Abstract
Among the three nonmuscle myosin 2 (NM2) paralogs, NM 2A and 2B, but not 2C, are detected in endothelial cells. To study the role of NM2 in vascular formation, we ablate NM2 in endothelial cells in mice. Ablating NM2A, but not NM2B, results in reduced blood vessel coverage and increased vascular branching in the developing mouse skin and coronary vasculature. NM2B becomes essential for vascular formation when NM2A expression is limited. Mice ablated for NM2B and one allele of NM2A develop vascular abnormalities similar to those in NM2A ablated mice. Using the embryoid body angiogenic sprouting assay in collagen gels reveals that NM2A is required for persistent angiogenic sprouting by stabilizing the endothelial cell cortex, and thereby preventing excessive branching and ensuring persistent migration of the endothelial sprouts. Mechanistically, NM2 promotes focal adhesion formation and cortical protrusion retraction during angiogenic sprouting. Further studies demonstrate the critical role of Rho kinase–activated NM2 signaling in the regulation of angiogenic sprouting in vitro and in vivo.
INTRODUCTION
Collective cell migration requires highly orchestrated, mechanically coupled migratory behavior which coordinates regulation of cell–cell adhesion, intercellular communication, and cell contractility to ensure efficient directional cell migration (Mayor and Etienne-Manneville, 2016; Park et al., 2016). The leader cells positioned at the front of the sprout generate lamellipodial and filapodial protrusions that sense, lead, and power the migration. The follower cells proceed by cell–cell contact. In addition, cell–cell contact inhibition of locomotion prevents the follower cells from scattering. Angiogenesis, the process of neovascular formation from preexisting blood vessels through collective endothelial cell sprouting in response to various angiogenic stimuli, is essential for many physiological and pathological conditions, including animal development, wound healing, and tumor formation (Phng and Gerhardt, 2009; Eilken and Adams, 2010; Carmeliet and Jain, 2011). Collective endothelial cell migration is a critical event for angiogenesis. In response to angiogenic stimuli, endothelial cells polarize, initiate sprouts, and migrate toward the angiogenic stimulus (such as vascular endothelial growth factor [VEGF] and fibroblast growth factor [FGF]). All these aspects rely on the integrated dynamic regulation of cell adhesion and the cytoskeleton. VEGF induces polarized cell elongation by decreasing VE–cadherin concentration at junctions, triggering polarized formation of actin-driven, junction-associated intermittent lamellipodia (JAIL) and thereafter endothelial cell sprouting (Cao et al., 2017). In vivo studies from both mice and zebrafish show that loss of VE-cadherin at endothelial cell-cell junctions results in excessive angiogenic sprouting associated with disrupted actin filaments at these junctions (Abraham et al., 2009; Gaengel et al., 2012; Sauteur et al., 2014). Dynamic endothelial cell–cell adhesions during angiogenic sprouting are associated with the activity of nonmuscle myosin 2 (NM2), a member of the conventional myosin 2 family, which is ubiquitously expressed both in muscle and nonmuscle cells in vertebrates. Inhibition of myosin ATPase activity by the NM2 inhibitor blebbistatin or the Rho kinase inhibitor Y27632 disrupts VE–cadherin mediated endothelial cell–cell adhesion associated with increased sprouting activity (Abraham et al., 2009; Wimmer et al., 2012; Cao et al., 2017). NM2 activity is also involved in endothelial cell rearrangement during sprout elongation (Angulo-Urarte et al., 2018). Recently, it was shown that application of blebbistatin or Y27632 and deletion of the NM2A paralog in endothelial cells disrupts cell–cell adhesion and results in endothelial cell scattering in a 3D angiogenic sprouting culture of human umbilical vein endothelial cells (Yoon et al., 2019). In addition, the acute injection of blebbistatin into the developing postnatal mouse retina leads to increased numbers of detached endothelial cells at the migrating front of the developing retina vasculature (Yoon et al., 2019). The role of NM2 in angiogenesis, however, has not been tested directly in vivo during animal development.
NM2 is composed of a pair of coiled-coil heavy chains with globular heads, and two pairs of myosin light chains (Heissler and Manstein, 2013; Ma and Adelstein, 2014). NM2 constitutes one of the most abundant cytoplasmic motor proteins, supporting various cellular functions in eukaryotic cells, such as cell division, cell migration, and cell adhesion. Three paralogs of NM2, namely NM2A, 2B, and 2C, have been identified based on differences in their heavy chains, which are the products of three different genes (Myh9, Myh10, and Myh14, respectively) located on different chromosomes in humans and mice (Berg et al., 2001; Golomb et al., 2004). The expression level of each NM2 varies in different organs and cells and is also regulated during development in mice. The three NM2s exert overlapping as well as unique functions in mouse development. Mice ablated for NM2A die very early in development by embryonic day (E)6.5 before gastrulation, due to a failure in visceral endoderm formation (Conti et al., 2004). Mice ablated for NM2B die by E14.5 from severe defects in brain and heart development (Tullio et al., 1997, 2001). NM2C-ablated mice have no obvious detectable abnormalities (Ma et al., 2010). Both NM2B and 2C can replace NM2A in vivo, supporting cell–cell adhesion and functional visceral endoderm formation, but cannot replace NM2A during placental vascular formation in mouse embryonic development (Wang et al., 2010; Zhang et al., 2018). In addition, NM2 paralogs may function together by forming heteromyosin filaments (Beach et al., 2014; Shutova et al., 2014). In most cases, mammalian cells express at least two NM2 paralogs. In developing mouse endothelial cells, NM2A and 2B, but not NM2C, are detected. Whether and how NM2 paralogs function together in the regulation of developmental processes such as blood vessel formation is not clear.
This study was designed to investigate the role of NM2A and 2B in vascular network formation during mouse development. The data presented in this report demonstrate that both NM2A and 2B are involved during embryonic blood vessel formation. This is accomplished using serial, endothelial cell–specific, and temporal ablations of various combination of NM2A and 2B during embryonic mouse development. These data also make use of an in vitro embryoid body angiogenic sprouting assay from NM2A and NM2B ablated mouse embryonic stem cells grown in 3D culture. Mechanistically, NM2A is essential for sprouting angiogenesis by maintaining the integrity of the cortical membrane through retraction of membrane protrusions, thereby ensuring persistent collective endothelial cell sprouting.
RESULTS
Ablation of nonmuscle myosin 2A, but not 2B, impairs vascular network formation in mice
To illustrate the expression of NM2A and 2B in the developing mouse vasculature, the back skins from mouse embryos that expressed EGFP-fused NM2A or 2B were dissected at embryonic day (E)14.5 and then wholemount stained with antibodies against GFP together with CD31 (a marker for endothelial cells). Supplemental Figure S1A displays the distribution of NM2A and 2B in the developing E14.5 mouse back skin. NM2A is predominantly detected in vascular endothelial cells (Supplemental Figure S1Aa) that costain with CD31 (Supplemental Figure S1Ab). NM2B is also detected in vascular endothelial cells, but is not as enriched as in endothelial cells (Supplemental Figure S1Ae) compared with other cells such as hair follicle cells (Supplemental Figure S1Ad, arrows). Panels S1Ac and S1Af show merged images of two channels. Staining using GFP antibodies also allows us to estimate the relative expression levels of NM2A and 2B in vascular endothelial cells in vivo. Supplemental Figure S1A shows that vascular endothelial cells expresses significantly more NM2A (Supplemental Figure S1a) than NM2B (Supplemental Figure S1d). Because NM2C is not detected in endothelial cells, our studies focus on the role of NM2A and 2B in vascular network formation.
We first individually ablated NM2A and 2B from endothelial cells in mice by crossing NM2A or NM2B floxed mice with Tie2-cre mice where the cre recombinase is expressed in all endothelial cells. E14.5 embryos ablated for NM2A (ATie2/ATie2) develop edema (Supplemental Figure S1Bb, red arrows) and hemorrhage (Supplemental Figure S1Bb, white arrows), indicating a leakage of the blood vessels in ATie2/ATie2 mice, which is not observed in control Aflox/Aflox embryos (Supplemental Figure S1Ba). To evaluate the vascular phenotype of endothelial NM2A or 2B ablated mice, the vasculature in E14.5 mouse back skins was visualized by wholemount staining using antibodies to CD31. As shown in Figure 1, the vascular network in ATie2/ATie2 mice is less mature, showing only a “chicken wire” capillary plexus (Figure 1b), and covers a reduced area of the back skin as compared with Aflox/Aflox littermates, which show a hierarchical vascular network (Figure 1a, arrows). To quantitate the extent of the blood vessel coverage, we measured the angle between the lines bordering the front edges of vascular sprouts in an open-book configuration of the back skin (a, b, dashed lines). The larger the angle, the lower the coverage. The average angle for control mice is 23 ± 6° (e, n = 4 mice). The average angle for ATie2/ATie2 mice is 40 ± 4° (e, n = 4 mice), which is significantly larger than for the control mice (p < 0.01). In addition, ATie2/ATie2 mice show abnormal clusters of endothelial cells at the middle of the back skin that are disconnected from centrally growing vascular sprouts (Figure 1d, yellow arrows). These clusters are not normally seen in control mice. Closer examination of the front of the vascular sprouts shows that wild-type sprouts are smooth, without obvious branches (Figure 1c, white arrows); ATie2/ATie2 sprouts, however, contain multiple branches (Figure 1d, white arrows). Thus loss of NM2A results in vascular overbranching. Quantitation of branch points of the vascular networks from Aflox/Aflox and ATie2/ATie2 mice using the AngioTool reveals a moderate, but significant increase in branch points in ATie2/ATie2 mice (27 ± 1.5 per mm length) compared with the Aflox/Aflox mice (24.5 ± 2.7 per mm length; Supplemental Figure S2, n = 4 mice each, p < 0.05). Note that the developing back skin vascular sprouts remain in a centrally migrating pattern in the open-book configuration in both ATie2/ATie2 and Aflox/Aflox embryos. This indicates that ablation of NM2A does not affect the directionality of the migrating vascular sprouts.
