Abstract
Objectves:
To comparatively explore the differences between 18F-Flurpiridaz and 13N-NH3·H2O PET/CT myocardial perfusion imaging in miniature pigs.
Methods:
Ten Bama minipigs were divided into normal group and myocardial infarction group. The changes of the ratio of left ventricular myocardium to main organs with time were calculated and the best imaging time was confirmed for 18F-Flurpiridaz imaging in normal group. The image quality score, summed rest score(SRS), Extend, total perfusion deficit(TPD) and left ventricle ejection fraction(LVEF) were respectively compared for 18F-Flurpiridaz and 13N-NH3·H2O in infarction group.
Results:
18F-Flurpiridaz was rapid distributed in myocardium, and the background counts of cardiac cavity were very low, and no obvious interference extracardiac radioactivity was observed. The radioactive ratio of the left ventricular myocardium to cardiac blood pool and adjacent liver were high. Compared with 13N-NH3·H2O, there were no significant differences in functional parameters, including SRS, Extend, TPD and LVEF.
Conclusion:
The results preliminaryly show that 18F-FIurpiridaz is a promising positron MPI agent with good image quality, ability of accurately evaluating cardiac function, and also convenience for application.
Keywords: 18F-Flurpiridaz, 13N-NH3·H2O, 13N-ammonia, myocardial perfusion imaging, MPI, Positron emission tomography/PET;x-ray computed tomography/CT, animal experiment
Introduction
Cardiovascular disease is a major disease that seriously endangers human health.1 The prevalence of cardiovascular disease in China continues to rise and has a younger development trend. Myocardial perfusion imaging (MPI) is an internationally recognized noninvasive imaging technique for early diagnosis, risk stratification and prognostic assessment of myocardial ischemia in patients with coronary artery disease (CAD).2 In particular, with the application of positron emission tomography/computed tomography(PET/CT), it has the advantages of high imaging speed, clear image, high diagnostic accuracy, absolute quantification and low radiation dose, and is gradually being used for the diagnosis and evaluation of CAD.3 13N-NH3·H2O(13N-ammonia) is the earliest and most widely used positron MPI agent, but its physical half-life is only about 9.96 minutes, which must be equipped with expensive medical cyclotron for clinical application, and also it is difficult to be used for exercise-stress imaging, which can not meet the practical needs of clinical convenience. Therefore, the development of positron MPI agents with relatively long half-life and optimal imaging performance has become the focus of current research.4
2-(1, 1-dimethyl ethyl)-4-chloro-5 [4-[2-fluoro (18F) ethoxy] methyl] phenyl]-3-piperidone, 18F-Flurpiridaz is a new type of positron MPI agent with a half-life of 109.7 minutes. It is suitable for exercise-stress imaging and long distance distribution, and has the potential of quantitative detection of myocardial blood flow. It is deemed to have a broad clinical application prospect.2,5 According to literature retrieval and to our knowledge, there are not any study reports on 18F-Flurpiridaz PET MPI in China until now. The main purpose of this study is to determine the distribution characteristics of 18F Flurpiridaz in vivo and the best imaging time of myocardium through PET imaing of animal experiment, and compared with traditinal 13N-ammonia imaging to clarify its accuracy in image quality, infarction area, left ventricular function parameters.
Materials and Methods
Experimental Animals
Ten Bama miniature pigs of either gender, were divided into normal group (n = 5) and infarction group (n = 5). No treatment was done in the normal group, and the model group was established by thoracotomy and ligating the coronary artery in the infarction group.
Estabolishment of Infarction Group Model
Fasting for 12 hours and drinking for 4 hours before the experiment. Five Bama miniature pigs were anesthetized, the skin of the ear was prepared and disinfected, and the middle ear vein pathway was established. The myocardial infarction model was established by thoracotomy, the location of the model was selected, the coronary artery was ligated in the mid-distal segament of left anterior descending(LAD), and the myocardial infarction model was established for more than 1 week after operation. The results of sucessful estabolishment of infarcted model were confirmed by ECG and echocardiography that the ST segment was significantly elevated in a unidirectional curve, and the local wall motion was significantly decreased or no movement was shown by echocardiography.
Equipments, Instruments and Reagents
Main equipments and instruments
Discovery Elite PET/CT(GE company, USA), MINI trace medical cyclotron(GE company, USA), Tracer Lab FN positron drug synthesizer (GE company, USA); CRC-25 R radioactivity meter (CAPINTEC, USA); MINI scan thin layer scanner (Bioscan, USA).
