Abstract
Objective
The aim of the present study was to assess the expression of the Ikaros transcription factor (IKZF1) in lung adenocarcinoma and investigate whether expression levels of Ikaros are correlated with lung adenocarcinoma progression.
Methods
We conducted a retrospective study of 325 cases of resected stage I pulmonary adenocarcinoma, in which histological subtyping was performed according to the 2015 World Health Organization classification. We performed immunohistochemical examinations to assess expression of Ikaros in pulmonary adenocarcinomas and evaluated the correlation between Ikaros expression and cancer progression.
Results
Immunohistochemical staining was heterogeneous, with the majority of well-differentiated and moderately differentiated lung adenocarcinomas being weakly positive and the majority of the poorly differentiated lung adenocarcinomas exhibiting strong positive staining. Higher expression of Ikaros was associated with tumor recurrence or metastasis.
Conclusions
Ikaros is heterogeneously expressed in different subtypes of lung adenocarcinoma; higher expression of Ikaros was found to be associated with cancer progression.
Keywords: Ikaros, transcription factor, immunohistochemistry, stage I pulmonary adenocarcinoma, recurrence, metastasis
Introduction
Ikaros (IKZF1) is a member of the Kruppel family of zinc finger transcription factors and it is encoded by the IKZF1 gene. The IKZF1 gene comprises eight exons and can generate multiple Ikaros isoforms by alternative splicing of exons 3 to 5.1,2 Ikaros was originally found to play a pivotal role in regulating the differentiation of lymphocytes, hematopoietic stem cells, and some myeloid cells.3–6 Subsequent studies revealed that Ikaros was also expressed in several other organs and tissues, including lung, liver, prostate, brain, heart, colon, ovary, thymus, mammary gland, bone marrow, and intestine, but the role of Ikaros in these organs and tissues was unclear. A recent study demonstrated that Ikaros may be associated with the prognosis of certain types of cancer, including colorectal, ovarian, and bladder cancers.7 The above mentioned results suggest that Ikaros may play an important role in development or progression of solid tumors. However, the expression and function of Ikaros in these cancers have not been extensively investigated.
Lung cancer is the leading cause of cancer-related mortality worldwide, and adenocarcinoma is currently the most common histological subtype, accounting for almost half of all lung cancers.8 To date, very few studies have investigated the expression and functional role of Ikaros in lung cancers. The aim of the present study was to examine the expression of Ikaros in lung adenocarcinoma and to analyze the association between Ikaros expression and clinicopathological characteristics such as tumor recurrence and distant metastasis.
Patients and methods
Ethical approval
The protocol of the present study was approved by the Ethics Committee of Soochow University, Changzhou, China. Verbal informed consent was obtained from participants in the study.
Patients
We evaluated a retrospective series of patients with resected stage I lung adenocarcinomas (n = 325) who had received no preoperative treatment. Patient information was retrieved from the database of the First People’s Hospital of Changzhou, China, between 2010 and 2015. All patients were required to have a diagnosis of primary lung adenocarcinoma and were confirmed to have no distant metastases by computed tomography and radionuclide bone scanning before surgery. Patients with mucinous adenocarcinoma and colloid adenocarcinoma were excluded because the number of cases with these two subtypes was very limited. The follow-up period ranged from 6 to 110 months. Clinical data, including age, sex, tumor recurrence, and distant metastasis, were obtained from detailed prospective clinical databases. Clinical staging was performed according to the eighth edition of the American Joint Committee on Cancer TNM Staging Manual for lung cancers.9
Histological evaluation
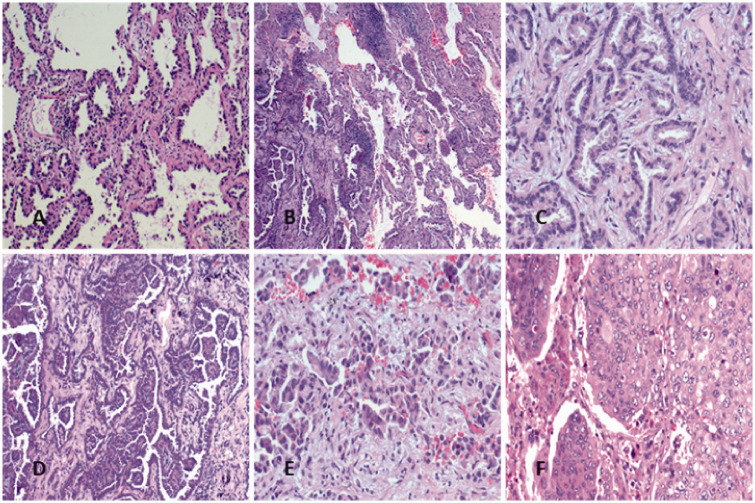
All resected specimens were routinely fixed in 10% formalin and stained with hematoxylin and eosin. The location, number, and size of the tumors were recorded as reported in the original pathology report. The slides were evaluated microscopically for histological patterns by two pathologists according to the 2015 World Health Organization (WHO) lung adenocarcinoma classification system. Comprehensive histological evaluation was performed in each tumor, recording the percentage of each histological component in five-percentage-point increments, totaling 100% for a tumor.10,11 Tumors were classified by their predominant morphological percentage as follows (Figure 1): (1) adenocarcinoma in situ; (2) minimally invasive adenocarcinoma (<5 mm); and (3) invasive adenocarcinoma, which was further subdivided into acinar-, papillary-, micropapillary-, and solid-predominant subtypes.12
Figure 1.
