Abstract
Background:
Contact with livestock wastewater on farms and in communities can pose a risk to human and animal health.
Methods:
This cross-sectional study was conducted in 180 households and 24 pig farms (96 wastewater samples) to explore information about pig production, livestock waste management, antibiotic use, and to analyze antibiotic residues and microbial contamination, respectively.
Results:
Of the 120 households raising pigs, biogas systems were the most commonly used to treat animal waste (70%), followed by compositing (19%), and the remaining respondents discharged waste directly into drains or ponds (11%). The majority of respondents (78%) used antibiotics to treat and prevent disease in pigs, but 32% of them did not know of any disadvantages of antibiotic abuse. ELISA assays were performed on half of the wastewater samples (n = 48), demonstrating that residues of flouroquinolones and sulfonamides were present in 6.3% (3/48) and 22.9% (11/48) of tested samples, respectively. The average residual level of sulfamethazine was 27.8 ug/l. Further, E. coli concentrations exceeding regulatory levels in Vietnam were found in nearly all samples. Salmonella spp. was also found in 57.3% of samples, though prevalence rates varied across the different sites. Finally, G. lamblia was found in 8.4% of samples, and C. parvum was found in 5.2% of samples.
Conclusions:
This study suggests that livestock wastewater carried potential harmful pathogens and antibiotic residues that could come into contact with humans in the community. Thus, appropriate operation and application of livestock wastewater treatment (such as biogas or composting) and management should be a continued focused.
Keywords: Antibiotic residue, microbial contamination, swine farms, wastewater, vietnam
Introduction
Agricultural activities make an important contribution to the livelihoods of households in developing countries. Utilization of wastewater is common in agriculture and aquaculture,1 though contact with wastewater from livestock farms, communities, and agricultural fields can pose a risk to human and animal health.2-6 Potential health risks derive from the presence of harmful bacteria and antimicrobial residues in the wastewater, which can result in infection and promote antimicrobial resistance.
The use of antibiotics in agriculture is known to be a major driver behind increased resistance profiles in bacteria,7,8 and untreated livestock waste is a source of antibiotic pollution in the environment. The level of the antibiotic residues in the environment as a result of wastewater varies upon the source from which it is discharged. However, antibiotic pollution and antimicrobial use in food animals often lacks regulation.7 As livestock production intensifies, there are mounting challenges to managing livestock waste discharged into the environment, particularly from pig husbandry. As such, livestock wastewater management is becoming a major concern for both public and environmental health, and is of particular concern in developing countries where water treatment infrastructure is less established.9
In Vietnam, animal husbandry is the fastest growing field within the agricultural sector,10 leading to large accumulation of animal waste in communities. Farmers and their surrounding communities are in regular contact with wastewater from both livestock and households. At the community level, waste treatment systems are often underdeveloped or lack proper management, so livestock waste and sewage are not adequately treated. For instance, biogas systems and composting have been applied to treat livestock manure and waste in some areas. However, many of these systems are limited in functionality or are poorly operated.3,11 Therefore, the potential for release of harmful pathogens and substances remains.
Like other countries in the South East Asian region, Vietnam has a relatively high prevalence of preventable and emerging infectious diseases.12 An epidemiological study in Vietnam showed that close contact with wastewater was associated with increased risk of diarrheal disease in adults.13 The most common infectious disease is diarrhea, which is predominantly caused by Salmonella spp., Escherichia coli, Giardia lamblia, and Cryptosporidium parvum.3,4 A known source of these harmful pathogens is untreated wastewater from drainage, ponds, or agricultural fields.
The presence of antibiotics in livestock wastewater generates resistance to the drugs in commensal and environmental bacteria, which can reduce effectiveness of the drug to treat humans and animals. However, little is known about how antibiotic residues and pathogens in wastewater flow from pig farms and affect agricultural fields. In addition, there is little knowledge around the practices and knowledge of Vietnamese farmers with regards to antibiotics and wastewater use in agriculture and aquaculture. The objective of this study was to quantify the level of antibiotic residues and pathogen contamination present in wastewater that travelled from pig farms to agricultural fields. In addition, this study explores farmers’ knowledge and practices on antibiotic and wastewater use and management.
Materials and methods
Study site
The location of the study, Hoang Tay commune (Kim Bang district, Ha Nam province), has characteristics typical of the geographic regions spanning the Red River delta and Nhue River (Figure 1). Hoang Tay has about 485 ha of total natural land area, of which approximately 334 ha (68.8%) is agricultural land. In 2015, there were 1862 households holding 5325 inhabitants in Hoang Tay commune. Agriculture is widely practiced in the commune, with the percentage of households engaged in the agricultural industry estimated at 81 percent.14 Hoang Tay is a growing peri-urban area close to urban markets, though socioeconomic conditions are generally low.