Main reagents
H2 18O(RCTEM Company, Israel); toluene 4-sulfonic acid 2-[4-(1-tert-butyl-5-chloro-6-oxo-1, 6-dihydro-pyridazin-4-oxymethyl)-benzyloxy]-ethyl ester (referred to as precursor) (Tianjin Heans Opd Technology Co., Ltd. China); Sep-Pak QMA column (QMA column) (Waters Co., Ltd. USA), Sep-Pak C18 solid phase extraction column (C18 column) (Waters Co., Ltd. USA), amino polyether (K2.2.2) (ABX Company, Germany), potassium carbonate (Sigma Co., Ltd. USA), Anhydrous acetonitrile (Sigma Co., Ltd. USA), anhydrous ethanol and other reagents (Sigma Co., Ltd. USA).
Preparations of Imaging Agents
Preparations of 18F-flurpiridaz
The synthesis of 18F-Flurpiridaz was based on the method reported in the literature.6 The 18F produced by the accelerator was transferred to the synthesizer, 18F was enriched on an anion chromatographic column, 18F was eluted to the reaction bottle by K2CO3/K2.2.2 solution, the water was heated, and the precursor solution was added to the reaction vessel. After the reaction, the product was transferred to semi-preparation high performance liquid chromatography for purification, and the final product could not be used until it was sterilized and filtered. The radiochemical purity was more than 95%.
Preparation of 13N-NH3·H2O
13N-NH3·H2O was prepared by on-line reduction method. Highly oxidized nitrogen oxides were formed in the target by cyclotron bombardment and 13N-NH3·H2O was prepared with methane as reducing agent. After bombardment, the target water was sent out, and the unreduced 13N-NO2-and 13N-NO3- were removed by anion chromatographic column, and the 13N-NH3·H2O for injection was obtained after sterilization and filtration.
Imaging Scheme
PET/CT MPI
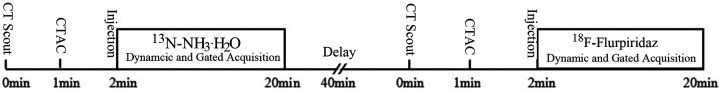
Normal group: minipigs were fixed on the examination bed after sucessful anesthesia. 13N-NH3·H2O rest imaging was performed at first, 40 minutes later followed by 18F-Flurpiridaz rest imaging. The dynamic acquisition program was initiated immediately after rapid injection of 185∼370 MBq (3∼5 mCi) 13N-NH3·H2O through the ear vein, and flushed by 10 ml saline. The imaging acquisition parameters were as follows: the scanning voltage and current of CT was 120kVand 20 mA, and the dynamic data of PET in 3D dynamic list model form: 1 frame/10 s acquisition plus 12 frames, 1 frame/30 s acquisition plus 2 frames, 1 frame/60 s acquisition plus 1 frame, 1 frame/6 min acquisition plus 1 frame; After dynamic acquisition, 1 bed 3D rest gated myocardial perfusion was performed, including 8min/ bed, 8 frames/cardiac cycle, heart rate window was ±15%. After 40 min of 13N-NH3·H2O imaging, 185∼370 MBq (1∼2 mCi) 18F-Flurpiridaz was injected intravenously and started dynamic acquisition, whose parameters were the same as above. The flow chart of imaging process was shown in Figure 1.
Figure 1.
Flow chart of 13N-NH3·H2O and 18F-Flurpiridaz PET/CT imaging.
Infarction group: 13N-NH3·H2O and 18F-Flurpiridaz rest MPIs were performed at 15-20 min after injection of imaging agent, respectively. The specific imaging sequence, interval time and gated acquisition parameters were the same as above. The imaging of infarction group was performed 1 week after infarct.
18F-Flurpiridaz PET/CT Whole Body Scan
In the normal group, about 74∼185 MBq (1∼2 mCi) 18F-Flurpiridz was injected intravenously after anesthesia, and 1 min, 5 min, 10 min, 20 min, 40 min, 60 min, 90 min and 120 min whole body scan data were collected at different time points.Acquisition parameters were as followings:120kv and 20 mA for CT scan, 2 min of 1 bed for PET.Scan range covered from skull to thigh.
Analysis of the Results of PET/CT Imaging
All PET/CT data were controlled by ACQC (attenuation correction quality control) in acquisition workstation before tranferred to post-processing workstation. Finally, all the data were transferred to the dedicated post-processing workstation (Xeleris workstation, GE company, USA).
Distribution of 18F-Flurpiridaz in Normal Animals
The original collected data were reviewed on the Xeleris workstation, the left ventricular myocardium and blood pool, liver, lung, spleen and kidney ROI(Region of Interest) were drawn, and the radioactivity ratio of left ventricular myocardium to each organ was calculated at each time point after injection of imaging agent. The changes of the ratios with time were observed, and the biological distribution of 18F-Flurpiridaz in normal pigs, the maximum time point of cardiac uptake and the best imaging time were evaluated.