Histological subtypes of lung adenocarcinoma. (A) Adenocarcinoma in situ (well differentiated); (B) minimally invasive adenocarcinoma (well differentiated); (C) acinar-predominant (moderately differentiated); (D) papillary-predominant (moderately differentiated); (E) micropapillary-predominant (poorly differentiated); (F) solid-predominant (poorly differentiated).
Immunohistochemical examination
Immunohistochemical studies were performed on 4-μm-thick sections from formalin-fixed and paraffin-embedded tissue blocks. The sections were deparaffinized and subjected to antigen retrieval. The tissue sections were then incubated with rabbit polyclonal anti-Ikaros antibody (1:100, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). All slides were stained on a Benchmark Autostainer (Ventana Medical System, Tucson, AZ, USA) with the EnVision detection system (Dako, Glostrup, Denmark). Unequivocal nuclear staining in >10% of the tumor cells was defined as a positive response. Nuclear staining for Ikaros was graded as weak or strong.
Statistical analysis
Differences in recurrence or distant metastasis between different expression levels of Ikaros in lung adenocarcinoma were evaluated using the Chi-square test. Survival rate was estimated by the Kaplan–Meier method. Comparisons were made using the log-rank Mantel–Cox test. All statistical analyses were performed using SPSS for Windows, version 17.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered to indicate a statistically significant difference.
Results
Clinicopathological characteristics
The clinicopathological characteristics, including age, sex, histological subtype, and stage are summarized in Table 1. The cohort included 212 women (65%) and 113 men (35%), with a mean age of 62 years (range, 26–88 years). In total, 219 of the tumors (67%) were located in the right lung and 106 (33%) in the left lung. According to the 2015 WHO classification, the majority of the cases were classified as mixed subtype. Six cases of adenocarcinoma in situ and 15 cases of minimally invasive adenocarcinoma were nonmucinous. A total of 137 cases were acinar-predominant, which was the most common histological subtype, followed by 83 cases of solid-predominant, 66 cases of papillary-predominant, and 18 cases of micropapillary-predominant. The majority of the cases were stage IA (253 cases, 79%) and the remaining 72 cases were stage IB (21%). The follow-up period ranged from 3 to 9 years. Sixty-seven cases of recurrence or distant metastasis were detected during the follow-up period.
Table 1.
Characteristics of patients with stage I lung adenocarcinoma.