FIGURE 1:
Abnormal blood vessel formation in ATie2/ATie2 mouse back skins at E14.5. Wholemount confocal images of back skins dissected from ATie2/ATie2 (b, enlarged in d) and Aflox/Aflox control (a, enlarged in c) mice at E14.5 stained with CD31 antibodies to reveal the developing vasculature (red) show that ATie2/ATie2 mice have reduced blood vessel coverage, b, compared with Aflox/Aflox mice, a. Aflox/Aflox mice develop mature blood vessels—a, arrows, which are not seen in ATie2/ATie2 mice, b. The dashed white lines in a and b depict a V-shaped area that has not been fully covered by blood vessels. Aflox/Aflox back skins develop smooth straight vascular sprouts toward the middle of the back—c, arrows. ATie2/ATie2 back skins show vascular sprouts that contain multiple branches—d, white arrows. Isolated clusters of endothelial cells are observed in the middle of ATie2/ATie2 back skins—d, yellow arrows—which are not seen in Aflox/Aflox mice, c. Panel e shows the quantification of average angles from Aflox/Aflox and ATie2/ATie2 mouse back skins, n = 4 for each genotype.
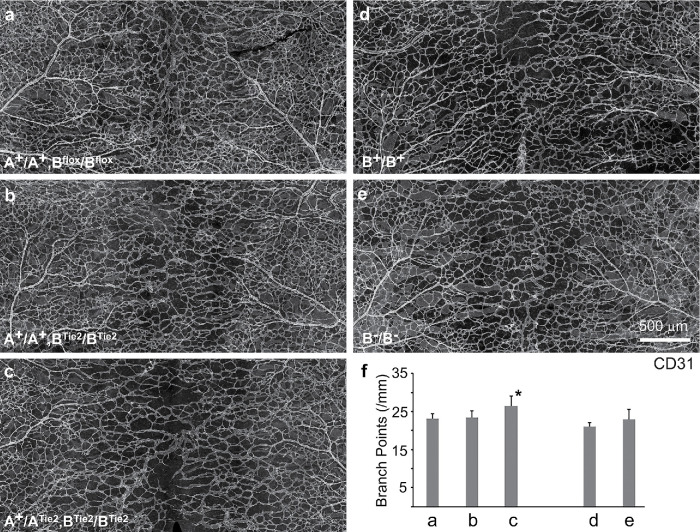
In contrast, ablation of NM2B alone in endothelial cells shows no edema, no hemorrhage, and no obvious defects in blood vessel formation in the back skin at E14.5 (Figure 2b) compared with the control littermate (Figure 2a). As previously shown (Tullio et al., 1997; Takeda et al., 2003), although B–/B– mice (germline ablation of NM2B) develop severe defects in the heart and die by E14.5 during embryonic development with heart failure, no significant defects in blood vessel formation are found in these mice at E14.5 (Figure 2, e and f) compared with their control littermates (Figure 2, d and f). These data suggest that defective heart development does not affect the vascular network in the B–/B– back skin. Taken together these results show that NM2A, but not NM2B, is essential for vascular network formation in the developing mouse skin. Additionally, NM2A functions to prevent angiogenic sprouts from excessive branching during vascular network formation.
FIGURE 2:
Ablation of NM2B impairs blood vessel formation when NM2A is not fully expressed. Wholemount confocal images of mouse back skins at E14.5 from various genotypes (as indicated) stained with CD31 antibodies show that mice ablated for NM2B in endothelial cells (b, A+/A+;BTie2/BTie2) or in the germline (e, B–/B–) have no defects in blood vessel formation as compared with their control littermates A+/A+;Bflox/Bflox (a) and B+/B+ (d), respectively. Ablation of one copy of NM2A in BTie2/BTie2 mice (c, A+/ATie2;BTie2/BTie2) results in immature back skin blood vessels together with moderately reduced blood vessel coverage compared with the control littermate, a. Panel f quantifies average branch points of vascular networks for each genotype. Bars a to e correspond to panels a to e, respectively. A+/ATie2;BTie2/BTie2 vasculature, c, shows a moderate increase in branch points (27 ± 3, n = 4, p < 0.05) from control A+/A+;Bflox/Bflox mice, a (23 ± 1, n = 4). *p < 0.05 (one way ANOVA, Post Turkey).
An auxiliary role for NM 2B in blood vessel formation during mouse development
As shown above, the development of blood vessels is compromised but not drastically disrupted in ATie2/ATie2 mice. We thus hypothesize that NM2B is also functioning during vascular network formation, especially in ATie2/ATie2 mice. To test this idea, we generated NM2A and 2B compound endothelial cell–ablated mice. Crossing a Tie2-Cre male with an Aflox/Aflox;Bflox/Bflox female generated healthy heterozygous A+/ATie2;B+/BTie2 mice. Figure 2 shows the back skin vasculatures from littermates obtained by crossing an A+/ATie2;B+/BTie2 male with an A+/A+;Bflox/Bflox female. A+/ATie2;BTie2/BTie2 mice, which express one copy of NM2A but no 2B, show abnormalities in vascular network formation. The back skin vasculature in these mice at E14.5 (Figure 2c) appears less mature and shows reduced coverage compared with control littermates (Figure 2a). The average branch points for A+/ATie2;BTie2/BTie2 vasculatures is 27 ± 3 per mm length (Figure 2f, n = 4, p < 0.05), which is moderately increased compared with control A+/A+;Bflox/Bflox vasculatures (Figure 2f, 23 ± 1, n = 4). Note that defects in A+/ATie2;BTie2/BTie2 vasculatures are not as severe as those seen in ATie2/ATie2 vasculatures. These results indicate that expression of one copy of NM2A is not sufficient to support normal blood vessel formation when NM2B is not expressed, while expression of one copy of NM2A plus one copy of NM2B is sufficient. Crossing A+/ATie2;B+/BTie2 males with Aflox/Aflox;Bflox/Bflox females generated no live ATie2/ATie2;B+/BTie2 and ATie2/ATie2;BTie2/BTie2 embryos at E12.5. At E10.5 the ATie2/ATie2;B+/BTie2 embryo is smaller than the controls (Supplemental Figure S3c), and the ATie2/ATie2;BTie2/BTie2 embryo is significantly delayed in development with pericardial effusion (Supplemental Figure S3d, arrow). These results further support the importance of NM2B in endothelial cells during mouse development. Early embryonic death of ATie2/ATie2; BTie2/BTie2 double homozygous mice prevents further analyses of the vascular phenotypes in the skin.
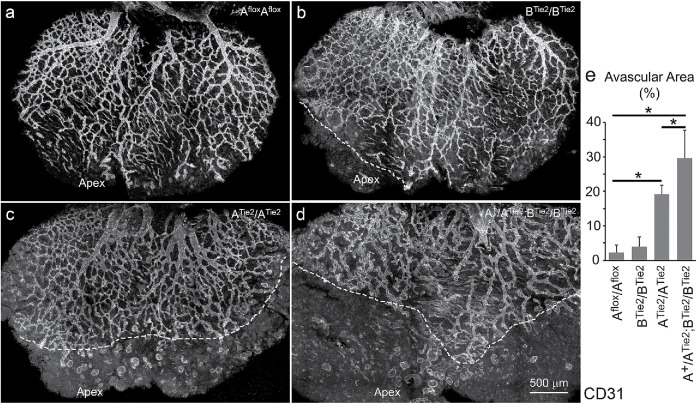
Defects in blood vessel formation are not limited to the back skin. Mice ablated for NM2 in endothelial cells also show abnormalities in coronary vessel formation during heart development (Figure 3). By E14.5, the entire dorsal heart surface is covered by a hierarchical coronary vasculature in control Aflox/Aflox littermates (Figure 3a); the coronary vasculature fails to cover the apex area in ATie2/ATie2 hearts (Figure 3c). Despite a very limited avascular area at the apex, BTie2/BTie2 hearts have no major defect in the coronary vasculature (Figure 3b). However A+/ATie2;BTie2/BTie2 hearts show a major reduction of coronary coverage (Figure 3d) which is even more severe than that seen in ATie2/ATie2 hearts. Avascular areas were measured using ImageJ (Figure 3e). The average percentages of the avascular area over the entire dorsal heart surface are 2 ± 2%, 4 ± 3%, 19 ± 3%, and 30 ± 8% for Aflox/Aflox, BTie2/BTie2, ATie2/ATie2, and A+/ATie2;BTie2/BTie2 hearts, respectively (n = 3 for each genotype, p < 0.05 between two of each groups by one way ANOVA, Post Turkey). In addition, coronary vasculature in both ATie2/ATie2 (c) and A+/ATie2;BTie2/BTie2 (d) hearts appears immature compared with control and BTie2/BTie2 hearts (a, b). These results indicate that coronary vascular formation relies more on the function of NM2B compared with skin vascular formation. Again, we find no changes in vascular patterning in the developing coronary vessels with various combinations of NM2A and 2B ablation.
FIGURE 3:
Abnormal coronary vessel formation in endothelial specific NM2–ablated mouse hearts. Wholemount confocal images of coronary vessels in the dorsal surface of E14.5 mouse heart stained with CD31 antibodies show that the coronary vasculature completely covers the entire dorsal surface in control cre-negative mice (a). BTie2/BTie2 hearts have a minor defect in coronary vasculature (b) compared with control hearts (a). ATie2/ATie2 hearts lack coronary vasculature covering the apex of the hearts (c). A+/ATie2;BTie2/BTie2 hearts show a marked reduction in coronary coverage (d). Dashed white lines in panels b, c, and d depict the front of vascular sprouts in mutant hearts. Panel e quantifies avascular areas for each genotype. n = 3 for each genotype. *p < 0.05 between two groups (one-way ANOVA, post Tukey).
