Publically available internet programs or cell phone apps, such as SparkPeople and Fitbit, show promise for weight loss in African American women with breast cancer.
Keywords: African Americans, Breast cancer survivors, Fitness trackers, Health status disparities, Internet, Weight loss programs
Abstract
Weight management after breast cancer (BC) treatment in African American (AA) women is crucial to reduce comorbid conditions and health disparities. We examined feasibility and potential efficacy of commercial eHealth/mHealth tools for weight management in AA BC survivors in New Jersey. Participants (N = 35) were randomized to an intervention (SparkPeople) plus activity tracker, Fitbit Charge (n = 18), or wait-list active control group (Fitbit only, n = 17). Anthropometric, behavioral, and quality of life (QOL) outcomes were collected at baseline, 3, 6, and 12 months. Differences in outcomes were assessed using intent-to-treat analysis. Retention was 97.1%. Both groups lost weight, with no significant differences between groups. At month 6, mean weight change was: intervention: −1.71 kg (SD 2.33; p = .006), 33.3% lost ≥3% of baseline weight; control: −2.54 kg (SD 4.00, p = .002), 23.5% lost ≥3% weight. Intervention participants achieved significant improvements in waist circumference (−3.56 cm, SD 4.70, p = .005), QOL (p = .030), and use of strategies for healthy eating (p = .025) and decreasing calories (p < .001). Number of days logged food per week was associated with decreases in waist circumference at 6 months (β −0.79, 95% CI, −1.49, −0.09, p = .030) and 12 months (β −2.16, 95% CI, −4.17, −0.15, p = .038). Weight loss was maintained at 12 months. This is the first study to demonstrate potential efficacy of commercial eHealth/mHealth tools for weight loss in AA BC survivors, without additional counseling from the research team. If effective, they may be convenient weight loss tools that can be easily and widely disseminated.
Clinical Trials registration: ClinicalTrials.gov NCT02699983
Implications
Practice: Commercial mHealth/eHealth programs are feasible and acceptable for improving self-regulation, quality of life, and weight management in African American (AA) breast cancer (BC) survivors.
Policy: Publically available mHealth/eHealth tools may be convenient, efficacious, and easily disseminated weight loss interventions for vulnerable populations.
Research: Future research is needed to confirm effectiveness of these commercial mHealth/eHealth tools for weight loss in AA BC survivors.
INTRODUCTION
African American (AA) breast cancer (BC) survivors are disproportionately affected by obesity and obesity-related comorbidities (e.g., diabetes, hypertension, cardiovascular mortality) [1–3]. For example, 57.2% of AA BC survivors have obesity compared with 27.4% of White BC survivors [1]. Additionally, AA women gain more weight following chemotherapy for BC than other racial/ethnic groups [4]. Many AA BC survivors do not adhere to nutrition or physical activity guidelines [5], and they are more likely than their white counterparts to die from comorbid conditions [6]. Weight loss in BC survivors improves cardiovascular disease (CVD) risk factors, physical function, fatigue, and quality of life (QOL) [7–9]. As little as 3%–5% loss of baseline weight can reduce risk of diabetes, dyslipidemia and hypertension, all precedents of CVD, the number one cause of death in AA women and BC survivors [10–12]. Therefore, weight management after cancer diagnosis and treatment in AA BC survivors is crucial to improve their QOL and reduce their CVD risk factors and health disparities.
Intensive lifestyle interventions have shown promise in promoting weight loss and improving lifestyle behaviors, functional status, and QOL in BC survivors [13]. However, many patients lack access, financial resources, or time to participate in these face-to-face weight loss interventions [14], and the substantial resources and time needed for intensive weight loss counseling limit their reach, sustainability, and translation. Successful weight loss outcomes have been achieved in randomized controlled trials (RCTs) using web (eHealth) or mobile phone technology (mHealth) as adjuncts or replacements to face-to-face or telephone-based behavioral counseling; however, replication and scalability are limited by reliance on privately developed programs [15] or the inclusion of resource-intensive in-person or telephone weight loss counseling [16,17]. Popular commercial eHealth weight loss programs may be ideal platforms for delivering weight loss interventions due to their convenience and widespread accessibility. To our knowledge, only two of four published RCTs to date that compared a commercial eHealth/mHealth program to control found significant weight loss, but these did not include AA women [18,19]. Therefore, it is unknown if commercial eHealth/mHealth programs are feasible or efficacious among AA women, in particular, AA BC survivors.
One popular free commercial eHealth/mHealth weight loss program is SparkPeople.com, with over 13 million monthly hits. SparkPeople, which is underpinned by social cognitive theory [20], has the following features: (a) educational and motivational articles and videos on nutrition, fitness, wellness, and stress management; (b) self-monitoring nutrition and weight tracking tools; (c) direct integration with many popular physical activity trackers; (d) recipes and daily meal plans; (e) incentives for engagement (SparkPoints); (f) social support communities, including discussion forums, teams, challenges, and expert blogs, (g) options for daily or weekly content delivered to members’ email; and (h) exercise videos from certified personal trainers and fitness instructors. Retrospective analyses of SparkPeople found it provides accurate information and valuable social support [21,22], and that more active usage is associated with greater weight loss [23]. However, prospective evaluations are lacking. This study examines feasibility and potential efficacy of SparkPeople plus an activity tracker for weight loss in AA BC survivors in New Jersey. To our knowledge, we are the first to test a free commercial eHealth/mHealth weight loss program in AA BC survivors.