| Characteristic | n (%) |
|---|---|
| Sex | |
| Female | 212 (65) |
| Male | 113 (35) |
| Mean age at diagnosis (years) | |
| Overall | 61 |
| Female | 59 |
| Male | 64 |
| Histological subtype by the 2015 WHO Lung Adenocarcinoma Classification | |
| Adenocarcinoma in situ | 6 (2) |
| Minimally invasive adenocarcinoma | 15 (5) |
| Acinar-predominant | 137 (42) |
| Papillary-predominant | 66 (20) |
| Micropapillary-predominant | 18 (6) |
| Solid-predominant | 83 (25) |
| Stage | |
| IA | 253 (79) |
| IB | 72 (21) |
Immunohistochemical staining
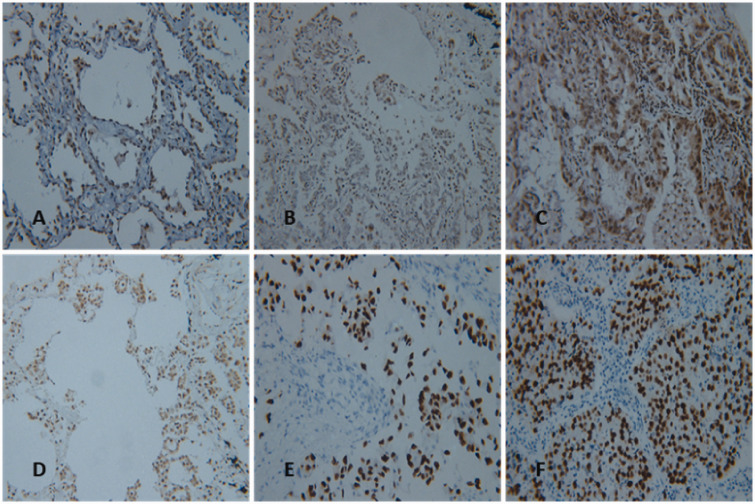
Immunohistochemical results are shown in Figure 2 and Table 2. Weak positive staining for Ikaros was observed in normal lung tissues surrounding the lung tumor. Significantly higher expression of Ikaros was observed in most lung adenocarcinoma tissues compared with that in normal lung tissues, and significant differences were observed among the different histological subtypes. Ikaros was weakly positive in six cases of adenocarcinoma in situ. All 15 cases of minimally invasive adenocarcinoma exhibited weak staining. Among the acinar-predominant cases, 36 exhibited strong staining and 101 exhibited weak staining. Among papillary-predominant cases, 14 exhibited strong staining and the remaining 52 exhibited weak staining. Among solid-predominant cases, 54 exhibited strong staining and 29 exhibited weak staining. Finally, among micropapillary-predominant cases, 13 exhibited strong staining and 5 exhibited weak staining.
Figure 2.
Immunohistochemical staining of Ikaros (IKZF1) in the subtypes of lung adenocarcinoma. Weak positive staining for Ikaros was observed in (A) adenocarcinoma in situ (well differentiated), (B) minimally invasive adenocarcinoma (well differentiated), (C) acinar-predominant (moderately differentiated), and (D) papillary-predominant (moderately differentiated) subtypes; strong positive staining for Ikaros was observed in (E) micropapillary-predominant (poorly differentiated) and (F) solid-predominant (poorly differentiated) subtypes.
Table 2.
Expression of Ikaros (IKZF1) in the histological subtypes of lung adenocarcinoma.
| Histologic subtype | No. of cases (%) | Immunohistochemistry (%) |
|
|---|---|---|---|
| Weak | Strong | ||
| Adenocarcinoma in situ | 6 (2) | 6 (2) | 0 |
| MIA | 15 (5) | 15 (5) | 0 |
| Acinar-predominant | 137 (42) | 101 (30) | 36 (12) |
| Papillary-predominant | 66 (20) | 52 (16) | 14 (4) |
| Micropapillary-predominant | 18 (6) | 5 (2) | 13 (4) |
| Solid-predominant | 83 (25) | 29 (9) | 54 (16) |
MIA = minimally invasive adenocarcinoma.
Correlation between Ikaros expression and recurrence or distant metastasis of lung adenocarcinoma
Further analyses demonstrated that Ikaros expression was significantly higher in patients with in lung adenocarcinomas that recurred or metastasized during the follow-up period. Of the 67 cases of recurrence or distant metastasis, 48 exhibited strong staining and 19 exhibited weak staining; of the 258 cases without recurrence or distant metastasis, 69 exhibited strong staining and 189 exhibited weak staining (P < 0.001) (Table 3). These data suggest that Ikaros may be involved in the recurrence and metastasis of lung adenocarcinoma.
Table 3.
