Women who were treated with chemotherapy for breast cancer became less active throughout the first half of chemotherapy, but then activity leveled off and did not reduce more in the second half of chemotherapy.
Keywords: Cancer, Oncology, Breast cancer, Exercise, Physical activity, Chemotherapy
Abstract
Despite many potential benefits of physical activity during and after breast cancer treatment, activity levels typically decline from pre- to posttreatment. Most previous research has relied on self-reported activity. The purpose of this study were to assess patterns of daily, to objectively measured physical activity throughout chemotherapy for breast cancer, and to identify predictors of physical activity patterns. Participants were given a Fitbit before starting chemotherapy and asked to wear it throughout chemotherapy. Restricted cubic splines assessed nonlinear patterns of Fitbit measured total physical activity (TPA) and moderate-to-vigorous physical activity (MVPA) throughout the duration of chemotherapy (mean = 17 weeks, standard deviation [SD] = 6.3). Mixed-effects regression models assessed the rate of physical activity decline. Regressions of subject-level random slope assessed predictors of the rate of physical activity decline on participant and cancer characteristics and self-reported physical and cognitive functioning. Participants (n = 32) were on average 50 years old; the majority had stage II breast cancer. MVPA declined linearly at a mean rate of 1.4 min/day (p = .002) for every 10% of chemotherapy completed, whereas TPA declined linearly at an average rate of 13.4 min/day (p = .0007) for every 10% of chemotherapy completed, until around halfway through chemotherapy, when activity rates leveled off. HER+ receptor status was associated with a greater rate of MVPA decline, β = 13.3, p = .04. This novel study of objectively measured daily MVPA throughout chemotherapy showed that most reductions in activity occurred during the first half of a course of chemotherapy. Targeting this early period of chemotherapy may be important for preventing declines in activity levels throughout chemotherapy.
Implications.
Practice: Exercise interventions during chemotherapy for breast cancer, particularly in the first half of treatment, may be important for preventing declines in physical activity often observed from pre- to post-chemotherapy.
Policy: Policymakers who want to improve the health and well-being of the growing number of women diagnosed with breast cancer should consider supporting legislation that allocates funding for physical activity interventions during chemotherapy treatment, when women may need extra support to stay active.
Research: Future research should recruit women early enough to objectively measure trends in activity starting before breast cancer diagnosis, which could improve our understanding of when to intervene to prevent declines in physical activity.
INTRODUCTION
More than 3.1 million women are currently living with a diagnosis of breast cancer in the United States [1]. The increased breast cancer survival rate has necessitated a shift in cancer care toward supporting quality of life during and after treatment. About 40%–75% of women diagnosed with breast cancer receive chemotherapy [1], which is associated with negative side effects, including fatigue, nausea, disturbed sleep, decreased activity, and weight gain [2–4]. Patients with breast cancer treated with chemotherapy often report poorer quality of life, including impaired cognitive and physical functioning, compared to patients with breast cancer not treated with chemotherapy [5–8]. Data from animal and human studies indicate that physical activity after a diagnosis of breast cancer may counteract some of these negative effects and increase disease-free survival, thus improving quality of life for breast cancer survivors [9–14].
Despite many potential benefits of physical activity during and after breast cancer treatment, activity levels typically decline from pre- to posttreatment [15–19]. Most existing research on physical activity in breast cancer has focused on the posttreatment period. These studies have found activity levels to be lower than healthy controls and well below recommended levels [20–24]. Numerous observational studies have examined patterns of physical activity during active treatment for breast cancer [15–19,25–27]. However, to our knowledge, all studies but one have relied on self-reported activity levels and therefore could only capture periodic, often retrospective, snapshots of activity. Despite these limitations, most studies agree that physical activity decreases from pre- to posttreatment [15–19] and that women who receive chemotherapy have greater declines [15,19,26].
Numerous patient-related factors have been assessed as predictors of physical activity decline during chemotherapy, including age and obesity, but findings are mixed [15,17–19]. Impairments in cognitive [28] and physical functioning [29] are highly prevalent among women undergoing treatment for breast cancer and have been associated with physical activity levels [30,31]. To date, no published studies have examined the relationship of pretreatment cognitive or physical functioning with trajectory of objectively measured physical activity during the treatment for breast cancer. Given that physical activity during treatment may increase the efficacy of chemotherapy [32,33], identifying patterns and predictors of physical activity during chemotherapy can have a significant public health impact by facilitating a deeper understanding of key points and populations for intervention.
The Activity in Treatment pilot study assessed breast cancer patients’ daily physical activity levels using objective measurement throughout chemotherapy. Women were recruited before starting chemotherapy. Participants were given a wrist-worn activity tracker (Fitbit Charge HR) to wear throughout the duration of chemotherapy. Daily activity data and chemotherapy information were gathered to assess natural trends in free-living physical activity throughout chemotherapy, and health and participant characteristics associated with changes in activity. Building on evidence that physical activity is beneficial during chemotherapy [14], yet is decreased in breast cancer survivors [20–24], these in-depth data will identify critical times for intervention to prevent physical activity declines.