METHODS
Study design
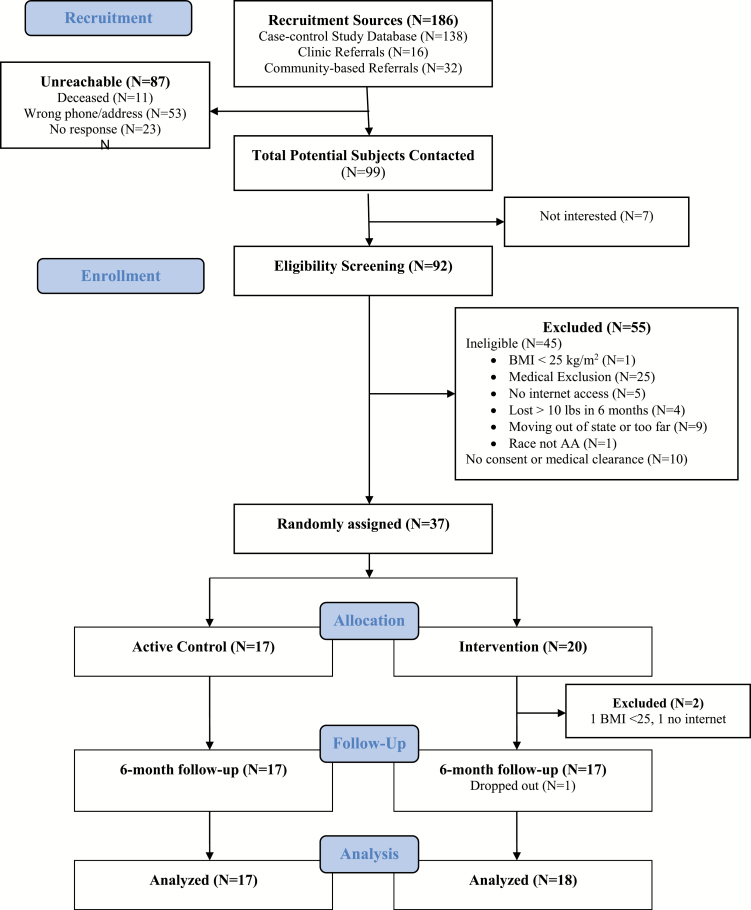
This 6-month pilot weight loss trial randomized 37 AA BC survivors to an intervention group, SparkPeople plus a wrist-worn physical activity tracker (Fitbit), versus an active wait-list control group (tracker only; Figure 1). Because physical activity in cancer survivors is important for improving cognition, mental health, and physical function irrespective of weight loss [24,25], we chose an active comparison group (tracker only) to objectively measure physical activity in all participants and to enhance accrual in this vulnerable population [26]. After randomization, two participants in the intervention group were found to not meet inclusion criteria; therefore, they were excluded from analyses (analytic sample = 35). Treatment outcomes were assessed at baseline, 3, and 6 months via in-person interviewer-administered questionnaires and anthropometric measurements. After 6 months, the wait-list control group received the Sparkpeople intervention, while the original treatment group was followed for an additional 6 months to assess adherence and sustainability. Participants received $25 after each completed in-person visit. Recruitment and data collection occurred between January 2016 and October 2017. The Rutgers University Institutional Review Board approved all study procedures (Pro20150001595), and we obtained informed consent from all participants prior to enrollment.
Fig 1.
Consort diagram.
Participants, recruitment, and randomization
AA BC survivors were recruited via: (a) letters to participants in a population-based case–control study of BC in AA women (Women’s Circle of Health Study) [27] who agreed to be contacted for future studies; and (b) flyers at cancer support group organizations and oncology practices. Telephone screening determined eligibility, which included: self-identification as AA; age 21–75 years; body mass index (BMI) at least 25 kg/m2; Stage 0-III BC at least 2 years from diagnosis; ability to read English; and home Internet access via computer or smartphone. Exclusion criteria included: serious medical or psychiatric conditions or disability limiting moderate physical activity; use of weight loss medications/supplements in past 3 months; bariatric surgery or successful loss of 5% of body weight in previous 6 months; pregnancy, breast feeding, or postpartum within 3 months; or leaving area in the next 6 months.
We sent eligible individuals an informed consent form and release of medical information form to obtain medical clearance from their physician. Research assistants followed up with a telephone call to confirm receipt, review the consent form, and answer any questions. After receipt of signed informed consent and medical clearance, participants were scheduled for a baseline visit, where all anthropometric measurements and surveys were completed. Participants were then randomly assigned 1:1 to the intervention or wait-list control group stratified by age (60 years or older vs. younger than 60 years). A researcher not involved in data collection prepared two randomization schedules, one for each age strata (< or ≥60 years), using a computer-based random number generator, with assignment kept in separately sealed sequentially numbered envelopes.
Procedures
All participants received a handout of their goals for weight loss (5% weight loss over 6 months), caloric intake (1200–1500 kcal daily), and physical activity (starting with mild–moderate exercise 10 minutes per day with stepwise increase in time and intensity to 30 minutes per day of moderate activity and 10,000 steps per day) [11]. We provided and trained everyone in the use of the wrist-worn physical activity tracker, Fitbit Charge, which was later replaced with Fitbit Alta for future participants and when tracker was lost or damaged. Research staff downloaded data from the Fitabase research platform (Small Steps Labs, CA) weekly to collect physical activity and Fitbit adherence data.
Treatment group participants received one 30-minute session on the SparkPeople website, with additional training if requested. Participants were instructed to self-monitor their diet at least weekly using the SparkPeople tool, and their physical activity levels daily using the Fitbit, which automatically transmitted data to their SparkPeople profile. Treatment group participants also received weekly text message reminders for the first 3 months to log onto SparkPeople. At month 6, control participants received the SparkPeople treatment (delayed intervention), while treatment group participants were monitored for adherence for an additional 6 months. The trial ended after all participants completed the 12-month assessment. There was no other education or counseling provided by the research team.
Measurements
At baseline, participants self-reported demographic information. BC history and comorbid conditions were obtained from the medical clearance form and by self-report. The following primary anthropometric and secondary outcomes and process measures were collected at baseline, 3, 6, 9, and 12 months.
Weight was measured in light clothing, without shoes, on a calibrated digital scale (Tanita BF-684W scale). Height was measured to the nearest 1 mm at the baseline visit. Weight and height were used to calculate BMI (weight [kg]÷height [m2]). Waist circumference was measured to the nearest 0.1 cm using a tape measure around the waist covering the umbilicus at the end of normal expiration. Blood pressure was assessed by standard protocol while seated after 5 minutes. Caloric intake was quantified by 24-hour diet recall administered by the research assistant using the Sparkpeople.com food diary tool. Direct data downloads from the Fitabase research platform provided physical activity levels. Similar to other studies using activity trackers and to account for the novelty of the tracking device from affecting baseline physical activity levels, we used 7 days of Fitbit data starting on day 8 as baseline [28,29], excluding days with less than 1,000 steps (an indication of incomplete wear or data capture). To be conservative, we made the a priori decision to exclude days with less than 1,000 steps (6.07% of total days), based on the typical average of 3,500–5,500 steps/day in the elderly and those with chronic diseases [30], and prior literature defining a valid wear day as at least 1,500 steps [31]. Fitbit data from the 2nd week after each study visit was processed using the same procedures. Cardiopulmonary fitness was estimated by the total distance walked, rounded to the nearest meter, during a 6-minute walk test [32]. QOL was measured using the Quality of Life in Adult Cancer Survivors Scale (QLACS); a lower score represents better QOL [33]. The QLACS has good reliability (ICC 0.98) in BC survivors and concurrent validity with other health-related QOL measures (e.g., SF-36, ρ = −0.70; FACT BC-specific concerns subscale, ρ = −0.62) [33,34]. Social cognitive theory variables, including nutrition and physical activity-related self-regulation, self-efficacy, and social support, were assessed using the Health Beliefs Survey, which has good reliability (Cronbach’s alpha 0.72–0.96), predictive validity, and sensitivity to change [35,36].
