Abstract
As a period of heightened plasticity, puberty may provide a window of opportunity for recalibration of the hypothalamic-pituitary-adrenal (HPA) axis to current conditions. Our group has recently documented evidence for pubertal recalibration of HPA axis reactivity among children internationally adopted as infants from institutions into supportive, well-resourced homes. As a first step at examining potential mechanisms by which puberty may facilitate recalibration of the HPA axis, the current study assessed whether previously-institutionalized (PI) children differed from non-adopted (NA) comparison children in levels of the adrenal steroid hormone dehydroepiandrosterone (DHEA) and in its intra-individual covariation (coupling) with cortisol by adrenal pubertal stage. In an accelerated longitudinal design, 7- to 15-year-olds completed up to 3 annual assessments, which included nurse-conducted pubertal staging and the Modified Trier Social Stress Test for Children (TSST-M). Adrenal (pubic hair) rather than gonadal pubertal stage scores were used in the analysis. Paired salivary cortisol-DHEA samples were available at 60-80 minutes post-TSST-M. NA and PI children did not differ in DHEA levels, which were higher among children at more advanced pubertal stages (averaged across the sessions) for both groups. For NA children, post-stressor cortisol and DHEA were positively coupled across sessions at all average adrenal pubertal stages. For PI children who were, on average, at earlier adrenal pubertal stages, post-stressor cortisol and DHEA were not coupled, but PI children who were at later pubertal stages demonstrated positive cortisol-DHEA coupling similar to that of the NA children. We suggest that these findings provide insights into processes which may underlie pubertal recalibration of the HPA axis.
Keywords: HPA axis, puberty, recalibration, early life stress, cortisol, DHEA
1. Introduction
Accumulating cross-species evidence supports the hypothesis that, during sensitive periods1 in development, stress response systems are adaptively calibrated to match social and physical environmental conditions (Del Giudice et al., 2011). The hypothalamic-pituitary-adrenal (HPA) axis is a key stress-mediating system which mobilizes the body’s physiological and psychological resources to cope with stressors and maintain homeostasis (Smith and Vale, 2006; Tsigos and Chrousos, 2002). The quality of the early life environment appears to be an important determinant of HPA axis activity over the lifespan. Studies of children reared in institutions indicate that social deprivation and neglect are associated with hypoactivity of the HPA axis as measured by the activity of its end-product, the adrenal steroid hormone cortisol (Hostinar et al., 2015; Koss et al., 2016; McLaughlin et al., 2015). Previously institutionalized (PI) children exhibit blunted cortisol reactivity to social stressors, lower cortisol awakening responses, and flatter diurnal cortisol slopes. These patterns are observed for years after children are adopted or fostered into supportive, well-resourced homes (Hostinar et al., 2015; Kumsta et al., 2017; Leneman et al., 2018; McLaughlin et al., 2015).
Beyond early life, sensitive periods later in development may open windows for recalibration of the stress system to current conditions (Del Giudice et al., 2011; Gunnar et al., 2019; Romeo and McEwen, 2006). Puberty is one such period of heightened plasticity, marked by dynamic changes in hormonal and brain systems (Byrne et al., 2017; Sisk and Zehr, 2005). Our group has recently documented evidence for pubertal recalibration of HPA axis reactivity among children internationally adopted as infants from institutions into generally supportive, well-resourced homes. Using an accelerated longitudinal design, PI children and non-adopted (NA) comparison children ages 7-15 years completed a social evaluative stressor (Modified Trier Social Stress Test for Children; Yim et al., 2010) at three annual visits. With advancing pubertal stage, PI children exhibited increasing cortisol reactivity which became comparable to the reactivity of NA children at later pubertal stages. Pubertal development appeared to reorganize the stress systems of these PI children to match their more supportive current life conditions. This finding was initially documented cross-sectionally (DePasquale et al., 2019) and then longitudinally (Gunnar et al., 2019). Consistent with these findings are two cross-sectional studies suggesting that peripubertal children who experienced early adversity may transition from a blunted cortisol awakening response to either a more normative (Quevedo et al., 2012) or an exaggerated response (King et al., 2017; however, also see Leneman et al., 2018). Our recalibration finding is also consistent with rodent models indicating that environmental enrichment during the peripubertal period functionally reverses the effects of early life stress on HPA axis responsivity (Francis et al., 2002; Morley-Fletcher et al., 2003).
Mechanisms by which puberty may facilitate recalibration of the HPA axis are currently unknown. Of potential relevance is the activity of another HPA end-product, the adrenal steroid hormone dehydroepiandrosterone (DHEA). The hormonal changes underlying pubertal development occur as two separate but related processes – adrenarche and gonadarche (Auchus and Rainey, 2004; Bordini and Rosenfield, 2011). Adrenarche involves maturational processes in the zona reticularis of the adrenal cortex, which initiate increased release of DHEA, typically between ages 6 to 9 (Auchus and Rainey, 2004; Nguyen and Conley, 2008). Gradually rising basal levels of DHEA play a role in some aspects of pubertal physical maturation (Shirtcliff et al., 2009; Vijayakumar et al., 2018). As an HPA axis end-product, DHEA is simultaneously released with cortisol in response to their common secretagogue adrenocorticotropic hormone (ACTH), so it similarly demonstrates a diurnal decline from a morning acrophase (Hucklebridge et al., 2005) and responds to stress (Eatough et al., 2009; Lennartsson et al., 2012; Marceau et al., 2012; Shirtcliff et al., 2007). While not as dramatic as the rise in DHEA, puberty also appears to involve normative increases in cortisol levels (Gunnar et al., 2009; Shirtcliff et al., 2012; Stroud et al., 2009).
Assessing the activity of DHEA in addition to cortisol may advance our understanding of processes underlying pubertal recalibration of the axis following early life institutional care. Given their shared role as stress-reactive adrenal hormones, joint consideration of cortisol and DHEA has been of increasing interest in the broader literature (Kamin and Kertes, 2017; Marceau et al., 2015a). Parallel examination of both hormones can enhance interpretation of aberrant patterns of HPA axis activity by exploring whether dysregulation is evident in both hormones (reflecting overall steroidogenic activity) or in cortisol alone (Oskis et al., 2012). If both cortisol and DHEA transition from blunted to normally reactive in response to stress with increasing pubertal stage in PI children, it would be expected that mechanisms higher up in the axis which regulate the production of ACTH are recalibrated. However, if cortisol but not DHEA activity is associated with early life stress, this would seem to implicate mechanisms within the adrenal itself as playing a role in the observed blunting or down-regulation of cortisol.