METHODS
Study design
This longitudinal pilot study recruited patients with breast cancer who were scheduled for but had not yet started chemotherapy at a comprehensive cancer center. Data were collected from December 2015 to February 2018. Potential participants were first telephone-screened for eligibility based on the following inclusion criteria: (1): diagnosed with breast cancer; (2) scheduled to receive chemotherapy, but had not yet started; (3) receiving chemotherapy at the cancer center; (4) willing to wear a Fitbit throughout chemotherapy; (5) access to Fitbit-compatible computer, phone, or tablet; (6) able to read and communicate in English; (7) aged 21–85 years; and (8) no serious physical limitations that greatly limited mobility.
Eligible participants attended one in-person visit before initiating chemotherapy. Participants were provided a wrist-worn activity tracker, the Fitbit Charge HR. They were asked to wear the Fitbit for 24 hr/day throughout chemotherapy and to sync it at least once a week. Participants were asked not to alter their activity in any way. At this visit, baseline questionnaires were completed. At study completion, data from electronic medical records were collected, including cancer characteristics at diagnosis, surgery type, chemotherapy regimen, and date of each infusion visit. The study was approved by the institutional review board and all participants provided written informed consent.
Measures
Fitbit measured physical activity
Physical activity data were collected through the Fitbit Charge HR, an accelerometer-based activity meter that collects data on intensity of physical activity at the minute level. Initial validation studies of the Fitbit Charge HR have shown strong agreement with ActiGraph GT3X-measured moderate-to-vigorous physical activity (MVPA) [34,35]. The Fitbit is watch-sized, provides a digital clock, is wrist-worn, and holds 20 days of data. Fitbit data (physical activity and heart rate) were accessed through Fitabase, a web-based database program that collects physical activity, heart rate, and sleep data from the Fitbit cloud. Study staff monitored Fitabase weekly to ensure that the Fitbit was being synced and charged. Participants were contacted if they had not synced for at least 1 week or if battery charge was low. Fitbit’s proprietary algorithm classifies the types of activity over a 24-hr day, with each minute classified as being asleep, sedentary, or in light, moderate, or vigorous activity. Activity level by minute was downloaded and cleaned using R [36] to calculate daily total physical activity (TPA) minutes, MVPA minutes, and total non-sleep wear minutes. Non-wear time was determined by lack of heart rate and activity (steps or intensity) at any given minute. Days were considered “valid” if there was any wear recorded (defined as >5 min of wear). This 5-min threshold for a valid Fitbit wear day was used to avoid recording days where the Fitbit had simply been picked up and moved from one place to another, while simultaneously avoiding excluding days where the participant had purposefully worn the Fitbit. Analyses used inverse wear time weighting, which has been validated to account for missing wear without excluding days based on minimum wear hours in a day [37].
Chemotherapy
We defined chemotherapy time in terms of the original chemotherapy plan. Participants who, after completing or stopping their original planned chemotherapy, were switched to a different chemotherapy regimen and given a new chemotherapy plan, were considered finished with the original chemotherapy plan for the purposes of our analyses. The first day of chemotherapy was defined as the first day of infusions and the final day was defined as 14 days after the last infusion of the original chemotherapy plan. Fourteen days was chosen because it was the mean length of time between infusions.
Other measures
Medical charts were abstracted to obtain information regarding cancer diagnosis and treatment (cancer stage at diagnosis, receptor status, date of each infusion), height, and weight (to calculate body mass index [BMI]). Baseline questionnaires were used to collect demographic data as well as self-reported cognitive and physical functioning from the Patient-Reported Outcomes Measurement Information System (PROMIS) short form questionnaires. The PROMIS short form questionnaires are fixed-length measures that assess the full range of symptom severity [38]. Questions evaluate symptoms over the past 7 days on a 5-point ordinal Likert scale (never, rarely, sometimes, often, always). Cognitive functioning was assessed with six items and physical functioning was assessed with four items. PROMIS scores are standardized with a mean of 50 and an SD of 10, with higher scores representing higher cognitive or physical functioning [39].
Data analyses
Descriptive statistics (mean/SD or n/%) were calculated for demographics, self-reported functioning, and cancer-related variables. We also calculated mean and standard deviation (SD) for the number of days participants wore the Fitbit and the percentage of chemotherapy days with Fitbit wear. We [1] calculated the mean and SD for each participant’s average minutes/day of MVPA, TPA, and hours/day of non-sleep wear overall; and [2] calculated these values during the time pertaining to 0%–10%, 45%–55%, and 90%–100% of chemotherapy completed. Given variation in chemotherapy lengths among participants, the percentage of treatment that had been completed at each day (percentage chemotherapy completed) was used as the time variable to standardize the time axis, with all analyses controlling for the total number of chemotherapy infusions. Patterns of physical activity were examined graphically by plotting the average day level physical activity (MVPA or TPA) by percentage of chemotherapy completed, with a rolling average of physical activity overlaid as a line to help visualize trends.