To further demonstrate the importance of NM2B in vascular network formation during embryonic development, we ablated NM2A and 2B together in mice using crosses to an inducible VE–cadherin–Cre mouse (Cdh5–CreER). Introduction of tamoxifen at E10.5 circumvents the early embryonic lethality found in Tie2–cre–mediated NM2A and 2B doubly ablated mice. Crossing A+/ACdh5;B+/BCdh5 males with Aflox/Aflox;Bflox/Bflox females generates various combinations of Cdh5–CreER–mediated NM2A and NM2B compound ablated mice. Supplemental Figure S4 demonstrates the efficiency of NM2 ablation from these crossing. Figure 4, a–d displays embryos of littermates at E13.5 following a single intraperitoneal injection of tamoxifen at E10.5. A+/ACdh5;BCdh5/BCdh5 mice, which express one copy of NM2A and no 2B, show no obvious abnormalities, in contrast to Aflox/Aflox;Bflox/Bflox control littermates (Figure 4, a and b). ACdh5/ACdh5;B+/BCdh5 mice, which express one copy of NM2B and no 2A, develop obvious hemorrhages (Figure 4c, yellow arrows). ACdh5/AChd5;BCdh5/BCdh5 mice, which express no NM2A and no 2B, develop severe hemorrhages (Figure 4d, yellow arrows). Thus both NM2A and 2B are functioning to maintain the integrity of blood vessels during mouse embryonic development. A similar graded severity of vascular network defects in the back skin is observed among these genotypes (Figure 4, e–h). Compared with that in the control floxed littermates (Figure 4e), the vascular network in the back skin of A+/ACdh5;BCdh5/BCdh5 mice appears normal (Figure 4f). ACdh5/ACdh5;B+/BCdh5 back skin shows obvious abnormalities in vascular network formation and coverage (Figure 4g), and ACdh5/ACdh5;BCdh5/BCdh5 back skin shows the most severe defects (Figure 4h). The average branch points per millimeter length are 24 ± 3 (n = 5) for controls, 25 ± 1 (n = 5) for A+/ACdh5;BCdh5/BCdh5 mice, 33 ± 2 (n = 5) for ACdh5/ACdh5;B+/BCdh5 mice, and 66 ± 4 (n = 5) for ACdh5/ACdh5;B Cdh5/BCdh5 mice (Figure 4i). Both ACdh5/ACdh5;B+/BCdh5 and ACdh5/ACdh5;B Cdh5/BCdh5 mice show significant increases in vascular branch points as compared with the control mice (p < 0.01, one-way ANOVA). Moreover, branch points in ACdh5/ACdh5;B Cdh5/BCdh5 mice are also significantly more numerous than those in ACdh5/ACdh5;B+/BCdh5 mice (p < 0.01). Thus, NM2B is important when the expression of NM2A is not sufficient (heterozygous and homozygous) during vascular network formation. Importantly, when only a single allele of NM2A is expressed, the expression of NM2B is essential and sufficient to support normal development of blood vessels in mice. All these results further support the role of NM2 in the prevention of vascular overbranching during embryonic development.
FIGURE 4:
NM2B is essential for endothelial cells when NM2A is not expressed. (a–d) Images of E13.5 mouse embryos with various combinations of tamoxifen-induced (injection at E10.5) NM2A and 2B ablation after crosses with Cdh5–CreER mice show that with total ablation of NM2A from endothelial cells, an additional ablation of one copy of NM2B results in minor hemorrhages in ACdh5/ACdh5;B+/BCdh5 mice, c. Complete ablation of NM2A and 2B together leads to severe hemorrhages in ACdh5/ACdh5;BCdh5/BCdh5 mice, d. Mice ablated for one copy of NM2A together with both copies of NM2B, b (A+/ACdh5;BCdh5/BCdh5) do not show obvious abnormalities compared with control mice, a. (e–h) Wholemount confocal images of back skin vasculature from mouse embryos described above, a–d, show that with total ablation of NM2A from endothelial cells, additional ablation of one copy of NM2B results in reduced coverage of vasculature in ACdh5/ACdh5;B+/BCdh5 mice, g. Ablation of both copies of 2B causes a severe decrease in vascular coverage in ACdh5/ACdh5;BCdh5/BCdh5 mice, h. Mice ablated for one copy of NM2A together with both copies of NM2B, f (A+/ACdh5;BCdh5/BCdh5) show no defect in blood vessels compared with control mice, a. Panel i quantifies average branch points for each genotype. n = 5 for each genotype. *p < 0.01 between two groups (one-way ANOVA, post Tukey).
We have previously demonstrated that mice expressing NM2B or NM2C in place of NM2A under the control of the endogenous Myh9 promotor die early by E9.5 or E10.5, respectively, with abnormalities in blood vessel formation (Wang et al., 2010; Zhang et al., 2018). Therefore, the total amount of NM2 is not the single most important factor in blood vessel formation.
Loss of NM2A causes excessive branching in embryoid body sprouting angiogenesis
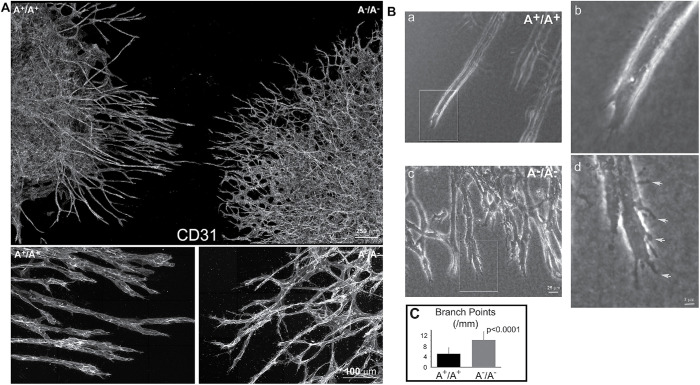
As shown above, ablation of NM2 expression from endothelial cells in mice resulted in an increased branching morphogenesis during embryonic vascular network formation. Because NM2A plays the major role, we next focused on the role of NM2A in 3D sprouting angiogenesis. We employed an in vitro 3D sprouting angiogenesis assay using embryoid bodies (EBs) generated from wild-type (A+/A+) and NM2-ablated (A–/A–) mouse embryonic stem cells. A–/A– embryonic stem cells were generated by homologous recombination as previously described (Conti et al., 2004). A+/A+ and A–/A– EBs were embedded and cultured side by side in pairs in a 1.5-mg/ml collagen gel containing 30 ng/ml VEGF-A. Immunofluorescence staining of EB sprouts with antibodies against the heavy chain of NM2A (NMHC2A) confirms the ablation of NM2A in A–/A– EBs (Supplemental Figure S5, c and d). NM2A is detected in A+/A+ EBs (Supplemental Figure S5, a and b). Figure 5A shows the endothelial sprouts stained with the CD31 antibody following 7 d in culture. A+/A+ sprouts in general appear straight and smooth (left, magnified below), A–/A– sprouts are much thinner with a marked increase in branches (right, magnified below). We quantified the density of the branching points and measured the length of the branches from cultured EB sprouts using the AngioTool (Figure 5C). The density of branch points is significantly higher in A–/A– sprouts (10 ± 3 per millimeter of sprout, n = 32) than in the control A+/A+ sprouts (5 ± 2 per millimeter of sprout, n = 44, p < 0.0001). Correspondingly, A–/A– sprouts show a marked decrease in average length of the branch (0.86 ± 0.26 mm, n = 32) compared with A+/A+ sprouts (1.26 ± 0.82 mm, n = 44, p < 0.05). Figure 5B shows phase-contrast images of the sprouts. Compared with the wild-type sprouts (Figure 5B, a and b), A–/A– sprouts develop excessive branches and cortical protrusions (Figure 5B, c and d, arrows). Panels b and d are enlarged from the boxed areas of panels a and c, respectively. Time-lapse videos of growing sprouts further reveal that in A+/A+ growing sprouts, the endothelial cells migrate collectively, and the tip of the sprout develops one dominant protrusion that leads to a persistent growth of sprouts (Supplemental Movie S1a). A–/A– sprouts, however, often develop multiple protrusions at the tip causing frequent splitting and/or turning of the sprouts (Supplemental Movie S1b). Together, these results demonstrate that loss of NM2A results in an increase in vascular branching during sprouting angiogenesis due to a defect in persistent sprouting. To further confirm these results, a second line of NM2A ablated mouse embryonic stem cells was generated using CRISPR and the 3D sprouting angiogenesis studies were repeated. Supplemental Figure S5, e and f demonstrates the ablation of NM2A in CRISPR A–/A– sprouts. Again, CRISPR-NM2A–ablated EBs show excessive vascular branching (Supplemental Figure S6b) as compared with the isogenic wild-type EBs (Supplemental Figure S6a). The average branch points of CRISPR A–/A– sprouts (10 ± 1 per mm sprouts, n = 5, p < 0.01) are significantly more numerous than those of the wild-type sprouts (4 ± 2 per mm sprouts, n = 6; Supplemental Figure S6d). Using CRISPR, we also generated NM2B-ablated mouse embryonic stem cells. Supplemental Figure S5, g–j confirms the ablation of NM2B expression in CRISPR B–/B– sprouts (i, j). NM2B is detected in wild-type (B+/B+) sprouts (g, h). Ablation of NM2B does not affect EB sprouting angiogenesis (Supplemental Figure S6c; Supplemental Movie S1c). The average number of branch points of CRISPR B–/B– sprouts is 4 ± 2 per mm of sprout (n = 5), which is no different from that for the wild-type sprouts (Supplemental Figure S6d). These results are consistent with our findings from NM2 conditionally ablated mice, suggesting that defects of blood vessel formation in NM2 conditionally ablated mice are primarily due to the loss of NM2 function during sprouting angiogenesis.
FIGURE 5:
Excessive branching of A–/A– angiogenic sprouts from EBs cultured in 3D collagen. (A) Z-projections of confocal images from A+/A+ and A–/A– angiogenic sprouts stained with CD31 antibodies for endothelial cells show that A–/A– sprouts (Right) develop more branches than A+/A+ sprouts (Left). The average densities of branch points are 10 ± 3 (n = 32) and 5 ± 2 (n = 44) per millimeter of sprout for A–/A– and A+/A+ sprouts, respectively (p < 0.0001). The average lengths of branches are 0.86 ± 0.26 mm (n = 32) and 1.26 ± 0.82 mm (n = 44) for A–/A– and A+/A+ sprouts, respectively (p < 0.05). The panels at the bottom show a magnified view of the upper panel. (B) Phase contrast images of A+/A+ (a, magnified in b) and A–/A– (c, magnified in d) angiogenic sprouts show that A+/A+ sprouts in general are straight and smooth, b, while A–/A– sprouts have excessive branches and cortical protrusions—d, arrows. (C) Quantification of average branch points for A+/A+ (n = 44) and A–/A– (n = 32) sprouts. p < 0.0001.