In addition to measuring levels of cortisol and DHEA (either separately or as a ratio),_the within-individual covariation (‘coupling’) of the two hormones can be assessed to index their co-activity (Marceau et al., 2015b). Positive coupling indicates that within an individual, when one hormone is higher, the other hormone is also higher (e.g., their release is coordinated). Several studies have documented positive cortisol-DHEA coupling across time in adolescent samples (Marceau et al., 2015b, 2014) and moderation of cortisol-DHEA coupling by early adverse experiences (Black et al., 2018; Ruttle et al., 2015; Simmons et al., 2015). If blunting and subsequent normalization of HPA axis reactivity occurs higher up in the axis (e.g., at the level of ACTH production), we would anticipate that the co-release of cortisol and DHEA would not be affected in PI children (would not differ from that of NA children). However, if the coupling of the two hormones differs in PI children by pubertal stage, this again would point to the possibility that activity at the level of the adrenal gland may, at least in part, underlie pubertal recalibration of the axis.
Our goal in the current analysis was to further explore our finding of pubertal recalibration of cortisol reactivity to stress in previously-institutionalized children (reported in Gunnar et al., 2019) by examining DHEA levels, as well as within-individual coupling of cortisol and DHEA, across up to three annual sessions in this same sample of children. Paired cortisol-DHEA samples were only available at 60-80 minutes post-stressor, so we examined DHEA levels and the coupling of post-stressor cortisol and DHEA across sessions (rather than within sessions). To determine whether PI and NA children differed in cortisol-DHEA coupling by average pubertal stage, we included group (previously-institutionalized versus non-adopted comparison), average pubertal stage across sessions, and their interaction as predictors of cortisol-DHEA coupling.
2. Methods
2.1. Study Overview
Using an accelerated longitudinal design (the same study and participants as in Gunnar et al., 2019), salivary cortisol, salivary DHEA, and pubertal stage were assessed in previously-institutionalized (PI) and non-adopted (NA) children and adolescents across up to three annual sessions, beginning at 7-15 years of age.
2.2. Participants
Participants in the current study were 294 children ages 7 – 15 at the beginning of the study (M age at session 1 = 11.29, SD = 2.31). 125 children (68% female) were previously institutionalized in other countries as infants and toddlers and adopted to the United States (previously-institutionalized; PI), and 169 children (53% female) were born and raised in their families of origin in the United States (non-adopted comparison; NA). PI children were recruited from a registry for families adopting children internationally; a majority of families joined this registry through an online survey sent to them after adopting internationally via a local agency. Children in the NA group were recruited from a registry of families who previously demonstrated interested in participating in research, most of whom joined this registry through a letter with return postcard received soon after the child’s birth using a list of all live births in a large metropolitan area. Initial exclusion criteria were any major congenital abnormality or regular steroid or hormone medication use. PI children were selected to have been adopted prior to the age of 5 years (M age of adoption = 19.30 months, SD = 12.72 months, range = 5.5 – 59 months, 74% before 24 months) and spent at least 50% of their pre-adoptive life in an institution (M = 95%, SD = 9%, range = 63% – 100%). PI and NA families were comparable in socioeconomic status (see Table 1 for demographic information and tests of group differences). The 294 children included in the current study were drawn from the overall study sample of 318 children, from which children were excluded due to suspected or diagnosed Fetal Alcohol Syndrome (n = 6), diagnosed autism (n = 2), no usable DHEA data (n = 14), or no pubertal staging data (n = 2).
Table 1.
Participant characteristics by group (overall N = 294).
| PI (n = 125) | NA (n = 169) | Test of difference | |
|---|---|---|---|
| Session 1 child age in years (M(SD)) | 11.4 | 11.2 | t(292) = .89, p = .38 |
| Child sex: female, n (%) | 85 (68) | 89 (53) | χ2(1) = 6.99, p = .008 |
| Child racea, n (%) | |||
| African American | 5 (4) | 8 (2) | |
| Asian | 51 (42) | 1 (1) | White vs. non-White: |
| Caucasian | 50 (41) | 151 (90) | χ2(1) = 79.44, p < .001 |
| Latin American | 12 (10) | 0 (0) | |
| Mixed race or other | 4 (3) | 13 (8) | |
| Child region of origin, n (%) | |||
| Russia/Eastern Europe | 76 (61) | – | |
| China/Southeast Asia | 31 (25) | – | – |
| Latin America | 13 (10) | – | |
| Africa/Haiti | 5 (4) | – | |
| Session 1 primary caregiver education, n (%) Bachelor’s degree or higher |
101 (84) | 135 (83) | χ2(1) = 0.09, p = .76 |
| Session 1 annual household income, n (%) $100,000 or higher |
76 (62) | 99 (59) | χ2(1) = 0.34, p = .56 |
| Mean medication usage across sessions, M (SD) | 0.79 (1.28) | 0.41 (0.88) | t(208) = 2.87, p = .005 |
| Mean TSST-M subjective stress across sessions, M (SD) | 3.38 (0.85) | 3.42 (0.73) | t(292) = −0.47, p = .64 |
| Mean Youth Life Stress Interview across sessions, M (SD) | 2.45 (0.50) | 2.20 (0.42) | t(292) = 4.79, p < .001 |
| Mean adrenal pubertal stage across sessions, M (SD) | 2.93 (1.45) | 2.73 (1.47) | t(292) = 1.15, p = .25 |
| Mean raw salivary cortisol across sessions, M (SD) | 0.07 (0.04) | 0.08 (0.05) | t(292) = 1.40, p = .16 |
| Mean log10 salivary cortisol across sessions, M (SD) | 0.03 (0.02) | 0.03 (0.02) | t(292) = −1.44, p = .15 |
| Mean raw salivary DHEA across sessions, M (SD) | 188.81 (191.58) | 173.02 (162.12) | t(292) = −0.76, p = .45 |
| Mean log10 salivary DHEA across sessions, M (SD) | 2.07 (0.42) | 2.01 (0.44) | t(292) = 1.06, p = .29 |
Note. PI = previously-institutionalized. NA = non-adopted.
Child race was missing for 3 PI children and 1 NA child.
2.3. Procedures
We used an accelerated longitudinal design with multiple age cohorts and followed children once per year for three annual assessments. This design allowed for a wider sampling window (approximately 7-17 years of age, from the youngest children at session 1 to the oldest children at session 3) in a shorter span of time than a traditional longitudinal design. Each annual assessment included two laboratory visits completed approximately one week apart. At one visit, nurses performed physical exams to determine pubertal stage; parents and children also completed the Petersen Pubertal Development scale (Petersen et al., 1988). At the other visit, children performed the Modified Trier Social Stress Test for Children (TSST-M; Yim et al., 2010), which involved a socially evaluative public speaking task and verbal math performance (see DePasquale et al., 2019 and Gunnar et al., 2019 for additional details on this procedure). Saliva samples were collected across the visit. Of the 294 total participating children, 145 provided pubertal stage and salivary hormone data for all three annual assessments, 101 for two annual assessments, and 48 for one annual assessment.
