologic manifestations, skin affections, respiratory failure, liver and renal disorders, and pneumonia, although these are typically associated with immune suppression or various co-morbidities (Economopoulou et al., 2009). Although the majority of disease cases are self-limiting and quickly resolve, some patients may develop chronic disease that can last months to years. More rare manifestations of CHIKV infection include neurological, gastrointestinal or ocular issues, which may be misdiagnosed. Although mortality is rare, it may occur in patients with immune deficiency. It has also been demonstrated that infection of mice lacking key interferon pathway intermediates, including interferon receptors, are acutely susceptible to CHIKV infection and succumb to disease within one week of infection, while mortality does not typically occur in immune competent mice (Couderc et al., 2008; Gardner et al., 2012). This underscores the importance of the interferon response in controlling CHIKV infection.
There are no currently approved countermeasures for the treatment or prevention of CHIKV. Several compounds have shown promise in cell culture and in animal models, including favipiravir (T-705). This compound is broadly active against a host of RNA viruses (Furuta et al., 2009). The mechanism of favipiravir is through the inhibition of the RNA polymerase (Furuta et al., 2013). This compound is active against CHIKV in cell culture and in animal models of disease (Abdelnabi et al., 2018; Delang et al., 2014; Franco et al., 2018). Treatment of mice experimentally infected with an Indian Ocean strain of CHIKV with 300 mg/kg/d of favipiravir resulted in a reduction of viral particles during the acute stage of infection, but not during the chronic phase (Abdelnabi et al., 2018), but it is unknown if this would be the case for the treatment of human infection. Escape mutants have also been observed (Delang et al., 2014), so combination therapy would be useful if favipiravir was considered for human treatment. Favipiravir is used for the treatment of influenza infection in Japan and is undergoing clinical evaluation or off-label use to treat disease associated with various viral infections (Delang et al., 2018).
Various mouse models of CHIKV have been developed, including those that model joint swelling as well as the lethal mouse models mentioned above. We have utilized a mouse strain that demonstrates footpad swelling at the site of virus challenge. The purpose of the present study is to characterize and compare infection of various CHIKV strains in DBA/1J or in AG129 mice with representative strains of the 3 clades of CHIKV (as well as the two subclades) and to compare the effect of favipiravir on the treatment of each strain.
Materials and Methods
Viruses and cells:
Chikungunya virus isolates from British Virgin Islands (BVI, R99659 TVP 20811), Indonesia (IND, RSUI TVP-1336), Nigeria (NGR, IbH 35 TVP-1337), Senegal (SEN, PM 2951) and Reunion Island (LR06, LR2006-OPYI) were obtained from Bob Tesh (WRCEVA) or from BEI Resources (Manassas, VA). Virus stocks were prepared by passaging LR06 twice in C6/36 cells (ATCC, CRL-1660). Infected flasks were observed daily after infection with virus and frozen when initial CPE was observed, followed by thawing, clarificiation via low-speed centrifugation to pellet the cells and aliquoting of supernatant. The passage 2 of the virus stocks were used in these studies and had titers of 108.2, 108.5 and 109.5 50% cell cultures infectious doses (CCID50)/ml for BVI, IND, NGR, SEN and LR06, respectively. Human rhabdosarcoma muscle cells (RD cells, ATCC CCL-136) were used to evaluated growth curves. Two independent runs were performed and an average curve was plotted. Green Monkey kidney cells (Vero, ATCC CCL-81) were used for other virus titrations.
Animals:
Female DBA/1J were obtained from Jackson Laboratories at an age of 4–6 weeks. Mice were randomly assigned to groups to model joint swelling after infection with various CHIKV strains. Male and female AG129, produced by an in-house colony, were used for disease and treatment efficacy studies. Mice were between 4–6 weeks when used. Groups of animals were randomly assigned to experimental groups and individually marked with ear tags.
Compounds:
Favipiravir (T-705) was provided by FUJIFILM Toyama Chemical Co., Ltd. (Toyama, Japan). Compound was prepared at various concentrations in 7.5% Sodium Bicarbonate solution in water. Methotrexate (MTX) was obtained from Sigma-Aldrich and prepared using sterile saline solution.
Infectious cell culture assay:
Growth curves were assessed by inoculating a human muscle cell line (RD, ATCC CCL-136) monolayer with various strains of CHIKV at a multiplicity of infection (MOI) of 0.01 and harvesting supernatant from individual wells of a microwell plate at various time-points. The virus titers in the cell culture supernatant samples, as well as tissues or serum from infected animals, were assessed using an infectious cell culture assay where a specific volume of either tissue homogenate or plasma was added to the first tube of a series of dilution tubes. Serial dilutions were made and added to Vero 76 cells (ATCC CRL-1587). Three days later cytopathic effect (CPE) was used to identify the end-point of infection. Four replicates were used to calculate the 50% cell culture infectious doses (CCID50) per mL of plasma or gram of tissues.