Movie S1a.
Effect of NM2A and 2B ablation on EB angiogenic sprouts over 6 hrs. Phase contrast time lapse videos of EB sprouts at 1min/frame with 10X objective. a. wild-type sprout, b. A−/A− sprouts, c. B−/B− sprouts. In general the wild-type and B−/B− sprouts develop one dominant protrusion resulting in a persistent growth of sprouts (a,c). A−/A− sprouts often develop multiple protrusions at the tip causing frequent splitting and/or turning of the knockout sprouts (b). 1min/frame (capture rate), 100fps (playback).
Movie S1b.
Effect of NM2A and 2B ablation on EB angiogenic sprouts over 6 hrs. Phase contrast time lapse videos of EB sprouts at 1min/frame with 10X objective. a. wild-type sprout, b. A−/A− sprouts, c. B−/B− sprouts. In general the wild-type and B−/B− sprouts develop one dominant protrusion resulting in a persistent growth of sprouts (a,c). A−/A− sprouts often develop multiple protrusions at the tip causing frequent splitting and/or turning of the knockout sprouts (b). 1min/frame (capture rate), 100fps (playback).
Movie S1c.
Effect of NM2A and 2B ablation on EB angiogenic sprouts over 6 hrs. Phase contrast time lapse videos of EB sprouts at 1min/frame with 10X objective. a. wild-type sprout, b. A−/A− sprouts, c. B−/B− sprouts. In general the wild-type and B−/B− sprouts develop one dominant protrusion resulting in a persistent growth of sprouts (a,c). A−/A− sprouts often develop multiple protrusions at the tip causing frequent splitting and/or turning of the knockout sprouts (b). 1min/frame (capture rate), 100fps (playback).
Loss of cortical stability in NM2A-ablated vascular sprouts
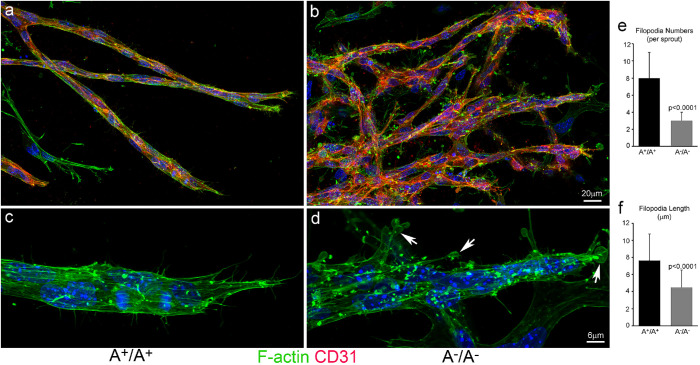
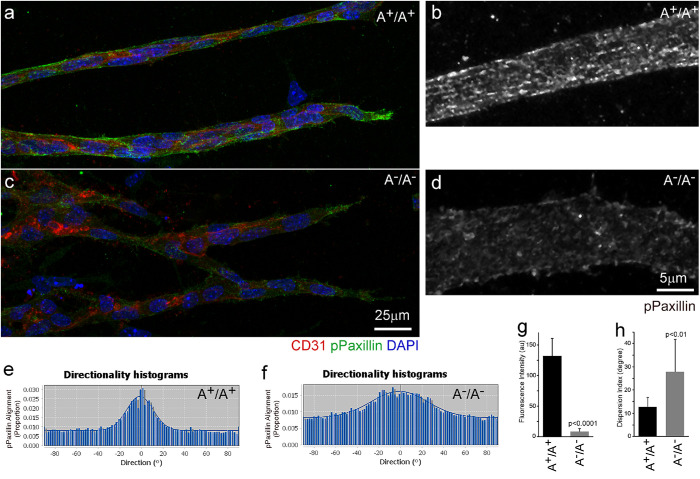
To understand how ablation of NM2A causes overbranching during sprouting angiogenesis, time-lapse videos of EB sprouting were captured at a frequency of 1 s per frame and carefully analyzed for the behavior of the sprouts. In A+/A+ EBs, the front of growing sprouts generates numerous cortical blebs with an average lifetime of 15 ± 3 s (n = 50, Supplemental Movie S2a, arrow). Typical blebbing consists of a fast phase of protrusion and growth followed by a slower phase of retraction (Supplemental Figure S7a, Front; enlarged in panel S7d). Blebs quickly form at the front of the sprouts following the protrusion of the tip cells. Blebs are also observed at the back of the sprouts but with decreased frequency (Supplemental Figure S7a, Back). In contrast, the front of A–/A– sprouts show no dynamic blebbing but develop marked cortical ruffles with multiple protrusions (Supplemental Movie S2b, arrow). Instead of blebbing, the backs of A–/A– sprouts show slow cortical fluctuations (Supplemental Figure S7b, Back), suggesting a reduction of cortical tension compared with that in the wild-type sprouts. Interestingly, cortical blebbing requires NM2A but not NM2B. NM2B-ablated sprouts show normal sprouting and blebbing similar to those in the wild-type sprouts (Supplemental Movie S1c; Supplemental Movie S2c, arrow; Supplemental Figure S7c). The actomyosin cytoskeletons in A–/A– and A+/A+ sprouts were examined by staining with antibody to CD31 and Alexa-488 phalloidin for F-actin. A+/A+ sprouts are generally encircled by thick actomyosin bundles around the entire sprout, manifesting a mechanically coupled migratory unit, and they develop numerous filopodia-like structures that are enriched at the tips of the sprouts during navigation (Figure 6a). The stress fibers in A+/A+ sprouts align along the long axis of the sprouts (Figure 6a, enlarged in c, green). In contrast, A–/A– sprouts are not as compact, show no obvious boundary of actin bundles surrounding the sprouts, and show a marked reduction in filopodia formation and stress fibers (Figure 6b, enlarged in d, green). Instead of filopodia-like structures, A–/A– sprouts develop numerous balloon-like protrusions (Figure 6d, arrows) reminiscent of membrane ruffles in cells under 2D culture. We counted the filopodia numbers at the tip of the sprouts and measured the lengths of the filopodia using ImageJ (Figure 6, e and f). The average numbers of filopodia per sprout for A+/A+ and A–/A– sprouts are respectively 8 ± 3 (n = 21) and 3 ± 1 (n = 28; p < 0.0001). The average lengths of filopodia for A+/A+ (n = 103) and A–/A– (n = 115) sprouts are 7.6 ± 3.1 μm and 4.5 ± 2.0 μm, respectively (p < 0.0001). These results suggest that loss of NM2A impairs the cortical membrane stability in sprouts. Fibroblast cells lacking NM2 function develop excessive lamellipodia ruffling associated with impaired focal adhesion maturation when cultured on a flat surface (Lo et al., 2004; Even-Ram et al., 2007; Ma et al., 2017). Focal adhesions in EB sprouts were examined by staining for phosphopaxillin (pPaxillin, a marker of mature focal adhesions). Figure 7 shows that mature focal adhesions in A+/A+ sprouts align parallel to the long axis of the sprouts (Figure 7a, enlarged in b, green), similarly to the focal adhesions seen in mesenchymal cells cultured in a 3D matrix (Cukierman et al., 2001). In A–/A– sprouts, however, pPaxillin signaling is markedly reduced and shows no regularity in alignment (Figure 7c, enlarged in d, green). The fluorescence intensity of pPaxillin staining was quantified using ImageJ (Figure 7g). The relative fluorescence intensity (arbitrary units) of A+/A+ sprouts is 132 ± 28 (n = 60), which is significantly greater than for A–/A– sprouts, for which it is 9 ± 5 (n = 48, p < 0.0001). The alignment of adhesion was analyzed using ImageJ Directionality. A+/A+ sprouts show an obvious peak corresponding to their major alignment along the long axis of the sprouts (Figure 7e), while the peak in A–/A– sprouts is markedly damped (Figure 7f). Accordingly, the Dispersion index (Figure 7h; a measurement of deviation from the Directionality analysis) is significantly larger for A–/A– sprouts (28 ± 14°, n = 11, p < 0.01) than for A+/A+ sprouts (13 ± 4°, n = 10). Thus, NM2A plays an important role in maintaining cortical integrity and in the regulation of focal adhesions during sprouting angiogenesis.
FIGURE 6:
Abnormal actin organization in A–/A– angiogenic sprouts. Confocal images of A+/A+ (a) and A–/A– (b) angiogenic sprouts stained with CD31 (red) and Alexa488-phaloidin for F-actin (green) show disruption of stress-fibers in A–A– sprouts (b, magnified in d) compared with A+/A+ sprouts (a, magnified in c). The stress fibers are aligned along the long axis in A+/A+ sprouts, c. A–/A– sprouts show a great reduction in stress -fibers and develop numerous balloonlike cortical membrane protrusions manifesting membrane ruffling—d, arrows. Panel e quantifies the average numbers of filopodia at the tip of sprouts (n = 21 and 23 for A+/A+ and A–A– sprouts, respectively, p < 0.0001). Panel f quantifies the average length of filopodia (n = 103 and 115 for A+/A+ and A–A– sprouts, respectively, p < 0.0001).
FIGURE 7:
Defects in focal adhesion formation in A–/A– angiogenic sprouts. (a–d) Confocal images of angiogenic sprouts stained with antibodies for phosphopaxillin (green, a marker for mature focal adhesions) and CD31 (red, a marker for endothelial cells) show that A+/A+ sprouts develop mature focal adhesions that align in parallel along the long axis of sprouts (a, magnified in b, green). Mature focal adhesions are infrequently observed in A–/A– sprouts and they are randomly distributed (c, magnified in d, green). (e, f) Representative directionality histograms of phosphopaxillin staining in A+/A+ and A–/A– sprouts obtained from the Directional analysis. A+/A+ sprouts show an obvious peak (e), which is consistent with a major alignment of focal adhesions along the long axis of the sprouts. A–/A– sprouts show a markedly damped peak (f) reflecting a loss of alignment. Panel g quantifies the average fluorescence intensity of pPaxillin staining (n = 60 and 48 for A+/A+ and A–A– sprouts, respectively, p < 0.0001). Panel h quantifies the average dispersion index (a measurement of deviation from the Directionality analysis) of pPaxillin alignments (n = 10 and 11 for A+/A+ and A–A– sprouts, respectively, p < 0.01).