Informed consent was obtained from one parent for each child participant, and all children provided written and verbal assent. This study was approved by the university’s Institutional Review Board.
2.4. Measures
2.4.1. Adrenal pubertal stage
Pubertal staging was conducted by trained nurses according to Marshall and Tanner criteria (Marshall and Tanner, 1970, 1969) on a different day from when children completed the TSST-M. Separate gonadal (breast and testicle) and adrenal (pubic hair) stage scores were given and ranged from 1 (pubertal development not yet begun) to 5 (pubertal development is complete). Gonadal and adrenal scores were correlated within sessions (r = .89 - .91, p < .001). As detailed elsewhere (DePasquale et al., 2019; Gunnar et al., 2019; Reid et al., 2017), a subset of children underwent the physical exam with two of the three nurses to examine nurse agreement (κ = 0.74-1.0 for all three annual sessions). If a child reached stage 5 before their third annual assessment, they were presumed to remain at stage 5 and did not complete pubertal staging again (session 2 n = 19 children, session 3 n = 35 children).
22 children refused the physical exam at session 1, 17 at session 2, and 23 at session 3. Missing Tanner stage values were imputed based on parent responses on the Pubertal Development Scale (PDS; Petersen et al., 1988), a pubertal stage questionnaire. The scale includes items on growth in height, body hair, and skin changes for both genders, as well as items on deepening of voice and facial hair for boys and on breast development and onset of menarche for girls. Because Tanner stage and PDS scores are on different scales, the PDS scores were converted to Tanner scores following guidelines from Shirtcliff and colleagues (2009). The previous year’s score was used in cases when estimation was lower (n = 4) under the assumption that children cannot regress in pubertal stage. Tanner and PDS scores were highly correlated at all sessions, r = .81 - .83, ps < .001.
Due to our focus on adrenal hormones in the current study, we used average adrenal stage (pubic hair) scores as our index of pubertal stage.
2.4.2. Cortisol and DHEA
Because the HPA axis has a diurnal rhythm, all TSST-M sessions began between 3:00 – 4:30 pm. Children were asked to refrain from eating and drinking (including water and especially dairy or caffeine) during the visit. Saliva samples were collected by passive drool and stored at −20°C until shipment to the University of Trier, Germany for assay (which involved only one freeze-thaw cycle). For the current study, salivary cortisol and DHEA levels from samples collected at 60 and 80 minutes post-TSST-M were examined, as only these samples were assayed for DHEA (assessment of DHEA was not a central aim of the primary study). Samples from each child within year were included in the same assay batch to control for inter-assay variability.
All cortisol samples were assayed in duplicate using a time-resolved fluorescence immunoassay (DELFIA). The test uses 25-50 μl of saliva per duplicate and has an analytical sensitivity of 0.01 μg/dL. Intra-assay coefficients of variation (CV) range from 4.0-6.7%, and inter-assay CVs are between 7.1% to 9.0%. Within our sample, duplicates had an average CV of 6.6% and were correlated .999, p < .001. Therefore, duplicates were averaged to create one cortisol value per time point per session. Because DHEA values were determined from the pooled remaining saliva from the 60 and 80 minutes post-TSST-M samples (see below), the 60- and 80-minute post-TSST-M cortisol values were averaged to match the one DHEA value available for this time interval. Cortisol values were log10-transformed to address positive skew.
Following assay for cortisol determination, the remaining saliva from the 60 and 80 minutes post-TSST-M samples were pooled and subjected to ELISA assay for DHEA determination (Demeditec, DES6666). Thawed samples were centrifuged, and tests were performed on the supernatant. The test uses 50-100μl of saliva per duplicate, is robust to repeated free-thaw cycles, has an analytical sensitivity of 3.7 pg/ml with a range of 10-2560 pg/ml, and has been demonstrated to have low (< .01%) cross-reactivity at 50% binding with other relevant hormones, such as cortisol, testosterone, estradiol, and DHEA-S. Intra-and inter-assay coefficients of variation range from 4.9-9.0% and 4.5-6.8%, respectively. Samples were tested in duplicate, and duplicates that varied by more than 20% were repeat tested; this high value was based on the relatively low levels of DHEA witnessed in our samples. DHEA levels were undetectable for 4 of 274 participants at session 1, 5 of 201 participants at session 2, and 4 of 228 participants at session 3. Within our sample, duplicates had an average CV of 8.26% and were correlated .99. Therefore, the duplicates were averaged to create one DHEA value per session. DHEA values also were log10-transformed to address positive skew.
2.4.3. Questionnaires
Parents completed questionnaires regarding child race, child medication use, primary caregiver education, and family income. An index of daily medication usage (following guidelines in Granger, Hibel, Fortunato, & Kapelewski, 2009) was created to assess potential impact on adrenal hormone production. Parents of PI children completed questions which asked about the child’s age at adoption, amount of pre-adoption time in an institution, and country of origin.
After completing the TSST-M, children were asked to rate their feelings of stress at five points throughout the laboratory visit (arrival, preparation, speech, math, after completion) on a scale from 1 (not at all) to 5 (a whole lot), which were averaged to create a single index of child-reported subjective stress during the TSST-M. During the Tanner Staging session, children also completed the chronic life stress portion of the Youth Life Stress Interview (Rudolph and Hammen, 1999), a semi-structured interview. The chronic life stress portion of this semi-structured interview assesses life challenges experienced over the past 12 months in eight domains. We included seven of these domains in our measure (academic, behavioral, same-sex peer, opposite-sex peer, sibling, parent-child, and marital); the eighth domain (romantic) was dropped due to lack of variation in our sample. The interviews were audiotaped, and trained coders rated each domain on a half-point scale from 1 (superior/no stress) to 5 (extreme/severe stress). Disagreements were conferenced to consensus. The conferenced scores were averaged to create one score of child-reported life stress in the past year. Pre-conference reliabilities (intra-class correlations) for each session’s scores ranged from .82 - .85.
2.5. Data Analysis
A linear mixed effects model (‘lmer’ package in R; Bates, Mächler, Bolker, & Walker, 2015) examined group (PI vs. NA) differences in 60-80 minutes post-TSST-M cortisol-DHEA coupling over sessions as a function of average adrenal pubertal stage. Marginal and conditional R2GLMM (‘MuMln’ package in R; Bartoń, 2019) and standardized fixed effects estimates ('sjstats’ package in R; Lüdecke, 2020) were used as measures of effect size. Session was used as the longitudinal ‘time’ variable. Session is an ordinal variable; however, because model assumptions were still met, it was analyzed continuously to simplify models. The model included random effects of intercept and session and adjusted for time of day of sample collection. Child sex, child race (White versus non-White or more than 1 race), primary caregiver education, family income, medication usage, child-reported past year life stress, and child-reported subjective stress during the TSST-M were also examined as potential covariates (entered into the model as main effects), but these variables were not significant predictors and did not change the pattern of results, so were not included in the final model (see Table 1 for group differences and Supplemental Table 1 for zero-order correlations). There were not sex differences in cortisol-DHEA coupling by average adrenal pubertal stage in either group. Age was highly correlated with adrenal pubertal stage (r = .84, p < .001) so age was not considered as a covariate, given that adrenal pubertal stage was a focal predictor. Additionally, if age was included as a covariate, we would then be indexing adrenal pubertal timing rather than adrenal puberty itself.