Cytokine and chemokine multiplex profiling:
Expression of various cytokines and chemokines was determined using the Q-plex mouse cytokine array (Quansys Biosciences, Logan, UT). Clarified hind leg homogenates taken 6 days post virus instillation (dpi) were analyzed per manufactures instructions. Cytokine levels obtained from infected mouse tissues were compared with a 50% effective dose (ED50) generated in vitro by the supplier (R&D Systems, Inc, Minneapolis, MN) using recombinant mouse cytokines/chemokines.
Experiment Design:
Groups of DBA1/J mice were challenged with 104.5 or 107.5 CCID50/mL via subcutaneous (s.c.) injection in the footpad and hock of the right leg with a total volume of 0.1 ml of the diluted (in minimal essential media) virus (0.05 ml each site) under isoflurane anesthesia. A separate study evaluating swelling after infection of mice with S27 at a dose of 108.0 CCID50/ml was included for comparative purposes. Animals were observed daily for morbidity and mortality for the experimental period of 9 days. Serum was obtained through cheek bleeds on day 2 post infection for titers. Footpad measurements were obtained every day for 9 days using a digital caliper. Measurement of the right footpad, which was the site of virus challenge, was compared against the contralateral footpad to calculate percent increase in footpad thickness. Two separate studies were conducted with BVI and NGR strains included in both studies for consistency purposes and to compare between studies. Study 1 included BVI, IND and NGR, while the second study included BVI, SEN, NGR, and LR06. Data for S27 was obtained from a separate study for comparative studies. Footpad swelling in mice infected with various CHIKV strains was compared with that of sham-infected mice. Lethal challenge studies utilized AG129 mice, which were challenged with 101.5 or 102.5 CCID50/0.1ml of each virus as described above. Survival was monitored twice a day through the critical period of disease to 10 dpi. Animals were observed daily for morbidity and mortality and weight change was monitored from 0–5 dpi. Serum was obtained through cheek bleeds for assessment of viremia.
Treatment studies tested various doses of favipiravir for efficacy in reducing footpad swelling and virus replication in DBA/1J mice after infection with various CHIKV strains as described above. Concentrations of 104.5 CCID50/0.1ml of each virus strain were inoculated as above. Favipiravir was prepared at doses of 400 mg/kg/day and 100 mg/kg/day in 7.5% sodium bicarbonate buffer (Life Technologies, Carlsbad, CA). In both studies, treatment began four hours (−4 h) before virus infection and was administered twice a day for eight days. Methotrexate was selected as a positive control due to the ready availability and mechanism that would be expected to reduce swelling after virus infection. Treatment was administered via intraperitoneal (i.p.) injection. Animals were observed daily for morbidity and mortality for the experimental period of 14 days. Serum was collected on 2 dpi for titration of viremia. Footpad measurements were obtained every day for 9 days to check for footpad swelling induced by the CHIKV virus strains in this model.
Treatment of lethal disease with 400 mg/kg/d of favipiravir was evaluated in AG129 mice infected with 102.5 CCID50 of various strains of CHIKV, including BVI, NGR and S27. Treatment and infection routes were as above. Morbidity and mortality were monitored for 10 days. Weight change was monitored from 0–5 dpi. Serum was obtained on 2 dpi for viral titers. Serum was also collected on 14 dpi to measure antibody in mice that survived the experiment.
Statistical analysis:
Survival data were analyzed using the Wilcoxon log-rank survival analysis and all other statistical analyses were done using one-way ANOVA using a Bonferroni group comparison (Prism 5, GraphPad Software, Inc).
Ethics regulation of Laboratory animals:
This study was conducted in accordance with the approval of the Institutional Animal Care and Use Committee of Utah State University (Protocol #2339). The work was done in the AAALAC-accredited Laboratory Animal Research Center of Utah State University. The facility is also approved by the National Institutes of Health (PHS Assurance no. A3801–01) in accordance with the NIH Guide for the Care and Use of Laboratory Animals (Revision; 2010).
Results
The growth curve of CHIKV isolates from British Virgin Isles (BVI), Indonesia (IND), Senegal (SEN), Nigeria (NGR), African prototype (S27) or Reunion Island (LR06) were assayed in RD cells. West African (SEN and NGR) and LR06 strains replicated rapidly, reaching peak titer by 24–36 hours post-virus inoculation (hpi) (Figure 1). The replication curves of BVI, IND and S27 strains did not enter exponential phase until after 24 hpi. Peak titers for these strains occurred around 48 hpi in RD cells (Figure 1). A general trend toward more robust replication was observed in the West African (SEN, NGR) and Reunion Island (LR06) isolates, whereas less robust replication was observed in the prototypical ECSA clade virus, S27, and the Asian-lineage strains (BVI, IND). Animal studies were planned to determine if these differences were also observed in animal models.
Figure 1.
Growth curves of representative Asian (BVI, IND), West African (SEN, NGR) and ECSA (S27, LR06) clade CHIKV strains in RD cells (*P<0.05, as compared with the growth curve of BVI).