Statistical analysis
Restricted cubic splines (RCS) were used to model patterns of change in physical activity (MVPA and TPA) throughout chemotherapy (modeled as the percentage of chemotherapy completed) while allowing for nonlinearity. RCS models included three knots as determined by comparison of the quasi-information criterion. The three knots were located at pre-specified locations according to the percentiles of the distribution of percentage of chemotherapy completed, the 5th, 50th, and 95th percentiles [40]. The RCS model was carried out using mixed-effects regression with a subject-level random intercept and slope. A test for nonlinearity was carried out by comparing the log likelihoods of the model containing the knots with the linear model. RCS were carried out using PROC GLIMMIX, SAS, version 9.4 (SAS Institute Inc., Cary, NC). Interpretation of nonlinear models was done graphically by plotting predicted physical activity (MVPA or TPA), based on the RCS model, on the y-axis by percentage of chemotherapy completed on the x-axis.
After visual inspection of the RCS model, we assessed the linear change in physical activity throughout chemotherapy (modeled as the percentage of chemotherapy completed) for the visually ascertained linear range (0%–60% for MVPA and 0%–50% for TPA) using linear mixed-effects models with a subject-level random intercept and slope on percentage of chemotherapy completed. All models controlled for the confounding effects of total number of chemotherapy infusions, cancer stage, receptor status, education, baseline BMI, and age. All models accounted for wear time using inverse wear time weighting [37] and used an unstructured covariance structure.
We assessed seven possible predictors of the rate of physical activity change: participant characteristics (age, baseline BMI), cancer-related variables (receptor status, cancer stage, mastectomy), and baseline self-reported functioning (cognitive and physical). Predictors were assessed by first determining the subject-specific slope. Slopes were determined using a linear mixed-effects model of physical activity measures (MVPA and TPA; separately) on percentage of chemotherapy completed, with a subject-level random intercept and slope, controlling for total number of chemotherapy infusions and using inverse wear time weighting. The linear mixed-effects model was carried out on only the portion of chemotherapy determined to have a linear decline in activity based on visual assessment of the RCS model. We then regressed the subject-level slope on each of seven possible predictors: age, baseline BMI, receptor status, cancer stage, mastectomy, and baseline self-reported cognitive and physical functioning.
RESULTS
Study population
Eighty-one women were telephone-screened for eligibility. Of these, 33 were eligible and 32 completed a baseline visit. Ineligibility reasons included: already started chemotherapy (n = 19), change in treatment plan/no longer receiving chemotherapy (n = 12), not receiving chemotherapy at the cancer center (n = 5), uncomfortable reading/communicating in English (n = 3), and not interested/unable to comply with study procedures (n = 42).
Baseline characteristics of the 32 participants are outlined in Table 1. Participants were on average 50 years old, had a BMI of 28 kg/m2, and reported roughly average self-reported cognitive and physical functioning. Most participants had stage II cancer and received a lumpectomy. Participants received a mean of 10 chemotherapy infusions and had a mean chemotherapy duration of 17 weeks.
Table 1.
Characteristics of the sample of breast cancer patients (N = 32)
| Mean (SD) or n/% | |
|---|---|
| Demographics | |
| Age, years | 49.6 (10.72) |
| Body mass index, kg/m2 | 27.5 (6.03) |
| White | 25/78.1% |
| Latina | 5/15.6% |
| Education (% college graduates) | 20/62.5% |
| Married of living with partner | 17/53.1% |
| Employed | 22/68.8% |
| Self-reported functioning | |
| Cognitive | 51.9 (10.92) |
| Physical | 54.0 (6.11) |
| Cancer characteristics | |
| Disease stage | |
| Stage I | 5/15.6% |
| Stage II | 19/59.4% |
| Stage III | 6/18.8% |
| Stage IV | 2/6.3% |
| Receptor type | |
| HER2+ | 9/28.1% |
| ER+ or PR+, HER2- | 12/37.5% |
| Triple-negative (ER–, PR–, HER2–) | 11/34.4% |
| Pathologically positive lymph nodes | 18/58.1% |
| Mastectomy | 9/28.1% |
| Number of chemotherapy infusions | 9.7 (6.02) |
| Chemotherapy duration, weeks | 17.3 (6.26) |
Physical activity trends
Table 2 provides the distribution of Fitbit wear and average physical activity (minutes/day of TPA and MVPA) overall and at segments of chemotherapy corresponding to 0%–10%, 45%–55%, and 90%–100% of chemotherapy completed. On average, women wore the Fitbit for 84% (SD = 20.1) of their chemotherapy days and wore the Fitbit on average 13 (SD = 2.7) waking hours/day. Participants averaged 11 (SD = 9.4) minutes/day of MVPA and 189 (SD = 77.2) minutes/day of TPA.