Figure 1.
Study site of Hoang Tay commune, Kim Bang district, Ha Nam Province.
In 2015, Hoang Tay was ranked as a commune with one of the highest rates of pig production in Kim Bang district. As in wider Vietnam, smallholder pig farms in Hoang Tay are established alongside homes, with modest pig pens constructed to keep a low number of fattening pigs or sows (typically less than 50 pigs or five sows). As such, most pig farms (88.8%) are located within the human residential areas.14 Farmers in Hoang Tay usually utilize manure and household livestock wastes for agricultural and aqua-cultural producing activities, such as irrigation of vegetables and rice, or feeding fish.
Study design and scope
This cross-sectional study was conducted between June and August 2015. The study scope included a quantitative survey of households, as well as wastewater sampling for biological and antibiotic residue analyses. The study also examined pig farms in proximity to communal and wider agricultural areas in Hoang Tay commune. Wastewater flow from pig pens outlets, household outlets, communal drainages, and agricultural fields were targeted for the study.
Household survey and sample size
A household survey was carried out using a structured questionnaire, which was developed by the research team. The questionnaire was piloted to finalize form and contents prior to collecting data used in the final study. The questionnaire collected information regarding: (i) farmer demographics, (ii) livestock and farm management, (iii) livestock waste use on farms, (iv) knowledge on antibiotics (v) attitudes towards the use of antibiotics in livestock production, and (vi) practices regarding antibiotic use in pig raising. Respondents were those who were primarily responsible for farming activities from each household approached.
The required number of participating households was calculated based on the determination of a single proportion, as per the below formula. The average proportion of households using antibiotics for disease prevention on pig farms (P) was 10.9 percent.15 The precision estimate (d) was set at 5%, confident interval of 95%, Z value = 1.96, and the design effect of the survey was set at 1.2. The total number of households interviewed was 180.
Sample collection
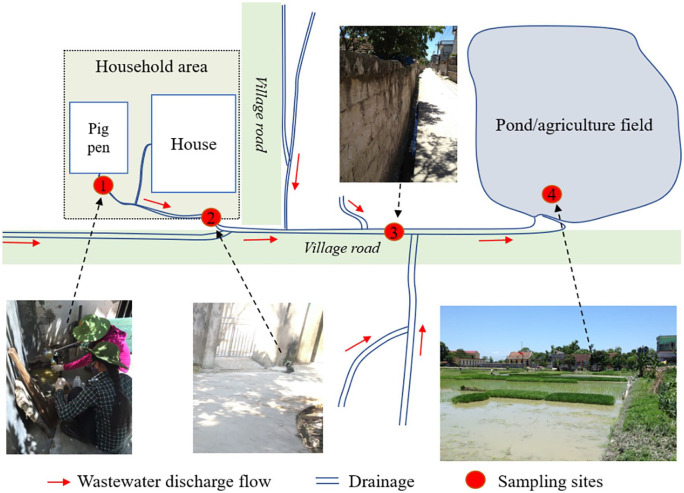
Following the survey of 180 households, a subset of 24 pig farms were selected for sampling based on the farms’ locations in different villages and their wastewater discharge flow within villages, residential areas, and agricultural fields. The selected farms were also identified based on connections between farms, drainage areas, and agricultural fields in Hoang Tay commune. From each selected farm, four sampling points were identified to get a total number of 96 wastewater samples (Figure 2).
Figure 2.
Wastewater discharge flow from pig farms and sampling sites.
Wastewater sampling was conducted following the guidelines for wastewater sampling techniques according to Vietnamese standards, TCVN 6663-10. Approximately 1 L of wastewater was taken to be shared across three separate sterile bottles using disinfected stainless steel dippers. Pond or canal water samples were taken at approximately 20 cm below the water’s surface. Bottles were then labelled with sample type, code, and location. These samples were used to test for presence and concentration of bacteria (Salmonella spp. and E. coli), protozoa (C. parvum and G. lamblia), and antibiotic residues. Fluoroquinolone and sulfonamide antibiotic groups were selected for analysis as these are commonly used in livestock, residues have recently been found in pork meat,16 and they are important for public health. All samples were kept in cool box (2-8°C) for transport to laboratories and tested on the same day.
Laboratory analysis
Antibiotic residue analysis: Two antibiotic groups selected for testing were fluoroquinolones and sulfonamides. The antibiotic residue test was conducted at the Laboratory of the Center for Veterinary Hygiene and Inspection No.1 (Hanoi, Vietnam). Samples were first analyzed using the enzyme-linked immunosorbent assay (ELISA, BIOOSCIENTIFIC, MaxSignalTM) method. Positive samples from ELISA screenings were quantitatively assessed using liquid chromatography–mass spectrometry/mass spectrometry (LCMSMS, following AOAC 2007.01) for specific antibiotics. Detection limits and procedure of the analytical method and for each specific antibiotic group were also reported by Tuyet-Hanh et al.16 Due to limited resources, one of every two constitute samples was randomly selected to analyze antibiotic residues. Therefore, only 48 out of 96 samples were used to carry out these analyses.
