Abstract
Objectives:
We investigated physical activity as a moderator of relations between sleep duration and quality and adolescents’ internalizing and externalizing problems.
Design:
The study used a cross-sectional design.
Setting:
Participants were recruited from small towns and semi-urban communities in Alabama.
Participants:
The sample was comprised of 235 adolescents (Mage = 15.78 years, SD = 9.60 months) who were diverse in sex (53% female), race/ethnicity (34% Black/African American, 66% White), and socioeconomic status.
Measurements:
Sleep duration (actual sleep minutes), efficiency (minutes/total sleep period), and latency (minutes from sleep attempt to onset) were examined with actigraphs for 1 week. Youth reported on their physical activity levels and internalizing and externalizing problems.
Results:
Interactions between sleep and physical activity emerged in the prediction of adolescents’ internalizing and externalizing problems. Supportive of moderation effects, adolescents with shorter or poorer-quality sleep in conjunction with less physical activity showed the highest levels of internalizing and externalizing problems. Demonstrative of protective effects, adolescents with more physical activity had lower levels of internalizing and externalizing problems regardless of their sleep duration or quality.
Conclusions:
Findings illustrate that not all youth are at equal risk for adjustment problems when they experience short or poor-quality sleep, suggesting the importance of examining both bioregulatory and environmental factors in understanding adolescent adjustment.
Keywords: Sleep, Actigraphy, Physical activity, Adolescents, Internalizing, Externalizing
Good-quality and sufficient-length sleep is necessary for mental health.1 However, the effects of poor sleep on youths’ adaptation and maladaptation are not the same for all individuals, and a growing body of research is demonstrating the importance of examining moderators of this association in order to explicate conditions under which sleep is particularly influential.2 At the same time, involvement in physical activity also is known to be related to better mental health.3 The current study is based on a moderation model and extends the literature by examining the novel question of whether sleep has a differential impact on adolescent mental health based on levels of physical activity. Examining physical activity as a moderator in this association helps to identify adolescents who are most at risk for mental health problems when they experience poor sleep, or who are protected by longer or better-quality sleep.
In adolescence, sleep duration tends to be shortened due to maturational changes in circadian rhythms and competing academic and social demands4 with ramifications for youths’ development.5 Indeed, only 5% to 20% of adolescents obtain the recommended 9 hours of sleep needed per night, and up to 25% sleep fewer than 6 hours per night.6,7 Internalizing and externalizing problems also are common during this development period, affecting up to 1 in 5 young people,8 and are related to sleep disturbances. Short sleep duration and poor sleep quality are associated with depression and anxiety in youth.9–11 Likewise, shorter sleep duration and lower sleep quality are related to aggressive behavior, delinquency, and social problems in adolescence.9,11,12
Past work has demonstrated a similar relationship between physical activity and adjustment. Higher levels of physical activity are associated with fewer symptoms of depression and anxiety in the teenage years.3,13,14 Participation in physical activity additionally is related to lower odds of adolescent involvement in substance use, vandalism, truancy, physical fighting, and other conduct problems.3,15
Existing literature also is supportive of positive direct relations between higher physical activity among adolescents and longer and better-quality sleep,16,17 findings that remain true despite age-related decreases in intensity and volume of physical activity in adolescence.18 Although both sleep and physical activity have been associated with adjustment, no studies to our knowledge have assessed the interaction between sleep and physical activity as predictors of adjustment in adolescence.
Consistent with recent calls in the literature,19 emerging research has been expanding on assessments of direct relations between sleep and adaptation in youth to consider the role of moderators of risk. For example, it has been demonstrated that poor-quality sleep is associated with higher instance of externalizing problems and lower self-esteem in adolescence—but this risk is increased among individuals who report less-secure attachment to their mothers and fathers.2 In late childhood, the association between poor sleep and higher BMI is stronger for youth with more cumulative familial risk factors such as family poverty, low maternal education, and recent stressful life events.20 Further, in addition to studies that have considered sleep as the predictor and other variables (e.g., secure attachment, poverty) as moderators, sleep has been found to moderate risk for various developmental outcomes. In early childhood, maternal sensitivity predicts higher emotional security over time, but only in the context of longer sleep.21 In middle childhood and early adolescence, aggression is predicted by high interparental conflict in combination with greater variability in sleep duration between weekday and weekends.22 In addition, in middle-to-late adolescence, peer victimization is associated with higher risk for internalizing symptoms among individuals with poorer-quality sleep.23 Collectively, these studies are supportive of further assessments of interactions between sleep and other variables as predictors of adaptation and maladaptation in youth.
The Present Study
The current investigation is consistent with these recent developments in the literature that attempt to explicate for whom and under which conditions sleep problems may be associated with adjustment.19,21,22 Toward this endeavor, we examined the interactive effects of sleep and physical activity as predictors of adolescents’ internalizing symptoms and externalizing behavior. To the best of our knowledge, this is the first such investigation in the field.
In accord with the increasing recognition of the importance of investigating multiple sleep parameters with objective methods,24 we examined multiple actigraphy-derived sleep duration and quality variables: sleep minutes (number of minutes scored as sleep), sleep efficiency (percentage of total sleep period scored as sleep), and sleep latency (number of minutes between sleep attempt and onset). Physical activity was assessed with a widely used weekly recall instrument. Mental health and adjustment problems were examined across internalizing (e.g., depression, anxiety) and externalizing (e.g., acting-out behaviors, substance use) domains.
