Abstract
As COVID-19 (coronavirus disease 2019) spreads across the world multiple therapeutic interventions have been tried to reduce morbidity and mortality. We describe a case of collapsing focal sclerosing glomerulosclerosis (FSGS) and acute oxalate nephropathy in a patient treated with high-dose intravenous vitamin C for severe COVID-19 infection. Collapsing FSGS has been described in patients with COVID-19 infection associated with APOL-1; however, this case had collapsing FSGS developing in low-risk heterozygous APOL-1 variant, and we postulate that the intensity of the COVID-19 cytokine storm overwhelmed the protective state of APOL-1 heterozygosity. This case illustrates the importance of assessing the risk and benefit of planned therapeutic interventions on a case-by-case basis especially when there are still so many unknowns in the management of COVID-19 infection. Strong consideration should be given for performing a renal biopsy in patients who develop multifactorial acute kidney injury.
Keywords: COVID, collapsing, glomerulopathy, FSGS, SARS, AKI, oxalate
Introduction
COVID-19 (coronavirus 2019) has stormed across the globe without a thorough understanding of its pathogenesis or accepted therapeutic approaches. All around the world multiple therapeutic interventions have been tried to mitigate the disease burden associated with COVID-19 infection. One of these treatment strategies involves high-dose vitamin C (ascorbic acid). This group and others have described the development of collapsing focal sclerosing glomerulosclerosis (FSGS) in patients with COVID-19 infection associated with APOL-1.1,2 We present a case of collapsing FSGS and acute oxalate nephropathy in a patient treated with high-dose intravenous (IV) vitamin C (ascorbic acid) for severe COVID-19 infection.
Case Report
A 64-year-old African American male with past medical history of hypertension, diabetes mellitus, chronic kidney disease stage III (without proteinuria), and HIV controlled on HAART (highly active antiretroviral therapy) presented to the emergency department from a nursing home for shortness of breath and fever. There was no past medical history of gastric bypass, bariatric surgery, small bowel resection, chronic pancreatitis, malabsorption syndromes, or prior history of vitamin C intake. His home medications included lantus, sitagliptin, metoprolol, amlodipine, HAART regimen, and allopurinol. Initial temperature was 101.9 °F, pulse was 76 beats per minute, respiratory rate was 26 breaths per minute, Blood Pressure was 132/72 mm Hg, and O2 saturation was 96% on 4 L/m oxygen. See Table 1 for laboratory results on presentation. He was started on broad-spectrum antibiotics and COVID-19 RNA polymerase chain reaction was positive. Chest X-ray revealed airspace opacity in the right upper lobe, peripheral right lower lobe, and left perihilar area. Computed tomography angiogram of the chest had no evidence of pulmonary embolism, but multifocal bilateral diffuse ground glass opacities were seen. He had worsening respiratory status and increasing oxygen needs and was eventually placed on non-rebreather oxygen and transferred to the intensive care unit within 24 hours of admission. He was treated with IV solumedrol, zinc, and vitamin C 3 g every 6 hours from hospital admission day 2 through 9 (total vitamin C dose 84 g). Initial urine studies had new proteinuria and hematuria (Table 1). He had acute kidney injury (AKI) with worsening azotemia, oliguria, and renal ultrasound of kidneys was unremarkable. On day 3, he was initiated on continuous renal replacement therapy (CRRT) due to anuric AKI with metabolic acidosis. CRRT with Oxiris filter (Baxter International Orixis filter, emergency use authorization by the Food and Drug Association to reduce pro-inflammatory cytokine levels in patients with confirmed COVID-19 infection) was used for 2 days and then CRRT with standard filter was continued for another 4 days. Worsening respiratory status on the fourth day required intubation and mechanical ventilation. He was switched to intermittent hemodialysis on hospital admission day 9. On day 11, he had a renal biopsy due to AKI with proteinuria and hematuria.
Table 1.
Laboratory Results on Presentation.
| Reference | On admission | |
|---|---|---|
| Hemoglobin, g/dL | 12.0-16.0 | 11.4 |
| WBC count, K/µL | 4.6-13.2 | 7.5 |
| Serum sodium, mmol/L | 136-145 | 143 |
| Potassium, mmol/L | 3.5-5.5 | 3.9 |
| BUN, mg/dL | 7.0-18 | 32 |
| Creatinine, mg/dL | 0.6-1.3 | 2.3 |
| eGFR, mL/min | >60 | 34 |
| Urinalysis protein | Negative | 500 |
| Urinalysis blood | Negative | Moderate |
| Urine RBC | 0-2/hpf | 20-50 |
| Urine WBC | 0-2/hpf | 5-10 |
| Urinalysis leucocyte esterase | Negative | Negative |
| Urine microscopy | 20-50 RBCs, but none were dysmorphic | |
| Urine protein: creatinine ratio | <0.2 | 2.74 |
| HbA1c | 4.8-5.6 | 5.7 |
| CD4 count | 404-1308 | 291 |
| HIV RNA | <20 copies | Target not detected |
Abbreviations: WBC, white blood cell; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; RBC, red blood cell; hpf, high-power field; HbA1c, hemoglobin A1c; HIV RNA, human immunodeficiency virus ribonucleic acid.