Feasibility process measures included accrual (percentage of patients recruited and completing baseline assessments) and retention (percentage of patients completing follow-up assessments at 6 and 12 months). We considered the trial to be feasible and to warrant a future definitive trial if the accrual rate was at least 75% and retention was at least 80%, based on the following. Other web-based obesity interventions found a recruitment rate of 50% [37]; however, we expected our rate to be higher as the study sample was an activated population who had already participated in a previous study, met eligibility criteria, and expressed interest in future studies. Additionally, other weight loss trials in AA BC survivors had retention rates of 71%–97% [26,38–40], and trials testing commercial web-based weight loss programs had retention rates of 63%–95% [18,19,41–46].
We determined intervention adherence based on SparkPeople usage. This was provided by SparkPeople and included number of days participants logged into the website, number of days they logged food, and total SparkPoints earned (an indication of website engagement). Adherence to Fitbit was determined by number of days of recorded steps. Missing Fitbit data were recorded as 0. Acceptability and satisfaction to SparkPeople and Fitbit were assessed by structured (ease of use, usefulness of site, need for extra training) and open-ended questions (type of assistance needed, other comments) at each visit. Ease of use questions were scored on a Likert scale from 1= “very difficult” to 4 = “very easy”; usefulness questions were scored from 1 = “not useful at all” to 4 = “very useful.”
Analyses
Descriptive statistics show baseline participant characteristics and outcomes. We used intent-to-treat analysis, imputing missing data with last observation carried forward for participants who dropped out (N = 1 after baseline visit, N = 2 after 9 months) or were lost to follow-up (N = 1 after 9 months); all were from the intervention group. Data at 3 months were also imputed for two participants (1 intervention, 1 control), who were out of the area at 3 months. Sensitivity analyses were conducted including only the participants who had 6 months of follow-up, and those with baseline BMI over 30 kg/m2. Paired t-test was used to compare change in outcomes within each participant from baseline to 3, 6, and 12 months. Independent sample t-tests (or chi-square and two-tailed Fisher’s Exact Test for categorical data) assessed significance of differences between groups. We evaluated association of SparkPeople adherence (days logged in, days logged food, total SparkPoints earned) with main outcomes at 3, 6, and 12 months using correlations and linear regression, with 95% confidence intervals. For the open-ended questions, members of the team summarized and aggregated answers into themes, and representative quotes are presented. All analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC), and an overall significance level of 0.05 was used.
RESULTS
Accrual, Retention, and Baseline Characteristics
We enrolled and randomized 37 women. Out of 92 AA BC survivors screened for eligibility, 45 were ineligible (see Fig. 1 for reasons for ineligibility). Of the 47 eligible women, 37 completed the baseline visit (78.7% accrual). Retention rate was 97.1% and 88.6% at 6 and 12 months, respectively, demonstrating feasibility. Our analytic sample included 35 participants, after excluding two participants who were found to not meet inclusion criteria after randomization.
On average, participants were 61.54 (SD 8.83) years old, 6.60 (SD 4.28) years since diagnosis, with BMI 36.73 kg/m2 (SD 6.84). The majority of participants were married, retired, and college graduates (Table 1). Baseline characteristics and outcome measures were not significantly different between groups except for smoking status.
Table 1.
Baseline characteristics of African American breast cancer survivors enrolled in study (N = 35)
| Characteristics | Total Na (%) |
Control Na (%) |
Intervention Na (%) |
p-value |
|---|---|---|---|---|
| N (%) | 35 (100) | 17 (48.6) | 18 (51.4) | — |
| Age (in years) | ||||
| <60 | 14 (40.0) | 7 (41.18) | 7 (38.89) | .890 |
| ≥60 | 21 (60.0) | 10 (58.82) | 11 (61.11) | |
| Employment status | ||||
| Employed | 10 (28.57) | 6 (35.29) | 4 (22.22) | .742 |
| Unemployed | 7 (20.0) | 3 (17.65) | 4 (22.22) | |
| Retired | 18 (51.43) | 8 (47.06) | 10 (55.56) | |
| Educational attainment | ||||
| HS or less | 6 (17.14) | 4 (23.53) | 2 (11.11) | .487 |
| Some college | 11 (31.43) | 6 (35.29) | 5 (27.78) | |
| College grad | 18 (51.43) | 7 (41.18) | 11 (61.11) | |
| Marital status | ||||
| Married | 13 (38.24) | 3 (18.75) | 10 (55.56) | .081 |
| Never married | 12 (35.29) | 7 (43.75) | 5 (27.78) | |
| Divorced/Widowed/Separated | 9 (26.47) | 6 (37.50) | 3 (16.67) | |
| Smoking status | ||||
| Never | 23 (65.71) | 8 (47.06) | 15 (83.33) | .023 |
| Current | 4 (11.43) | 2 (11.76) | 2 (11.11) | |
| Former | 8 (22.86) | 7 (41.18) | 1 (5.56) | |
| Number of chronic conditions | ||||
| 0–2 | 12 (34.29) | 4 (23.53) | 8 (44.44) | .484 |
| 3–4 | 12 (34.29) | 7 (41.18) | 5 (27.78) | |
| ≥5 | 11 (31.42) | 6 (35.29) | 5 (27.78) | |
| Years since diagnosis | ||||
| 2–5 | 19 (54.29) | 10 (58.82) | 9 (50.00) | .870 |
| 6–10 | 9 (25.71) | 4 (23.53) | 5 (27.78) | |
| >10 | 7 (20.00) | 3 (17.65) | 4 (22.22) | |
| Breast cancer stage | ||||
| 0–1 | 13 (41.94) | 9 (52.94) | 4 (28.57) | .463 |
| 2 | 11 (35.48) | 5 (29.41) | 6 (42.86) | |
| 3 | 7 (22.58) | 3 (17.65) | 4 (28.57) | |
| Treatmentb | ||||
| Surgery | 34 (97.14) | 16 (94.12) | 18 (100.0) | .297 |
| Radiation | 24 (68.57) | 14 (82.35) | 10 (55.56) | .088 |
| Chemotherapy | 27 (77.14) | 14 (82.35) | 13 (72.22) | .476 |
| Immunotherapy | 7 (20.59) | 3 (18.75) | 4 (22.22) | .554 |
| Hormonal therapy | 20 (57.14) | 10 (58.82) | 10 (55.56) | .976 |
| Currently receiving hormonal therapy | ||||
| Yes | 6 (17.00) | 3 (18.00) | 3 (17.00) | 1.00 |
aNumbers may not add to total due to missing data.
bParticipants may have received more than one treatment.