In the context of poor sleep (fewer sleep minutes, lower sleep efficiency, longer sleep latency), we expected that lower levels of physical activity would intensify the risk for internalizing and externalizing problems. The exacerbation of risk for negative outcomes for youth with both poor sleep in conjunction with an additional risk factor (lower levels of physical activity in our study) is consistent with the dual-risk and stress-diathesis frameworks, which propose that risk for various outcomes is amplified in the context of multiple stressors and vulnerabilities.25 Our expectations also are consistent with empirical evidence demonstrating that poor sleep in conjunction with other risk factors (e.g., family conflict, peer victimization) are associated with increased problems across several child development domains.22,23 On the other side of the coin, and consistent with literature demonstrating protection in the context of risk,2,19 higher levels of physical activity may function as a protective factor against adjustment problems in the context of poor sleep. Of course, increased risk in the context of lower levels of physical activity and protection in the context of higher levels of such activity are not mutually exclusive.
Method
Participants
The current study analyzed data from the Family Stress and Youth Development Study, a longitudinal investigation of child and adolescent development within the context of bioregulation and family processes. At the initial wave of the study in 2005, a total of 251 children and their families participated after being recruited through rural and semi-urban elementary schools in east Alabama. Children qualified for enrollment if they were from two-parent homes and had not been diagnosed with attention-deficit/hyperactivity disorder, a developmental delay, or a chronic illness. Utilizing the same inclusion and exclusion criteria, an additional 53 adolescents from the same school districts were recruited into the study at the fourth wave of data collection in 2012 to 2013 in order to increase the sample size.
The analytic sample for the present investigation, composed of 235 adolescents between the ages of 14 and 18 (M = 15.78 years, SD = 9.58 months), was drawn from this fourth study wave; prior time points did not include actigraphy-based sleep assessments or physical activity information. Participants in the analytic sample were representative of the recruitment area and were diverse in sex (53% female), race/ethnicity (34% Black/African American, 66% White), and socioeconomic status (SES; 43% living at or below the federal poverty line, 22% middle class, and 35% upper middle class). Fifty-eight percent of adolescents lived with both of their parents, 24% lived in reconstituted families primarily with the mother and her partner, and the rest resided mostly with a single mother. SES was measured using the U.S. Department of Commerce’s (2013) family income-to-needs, a ratio derived through dividing annual family income by the poverty threshold accounting for household size.
Procedure
The Auburn University institutional review board approved the study protocol. Adolescents gave assent and their parents gave consent to participate. Youth wore actigraphs on their non-dominant wrists while sleeping at home for 1 week during the school year. An average of 3.96 days (SD = 12.25) later, families came to the university campus for a laboratory visit that lasted approximately 3.5 to 4 hours; 79% of participants completed the laboratory assessment on the day following the last night of actigraphy-based sleep assessment. Parents provided demographic data for the family, and adolescents reported on their physical activity over the previous week, as well as their adjustment problems over the previous 6 months.
Measures
Sleep
Octagonal Basic Motionloggers measured adolescent sleep for up to seven nights (Ambulatory Monitoring, Ardsley, NY, USA). Sadeh’s scoring algorithm26 and zero crossing mode were used to derive three sleep parameters. Sleep minutes represent the number of 1-minute epochs scored as sleep between sleep onset and wake time. Sleep efficiency is the percentage of time slept, calculated by dividing sleep minutes by the length of the total sleep period from onset to offset. Sleep latency is the number of minutes from initial sleep attempt in bed until sleep onset. Each sleep parameter was averaged across all available nights, excluding nights on which adolescents used medication for allergy relief or as a sleep aid. Sleep minutes, efficiency, and latency were stable across the week (α = .75, .92, .62, respectively).
One-third of adolescents (35.3%) had sleep data for the entire week, 27.7% had data for six nights, 19.1% for five nights, 9.8% for four nights, and 5.1% for three nights. Consistent with recommendations in the field, sleep data for 3% of youth were not used in analyses because the participants had fewer than three nights of actigraphy.27
Physical Activity
Youth reported on their physical activity levels by completing the frequently used weekly activities subscale of the Physical Activity Questionnaire for Adolescents (PAQ), a psychometrically sound, seven-day recall instrument with strong content and convergent validity.28 Self-report levels of activity on the PAQ are found to correspond accurately within 10% to 15% of total moderate-to-vigorous activity measured objectively by accelerometer, further supportive of the PAQ’s validity to measure physical activity.29 Participants used a rating scale of 1 (“None”) to 5 (“Very often”) to describe how often they engaged in activities like sports, dancing, games, or “any other physical activity” on each day of the previous week, and an average of the seven days was used in analyses. Adolescents’ physical activity levels were stable across the week (α = .91).
Adjustment
Adolescents completed the widely used and well-validated internalizing (α = .92) and externalizing (α = .92) scales of the Youth Self-Report (YSR).30 The YSR surveys a wide range of adjustment problems experienced over the previous six months, such as depression and anxiety (internalizing), and alcohol use and fighting with peers (externalizing). Approximately one-fifth of participants met borderline (T score between 60 and 62) or clinical (≥ 63) cutoffs for internalizing (20.70%) or externalizing (19.82%) problems.