Evaluation of MPI Quality in 18F-Flurpiridaz Study
The quality of 18F-Flurpiridaz image was scored by 2 experienced nuclear medicine physicians, using the left ventricular 17 segment and 5 point score. 0 point: very poor, can not explain; 1 point: poor, due to a variety of reasons, including the counting rate is too low or adjacent organs covered can not clearly explain the diagnostic results; 2 point: qualified, refers to the poor counting rate or high background radioactivity and other factors leading to the decline of image quality, but it did not affect the judgment of the diagnosis results; 3 point: good, the image was clear, no obvious background interference; 4 point: very good, the image was very clear, no background interference, the boundary of epicardial membrane of myocardium is clear and sharp. At the same time, the 13N-NH3·H2O image was used to be compared. When there was a disagreement for evaluation, it was finally determined by consultation.
Determination of Cardiac Function Parameters in Infarction Group
The data of PET/CT rest gated MPIs were analyzed by QGS/QPS software (Cedars-Sinai Company, USA) in Xeleris workstation. The summed rest score(SRS), the percentage of left ventricular myocardial defect area (Extend), the percentage of total perfusion deficit (TPD), the left ventricular ejection fraction (LVEF) of 18F-Flurpiridaz and 13N-NH3·H2O MPIs in infarction group were obtained and comparatively analyzed.
Pathological Examination
At the end of the experiment, respective 2 animals in the normal group and the infarction group were sacrificed, and the normal and myocardial infarction model tissues were taken for pathological examination (HE staining).
Statistical Analysis
SPSS 19.0 was used for all statistical analysis, and the measurement results were expressed by mean ± standard deviation. Mann Whitney U-test was used for comparison between the 2 groups. P values less than 0.05 were considered statistically significant.
Results
Distribution of 18F-Flurpiridaz in Normal Animals
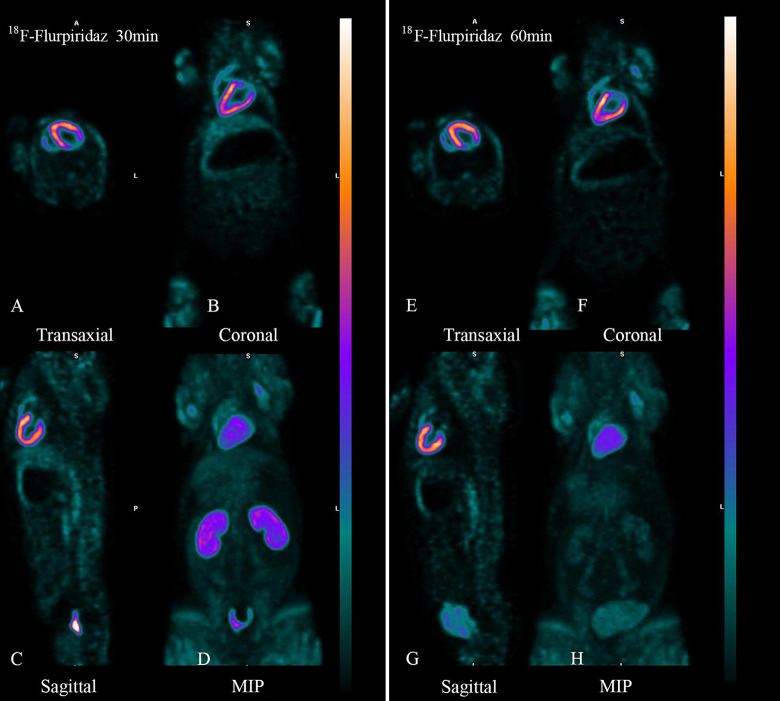
The typical whole body 18F-Flurpiridaz PET/CT imaging in the normal group of miniature pigs was shown in Figure 2, the left ventricular myocardium was clearly delineated and maintained high radioactivity uptake within 2 hours after injection, and there was no significant high radioactivity uptake in the non-target organs outside the heart. At 30 min after injection, the heart and kidney was clearly delineated, and the radioactivity count was high, and the radioactivity count of other tissues and organs was close to the background level. At 60 min, the heart still maintained high radioactivity uptake, and the renal radioactivity uptake decreased.
Figure 2.
Body imaging of 18F-Flurpiridaz 30 min (left) and 60 min (right) in normal miniature pigs. The left and right of the images were 30min and 60min body images with 18F-Flurpiridaz PET/CT, respectively. A and E are transverse slices, B and F are coronal slices, C and G are sagittal slices, and D and H were maximum intensity projections(MIPs). No matter whether 30min or 60 minutes, left ventricular myocardium was clearly delineated and there was no radioactive interference outside the heart, and the radioactivity in cardiac cavity, lung tissue and liver was very low, and the radioactivity in kidney was significantly decreased in 60min.