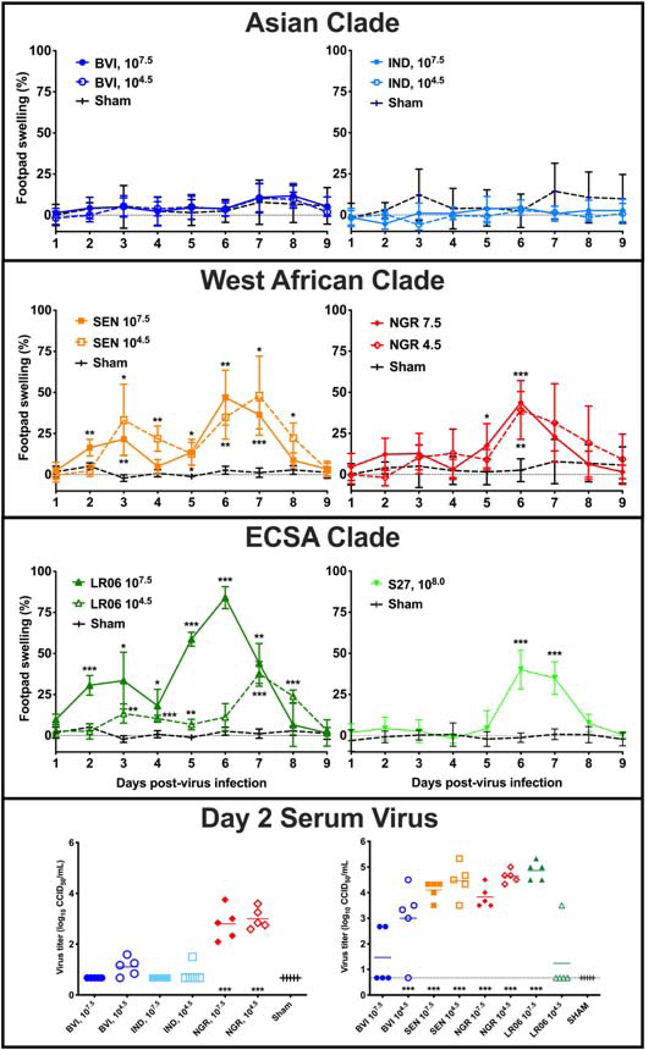
DBA/1J mice are susceptible to CHIKV infection and exhibit joint swelling at the site of virus inoculation. Groups (n=5) of DBA/1J mice were inoculated via s.c. injection into the footpad and hock of the rear hind leg with two challenge doses of each strain of CHIKV. Footpad swelling was assessed between 1 and 9 dpi by comparing the percent swelling in the foot inoculated with virus with that of the contralateral footpad to determine the difference in disease manifestation between strains within different clades. Mice infected with Asian lineage strains BVI and IND did not exhibit appreciable swelling (Figure 2). By contrast, inoculation with both low and high doses of West African clade CHIKV strains SEN and NGR resulted in a similar degree of swelling with an approximate 50% increase in footpad thickness around 6 or 7 dpi (Figure 2). A higher challenge dose of SEN resulted in an earlier peak in swelling compared to the lower challenge dose that peaked around 24 later, although the peak swelling did not differ substantially between the doses (Figure 2). There was a substantial difference between swelling of mice inoculated with high and low doses of LR06, with over 75% peak swelling associated with the high dose as compared with around 40% swelling observed after infection with a lower dose (Figure 2). Previous studies demonstrated a lack of swelling after inoculation with a low dose of S27, while high dose inoculation results in around a 50% increase in swelling in DBA/1J mice. Sham-infected mice were inoculated with culture media from uninfected cells to mimic injection with the virus preparation. Although minor footpad swelling is occasionally observed, these mice typically demonstrate less than ±10% swelling. Occasionally swelling between 20 and 35% is observed, but this occurs in relatively few individuals and is only rarely seen. The reason for this swelling has not been conclusively determined, but is likely due to damage during inoculation or an overreactive immune response to the inoculum.
Figure 2.
Footpad swelling of DBA/1J mice infected with various strains of CHIKV representing the three viral clades. Mice were inoculated and footpad swelling was assessed between 1 and 9 dpi using a digital caliper in two separate studies. Virus titers for the two titration studies are also shown (***P<0.001, **P<0.01, *P<0.05, as compared with footpad swelling of sham-infected mice).
Serum was collected 2 dpi from infected mice in two separate studies. BVI and NGR strains were included in both and had a similar profile, although titers were generally higher in the second study. Generally, infection with West African strains resulted in higher titers as compared with Asian strains (Figure 2). There was also a trend towards higher titers associated with lower challenge doses of BVI and LR06. No virus was observed in sham-infected animals. The swelling and viremia profiles observed in these studies correspond with the differences in growth curves observed in cell culture and reiterate the lower pathogenicity of the Asian clade and S27 infection in DBA/1J mice as compared with the West African and LR06 viruses in these model systems.