The linear mixed-effects model (see below equation) examined potential differences in the coupling of 60-80-minute post-TSST-M cortisol and DHEA across sessions as a function of average adrenal pubertal stage and whether this association differed by group. Since our previous studies (DePasquale et al., 2019; Gunnar et al., 2019) documented group by pubertal stage differences in cortisol, we included DHEA as the outcome variable in our primary model so that we could assess whether group, average (mean) adrenal pubertal stage, and their interaction (γ01, γ02, and γ05) were associated with DHEA levels. Similar to the approach of Marceau et al. (2014), we isolated within-individual variation in cortisol and DHEA (coupling) by adjusting for between-individual variation in these hormones. Two cortisol variables were included as predictors: each child’s average cortisol across all sessions (mean log10cortisol; γ03), and children’s individual-mean-centered cortisol values (IMC log10cortisol; γ10). The latter of these is the coupling parameter and represents the extent to which the two hormones covary within individuals across the sessions (i.e., if hormones are positively coupled within an individual, when one hormone is higher, the other hormone also tends to be higher). Because session is included (i.e., controlled for) in the model, the coupling parameter can be interpreted as the extent to which the two hormones covary within an individual after adjusting for changes in DHEA as a function of time/advancing age. With only one paired cortisol and DHEA value per session (for up to three data points per individual), we were not able to isolate within- from between-individual differences in pubertal stage as in Gunnar et al. (2019). Instead, we examined whether cortisol-DHEA coupling differed as a function of average adrenal pubertal stage (γ11). We further examined if there were group differences in cortisol-DHEA coupling by average adrenal pubertal stage (γ13). To confirm that associations were specific to the intra-individual covariation of cortisol and DHEA and not explained by between-individual levels of cortisol and DHEA, we additionally tested a three-way interaction between average log10cortisol, group, and average adrenal pubertal stage. This interaction was not statistically significant (t = −0.01, p = .99) so was not included in the final model. The equation for the final model is as follows:
Level 1 (session):
Level 2 (individual):
In Gunnar et al. (2019), we documented group by pubertal stage differences in cortisol reactivity (measured with repeated cortisol sampling) over the TSST-M. To examine specifically whether there were differences in 60-80-minute post-TSST-M cortisol levels for PI children at different average pubertal stages, we fit an additional linear mixed effects model to assess whether group, average adrenal pubertal stage, and their interaction were associated with cortisol at this time point (γ01, γ02, and γ05):
Level 1 (session):
Level 2 (individual):
3. Results
3.1. Descriptive Statistics
Descriptive statistics for study variables are displayed in Table 1, and zero-order correlations among study variables can be found in Supplemental Table 1. Groups significantly differed by sex and race: PI children were more likely to be female (χ2 = 6.99, p = .008) and more likely to be non-White than White (χ2 = 79.44, p < .001). PI children also had higher average medication use across sessions (t(208) = 2.87, p = .005) and reported higher levels of average past year life stress across sessions (t(292) = 4.79, p < .001). As stated in the Data Analysis section, when included in the linear mixed effects model, these variables did not change the pattern of results. Groups did not differ in annual household income (χ2 = 0.34, p = .56) or primary caregiver education (χ2 = 0.09, p = .76). Furthermore, groups did not differ in average adrenal pubertal stage (t(292) = 1.15, p = .25), average log10cortisol (t(292) = −1.44, p = .15), or average log10DHEA (t(292) = 1.06, p = .29) across sessions.
3.2. Cortisol
Replicating findings in Gunnar et al. (2019), 60-80-minute post-TSST-M cortisol levels differed as a function of average pubertal stage for PI children (see Supplemental Figure 1 for visualization). Among children who were, on average, at earlier adrenal pubertal stages, PI children had lower average levels of cortisol compared to NA children (centered at Stage 1: t = −2.29, p = .02). Among children at later average adrenal pubertal stages, PI children’s cortisol levels were comparable to those of NA children (centered at Stage 5: t = 0.51, p = .61).
3.3. DHEA
Model results can be found in Table 2. Across groups, 60-80-minute post-TSST-M DHEA levels were higher among children who were, on average, at more advanced adrenal pubertal stages (t = 9.90, p < .001). PI and NA children did not differ in average levels of DHEA across the sessions (t = 0.80, p = .43), and the association between average adrenal pubertal stage and DHEA levels did not differ by group (t = −0.43, p = .67). See Supplemental Figure 2 for visualization of associations between adrenal pubertal stage and DHEA by group.
Table 2.
Linear mixed-effects model examining the coupling of cortisol and DHEA as moderated by mean adrenal pubertal stage and Group (N = 294).
| Fixed effects | Estimate (SE) |
Standardized Estimate |
t | p |
|---|---|---|---|---|
| Intercept, γ00 | 1.96 (0.03) | 0.03 | 69.45 | < .001 |
| Session, γ20 | 0.08 (0.01) | 0.14 | 6.44 | < .001 |
| Sample timea, γ30 | −0.02 (0.02) | −0.03 | −1.10 | .27 |
| Group (1 = PI), γ01 | 0.03 (0.04) | 0.06 | 0.80 | .43 |
| Mean adrenal pubertal stage (across sessions)a, γ02 | 0.17 (0.02) | 0.54 | 9.90 | < .001 |
| Mean log10cortisol (across sessions)a, γ03 | 4.39 (1.45) | 0.15 | 3.04 | .003 |
| Mean adrenal pubertal stagea x Group, γ04 | −0.01 (0.03) | −0.04 | −0.43 | .67 |
| Group x Mean log10cortisola, γ05 | 1.99 (2.40) | 0.07 | 0.83 | .41 |
| Individual-mean-centered (IMC) log10cortisol, γ10 | 3.82 (0.95) | 0.11 | 4.02 | < .001 |
| Mean adrenal pubertal stagea x IMC log10cortisol, γ11 | −0.39 (0.59) | −0.02 | −0.67 | .50 |
| IMC log10cortisol x Group, γ12 | −1.82 (1.47) | −0.05 | −1.23 | .22 |
| Mean adrenal pubertal stagea x IMC log10cortisol x Group, γ13 | 2.24 (1.01) | 0.09 | 2.23 | .03 |
| Random effects | Variance | SD | ||
| Intercept | 0.10 | 0.32 | ||
| Session | 0.01 | 0.08 | ||
| Residual | 0.04 | 0.21 |
Note. DHEA is the dependent variable.
