Abstract
Allergic contact dermatitis is a skin inflammatory disease manifested with itch and pain symptom around the inflamed area. Chemokines such as CXCL12 are involved in the pathophysiology of allergic contact dermatitis, but little has been known about the effect of CXCL12/CXCR4 signaling for nociceptive sensation accompanying allergic contact dermatitis. Our study showed that CXCL12 and CXCR4 were upregulated in trigeminal ganglion with the progression of allergic contact dermatitis through western blotting and immunofluorescence. CXCL12 and CXCR4 were mainly upregulated in small-diameter neurons, which were co-localized with nociceptive markers in trigeminal ganglion. CXCR4 and CXCL12 were also expressed in trigeminal ganglion neurons retrograded from the skin lesion. Intradermal injection of CXCL12 enhanced the itch- and pain-like behavior which could be relieved by AMD3100, a CXCR4 antagonist, without changes of mast cells. Our findings suggested that blockade of CXCL12/CXCR4 signaling pathway might be beneficial to relieve itch and pain sensation accompanying allergic contact dermatitis.
Keywords: CXCR4, CXCL12, chemokine, itch, pain, primary sensory neurons
Introduction
Allergic contact dermatitis (ACD) is a major cause of occupational skin disease, and accounts for at least 20% of the general population who are contact-allergic to common environmental allergens.1 ACD, a type IV delayed immunological reaction in response to contacting with an allergen in sensitized individuals, manifests a hypersensitivity reaction to antigens in contact with skin.2 Currently, clinical manifestations of ACD are itch, pain, burning, and stinging.3 Classically, most research on itch mechanisms has focused on the canonical immunoglobulin E (IgE)-mast cell-histamine axis. However, therapies aiming at blocking histaminergic itch pathway have been largely ineffective, suggesting the existence of nonhistaminergic itch pathways.4,5
Over a long period, ACD has been regarded as an IgE-mediated T-helper-1(Th1)-driven allergic reaction with multiple cytokines and chemokines involved.5–7 Of these chemokines, CXCL12, also known as stromal cell-derived factor-1α (SDF-1 α) or preB-cell growth-stimulating factor (PBSF), functions as the ligand for chemokine (C-X-C motif) receptor 4 (CXCR4). CXCL12/CXCR4 signaling plays a role in many diverse cellular functions, including embryogenesis, immune surveillance, inflammation response, tissue homeostasis, tumor growth and metastasis. CXCR4 acts with CD4 protein to facilitate the entry of human immunodeficiency virus (HIV) into cells, and mutations in CXCL12 are associated with resistance to HIV-1 infection. In ACD inflammation, CXCL12 acts on resting regulatory CD4+ T cells in terms of calcium mobilization and in vitro migration, it also attracts dendritic cells and memory T cells to random and directed motion.8–10 Despite the proinflammatory effects of CXCL12 in the pathogenesis of ACD, a possible contribution of CXCL12 to itch and pain accompanying ACD has not been explored.
Itch and pain are usually caused by nociceptive primary afferent nerves and their cell bodies in the dorsal root ganglia or trigeminal ganglia. The interaction between immune and neuronal system has been considered as the major mechanism of pain and itch, that is, the activation of immune procedure is based on neuronal activity, and immune activation affects neuronal activity in return. Multiple immune factors have been identified to activate sensory neurons directly through corresponding receptors expressed in sensory neurons. These immune factors and chemokines, such as CCL2 and CXCL10, arouse great attention as they may contribute to itch associated with ACD.
Traditionally, CXCL12 and its receptor CXCR4 are expressed in immune cells, but studies have confirmed that CXCR4 is expressed in nervous tissue also, including primary sensory neurons, and it contributes to the development and maintenance of neuropathic pain. Furthermore, CXCL12 can directly activate primary sensory neurons through neuronal CXCR4. Despite that CXCL12 is upregulated in affected human skin of ACD, its function in sensory neurons under the condition of ACD and its effect to relatively nociceptive sensations are still unknown.8,11–13 In this study, we examined the pattern of CXCL12 and CXCR4 expression in trigeminal ganglion (TG) and skin under the condition of ACD and explored the potential role of CXCL12/CXCR4 axis between skin and TG in mediating allergic itch and pain.
Materials and methods
Animals and chemicals
Adult male C57BL/6 mice (2–3 months’ old, 20–30 g, provided by HFK Bioscience Co., Ltd, Beijing, China) were housed in a controlled environment (21 ± 4°C, standard 12-h light/dark cycle, 4–5 mice per cage). All mice had ad libitum access to food and water. All experimental procedures were approved by the Animals Ethics Committee of our institution.
AMD3100, the first U.S. Food and Drug Administration (FDA)-approved CXCR4 antagonist drug Plerixafor (Selleck, China), was dissolved in saline. The purpose of AMD3100 treatment in this study was to specifically block the biological activity of CXCR4.
CXCL12 (R&D Systems, Minneapolis, MN, USA), a ligand for CXCR4 that functioned as an activator of CXCR4 was used.
Model of ACD
The murine model of ACD was constructed by applying the contact sensitizer squaric acid dibutylester (SADBE; Sigma, USA) referring to previous studies.7,14 The abdomen of mice was shaved without any skin lesion on day 1 under the brief anesthesia with isoflurane (2% in oxygen). Later, “ACD” mice were sensitized with 25 µL of 1% SADBE in acetone solution superficially applied to shaved abdomen for three consecutive days (Days 2–4). On day 7, the right cheek of mice was shaved without any skin lesion. Later on Days 8–10, the mice were challenged for three consecutive days with 25 µL 1% SADBE to the shaved cheek. Control mice only received acetone of equal volume on abdomen and cheek in the same time period. For the sensitized group represented the sensitization phase, mice were sensitized with 1% SBADE of equal volume on abdomen only, while ipsilateral cheek was treated with 25 µL of acetone instead of SADBE challenge.