Microbiology analysis: Microbiological analysis was performed by the National Institute of Veterinary Research, Vietnam. Depending on the wastewater sample types, wastewater samples were prepared at appropriate tenfold dilutions (such as 10–1, 10–2, etc.) using sterile Peptone Water (Merck, Darmstadt, Germany). Brilliance E. coli/coliform Selective Agar (CM1046, Oxoid) was then used to isolate and enumerate E. coli from wastewater samples. The surface of the agar plates was dried before pipetting 0.1 ml of the prepared sample onto each plate and spread over the surface with a sterile spreader. The plates were then incubated for 24 hours at 37°C. The growth of dark purple to indigo blue colonies on agar plates indicated presence of E. coli. Isolation and enumeration of E. coli procedure was followed, as described by Le-Thi et al.3 According to Vietnamese standards for livestock wastewater,17 when the concentration of E. coli in a sample was above 5000 CFU/100 ml, the sample was considered positive.
Salmonella spp. detection was carried out according to the ISO 6579:2002. The wastewater samples were diluted 1:10 in buffered peptone water medium (Merck, Darmstadt, Germany). Then, first and second selective enrichment was consequently incubated in MSRV (Merck, Darmstadt, Germany) agar and MKTTn broth (Merck, Darmstadt, Germany), then in XLT (Merck, Darmstadt, Germany) and Rambach (Merck, Darmstadt, Germany) agar, respectively. Biochemical confirmation of Salmonella was done by selecting typical Salmonella colonies from both XLT and Rambach agar plates to test for Lactose, Indol, Lysine, H2S, and Urease phenotypes, etc. Another one to two colonies were inoculated onto Nutrient Agar (NA; Merck, Germany) to grow Salmonella for serological confirmation, using Antiserum Salmonella Polyvalent-O (Bio-Rad, France). Both biochemical and serological tests were performed as described previously by Dang-Xuan.18
G. lamblia and C. parvum were detected and quantified using immunofluorescent kits Crypto/Giardia CEL; Cellabs Pty Ltd, Australia, which were used in accordance with manufacturers’ instructions. Testing procedure was described previously by Le-Thi.3 These tests were done at the National Institute for Hygiene and Epidemiology (Hanoi, Vietnam). Sample types and number of collected and tested samples was shown in Table 1.
Table 1.
Sample types and number of collected samples and laboratory tests.
| Sample type | Salmonella (yes/no, MPN/100 ml) | E. coli (yes/no, logCFU/100 ml) | G. lamblia (yes/no, cyst/100 ml) | C. parvum (yes/no, cyst/100 ml) | Fluoroquinolone/ Sulfonamide* |
|---|---|---|---|---|---|
| Pig pen outlet | 24 | 24 | 24 | 24 | 12 |
| Household wastewater outlet | 24 | 24 | 24 | 24 | 12 |
| Communal drainage | 24 | 24 | 24 | 24 | 12 |
| Agricultural field | 24 | 24 | 24 | 24 | 12 |
| Total | 96 | 96 | 96 | 96 | 48 |
Screening first by ELISA (yes/no), ELISA positive samples were confirmed by LSMSMS.
Data analysis
Data obtained from the questionnaire and laboratory tests was encoded and entered using EpiInfo 6.0 (USA cop.) with quality checks to ensure accuracy. The data was then exported to .csv files and R software (R core Team, 2015)19 was used for analysis. Frequency tables were used to describe tendencies, and cross tabulations were used to compare sub-groups. Descriptive statistics were used to gain statistically significant (P ⩽ .05) conclusions from the data.
Ethical clearance
This study received ethical clearance from the Institutional Review Board of Hanoi University of Public Health, No. 041/2013/YTCC-HD3, with collaboration document No. 3774/BNN-HTQT-2015 allowing collaboration between the Ministry of Agriculture and Rural Development and the local authority in Ha Nam province in 2015.
Results
General information of surveyed households
A total of 180 households were recruited in the study; no respondents refused the interview. Households involved in the study had an average of four members (R: 1-8). The majority of respondents were female (57.2%) and were an average of 52 years old (R: 20-84). Most respondents (83.9%) had an education at or below secondary school levels. Levels of education did not significantly differ between males and females enrolled in the study.
In this study, 146 out of 180 households surveyed (81.1%) were involved livestock activities. Most of the households breeding or raising livestock primarily produced pigs (120/146, 82.2%), with other households raising livestock including chicken, cattle, and ducks. Livestock farming was the highest reported primary occupation amongst respondents (40%), followed by paddy farming (22.8%). Among households producing pigs, most of the households (94.2%) kept sows to breed piglets for sale or fattening. The average number of pigs per household was 8 (R: 1-130), though this was a mixture of sows, piglets, and fattening pigs.