Grand-mean-centered. Marginal R2GLMM = .37. Conditional R2GLMM = .79
3.4. Cortisol-DHEA coupling
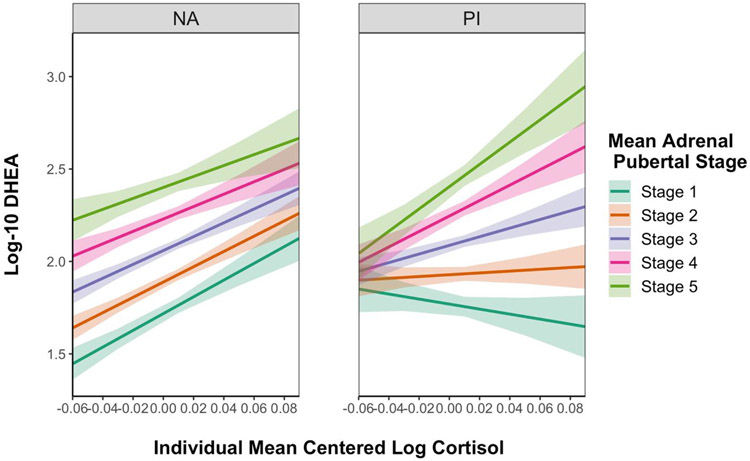
Cortisol-DHEA coupling results are presented in Table 2. Across groups and average adrenal pubertal stages, 60-80-minute post-TSST-M cortisol and DHEA levels were positively coupled within individuals (t = 4.23, p < .001), after adjusting for between-individual differences in cortisol (t = 3.04, p = .003). PI and NA children demonstrated different patterns of cortisol-DHEA coupling across sessions as a function of average adrenal pubertal stage (t = 2.23, p = .03; see Figure 1). Specifically, for NA children, cortisol and DHEA were positively coupled regardless of average adrenal pubertal stage. For PI children who were, on average, at earlier adrenal pubertal stages, cortisol and DHEA were not positively coupled, but PI children who were, on average, at later adrenal pubertal stages demonstrated positive cortisol-DHEA coupling similar to that of the NA children. In follow-up reduced models, among children at earlier average adrenal pubertal stages (stages 1–3), PI children had less positive cortisol-DHEA coupling compared to NA children (t = −2.22, p = .03), whereas there were not group differences in coupling among children at later average adrenal pubertal stages (stages 4–5; t = 0.79, p = 0.43).
Figure 1.
Model-implied results of a linear-mixed effects model examining differences in cortisol-DHEA coupling by average adrenal pubertal stage and by group (non-adopted, left; previously-institutionalized, right). The intra-individual covariation (coupling) of cortisol and DHEA is represented by the association between individual-mean-centered log10cortisol values (shown on the x-axis) and log10DHEA values (on the y-axis). For non-adopted children, cortisol and DHEA were positively coupled regardless of average adrenal pubertal stage. For PI children, cortisol and DHEA were not coupled among children who were, on average, at earlier stages, and were positively coupled only among children at later adrenal pubertal stages. Envelopes represent standard errors.
4. Discussion
The results of this study suggest that pubertal recalibration of HPA axis activity in previously-institutionalized children, reported in Gunnar et al., 2019, is also evident in the intra-individual covariation (coupling) of post-stressor cortisol and DHEA in these same children. Cortisol and DHEA were coupled within-individuals at 60-80 minutes post-TSST-M across annual sessions (e.g., within a child, when one hormone was higher, the other hormone also tended to be higher). Non-adopted (NA) comparison children demonstrated positive post-stressor cortisol-DHEA coupling regardless of average adrenal pubertal (pubic hair) stage, whereas for previously-institutionalized (PI) children, post-stressor cortisol and DHEA were positively coupled only among children who were, on average, at later adrenal pubertal stages. This finding was not explained by between-individual differences in cortisol and DHEA levels and was specific to the intra-individual covariation among these hormones across sessions. The lack of group differences in post-stressor DHEA levels by average adrenal pubertal stage is also of note and suggests that PI youth demonstrate normative increases in DHEA across pubertal stages. Consistent with our findings in this sample reported in Gunnar et al. (2019), we documented group by average pubertal stage differences in post-TSST-M cortisol levels, with PI children at more advanced adrenal pubertal stages demonstrating more normative cortisol levels.
Consistent with our finding of pubertal recalibration of cortisol reactivity (DePasquale et al., 2019; Gunnar et al., 2019), our results suggest that changes in the coupling of post-stressor cortisol and DHEA in PI children do not occur until well after adoption into a supportive, well-resourced environment. The majority of PI children in this study were adopted by the age of 2, yet PI children in the early stages of adrenal puberty (at least 5 to 7 years post-adoption) differed from NA comparison children in cortisol-DHEA coupling. This finding provides additional evidence that the physiological changes associated with puberty may open the HPA axis for recalibration.
The primary purpose of the current study was to take an initial step at exploring which mechanisms associated with puberty may result in recalibration of HPA axis activity. Changes in the HPA axis during puberty may occur at the level of the adrenal, pituitary, hypothalamus, and/or limbic inputs to the hypothalamus (Romeo, 2010). Taken in isolation from the coupling finding, our findings that post-stressor cortisol activity but not DHEA activity appears to differ among PI and NA children who were, on average, at earlier adrenal pubertal stages may provide some, although not definitive, insight. Cortisol and DHEA are both released from the adrenal cortex in response to their common secretagogue adrenocorticotropic hormone (ACTH). The finding that PI children in earlier pubertal stages differed from NA children in cortisol but not DHEA levels following the TSST-M suggests that in PI children, the DHEA-secreting zone of the adrenal was responding typically to ACTH, while the cortisol-secreting zone was not. Additionally, among both PI and NA children, post-stressor DHEA was higher for children at more advanced adrenal pubertal stages. In contrast, post-stressor cortisol levels were blunted among PI children at earlier average adrenal pubertal stages, whereas PI children at later adrenal pubertal stages had levels comparable to NA children. Cortisol levels among NA children did not differ by adrenal pubertal stage. Cumulatively, these findings also suggest that early life stress had a particular impact on the mechanisms specific to cortisol and not DHEA production and release.
The overall rise in DHEA levels during adrenarche is due to maturational changes in the zona reticularis of the adrenal cortex (Rainey and Nakamura, 2008; Rich et al., 1981), where DHEA, but not cortisol, is synthesized and secreted (cortisol is produced in the zona fasciculata). The lack of apparent difference in post-stressor DHEA levels between PI and NA children suggests that adrenal maturational processes specific to the zona reticularis occurs normatively for PI children. Albeit controversial (e.g., due to the apparent dissociation of cortisol and DHEA secretion in conditions like Cushing’s syndrome; Bornstein and Chrousos, 1999; Cunningham and McKenna, 1994), there is evidence that ACTH plays a significant role in regulating adrenarche (Lappalainen et al., 2008; Weber et al., 1997).