Behavioral testing
Before behavioral testing, mice were fully acclimated by being placed in the test chamber 1 h quaque die (QD) for three consecutive days. According to previous study, the test chamber was surrounded by four mirrors to provide a top view of all sides.7 Behavioral responses were recorded by camera, and quantified at a slow-motion. One single scratching bout was defined as the mice lifting its hindpaw, scratching the concerned face for multiple times within seconds, and putting the hindpaw to the floor or to its mouth. One wiping bout was defined as the mice lifting its forepaw, wiping the face area, and returning to its original start position. Pain- and itch-like behaviors were quantified by counting the number of forepaw wiping and hindpaw scratching bouts during the 30 min observation period.
Next, 200 µL AMD3100 (5 mg/kg) or saline of equal volume alone (Veh1) were injected intraperitoneally 120 min before behavioral testing in corresponding groups. And CXCL12 (2 µg/10 µL in PBS; R&D Systems) or PBS (Veh2, 10 µL) was injected intracutaneously into ipsilateral cheek before recording. All records were performed after 24 h of second SABDE challenge. Chemicals were previously prepared and then coded by a laboratory administrator and not the experimenter. The operator and observer were blind to the code.
Histology and immunohistochemistry
Under deep anesthesia with isoflurane, ACD mice were perfused through the left ventricle with sterile 0.1 M phosphate-buffered saline (PBS). An outlet as large as possible in right atrium was incised by iris scissors to remove blood and then flowed by pre-cooled 4% paraformaldehyde. Inducing any air bubbles were avoided during the period of perfusion. After perfusion, ipsilateral skin was dissected, post-fixed overnight in buffered 10% formalin at room temperature, transferred to sequential 70%, 80%, 90%, and 100% methanol, embedded in paraffin and sectioned (3 µm). Slices were immersed into hematoxylin solution for 5 min, and rinsed with PBS for 10 min, followed by 70% and 90% alcohol for 10 min for dehydrating, respectively. Slices were dyed in eosin staining solution for 2 to 3 min. After dehydrated by pure alcohol and penetrated by xylene, the slices were dropped with gum and sealed with a cover glass. Sections were stained with hematoxylin and eosin (H&E) and examined microscopically by two pathologists. Images were acquired using fluorescence microscopy imaging system (Ti-S, Olympus FluoView software, Olympus, Japan). For immunohistochemistry staining, unstained serial sections were transferred into xylene I (5 min) and xylene II (10 min) for deparaffinage, and sequential 100%, 95%, 95%, and 80% ethanol for rehydrating (5 s, respectively). Sections were treated with microwave heat-induced antigen retrieval (121°C, 15 min) to reveal the activity of endogenous biotin and were transferred into 5% hydrogen peroxide to block endogenous peroxidase. After blocking the non-specific binding of the antibody with 5% normal horse serum, sections were incubated with primary antibody (Rabbit anti-CXCL12, 1:400, Abcam, USA) at 4°C overnight and then rinsed with PBS. Then sections were incubated with proper secondary antibodies (Goat Anti-Rabbit IgG H&L, 1:1000, Abcam). Images were acquired in the same manner as above.
Retrograde labeling of cutaneous neurons
Dil Stain (1,1′-Dioctadecyl-3,3,3′,3′-Tetramethylindocarbocyanine Perchlorate (‘DiI’; DiIC18(3)), Invitrogen, USA) is a lipophilic membrane dye which is weakly fluorescent until incorporated into membranes presenting red fluorescence when entering the cell membrane. It can diffuse laterally and gradually stain the cell membrane of the entire cell. Before the first challenge with either the acetone- or SADBE-treated skin, Dil (1.7 mg/ml in 1% DMSO) was injected intracutaneously into ipsilateral cheek for at least 3 sites (10 µL per site) under the brief anesthesia with isoflurane (2% in oxygen).
Western blotting
Under deep anesthesia with isoflurane, right TGs were collected from mice transcardially perfused with sterile PBS to remove blood and homogenized in T-PER Tissue Protein Extraction Reagent (Thermo Fisher Scientific, USA) with protein phosphatase inhibitors (Solarbio, China) and protease inhibitors (Thermo Fisher Scientific, USA) at 1:100. Denaturation of proteins on heating was separated by 12% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, France). Before incubation at 4°C for 6–8 h with primary antibodies (Rabbit Anti-CXCR4 antibody,1:1000, Abcam, USA; Rabbit Anti-CXCL12 antibody, 1:1000, Abcam, USA; Rabbit Anti-β-Tubulin, 1:1000, Proteintech, China), the membranes were blocked with 5% (w/v) bovine serum albumin (Solarbio, China) for 1 h at room temperature. Then, the membranes were cut according to the distribution of the target protein, washed three times and incubated with a corresponding secondary antibody (Goat Anti-Rabbit IgG H&L, 1:1000, Abcam) for 1 h at room temperature. The bands were visualized with western blot detection system (Tanon, China) by High-sig ECL Western Blotting Substrate (Tanon, China) and analyzed with ImageJ Software.