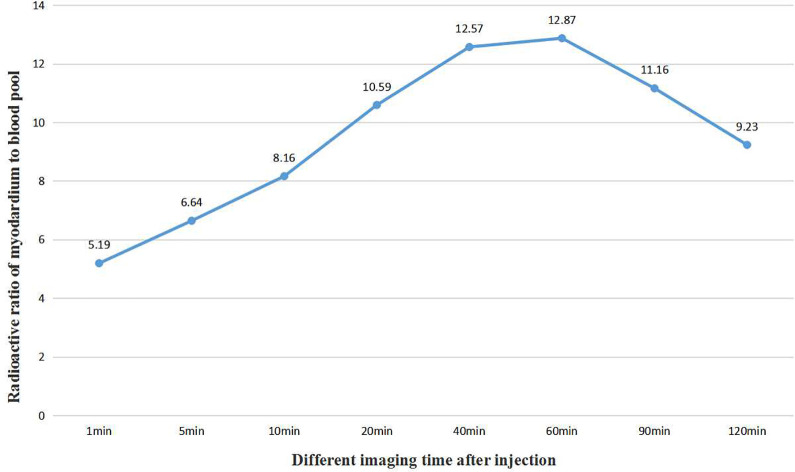
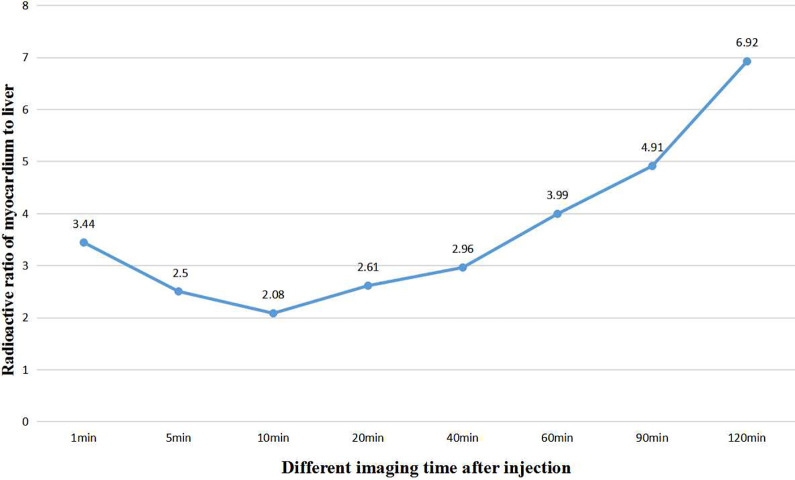
By drawing the region of interest, the radioactivity ratios of left ventricular myocardium to blood pool, liver, lung, spleen and kidney were obtained at different time points after injection of imaging agents, such as 1 min, 5 min, 10 min, 20 min, 40 min, 60 min, 90 min and 120 min, as shown in Table 1.Among them, the radioactivity ratio of left ventricular myocardium to blood pool gradually increased from 5.19 to 12.87 in 60 min, and began to decrease to 9.23 60 minutes later, as shown in Figure 3. The radioactivity ratio of left ventricular myocardium to liver decreased from 3.44 to 2.08 in 10 min and increased from 2.08 to 6.92 in 10 min, as shown in Figure 4.
Table 1.
Radioactive Ratio of 18F-Flurpiridaz in Left Ventricular Myocardium to Blood Pool and Major Organs in Normal Miniature Pigs at Different Time Points.
| Target/non-target ratio | Target/non-target radioactive ratio at different time points | |||||||
|---|---|---|---|---|---|---|---|---|
| 1min | 5min | 10min | 20min | 40min | 60min | 90min | 120min | |
| Myocardial/Liver Ratio | 3.44 ± 0.34 | 2.5 ± 0.25 | 2.08 ± 0.68 | 2.61 ± 0.37 | 2.96 ± 0.61 | 3.99 ± 1.20 | 4.91 ± 1.02 | 6.92 ± 2.10 |
| Myocardial/Lung Ratio | 4.17 ± 0.46 | 9.17 ± 1.25 | 10.76 ± 1.78 | 14.79 ± 2.50 | 21.75 ± 2.96 | 32.03 ± 7.64 | 50.51 ± 8.49 | 49.61 ± 7.84 |
| Myocardial/Spleen Ratio | 4.92 ± 0.22 | 5.68 ± 0.62 | 5.69 ± 0.97 | 7.10 ± 0.76 | 13.93 ± 3.52 | 16.30 ± 2.39 | 17.00 ± 2.18 | 27.97 ± 6.72 |
| Myocardial/Kidney Ratio | 0.27 ± 0.03 | 0.34 ± 0.11 | 0.37 ± 0.09 | 0.51 ± 0.02 | 0.86 ± 0.10 | 1.15 ± 0.09 | 1.70 ± 0.33 | 2.60 ± 0.59 |
| Myocardial/Blood Pool Ratio | 5.19 ± 1.38 | 6.64 ± 1.02 | 8.16 ± 0.68 | 10.59 ± 2.12 | 12.57 ± 2.42 | 12.87 ± 2.01 | 11.16 ± 2.63 | 9.23 ± 1.50 |
Figure 3.