Covariates
The effects of adolescents’ age, sex, race/ethnicity, family income, and body mass index were controlled in all analyses. Participants’ body mass index was computed from weight and height measured by research assistants on a Tanita digital weight scale (Model BC-418) and wall-mounted stadiometer (Arlington Heights, IL). The Centers for Disease Control and Prevention’s31 online calculator derived a standardized body mass index score (zBMI) that was used in analyses. A majority (56%) of adolescents had a healthy weight (5th-84th percentile for age and sex), and 44% were overweight or obese (≥85th percentile).
Plan of Analysis
Values greater than 4 SDs from the mean for each variable were recoded as the value equal to 4 SDs.32 A total of 6 values were recoded, including 2 for sleep latency, 2 for internalizing symptoms, and 2 for externalizing behavior. Skewness was assessed before and after the variables were recoded, and all study variables had distributions acceptably close to normal. Separate path models were fit for internalizing and externalizing problems in Amos 23.33 Study variables were missing between 2.98% and 4.26% of data, which was handled with full information maximum likelihood (FIML) estimation in order to make use of all available data to construct the least-biased estimates.34,35 Continuous predictor variables and covariates were mean-centered, and within each model, significantly correlated exogenous variables were covaried with one another. Covariates (age, sex, race, SES, and zBMI) were entered in the first step, followed by sleep and physical activity in the second step, and the two-way interactions between sleep and physical activity in the third step. All models were a good fit to the data, with a non-significant χ,2 a χ2/df ratio ≤1.38, comparative fit index ≥.90, and a non-significant root mean square error of approximation (RMSEA) acceptably close to zero (RMSEA ≤.035).36 Significant interactions were plotted at −1 and+ 1 SD from the mean for levels of sleep and physical activity using Preacher, Curran, and Bauer’s37 online interaction utility that also tests simple slopes within regions of significance. In the reporting of results that follows, prediction is used in the statistical versus causal sense.
Results
Preliminary Analyses
Table 1 includes descriptive statistics and correlations among study variables. Sleep minutes were associated negatively with internalizing symptoms, and sleep latency was associated positively with both internalizing and externalizing problems. Physical activity was associated negatively with internalizing and externalizing problems. Independent-samples t-tests revealed sex and race differences for sleep parameters and physical activity. On average, females slept 22 min longer than males (Mfemale = 413.48 min; Mmale = 391.91 min), and White adolescents slept 27 min longer than their Black counterparts (MWhite = 412.46 min; MBlack = 385.30 min). Females also had more efficient sleep (Mfemale = 91.87%; Mmale = 89.42%) and shorter sleep latency (Mfemale = 9.95 min; Mmale = 12.61 min) compared to males. Males, on the other hand, reported more physical activity than females (Mmale = 3.24; Mfemale = 2.73).
Table 1.
Descriptive statistics and correlations among study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | – | ||||||||||
| 2. Sex | .24*** | – | |||||||||
| 3. Race/ethnicity | −.08 | −.06 | – | ||||||||
| 4. Income-to-needs | −.04 | .00 | −.27*** | – | |||||||
| 5. zBMI | −.02 | −.04 | .14* | −.09 | – | ||||||
| 6. Sleep minutes | −.09 | −.19** | −.23*** | .16* | −.01 | – | |||||
| 7. Sleep efficiency | .05 | −.17** | −.12 | .12 | .03 | .59*** | – | ||||
| 8. Sleep latency | .03 | .17* | −.03 | −.04 | .00 | −.15* | −.23*** | – | |||
| 9. Physical activity | −.03 | .21*** | .06 | .09 | .08 | .05 | −.01 | −.12 | – | ||
| 10. Internalizing symptoms | −.01 | −.05 | .04 | −.08 | −.10 | −.17* | −.08 | .14* | −.26*** | – | |
| 11. Externalizing behavior | .07 | −.03 | −.04 | −.15* | −.06 | −.07 | −.04 | .14* | −.16*** | .58*** | – |
| Mean | 15.78 | – | – | 2.39 | .87 | 403.64 | 90.75 | 11.17 | 2.97 | 50.49 | 50.83 |
| (SD) | (.80) | – | – | (1.31) | (.98) | (56.28) | (7.02) | (7.96) | (1.23) | (11.20) | (10.91) |
Age reported in years, but was used in months for analyses; sex: 0 = female, 1 = male; race: 0 = White, 1 = Black; zBMI = standardized body mass index score; internalizing symptoms and externalizing behavior are represented in T scores; 403.64 minutes = 6.73 hours.
p < .05.
p < .01.
p < .001.