Movie S2a.
Effect of NM2A and 2B ablation on tip cell dynamics in EB sprouts. Phase contrast time lapse videos of EB sprouts at 1sec/frame with 40X objective. (a) wild-type sprout, (b) A−/A− sprout, (c) B−/B− sprouts. Wild-type and B−/B− tips cells generate numerous cortical blebs (a,c, arrow). A−/A− tip cells develop cortical ruffles (b, arrow), but no blebbing. 1sec/frame (capture rate), 100fps (playback).
Movie S2b.
Effect of NM2A and 2B ablation on tip cell dynamics in EB sprouts. Phase contrast time lapse videos of EB sprouts at 1sec/frame with 40X objective. (a) wild-type sprout, (b) A−/A− sprout, (c) B−/B− sprouts. Wild-type and B−/B− tips cells generate numerous cortical blebs (a,c, arrow). A−/A− tip cells develop cortical ruffles (b, arrow), but no blebbing. 1sec/frame (capture rate), 100fps (playback).
Movie S2c.
Effect of NM2A and 2B ablation on tip cell dynamics in EB sprouts. Phase contrast time lapse videos of EB sprouts at 1sec/frame with 40X objective. (a) wild-type sprout, (b) A−/A− sprout, (c) B−/B− sprouts. Wild-type and B−/B− tips cells generate numerous cortical blebs (a,c, arrow). A−/A− tip cells develop cortical ruffles (b, arrow), but no blebbing. 1sec/frame (capture rate), 100fps (playback).
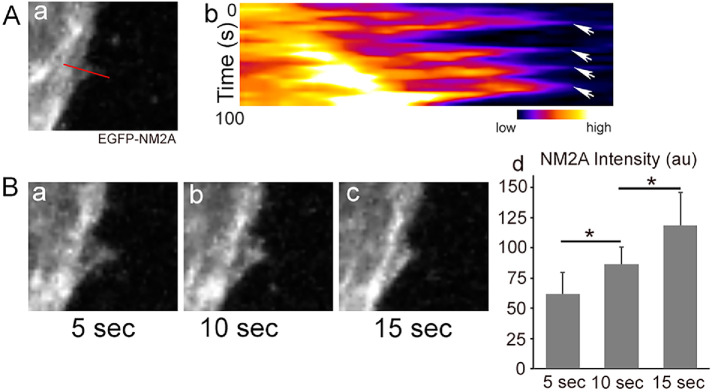
NM2A dynamics during cortical protrusion and retraction during EB sprouting were investigated next. Using EGFP–NM2A embryonic stem cells (where EGFP is fused to the N-terminal of the endogenous nonmuscle myosin heavy chain 2A), we recorded time-lapse videos of EGFP–NM2A signals during EB sprouting. As shown in Supplemental Movie S3, the cortical protrusions initiate at an area with reduced NM2A signal; however, an increase of NM2A intensity is observed during cortical retraction. Figure 8Ab shows the kymograph of EGFP–NM2A dynamics along the line shown in Figure 8Aa. The relative NM2A intensity is shown in a “Fire” scale to better visualize the differences. NM2A intensity increases during retraction from a low “blue” to a high “orange” in all four retraction events (Figure 8Ab, arrows). Figure 8B, a–c shows a timed series of images at 5, 10 and 15 s of a single retraction, which further demonstrates increased NM2A intensity during cortical retraction. EGFP–NM2A fluorescence intensity (au, arbitrary units) was quantified using ImageJ (Figure 8Bd). The average EGFP–NM2A intensities (n = 10 events) are 62 ± 18, 84 ± 14, and 119 ± 28 au, respectively. All these results are consistent with the role of NM2A in cortical retraction. Actomyosin therefore provides the mechanical machinery that is required for maintaining cortical stability and prevents excessive protrusion during sprouting angiogenesis.
FIGURE 8:
Increase of NM2A intensity during cortical retraction in angiogenic EB sprouting. (A) Kymograph of NM2A dynamics (b) along the line indicated in panel a during angiogenic EB sprouting. NM2A intensity is shown in a “Fire” scale. NM2A intensity increases from a low “blue” to a high “orange” in all four retraction events—b, arrows. (B) A time series of a retraction event (a–c) demonstrates a gradual increase of NM2A from panel a to panel c during cortical retraction. NM2A intensity is lowest in panel a and highest in panel c among the three time points. Panel Bd quantifies the average EGFP–NM2A fluorescence intensity (au, arbitrary units). n = 10, *p < 0.05 between two groups (one-way ANOVA, post Tukey). Note that the sprout shows a general shift from left to right during imaging as shown both from the kymograph, A, and the time series, B.
Movie S3.
NM2A dynamics in EB sprouting and cortical ruffling. A time lapse video of EGFP-NM2A in an EB sprout. NM2A accumulates during the retraction of the cortical protrusion in angiogenic sprouts.
ROCK-activated NM2A activity is required during sprouting angiogenesis
To further confirm that sprouting angiogenesis requires NM2 enzymatic ATPase activity, we introduced the myosin inhibitor blebbistatin into the EB sprouting assay. Blebbistatin inhibits NM2 MgATPase activity by blocking phosphate release from ADP. Movie Supplemental S4a shows that the application of 10 μM blebbistatin results in excessive branching. The sprouts develop multiple protrusions and no dynamic cortical blebbing, which phenocopies the abnormities of the NM2A ablated sprouts. We next examined the upstream activator of NM2 during angiogenesis. NM2 myosin MgATPase activity is activated by two major kinases, Rho kinase and Ca-calmodulin–dependent myosin–light chain kinase, which phosphorylate the regulatory myosin light chain. Inhibition of Rho kinase by Y27632 (10 μM) results in abnormal branching and the loss of cortical blebbing, similarly to blebbistatin inhibition of NM2 activity (Supplemental Movie S4b). However, inhibition of myosin–light chain kinase by ML-7 (20 μM) has no effect on sprouting (Supplemental Movie S4c). Applications of blebbistatin (10 μM) and Y27632 (10 μM), but not ML-7 (20 μM), during EB-sprouting angiogenesis assays overnight at day 6 significantly increases sprout branching morphogenesis (Supplemental Figure S8). The average branch points of blebbistatin- and Y27632-treated sprouts are 7 ± 2 (n = 14) and 7 ± 1 (n = 14), respectively; those are significantly more numerous than those of the sprouts treated with the same amount of solvent DMSO (4 ± 2, n = 14, p < 0.01). ML-7 does not increase the branch points of the sprouts (5 ± 1, n = 9). Supplemental Figure S8e shows the quantification of the average branch points. All of these results demonstrate that Rho kinase–activated NM2 activity is essential for sprouting angiogenesis.
Movie S4a.
Effect of NM2 inhibition on tip cell dynamics in EB Sprouts. Phase contrast time lapse videos of EB sprouts at 1sec/frame with 40X objective. (a) 10μM blebbistatin, (b) 10μM Y27632, (c) 20μM ML-7. Blebbistatin inhibition of NM2 activity (a) and Y27632 inhibition of Rho kinase (b) result in abnormal cortical protrusion (arrows) and blocked tip cell blebbing. ML-7 inhibition of MLCK has no effect on blebbing (c, arrow) in EB sprouting angiogenesis. 1sec/frame (capture rate), 100fps (playback).
Movie S4b.
Effect of NM2 inhibition on tip cell dynamics in EB Sprouts. Phase contrast time lapse videos of EB sprouts at 1sec/frame with 40X objective. (a) 10μM blebbistatin, (b) 10μM Y27632, (c) 20μM ML-7. Blebbistatin inhibition of NM2 activity (a) and Y27632 inhibition of Rho kinase (b) result in abnormal cortical protrusion (arrows) and blocked tip cell blebbing. ML-7 inhibition of MLCK has no effect on blebbing (c, arrow) in EB sprouting angiogenesis. 1sec/frame (capture rate), 100fps (playback).
Movie S4c.
Effect of NM2 inhibition on tip cell dynamics in EB Sprouts. Phase contrast time lapse videos of EB sprouts at 1sec/frame with 40X objective. (a) 10μM blebbistatin, (b) 10μM Y27632, (c) 20μM ML-7. Blebbistatin inhibition of NM2 activity (a) and Y27632 inhibition of Rho kinase (b) result in abnormal cortical protrusion (arrows) and blocked tip cell blebbing. ML-7 inhibition of MLCK has no effect on blebbing (c, arrow) in EB sprouting angiogenesis. 1sec/frame (capture rate), 100fps (playback).