Immunofluorescence
Ipsilateral TGs were collected from mice transcardially perfused with sterile 0.1 M PBS, followed by pre-cooled 4% paraformaldehyde (Sigma) in 0.1 M PBS. Primary tissues of interest, TGs and skin were dissected, post-fixed in 4% paraformaldehyde at 4°C for 2 h, and then dehydrated with 20% sucrose solution for 24 h then 30% sucrose for 24 h at 4°C. Tissues were sectioned in a freezing microtome (Leica 2000, Germany) at 12 µm thickness after embedded in OCT. Slices were permeabilized with 0.3% Triton X-100 for 15 min (TGs) or 1 h (skin) and blocked in 5% horse sera for 1 h at room temperature, followed by overnight incubation at 4°C with primary antibodies (Rabbit Anti-CXCR4 antibody, 1:400, Abcam; Rabbit Anti-CXCL12, 1:400, Abcam; Goat Anti-CGRP antibody, 1:2000, Abcam; Chicken Anti-Neurofilament antibody, 1:1000, Abcam; Guinea pig Anti-VR1, 1:400, Abcam). Slides were incubated with proper secondary antibodies (Alexa Fluor 488-conjugated donkey anti-rabbit, 1:400; Alexa Fluor 594-conjugated donkey anti-guinea pig, 1:400, Jackson ImmunoResearch; Alexa Fluor 594-conjugated IB4, 1:200, Invitrogen; Alexa Fluor 594-conjugated donkey anti-goat, 1:400, Jackson ImmunoResearch; Alexa Fluor 594-conjugated donkey anti-chicken, 1:400, Abcam). After three rinses in the dark, all of the sections were cover-slipped with Mounting Medium with DAPI (ZSJB-Bio, China). Mast cell staining was different from normal immunostaining, which were incubated with FITC avidin for 15 min at room temperature, then cover-slipped with Mounting Medium with DAPI imaged after drying. Images were acquired with laser confocal microscopic imaging system (FV1000 and Olympus FluoView software, Olympus, Japan). According to the previous study, after taking into account the approximately 10% decrease in size because of the fixation procedure, TG neurons were classified as small- (cross-sectional area, CSA < 442 µm2), medium- (CSA 443–865 µm2), and large-sized (CSA > 865 µm2).15 Only neurons with nucleus profile in the cross-section were counted and data from TGs were pooled throughout the study.
Statistical analyses
All data are presented as the mean and its standard error (mean ± SEM). Differences between two groups were analyzed using Student’s t-test. Differences among multiple groups were analyzed using one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test. A statistically significant difference was defined as a two-sided P value <0.05. IBM SPSS Statistics 21.0 for Windows (Armonk, NY, USA) was used for statistical analysis.
Results
ACD-induced spontaneous pain and itch behavior
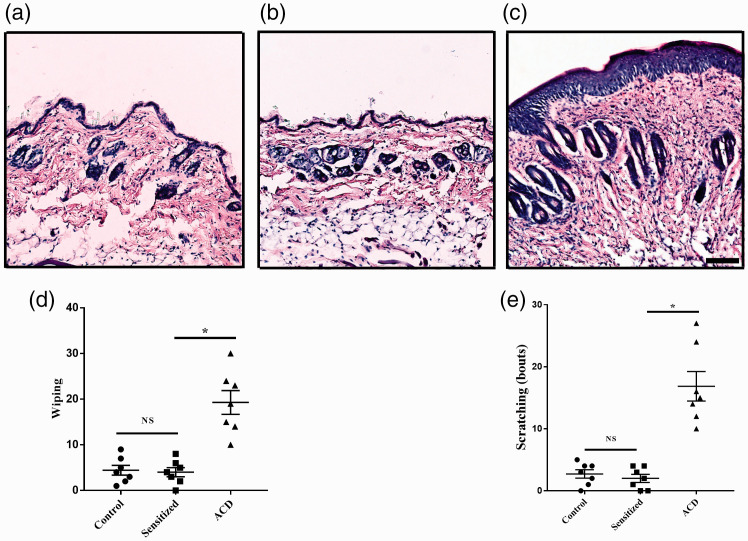
H&E staining showed typical pathology of ACD in ACD group used in our study: thickening of the epidermis, aggregation of immune cells, spinous cells proliferation, and obvious edema. However, no difference was detected in skin from mice which were only sensitized but not challenged (Figure 1(a) to (c)). The cheek model of ACD was used to investigate the behavioral effects evoked by SBADE challenge. During the elicitation phase, the forepaw wipings and hindpaw scratchings increased significantly compared with Control group and Sensitized group without challenging (n = 7, P <0.05, Figure 1(d) and (e)).
Figure 1.
Effects of ACD on the pathologic and behavioral response. (a)–(c) Representative skin biopsies for control, sensitized, and ACD mice, respectively. The change of skin in sensitization phase was not obvious. ACD skin showed prominent proliferation of cells in the stratum spinosum. And dermis was infiltrated by immune cells conforming with the swollen and thickened appearance. Scale bar: 200 µm. (d) and (e). During the period of sensitization (Sensitized group), pain-like behavioral responses (forepaw wiping) and itch-like behavioral responses (hindpaw scratching) were not increased. By contrast, forepaw wiping and hindpaw scratching increased significantly in the period of elicitation (ACD group). n = 7 for each group. Statistical analysis by one-way ANOVA, *P < 0.05, Sensitized versus Control group, and ACD versus Control group. ACD: allergic contact dermatitis; NS: not significant.