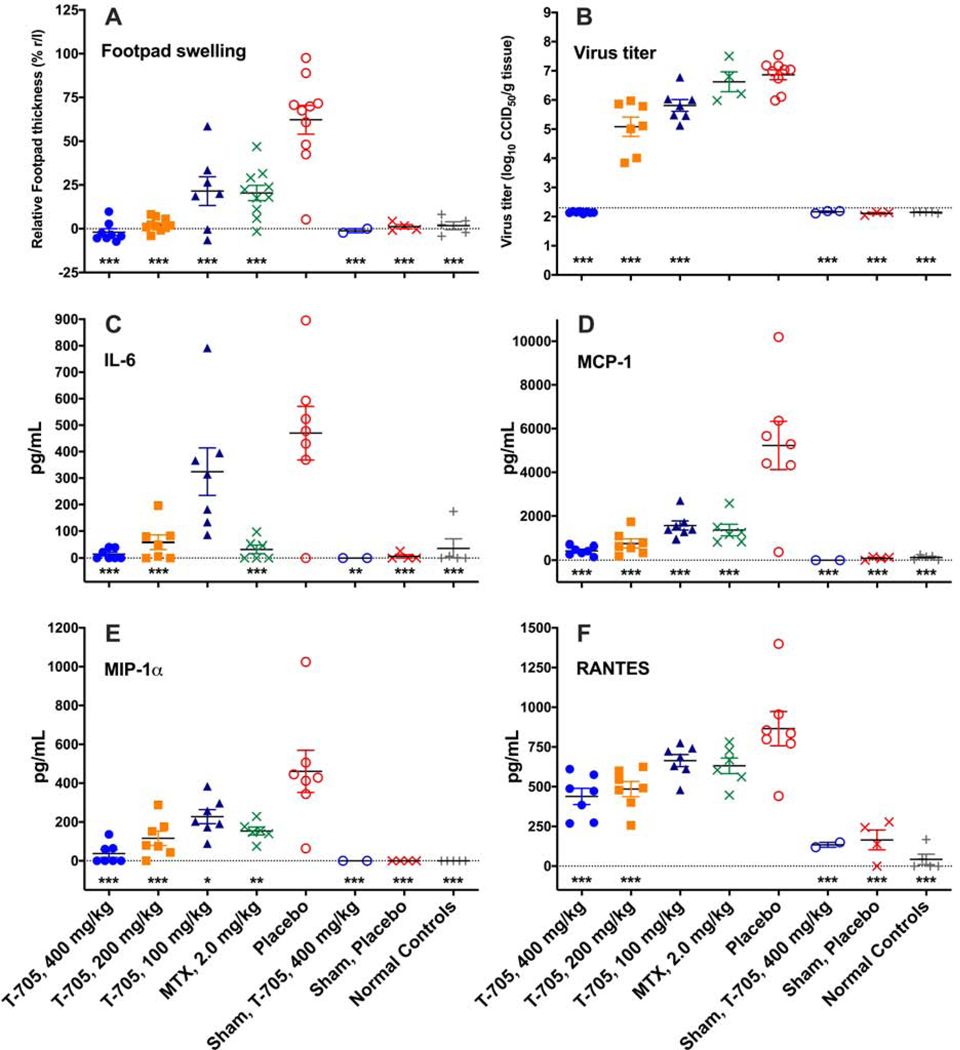
In initial efforts to evaluate the effect of a broadly active antiviral, favipiravir (T-705), we tested various doses of this compound in DBA/1J mice (n=10/group) inoculated with LR06 CHIKV. Treatment effectively reduced or prevented footpad swelling in CHIKV-infected mice, reducing footpad swelling to baseline at doses of 400 and 200 mg/kg/d (Figure 3A). Significantly reduced footpad swelling was also observed in mice treated with the lowest dose of 100 mg/kg/d as well as with 2 mg/kg/d of the positive control compound, methotrexate (MTX) (Figure 3A). Reduced footpad swelling also corresponded with a significant decrease in virus titer, which appeared to be reduced in a dose-dependent manner (Figure 3B). The positive control compound MTX did not improve virus titer, which would not be expected for this compound that likely exerts its effect by reducing swelling through a host-targeted immunological mechanism rather than through direct inhibition of virus.
Figure 3.
Favipiravir (T-705) treatment of DBA/1J mice infected with 104.5 CCID50 LR06 CHIKV results in a dose-dependent improvement in footpad swelling (A), virus titer in the hindlimb on 6 dpi (B), and various cytokines and chemokines, including IL-6 (C), MCP-1 (D), MIP-1α (E) and RANTES (F) were evaluated in hind leg homogenates at the site of virus challenge (***P<0.001, **P<0.01, *P<0.05 as compared with virus infection controls. Dashed line in Figure 3B represents the lower limit of detection).