Bold values indicate statistically significant (p = 0.05).
Adherence and acceptability
Participants logged into SparkPeople more than once weekly throughout the study. Activity on the website decreased over time, particularly for logging in food. Overall, the delayed intervention group engaged less than the intervention group, and this was statistically significant during months 4–6 (Table 2). There were no significant differences found in SparkPeople adherence by age, employment, marital status, cancer stage, baseline BMI, or baseline QOL (data not shown). However, days logged in during months 1–3 were significantly different across educational attainment, with college graduates having fewer mean days logged in per week (1.79, SD 1.50) compared with those with high school or less education (mean 2.17, SD 1.93) and some college (mean 4.25, SD 2.39), p = .039.
Table 2.
Adherence and acceptability of SparkPeople and Fitbit over time
| Variable | Months 1–3 Mean (SD) |
Months 4–6 Mean (SD) |
Months 7–9 Mean (SD) |
Months 10–12 Mean (SD) |
|---|---|---|---|---|
| Days logged in per week | ||||
| Intervention | 3.01 (2.07) | 2.30 (2.30)* | 1.86 (2.32) | 1.46 (2.29) |
| Delayed interventiona | 2.30 (2.27) | 1.14 (1.64)* | — | — |
| Days logged food per week | ||||
| Intervention | 1.69 (1.84) | 0.60 (0.87) | 0.34 (0.72) | 0.11 (0.26) |
| Delayed interventiona | 1.50 (1.85) | 0.71 (1.17) | — | — |
| SparkPoints earned per week | ||||
| Intervention | 67.23 (29.07) | 46.76 (26.35) | 41.99 (30.90) | 33.86 (26.57) |
| Delayed interventiona | 50.61 (37.00) | 40.79 (37.29) | — | — |
| Acceptability of SparkPeople, ease of useb | ||||
| Intervention | 3.27 (0.88) | 3.35 (0.70) | 3.13 (0.74) | 2.93 (0.73) |
| Delayed interventiona | 3.10 (0.57) | 3.00 (0.74) | — | — |
| Acceptability of SparkPeople, usefulnessc | ||||
| Intervention | 3.53 (0.83) | 3.53 (0.80) | 3.33 (0.82) | 3.08 (0.86) |
| Delayed interventiona | 3.60 (0.52) | 3.42 (0.79) | — | — |
| Days used Fitbit per week | ||||
| Intervention | 6.24 (0.84) | 5.57 (1.83) | 5.57 (1.80) | 5.47 (2.05) |
| Delayed interventiond | 5.96 (1.63) | 5.61 (1.77) | 5.43 (2.00) | 4.97 (2.52) |
| Acceptability of Fitbit, ease of useb | ||||
| Intervention | 3.46 (0.73) | 3.76 (0.44) | 3.75 (0.45) | 3.41 (0.62) |
| Delayed interventiond | 3.56 (0.51) | 3.47 (0.51) | 3.56 (0.51) | 3.41 (0.80) |
| Acceptability of Fitbit, usefulnessc | ||||
| Intervention | 3.93 (0.26) | 4.00 (0.00) | 4.00 (0.00) | 3.94 (0.24) |
| Delayed interventiond | 3.93 (0.26) | 4.00 (0.00) | 3.88 (0.34) | 3.94 (0.24) |
aWait-list control group received same 6-month intervention starting month 6 of the randomized controlled trial; therefore, they have no data on SparkPeople adherence for months 7–9 and 10–12.
bScale range: 1 = very difficult, 2 = difficult, 3 = easy, 4 = very easy.
cScale range: 1 = not useful at all, 2 = slightly useful, 3 = somewhat useful, 4 = very useful.
dWait-list control group also received Fitbit at baseline.
*p = .053 (two group t-test) for difference in means between groups.
Participants found SparkPeople easy to use and somewhat to very useful, and many provided positive comments regarding the educational and inspirational articles, recipes, videos, incentives, and social groups. Several participants found tracking food to be beneficial, and they lauded the nutritional report, convenience of the phone app, and ability to scan food labels. However, many others made comments like, “tracking food is tedious” and “it is too time consuming,” particularly with estimating portion size and entering home-cooked meals. Eleven participants needed extra training with the following: (a) tracking foods; (b) adding own recipes; (c) installing the app on a new phone; and (d) using exercise videos and message boards. A few participants stated, “[The website] is daunting. There’s just so much there,” and “it’s a little confusing to me.” Two participants wanted access to a nutritionist and a more structured plan, e.g., for SparkPeople to “send you a menu everyday of what to eat.”
Adherence with Fitbit was high, with mean of 5–6 days of use per week throughout the study period with no difference between groups. Participants universally felt it was a “wonderful tool.” Eight participants needed extra training with the following: (a) using the computer dashboard; (b) syncing data; (c) updating the Fitbit app; and (d) connecting the tracker to a new phone. Other problems included broken wristbands (replacement Fitbits were given) and incompatibility with certain smartphones (the one participant was able to use her computer to sync data).
Main effects at 6 months
Anthropometric outcomes
Within both groups, weight and BMI decreased significantly, with no significant differences between groups. Six participants (33.3%) of intervention group and four participants (23.5%) of control group lost at least 3% of baseline weight, and three participants in each group lost at least 5% of baseline weight. Only the intervention group had significant decreases in waist circumference (Table 3). For the 17 intervention participants with 6-month visit data, mean weight loss was −1.81 kg (SD 2.36, p = .006). Weight loss was greater among women with baseline BMI over 30 kg/m2 (intervention −1.98 kg, SD 2.42, p = .009; control −2.90 kg, SD 4.32, p = .026). The intervention group maintained weight loss (mean −1.98 kg, SD 2.41, p = .003; 33% lost at least 3% baseline weight) and decreases in waist circumference (−4.01 cm, SD 5.76, p = .011) at 12 months.
Table 3.