We suggest that our coupling finding provides additional insights into processes which may underlie pubertal recalibration of the axis. The lack of coupling of post-stressor cortisol and DHEA among PI children in earlier pubertal stages suggests that the activity of these hormones in response to ACTH is not coordinated in these children, whereas the activity of these hormones is coordinated even at earlier pubertal stages among NA comparison children. This points to the possibility that maturational changes specific to the adrenal cortex during the pubertal transition (e.g., patterning of specific adrenal steroid metabolizing enzymes, ACTH sensitivity; Rainey and Nakamura, 2008) and/or changes in intra- or extra-adrenal, non-ACTH factors regulating cortisol production (e.g., catecholamines; see Bornstein and Chrousos, 1999; Ehrhart-Bornstein et al., 1998) may at least in part underlie pubertal recalibration of the HPA axis, resulting in more a coordinated release of the two adrenal hormones. As stated above, studies have indicated several physiological conditions in which there is a dissociation of adrenal cortisol and DHEA production. Mechanisms associated with puberty may allow adrenal production of cortisol and DHEA to shift from a profile of dissociation to one of association. What these puberty-associated mechanisms are, and which levels of the axis are involved, requires future exploration and could be investigated more closely in non-human animal models. For example, it is possible that pubertal changes in levels of cortisol and DHEA as a result of adrenal maturational processes further shape activity at higher levels of the axis via feedback mechanisms. Evidence that cortisol and DHEA have opposing (catabolic versus anabolic) effects in the body may also be relevant to consider (Kamin and Kertes, 2017; Maninger et al., 2009). Puberty-related increases in DHEA could act to alter the effects of cortisol, and vice versa. This possibility is supported by findings from rodent models which demonstrate that DHEA induces a shift from 11β-hydroxysteroid dehydrogenase (11β-HSD) type 1 to 11β-HSD type 2 expression, increasing the conversion of active cortisol into its metabolite cortisone, which does not act on glucocorticoid receptors (Apostolova et al., 2005; Balazs et al., 2008).
4.1. Limitations and Future Directions
A primary limitation of the current study was that only one paired cortisol-DHEA sample (measured post-stressor) was available per session. Because of this, we were not able to examine intra-individual coupling of cortisol and DHEA within-sessions in response to the TSST-M and rather assessed coupling across sessions within an individual. This meant that we could not examine changes in the coupling of cortisol and DHEA within-individuals with advancing pubertal development. Our coupling finding awaits additional replication with more intensive measurement of both hormones. We were additionally limited by measuring DHEA only within our TSST-M session. Our DHEA finding should be qualified by previous research and theory suggesting complexity with respect to interpreting the activity of DHEA during puberty, given its dual role as a pubertal and stress reactive hormone (Shirtcliff et al., 2007). More fine-grained measurement of DHEA levels (e.g., both diurnal output and reactivity to stress) in PI youth could assist in disentangling potential effects of early life institutional care on DHEA’s pubertal versus stress reactive functions. Measurement of other HPA axis hormones could also further our understanding of mechanisms of recalibration. Assessment of ACTH levels in addition to cortisol and DHEA would provide a stronger test of adrenal versus pituitary involvement. However, this would require obtaining blood, as ACTH cannot be measured in saliva. Adrenal activity could be further probed through examination of metabolites of cortisol (cortisone) and DHEA (dehydroepiandrosterone sulfate).
As stated in Gunnar et al. (2019), it will be important to examine the extent to which variations in early and current environmental conditions might relate to the magnitude of pubertal recalibration, and whether our finding of recalibration of the HPA axis in PI children will translate to children who have experienced other forms of adversity. Other studies have found persisting HPA axis dysregulation in individuals whose experiences of stress and adversity were not limited to early life but ongoing (e.g., Young et al., 2019), suggesting that conditions during puberty likely need to change markedly for recalibration to occur. Further research is also needed to determine whether recalibration of HPA axis activity in PI children is predictive of psychological and physical health. The results of the current study suggest that it may be fruitful to specifically examine the implications of increased positive coupling of cortisol and DHEA in these children. Similar to cortisol, it has been suggested that DHEA has heightened effects on brain development more broadly during adrenarche (Byrne et al., 2017; Campbell, 2011). In addition to its apparent protective actions that offset the neurotoxic effects of cortisol in the brain (Kamin and Kertes, 2017; Maninger et al., 2009), DHEA is broadly involved in neuroprotection, neurite growth, neurogenesis, neuronal survival, apoptosis and catecholamine secretion and has anti-oxidant and anti-inflammatory effects (see Maninger et al., 2009). Further, there is a growing literature which indicates that the coupling of cortisol and DHEA, as well as the relative levels of the two hormones (reflected in their ratio), are associated with psychological and physical health (Cicchetti and Rogosch, 2007; Kamin and Kertes, 2017; Marceau et al., 2015a).
4.2. Conclusions
The current study provides additional support that the pubertal transition constitutes a window of opportunity for changes in HPA axis activity for children who experienced early life stress. Expanding upon our previous finding of pubertal recalibration of cortisol reactivity in these children, our results suggest that there also is recalibration of cortisol-DHEA coupling with advancing puberty. Examination of the coupling of cortisol and DHEA provides additional insight into potential mechanisms underlying pubertal recalibration of the HPA axis. While we focused on recalibration at the adrenal level in our discussion, this does not rule out mechanisms of recalibration at higher levels of the axis. It is possible that recalibration of the HPA axis to a more typical pattern of functioning may be associated with resilience in these high-risk adolescents, but this remains to be tested and represents an exciting direction for future research. Our findings more broadly support the notion that puberty represents a period of heightened developmental plasticity and suggest that the pubertal transition may be an optimal period to intervene in the lives of children who have experienced adversity.
Supplementary Material
Highlights.
Puberty may allow for recalibration of HPA axis reactivity to current conditions.
Previously-institutionalized and non-adopted youth differed in cortisol but not DHEA levels by adrenal pubertal stage.
These children displayed different patterns of cortisol-DHEA coupling by adrenal pubertal stage.
Results suggest that adrenal maturational changes may at least in part underlie HPA axis recalibration.
Puberty may be an optimal period for intervention following early adversity.
Acknowledgements:
This research was supported by the NICHD (R01 HD075349 to MRG) and the National Science Foundation (Graduate Research Fellowship to MAH). We would like to express our gratitude to the families who make our research possible, the Minnesota International Adoption Project, and the Center for Neurobehavioral Development at the University of Minnesota. We also thank Tori Simenec, Bao Moua, Lea Neumann, and Heather Taylor, and Dr. Chris Desjardins for their assistance with the study; our nurses Janet Goodwalt, Terri Jones, and Melissa Stoll for Tanner staging; and Dr. Lorah Dorn for providing training in pubertal assessment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None.