The levels of various cytokines in the hind leg of mice infected with LR06 CHIKV and treated with favipiravir were evaluated to determine the effect of treatment on the host immune response. A dose-dependent reduction in IL-6, MCP-1, MIP-1α, and RANTES was observed after treatment with favipiravir as compared with placebo treatment (Figure 3C-F). As expected, MTX treatment also significantly reduced IL-6, MCP-1 and MIP-1α levels in the hind legs of infected mice (Figure 3C-F). This study demonstrated efficacy of favipiravir in the DBA/1J mouse model of CHIKV swelling after infection with LR06 CHIKV. With strain-dependent differences in viral pathogenicity, the next objective was to determine the effect of favipiravir against different CHIKV strains in a mouse model of footpad swelling.
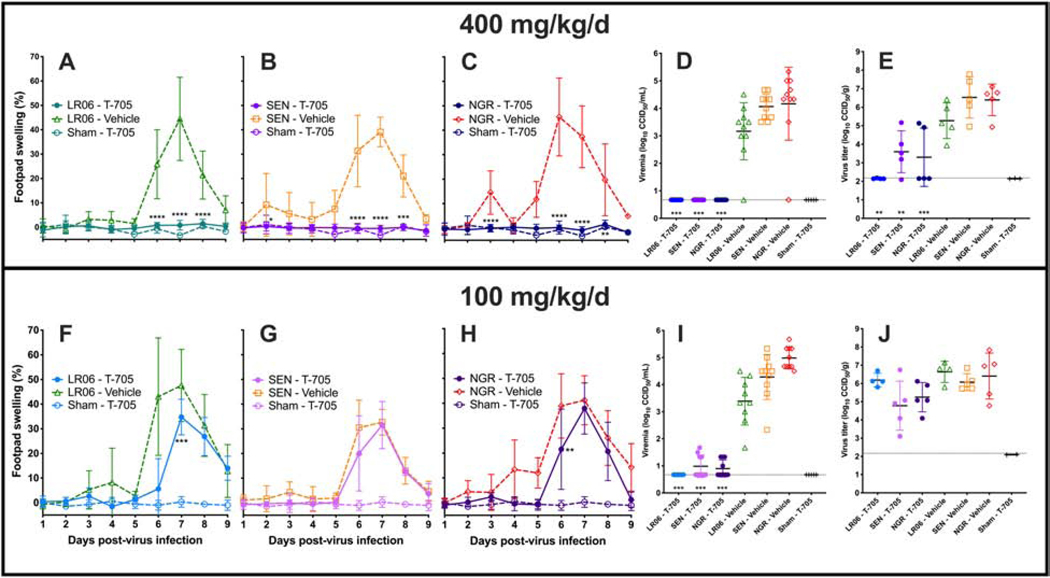
Robust footpad swelling was observed in DBA/1J mice after virus challenge with LR06, SEN and NGR strains of CHIKV. Therefore, the efficacy of favipiravir was tested against these CHIKV isolates. Treatment of CHIKV-infected DBA/1J mice (n=10/group) with 400 m/kg/d of favipiravir significantly improved footpad swelling, viremia on 2 dpi and virus titer in the rear hind leg at the site of virus challenge for all CHIKV strains (Figure 4A-E). Footpad swelling and viremia were reduced to baseline levels, regardless of challenge strain, while some virus was detected in the hind legs of mice infected with SEN or NGR, but not LR06 (Figure 4E). A dose of 100 mg/kg/d of favipiravir was less effective against infection with LR06 as compared with the higher dose and resulted in significantly reduced footpad swelling on 7 dpi, a delayed swelling curve and a trend towards reduced footpad swelling at later time-points (Figure 4F). While no improvement was observed with this dose in mice infected with SEN CHIKV, a similar profile of significantly reduced footpad swelling on 6 dpi and a delayed footpad swelling curve were observed in mice infected with NGR CHIKV (Figure 4G and H). As with the higher dose, viremia was significantly reduced (Figure 4I), while no significant improvement was observed in hind legs of infected mice (Figure 4J). Prophylactic treatment with favipiravir was effective in significantly improving disease in mice infected with various pathogenic strains of CHIKV.
Figure 4.
Favipiravir (T-705) effectively reduces footpad swelling in CHIKV-infected mice at doses of 400 mg/kg/d (A-E), while it is less effective at a dose of 100 mg/kg/d (F-J). There appeared to be equivalent efficacy in mice infected with LR06, SEN, or NGR. Footpad swelling (A-C, F-H), viremia (D, I) and virus titer in the hind limb on 6 dpi (E, J) were used as disease parameters (***P<0.001, **P<0.01, *P<0.05 as compared with virus infection controls).
Since no footpad swelling was observed in mice infected with Asian clade strains, we utilized AG129 mice, which are acutely sensitive to CHIKV infection, to determine the relative pathology of CHIKV strains from the different clades. Initial studies in AG129 mice demonstrated lethal outcome after infection with S27 CHIKV (data not shown). Infection of AG129 mice (n=5/group) with BVI, NGR or LR06 CHIKV was uniformly lethal at a challenge dose of 102.5 CCID50 (Figure 5A-C). A 10-fold lower dose caused complete mortality in mice infected with BVI and NGR, but the majority of mice infected with this dose of LR06 survived infection. There was a longer mean survival time in mice infected with BVI CHIKV as compared with NGR infection, while LR06 infection fell between the two. Weight change of CHIKV infected mice was similar to sham-infection, except for a precipitous drop in weight just prior to succumbing to virus infection (Figure 5D). Viremia was detected 2 days after virus challenge in all groups except for mice infected with the higher infectious dose of NGR CHIKV (Figure 5E). It is likely that peak viremia occurred prior to this collection time, as titers higher than 9 log10 were observed on 2 dpi in mice infected with the lower challenge dose of NGR (Figure 5E).