Table 2.
Physical activity and Fitbit wear distribution in the sample of breast cancer patients (N = 32)
| Variable | Mean (SD) |
|---|---|
| Days with Fitbit weara | 102.5 (48.42) |
| Percent of chemotherapy days with Fitbit wear | 84.0 (20.10) |
| Chemotherapy overall | |
| Fitbit wear hr/dayb | 12.9 (2.69) |
| MVPA min/day | 10.5 (9.43) |
| Total PA min/day | 189.1 (77.15) |
| 0%–10% of chemotherapyc | |
| Fitbit wear hr/dayb | 13.9 (2.80) |
| MVPA min/day | 15.2 (15.42) |
| Total PA min/day | 203.5 (89.75) |
| 45%–55% of chemotherapyc | |
| Fitbit wear hr/dayb | 12.4 (4.11) |
| MVPA min/day | 9.7 (11.00) |
| Total PA min/day | 183.8 (82.82) |
| 90%–100% of chemotherapyc | |
| Fitbit wear hr/dayb | 12.8 (3.57) |
| MVPA min/day | 7.2 (8.72) |
| Total PA min/day | 172.4 (93.01) |
aDays with > 5 min of wear
bNon-sleep wear
cDistribution at proportion of chemotherapy completed
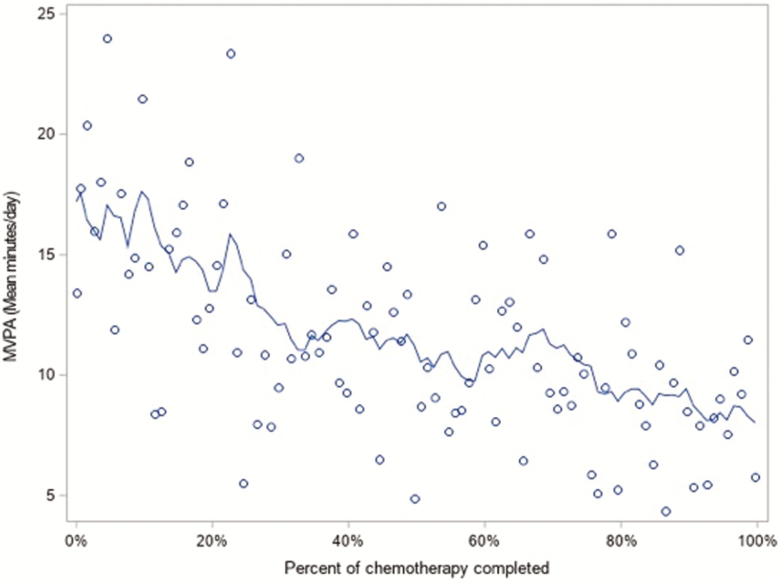
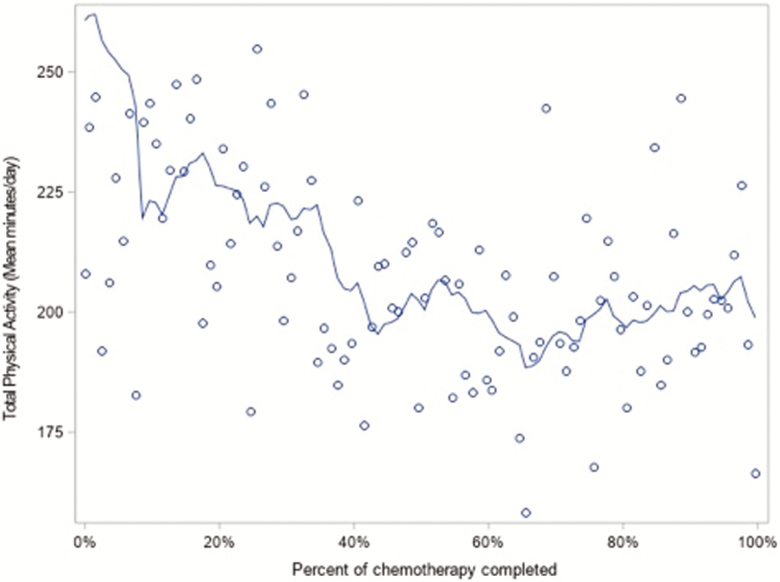
Figures 1 and 2 provide graphical representations of average day level physical activity by percentage of chemotherapy completed. Figure 1 shows that MVPA declines throughout chemotherapy, whereas Fig. 2 shows that TPA declines until about halfway through chemotherapy and then levels off. Because these figures appear to suggest a tapering off in the decline in activity, we assessed the significance of a nonlinear association between activity and percentage of chemotherapy completed.