Changes in outcomes across groups at 3 and 6 months, N = 35 (18 intervention, 17 control)
| Outcome variable and group | Baseline Mean (SD) |
3-Month change Mean (SD) |
p-value within groupa |
p-value intervention vs. controlb |
6-Month change Mean (SD) |
p-value within groupa |
p-value intervention vs. controlb |
|---|---|---|---|---|---|---|---|
| Weight (kg) | |||||||
| Intervention | 91.98 (15.35) | −2.10 (2.64) | .004 | .848 | −1.71 (1.88) | .006 | .461 |
| Control | 104.06 (22.65) | −2.26 (2.16) | <.001 | −2.53 (4.00) | .002 | ||
| % Weight change | |||||||
| Intervention | Ref | −2.33 (2.62) | .882 | −1.91 (2.42) | .718 | ||
| Control | Ref | −2.21 (2.04) | −2.27 (3.38) | ||||
| BMI, kg/m2 | |||||||
| Intervention | 35.64 (6.64) | −0.74 (0.83) | .002 | .868 | −0.74 (0.99) | .006 | .692 |
| Control | 37.88 (7.06) | −0.69 (0.83) | .003 | −0.91 (1.39) | .012 | ||
| Waist circumference (cm) | |||||||
| Intervention | 110.59 (11.38) | −1.98 (5.39) | .136 | .304 | −3.56 (4.70) | .005 | .113 |
| Control | 115.42 (18.06) | −0.05 (5.56) | .970 | −0.84 (5.21) | .518 | ||
| Calories per day (kcal) | |||||||
| Intervention | 1563.71 (651.84) | −62.06 (589.27) | .670 | .103 | −216.65 (606.09) | .160 | .860 |
| Control | 1610.88 (573.01) | −386.38 (514.95) | .009 | −173.06 (805.40) | .389 | ||
| Steps per day | |||||||
| Intervention | 5622.33 (2571.32) | +466.36 (2203.49) | .382 | .223 | −107.07 (2184.94) | .838 | .258 |
| Control | 8092.54 (4814.03) | −609.83 (2898.36) | .399 | −205.47 (2147.79) | .699 | ||
| Total fairly/very active minutes per week | |||||||
| Intervention | 71.94 (96.0) | −10.89 (104.58) | .664 | .352 | −34.89 (98.49) | .151 | .044 |
| Control | 210.18 (282.86) | −65.82 (222.30) | .240 | +11.35 (110.87) | .679 | ||
| 6-Minute walk test (m) | |||||||
| Intervention | 413.22 (106.85) | +0.81 (51.13) | .947 | .281 | +26.64 (35.88) | .006 | .620 |
| Control | 370.25 (72.72) | +20.07 (52.81) | .137 | +31.97 (26.27) | <.001 | ||
| Quality of lifec | |||||||
| Intervention | 109.78 (39.26) | −5.44 (18.11) | .219 | .854 | −9.44 (16.97) | .031 | .500 |
| Control | 108.76 (36.17) | −4.35 (16.69) | .298 | −4.65 (24.21) | .440 | ||
| Plan and track nutritiond | |||||||
| Intervention | 2.19 (0.73) | +1.03 (0.79) | <.001 | .330 | +0.81 (0.76) | <.001 | .247 |
| Control | 2.65 (0.93) | +0.76 (0.88) | .003 | +0.52 (0.70) | .007 | ||
| Strategies to increase fruits/vegetables/ grainsd | |||||||
| Intervention | 3.49 (0.70) | +0.53 (0.58) | .002 | .194 | +0.39 (0.65) | .025 | .260 |
| Control | 3.69 (0.63) | +0.24 (0.64) | .152 | +0.13 (0.63) | .412 | ||
| Strategies to decrease fat and caloriesd | |||||||
| Intervention | 3.14 (0.72) | +0.66 (0.55) | <.001 | .318 | +0.58 (0.42) | <.001 | .141 |
| Control | 3.45 (0.63) | +0.48 (0.48) | .001 | +0.30 (0.62) | .071 | ||
| Physical activity goal setting and planningd | |||||||
| Intervention | 2.33 (1.12) | +1.08 (1.22) | .003 | .293 | +0.84 (1.10) | .006 | .813 |
| Control | 2.83 (1.20) | +0.65 (1.05) | .025 | +0.75 (1.17) | .022 | ||
| Track walkingd | |||||||
| Intervention | 1.87 (0.91) | +1.46 (1.29) | <.001 | .945 | +1.37 (0.99) | <.001 | .995 |
| Control | 2.28 (1.07) | +1.48 (0.95) | <.001 | +1.36 (0.98) | <.001 | ||
| Strategies to increase physical activity enjoymentd | |||||||
| Intervention | 2.09 (1.11) | +0.65 (1.38) | .081 | 1.00 | +0.65 (0.91) | .010 | .864 |
| Control | 2.59 (1.17) | +0.65 (1.14) | .038 | +0.58 (1.20) | .070 | ||
| Self-efficacy, eating healthy foodse | |||||||
| Intervention | 79.16 (12.42) | +2.14 (8.17) | .282 | .501 | +5.59 (12.63) | .078 | .015 |
| Control | 75.86 (16.20) | −0.41 (13.47) | .903 | −6.56 (15.36) | .097 | ||
| Self-efficacy, increasing physical activitye | |||||||
| Intervention | 71.03 (16.26) | −3.34 (14.05) | .328 | .345 | −0.46 (13.97) | .890 | .975 |
| Control | 65.37 (17.74) | +0.84 (11.57) | .768 | −0.61 (14.55) | .865 | ||
| Social support for healthy nutritionf | |||||||
| Intervention | 2.67 (0.93) | +0.40 (0.58) | .009 | .147 | +0.39 (0.85) | .069 | .300 |
| Control | 2.94 (0.60) | +0.11 (0.56) | .413 | +0.11 (0.71) | .531 | ||
| Social support for physical activityf | |||||||
| Intervention | 3.42 (0.64) | +0.02 (0.57) | .881 | .926 | −0.05 (0.60) | .738 | .767 |
| Control | 3.49 (0.77) | 0.0 (0.72) | 1.000 | −0.13 (0.97) | .594 | ||
BMI body mass index.
a p-values within group estimated using paired t-test.
b p-values between intervention and control group estimated using two group t-test.
cQuality of life in adult cancer survivors, scale range 1–7 (never-always; lower score is better quality).
dHealth Beliefs Survey, scale range 1–5 (never-repeatedly).
eHealth Beliefs Survey, scale range 0–100 (certain I cannot–certain I can).
fHealth Beliefs Survey, scale range 1–5 (strongly disagree–strongly agree).
Diet and physical activity
Neither group had significant within-group changes in caloric intake or physical activity, but change in total active minutes per week was significantly different between groups (control group increased while intervention group decreased activity).
Cardiopulmonary fitness
Both groups showed significant within-group improvements on the 6-minute walk test with no between group differences.
Quality of life
Only the intervention group reported significant improvements in QOL.
Social cognitive theory variables
Both groups had significant improvements in planning and tracking nutrition and physical activity. However, only the intervention group had significant improvements in strategies for healthy eating, strategies to decrease fat and calories, and strategies to increase physical activity enjoyment. There was a significant between-group difference in self-efficacy to eat healthy foods (intervention group increased while control group decreased self-efficacy).