Antibiotic residue in wastewater from pig farms to community
Of the 48 water samples tested for antibiotic residues, three (3/48, 6.3%) were positive for fluoroquinolones and eleven (11/48, 22.9%) were positive for sulfonamides. Fluoroquinolones were found in household wastewater outlets (2/12, 16.7%), communal drainage (2/12, 16.7%) and agricultural field (7/12, 58.3%) samples. However, none of these samples were above level of detection (LOD: 5 μg/l) for fluoroquinolones in confirmation analysis. There was 27.8 µg/L sulfamethazine (a sub-group of sulfonamides) residues detected in household wastewater and 14.6 µg/L in agricultural field samples. As such, sulfonamides were detected at a higher rate than fluoroquinolones, however there was no sample that tested positive for both antibiotic groups (Table 2).
Table 2.
Prevalence and level of fluoroquinolone and sulfonamide residues in wastewater samples.
| Sample type |
Screening
result by ELISA (No. positive (%)) |
Confirmation test by
LSMSMS (mean (μg/l)) |
||||||
|---|---|---|---|---|---|---|---|---|
| Flouroquinolones | Sulfonamides | |||||||
| Flouroquinolones | Sulfonamides | Enroflorxacine | Norflorxacine | Flumequine | Sulfamethazine | Sulfadiazine | Sulfaquinoxaline | |
| Pig pen outlet (n = 12) | 0 (0) | 0 (0) | na | na | na | na | na | na |
| Household wastewater outlet (n = 12) | 2 (16.7) | 2 (16.7) | –** | – | – | 27.8 | – | – |
| Communal drainage (n = 12) | 0 (0) | 2 (16.7) | na | na | na | – | – | – |
| Agricultural field (n = 12) | 1 (8.3) | 7 (58.3)* | – | – | – | 14.6 | – | – |
Note: (*) not significant difference (P = .09, Fisher exact test); na: not available; (**) below level of detection (LOD: <5 μg/l for flouroquinolones and < 10 μg/l for sulfonamides).
Microbial prevalence in wastewater flow
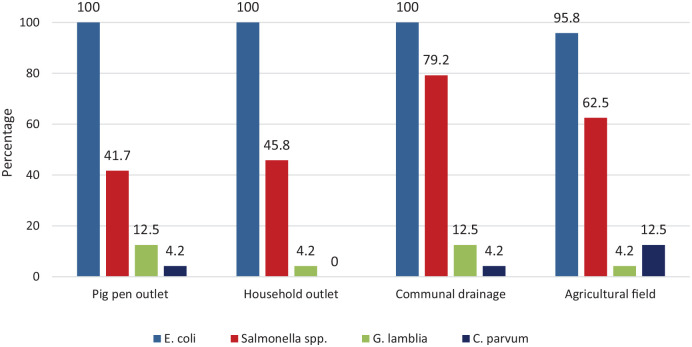
E. coli was detected in most samples, with all samples collected from pig pens, household wastewater outlets and communal drainage areas testing positive. A further 95.8% of samples collected from agricultural fields tested positive for E. coli. Positive samples exceeded regulatory standards for E. coli concentration in treated wastewater (<5000 CFU/100 ml). The prevalence of Salmonella spp. was also relatively high, with 41.7% of pig pen, 45.8% of household wastewater outlet, 79.2% of communal drainage area, and 62.5% of agricultural field samples testing positive. G. lamblia was detected in 12.5% of pig pen and communal drainage samples, as well as 4.2% of household wastewater outlets and agricultural field samples. C. parvum was detected in 12.5% of agricultural fields, 4.2% of pig pens and communal drainage areas, and was not detected in samples from household outlets (Figure 3).
Figure 3.
Prevalence of E. coli, Salmonella spp., G. lamblia and C. parvum in wastewater samples.
Microbial concentration in wastewater flow from smallholder pig farms
Concentration of Salmonella spp., E. coli, G. lamblia and C. parvum in wastewater samples is shown in Table 3. The area with the highest prevalence of Salmonella was community drainage areas, with the lowest concentrations found in household outlets. Concentration of G. lamblia and C. parvum were found highest in pig pen outlet samples, average of 85 and 7 cyst/100 ml, respectively.
Table 3.