Figure 5.
Lethality is observed after footpad challenge of AG129 mice with various strains of CHIKV (A-C). Mice also typically lost weight after infection (D) and typically had detectable virus titer in the serum 2 days after virus challenge (E).
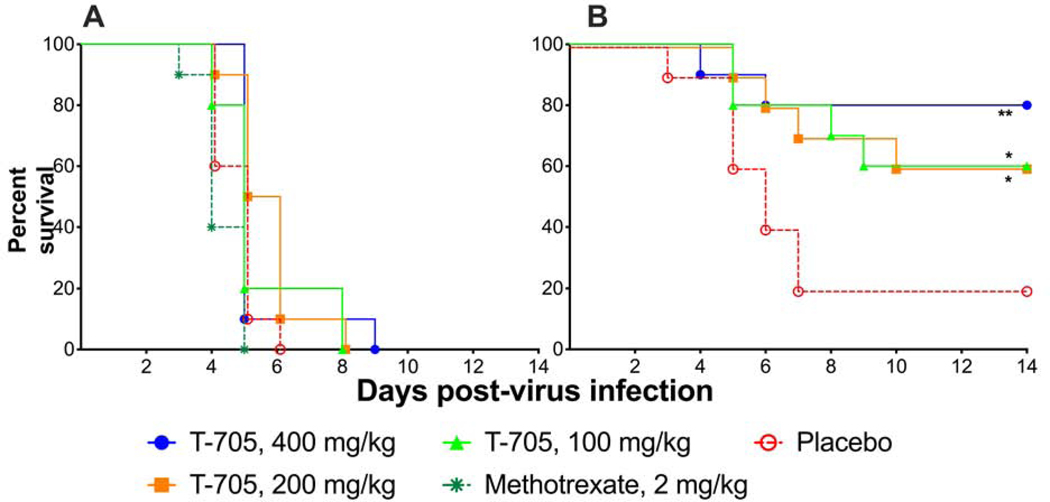
Since favipiravir was active in the DBA/1J swelling model against several CHIKV strains, we wanted to determine if this compound would be active against a lethal infection. AG129 mice (n=10/group) were treated with favipiravir and treatment was initiated just prior to challenge with LR06 CHIKV. Treatment with up to 400 mg/kg/d was ineffective in this model and did not protect mice from mortality after LR06 CHIKV infection (Figure 6A). In a subsequent experiment, mice infected with S27 CHIKV were treated with favipiravir at the same doses and timing as the study with LR06. Favipiravir was effective in preventing lethal disease in mice infected with S27 CHIKV with significant improvement in survival at doses as low as 100 mg/kg/d (Figure 6B). This demonstrated a difference in response to antiviral treatment based on the virus pathogenicity.
Figure 6.
Favipiravir (T-705) did not significantly improve survival in AG129 mice infected with a lethal challenge of LR06 CHIKV (A), while mice infected with S27 CHIKV were protected by various doses of T-705 (B) (**P<0.01, *P<0.05 as compared with placebo treatment).
A further study was designed to test favipiravir against representative CHIKV strains from different clades. We included treatment of S27 CHIKV-infected mice with favipiravir as a positive control in this study and to verify the previous results. Infection with BVI and NGR were included to compare favipiravir efficacy in less and more pathogenic models, respectively. AG129 mice (n=7/group) were treated 4 hours prior to infection with 102.5 CCID50 of BVI, S27 or NGR CHIKV via footpad injection. A significant improvement in survival was observed in mice infected with BVI or S27 after treatment with 400 mg/kg/d of favipiravir (Figure 7A-B). A non-significant delay in the mortality curve of mice infected with NGR CHIKV was observed in AG129 mice treated with compound, although complete mortality was observed (Figure 7C). Average weight change curves of infected mice treated with favipriavir were improved as compared with those of the vehicle-treated controls (Figure 7D). Viremia on 2 dpi was significantly reduced by an average of approximately 5 log10 in all mice treated with 400 mg/kg/d of favipiravir (Figure 7E). Higher titers were observed in mice infected with NGR, while those of mice infected with BVI were the lowest and S27 infected animals had intermediate virus levels in the serum. These results are consistent with the severity of disease in AG129 mice where animals infected with NGR CHIKV were more difficult to treat as compared with those infected with S27 or BVI CHIKV.
Figure 7.
Prophylactic treatment with favipiravir (T-705) was effective in significantly improving survival in AG129 mice after infection with BVI (A) or S27 (B), but not NGR (C) CHIKV. Survival curves (D) and viremia on 2 dpi (E) were also used to assess the efficacy of favipiravir treatment (***P<0.001 as compared with vehicle-treated controls).