Fig 1.
Distribution of moderate-to-vigorous physical activity by percent of chemotherapy completed with rolling average trend line, N = 32.
Fig 2.
Distribution of total physical activity by percent of chemotherapy completed with rolling average trend line, N = 32.
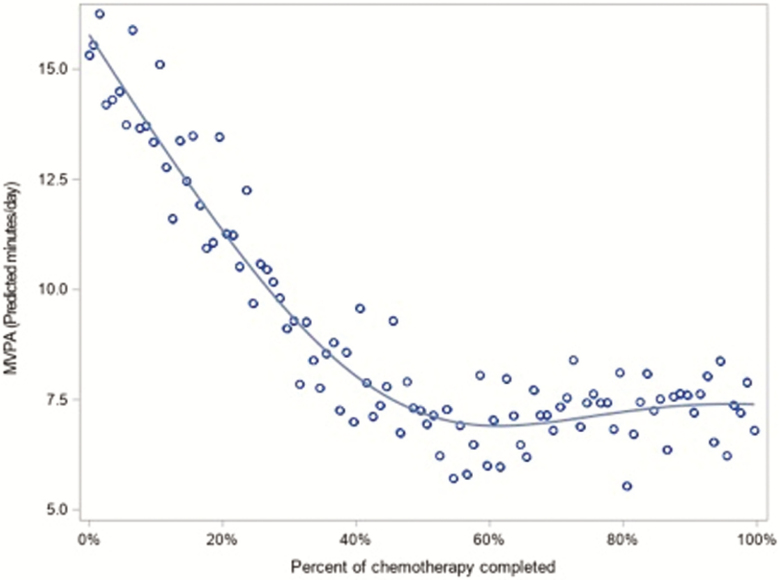
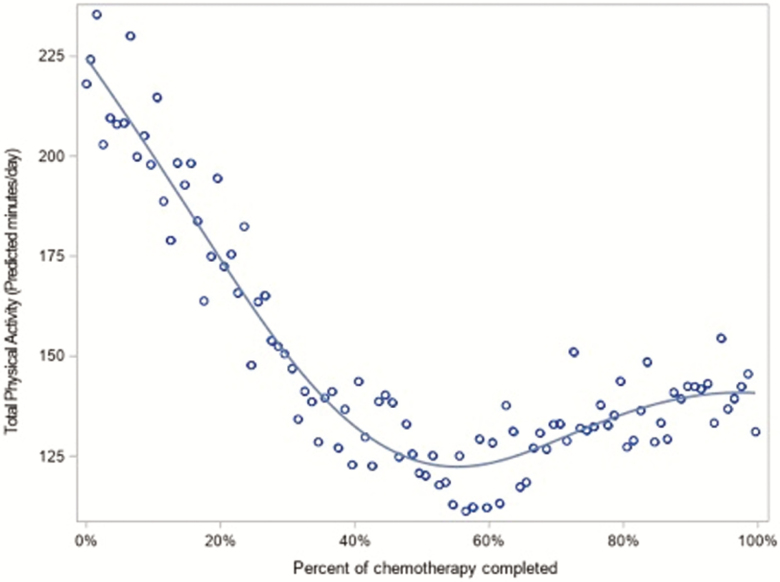
Nonlinear analysis of activity by percentage of chemotherapy completed shows a significant nonlinear association for both MVPA and TPA (both p < 0.001). Figures 3 and 4 provide graphical interpretations of the nonlinear model. In Fig. 3, MVPA declines roughly linearly until around 60% of chemotherapy and then levels off. In Fig. 4, TPA declines until around 50% of chemotherapy and then rebounds slightly.
Fig 3.
Nonlinear association between percent of chemotherapy completed and moderate-to-vigorous physical activity, N = 32.
Fig 4.
Nonlinear association between percent of chemotherapy completed and total physical activity, N = 32.
On the basis of our nonlinear analysis, we carried out statistical analysis of the linear change in activity by percentage of chemotherapy completed, during the portion of chemotherapy with a linear trend (0%–60% for MVPA and 0%–50% for TPA based on visual inspection of the nonlinear analysis). Linear analysis shows a significant decrease in MVPA and TPA as chemotherapy progresses (β = −14.2, p = .002 and β = −133.9, p = .0007). This can be interpreted as a 1.42 min/day decline in MVPA and a 13.39 min/day decline in TPA for every 10% of chemotherapy that elapses, until the physical activity decline plateaus, equating to a total decline of 8.5 min/day (1 hr/week) in MVPA and 67 min/day (7.8 hr/week) in TPA during the period of linear decline.