Association of SparkPeople adherence with outcomes
Mean days logged food was significantly correlated with several outcome changes, for example, 3-month calories consumed per day, 6-month waist circumference change, and 12-month waist circumference change (Table 4). Regression models reinforced the correlations. For example, number of days logged food per week was associated with decreases in waist circumference at 6 months (β −0.79, 95% CI, −1.49, −0.09, p = .030) and 12 months (β −2.16, 95% CI, −4.17, −0.15, p = .038), that is, over 6 months, for every 1 SD increase of 1.25 days logged food per week, waist circumference decreased by 2.51 cm, 95% CI −4.72, −0.28, p = .030. Over 12 months, for every 1 SD increase of 0.80 days logged food per week, waist circumference decreased by 3.3 cm, 95% CI −6.22, −0.23, p = .038. Similar associations were seen between number of SparkPoints earned and change in waist circumference (data not shown).
Table 4.
Correlation of days logged food per week with changes in outcomes (intervention group, N = 17)
| Outcomes | Mean change in outcome (SD) |
Mean days logged food (SD) | Correlation, days logged food with outcome r (95% CI) |
p-value |
|---|---|---|---|---|
| 3 months | ||||
| Waist circumference (cm) | −1.981 (5.385) | 1.687 (1.844) | −0.456 (−0.945, 0.034) | .066 |
| Calories consumed per day (kcal) | −62.059 (589.269) | −0.647 (−1.00, −0.228) | .005 | |
| 6 months | ||||
| Waist circumference (cm) | −3.556 (4.699) | 1.145 (1.249) | −0.526 (−0.994, −0.057) | .030 |
| Generic quality of lifea | −8.647 (16.428) | −0.518 (−0.989, −0.047) | .033 | |
| Calories consumed per day (kcal) | −216.647 (606.086) | −0.465 (−0.952, 0.022) | .060 | |
| 12 months | ||||
| Waist circumference (cm) | −4.006 (5.756) | 0.688 (0.802) | −0.560 (−1.00, −0.105) | .038 |
aQuality of life in adult cancer survivors (lower score is better quality).
Delayed intervention outcomes
The control group continued to lose weight after receiving the delayed intervention, with 6 participants (35.3%) achieving additional 3% weight loss or more from month 6 to 12 of the study (Supplementary Table S1). Overall, effect sizes were greater in the intervention versus delayed intervention group. During the first 3 months of delayed intervention, logging into SparkPeople (β 0.11, 95% CI 0.03, 0.19; p = .011) and logging in food (β 0.11, 95% CI 0.02, 0.22, p = .046) was significantly associated with using strategies to reduce fat and calories.
DISCUSSION
To date, there have been eight published weight loss studies (four RCTs) focusing on AA BC survivors [26,38–40,47–50]. Most studies have been small (N = 8–31), used individual or group face-to-face with or without telephone counseling, and achieved modest weight loss results (mean at 6 months −0.77 to −2.53 kg) [38,39,47–50]. One larger weight loss RCT in 246 AA BC survivors achieved more substantial weight loss at 6 months (mean −3.49 kg, SD 0.39), but it was resource and time-intensive, requiring twice weekly in-person classes throughout 6 months [26]. The only other technology and distance-based weight loss RCT in AA BC survivors (N = 35) evaluated a 6-month self-regulation intervention, consisting of a 1-hour in-person nutritional counseling session followed by daily weighing via a smart scale, weekly emailed lessons and tailored feedback with or without a wearable activity tracker [40]. While it had a high retention rate similar to ours of 97.1%, that program achieved no significance within or between group weight loss. Our randomized pilot study demonstrates feasibility and acceptability of a self-regulated eHealth/mHealth weight loss program in AA BC survivors. Furthermore, our study is the first to report significant weight loss in AA BC survivors without requiring any additional education, counseling or tailored feedback from the research team.
Unlike other studies [25,51], our Fitbit only group also lost significant amount of weight, probably due to the novelty of a free activity tracking device given to this group of motivated AA BC survivors who selectively chose to participate in a weight loss study. Additionally, the simplicity of having only physical activity to track (Fitbit group) versus tracking diet and physical activity (SparkPeople plus Fitbit) may have motivated our active control group more to lose weight. Another exercise only study in 22 AA BC survivors, which included 75 minute weekly sessions for 8 weeks, also resulted in significant mean weight loss, albeit less than ours, of −0.91 kg (p = .005) immediately after the intervention, but mean 0.31 kg weight regain by 3 months [50]. Our study is the first to show that giving a wrist-worn activity tracking device to AA BC survivors, without any other intervention, resulted in weight loss. While the mean weight loss in our study was modest in both groups, it is comparable to weight loss in other internet or mobile phone delivered intervention trials that included minority persons (−1.0 kg to −2.57 kg) [52], and greater than or equal to that achieved in two other commercial internet weight loss RCTs that included at least 30 AA women (−0.3 kg and −1.8 kg) [42,53]. One-third of intervention group participants achieved clinically significant weight loss of at least 3% [11], and this was maintained at 12 months, which makes this low-resource and widely available intervention promising.
The Fitbit only group had higher absolute mean weight loss compared with the intervention group; this may have been due to their higher baseline weight. However, only the intervention group had significant improvements in QOL and in using strategies to eat healthy, which is particularly important for cancer survivors [54]. More importantly, the intervention group achieved significant decreases in waist circumference, which is an independent risk factor for type 2 diabetes, dyslipidemia, hypertension, and CVD [55], as well as all-cause mortality in AA BC survivors [56]. While tracking food seems to be the strongest adherence predictor of positive outcomes, it was found cumbersome and difficult to maintain by most, similar to other studies in this population [39]. Future programs will need to consider how to make tracking food intake easier and more convenient for AA BC survivors trying to lose weight.
Despite our study’s strengths, several limitations must be considered. Our small sample size limited our ability to detect between group differences and conduct multivariate analyses; therefore, the potential for confounding is great, and the results should be interpreted with caution. For example, the control group had a higher proportion of stage 0/1 cancers then the intervention group; however, there were similar numbers of participants who received chemotherapy or were currently receiving hormonal treatments, both factors that may affect weight and adiposity [4,57]. Additionally, more women in the intervention group were married and may have had greater social support. However, the control group reported higher social support for healthy nutrition at baseline, and both groups equally reported social support for physical activity. Moreover, the control group had more chronic conditions than the intervention group, which may have increased or decreased their motivation for weight loss. Nevertheless, our sample size was adequate to assess feasibility and significant within-group changes. While an adequately powered RCT is needed to establish efficacy, the positive within-group weight loss results and high retention rate suggest that both interventions (SparkPeople and Fitbit) hold promise for AA BC survivors. The high educational status of our participants (over 50% college graduates) limits generalizability and may have contributed to adherence and success of both programs. However, participants who were college graduates demonstrated less engagement with SparkPeople, so our findings may be conservative. Additionally, although physical activity data were collected via objective monitoring, the baseline activity levels were very high, particularly for the control group, which limited further improvements.