Discussion
Infection studies in two different mouse strains with two representative strains from each of the three clades of CHIKV demonstrated clade-dependent pathogenesis. Infection with West African clade viruses, including isolates from Nigeria and Senegal, caused significant footpad swelling in DBA/1J mice and lethality in AG129 mice by 3–4 dpi. East, Central and South African clade viruses appeared to have an intermediate level of pathogenesis with the mutant emergent virus isolated in La Reunion Island (LR06), which belongs to the IOL subclade, causing fairly severe disease, while the prototypic S27 strain caused footpad swelling in DBA/1J mice at a higher challenge dose and lethality in AG129 mice by 3–5 dpi. The least pathogenic viruses were those from the Asian clade of CHIKV.
Virus isolates from British Virgin Isles (belonging to the Asian/American sublineage) and Indonesia failed to induce footpad swelling in DBA/1J mice and had a delayed mortality curve in AG129 mice as compared with strains from the two other clades. A similar pattern of pathogenesis was observed in a previous study with the West African clade causing more severe disease in immune compromised mice (Langsjoen et al, 2018). The same study demonstrated a less pathogenic phenotype associated with challenge with an American isolate of CHIKV as compared with previous infection with LR06 in cynomolgus macaques.
Infection with CHIKV does not typically cause lethality, and there is little information regarding comparative disease pathogenesis associated with different virus outbreaks, so it is difficult to say if these mouse models are predictive in regard to human infection. A high percentage of patients experienced arthralgia and swelling after natural infection with strains circulating in the Americas during the 2014–2015 epidemic (Chang et al., 2018), so in regard to human pathogenesis, there is no indication that Asian clade strains are less pathogenic. Our results provide comparative pathogenicity data in an immune competent mouse model of joint swelling and confirm the results previous studies in mice and macaques regarding comparative pathogenicity between viruses from the different CHIKV clades (Langsjoen et al., 2018).
After demonstrating clade-dependent pathogenicity of CHIKV isolates, we wanted to determine if these differences would be a factor for antiviral treatment. We selected the broadly active antiviral compound favipiravir, which has been shown to be active against CHIKV in cell culture and in animal models (Abdelnabi et al., 2018; Delang et al., 2014). We confirmed the efficacy of favipiravir against CHIKV infection in a mouse model and expanded the findings to include a comparison of compound efficacy of this compound against several different strains of CHIKV from the 3 clades. Favipiravir was effective in a dose-dependent manner in reducing footpad swelling, virus titer and inflammatory cytokine levels in mice infected with LR06 CHIKV, an IOL subclade virus within the ECSA clade. Efficacy was also observed in DBA/1J mice infected with West African clade virus strains from Nigeria (NGR) and Senegal (SEN). Since mutations in the nsP4 provides resistance of CHIKV to favipiravir, it is conceivable that differences in this protein among the different clades could explain potential differences in response to treatment (Delang et al., 2014). This study demonstrated, however, that mutations in the polymerase occur in a very conserved region of the protein.
Since DBA/1J mice do not display footpad swelling after infection with Asian/American clade strains (BVI and IND), a lethal mouse model in AG129 mice was used to test the efficacy of favipiravir against these viruses and to compare with more virulent strains at equivalent titers. Favipiravir, administered at a dose of 400 mg/kg/d, was effective in preventing mortality of mice infected with BVI and S27, but not after infection with NGR. From the present study as well as previous studies, we have identified differences in response to antiviral treatment based on the clade or subclade of challenge virus. We also know that joint swelling and pain have been observed in patients infected with Asian/American (sub)clade viruses, suggesting some disconnect between the mouse and clinical infection. There are also no approved antiviral countermeasures for the treatment or prevention of CHIKV, so such comparative studies are not possible at this time. The results of this study, however, may be instructive for future compound development for clinical use against CHIKV and a potential need to test for activity against viruses from different clades.
Highlights.