We use the term sensitive period to refer to a time or stage in development when a system is more responsive to certain stimuli and quicker to establish patterns of functioning that are then relatively resistant to change following that period.
5. References
- Apostolova G, Schweizer RAS, Balazs Z, Kostadinova RM, Odermatt A, 2005. Dehydroepiandrosterone inhibits the amplification of glucocorticoid action in adipose tissue. Am. J. Physiol. - Endocrinol. Metab 10.1152/ajpendo.00442.2004 [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Rainey WE, 2004. Adrenarche - Physiology, biochemistry and human disease. Clin. Endocrinol. (Oxf) 10.1046/j.1365-2265.2003.01858.x [DOI] [PubMed]
- Balazs Z, Schweizer RAS, Frey FJ, Rohner-Jeanrenaud F, Odermatt A, 2008. DHEA induces 11β-HSD2 by acting on CCAAT/enhancer-binding proteins. J. Am. Soc. Nephrol 10.1681/ASN.2007030263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoń K, 2019. MuMIn: multi-model inference [WWW Document].
- Bates D, Mächler M, Bolker BM, Walker SC, 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Black SR, Lerner MD, Shirtcliff EA, Klein DN, 2018. Patterns of neuroendocrine coupling in 9-year-old children: Effects of sex, body-mass index, and life stress. Biol. Psychol 10.1016/j.biopsycho.2017.11.004 [DOI] [PMC free article] [PubMed]
- Bordini B, Rosenfield RL, 2011. Normal pubertal development: Part I: The endocrine basis of puberty. Pediatr. Rev 10.1542/pir.32-6-223 [DOI] [PubMed]
- Bornstein SR, Chrousos GP, 1999. Adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: Neural and immune inputs. J. Clin. Endocrinol. Metab 10.1210/jcem.84.5.5631 [DOI] [PubMed] [Google Scholar]
- Byrne ML, Whittle S, Vijayakumar N, Dennison M, Simmons JG, Allen NB, 2017. A systematic review of adrenarche as a sensitive period in neurobiological development and mental health. Dev. Cogn. Neurosci 10.1016/j.dcn.2016.12.004 [DOI] [PMC free article] [PubMed]
- Campbell B, 2011. Adrenarche in comparative perspective. Am. J. Hum. Biol 10.1002/ajhb.21111 [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, 2007. Personality, adrenal steroid hormones, and resilience in maltreated children: A multilevel perspective. Dev. Psychopathol 10.1017/S0954579407000399 [DOI] [PMC free article] [PubMed]
- Cunningham SK, McKenna TJ, 1994. Dissociation of adrenal androgen and cortisol secretion in Cushing’s syndrome. Clin. Endocrinol. (Oxf) 10.1111/j.1365-2265.1994.tb02795.x [DOI] [PubMed]
- Del Giudice M, Ellis BJ, Shirtcliff EA, 2011. The Adaptive Calibration Model of stress responsivity. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2010.11.007 [DOI] [PMC free article] [PubMed]
- DePasquale CE, Donzella B, Gunnar MR, 2019. Pubertal recalibration of cortisol reactivity following early life stress: a cross-sectional analysis. J. Child Psychol. Psychiatry Allied Discip 10.1111/jcpp.12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatough EM, Shirtcliff EA, Hanson JL, Pollak SD, 2009. Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology. 10.1016/j.psyneuen.2009.03.006 [DOI] [PMC free article] [PubMed]
- Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP, 1998. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr. Rev 10.1210/edrv.19.2.0326 [DOI] [PubMed]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ, 2002. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J. Neurosci 10.1523/jneurosci.22-18-07840.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH, 2009. Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 10.1016/j.psyneuen.2009.06.017 [DOI] [PubMed]
- Gunnar MR, DePasquale CE, Reid BM, Donzella B, 2019. Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proc. Natl. Acad. Sci. U. S. A 10.1073/pnas.1909699116 [DOI] [PMC free article] [PubMed]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C, 2009. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Dev. Psychopathol 10.1017/S0954579409000054 [DOI] [PMC free article] [PubMed]
- Hostinar CE, Johnson AE, Gunnar MR, 2015. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Dev. Sci 10.1111/desc.12195 [DOI] [PMC free article] [PubMed]
- Hucklebridge F, Hussain T, Evans P, Clow A, 2005. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology. 10.1016/j.psyneuen.2004.04.007 [DOI] [PubMed]
- Kamin HS, Kertes DA, 2017. Cortisol and DHEA in development and psychopathology. Horm. Behav 10.1016/j.yhbeh.2016.11.018 [DOI] [PubMed]
- King LS, Colich NL, LeMoult J, Humphreys KL, Ordaz SJ, Price AN, Gotlib IH, 2017. The impact of the severity of early life stress on diurnal cortisol: The role of puberty. Psychoneuroendocrinology. 10.1016/j.psyneuen.2016.11.024 [DOI] [PMC free article] [PubMed]
- Koss KJ, Mliner SB, Donzella B, Gunnar MR, 2016. Early adversity, hypocortisolism, and behavior problems at school entry: A study of internationally adopted children. Psychoneuroendocrinology. 10.1016/j.psyneuen.2015.12.018 [DOI] [PMC free article] [PubMed]
- Kumsta R, Schlotz W, Golm D, Moser D, Kennedy M, Knights N, Kreppner J, Maughan B, Rutter M, Sonuga-Barke E, 2017. HPA axis dysregulation in adult adoptees twenty years after severe institutional deprivation in childhood. Psychoneuroendocrinology. 10.1016/j.psyneuen.2017.09.021 [DOI] [PubMed]
- Lappalainen S, Utriainen P, Kuulasmaa T, Voutilainen R, Jääskeläinen J, 2008. ACTH receptor promoter polymorphism associates with severity of premature adrenarche and modulates hypothalamo-pituitary-adrenal axis in children. Pediatr. Res 10.1203/PDR.0b013e3181659c14 [DOI] [PubMed]
- Leneman KB, Donzella B, Desjardins CD, Miller BS, Gunnar MR, 2018. The slope of cortisol from awakening to 30 min post-wake in post-institutionalized children and early adolescents. Psychoneuroendocrinology. 10.1016/j.psyneuen.2018.06.011 [DOI] [PMC free article] [PubMed]
- Lennartsson AK, Kushnir MM, Bergquist J, Jonsdottir IH, 2012. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biol. Psychol 10.1016/j.biopsycho.2012.03.003 [DOI] [PubMed]
- Lüdecke D, 2020. sjstats: Collection of convenient functions for common statistical computations [WWW Document].