Concentration of E. coli, Salmonella spp., G. lamblia and C. parvum in wastewater samples.
| Pathogens and locations | Mean | SD | Min | Max | P-value |
|---|---|---|---|---|---|
| E. coli (LogCFU/100 ml) | |||||
| Pig pen outlet | 7.7 | 0.9 | 5.0 | 8.7 | Ref |
| Household outlet | 7.1 | 0.8 | 5.0 | 8.7 | .03 |
| Communal drainages | 7.1 | 1.0 | 5.4 | 9.3 | .03 |
| Agricultural field | 5.0 | 0.9 | 3.0 | 6.3 | <.01 |
| Salmonella spp. (MPN/100 ml) | |||||
| Pig pen outlet | 2497 | 4540 | 30 | 11000 | Ref |
| Household outlet | 164 | 263 | 30 | 930 | .10 |
| Communal drainages | 1441 | 1966 | 30 | 4600 | .52 |
| Agricultural field | 379 | 822 | 30 | 2400 | .09 |
| G. lamblia (Cyst/100 ml) | |||||
| Pig pen outlet | 136 | 229 | 4 | 400 | NA |
| Household outlet | 28 | 0 | 0 | 28 | NA |
| Communal drainages | 5.3 | 2.3 | 4 | 8 | NA |
| Agricultural field | 12 | 0 | 0 | 12 | NA |
| C. parvum (Cyst/100 ml) | |||||
| Pig pen outlet | 32 | 0 | 0 | 32 | NA |
| Household outlet | 0 | 0 | 0 | 0 | NA |
| Communal drainages | 8 | 0 | 0 | 8 | NA |
| Agricultural field | 9.3 | 2.3 | 8 | 12 | NA |
Abbreviations: *SD, standard deviation; MPN, Most probable number; CFU, colony forming unit.
Knowledge on animal feed, antibiotics, and livestock waste use at farms
The rate of smallholder farmers who indicated they knew antibiotics are present in commercial feed was 17.8 percent. Nearly half of farmers (47.2%) did not know about the presence of antibiotics in commercial feed. Responses showed that most participants (77.8%) knew antibiotics were used to treat and prevent pig disease. However, approximately 20% of respondents did not report to know of any advantages in using antibiotics. Further, approximately 32% did not report to know any disadvantages of antibiotic use (Table 4).
Table 4.
Knowledge on antibiotics in feed, using antibiotics, and utilize livestock waste.
| Information | Percentage |
|---|---|
| Knowledge of the presence of antibiotics in commercial feed (n = 180, single choice) | |
| Yes | 17.8 |
| No | 35 |
| Don’t know | 47.2 |
| Advantages of antibiotics (n = 180, multiple choice) | |
| Disease prevention and treatment for pigs | 77.8 |
| Enhance the immune system of pigs | 4.4 |
| Improve weight gain of pig | 1.7 |
| Other (no advantage) | 0.6 |
| Don’t know | 19.9 |
| Effect of antibiotics abuse (n = 180, multiple choice) | |
| No harmful effects | 2.8 |
| Digestive disorders | 41.7 |
| Antibiotic resistance | 5 |
| Antibiotic residues in pork | 18.9 |
| Others (death, abortion, no deliveries. . .) | 8.9 |
| Don’t know | 31.7 |
| Advantage of utilizing livestock waste in agricultural production (n = 159, multiple-choice) | |
| Crops/fish get more nutrients and grow faster | 96.9 |
| Crops/fish prevent diseases better | 10.1 |
| Restrict harmful insects | 1.3 |
| Reduce cost for fertilizers | 5 |
| No benefits | 1.26 |
| Disadvantages of livestock waste use in agricultural production (n = 159, multiple-choice) | |
| Safe, not any problems | 52.2 |
| Crops can get disease/insects | 17 |
| Unsafe for consumer | 10.1 |
| Pollution to environment | 5 |
| Don’t know | 15.7 |
Attitude towards the use of antibiotics in livestock production
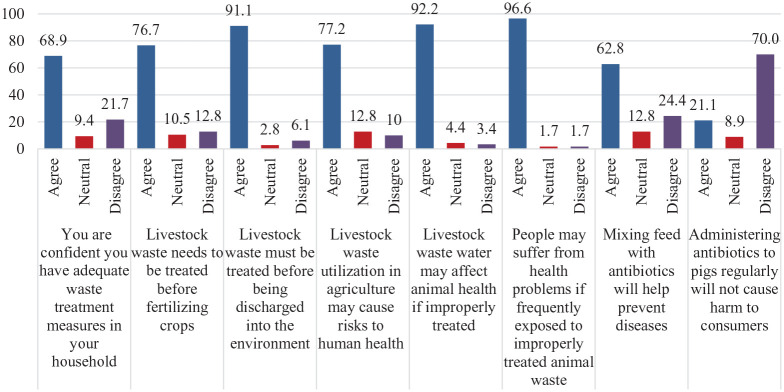
Almost two-thirds of respondents (63%) agreed that mixing feed with antibiotics would help to prevent diseases. Of those participants, about 24% thought that using the appropriate type and dosage of drugs might not be enough to prevent disease in their herds. Seventy percent (70%) of respondents agreed that using antibiotics while raising pigs can affect humans. Approximately 69% of surveyed households self-reported that waste treatment in their family was reasonable, though data on actual practices was not captured. The vast majority (91%) agreed that livestock waste must be treated before being discharged into the environment. Regarding health risk, 77% of respondents reported that the reuse of livestock waste for agricultural activities might cause risks to human health, and almost all participants (96.6%) agreed that people may suffer from health problems when they are frequently exposed to improperly treated animal waste (Figure 4).