ACD upregulated neuronal CXCR4 in nociceptive sensors
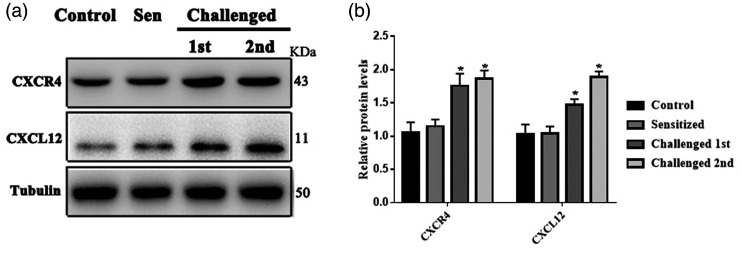
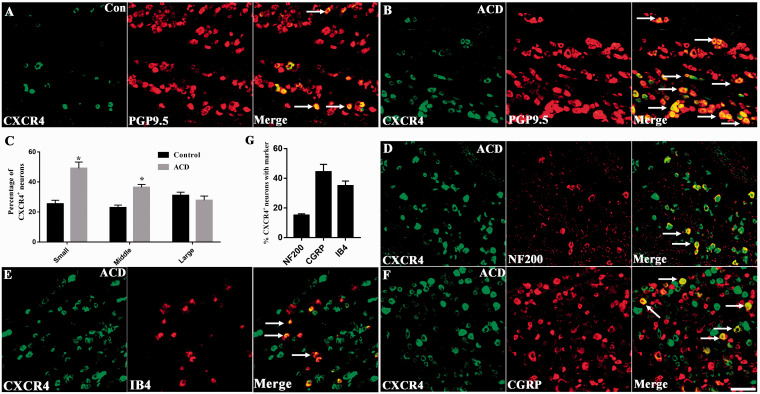
Compared with the Control group treated with acetone and Sensitized group without challenging, the protein expression of CXCL12 and CXCR4 increased significantly in TG of ACD mice (n = 3, P < 0.05, Figure 2(a) and (b)). Immunofluorescent staining revealed that CXCR4+ neurons were less distributed in TG neurons of control group while under the condition of ACD, the percentage of small-diameter and middle-diameter neurons increased (n = 3, P <0.05, Figure 3(a) to (c)). In addition, CXCR4 immunopositive TG neurons were abundantly co-expressed with calcitonin gene-related peptide (CGRP) and IB4, markers for peptidergic and nonpeptidergic sensors, at percentage of 44.63% and 34.55% in the ACD group. However, CXCR4+ was also co-localized with NF200, a marker for myelinated neuron, at a percentage of 14.88% in the ACD group (Figure 3(d) to (g)). We also analyzed the percentages of CXCR4 in neurons with markers including NF200, CGRP, and IB4 to compare the expression pattern of CXCR4 between Control and ACD. Our results indicated that the subpopulation of neurons with the three markers all increased the expression of CXCR4 (Figure S1).
Figure 2.
Effects of ACD on the expression of CXCR4 and its ligand CXCL12 in TG. (a) For the protein expression level of CXCL12 and CXCR4 in TG, the representative protein bands of CXCL12 and CXCR4 protein from Control, Sensitized, and ACD mice with first and second challenge respectively. n = 4 mice/group B. There was no significant difference between control and sensitized group. Both CXCL12 and CXCR4 were significantly upregulated in ACD mice. n = 4 mice, one-way ANOVA, *P < 0.05, Sensitized versus Control group, and Challenged versus Control group.
Figure 3.
Upregulation of CXCR4 expression in TG neurons of ACD mice. (a) CXCR4+ detected by immunofluorescence staining existed in neurons of control mice (n = 3). (b) CXCR4+ was upregulated in ACD neurons (n = 3). (c) CXCR4+ expression was significantly increased in small-diameter neurons (n = 3). (d)–(f) Co-expression of CXCR4+ with NF200, IB4 and CGRP in TG neurons of ACD mice. (n = 3). (g) Percentages of CXCR4+ TG neurons that expressed NF200, IB4 and CGRP in ACD mice. Immunopositive neurons were indicated by arrows (n = 3). Scale bar: 50 μm. Statistical analysis by Student’s t-test (*P < 0.05), ACD versus control (c and g). ACD: allergic contact dermatitis; CGRP: calcitonin gene-related peptide.
ACD upregulated CXCL12 in TG and inflamed skin
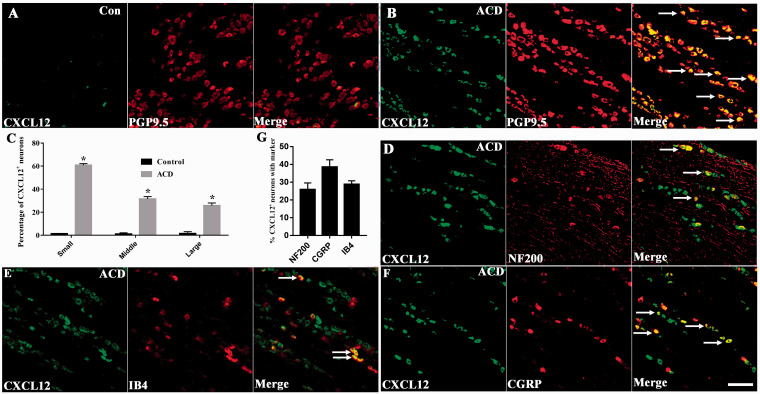
Immunofluorescence showed that ACD upregulated CXCL12 in small-diameter neurons compared with Control group in TG from 33.90% to 67.62%. However, there was no difference in distribution of middle-diameter and large-diameter neurons compared to Control group (Figure 4(a) to (c)). CXCL12 co-expressed with CGRP (39.86%) and IB4 (28.87%). However, CXCL12 was also detected in NF200+ neurons at a proportion of 25.37% (Figure 4(d) to (g)). Immunohistochemistry showed elevated expression of CXCL12 in affected skin, mainly in hair follicles, compared with control mice (Figure S2). In short, under the condition of ACD, CXCL12 was upregulated in nociceptive sensors in TG and the inflamed skin.