The trend of radioactive ratio of left ventricular myocardium to cardiac blood pool with the time change after injection in normal miniature pigs.
Figure 4.
The trend of radioactive ratio of left ventricular myocardium to liver with the time change after injection in normal miniature pigs.
The left and right of the images were 30 min and 60 min body images with 18F-Flurpiridaz PET/CT, respectively. A and E are transverse slices, B and F are coronal slices, C and G are sagittal slices, and D and H were maximum intensity projections(MIPs). No matter whether 30 min or 60 minutes, left ventricular myocardium was clearly delineated and there was no radioactive interference outside the heart, and the radioactivity in cardiac cavity, lung tissue and liver was very low, and the radioactivity in kidney was significantly decreased in 60 min.
Evaluations of 18F-Flurpiridaz Image Quality
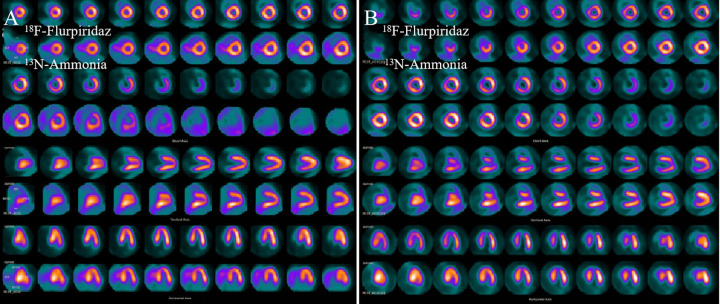
The scores for MPI quality of 18F-Flurpiridaz and 13N-NH3·H2O were both 4 points, indicating the image qualities were both excellent. 18F-Flurpiridaz MPI clearly revealed the myocardium, uniform distribution in each segment, low background count of cardiac cavity, no extracardiac radioactive interference, and had excellent imaging quality compared with 13N-NH3·H2O MIPs. The image quality of 18F-Flurpiridaz and 13N-NH3·H2O were compared and showed in Figure 5, and the image quality scores were compared and listed in Table 2.
Figure 5.
Comparison of MPI between 18F-Flurpiridaz and 13N-NH3·H2O in normal and infarct miniature pigs. From top to bottom, there were short axis (1 to 4 rows), vertical long axis (5 to 6 rows) and horizontal long axis (7 to 8 rows). The odd row was 18F-Flurpiridaz images and the even row was 13N-NH3·H2O images. a (normal group): the myocardium of each wall of the left ventricle was clearly delineated. Compared with 13N-NH3·H2O imaging, the 18F-Flurpiridaz distribution in the myocardium was very uniform and the myocardial walls were well displayed. b (infartion group): 18F-Flurpiridaz clearly delineated apical and part of anterior wall apical infarct area (radioactive defect), myocardium and infarction boundary were clearly delineated, 13N-NH3·H2O image showed a small amount of radioactivity distribution outside the heart.
Table 2.
Comparison of Image Quality Between 18F-Flurpiridaz and 13N-NH3·H2O MPIS in Normal and Infarction Groups.
| Image quality score | Normal group | Infarction group | ||
|---|---|---|---|---|
| 13N-NH3·H2O | 18F-Flurpiridaz | 13N-NH3·H2O | 18F-Flurpiridaz | |
| 0 Point | 0 | 0 | 0 | 0 |
| 1 Point | 0 | 0 | 0 | 0 |
| 2 Point | 0 | 0 | 0 | 0 |
| 3 Point | 0 | 0 | 0 | 0 |
| 4 Point | 5 | 5 | 5 | 5 |
Comparison of Cardiac Function Parameters Between 18F-Flurpiridaz and 13N-NH3·H2O Imaging in Infarction Group
The main cardiac function parameters of myocardial perfusion imaging of 18F-Flurpiridaz and 13N-NH3·H2O in infarction group were obtained by QGS/QPS software, including SRS, Extend, TPD and LVEF. Mann Whitney U-test of SRS, Extend, TPD and LVEF of 18F-Flurpiridaz and 13N-NH3·H2O imaging showed that the P values were all > 0.05, indicating that there was no significant difference between them in Table 3.
Table 3.