Figure 4.
Attitude towards livestock waste and using antibiotics in livestock production
Practices with regards to using antibiotic at smallholder pig farm
Of 120 households raising pigs, 101 households (84.2%) reporting administering antibiotics to pigs. Responses indicate that reasons behind antibiotic use were the treatment of sick pigs (99%) or because pigs had refused to eat (21.8%). For 74.3% of respondents, a local veterinarian sold the drugs, and for the remaining 25.7% of households, other drug retailers were consulted. Respondents often bought drugs to self-administer to their pigs (Table 5).
Table 5.
Practices including administering antibiotics to pigs, disposing of dead pigs and frequency of farm disinfection (n = 101).
| Information | % |
|---|---|
| When do you use antibiotics (multi-choice questions) | |
| Pigs are sick (with abnormal signs/symptoms) | 99.0 |
| Pigs refuse to eat | 21.8 |
| Other (at least one individual pig is ill) | 2.0 |
| Action when your pigs are sick (multi-choice questions) | |
| Call veterinarian for advice and treatment | 80.8 |
| Consult drug retailers and buying drugs to self-treat | 40.8 |
| Self-diagnosis and medication when pigs suffer with less severity diseases | 33.3 |
| Action when your pigs are dead (multi-choice questions) | |
| Sell for relatively low price | 34.2 |
| Throw into landfill, lake, or pond (or feeding fish) | 52.5 |
| Bury or put in the biogas system | 40.8 |
| No answer | 3.3 |
| Frequency of farm disinfection | |
| At least once a week | 14.2 |
| At least once a month | 21.7 |
| Only after selling of pigs | 32.1 |
| Whenever outbreaks occur | 2.8 |
| Sometimes when feel needed | 29.2 |
Discussion
This study showed a high contamination of E. coli and Salmonella in the wastewater flow from smallholder pig farms to agricultural fields. However, contamination in the community and agricultural field pollution was also a result of household wastewater. Findings of this study also demonstrate that fluoroquinolone and sulfonamide residues are present in wastewater in communities and agricultural fields.
According to Vietnamese standards regarding livestock wastewater, wastewater should contain less than 5000 CFU/100 ml of E. coli. Therefore, the levels of E. coli detected in wastewater output and drainage samples are all over this threshold. In addition, a 50 ml sample of livestock wastewater is not allowed to contain Salmonella according to the Vietnamese standard for livestock wastewater (QCVN_2010-1-15/BNN).17 However, in this study, most of wastewater samples did not met this criterion for Salmonella contamination, especially for water samples from communal drainages and agricultural fields.
The antibiotic residue levels found in tested samples showed that there is evidence of antibiotics in wastewater, which might induce antibiotic resistance in environmental microflora. The frequency of sulfamethazine detected in wastewater demonstrated the use of this antibiotic in the community and overuse or abuse this antibiotic in livestock. However, concentrations present in wastewater were within range of the Vietnamese maximum residue standards. Fluoroquinolones are frequently used in veterinary medicine to treat infections of E. coli, Salmonella, Pasteurella, Mycoplasma, and Hemophilus spp. On the other hand, sulfonamide is widely used as a feed additive, mainly for fattening of calves and pigs. Sulfonamides are also used in veterinary medicine for the treatment of intestinal infections, mastitis, and other diseases. With improper use, fluoroquinolone and sulfonamide residues may persist in food of animal origin (fish, shrimp and pork) and the environment (wastewater, soil). In order to help address this risk, the Vietnamese government has banned the use of flouroquinolones in aquaculture.
E. coli, Salmonella, and protozoan contaminated wastewater, which is used for agriculture irrigation, can affect to farmers.20,21 However, there is evidence to show that farmers only consider health risks to result from pungent faeces, and adverse health effects to be a result of ‘polluted’ air.22 Le-Thi et al reported that the annual diarrhea risk due to exposure to biogas effluent via irrigation activities ranged from 17.4% to 21.1% (E. coli O157:H7), 1.0% to 2.3% (G. lamblia), and 0.2% to 0.5% (C. parvum).3 In addition, utilizing wastewater for agriculture has also contributed to increased risks of helminth infections, for example Ascaris (round worm), Trichuris (whipworm), and hookworm.23 Therefore, proper use of biogas systems and treatment of wastewater before using it in agriculture should be a focus in Vietnam, with guidance provided on how farmers can ensure water meets treatment standards.