Path Models
Sleep Minutes
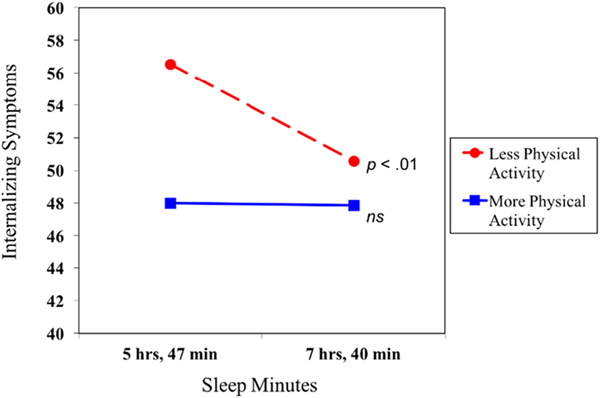
After covarying age, race, sex, income, and zBMI, sleep minutes and physical activity were negatively associated with internalizing symptoms (Table 2). Central to this investigation, physical activity moderated the association between sleep minutes and internalizing symptoms at the statistical-trend level (p = .056). A test of simple slopes revealed a negative association between sleep minutes and internalizing symptoms for less active youth (Fig. 1). No such association emerged for adolescents with higher levels of physical activity, who tended to have relatively low levels of internalizing symptoms regardless of their sleep duration. Predicted means provide further clarification of the nature of physical activity as a moderator of associations. As shown in Fig. 1, for adolescents with shorter sleep, those who were more sedentary (M = 56.52) had higher levels of internalizing symptoms compared to those with higher levels of physical activity (M = 48.00), a difference of 0.76 SD. Youth with longer sleep, on the other hand, had predicted levels of internalizing symptoms near or below the mean regardless of physical activity level (M = 50.58 for lower physical activity; M = 47.87 for higher). The full model with covariates, sleep minutes, physical activity, and the interaction between sleep minutes and physical activity explained about 12% of the variance in adolescents’ internalizing symptoms. No moderation effect emerged for externalizing behavior.
Table 2.
Unstandardized and standardized coefficients of associations linking sleep minutes, physical activity, and adjustment in adolescence
| Internalizing symptoms |
Externalizing behavior |
|||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | β | R2 | B | SE | β | R2 | |
| Step 1 | 2.5% | 4.3% | ||||||
| Age | −0.02 | 0.08 | −.02 | 0.06 | 0.08 | .06 | ||
| Sex | −0.66 | 1.51 | −.03 | −0.95 | 1.52 | −.04 | ||
| Race/ethnicity | 0.09 | 1.58 | .004 | −1.81 | 1.59 | −.08 | ||
| Socioeconomic status | −0.49 | 0.57 | −.06 | −1.40* | 0.57 | −.17 | ||
| zBMI | −0.94 | 0.72 | −.08 | −0.61 | 0.72 | −.06 | ||
| Step 2 | 10.8% | 5.4% | ||||||
| Sleep minutes | −0.03* | 0.01 | −.14 | −0.01 | 0.01 | −.04 | ||
| Physical activity | −2.29*** | 0.59 | −.25 | −1.12~ | 0.60 | −.13 | ||
| Step 3 | 12.1% | 6.2% | ||||||
| Minutes × Physical activity | 0.02~ | 0.01 | .13 | 0.01 | 0.01 | .07 | ||
Path models covaried age, sex, race/ethnicity, socioeconomic status, and standardized body mass index score (zBMI). Path coefficients reported are from the final model. R2 reported is from the step of entry. SE = standard error. Sex: 0 = female, 1 = male; race: 0 = White, 1 = Black.
p < .10.
p < .05.
p < .001
Fig. 1.
Associations between sleep minutes and adjustment at lower and higher levels of physical activity. Internalizing symptoms are represented in T scores. ns = not significant.
Sleep Efficiency
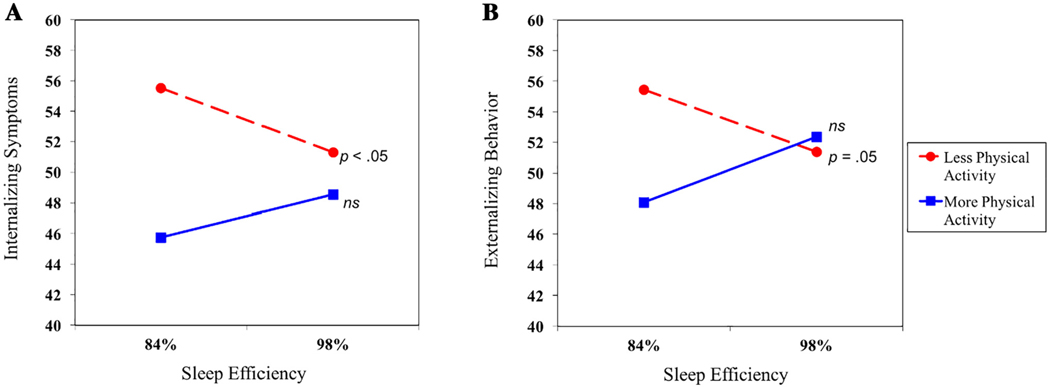
After accounting for covariates, physical activity was negatively associated with internalizing symptoms (Table 3). In addition, physical activity moderated the association between sleep efficiency and internalizing symptoms. A test of simple slopes revealed that sleep efficiency was significantly associated with internalizing symptoms for youth with lower but not higher levels of physical activity (Fig. 2A). Further, at lower levels of sleep efficiency, more sedentary adolescents had higher levels of internalizing symptoms (M = 55.51) compared to more physically active adolescents (M = 45.75), a difference of 0.87 SD. At higher levels of sleep efficiency, internalizing symptoms were roughly similar regardless of physical activity level—and near the mean (M = 51.31 for lower physical activity; M = 48.55 for higher physical activity). For youth with higher levels of physical activity, the slope representing the association between sleep efficiency and internalizing symptoms did not differ significantly from zero, and the predicted means for internalizing symptoms were modest at both lower and higher sleep efficiency (M = 45.75 for lower; M = 48.55 for higher). Together with covariates, main predictors, and the interaction between sleep efficiency and physical activity, the full model explained 11% of the variance in internalizing symptoms.