Correlations among the recurrence or distant metastasis of lung adenocarcinoma and the expression levels of Ikaros (IKZF1).
| Recurrence or distant metastasis | No. (%) of cases | Expression level of Ikaros (%) |
P-value | |
|---|---|---|---|---|
| Strong | Weak | |||
| Negative | 258 (79) | 69 (21) | 189 (58) | <0.001 |
| Positive | 67 (21) | 48 (15) | 19 (6) | |
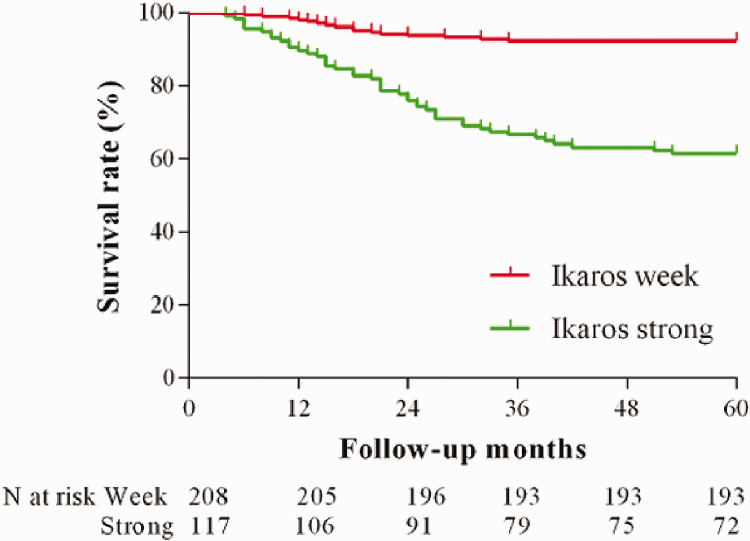
Association between Ikaros expression and survival rate of patients with lung adenocarcinoma
Our data showed a correlation between Ikaros expression and survival rate of the patients with lung adenocarcinoma (Figure 3). The survival curves for patients whose tumors showed strong expression of Ikaros were significantly different from those whose tumors showed weak expression (P < 0.01).
Figure 3.
Association between Ikaros (IKZF1) expression and survival rate in patients with stage I lung adenocarcinomas. Kaplan–Meier curve for stage I tumors was separated by expression of Ikaros (P < 0.01; log-rank Mantel–Cox test).
Discussion
The development of recurrence or distant metastasis makes lung adenocarcinoma a fatal tumor, and a significant number of patients undergoing surgical intervention ultimately present with recurrence or metastasis. A better understanding of the molecular targets contributing to tumor invasion and metastasis is crucial for developing novel therapeutic methods for lung adenocarcinoma.
Ikaros is a zinc finger transcriptional regulator, which is essential for normal hematopoiesis and the development of lymphoid, myeloid, and erythroid lineages.13 Although the majority of studies on Ikaros are limited to the hematopoietic system, aberrant expression of Ikaros has been observed in other tissues and organs.14–18 Ikaros may be involved in the normal physiological functions of these tissues or organs. Ikaros has been found to be expressed in several types of solid cancers, including bladder, breast, skin, colorectal, ovarian, and lung cancers,7 and the expression of Ikaros may be associated with the prognosis of these cancers.
Originally, Ikaros was thought to be a tumor suppressor, but it was demonstrated recently that higher expression of Ikaros was significantly associated with advanced stage and low differentiation of ovarian cancer; thus, Ikaros may be involved in the metastasis and invasion of ovarian cancer cells.19 Yamamoto et al.16 reported that Ikaros is involved in the migration and invasion of extravillous trophoblast in early placentation.
In the present study, we demonstrated that expression of Ikaros was heterogeneous in lung adenocarcinomas. According to the 2015 WHO lung adenocarcinoma classification system, adenocarcinoma in situ and minimally invasive adenocarcinoma are considered to be well differentiated; acinar-predominant and papillary-predominant are considered to be moderately differentiated; and micropapillary-predominant and solid-predominant are considered to be poorly differentiated. In our results, compared with the weak positive staining of Ikaros observed in well- and moderately differentiated lung adenocarcinomas, higher expression of Ikaros was observed in poorly differentiated lung adenocarcinomas. Consistent with the results reported by Yamamoto et al., 16 our data demonstrated that Ikaros may be involved in the migration and invasion of lung adenocarcinoma cells, thereby resulting in cancer recurrence or metastasis.