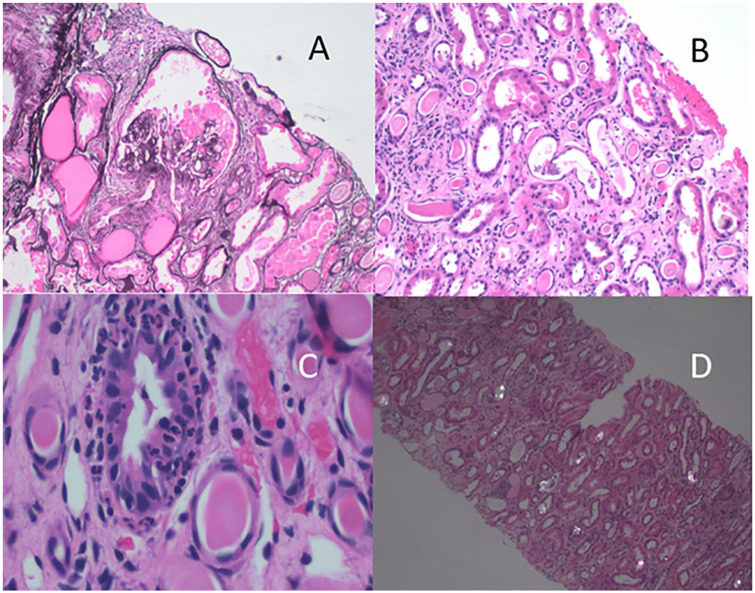
Renal biopsy had 6 glomeruli available, 3 were globally sclerosed and 1 of the remaining glomeruli showed epithelial hyperplasia, foam cells, and compression of glomerular capillaries (Figure 1A). The remaining glomeruli showed mesangial expansion. There was flattening of tubular epithelial cells with debris and cells within tubular lumena (Figure 1B). Occasional tubules showed neutrophils infiltrating among tubular epithelial cells (Figure 1C). These findings support the diagnosis of collapsing glomerulopathy.3 There was widely distributed polarizable material within tubular lumens (Figure 1D). There was interstitial fibrosis and tubular atrophy involving 25% to 30% of the tissue. Electron microscopy was negative for electron dense deposits and there was 80% effacement of epithelial cell foot processes. Basement membranes were of normal thickness. An instance of an epithelial cell retracted from basement membranes with condensed cytoplasm was seen. Additionally, there was evidence of acute tubular injury with focal pyelonephritis. No viral inclusions were seen.
Figure 1.
(A) Periodic acid–Schiff (PAS) stain showing epithelial proliferation and compression of the glomerulus. (B) Hematoxylin and eosin (H&E)-stained section showing dilated tubules with flattened epithelial cells; and cells and debris within tubular lumena. (C) H&E-stained section showing intraepithelial neutrophils. (D) H&E-stained section photographed with polarized light showing crystalline material within tubules.
Follow-up
COVID-19 symptoms have fully improved by 30 days after presentation, but the patient remains dialysis dependent. Patient’s APOL-1 risk variants were tested on serum sample at Labcorp and patient has heterozygous expression of wild type and G1 variants, which are considered lower risks for renal failure from collapsing FSGS compared with heterozygous or homozygous expression of higher risk variants.
Discussion
This case demonstrates 2 different renal pathologies in the setting of COVID-19 infection. The first is collapsing glomerulopathy due to COVID-19 infection. HIV was not considered as a cause of collapsing FSGS because HIV viral load was undetectable and CD4 count was more than 200. The second is oxalate nephropathy because of high-dose vitamin C use. Although occurring in the same patient these 2 findings are unrelated.
Collapsing glomerulopathy in COVID-19 infection is now well reported in patients homozygous for APOL-1.1,2,4 Of the cases reported so far, only one had evidence of direct viral infection from SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) whereas in the rest direct viral trophism was ruled out as possible etiology for renal injury. Collapsing FSGS in these cases is thought to be due to cytokine storm as a second hit in genetically predisposed patients. All reported cases were either homozygous or had 2 high-risk heterozygous variants of APOL-1. To our knowledge, this is the first case of collapsing FSGS associated with heterozygous expression of wild type and G1 variants, which are considered lower risks for renal failure from collapsing FSGS. It is possible that the intensity of the COVID-19 cytokine storm overwhelmed the protective state of APOL-1 heterozygosity for low-risk variants.