As in other developing countries, Vietnamese farmers often directly buy drugs or antibiotics to use for their animals, since veterinary drugs are sold over-the-counter at many places in the province and commune.24,25 Our study indicated that almost all respondents (99%) reported using antibiotics as soon as their pigs showed signs of ill health. Though farmers seek advice and support from local veterinarians, they tend to treat their pigs themselves without prescription or oversight. With low awareness and knowledge on antibiotics, this practice can facilitate antibiotic resistance.25-27 As such, relevant stakeholders in antibiotic management (such as local veterinarians) should raise awareness on proper use of antibiotics in agriculture. Relevant information could include the need to use recommended dosages, recommended administration of antibiotics, and to not combine several antibiotics if not necessary.
This study has several limitations. First, due to non-random sampling employed in this study, the samples might not reflect or represent any particular pig farming practices or waste management scheme. Second, this study only examined the residues of two groups of antibiotics and did not examine the antimicrobial resistance of the isolated strains from collected samples. Samples in this study were collected over a short period without significant seasonal variation, spanning June to August 2015. To assess the potential for seasonal factors to alter levels of antibiotic residues and pathogen load in wastewater, further research is required. However, the findings of this survey have indicated the load of harmful pathogens and antibiotic residues in wastewater at a point in time.
Conclusion
Results of this study suggest that wastewater in the community carried potential harmful pathogens and antibiotic residues that could come into contact with humans. The wastewater flow quality needs to be improved considerably to protect farming communities downstream of Hanoi. Antibiotic contamination of water represents a further public health threat as it facilitates antibiotic resistance in microbial flora. As such, wastewater needs better management to reduce bacterial contamination to safe levels prior to use in aquaculture and agriculture. Control measures to treat wastewater are available, including composting and biogas treatment systems. Thus, appropriate operation and application of waste, especially wastewater, should be a continued focused.
Acknowledgments
The authors thank researchers at the Institute of Sustainable Environmental Health and Development and at the Center for Public Health and Ecosystem Research (CENPHER), Hanoi University of Public Health (HUPH) to assist in data collection. We thank Dr. Nhiem Duong-Van (Vietnam national Agriculture University), Mr. Thanh Nguyen-Tien and Ms. Nguyen Thi Mai Huong (CENPHER, HUPH) for their assistance in the field work, and Ms. Nguyen Thi Thu Thao (CENPHER, HUPH) for her assistance in data analysis.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contributions: Conceptualization: PP-D, HN-V, TL-Q, CP-D, DP, TD-T, SD-X; Formal analysis: SD-X, PT-T-M ; Investigation: TL-Q, PT-T-M, SD-X ; Methodology: PP-D, HN-V, CP-D, TD-T ; Project administration: PP-D, TD-T ; Interpretation of Data: HN-V, MAC, CP-D, DP, DG, SD-X; Writing- original draft, review and editing: PP-D, HN-V, TL-Q, MAC, DP, TD-T, DG, SD-X. All the authors reviewed the final manuscript and approved it for submission.
ORCID iDs: Meghan A. Cook  https://orcid.org/0000-0002-7964-6781
https://orcid.org/0000-0002-7964-6781
Sinh Dang-Xuan  https://orcid.org/0000-0002-0522-7808
https://orcid.org/0000-0002-0522-7808
References
- 1. Qadir M, Wichelns D, Raschid-Sally L, et al. The challenges of wastewater irrigation in developing countries. Agric Water Manag. 2010;97:561-568. [Google Scholar]
- 2. Hanjra MA, Blackwell J, Carr G, Zhang F, Jackson TM. Wastewater irrigation and environmental health: implications for water governance and public policy. Int J Hyg Environ Health. 2012;215:255-269. [DOI] [PubMed] [Google Scholar]
- 3. Le-Thi T, Pham-Duc P, Zurbrugg C, et al. Diarrhea risks by exposure to livestock waste in Vietnam using quantitative microbial risk assessment. Int J Public Health. 2017;62:83-91. [DOI] [PubMed] [Google Scholar]