Figure 4.
Upregulation of the expression of CXCL12 in the TG of ACD mice. (a) Immunofluorescence staining showed that CXCL12+ existed in TG neurons of control group (n = 3). (b) The distribution of CXCL12+ was more abundant in ACD mice (n = 3). (c) Classified by the diameter of the neuron, neurons are divided into small-, middle-, and large-diameter neurons. CXCL12+ expression was significantly increased in small-diameter neurons (n = 3). (d)–(f) Co-expression of CXCL12+ with NF200, IB4, and CGRP in TG neurons of ACD mice. (n = 3). (g) Percentages of CXCL12+ TG neurons that expressed NF200, IB4 and CGRP in ACD mice (n = 3). Immunopositive neurons were indicated by arrows. Scale bar: 50 μm. Statistical analysis by Student’s t-test (*P < 0.05), ACD versus Control group (c and g). ACD: allergic contact dermatitis; CGRP: calcitonin gene-related peptide.
CXCR4+ and CXCL12+ neurons innervated the affected skin
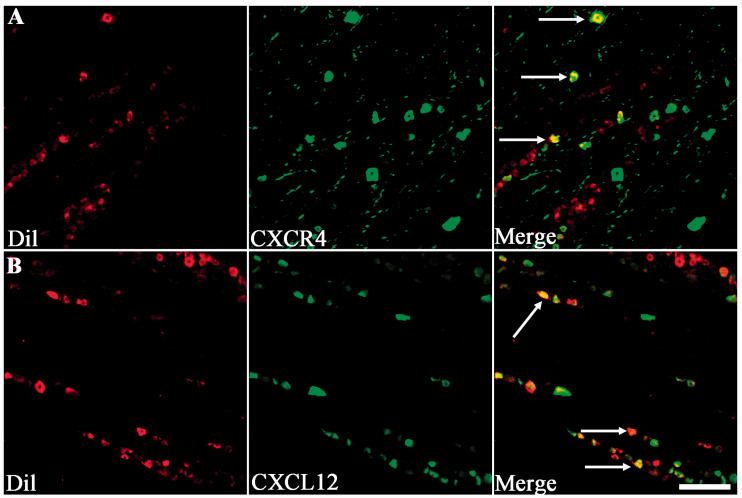
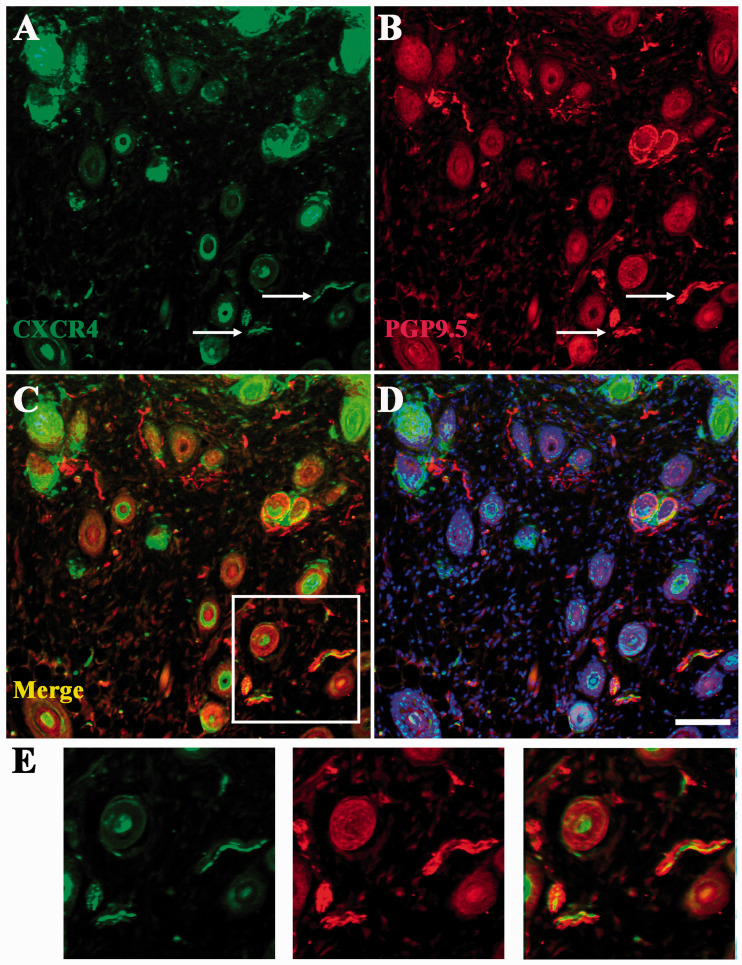
Dil was used to detect the expression of CXCR4 and CXCL12 in sensory neurons that innervated the affected skin. We found co-expression of CXCR4 with Dil (17/48) and CXCL12 with Dil (42/76) mainly in V2 zone of TG sections (Figure 5). The CXCR4+ nerve fiber was also detected in affected skin which was certified by co-localization between CXCR4 and PGP 9.5, a general neuronal marker (Figure 6).
Figure 5.
The involvement of CXCL12 and CXCR4 co-localized with Dil. (a) and (b) Images of Dil-labeled neurons in the TG after a subcutaneous injection of Dil into the right cheek, with the CXCR4+ and CXCL12+ detected by immunolabeling with Dil. Arrow indicated CXCR4+ ((a), green) and CXCL12+ ((b), green) was all co-localized with Dil (red), presenting an orange color. Scale bar: 50 µm.