Comparison of Cardiac Function Parameters between 18F-Flurpiridaz and 13N-NH3·H2O Myocardial Perfusion Imaging in Infarction Group.
| Parameters | Infarction group | P values | ||
|---|---|---|---|---|
| 18F-Flurpiridaz | 13N-NH3.H2O | Z values | ||
| SRS | 10.6 ± 4.1 | 9.20 ± 4.6 | −0.75 | 0.45 |
| Extend | 15.2 ± 9.0% | 12.6 ± 6.6% | −0.525 | 0.60 |
| TPD | 11.6 ± 6.3% | 9.6 ± 3.9% | −0.424 | 0.67 |
| LVEF | 68.6 ± 11.1% | 71.4 ± 11.3% | −0.529 | 0.60 |
Pathological Examination
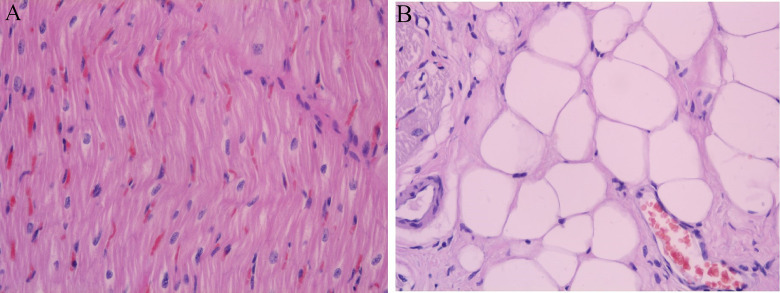
At the end of the experiment, the respective 2 animals in the normal and infarction group were sarificed, and the pathological examination (HE staining) was performed to verify myocardial changes, showed in Figure 6.
Figure 6.
(A) normal group HE staining, magnified 400 fold, normal myocardial HE staining was pink, cytoplasm, nucleus and tissue structure was clear; (B) Myocardial infarction group HE staining, magnified 400 fold, cytoplasmic transverse lines disappeared, forming different depth of transverse band, nuclear pyknosis, fragmentation dissolution.
Discussion
18F-Flurpiridaz is the latest generation of PET positron myocardial perfusion imaging agent which is a structural analog of the insecticide pyridaben, a known inhibitor of the NADH: ubiquinone oxidoreductase also known as mitochondrial complex-1 (MC-1) of the electron transport chain.7 The mitochondrial complex I (MC-I) is the beginning of the electron transport chain in the mitochondrial intima. The electrons produced by glycolysis, tricarboxylic acid cycle and fatty acid oxidation are introduced into the electron transfer chain to produce adenosine triphosphate (ATP). 18F-Flurpiridaz can competitively bind to MC-I, without affecting the activity of cardiomyocytes.In early publications, 18F-Flurpiridaz, known as RP10128 or BMS747158,9 is currently in phase III clinical trials.10 In this study, PET MPI was performed by using 18F-Flurpiridaz prepared by our department, and compared with the classical positron myocardial perfusion imaging agent, in order to determine the best imaging time and the ability of diagnosis of the lesion.In this study, it was found that left ventricular myocardium was clearly delineated in the whole body imaging with 18F-Flurpiridaz PET/CT in normal miniature pigs and the radioactive ratios of myocardium to surrounding tissue were high. After the injection of 18F-Flurpiridaz, the left ventricular myocardium of 1-5 min could be clearly displayed and maintained high radioactive uptake and stability within 2 hours, and the extra-cardiac adjacent non-target organs, such as the lungs and liver, had no obvious high radioactivity uptake, and no effects on myocardial imaging.
By calculating the radioactive ratio of left ventricular myocardium to blood pool, liver, lung, spleen and kidney at different time points after imaging agent injection, we found that the radioactive count ratios of left ventricular myocardium to blood pool increased gradually from 5.19 to 12.87 in 60 min, and began to decrease to 9.23 60 minutes later. The radioactive count in myocardium after injection of imaging agent 1 min was 5.19 times that of cardiac blood pool. Myocardial development was not affected by blood pool at all.The radioactive ratio of left ventricular myocardium to liver decreased gradually from 3.44 to 2.08 in 10 min, and increased gradually from 2.08 to 6.92 10 minutes later, indicating that the imaging agent had a slight uptake in the liver, gradually increased in a short period of time and was rapidly excreted, and the myocardial imaging was not affected by the liver count.
As we all know, 13N-NH3·H2O is not only myocardial uptake, but also high liver uptake. The inferior wall myocardial display is sometimes severely affected by the high radioactive counts of left lobe of liver, and has a certain impact on the diagnosis adn quantitative analysis. The higher cardiac uptake rate and uptake ratio of 18F-Flurpiridaz to adjacent organs can be converted into higher quality myocardial images, reduce artifacts and the impact on quantitative analysis.