Table 3.
Unstandardized and standardized coefficients of associations linking sleep efficiency, physical activity, and adjustment in adolescence
| Internalizing symptoms |
Externalizing behavior |
|||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | β | R2 | B | SE | β | R2 | |
| Step 1 | 2.5% | 4.3% | ||||||
| Age | −0.01 | 0.08 | −.01 | 0.07 | 0.08 | .07 | ||
| Sex | −0.02 | 1.52 | −.001 | −0.81 | 1.50 | −.04 | ||
| Race/ethnicity | 0.77 | 1.56 | .03 | −1.74 | 1.54 | −.08 | ||
| Socioeconomic status | −0.61 | 0.57 | −.07 | −1.55** | 0.56 | −.19 | ||
| zBMI | −0.83 | 0.72 | −.07 | −0.47 | 0.72 | −.04 | ||
| Step 2 | 8.9% | 5.4% | ||||||
| Sleep efficiency | −0.05 | 0.11 | −.03 | 0.01 | 0.11 | .01 | ||
| Physical activity | −2.55*** | 0.60 | −.28 | −1.30* | 0.60 | −.15 | ||
| Step 3 | 11.3% | 9.1% | ||||||
| Efficiency × Physical activity | 0.20* | 0.10 | .14 | 0.24* | 0.10 | .17 | ||
Path models covaried age, sex, race/ethnicity, socioeconomic status, and standardized body mass index score (zBMI). Path coefficients reported are from the final model. R2 reported is from the step of entry. SE = standard error. Sex: 0 = female, 1 = male; race: 0 = White, 1 = Black.
p < .05.
p < .01.
p < .001.
Fig. 2.
Associations between sleep efficiency and adjustment at lower and higher levels of physical activity. Internalizing symptoms and externalizing behavior are represented in T scores. ns = not significant.
Similar to the effects observed for internalizing symptoms, physical activity was negatively associated with externalizing behavior and functioned as a moderator of relations between sleep efficiency and externalizing behavior (Table 3). As evidenced by a test of simple slopes, sleep efficiency was significantly associated with externalizing symptoms for more sedentary—but not more active—youth (Fig. 2B). As with internalizing problems, more sedentary youth with lower sleep efficiency had higher levels of externalizing behavior (M = 55.46) than their more physically active peers with low sleep efficiency (M = 48.08)—a difference of two-thirds of an SD. For adolescents with more efficient sleep, externalizing behavior scores differed by just .09 SD (M = 51.38 for lower physical activity; M = 52.36 for higher). For more physically active youth, the simple slope representing the association between sleep efficiency and externalizing behavior did not differ significantly from zero, suggesting that adjustment does not vary by sleep efficiency for these adolescents. The full model accounted for 9% of the variance in externalizing behavior.
Sleep Latency
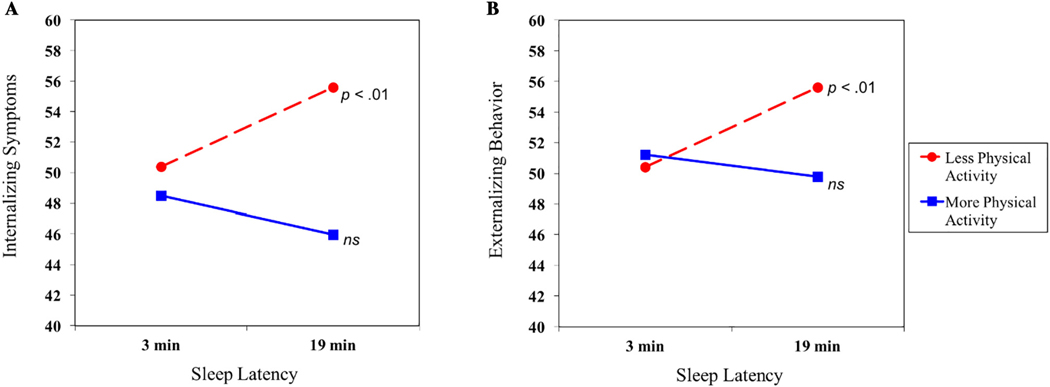
After accounting for covariates, higher levels of physical activity predicted fewer internalizing symptoms (Table 4). Further, physical activity moderated relations between sleep latency and internalizing symptoms. At lower levels of physical activity, the slope depicting the association between sleep latency and internalizing symptoms was statistically significant (Fig. 3A). In the context of longer sleep latency, the predicted mean for internalizing symptoms was higher among adolescents with lower (M = 55.57) compared with higher (M = 45.98) levels of physical activity—a difference of 0.86 SD. Shorter sleep latency was associated with similar levels of internalizing symptoms regardless of physical activity level (M = 50.38 for lower physical activity; M = 48.50 for higher). At higher levels of physical activity, the pertinent slope was not significantly different from zero, suggesting that the predicted means for internalizing symptoms do not vary by youths’ sleep latency. As shown in Fig. 3A, the more active youth had levels of internalizing symptoms that were relatively low—below the mean for the sample (M = 48.50 for shorter sleep latency; M = 45.98 for longer sleep latency). The full regression model explained 13% of the variance in internalizing symptoms.