Predictors of physical activity
Age, BMI, receptor status, cancer stage, mastectomy, and baseline cognitive and physical functioning were assessed as predictors of the rate of physical activity decline. There were no significant predictors for TPA, although higher BMI did trend toward greater declines in TPA (β = −7.9, p = .09). For MVPA, receptor status was significant: those with a HER2+ receptor status had a significantly greater rate of decline than those with a HER2– and ER or PR+, or triple negative receptor status (β = 13.3, p = .04). However, when compared individually, the difference between HER2+ and those who were HER2- /ER+ or PR+ and between HER2+ and triple negative did not achieve significance (β = 14.1, p = .05, and β = 12.5, p = .09). This result is likely due to the relatively small sample size when split into three groups. No other variables were significant predictors for MVPA, although greater age and higher BMI did trend toward greater declines in MVPA (β = −0.42, p = .12 and β = −0.68, p = .16).
DISCUSSION
Using daily objective measurement of physical activity throughout chemotherapy, we observed a significant decline in MVPA and TPA from the start of chemotherapy until roughly halfway through treatment. Overall, participants decreased their activity from pre- to post-chemotherapy by 1 hr/week in MVPA and 8 hr/week in TPA during the decline. We also found that HER2+ receptor status, greater age, and higher BMI were the strongest predictors of decline in MVPA.
To our knowledge, this is the first published study to objectively measure physical activity for the entire duration of chemotherapy outside of a physical activity intervention [41]. The only other published observational study we are aware of that used objective measures of physical activity during chemotherapy [27] compared a snapshot of the first 5 days of a chemotherapy cycle to the second 5 days of a chemotherapy cycle among 28 patients with breast cancer. Our findings are consistent, as they also found significant decreases in minutes per day of MVPA (23.3 min/day to 21.9 min/day), but in contrast, they did not see any change in light activity (310 min/day to 313 min/day) [27]. Our results are also consistent with the largest published study to examine physical activity during treatment for breast cancer [19], (n = 19,696) which assessed retrospectively self-reported pre-diagnosis activity and activity at 6 months post-diagnosis. Our use of objective measures also expands upon previous findings by permitting daily measurement, allowing us to identify precise timing of changes in physical activity levels during chemotherapy.
Several studies have reported that the greatest declines in physical activity were seen in women who received chemotherapy [15,19,25]. Our study focused on breast cancer patients receiving chemotherapy and, unlike earlier studies, examined factors within this population that might signal an opportunity for increased intervention to thwart decline. We found that those with a HER2+ receptor status had significantly greater declines in MVPA, in contrast to other studies that examined this predictor [19]. This may be because we restricted enrollment to those receiving chemotherapy and/or due to the chemotherapy used for HER2+ cancers [42]. We also found that increased age and BMI were associated with greater, although nonsignificant, decreases in MVPA, both of which are consistent with previous findings [15,18,19]. Studies assessing activity levels in women only after the completion of treatment have found that physical activity in breast cancer survivors is below recommended levels [20,21,24,43] and below that of matched controls [22,23]. These previous studies report only small further decreases in activity following completion of treatment [21,24]. Coupled with our findings, this suggests that the decline in activity is occurring during treatment.
There are several limitations to our study. As this was a pilot study, the chief limitation is the small sample size. Despite this, the long duration of daily measurements allowed us to assess significance of the decline in activity. The assessment of physical activity throughout chemotherapy is also limited by the fact that women undergo chemotherapy regimens of varying lengths. We attempted to mitigate the impact of this variation on our analyses by changing the time scale from days to percent of chemotherapy completed at each day. In this way, we could avoid having extremes of time with only a few, potentially sicker, participants still completing their chemotherapy. In addition, because of the varied chemotherapy regimens in this small sample, we could not include type of chemotherapy as a variable. We also did not collect treatment-related side effects such as nausea and neuropathy. Future studies should consider including type of chemotherapy and treatment-related side effects as predictors of physical activity during chemotherapy.
Another possible limitation is the use of the Fitbit to measure activity. Because the Fitbit provides the user immediate feedback on activity and steps taken, women may have been more active than usual, potentially attenuating our findings. However, a previous trial found that a Fitbit alone did not significantly increase physical activity beyond that seen in controls [44]. A final limitation is the possibility that selection bias may have occurred. Because participants knew they would be provided a Fitbit, women more interested in/accustomed to physical activity may have self-selected to participate. One previous study showed that women with higher starting levels of activity had greater declines during treatment [19], thus our study may have seen greater declines than if no self-selection had occurred.
Despite limitations, using Fitbits to record activity offered considerable advantages. First, using an objective, versus self-report, measure to capture physical activity reduced possible bias due to recall or social desirability [45]. Second, the Fitbit monitors heart rate, making calculating wear time more accurate. Finally, the Fitbit is designed to be small, attractive, and acceptable for long-term use, which may have contributed to high compliance with wearing the device for the entirety of chemotherapy.