Oxalate nephropathy is an uncommon condition resulting in calcium oxalate deposition in renal tubules, which results in kidney injury. Primary forms are due to genetic mutation but more commonly seen are secondary forms like those related to gastric bypass, small bowel resection, Crohn’s disease, and chronic pancreatitis.5 High-dose vitamin C has also been implicated in secondary oxalate nephropathy. In 1985, Lawton and colleagues6 reported a case of secondary oxalate nephropathy after a single administration of 45 g of IV ascorbic acid as an adjuvant therapy for primary amyloidosis with nephrotic syndrome. Clinical presentation can be nonspecific with AKI and hematuria. Lumlertgul and colleagues5 reviewed 108 patients with secondary oxalate nephropathy and found that proteinuria was the most common urinary finding at 69%, followed by hematuria at 32%, oxalate crystal deposition was universally found in the tubules and/or the interstitium. High-dose IV vitamin C has shown some benefit in sepsis-related acute respiratory distress syndrome (ARDS). Marik and colleagues7 observed that in patients with sepsis and ARDS, high-dose IV vitamin C, hydrocortisone, and thiamine resulted in mortality reduction when compared with patients treated with standard procedures (mortality of 8.5% [4 of 47] in the treatment group compared with 40.4% [19 of 47] in the control group [P < .001]). In addition, less vasopressor support was needed in the treatment group compared with the control group. In a randomized control trial by Fowler and colleagues8 in 167 patients with sepsis-related ARDS, group treated with IV infusion of vitamin C (50 mg/kg) every 6 hours for 96 hours had lower mortality 29.8% (25/84) than the placebo group 46.3% (38/82; χ2 = 4.84; P = .03; between-group difference, 16.58% [95% confidence interval = 2% to 31.1%]).8 Consequently in several centers, vitamin C is part of treatment protocols for severe respiratory illness caused by COVID-19 infection. However, Fontana and colleagues9 just recently reported 2 cases of oxalate nephropathy caused by high-dose vitamin C in COVID-19 infection. Our patient developed AKI on chronic kidney disease due to multifactorial causes; factors related to COVID-19 infection, potential renal hypoperfusion, and therapeutic intervention, all of which provided milieu for secondary oxalate deposition due to high-dose vitamin C therapy. Serum and urine oxalate levels were not measured during IV vitamin C therapy to show causative effect of vitamin C toxicity on AKI, which is a limitation of our case report.
The prognosis of secondary forms of oxalate nephropathy is somewhat guarded as described in the report by Lumlertgul and colleagues,5 with 55% of patients requiring renal replacement therapy. None of the patients had complete recovery and 58% progressed to end-stage renal disease. Some cases of oxalate nephropathy have been treated empirically with prednisone with varied results. Our patient received IV solumedrol for 7 days as part of COVID-19 treatment protocol, but we did not continue steroids beyond that due to limited evidence of its benefit.
This case highlights 4 important considerations. First, collapsing FSGS may occur even in lower risk heterozygous APOL-1 variants when the second hit is as overwhelming as seen in severe COVID-19 infection. Second, high-dose IV vitamin C therapy in COVID-19 should be evaluated in prospective randomized studies. Third, high-dose IV vitamin C therapy in COVID-19 should be used only after assessing for risk factors for oxalate nephropathy and if patient develops renal insufficiency vitamin C should be abruptly discontinued and serum oxalate levels measured to determine if vitamin C toxicity is the possible cause of renal failure. Finally, the importance of renal biopsy to determine the exact etiology of AKI cannot be overemphasized in a complex case like this.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Dean A. Troyer  https://orcid.org/0000-0002-0337-5890
https://orcid.org/0000-0002-0337-5890
References
- 1. Larsen CP, Bourne TD, Wilson JD, Sagga O, Sharshir MA. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19). Kidney Int Rep. 2020;5:935-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Magoon S, Bichu P, Malhotra V, et al. COVID-19-related glomerulopathy: a report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med. 2020;2:488-492. doi: 10.1016/j.xkme.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. D’Agati VD, Alster JM, Jennette JC, et al. Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol. 2013;8:399-406. doi: 10.2215/CJN.06100612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peleg Y, Kudose S, D’Agati VD, et al. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;5:940-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lumlertgul N, Siribamrungwong M, Jaber BL, Susantitaphong P. Secondary oxalate nephropathy: a systematic review. Kidney Int Rep. 2018;3:1363-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lawton JM, Conway LT, Crosson JT, Smith CL, Abraham PA. Acute oxalate nephropathy after massive ascorbic acid administration. Arch Intern Med. 1985;145:950-951. [PubMed] [Google Scholar]
- 7. Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151:1229-1238. [DOI] [PubMed] [Google Scholar]
- 8. Fowler AA, 3rd, Truwit JD, Hite RD, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322:1261-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fontana F, Cazzato S, Giovanella S, et al. Oxalate nephropathy caused by excessive vitamin C administration in 2 patients with COVID-19. Kidney Int Rep. Published online July 15, 2020. doi: 10.1016/j.ekir.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]