Table 4.
Unstandardized and standardized coefficients of associations linking sleep latency, physical activity, and adjustment in adolescence
| Internalizing symptoms |
Externalizing behavior |
|||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | β | R2 | B | SE | β | R2 | |
| Step 1 | 2.5% | 4.3% | ||||||
| Age | −0.003 | 0.08 | −.003 | 0.08 | 0.07 | .07 | ||
| Sex | −0.23 | 1.50 | −.01 | −1.22 | 1.49 | −.06 | ||
| Race/ethnicity | 1.08 | 1.54 | .05 | −1.46 | 1.53 | −.06 | ||
| Socioeconomic status | −0.52 | 0.56 | −.06 | −1.42* | 0.56 | −.17 | ||
| zBMI | −1.14 | 0.72 | −.10 | −0.78 | 0.71 | −.07 | ||
| Step 2 | 9.6% | 6.9% | ||||||
| Sleep latency | 0.08 | 0.10 | .06 | 0.12 | 0.10 | .09 | ||
| Physical activity | −2.33*** | 0.59 | −.26 | −1.02~ | 0.60 | −.12 | ||
| Step 3 | 13.0% | 9.8% | ||||||
| Latency × Physical activity | −0.20** | 0.08 | −.19 | −0.17* | 0.08 | −.16 | ||
Path models covaried age, sex, race/ethnicity, socioeconomic status, and standardized body mass index score (zBMI). Path coefficients reported are from the final model. R2 reported is from the step of entry. SE = standard error. Sex: 0 = female, 1 = male; race: 0 = White, 1 = Black.
p < .10.
p < .05.
p < .01.
p < .001.
Fig. 3.
Associations between sleep latency and adjustment at lower and higher levels of physical activity. Internalizing symptoms and externalizing behavior are represented in T scores. ns = not significant.
Physical activity also emerged as a moderator in the association between sleep latency and externalizing behavior (Table 4). The slope of externalizing behavior at lower levels of physical activity was statistically significant (Fig. 3B). In the context of longer sleep latency, less active youth had higher levels of externalizing behavior (M = 55.61) than their more active counterparts (M = 49.80), a difference of half an SD. Consistent with other interactions between the aforementioned sleep variables and physical activity, youth with shorter sleep latency had similar levels of externalizing behavior at lower (M = 50.42) and higher (M = 51.23) levels of physical activity. Furthermore, for adolescents with higher levels of physical activity, the slope representing the association between sleep latency and externalizing behavior was not significantly different from zero and indicated relatively low levels of such behavior problems, below the mean for the sample. The full model of sleep latency and physical activity explained almost 10% of the variance in externalizing behavior.
Discussion
Adolescent adjustment is influenced by a complex web of biological, psychological, and social factors. This study examined physical activity as a moderator of relations between multiple sleep parameters and mental health in adolescents. Specifically, we assessed whether low levels of physical activity intensify, or high levels of such activity protect against, the risk for adjustment problems that may be associated with shorter and poorer-quality sleep. Three objective measures of sleep duration and quality were considered in interactions with physical activity: sleep minutes, sleep efficiency, and sleep latency.
Consistent with our hypothesis, higher levels of adjustment problems were observed for youth who had shorter or poorer-quality sleep (efficiency, latency) in combination with lower levels of physical activity. This finding places the current study within the broader lens of dual-risk and stress-diathesis perspectives.25 As individuals experience cumulative risk in the presence of existing vulnerabilities, risk is aggregated and the likelihood of negative outcomes increases.38 Our analyses demonstrated that sleep problems interact with low physical activity to increase the likelihood of both internalizing and externalizing symptoms in adolescence. This dual-risk pattern is similar to others in the literature demonstrating that poor sleep interacts with familial and other environmental factors (e.g., interparental conflict, peer victimization) to exacerbate negative outcomes.22,23
Adolescents in this study had lower predicted means for internalizing and externalizing problems when they had either more physical activity or longer or higher-quality sleep. It appears that higher levels of physical activity provide protection from the risk accrued by insufficient or lower-quality sleep, and vice versa—the presence of longer or higher-quality sleep buffers against the ill effects of low physical activity. Such findings may be welcomed by the parents and clinicians of teenagers whose lives become increasingly busy in high school through the demands of course work, extracurricular involvement, social activities, and, for some, employment. Adolescents who are sleep deprived or experience poor-quality sleep might have their risk for adjustment problems mitigated through involvement in physical activities, and other teens may have the risk of a sedentary lifestyle lessened by improving the quality and duration of their sleep.