Given the World Health Organization’s recommendation of 150 min/week of MVPA [46], the decrease of 60 min/week of MVPA found in this study is alarming and likely contributes to the low activity levels seen among breast cancer survivors. These results indicate that interventions are needed early in chemotherapy to stem this decrease in activity and that Fitbits can be used to objectively measure activity long term. The next step, beyond replicating these findings in a larger cohort, is to recruit women early enough to objectively measure trends in activity starting before diagnosis. This would expand our understanding of the decline in objectively measured physical activity and provide a greater understanding of when to intervene. Finally, there is a need to design and evaluate tailored, targeted interventions to support patients that can be disseminated and implemented within healthcare systems.
To our knowledge, this is the first published study to objectively measure physical activity throughout the duration of chemotherapy for breast cancer. This study examined patterns in activity and identified when during chemotherapy declines in physical activity occur, a critical finding that could help clinicians assess when their patients are most likely to need extra support to stay active and counteract this drop in physical activity.
Funding:
This study was funded by the National Cancer Institute of the National Institutes of Health (U54CA155435) and a gift from Carol Vassiliadis and family. Dr. Hartman was supported by the National Cancer Institute (K07CA181323). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
Conflicts of interest: Sandahl H. Nelson, Lauren S. Weiner, Loki Natarajan, Barbara A. Parker, Ruth E. Patterson, and Sheri J. Hartman declare that they have no conflicts of interest.
Human Rights: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–271. [DOI] [PubMed] [Google Scholar]
- 2. Byar KL, Berger AM, Bakken SL, Cetak MA. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol Nurs Forum. 2006;33(1):E18–E26. [DOI] [PubMed] [Google Scholar]
- 3. Demark-Wahnefried W, Peterson BL, Winer EP, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19(9):2381–2389. [DOI] [PubMed] [Google Scholar]
- 4. de Jong N, Courtens AM, Abu-Saad HH, Schouten HC. Fatigue in patients with breast cancer receiving adjuvant chemotherapy: a review of the literature. Cancer Nurs. 2002;25(4):283–297; quiz 298. [DOI] [PubMed] [Google Scholar]
- 5. Ganz PA, Rowland JH, Meyerowitz BE, Desmond KA. Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results Cancer Res. 1998;152:396–411. [DOI] [PubMed] [Google Scholar]
- 6. Park BW, Lee S, Lee AR, Lee KH, Hwang SY. Quality of life differences between younger and older breast cancer patients. J Breast Cancer. 2011;14(2):112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kayl AE, Meyers CA. Side-effects of chemotherapy and quality of life in ovarian and breast cancer patients. Curr Opin Obstet Gynecol. 2006;18(1):24–28. [DOI] [PubMed] [Google Scholar]
- 8. Jim HS, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012;30(29):3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Courneya KS, Segal RJ, Mackey JR, et al. Effects of exercise dose and type on sleep quality in breast cancer patients receiving chemotherapy: a multicenter randomized trial. Breast Cancer Res Treat. 2014;144(2):361–369. [DOI] [PubMed] [Google Scholar]
- 10. Courneya KS, Segal RJ, McKenzie DC, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc. 2014;46(9):1744–1751. [DOI] [PubMed] [Google Scholar]
- 11. Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25(7):1293–1311. [DOI] [PubMed] [Google Scholar]
- 12. Carter SJ, Hunter GR, McAuley E, Courneya KS, Anton PM, Rogers LQ. Lower rate-pressure product during submaximal walking: a link to fatigue improvement following a physical activity intervention among breast cancer survivors. J Cancer Surviv. 2016;10(5):927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kang DW, Lee J, Suh SH, Ligibel J, Courneya KS, Jeon JY. Effects of exercise on insulin, igf axis, adipocytokines, and inflammatory markers in breast cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2017;26(3):355–365. [DOI] [PubMed] [Google Scholar]
- 14. Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9:CD005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Irwin ML, Crumley D, McTiernan A, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97(7):1746–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Demark-Wahnefried W, Hars V, Conaway MR, et al. Reduced rates of metabolism and decreased physical activity in breast cancer patients receiving adjuvant chemotherapy. Am J Clin Nutr. 1997;65(5):1495–1501. [DOI] [PubMed] [Google Scholar]
- 17. Littman AJ, Tang MT, Rossing MA. Longitudinal study of recreational physical activity in breast cancer survivors. J Cancer Surviv. 2010;4(2):119–127. [DOI] [PubMed] [Google Scholar]
- 18. Devoogdt N, Van Kampen M, Geraerts I, et al. Physical activity levels after treatment for breast cancer: one-year follow-up. Breast Cancer Res Treat. 2010;123(2):417–425. [DOI] [PubMed] [Google Scholar]