Previous studies11 have reported that the uptake and kinetics of 18F-Flurpiridaz in rat cardiomyocytes showed a very fast uptake rate, the maximum uptake time was 35 seconds, and the eluting time was more than 120 minutes.The biodistribution study12 in mice showed that the accumulation of 18F-Flurpiridaz in the heart was the highest. At 60 minutes, the myocardial uptake was 9.5% per gram, and the heart-lung ratio and heart-liver ratio were 14.1 and 8.3, respectively. Micro-PET images of 5 to 15 minutes and 55 to 65 minutes showed clear left ventricular myocardium and low background activity. Other experimental pig imaging studies13 showed that compared with 13N-NH3·H2O, the image contrast between heart and blood, lung and liver was generally higher in 18F-Flurpiridaz under stress and rest conditions. The increase in contrast observed in the study was due to the removal of radioactivity from non-target organs.In addition, the time-activity curve obtained by 18F-Flurpiridaz imaging showed that its activity in the myocardium was stable for at least 1 hour, while the activity of liver decreased by 49%. It was confirmed that the drug was not redistributed in myocardium.6 The results of this study are basically consistent with those reported in previous studies.
In this study, 18F-Flurpiridaz myocardial perfusion imaging showed clear myocardial uptake, uniform distribution of myocardial imaging agents in all segments, low background count of cardiac cavity, no extracardiac radioactive interference, and excellent imaging quality compared with 13N-NH3·H2O. This is mainly the result of the high first-pass extraction of 18F-Flurpiridaz and the high spatial resolution of 18F positrons.It has been reported14-16 that the first-pass extraction fraction of 18F-Flurpiridaz in an isolated rat heart is about 94%, which is higher than that of myocardial perfusion imaging agent available at present, in which the extraction fraction of 13N-NH3·H2O is 82%. Moreover, under adenosine stress, the extraction fraction of 94% of 18F-Flurpiridaz has no significant change, which is due to the high density of myocardial mitochondria (20% to 30% of the volume of cardiomyocytes). The lipophilicity of 18F-Flurpiridaz and its binding to MC-1 with high density distribution significantly increased the extraction fraction.17 In addition, the positron range of 18F-Flurpiridaz is only 1.03 mm, which is significantly shorter than 2.53 mm of 13N, so the spatial resolution of PET imaging is better than that of the latter, and the uptake contrast between the heart and its surrounding organs is higher.6,9,11 In the study of mice, rats, rabbits and pigs, 18F-Flurpiridaz found that the image quality of heart PET was up to excellent grade.6,9,18-20
We compared the SRS, Extend, TPD and LVEF of left ventricular, and other main cardiac function parameters of 18F-Flurpiridaz gated myocardial perfusion imaging with 13N-NH3·H2O. It was found that there was no significant difference between them. Ming Yu6,9,11 et al reported the value of 18F-Flurpiridaz myocardial perfusion imaging in detecting myocardial perfusion defect in myocardial infarction model induced by ischemia-reperfusion injury or permanent coronary artery occlusion and myocardial ischemia model induced by transient coronary artery occlusion in rats and pigs. In each case, 18F-Flurpiridaz can clearly show the defect area. Sherif et al20 compared the size of myocardial infarction measured by 18F-Flurpiridaz imaging with histology in rat myocardial infarction model, and found that there was a good correlation between the 2 measurements.
Conclusion
In summary, the results of this study show that 18F-Flurpiridaz has excellent imaging performance, the radioactive ratios of myocardium to non-target tissue are high and stable, and high quality myocardial perfusion images can be obtained early after drug injection. The conventional functional parameters obtained by gated acquisition are accurate and reliable.
As a new generation of positron myocardial perfusion imaging agent, 18F-Flurpiridaz has a optimal physical half-life of 109.8 min. It is convenient for rest-exercise load imaging and very suitable for long-distance distribution to distant PET centers. Moreover, the spatial resolution of 18F-Flurpiridaz is better and its uptake in the liver is more less, which has superior performance compared with 13N-NH3·H2O, which is the most commonly used myocardial perfusion imaging agent, so it has a very broad clinical application prospect.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Tianjin Natural Science Foundation (No. 17JCYBJC28200) and Science and Technology Project of Tianjin Binhai New Area Health and Family Planning Commission (No. 2018BWKZ007).