Infection with representative strains of CHIKV from each clades caused differences in morbidity and mortality in mice
Infection of mice with West African clade viruses caused more severe disease, while Asian strains were less pathogenic
More pathogenic viruses were also less responsive to treatment with favipiravir
Acknowledgements
We thank Jason Fairbourn, Jean Maxwell, Jared Bennett, Rachelle Stanton, Devin Pfister, Michael Bertolio, Taylor Redding and Brittney Downs for their expert technical help during the animal studies. This study was supported by NIAID/NIH funding from the Virology Branch under contract HHSN272201000039I through task order A105.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelnabi R, Jochmans D, Verbeken E, Neyts J, Delang L, 2018. Antiviral treatment efficiently inhibits chikungunya virus infection in the joints of mice during the acute but not during the chronic phase of the infection. Antiviral Res 149, 113–117. [DOI] [PubMed] [Google Scholar]
- Chang AY, Martins KAO, Encinales L, Reid SP, Acuna M, Encinales C, Matranga CB, Pacheco N, Cure C, Shukla B, Ruiz Arteta T, Amdur R, Cazares LH, Gregory M, Ward MD, Porras A, Rico Mendoza A, Dong L, Kenny T, Brueggemann E, Downey LG, Kamalapathy P, Lichtenberger P, Falls O, Simon GL, Bethony JM, Firestein GS, 2018. Chikungunya Arthritis Mechanisms in the Americas: A Cross-Sectional Analysis of Chikungunya Arthritis Patients Twenty-Two Months After Infection Demonstrating No Detectable Viral Persistence in Synovial Fluid. Arthritis & rheumatology 70, 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couderc T, Chretien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, Despres P, Arenzana-Seisdedos F, Michault A, Albert ML, Lecuit M, 2008. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog 4, e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couderc T, Lecuit M, 2009. Focus on Chikungunya pathophysiology in human and animal models. Microbes Infect 11, 1197–1205. [DOI] [PubMed] [Google Scholar]
- Delang L, Abdelnabi R, Neyts J, 2018. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res 153, 85–94. [DOI] [PubMed] [Google Scholar]
- Delang L, Segura Guerrero N, Tas A, Querat G, Pastorino B, Froeyen M, Dallmeier K, Jochmans D, Herdewijn P, Bello F, Snijder EJ, de Lamballerie X, Martina B, Neyts J, van Hemert MJ, Leyssen P, 2014. Mutations in the chikungunya virus non-structural proteins cause resistance to favipiravir (T-705), a broad-spectrum antiviral. J Antimicrob Chemother 69, 2770–2784. [DOI] [PubMed] [Google Scholar]
- Dupuis-Maguiraga L, Noret M, Brun S, Le Grand R, Gras G, Roques P, 2012. Chikungunya disease: infection-associated markers from the acute to the chronic phase of arbovirus-induced arthralgia. PLoS neglected tropical diseases 6, e1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economopoulou A, Dominguez M, Helynck B, Sissoko D, Wichmann O, Quenel P, Germonneau P, Quatresous I, 2009. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005–2006 outbreak on Reunion. Epidemiol Infect 137, 534–541. [DOI] [PubMed] [Google Scholar]
- Franco EJ, Rodriquez JL, Pomeroy JJ, Hanrahan KC, Brown AN, 2018. The effectiveness of antiviral agents with broad-spectrum activity against chikungunya virus varies between host cell lines. Antivir Chem Chemother 26, 2040206618807580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL, 2013. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 100, 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, Barnard DL, Gowen BB, Julander JG, Morrey JD, 2009. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res 82, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner CL, Burke CW, Higgs ST, Klimstra WB, Ryman KD, 2012. Interferon-alpha/beta deficiency greatly exacerbates arthritogenic disease in mice infected with wild-type chikungunya virus but not with the cell culture-adapted live-attenuated 181/25 vaccine candidate. Virology 425, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langsjoen RM, Haller SL, Roy CJ, Vinet-Oliphant H, Bergren NA, Erasmus JH, Livengood JA, Powell TD, Weaver SC, Rossi SL, 2018. Chikungunya Virus Strains Show Lineage-Specific Variations in Virulence and Cross-Protective Ability in Murine and Nonhuman Primate Models. mBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maljkovic Berry I, Eyase F, Pollett S, Konongoi SL, Joyce MG, Figueroa K, Ofula V, Koka H, Koskei E, Nyunja A, Mancuso JD, Jarman RG, Sang R, 2019. Global Outbreaks and Origins of a Chikungunya Virus Variant Carrying Mutations Which May Increase Fitness for Aedes aegypti: Revelations from the 2016 Mandera, Kenya Outbreak. Am J Trop Med Hyg 100, 1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider AB, Ochsenreiter R, Hostager R, Hofacker IL, Janies D, Wolfinger MT, 2019. Updated Phylogeny of Chikungunya Virus Suggests Lineage-Specific RNA Architecture. Viruses 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sow A, Faye O, Diallo M, Diallo D, Chen R, Faye O, Diagne CT, Guerbois M, Weidmann M, Ndiaye Y, Senghor CS, Faye A, Diop OM, Sadio B, Ndiaye O, Watts D, Hanley KA, Dia AT, Malvy D, Weaver SC, Sall AA, 2018. Chikungunya Outbreak in Kedougou, Southeastern Senegal in 2009–2010. Open Forum Infect Dis 5, ofx259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S, 2007. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS pathogens 3, e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin KA, Weaver SC, 2011. Sequential adaptive mutations enhance efficient vector switching by Chikungunya virus and its epidemic emergence. PLoS pathogens 7, e1002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SK, Mavian C, Salemi M, Morris JG Jr., Elbadry MA, Okech BA, Lednicky JA, Dunford JC, 2018. A new “American” subgroup of African-lineage Chikungunya virus detected in and isolated from mosquitoes collected in Haiti, 2016. PLoS One 13, e0196857. [DOI] [PMC free article] [PubMed] [Google Scholar]