Although not a consistent finding, past work reported that sleep quality may be more influential than sleep duration for youths’ well-being. El-Sheikh, Tu, Erath, and Buckhalt39 found that sleep efficiency, but not sleep duration, moderated the relationship between family functioning and cognitive ability. Sadeh, Raviv, and Gruber40 found that sleep efficiency and number of night wakenings, but not sleep period, were related to family factors such as family stress and parent age and education. In a similar vein, findings from the current study revealed the differential effects of sleep quantity and quality on adolescents’ well-being. In combination with physical activity, sleep efficiency and latency were significantly predictive (in the statistical sense) of both internalizing and externalizing problems, while sleep duration, measured through minutes scored as asleep from onset to wake, was not associated with externalizing problems and related to internalizing problems only at the level of a statistical trend. Conclusions about if and which sleep parameter may be more influential for various developmental outcomes are far ahead.
There are several limitations in the present study that warrant delineation and findings that point to important future directions in this area of inquiry. First, our results from path analyses cannot infer causal relations between sleep and adjustment outcomes. Future work could explore pathways through which these effects occur, including mediation models testing the directionality of relationships among study variables. Second, none of our participants had been diagnosed with sleep disorders at the time of recruitment, and 80% of participants endorsed levels of symptoms below clinical levels for internalizing and externalizing problems. Thus, the results of analyses of data from our generally healthy community sample may not be generalizable to other groups, particularly, individuals with sleep disorders and/or those with clinical-level depression, anxiety, and externalizing problems. The moderation effects of physical activity in the association between sleep and adjustment in clinical samples may represent an important focus for future research. Third, it would be prudent to attempt to replicate this study with additional age groups, investigating whether there are developmentally sensitive time periods for the conjoint effects of sleep and environmental factors on adolescent adjustment. Finally, our study lacked an objective indicator of physical activity (e.g., accelerometry). Although subjective assessments of physical activity with validated measures have advantages, future work in this area would benefit from the increased methodological rigor of objective physical activity assessments.
This study reveals multiplicative risk for adolescents who have insufficient or low-quality sleep together with lower levels of physical activity. Such youth have substantially higher levels of internalizing and externalizing problems than their peers. The study highlights the need for adolescents to obtain sufficient, high-quality sleep and high levels of physical activity to promote mental health.
Acknowledgments
This research was supported by Grant R01-HD046795 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development awarded to Mona El-Sheikh. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health. We wish to thank our research laboratory staff, particularly Bridget Wingo, for data collection and preparation, as well as the children and parents who participated.
Footnotes
Disclosure
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- 1.Gregory AM, Sadeh A. Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Med Rev. 2012;16(2):129–136. [DOI] [PubMed] [Google Scholar]
- 2.Tu KM, Marks BT, El-Sheikh M. Sleep and mental health: the moderating role of perceived adolescent-parent attachment. Sleep Health. 2017;3(2):90–97. [DOI] [PubMed] [Google Scholar]
- 3.Spruit A, Assink M, van Vugt E, van der Put C, Stams GJ. The effects of physical activity interventions on psychosocial outcomes in adolescents: a meta-analytic review. Clin Psychol Rev. 2016;45:56–71. [DOI] [PubMed] [Google Scholar]
- 4.Crowley SJ, Tarokh L, Carskadon MA. Sleep during adolescence In: Sheldon SH, Kryger MH, Ferber R, Gozal D, editors. Principles and Practice of Pediatric Sleep Medicine. 2nd ed London, UK: Elsevier Saunders; 2014. p. 45–52. [Google Scholar]
- 5.Brand S, Kirov R. Sleep and its importance in adolescence and in common adolescent somatic and psychiatric conditions. Int J Gen Med. 2011;4:425–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Sleep Foundation. Sleep in America Poll. https://sleepfoundation.org/sites/default/files/2006_summary_of_findings.pdf; 2006.