CONCLUSIONS
Weight management is crucial for AA BC survivors to decrease their obesity-related comorbidities, all-cause mortality, and health disparities [7–9]. Publically available eHealth/mHealth programs and wrist-worn activity trackers may be convenient, efficacious, and easily disseminated interventions for AA BC survivors in need of weight management. Our results are supportive of moving to an efficacy trial. A Sequential, Multiple Assignment, Randomized Trial (SMART) design may be ideal to isolate the effects of each eHealth/mHealth tool (SparkPeople versus Fitbit), determine the best way to sequence the intervention components, and to evaluate which participant characteristics moderate the efficacy of these tools.
Supplementary Material
Acknowledgment
This work was supported by the National Cancer Institute of the National Institutes of Health under Award Number R21CA191431 (J.M.F.) and R01CA185623 (E.V.B.). This research was also supported by Rutgers Cancer Institute of New Jersey (J.M.F.), which is funded by National Cancer Institute of the National Institutes of Health under Award Number P30CA72720. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Preliminary results from this manuscript have been presented at the Cancer Survivorship Symposium: Advancing Care and Research, American Society of Clinical Oncology/American Academy of Family Physicians/American College of Physicians, San Diego, CA, January 27–28, 2017.
Compliance with Ethical Standards
Conflicts of Interest: The authors declare that they have no conflicts of interest.
Authors’ Contributions: JMF, KAD, PAO, EVB, KOH designed the study. JMF, AB, AR implemented the study and collected data. JMF, AB performed the analysis. JMF drafted the manuscript. All authors contributed to the interpretation of results and provided critical feedback on the final paper.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Welfare of Animals: This article does not contain any studies with animals performed by any of the authors.
References
- 1. Chandran U, McCann SE, Zirpoli G, et al. Intake of energy-dense foods, fast foods, sugary drinks, and breast cancer risk in African American and European American women. Nutr Cancer. 2014;66(7):1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Office of Minority Health, U.S. Department of Health and Human Services. Diabetes and African Americans 2016. Available at http://minorityhealth.hhs.gov/templates/content.aspx?lvl=3&lvlID=5&ID=3017. Accessed June 4, 2018.
- 3. Office of Minority Health, U.S. Department of Health and Human Services. Heart disease and African Americans 2014. Available at https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=19. Accessibility verified June 4, 2018.
- 4. Saquib N, Flatt SW, Natarajan L, et al. Weight gain and recovery of pre-cancer weight after breast cancer treatments: evidence from the women’s healthy eating and living (WHEL) study. Breast Cancer Res Treat. 2007;105(2):177–186. [DOI] [PubMed] [Google Scholar]
- 5. Smith SA, Claridy MD, Whitehead MS, et al. Factors associated with body mass index among African American breast cancer survivors. J Ga Public Health Assoc. 2016;5(3):259–265. [PMC free article] [PubMed] [Google Scholar]
- 6. Dignam JJ, Wieand K, Johnson KA, et al. Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res Treat. 2006;97(3):245–254. [DOI] [PubMed] [Google Scholar]
- 7. Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. J Am Med Assoc. 2009;301(18):1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kenzik KM, Morey MC, Cohen HJ, Sloane R, Demark-Wahnefried W. Symptoms, weight loss, and physical function in a lifestyle intervention study of older cancer survivors. J Geriatr Oncol. 2015;6(6):424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swisher AK, Abraham J, Bonner D, et al. Exercise and dietary advice intervention for survivors of triple-negative breast cancer: effects on body fat, physical function, quality of life, and adipokine profile. Support Care Cancer. 2015;23(10):2995–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jensen MD, Ryan DH, Apovian CM, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023. [DOI] [PubMed] [Google Scholar]
- 12. DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66(4):290–308. [DOI] [PubMed] [Google Scholar]
- 13. Demark-Wahnefried W, Rogers LQ, Alfano CM, et al. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors. CA Cancer J Clin. 2015;65(3):167–189. [DOI] [PubMed] [Google Scholar]
- 14. George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104(2):e16–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arem H, Irwin M. A review of web-based weight loss interventions in adults. Obes Rev. 2011;12(5):e236–e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cadmus-Bertram L, Wang JB, Patterson RE, Newman VA, Parker BA, Pierce JP. Web-based self-monitoring for weight loss among overweight/obese women at increased risk for breast cancer: the HELP pilot study. Psychooncology. 2013;22(8):1821–1828. [DOI] [PubMed] [Google Scholar]
- 17. Lin M, Mahmooth Z, Dedhia N, et al. Tailored, interactive text messages for enhancing weight loss among African American adults: the TRIMM randomized controlled trial. Am J Med. 2015;128(8):896–904. [DOI] [PubMed] [Google Scholar]
- 18. Collins CE, Morgan PJ, Jones P, et al. A 12-week commercial web-based weight-loss program for overweight and obese adults: randomized controlled trial comparing basic versus enhanced features. J Med Internet Res. 2012;14(2):e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hartman SJ, Nelson SH, Cadmus-Bertram LA, Patterson RE, Parker BA, Pierce JP. Technology- and phone-based weight loss intervention: pilot RCT in women at elevated breast cancer risk. Am J Prev Med. 2016;51(5):714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 21. Hwang KO, Farheen K, Johnson CW, Thomas EJ, Barnes AS, Bernstam EV. Quality of weight loss advice on internet forums. Am J Med. 2007;120(7):604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hwang KO, Ottenbacher AJ, Green AP, et al. Social support in an Internet weight loss community. Int J Med Inform. 2010;79(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hwang KO, Ning J, Trickey AW, Sciamanna CN. Website usage and weight loss in a free commercial online weight loss program: retrospective cohort study. J Med Internet Res. 2013;15(1):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hartman SJ, Nelson SH, Myers E, et al. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: the memory & motion study. Cancer. 2018;124(1):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stolley M, Sheean P, Gerber B, et al. Efficacy of a weight loss intervention for African American breast cancer survivors. J Clin Oncol. 2017;35(24):2820–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bandera EV, Chandran U, Zirpoli G, et al. Body fatness and breast cancer risk in women of African ancestry. BMC Cancer. 2013;13:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harkins KA, Kullgren JT, Bellamy SL, Karlawish J, Glanz K. A trial of financial and social incentives to increase older adults’ walking. Am J Prev Med. 2017;52(5):e123–e130. [DOI] [PubMed] [Google Scholar]