In conclusion, our results demonstrated that Ikaros is heterogeneously expressed in different subtypes of lung adenocarcinoma and that higher expression of Ikaros is associated with recurrence and metastasis. Although Ikaros acts as a tumor suppressor in acute lymphoblastic leukemia,20 it appears to promote cell invasion and metastasis in solid tumors. Therefore, the function of Ikaros may be multidimensional and the underlying mechanism requires further investigation.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by National Health and Family Planning Commission science and technology project of Chang Zhou (No. ZD201704).
ORCID iDs
References
- 1.Molnar A, Georgopoulos K. The Ikaros gene encodes a family of functionally diverse zinc finger DNA-binding proteins. Mol Cell Biol 1994; 14: 8292–8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koipally J, Georgopoulos K. A molecular dissection of the repression circuitry of Ikaros. J Biol Chem 2002; 277: 27697–27705. [DOI] [PubMed] [Google Scholar]
- 3.Georgopoulos K, Bigby M, Wang JH, et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell 1994; 79: 143–156. [DOI] [PubMed] [Google Scholar]
- 4.Dumortier A, Kirstetter P, Kastner P, et al. Ikaros regulates neutrophil differentiation. Blood 2003; 101: 2219–2226. [DOI] [PubMed] [Google Scholar]
- 5.Wang JH, Nichogiannopoulou A, Wu L, et al. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity 1996; 5: 537–549. [DOI] [PubMed] [Google Scholar]
- 6.Payne KJ, Huang G, Sahakian E, et al. Ikaros isoform x is selectively expressed in myeloid differentiation. J Immunol 2003; 170: 3091–3098. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Luo Y, Wei J. Integrative genomic analyses on Ikaros and its expression related to solid cancer prognosis. Oncol Rep 2010; 24: 571–577. [DOI] [PubMed] [Google Scholar]
- 8.Devesa SS, Bray F, Vizcaino AP, et al. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer 2005; 117: 294–299. [DOI] [PubMed] [Google Scholar]
- 9.Edge SB, Byrd DR, Compton CCL. AJCC cancer staging manual. 7th ed New York, NY: Springer, 2009, pp.253–270. [Google Scholar]
- 10.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011; 25: 653–664. [DOI] [PubMed] [Google Scholar]
- 11.Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol 2010; 34: 1155–1162. [DOI] [PubMed] [Google Scholar]
- 12.Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol 2008; 32: 810–827. [DOI] [PubMed] [Google Scholar]
- 13.Kishihara K, Penninger J, Wallace VA, et al. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell 1993; 74: 143–156. [DOI] [PubMed] [Google Scholar]
- 14.Ezzat S, Mader R, Yu S, et al. Ikaros integrates endocrine and immune system development. J Clin Invest 2005; 115: 1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezzat S, Asa SL. The emerging role of the Ikaros stem cell factor in the neuroendocrine system. J Mol Endocrinol 2008; 41: 45–51. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto E, Ito T, Abe A, et al. Ikaros is expressed in human extravillous trophoblasts and involved in their migration and invasion. Mol Hum Reprod 2005; 11: 825–831. [DOI] [PubMed] [Google Scholar]
- 17.Ezzat S, Yu S, Asa SL. Ikaros isoforms in human pituitary tumors: distinct localization, histone acetylation, and activation of the 5' fibroblast growth factor receptor-4 promoter. Am J Pathol 2003; 163: 1177–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott J, Jolicoeur C, Ramamurthy V, et al. Ikaros confers early temporal competence to mouse retinal progenitor cells. Neuron 2008; 60: 26–39. [DOI] [PubMed] [Google Scholar]
- 19.He LC, Gao FH, Xu HZ, et al. Ikaros inhibits proliferation and, through upregulation of Slug, increases metastatic ability of ovarian serous adenocarcinoma cells. Oncol Rep 2012; 28: 1399–1405. [DOI] [PubMed] [Google Scholar]
- 20.Virely C, Moulin S, Cobaleda C, et al. Haploinsufficiency of the IKZF1 (IKAROS) tumor suppressor gene cooperates with BCR-ABL in a transgenic model of acute lymphoblastic leukemia. Leukemia 2010; 24: 1200–1204. [DOI] [PubMed] [Google Scholar]