- 19. Kwan ML, Sternfeld B, Ergas IJ, et al. Change in physical activity during active treatment in a prospective study of breast cancer survivors. Breast Cancer Res Treat. 2012;131(2):679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lynch BM, Dunstan DW, Healy GN, Winkler E, Eakin E, Owen N. Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: findings from NHANES (2003-2006). Cancer Causes Control. 2010;21(2):283–288. [DOI] [PubMed] [Google Scholar]
- 21. Sabiston CM, Brunet J, Vallance JK, Meterissian S. Prospective examination of objectively assessed physical activity and sedentary time after breast cancer treatment: sitting on the crest of the teachable moment. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1324–1330. [DOI] [PubMed] [Google Scholar]
- 22. Broderick JM, Hussey J, Kennedy MJ, O’Donnell DM. Testing the ‘teachable moment’ premise: does physical activity increase in the early survivorship phase? Support Care Cancer. 2014;22(4):989–997. [DOI] [PubMed] [Google Scholar]
- 23. Yee J, Davis GM, Beith JM, et al. Physical activity and fitness in women with metastatic breast cancer. J Cancer Surviv. 2014;8(4):647–656. [DOI] [PubMed] [Google Scholar]
- 24. Harrison S, Hayes SC, Newman B. Level of physical activity and characteristics associated with change following breast cancer diagnosis and treatment. Psychooncology. 2009;18(4):387–394. [DOI] [PubMed] [Google Scholar]
- 25. Andrykowski MA, Beacham AO, Jacobsen PB. Prospective, longitudinal study of leisure-time exercise in women with early-stage breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(3):430–438. [DOI] [PubMed] [Google Scholar]
- 26. Emery CF, Yang HC, Frierson GM, Peterson LJ, Suh S. Determinants of physical activity among women treated for breast cancer in a 5-year longitudinal follow-up investigation. Psychooncology. 2009;18(4):377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tonosaki A, Ishikawa M. Physical activity intensity and health status perception of breast cancer patients undergoing adjuvant chemotherapy. Eur J Oncol Nurs. 2014;18(2):132–139. [DOI] [PubMed] [Google Scholar]
- 28. Janelsins MC, Heckler CE, Peppone LJ, et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol. 2017;35(5):506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sehl M, Lu X, Silliman R, Ganz PA. Decline in physical functioning in first 2 years after breast cancer diagnosis predicts 10-year survival in older women. J Cancer Surviv. 2013;7(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lucas AR, Focht BC, Cohn DE, Klatt MD, Buckworth J. Recruiting endometrial cancer survivors to studies examining lifestyle behaviors and quality of life: challenges faced and lessons learned. J Cancer Educ. 2018;33(4):857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mishra SI, et al. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev, 2012. 8:1465–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sturgeon K, Schadler K, Muthukumaran G, et al. Concomitant low-dose doxorubicin treatment and exercise. Am J Physiol Regul Integr Comp Physiol. 2014;307(6):R685–R692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schadler KL, Thomas NJ, Galie PA, et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget. 2016;7(40):65429–65440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Farina N, Lowry RG. The validity of consumer-level activity monitors in healthy older adults in free-living conditions. J Aging Phys Act. 2018;26(1):128–135. [DOI] [PubMed] [Google Scholar]
- 35. Hargens TA, Deyarmin KN, Snyder KM, Mihalik AG, Sharpe LE. Comparison of wrist-worn and hip-worn activity monitors under free living conditions. J Med Eng Technol. 2017;41(3):200–207. [DOI] [PubMed] [Google Scholar]
- 36.(2016), R.T., RStudio: Integrated Development for R. RStudio, Inc., Boston, MA: URL http://www.rstudio.com/. Accessibility verified October 1, 2018. [Google Scholar]
- 37. Xu SY, Nelson S, Kerr J, et al. Statistical approaches to account for missing values in accelerometer data: applications to modeling physical activity. Stat Methods Med Res, 2018;27(4):1168–1186. [DOI] [PubMed] [Google Scholar]
- 38. Cella D, Yount S, Rothrock N, et al. ; PROMIS Cooperative Group The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cella D, Riley W, Stone A, et al. ; PROMIS Cooperative Group The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–1057. [DOI] [PubMed] [Google Scholar]
- 41. Nyrop KA, Deal AM, Choi SK, et al. Measuring and understanding adherence in a home-based exercise intervention during chemotherapy for early breast cancer. Breast Cancer Res Treat. 2018;168(1):43–55. [DOI] [PubMed] [Google Scholar]
- 42. Diéras V, Bachelot T. The success story of trastuzumab emtansine, a targeted therapy in HER2-positive breast cancer. Target Oncol. 2014;9(2):111–122. [DOI] [PubMed] [Google Scholar]
- 43. Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484–1491. [PMC free article] [PubMed] [Google Scholar]
- 44. Finkelstein EA, Haaland BA, Bilger M, et al. Effectiveness of activity trackers with and without incentives to increase physical activity (TRIPPA): a randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(12):983–995. [DOI] [PubMed] [Google Scholar]
- 45. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 Suppl):S1–14. [PubMed] [Google Scholar]
- 46. World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. Geneva: WHO; 2013. [Google Scholar]