- 29. Wang JB, Cadmus-Bertram LA, Natarajan L, et al. Wearable sensor/device (fitbit one) and SMS text-messaging prompts to increase physical activity in overweight and obese adults: a randomized controlled trial. Telemed J E Health. 2015;21(10):782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tudor-Locke CE, Myers AM. Methodological considerations for researchers and practitioners using pedometers to measure physical (ambulatory) activity. Res Q Exerc Sport. 2001;72(1):1–12. [DOI] [PubMed] [Google Scholar]
- 31. Tudor-Locke C, Barreira TV, Schuna JM Jr. Comparison of step outputs for waist and wrist accelerometer attachment sites. Med Sci Sports Exerc. 2015;47(4):839–842. [DOI] [PubMed] [Google Scholar]
- 32. Enright PL. The six-minute walk test. Respir Care. 2003;48(8):783–785. [PubMed] [Google Scholar]
- 33. Avis NE, Ip E, Foley KL. Evaluation of the Quality of Life in Adult Cancer Survivors (QLACS) scale for long-term cancer survivors in a sample of breast cancer survivors. Health Qual Life Outcomes. 2006;4:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Avis NE, Smith KW, McGraw S, Smith RG, Petronis VM, Carver CS. Assessing quality of life in adult cancer survivors (QLACS). Qual Life Res. 2005;14(4):1007–1023. [DOI] [PubMed] [Google Scholar]
- 35. Anderson-Bill ES, Winett RA, Wojcik JR. Social cognitive determinants of nutrition and physical activity among web-health users enrolling in an online intervention: the influence of social support, self-efficacy, outcome expectations, and self-regulation. J Med Internet Res. 2011;13(1):e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson-Bill ES, Winett RA, Wojcik JR, Winett SG. Web-based guide to health: relationship of theoretical variables to change in physical activity, nutrition and weight at 16-months. J Med Internet Res. 2011;13(1):e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harvey-Berino J, West D, Krukowski R, et al. Internet delivered behavioral obesity treatment. Prev Med. 2010;51(2):123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Djuric Z, Mirasolo J, Kimbrough L, et al. A pilot trial of spirituality counseling for weight loss maintenance in African American breast cancer survivors. J Natl Med Assoc. 2009;101(6):552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sheppard VB, Hicks J, Makambi K, Hurtado-de-Mendoza A, Demark-Wahnefried W, Adams-Campbell L. The feasibility and acceptability of a diet and exercise trial in overweight and obese black breast cancer survivors: the stepping STONE study. Contemp Clin Trials. 2016;46:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Valle CG, Deal AM, Tate DF. Preventing weight gain in African American breast cancer survivors using smart scales and activity trackers: a randomized controlled pilot study. J Cancer Surviv. 2017;11(1):133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gold BC, Burke S, Pintauro S, Buzzell P, Harvey-Berino J. Weight loss on the web: a pilot study comparing a structured behavioral intervention to a commercial program. Obesity (Silver Spring). 2007;15(1):155–164. [DOI] [PubMed] [Google Scholar]
- 42. Laing BY, Mangione CM, Tseng CH, et al. Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients: a randomized, controlled trial. Ann Intern Med. 2014;161 (10 Suppl):S5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sturgeon KM, Dean LT, Heroux M, et al. Commercially available lifestyle modification program: randomized controlled trial addressing heart and bone health in BRCA1/2+ breast cancer survivors after risk-reducing salpingo-oophorectomy. J Cancer Surviv. 2017;11(2):246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thomas JG, Raynor HA, Bond DS, et al. Weight loss in weight watchers online with and without an activity tracking device compared to control: a randomized trial. Obesity (Silver Spring). 2017;25(6):1014–1021. [DOI] [PubMed] [Google Scholar]
- 45. Thomas JG, Raynor HA, Bond DS, et al. Weight loss and frequency of body-weight self-monitoring in an online commercial weight management program with and without a cellular-connected ‘smart’ scale: a randomized pilot study. Obes Sci Pract. 2017;3(4):365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Womble LG, Wadden TA, McGuckin BG, Sargent SL, Rothman RA, Krauthamer-Ewing ES. A randomized controlled trial of a commercial internet weight loss program. Obes Res. 2004;12(6):1011–1018. [DOI] [PubMed] [Google Scholar]
- 47. Chung S, Zhu S, Friedmann E, et al. Weight loss with mindful eating in African American women following treatment for breast cancer: a longitudinal study. Support Care Cancer. 2016;24(4):1875–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Griffith KA, Royak-Schaler R, Nesbitt K, et al. A culturally specific dietary plan to manage weight gain among African American breast cancer survivors: a feasibility study. Nutr Health. 2012;21(2):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stolley MR, Sharp LK, Oh A, Schiffer L. A weight loss intervention for African American breast cancer survivors, 2006. Prev Chronic Dis. 2009;6(1):A22. [PMC free article] [PubMed] [Google Scholar]
- 50. Wilson DB, Porter JS, Parker G, Kilpatrick J. Anthropometric changes using a walking intervention in African American breast cancer survivors: a pilot study. Prev Chronic Dis. 2005;2(2):A16. [PMC free article] [PubMed] [Google Scholar]
- 51. Finkelstein EA, Haaland BA, Bilger M, et al. Effectiveness of activity trackers with and without incentives to increase physical activity (TRIPPA): a randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(12):983–995. [DOI] [PubMed] [Google Scholar]
- 52. Bennett GG, Steinberg DM, Stoute C, et al. Electronic health (eHealth) interventions for weight management among racial/ethnic minority adults: a systematic review. Obes Rev. 2014;15 (Suppl 4):146–158. [DOI] [PubMed] [Google Scholar]
- 53. Allen JK, Stephens J, Dennison Himmelfarb CR, Stewart KJ, Hauck S. Randomized controlled pilot study testing use of smartphone technology for obesity treatment. J Obes. 2013;2013:151597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. [DOI] [PubMed] [Google Scholar]
- 55. Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med. 2002;162(18):2074–2079. [DOI] [PubMed] [Google Scholar]
- 56. Kwan ML, John EM, Caan BJ, et al. Obesity and mortality after breast cancer by race/ethnicity: the California breast cancer survivorship consortium. Am J Epidemiol. 2014;179(1):95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sheean PM, Hoskins K, Stolley M. Body composition changes in females treated for breast cancer: a review of the evidence. Breast Cancer Res Treat. 2012;135(3):663–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.