ORCID iD: Jiao Wang, MD  https://orcid.org/0000-0002-7008-1913
https://orcid.org/0000-0002-7008-1913
References
- 1. Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):143–152. doi:10.1161/CIR.0b013e318282ab8f [DOI] [PubMed] [Google Scholar]
- 2. Salata BM, Singh P. Role of cardiac PET in clinical practice. Curr Treat Options Cardio Med. 2017;19(12):93 doi:10.1007/s11936-017-0591-x [DOI] [PubMed] [Google Scholar]
- 3. Pan JA, Salerno M. Clinical utility and future applications of PET/CT and PET/CMR in cardiology. Diagnostics (Basel). 2016;6(3):32 doi:10.3390/diagnostics6030032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Werner RA, Chen X, Rowe SP, Lapa C, Javadi MS, Higuchi T. Moving into the next era of PET myocardial perfusion imaging: introduction of novel F-labeled tracers. Int J Cardiovasc Imaging. 2019;35(3):569–577. doi:10.1007/s10554-018-1469-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berman DS, Maddahi J, Tamarappoo BK, et al. Phase II safety and clinical comparison with single photon emission computed tomography myocardial perfusion imaging for detection of coronary artery disease: Flurpiridaz F18 positron emission tomography. J Am Coll Cardiol. 2013;61(4):469–477. doi:10.1016/j.jacc.2012.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Purohit A, Radeke H, Azure M, et al. Synthesis and biological evaluation of pyridazinone analogues as potential cardiac positron emission tomography tracers. J Med Chem. 2008;51(10):2954–2970. doi:10.1021/jm701443n [DOI] [PubMed] [Google Scholar]
- 7. Maddahi J, Packard RR. Cardiac PET perfusion tracers: current status and future directions. Semin Nucl Med. 2014;44(5):333–343. doi:10.1053/j.semnuclmed.2014.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu M, Mistry M, Guaraldi M, et al. [18F]-RP1012: a novel myocardial perfusion imaging agent for use with positron emission tomography (PET). Circulation. 2005;112(17):II–761. [Google Scholar]
- 9. Yu M, Guaraldi MT, Mistry M, et al. BMS-747158-02: a novel PET myocardial perfusion imaging agent. J Nucl Cardiol. 2007;14(6):789–798. doi:10.1016/j.nuclcard.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 10. Maddahi J, Udelson J, Heller GV, et al. The first phase 3 international multicenter clinical trial of Flurpiridaz F18, a new radiopharmaceutical for PET myocardial perfusion imaging (abstract). J Nucl Cardiol. 2015;22:744. [Google Scholar]
- 11. Yalamanchili P, Wexler E, Hayes M, et al. Mechanism of uptake and retention of F-18 BMS-747158-02 in cardiomyocytes: a novel PET myocardial imaging agent. J Nucl Cardiol. 2007;14(6):782–788. doi:10.1016/j.nuclcard.2007.07.009 [DOI] [PubMed] [Google Scholar]
- 12. Yalamanchili P, Wexler E, Hayes M, et al. Mechanism of uptake agent for myocardial imaging using positron emission tomography. Bioorg Med Chem Lett. 2012;22(5):319–322. [DOI] [PubMed] [Google Scholar]
- 13. Nekolla SG, Reder S, Saraste A, et al. Evaluation of the novel myocardial perfusion positron-emission tomography tracer 18F-BMS-747158-02: comparison to 13N-ammonia and validation with microspheres in a pig model. Circulation. 2009;119(17):2333–2342. doi:10.1161/CIRCULATIONAHA.108.797761 [DOI] [PubMed] [Google Scholar]
- 14. Leppo JA, Meerdink DJ. Comparison of the myocardial uptake of a technetium-labeled isonitrile analogue and thallium. Circ Res. 1989;65(3):632–639. doi:10.1161/01.res.65.3.632 [DOI] [PubMed] [Google Scholar]
- 15. Schelbert HR, Phelps ME, Huang SC, et al. N-13 ammonia as an indicator of myocardial blood flow. Circulation. 1981; 63(6):1259–1272. doi:10.1161/01.cir.63.6.1259 [DOI] [PubMed] [Google Scholar]
- 16. Mullani NA, Goldstein RA, Gould KL, et al. Myocardial perfusion with rubidium-82: I—measurement of extraction fraction and flow with external detectors. J Nucl Med. 1983;24(10):898–906. [PubMed] [Google Scholar]
- 17. Maddahi J, Bengel F, Huang S-C, et al. Phase 1 rest-stress study of F-18 labeled BMS747158 myocardial perfusion PET tracer: human safety, dosimetry, biodistribution, and myocardial imaging characteristics. J Nucl Med. 2009;50:184. [DOI] [PubMed] [Google Scholar]
- 18. Huisman MC, Higuchi T, Reder S, et al. Initial characterization of an 18F-labeled myocardial perfusion tracer. J Nucl Med. 2008;49(4):630–636. doi:10.2967/jnumed.107.044727 [DOI] [PubMed] [Google Scholar]
- 19. Higuchi T, Nekolla SG, Huisman MM, et al. A new 18F-labeled myocardial PET tracer: myocardial uptake after permanent and transient coronary occlusion in rats. J Nucl Med. 2008;49(10):1715–1722. doi:10.2967/jnumed.108.053967 [DOI] [PubMed] [Google Scholar]
- 20. Sherif HM, Saraste A, Weidl E, et al. Evaluation of a novel 18F-labeled positron-emission tomography perfusion tracer for the assessment of myocardial infarct size in rats. Circ Cardiovasc Imaging. 2009;2(2):77–84. [DOI] [PubMed] [Google Scholar]