Figure 6.
The involvement of CXCL12 and CXCR4 in affected skin. (a)–(d) The involvement of CXCR4 in nerve fiber in affected skin in ACD. Arrow indicated CXCR4+(green) or nerve fiber (red). Scale bar: 50 µm. (e) High magnification of CXCR4+ focusing on the affected skin. Scale bar: 25 µm.
CXCL12/CXCR4 signaling mediated nociceptive behavior in ACD mice
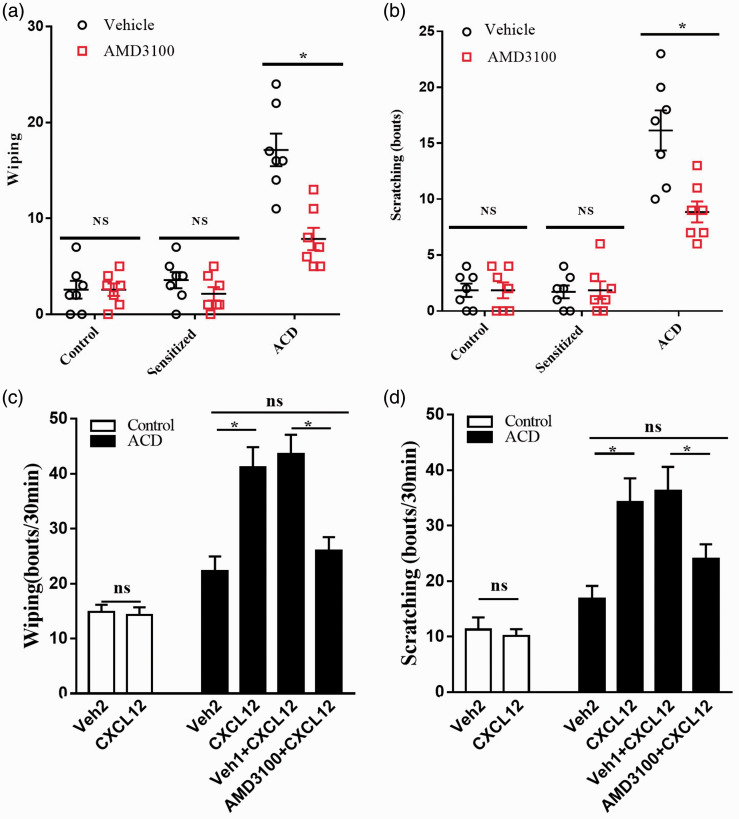
The spontaneous reaction, wiping and scratching, toward the challenged site by SBADE was decreased by application of AMD3100 in advance (n = 7, P < 0.05, Figure 7(a) and (b)). To further investigate whether CXCL12/CXCR4 signaling induced itch and pain sensation in ACD, artificially upregulating the expression of skin CXCL12 (intradermal injection 1 µg/10 µL) induced more forepaw wiping and hindpaw scratching in comparison with the equal volume vehicle (PBS). In contrast, artificially blocking the CXCR4 (intraperitoneal pre-injection of AMD3100, 5 mg/kg), the number of forepaw wiping and hindpaw scratching reduced (n = 7, P < 0.05, Figure 7(c) and (d)).
Figure 7.
CXCL12/CXCR4 signaling mediated nociceptive behaviors in ACD mice. (a) The AMD3100 did not attenuate the pain-like behavior in control and sensitized mice; however, it did reduce the pain-like behavior in ACD mice (after second challenge). (b) The AMD3100 did not attenuate the itch-like behavior in control and sensitized mice, but it did reduce the itch-like behavior in ACD mice (after second challenge). (c) CXCL12 or vehicle (PBS) was injected intracutaneously into ipsilateral cheek after 24 h of second challenge. The CXCL12 did not increase the pain-like behavior in control mice; however, it did increase pain-like behavior in ACD mice (after second challenge) which was relieved by pre-injection of AMD3100. (d) CXCL12 or vehicle (PBS) was injected intracutaneously into ipsilateral cheek after 24 h of second challenge. The CXCL12 did not increase the itch-like behavior in control mice, but it did increase itch-like behavior in ACD mice (after second challenge) which was relieved by pre-injection of AMD3100. n = 7 for each group. Statistical analysis by Student’s t-test (*P < 0.05). ACD: allergic contact dermatitis; NS: not significant.
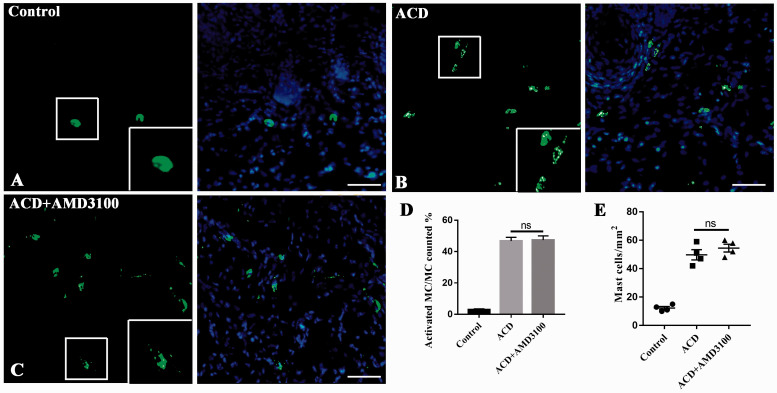
Indeed, SABDE challenging elicited significant mast cells degranulation and aggregation in the challenged site of skin. Importantly, we found that AMD3100 reduced nociceptive behavior in ACD mice without inhibiting mast cells degranulation and aggregation (n = 4, P > 0.05, Figure 8). These findings partly showed that the effect of AMD3100 to nociceptive reaction, especially the itch behavior, was not due to defects in mast cell activation because mast cell degranulation and aggregation were comparable between the group “ACD” and “ACD + AMD3100.”