- 7.Becker SP, Langberg JM, Byars KC. Advancing a biopsychosocial and contextual model of sleep in adolescence: a review and introduction to the special issue. J Youth Adolesc. 2015;44(2):239–270. [DOI] [PubMed] [Google Scholar]
- 8.Bor W, Dean AJ, Najman J, Hayatbakhsh R. Are child and adolescent mental health problems increasing in the 21st century? A systematic review. Aust N Z J Psychiatry. 2014;48(7):606–616. [DOI] [PubMed] [Google Scholar]
- 9.Kelly RJ, El-Sheikh M. Reciprocal relations between children’s sleep and their adjustment over time. Dev Psychol. 2014;50(4):1137–1147. [DOI] [PubMed] [Google Scholar]
- 10.McMakin DL, Alfano CA. Sleep and anxiety in late childhood and early adolescence. Curr Opin Psychiatry. 2015;28(6):483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadeh A, Tikotzky L, Kahn M. Sleep in infancy and childhood: implications for emotional and behavioral difficulties in adolescence and beyond. Curr Opin Psychiatry. 2014;27(6):453–459. [DOI] [PubMed] [Google Scholar]
- 12.Shochat T, Cohen-Zion M, Tzischinsky O. Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep Med Rev. 2014;18(1):75–87. [DOI] [PubMed] [Google Scholar]
- 13.Brand S, Gerber M, Beck J, Hatzinger M, Pühse U, Holsboer-Trachsler E. High exercise levels are related to favorable sleep patterns and psychological functioning in adolescents: a comparison of athletes and controls. J Adolesc Health. 2010;46(2): 133–141. [DOI] [PubMed] [Google Scholar]
- 14.Sigfusdottir ID, Asgeirsdottir BB, Sigurdsson JF, Gudjonsson GH. Physical activity buffers the effects of family conflict on depressed mood: a study on adolescent girls and boys. J Adolesc. 2011;34(5):895–902. [DOI] [PubMed] [Google Scholar]
- 15.Harrison PA, Narayan G. Differences in behavior, psychological factors, and environmental factors associated with participation in school sports and other activities in adolescence. J Sch Health. 2003;73(3):113–120. [DOI] [PubMed] [Google Scholar]
- 16.Lang C, Brand S, Feldmeth AK, Holsboer-Trachsler E, Pühse U, Gerber M. Increased self-reported and objectively assessed physical activity predict sleep quality among adolescents. Physiol Behav. 2013;120:46–53. [DOI] [PubMed] [Google Scholar]
- 17.Kalak N, Gerber M, Kirov R, et al. Daily morning running for 3 weeks improved sleep and psychological functioning in healthy adolescents compared with controls. J Adolesc Health. 2012;51(6):615–622. [DOI] [PubMed] [Google Scholar]
- 18.Madsen KA, McCulloch CE, Crawford PB. Parent modeling: perceptions of parents’ physical activity predict girls’ activity throughout adolescence. J Pediatr. 2009;154 (2):278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Sheikh M, Kelly RJ. Family functioning and children’s sleep. Child Dev Perspect. 2017;11(4):264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagley EJ, El-Sheikh M. Familial risk moderates the association between sleep and zBMI in children. J Pediatr Psychol. 2013;38(7):775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernier A, Bélanger M-È, Tarabulsy GM, Simard V, Carrier J. My mother is sensitive, but I am too tired to know: infant sleep as a moderator of prospective relations between maternal sensitivity and infant outcomes. Infant Behav Dev. 2014;37:682–694. [DOI] [PubMed] [Google Scholar]
- 22.Lemola S, Schwarz B, Siffert A. Interparental conflict and early adolescents’ aggression: is irregular sleep a vulnerability factor? J Adolesc. 2012;35(1):97–105. [DOI] [PubMed] [Google Scholar]
- 23.Tu K, Erath S, El-Sheikh M. Peer victimization and adolescent adjustment: the moderating role of sleep. J Abnorm Child Psychol. 2015;43(8):1447–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadeh A, El-Sheikh MXI. Sleep and development: conclusions and future directions. Monogr Soc Res Child Dev. 2015;80(1):177–181 [Serial No. 316]. [DOI] [PubMed] [Google Scholar]
- 25.Sameroff AJ. Development systems: Contexts and evolution In: Kessen W, editor. Handbook of Child Psychology: Vol I. History, Theory, and Methods. New York: Wiley; 1983. p. 237–294. [Google Scholar]
- 26.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. [DOI] [PubMed] [Google Scholar]
- 27.Acebo C, Sadeh A, Seifer R, et al. Estimating sleep patterns with actigraphy monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22(1):95–103. [DOI] [PubMed] [Google Scholar]
- 28.Kowalski KC, Crocker PR, Kowalski NP. Convergent validity of the physical activity questionnaire for adolescents. Pediatr Exerc Sci. 1997;9(4):342–352. [Google Scholar]
- 29.Saint-Maurice PF, Welk GJ, Bartee RT, Heelan K. Calibration of context-specific survey items to assess youth physical activity behaviour. J Sports Sci. 2017;35(9): 866–872. [DOI] [PubMed] [Google Scholar]
- 30.Achenbach TM, Rescorla L. ASEBA School-Age Forms & Profiles. Burlington, VT: Aseba; 2001. [Google Scholar]
- 31.Centers for Disease Control and Prevention. Body Mass Index (BMI) percentile calculator for child and teen. http://nccd.cdc.gov/dnpabmi/Calculator.aspx; 2013.
- 32.Cousineau D, Chartier S. Outliers detection and treatment: a review. Int J Psychol Res. 2010;3(1):58–67. [Google Scholar]
- 33.Arbuckle J. IBM SPSS Amos 23 user’s Guide. Chicago, IL: Amos Development Corp; 2014. [Google Scholar]
- 34.Acock AC. Working with missing values. J Marriage Fam. 2005;67(4):1012–1028. [Google Scholar]
- 35.Enders CK. The performance of the full information maximum likelihood estimator in multiple regression models with missing data. Educ Psychol Meas. 2001;61 (5):713–740. [Google Scholar]
- 36.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6(1):1–55. [Google Scholar]
- 37.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31(4):437–448. [Google Scholar]
- 38.McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 39.El-Sheikh M, Tu KM, Erath SA, Buckhalt JA. Family stress and adolescents’ cognitive functioning: sleep as a protective factor. J Fam Psychol. 2014;28(6):887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadeh A, Raviv A, Gruber R. Sleep patterns and sleep disruptions in school-age children. Dev Psychol. 2000;36(3):291–301. [DOI] [PubMed] [Google Scholar]