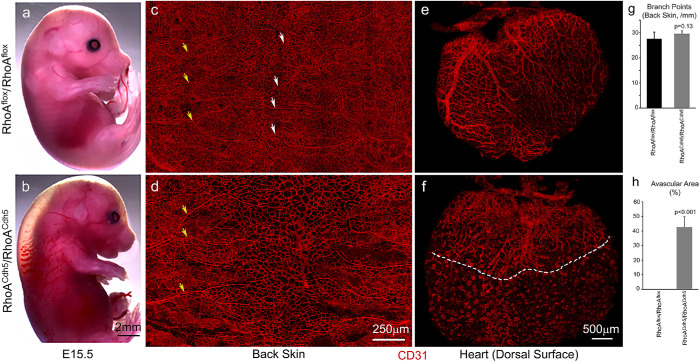
We next asked whether Rho kinase–activated NM2 was important in embryonic vascular development in vivo. There are many reports supporting the role of Rho kinase in the regulation of vascular network formation (Bryan et al., 2010; Kamijo et al., 2011; Liu et al., 2018). The importance of Rho kinase in vascular formation, however, is not well demonstrated during embryonic development in vivo. Two Rho kinases (ROCK1 and ROCK2) are expressed in endothelial cells and show overlapping roles during embryonic mouse development. The small GTPase RhoA is the major upstream activator of the Rho kinases. We therefore next ablated RhoA in mouse endothelial cells using Cdh5–CreER mice and examined the skin and cardiac vascular development in these mice. Figure 9 shows E15.5 mouse embryos following a tamoxifen injection at E10.5 to induce RhoA ablation. Embryos ablated for RhoA develop edema and hemorrhage (Figure 9b). Compared with the control mice (Figure 9c, RhoAflox/RhoAflox), mice ablated for RhoA in endothelial cells do not show obvious defects in the coverage of blood vessels and branching morphogenesis in the back skin at E15.5 (Figure 9d, RhoACdh5/RhoACdh5). The average branch points for RhoAflox/RhoAflox and RhoACdh5/RhoACdh5 back skin vasculatures are 28 ± 3 and 29 ± 1 per mm length, respectively (n = 4 each, p = 0.13, Figure 9g). The maturation of the vasculature, however, is delayed in RhoACdh5/RhoACdh5 back skin, which shows no remodeled blood vessels in the middle of the back (Figure 9d). These mature vessels are evident in the control littermate (Figure 9c, arrows). Interestingly, coronary vessel development in RhoA ablated mice is severely impaired, showing a marked reduction of coronary vessel coverage at the apex of the dorsal surface of the heart (Figure 9f, below the dashed line) compared with that in control mice, where the coronary vasculature completely covers the dorsal surface of the heart ventricle (Figure 9e). The average percentage of the avascular area for RhoACdh5/RhoACdh5 hearts is 43 ± 7% (n = 3, Figure 9h). There is no avascular area for RhoAflox/RhoAflox hearts. These results suggest that RhoA/RhoA activated kinase activation of NM2A plays an important role in blood vessel development in vivo, especially during mouse cardiac coronary vessel formation.
FIGURE 9:
Abnormal blood vessel formation in tamoxifen-induced (E10.5) endothelial RhoA–ablated mouse embryos at E15.5. (a, b) Images of control, a (RhoAflox/RhoAflox) and endothelial RhoA–ablated, b (RhoACdh5/RhoACdh5) mouse embryos at E15.5 show that endothelial specific ablation of RhoA in mice results in edema and hemorrhages, b, which are not seen in control littermates, a. (c, d) Wholemount confocal images of E15.5 mouse back skin stained with CD31 antibodies show that blood vessels cover the entire back skin in RhoAflox/RhoAflox, c, and RhoACdh5/RhoACdh5 mice, d. Mature remodeled blood vessels are observed in both lateral and middle regions in RhoAflox/RhoAflox mice—c, yellow and white arrows, respectively. Mature remodeled vessels are observed in the lateral region (yellow arrows), but are not found at the middle region in the RhoA ablated RhoACdh5/RhoACdh5 mice, d. (e, f) Wholemount confocal images of coronary vessels at the dorsal surface of E15.5 mouse hearts stained with CD31 antibodies show that the coronary vessels cover the entire dorsal surface in RhoAflox/RhoAflox hearts, e. The coronary vessels are only seen at the basal area, but fail to grow through the middle of the dorsal surface in RhoACdh5/RhoAChd5 hearts, f. Panel g quantified the average branch points of RhoAflox/RhoAflox and RhoACdh5/RhoACdh5 back skin vasculatures. n = 4 each, p = 0.13 (not statistically significantly different). Panel h quantifies the avascular areas of RhoAflox/RhoAflox and RhoACdh5/RhoACdh5 hearts. n = 3 each, p < 0.0001.
In summary, we show that NM2 plays a major role in persistent vascular sprouting and that NM2A functions to prevent excessive branching from sprouts by maintaining cortical stability through focal adhesions and facilitation of cortical membrane retraction.
DISCUSSION
Our results from singly NM2A or NM2B endothelial cell-ablated mice and 3D EB angiogenic sprouting show that NM2A, but not NM2B, is required for sprouting angiogenesis. Similar findings are reported for a 3D biomimetic model of angiogenesis using HUVECs by CRISPR ablation of NM2A and 2B individually (Yoon et al., 2019). Our analyses of endothelial NM2A and 2B compound ablated mice further show that NM2B also plays important roles in angiogenesis. Together with NM2A ablation, ablation of NM2B potentiates blood vessel abnormalities in mice, including a further decrease of blood vessel coverage and an increase of hemorrhage in mutant embryos. One possible explanation is that NM2A and 2B compensate for each other when one paralog is missing. A second possible explanation is that NM2A and 2B complement each other in regulating angiogenesis. Previously, results from NM2 paralog genetic swapping studies demonstrated that NM2A is uniquely required for placental vascular development in mice, which is not consistent with compensation between NM2A and 2B (Wang et al., 2010; Zhang et al., 2018). Our results here favor the notion that NM2A and 2B function complementarily in regulation of angiogenesis. Dynamic regulation of endothelial cell–cell adhesion plays key roles for angiogenic sprouting activity (Szymborska and Gerhardt, 2018). Weakening of cell–cell adhesion promotes sprouting. NM2 activity is required for filopodia-like bridge formation and recruitment of VE–cadherin in nascent adherens junctions in cultured endothelial cells (Liu et al., 2010; Hoelzle and Svitkina, 2012). In cultured epithelial MDCK cells, NM2A and NM2B have been shown to function complementarily in the formation and maintenance of the integrity of adherens junctions (Heuze et al., 2019). During adherens junction formation, NM2A and 2B are differentially localized to and stabilize the perijunctional contractile actin bundles and the juxtamembrane actin meshwork, respectively. NM2A provides the major tugging force for junction growth, while NM2B is required for E-cadherin clustering and coupling of perijunctional contractile actin to the plasma membrane, and provides the majority of intercellular stress (Heuze et al., 2019). In mature adherens junctions, junctional localization of NM2A and 2B is also differentially regulated. Rho/ROCK and myosin–light chain kinase activity are required for NM2A localization, whereas junctional NM2B depends on Rap1 (Smutny et al., 2010). All these findings support the idea that NM2A and 2B function complementarily but in different aspects of cell–cell adhesions. However, we failed to detect obvious abnormalities in NM2B endothelial cell–ablated mice and in NM2B-ablated sprouting angiogenesis in vitro using EBs.
NM2 promotes cortical protrusion retraction ensuring processive sprouting and preventing excessive branching. The back skin vasculature in mice ablated for NM2A develops increased branching morphogenesis. NM2A null endothelial sprouts from an in vitro angiogenic sprouting assay also show increased branching activity. Thus NM2 is important to inhibit excessive branching during angiogenic sprouting. This is consistence with the findings that inhibition of Rho kinase activity increases sprouting activity (Abraham et al., 2009; Kroll et al., 2009). This is also supported by the report that inhibition of NM2 activity by either the myosin inhibitor blebbistatin or the Rho kinase inhibitor Y27632 increases branching activity of endothelial cells cultured in a collagen gel (Fischer et al., 2009). Since the initiation of branching is usually observed in an area with reduced NM2 activity, it is proposed that the inhibition of NM2 activity by inhibition of ROCK promotes the initiation of branching. Here our study shows that tip endothelial cells in wild-type sprouts generate blebbing activities and that these blebs are highly dynamic, but usually do not form stable protrusions. Once protruded, they quickly retract. However when NM2A is ablated or NM2 activity is inhibited, the tip cells show no blebbing dynamics, instead they form excessive cortical protrusions. These results are consistent with the role of NM2 facilitating retraction of cortical protrusions, rather than inhibiting the initiation of cortical protrusions. This is further supported by our findings that the intensity of NM2A increases during retraction of cortical protrusion. The role of NM2 in the retraction of cortical protrusions has been proposed during border cell migration in Drosophila showing that loss of NM2 leads to the development of multiple cortical protrusions causing a defect in directional migration of the border cells (Mishra et al., 2019). Similar to NM2’s role in protrusion retraction in migrating cells, NM2 drives membrane integration into the apical plasma membrane of large secretory granules following exocytosis in the salivary gland (Milberg et al., 2017). Alternatively, instead of a role for NM2 in maintaining cortical stability, NM2 may in general regulate cytoskeletal structures. Ablation of NM2 disrupts actomyosin stress-fiber formation and stabilizes microtubules, thereby altering mechanical properties of the cell cortex. Disruption of actomyosin structures could lead to the release of monomeric actin from stress-fibers which then provides an extra pool available for actin nucleators to drive cortical protrusion (Lomakin et al., 2015; Rotty et al., 2015; Suarez et al., 2015; Beach et al., 2017). Delineating this possibility will be very important in the future.
Failure to prevent excessive cortical protrusions not only leads to the increased branching of blood vessels seen in NM2 ablated mice, it also impairs persistence of directed collective sprouting as manifested by frequent changes of migration directions. The impaired directional migration could contribute to the delayed vascular coverage seen in NM2 ablated mice. NM2 regulation of the direction of cell migration has been demonstrated both in vivo, such as in germ cell migration in zebrafish (Blaser et al., 2006) and in cultured MEF cells (Conti et al., 2004; Lo et al., 2004). Ablation of NM2 or inhibition of NM2 activity impairs the persistence of migration, but does not affect (often even increases) the speed of cell movement. During collective cell migration, the leader cells positioned at the front generate lamellipodial and filapodial protrusions, which sense, lead and power the migration (Reffay et al., 2014; Mayor and Etienne-Manneville, 2016). NM2 contributes to the traction forces at the front of migration, as well as to stabilization of the cortical surface to steer the direction of migration. Our results from EB angiogenic sprouting show that ablation of NM2 affects the directionality, but not the speed of sprouting migration. Thus NM2 plays a major role in maintaining the persistence of migration in vascular sprouting. In a 3D biomimetic model of angiogenesis using HUVECs, Yoon et al. (2019) reported that deletion of NM2A expression or inhibition of NM2 by blebbistatin led to scattering of HUVECs from sprouts, but does not affect cell invasion (Yoon et al., 2019). In our study of mice in vivo and in EB sprouting in culture, we do not see remarkable scattering of endothelial cells in NM2 ablated or blebbistatin treated sprouts. This may partially be due to the difference of our experimental models. Using 3D sprouting of HUVEC aggregates in collagen gels, we also find that inhibition of NM2 by blebbistatin or Y27632 results in cell scattering from sprouts and disruption of VE-cadherin junctions in sprouts (our unpublished data).