Figure 8.
AMD3100 reduced nociceptive behavior in ACD mice not by inhibiting mast cell degranulation in skin. (a)–(c) Representative images of mast cells (stained with FITC-avidin, green) in the right cheek skin of control group (a), ACD group (b), and ACD + AMD3100 group (c). Framed part showed the different formation of mast cells. Scale bar: 100 µm. (d) The proportion of activated mast cells closely associated with mast cells. There was no difference between ACD and ACD + AMD3100 group. ACD versus ACD + AMD3100, n = 4. Student’s t-test. (e) The proportion of mast cells in unit area. ACD and ACD + AMD3100 were not statistically different. Statistical analysis by Student’s t-test. ACD versus ACD + AMD3100, n = 4, ACD versus ACD + AMD3100, n = 4. ACD: allergic contact dermatitis; ns: not significant.
Discussion
Our study provided ample evidence that ACD upregulated CXCL12/CXCR4 signaling in small and middle-diameter nociceptive sensory neurons in TG. CXCL12 evoked itch and pain by activating neuronal CXCR4, and AMD3100 alleviated ACD-associated itch and pain sensation by blocking CXCR4.
Regulation of CXCL12 and CXCR4 has been increasingly paid attention to by researchers for its important role in immune system and in the pathophysiology of neuroinflammation, especially since FDA’s approval of the first CXCR4 antagonist Plerixafor.16,17 In the context of neuropathic pain induced by diabetes or chronic compression of dorsal root ganglion, CXCL12/CXCR4 pathway affects nociceptive sensation at the levels of dorsal root ganglion and spinal dorsal horn.18–20 CXCL12 can directly activate or participate in the activation of sensory neurons in the spinal dorsal horn.20 In our present study, upregulated CXCL12 in inflamed skin might be released by resident skin cells and monocytes. Sensory neurons in TG with upregulated CXCL12 expression might release CXCL12 to affected skin as well (Figure 3). CXCR4 was also detected in nerve fiber in affected skin, which made it possible for the interaction between CXCL12 and CXCR4 in the area of ACD (Figure 6).
Immunofluorescence revealed that not only CXCL12 but also CXCR4 were found in all size neurons (Figures 3 and 4). CXCL12 can be upregulated in small-diameter neurons and the expression of CXCR4 was increased in small- and middle-diameter neurons under the context of TG innervating ACD skin (Figures 3(c) and 4(c)), which was consistent with the expression of CXCR4 in the context of inflammatory and neuropathic pain.20,21 Double-labeling immunofluorescence was used to determine the nociceptive character of CXCL12+ and CXCR4+ neurons (Figures 3(d) to (g) and 4(d) to (g)). Both CXCL12 and CXCR4 showed co-expression with CGRP, a marker of peptidergic sensory neuron, and IB4. CGRP has been regarded as an itch-related neuropeptide in allergic conjunctivitis and a pain-related neuropeptide in dry eyes.22,23 Our study suggested that CGRP may be a vital neuropeptide in TG in multiple conditions that evoke itch or pain sensation. CXCL12 was also detected in F4/80 immunopositive cells in DRG under neuropathy of CCD and STAT3 immunopositve neurons in DRG under chemotherapeutic neuropathology.20,24 Therefore, the expression pattern of CXCL12 may be different in different types of pathology. As CXCL12 was also expressed in TG, it might challenge CXCR4 through autocrine or paracrine manners. However, The CXCL12 expressed by TG may also be transported to brainstem and affect the activity of microglia and astrocyte.
Previous study has detected abundant expression of CXCL12 in skin lesion, but did not demonstrate whether this chemokine could induce itch or pain accompanying ACD.25–27 In the present study, intradermal injection of CXCL12 into the site of affected skin significantly elicited site-directed wiping and scratching behaviors which represented drastic itch and pain sensation. Interestingly, CXCL12-induced nociceptive behaviors were almost obliterated by AMD3100, a specific CXCR4 antagonist. The itch- and pain-like behaviors were also alleviated by AMD3100 without changes of degranulation and aggregation of mast cells in the skin. Our results remaindered that the observed behavioral changes, especially the itch behavior, were not due to defects in mast cell activation and aggregation because the number of mast cells and percentage of degranulated mast cells were comparable between the group “ACD” and “ACD+AMD3100.”
Previous studies have shown that multiple chemokines could directly activate sensory neurons such as CCL2+, CCL10+, and CXCL12+ neurons.6,7,28 These chemokines may work together to contribute to itch and pain symptoms accompanying contact hypersensitivity in mice and humans by directly exciting primary sensory neurons through their neuronal receptors and/or indirectly activating immune cells to induce the release of inflammatory mediators that target primary sensory neurons.
Acknowledgments
The authors thank Huan Cui from the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, Beijing, China, for technical support and some constructive suggestions.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant no. 8167051700) and the Peking Union Medical College Innovation Fund for Graduates (grant no. 2019–1002-65).