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH, 2009. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocrinol 10.1016/j.yfrne.2008.11.002 [DOI] [PMC free article] [PubMed]
- Marceau K, Dorn LD, Susman EJ, 2012. Stress and puberty-related hormone reactivity, negative emotionality, and parent-adolescent relationships. Psychoneuroendocrinology. 10.1016/j.psyneuen.2012.01.001 [DOI] [PubMed]
- Marceau K, Ruttle PL, Shirtcliff EA, Essex MJ, Susman EJ, 2015a. Developmental and contextual considerations for adrenal and gonadal hormone functioning during adolescence: Implications for adolescent mental health. Dev. Psychobiol 10.1002/dev.21214 [DOI] [PMC free article] [PubMed]
- Marceau K, Ruttle PL, Shirtcliff EA, Hastings PD, Klimes-Dougan B, Zahn-Waxler C, 2015b. Within-person coupling of changes in cortisol, testosterone, and DHEA across the day in adolescents. Dev. Psychobiol 10.1002/dev.21173 [DOI] [PMC free article] [PubMed]
- Marceau K, Shirtcliff EA, Hastings PD, Klimes-Dougan B, Zahn-Waxler C, Dorn LD, Susman EJ, 2014. Within-adolescent coupled changes in cortisol with DHEA and testosterone in response to three stressors during adolescence. Psychoneuroendocrinology. 10.1016/j.psyneuen.2013.12.002 [DOI] [PMC free article] [PubMed]
- Marshall WA, Tanner JM, 1970. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child 10.1136/adc.45.239.13 [DOI] [PMC free article] [PubMed]
- Marshall WA, Tanner JM, 1969. Variations in pattern of pubertal changes in girls. Arch. Dis. Child 10.1136/adc.44.235.291 [DOI] [PMC free article] [PubMed]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA, 2015. Causal effects of the early caregiving environment on development of stress response systems in children. Proc. Natl. Acad. Sci. U. S. A 10.1073/pnas.1423363112 [DOI] [PMC free article] [PubMed]
- Morley-Fletcher S, Rea M, Maccari S, Laviola G, 2003. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur. J. Neurosci 10.1111/j.1460-9568.2003.03070.x [DOI] [PubMed] [Google Scholar]
- Nguyen A, Conley A, 2008. Adrenal androgens in humans and nonhuman primates: Production, zonation and regulation. Endocr. Dev 10.1159/000134765 [DOI] [PubMed]
- Oskis A, Clow A, Thorn L, Loveday C, Hucklebridge F, 2012. Differences between diurnal patterns of salivary cortisol and dehydroepiandrosterone in healthy female adolescents. Stress. 10.3109/10253890.2011.582529 [DOI] [PubMed]
- Petersen AC, Crockett L, Richards M, Boxer A, 1988. A self-report measure of pubertal status: Reliability, validity, and initial norms. J. Youth Adolesc 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Quevedo K, Johnson AE, Loman ML, Lafavor TL, Gunnar M, 2012. The confluence of adverse early experience and puberty on the cortisol awakening response. International Journal of Behavioral Development. 10.1177/0165025411406860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey WE, Nakamura Y, 2008. Regulation of the adrenal androgen biosynthesis. J. Steroid Biochem. Mol. Biol 10.1016/j.jsbmb.2007.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid BM, Miller BS, Dorn LD, Desjardins C, Donzella B, Gunnar M, 2017. Early growth faltering in post-institutionalized youth and later anthropometric and pubertal development. Pediatr. Res 10.1038/pr.2017.35 [DOI] [PMC free article] [PubMed]
- Rich BH, Rosenfield RL, Lucky AW, Helke JC, Otto P, 1981. Adrenarche: Changing adrenal response to adrenocorticotropin. J. Clin. Endocrinol. Metab 10.1210/jcem-52-6-1129 [DOI] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS, 2006. Stress and the adolescent brain. Annals of the New York Academy of Sciences. 10.1196/annals.1376.022 [DOI] [PubMed]
- Rudolph KD, Hammen C, 1999. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child Dev. 10.1111/1467-8624.00048 [DOI] [PubMed]
- Ruttle PL, Shirtcliff EA, Armstrong JM, Klein MH, Essex MJ, 2015. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Dev. Psychobiol 10.1002/dev.21138 [DOI] [PMC free article] [PubMed]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ, 2012. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Dev. Psychobiol 10.1002/dev.20607 [DOI] [PMC free article] [PubMed]
- Shirtcliff EA, Dahl RE, Pollak SD, 2009. Pubertal development: Correspondence between hormonal and physical development. Child Dev. 10.1111/j.1467-8624.2009.01263.x [DOI] [PMC free article] [PubMed]
- Shirtcliff EA, Zahn-Waxler C, Klimes-Dougan B, Slattery M, 2007. Salivary dehydroepiandrosterone responsiveness to social challenge in adolescents with internalizing problems. J. Child Psychol. Psychiatry Allied Discip 10.1111/j.1469-7610.2006.01723.x [DOI] [PubMed] [Google Scholar]
- Simmons JG, Byrne ML, Schwartz OS, Whittle SL, Sheeber L, Kaess M, Youssef GJ, Allen NB, 2015. Dual-axis hormonal covariation in adolescence and the moderating influence of prior trauma and aversive maternal parenting. Dev. Psychobiol 10.1002/dev.21275 [DOI] [PubMed]
- Sisk CL, Zehr JL, 2005. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol 10.1016/j.yfrne.2005.10.003 [DOI] [PubMed]
- Smith SM, Vale WW, 2006. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci [DOI] [PMC free article] [PubMed]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R, 2009. Stress response and the adolescent transition: Performance versus peer rejection stressors. Dev. Psychopathol 10.1017/S0954579409000042 [DOI] [PMC free article] [PubMed]
- Tsigos C, Chrousos GP, 2002. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research. 10.1016/S0022-3999(02)00429-4 [DOI] [PubMed] [Google Scholar]
- Vijayakumar N, Op de Macks Z, Shirtcliff EA, Pfeifer JH, 2018. Puberty and the human brain: Insights into adolescent development. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2018.06.004 [DOI] [PMC free article] [PubMed]
- Weber A, Clark AJL, Perry LA, Honour JW, Savage MO, 1997. Diminished adrenal androgen secretion in familial glucocorticoid deficiency implicates a significant role for ACTH in the induction of adrenarche. Clin. Endocrinol. (Oxf) 10.1046/j.1365-2265.1997.1580969.x [DOI] [PubMed]
- Yim IS, Quas JA, Cahill L, Hayakawa CM, 2010. Children’s and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrinology. 10.1016/j.psyneuen.2009.06.014 [DOI] [PubMed]
- Young ES, Farrell AK, Carlson EA, Englund MM, Miller GE, Gunnar MR, Roisman GI, Simpson JA, 2019. The dual impact of early and concurrent life stress on adults’ diurnal cortisol patterns: A prospective study. Psychol. Sci 10.1177/0956797619833664 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.