NM2 ablation in endothelial cells affects directional migration and sprouting activity during vascular development, however, the general pattern of the developing vasculature is not affected either in the back skin or the heart. Therefore NM2 is not required for sensing during vascular sprouting. It is believed that lamellipodial and filopodial structures function to sense the angiogenic stimulus thereby guiding vascular sprouts (Gerhardt et al., 2003; Lamalice et al., 2007; De Smet et al., 2009). NM2 is one of the major cytoplasmic motors regulating lamellipodial and filopodial structures (Burnette et al., 2011; Alieva et al., 2019). Ablation of NM2 or inhibition of NM2 activity greatly disrupts filopodium formation and lamellipodium dynamics in vascular sprouts, but does not affect vascular sprouting toward its destination. Therefore, NM2 regulation of endothelial filopodial and lamellipodial structures is not important for sensing the angiogenic stimulus during angiogenesis. Whether filopodia and lamellipodia are important or other regulators are required for sensing remains to be determined.
Our results also reveal that the relative contribution of NM2 paralogs to the development of the skin vasculature and cardiac coronary vasculature is not the same. Although NM2A in general plays the major role, the contribution of NM2B is more significant in coronary vascular development compared with skin vascular development. In addition, RhoA activated Rho kinase is important for coronary vascular development, but is not as important for the skin vasculature. Different contribution of NM2B in the regulation of the skin and coronary vasculatures may partially be related to the different mechanical environments of the developing vasculatures between the skin and heart. The cardiac coronary vasculature encounters a relative stiff environment compared with the skin vasculature. On the other hand, NM2B is more suitable to generate sustained tension and shows an increased mechanoresponsiveness due to its prolonged duty-ratio and increased load-dependent force generation, when compared with NM2A (Kovacs et al., 2007; Billington et al., 2013; Schiffhauer et al., 2019). This is consistent with the idea that vascular development in different organs is regulated by different signaling pathways and molecular motors.
MATERIALS AND METHODS
Animals
Aflox/Aflox, Bflox/Bflox, B–/B–, AGFP/AGFP, and BGFP/BGFP mice were generated as previously described (Tullio et al., 1997; Bao et al., 2007; Ma et al., 2009; Jacobelli et al., 2010; Zhang et al., 2012) and are available through the Mutant Mouse Regional Resource Centers (MMRRC: #032096, #016981, #16991, #037506, and #037053). RhoAflox/RhoAflox mice were generated as previously reported (Melendez et al., 2011). Tie2–cre mice were obtained from Jackson Laboratory (stock #004128). Cdk5–CreER mice were generated as described (Okabe et al., 2014). To obtain homozygous ablated mice (flox/flox;Cre+), a heterozygous male containing one allele of Cre (+/flox;Cre+/-) was crossed with a double-floxed female (flox/flox). Therefore the homozygous ablated mouse only expressed one Cre allele. All procedures were conducted using an approved animal protocol (H0053R3) in accordance with National Heart, Lung, and Blood Institute Animal Care and Use Committee guidelines.
Wholemount immunofluorescence staining of vasculature
Mouse embryos were collected in phosphate-buffered saline (PBS) and directly immersed in 4% paraformaldehyde (PFA) in PBS (pH 7.4) at 4°C overnight. Following fixation, the samples were washed with PBS and stored in PBS at 4°C for further analyses. Back skins and hearts were dissected from fixed embryos and then stained by wholemount immunofluorescence staining as previously described (Yamazaki et al., 2017). The following primary antibodies were used in this study: polyclonal antibodies against NMHC 2A (1:1,000, Biolegend), NMHC2B (1:3,000, Biolegend), p-paxillin (1:1,000; BD Transduction Laboratories); monoclonal antibodies against PECAM1 (CD31, 1:200, BD Pharmingen), GFP (1:200, ab183734, Abcam). Fluorescence secondary antibodies used were Alexa 488 goat anti-rabbit IgG or Alexa 594 goat anti-mouse IgG (1:250, Invitrogen, Carlsbad, CA). Confocal microscopy was carried out either on a Leica TCS SP5 or on a Zeiss LSM 880. In all cases, when possible, comparison was made among littermates. For each genotype we analyzed at least three to five mice. We found no abnormalities in NM2A and NM2B single heterozygous mice or NM2A/2B double heterozygous mice compared with the littermates that expressed no Cre. Littermates containing no cre recombinase were used as controls for conditional ablated mice.
In vitro EB angiogenic sprouting
EB angiogenic sprouting was performed as previously described (Jakobsson et al., 2010). Mouse embryonic stem (ES) cells were cultured in gelatin-coated culture dishes containing 100 U/ml leukemia inhibitory factor in ES culture medium. EB were prepared in a low-attachment 96-well plate. 500 ES cells (in 200 μl volume) were added into each well and cultured in differentiating medium (ES medium without LIF) for 4 d. EB were then collected and sandwiched in 1.5% collagen I gel (Purecol, INAMED biomaterials) to induce angiogenic sprouting in differentiation medium containing 30 ng/ml recombinant VEGFA-165 (Preprotech) in a 12-well culture plate. The sprouts were fixed in 4%PFA and analyzed by staining with the various antibodies at 7 d after culture. For live cell imaging, sprouts were analyzed between days 3 and 5 using an Olympus X-70 microscope.
Quantification and statistical analyses
AngioTool (Zudaire et al., 2011) was used to characterize the vasculature in the back skin and in vitro EB angiogenic sprouting following CD31 staining to reveal the vascular network. NIH ImageJ was used to analyze live cell imaging, fluorescence intensity of staining, and avascular areas. Data are expressed as means ± SD. Student’s t test was performed to compare two means. One-way ANOVA (post-Tukey) was used when comparing more than two means.
Supplementary Material
Acknowledgments
We thank Mary Anne Conti and Sachiyo Kawamoto and members of the Laboratory of Molecular Cardiology for their critical comments on the manuscript. We also thank Fang Zhang and Yubin Du (National Heart, Lung, and Blood Institute [NHLBI] Transgenic Core) and Christian A. Combs and Daniela Malide (NHLBI Light Microscopy Core). Dalton Saunders provided technical assistance.
Abbreviations used:
- E
embryonic day
- EB
embryoid body
- NM
nonmuscle myosin
- pPaxillin
phospho-paxillin.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E20-03-0175) on June 17, 2020.
REFERENCES
- Abraham S, Yeo M, Montero-Balaguer M, Paterson H, Dejana E, Marshall CJ, Mavria G. (2009). VE–cadherin-mediated cell–cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol , 668–674. [DOI] [PubMed] [Google Scholar]
- Alieva NO, Efremov AK, Hu S, Oh D, Chen Z, Natarajan M, Ong HT, Jegou A, Romet-Lemonne G, Groves JT, et al. (2019). Myosin IIA and formin dependent mechanosensitivity of filopodia adhesion. Nat Commun , 3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo-Urarte A, Casado P, Castillo SD, Kobialka P, Kotini MP, Figueiredo AM, Castel P, Rajeeve V, Mila-Guasch M, Millan J, et al. (2018). Endothelial cell rearrangements during vascular patterning require PI3-kinase-mediated inhibition of actomyosin contractility. Nat Commun , 4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Ma X, Liu C, Adelstein RS. (2007). Replacement of nonmuscle myosin II-B with II-A rescues brain but not cardiac defects in mice. J Biol Chem , 22102–22111. [DOI] [PubMed] [Google Scholar]
- Beach JR, Bruun KS, Shao L, Li D, Swider Z, Remmert K, Zhang Y, Conti MA, Adelstein RS, Rusan NM, et al. (2017). Actin dynamics and competition for myosin monomer govern the sequential amplification of myosin filaments. Nat Cell Biol , 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach JR, Shao L, Remmert K, Li D, Betzig E, Hammer JA., 3rd (2014). Nonmuscle myosin II isoforms coassemble in living cells. Curr Biol , 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JS, Powell BC, Cheney RE. (2001). A millennial myosin census. Mol Biol Cell , 780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington N, Wang A, Mao J, Adelstein RS, Sellers JR. (2013). Characterization of three full-length human nonmuscle myosin II paralogs. J Biol Chem , 33398–33410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser H, Reichman-Fried M, Castanon I, Dumstrei K, Marlow FL, Kawakami K, Solnica-Krezel L, Heisenberg CP, Raz E. (2006). Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev Cell , 613–627. [DOI] [PubMed] [Google Scholar]
- Bryan BA, Dennstedt E, Mitchell DC, Walshe TE, Noma K, Loureiro R, Saint-Geniez M, Campaigniac JP, Liao JK, D’Amore PA. (2010). RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J , 3186–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette DT, Manley S, Sengupta P, Sougrat R, Davidson MW, Kachar B, Lippincott-Schwartz J. (2011). A role for actin arcs in the leading-edge advance of migrating cells. Nat Cell Biol , 371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Ehling M, Marz S, Seebach J, Tarbashevich K, Sixta T, Pitulescu ME, Werner AC, Flach B, Montanez E, et al. (2017). Polarized actin and VE–cadherin dynamics regulate junctional remodelling and cell migration during sprouting angiogenesis. Nat Commun , 2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. (2011). Molecular mechanisms and clinical applications of angiogenesis. Nature , 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. (2004). Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem , 41263–41266. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. (2001). Taking cell–matrix adhesions to the third dimension. Science , 1708–1712. [DOI] [PubMed] [Google Scholar]
- De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P. (2009). Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. Arterioscler Thromb Vasc Biol , 639–649. [DOI] [PubMed] [Google Scholar]
- Eilken HM, Adams RH. (2010). Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol , 617–625. [DOI] [PubMed] [Google Scholar]
- Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. (2007). Myosin IIA regulates cell motility and actomyosin–microtubule crosstalk. Nat Cell Biol , 299–309. [DOI] [PubMed] [Google Scholar]
- Fischer RS, Gardel M, Ma X, Adelstein RS, Waterman CM. (2009). Local cortical tension by myosin II guides 3D endothelial cell branching. Curr Biol , 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaengel K, Niaudet C, Hagikura K, Lavina B, Muhl L, Hofmann JJ, Ebarasi L, Nystrom S, Rymo S, Chen LL, et al. (2012). The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell , 587–599. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. (2003). VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol , 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb E, Ma X, Jana SS, Preston YA, Kawamoto S, Shoham NG, Goldin E, Conti MA, Sellers JR, Adelstein RS. (2004). Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J Biol Chem , 2800–2808. [DOI] [PubMed] [Google Scholar]
- Heissler SM, Manstein DJ. (2013). Nonmuscle myosin-2: mix and match. Cell Mol Life Sci , 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuze ML, Sankara Narayana GHN, D’Alessandro J, Cellerin V, Dang T, Williams DS, Van Hest JC, Marcq P, Mege RM, Ladoux B. (2019). Myosin II isoforms play distinct roles in adherens junction biogenesis. Elife . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzle MK, Svitkina T. (2012). The cytoskeletal mechanisms of cell–cell junction formation in endothelial cells. Mol Biol Cell , 310–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobelli J, Friedman RS, Conti MA, Lennon-Dumenil AM, Piel M, Sorensen CM, Adelstein RS, Krummel MF. (2010). Confinement-optimized three-dimensional T cell amoeboid motility is modulated via myosin IIA-regulated adhesions. Nat Immunol , 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, et al. (2010). Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol , 943–953. [DOI] [PubMed] [Google Scholar]
- Kamijo H, Matsumura Y, Thumkeo D, Koike S, Masu M, Shimizu Y, Ishizaki T, Narumiya S. (2011). Impaired vascular remodeling in the yolk sac of embryos deficient in ROCK-I and ROCK-II. Genes Cells , 1012–1021. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Thirumurugan K, Knight PJ, Sellers JR. (2007). Load-dependent mechanism of nonmuscle myosin 2. Proc Natl Acad Sci USA , 9994–9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J, Epting D, Kern K, Dietz CT, Feng Y, Hammes HP, Wieland T, Augustin HG. (2009). Inhibition of Rho-dependent kinases ROCK I/II activates VEGF-driven retinal neovascularization and sprouting angiogenesis. Am J Physiol Heart Circ Physiol , H893–H899. [DOI] [PubMed] [Google Scholar]
- Lamalice L, Le Boeuf F, Huot J. (2007). Endothelial cell migration during angiogenesis. Circ Res , 782–794. [DOI] [PubMed] [Google Scholar]
- Liu J, Wada Y, Katsura M, Tozawa H, Erwin N, Kapron CM, Bao G, Liu J. (2018). Rho-associated coiled-coil kinase (ROCK) in molecular regulation of angiogenesis. Theranostics , 6053–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. (2010). Mechanical tugging force regulates the size of cell–cell junctions. Proc Natl Acad Sci USA , 9944–9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CM, Buxton DB, Chua GC, Dembo M, Adelstein RS, Wang YL. (2004). Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol Biol Cell , 982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakin AJ, Lee KC, Han SJ, Bui DA, Davidson M, Mogilner A, Danuser G. (2015). Competition for actin between two distinct F-actin networks defines a bistable switch for cell polarization. Nat Cell Biol , 1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Adelstein RS. (2014). The role of vertebrate nonmuscle Myosin II in development and human disease. Bioarchitecture , 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Jana SS, Conti MA, Kawamoto S, Claycomb WC, Adelstein RS. (2010). Ablation of nonmuscle myosin II-B and II-C reveals a role for nonmuscle myosin II in cardiac myocyte karyokinesis. Mol Biol Cell , 3952–3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Sung DC, Yang Y, Wakabayashi Y, Adelstein RS. (2017). Nonmuscle myosin IIB regulates epicardial integrity and epicardium-derived mesenchymal cell maturation. J Cell Sci , 2696–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Takeda K, Singh A, Yu ZX, Zerfas P, Blount A, Liu C, Towbin JA, Schneider MD, Adelstein RS, Wei Q. (2009). Conditional ablation of nonmuscle myosin II-B delineates heart defects in adult mice. Circ Res , 1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor R, Etienne-Manneville S. (2016). The front and rear of collective cell migration. Nat Rev Mol Cell Biol , 97–109. [DOI] [PubMed] [Google Scholar]
- Melendez J, Stengel K, Zhou X, Chauhan BK, Debidda M, Andreassen P, Lang RA, Zheng Y. (2011). RhoA GTPase is dispensable for actomyosin regulation but is essential for mitosis in primary mouse embryonic fibroblasts. J Biol Chem , 15132–15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberg O, Shitara A, Ebrahim S, Masedunskas A, Tora M, Tran DT, Chen Y, Conti MA, Adelstein RS, Ten Hagen KG, Weigert R. (2017). Concerted actions of distinct nonmuscle myosin II isoforms drive intracellular membrane remodeling in live animals. J Cell Biol , 1925–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AK, Mondo JA, Campanale JP, Montell DJ. (2019). Coordination of protrusion dynamics within and between collectively migrating border cells by myosin II. Mol Biol Cell , 2490–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe K, Kobayashi S, Yamada T, Kurihara T, Tai-Nagara I, Miyamoto T, Mukouyama YS, Sato TN, Suda T, Ema M, Kubota Y. (2014). Neurons limit angiogenesis by titrating VEGF in retina. Cell , 584–596. [DOI] [PubMed] [Google Scholar]
- Park JA, Atia L, Mitchel JA, Fredberg JJ, Butler JP. (2016). Collective migration and cell jamming in asthma, cancer and development. J Cell Sci , 3375–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phng LK, Gerhardt H. (2009). Angiogenesis: a team effort coordinated by notch. Dev Cell , 196–208. [DOI] [PubMed] [Google Scholar]
- Reffay M, Parrini MC, Cochet-Escartin O, Ladoux B, Buguin A, Coscoy S, Amblard F, Camonis J, Silberzan P. (2014). Interplay of RhoA and mechanical forces in collective cell migration driven by leader cells. Nat Cell Biol , 217–223. [DOI] [PubMed] [Google Scholar]
- Rotty JD, Wu C, Haynes EM, Suarez C, Winkelman JD, Johnson HE, Haugh JM, Kovar DR, Bear JE. (2015). Profilin-1 serves as a gatekeeper for actin assembly by Arp2/3-dependent and -independent pathways. Dev Cell , 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauteur L, Krudewig A, Herwig L, Ehrenfeuchter N, Lenard A, Affolter M, Belting HG. (2014). Cdh5/VE–cadherin promotes endothelial cell interface elongation via cortical actin polymerization during angiogenic sprouting. Cell Rep , 504–513. [DOI] [PubMed] [Google Scholar]
- Schiffhauer ES, Ren Y, Iglesias VA, Kothari P, Iglesias PA, Robinson DN. (2019). Myosin IIB assembly state determines its mechanosensitive dynamics. J Cell Biol , 895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutova MS, Spessott WA, Giraudo CG, Svitkina T. (2014). Endogenous species of mammalian nonmuscle myosin IIA and IIB include activated monomers and heteropolymers. Curr Biol , 1958–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. (2010). Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol , 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C, Carroll RT, Burke TA, Christensen JR, Bestul AJ, Sees JA, James ML, Sirotkin V, Kovar DR. (2015). Profilin regulates F-actin network homeostasis by favoring formin over Arp2/3 complex. Dev Cell , 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymborska A, Gerhardt H. (2018). Hold me, but not too tight–endothelial cell–cell junctions in angiogenesis. Cold Spring Harb Perspect Biol . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Kishi H, Ma X, Yu ZX, Adelstein RS. (2003). Ablation and mutation of nonmuscle myosin heavy chain II-B results in a defect in cardiac myocyte cytokinesis. Circ Res , 330–337. [DOI] [PubMed] [Google Scholar]
- Tullio AN, Accili D, Ferrans VJ, Yu ZX, Takeda K, Grinberg A, Westphal H, Preston YA, Adelstein RS. (1997). Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc Natl Acad Sci USA , 12407–12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullio AN, Bridgman PC, Tresser NJ, Chan CC, Conti MA, Adelstein RS, Hara Y. (2001). Structural abnormalities develop in the brain after ablation of the gene encoding nonmuscle myosin II-B heavy chain. J Comp Neurol , 62–74. [DOI] [PubMed] [Google Scholar]
- Wang A, Ma X, Conti MA, Liu C, Kawamoto S, Adelstein RS. (2010). Nonmuscle myosin II isoform and domain specificity during early mouse development. Proc Natl Acad Sci USA , 14645–14650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer R, Cseh B, Maier B, Scherrer K, Baccarini M. (2012). Angiogenic sprouting requires the fine tuning of endothelial cell cohesion by the Raf-1/Rok-alpha complex. Dev Cell , 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Nalbandian A, Uchida Y, Li W, Arnold TD, Kubota Y, Yamamoto S, Ema M, Mukouyama YS. (2017). Tissue myeloid progenitors differentiate into pericytes through TGF-beta signaling in developing skin vasculature. Cell Rep , 2991–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C, Choi C, Stapleton S, Mirabella T, Howes C, Dong L, King J, Yang J, Oberai A, Eyckmans J, Chen CS. (2019). Myosin IIA-mediated forces regulate multicellular integrity during vascular sprouting. Mol Biol Cell , 1974–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Conti MA, Malide D, Dong F, Wang A, Shmist YA, Liu C, Zerfas P, Daniels MP, Chan CC, et al. (2012). Mouse models of MYH9-related disease: mutations in nonmuscle myosin II-A. Blood , 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu C, Adelstein RS, Ma X. (2018). Replacing nonmuscle myosin 2A with myosin 2C1 permits gastrulation but not placenta vascular development in mice. Mol Biol Cell , 2326–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zudaire E, Gambardella L, Kurcz C, Vermeren S. (2011). A computational tool for quantitative analysis of vascular networks. PLoS One , e27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.