- 4. Pham-Duc P, Nguyen-Viet H, Hattendorf J, et al. Diarrhoeal diseases among adult population in an agricultural community Hanam province, Vietnam, with high wastewater and excreta re-use. BMC Public Health. 2014;14:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Utzinger J, Brattig NW, Leonardo L, Zhou XN, Bergquist R. Progress in research, control and elimination of helminth infections in Asia. Acta Trop. 2015;141:135-145. [DOI] [PubMed] [Google Scholar]
- 6. Van Vu T, Pham PD, Winkler MS, et al. Estimation of involuntary excreta ingestion rates in farmers during agricultural practices in Vietnam. Hum Ecol Risk Assess. 2019;25:1942-1952. [Google Scholar]
- 7. Landers TF, Cohen B, Wittum TE, Larson EL. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep. 2012;127:4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McEwen SA, Fedorka-Cray PJ. Antimicrobial use and resistance in animals. Clin Inf Dis. 2002;34 Suppl 3:S93-s106. [DOI] [PubMed] [Google Scholar]
- 9. Martinez J, Dabert P, Barrington S, Burton C. Livestock waste treatment systems for environmental quality, food safety, and sustainability. Bioresour Technol 2009;100:5527-5536. [DOI] [PubMed] [Google Scholar]
- 10. Dinh-Xuan T. An Overview of Agricultural Pollution in Vietnam: The Livestock Sector Washington, DC: The World Bank; 2017. [Google Scholar]
- 11. Yen-Phi VT, Clemens J, Rechenburg A, Vinneras B, Lenssen C, Kistemann T. Hygienic effects and gas production of plastic bio-digesters under tropical conditions. J Water Health. 2009;7:590-596. [DOI] [PubMed] [Google Scholar]
- 12. Coker RJ, Hunter BM, Rudge JW, Liverani M, Hanvoravongchai P. Emerging infectious diseases in southeast Asia: regional challenges to control. Lancet. 2011;377:599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Do TT, Bui TT, Molbak K, Phung DC, Dalsgaard A. Epidemiology and aetiology of diarrhoeal diseases in adults engaged in wastewater-fed agriculture and aquaculture in Hanoi, Vietnam. Trop Med Int Health. 2007;12 Suppl 2:23-33. [DOI] [PubMed] [Google Scholar]
- 14. Hoang Tay. Report on Health Care Program in First-half of Year 2015 and Planning for Second Half of Hoang Tay Commune. Ha Nam: Hoang Tay Health Station; 2015. [Google Scholar]
- 15. Dang Pham Kim CS, Douny Caroline, Dinh Ton Vu, et al. First survey on the use of antibiotics in pig and poultry production in the red river delta region of vietnam. Food Public Health. 2013;3:247-256. [Google Scholar]
- 16. Tuyet-Hanh TT, Sinh DX, Phuc PD, et al. Exposure assessment of chemical hazards in pork meat, liver, and kidney, and health impact implication in Hung Yen and Nghe An provinces, Vietnam. Inter J Public Health. 2017;62:75-82. [DOI] [PubMed] [Google Scholar]
- 17. BTNMT. QCVN 62-MT:2016/BTNMT. National Technical Regulation on the Effluent of Livestock. Hanoi; 2016. [Google Scholar]
- 18. Dang-Xuan S, Nguyen-Viet H, Unger F, et al. Quantitative risk assessment of human salmonellosis in the smallholder pig value chains in urban of Vietnam. Int J Public Health. 2017;62:93-102. [DOI] [PubMed] [Google Scholar]
- 19. RStudio Team. RStudio: Integrated Development for R. Boston, MA: RStudio, Inc; https://rstudio.com/products/team/. 2015. [Google Scholar]
- 20. Van der Hoek W, Tuan Anh V, Cam PD, Vicheth C, Dalsgaard A. Skin diseases among people using urban wastewater in Phnom Penh. Urban Agric Mag 2005;14:30-31. [Google Scholar]
- 21. Multistate outbreaks of Salmonella infections associated with live poultry—United States, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:25-29. [PubMed] [Google Scholar]
- 22. Knudsen LG, Phuc PD, Hiep NT, et al. The fear of awful smell: risk perceptions among farmers in Vietnam using wastewater and human excreta in agriculture. Southeast Asian J Trop Med Public Health. 2008;39:341-352. [PubMed] [Google Scholar]
- 23. Blumental U, Peasey A. Critical Review of the Epidemiological Evidence of the Health Effects of Wastewater and Excreta Use in Agriculture. London: London School of Hygeienc and Tropical Medicine; 2002. [Google Scholar]
- 24. Kinh N-V. Situation Analysis: Antibiotic Use and Resistance in Vietnam. Washington, DC: The Center for Diseases Dynamics, Economics and Policy; 2010. [Google Scholar]
- 25. Van TTH, Yidana Z, Smooker PM, Coloe PJ. Antibiotic use in food animals worldwide, with a focus on Africa: pluses and minuses. J Glob Antimicrob Resist. 2020;20:170-177. [DOI] [PubMed] [Google Scholar]
- 26. Manyi-Loh C, Mamphweli S, Meyer E, Okoh A. Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules. 2018;23:795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grace D. Review of Evidence on Antimicrobial Resistance and Animal Agriculture in Developing Countries. London: Evidence on Demand; 2015. [Google Scholar]