ORCID iD
Wenliang Su https://orcid.org/0000-0003-2464-7274
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Alinaghi F, Bennike NH, Egeberg A, Thyssen JP, Johansen JD. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Derm 2019; 80: 77–85. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan DH, Igyártó BZ, Gaspari AA. Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol 2012; 12: 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clinic Rev Allerg Immunol 2019; 56: 99–109. [DOI] [PubMed] [Google Scholar]
- 4.Ahlström MG, Thyssen JP, Wennervaldt M, Menné T, Johansen JD. Nickel allergy and allergic contact dermatitis: a clinical review of immunology, epidemiology, exposure, and treatment. Contact Derm 2019; 81: 227–241. [DOI] [PubMed] [Google Scholar]
- 5.Yang T-L, Kim BS. Pruritus in allergy and immunology. J Allergy Clin Immunol 2019; 144: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qu L, Fu K, Yang J, Shimada SG, LaMotte RH. CXCR3 chemokine receptor signaling mediates itch in experimental allergic contact dermatitis. Pain 2015; 156: 1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang H, Cui H, Wang T, Shimada SG, Sun R, Tan Z, Ma C, LaMotte RH. CCL2/CCR2 signaling elicits itch- and pain-like behavior in a murine model of allergic contact dermatitis. Brain Behav Immun 2019; 80: 464–473. [DOI] [PubMed] [Google Scholar]
- 8.Parr A, Anderson NR, Hammer DA. A simulation of the random and directed motion of dendritic cells in chemokine fields. PLoS Comput Biol 2019; 15: e1007295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meller S, Lauerma AI, Kopp FM, Winterberg F, Anthoni M, Müller A, Gombert M, Haahtela A, Alenius H, Rieker J, Dieu-Nosjean M-C, Kubitza RC, Gleichmann E, Ruzicka T, Zlotnik A, Homey B. Chemokine responses distinguish chemical-induced allergic from irritant skin inflammation: memory T cells make the difference. J Allergy Clin Immunol 2007; 119: 1470–1480. [DOI] [PubMed] [Google Scholar]
- 10.Sebastiani S, Allavena P, Albanesi C, Nasorri F, Bianchi G, Traidl C, Sozzani S, Girolomoni G, Cavani A. Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity. J Immunol 2001; 166: 996–1002. [DOI] [PubMed] [Google Scholar]
- 11.Fumagalli A, Zarca A, Neves M, Caspar B, Hill SJ, Mayor F, Smit MJ, Marin P. CXCR4/ACKR3 phosphorylation and recruitment of interacting proteins: key mechanisms regulating their functional status. Mol Pharmacol 2019; 96: 794–808. [DOI] [PubMed] [Google Scholar]
- 12.Radermecker C, Sabatel C, Vanwinge C, Ruscitti C, Maréchal P, Perin F, Schyns J, Rocks N, Toussaint M, Cataldo D, Johnston SL, Bureau F, Marichal T. Locally instructed CXCR4 neutrophils trigger environment-driven allergic asthma through the release of neutrophil extracellular traps. Nat Immunol 2019; 20: 1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heuninck J, Perpiñá Viciano C, Işbilir A, Caspar B, Capoferri D, Briddon SJ, Durroux T, Hill SJ, Lohse MJ, Milligan G, Pin J-P, Hoffmann C. Context-dependent signaling of CXC chemokine receptor 4 and atypical chemokine receptor 3. Mol Pharmacol 2019; 96: 778–793. [DOI] [PubMed] [Google Scholar]
- 14.Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, Oetjen LK, Wang F, Kim BS, Dong X. Activation of mast-cell-expressed Mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity 2019; 50: 1163–1171.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F, Oh SB, Ji RR. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med 2015; 21: 1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang F, Sun W, Yang Y, Wang Y, Li C-L, Fu H, Wang X-L, Yang F, He T, Chen J. SDF1-CXCR4 signaling contributes to persistent pain and hypersensitivity via regulating excitability of primary nociceptive neurons: involvement of ERK-dependent Nav1.8 up-regulation. J Neuroinflammation 2015; 12: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tahirovic YA, Pelly S, Jecs E, Miller EJ, Sharma SK, Liotta DC, Wilson LJ. Small molecule and peptide-based CXCR4 modulators as therapeutic agents. A patent review for the period from 2010 to 2018. Expert Opin Ther Pat 2020; 30: 87–101. [DOI] [PubMed] [Google Scholar]
- 18.Xu T, Zhang X-L, Ou-Yang H-D, Li Z-Y, Liu C-C, Huang Z-Z, Xu J, Wei J-Y, Nie B-L, Ma C, Wu S-L, Xin W-J. Epigenetic upregulation of CXCL12 expression mediates antitubulin chemotherapeutics-induced neuropathic pain. Pain 2017; 158: 637–648. [DOI] [PubMed] [Google Scholar]
- 19.Jayaraj ND, Bhattacharyya BJ, Belmadani AA, Ren D, Rathwell CA, Hackelberg S, Hopkins BE, Gupta HR, Miller RJ, Menichella DM. Reducing CXCR4-mediated nociceptor hyperexcitability reverses painful diabetic neuropathy. J Clin Invest 2018; 128: 2205–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y, Huang X, Di Y, Qu L, Fan N. Effect of CXCL12/CXCR4 signaling on neuropathic pain after chronic compression of dorsal root ganglion. Sci Rep 2017; 7: 5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai L, Wang X, Li Z, Kong C, Zhao Y, Qian J-L, Kan Q, Zhang W, Xu J-T. Upregulation of chemokine CXCL12 in the dorsal root ganglia and spinal cord contributes to the development and maintenance of neuropathic pain following spared nerve injury in rats. Neurosci Bull 2016; 32: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li F, Yang W, Jiang H, Guo C, Huang AJW, Hu H, Liu Q. TRPV1 activity and substance P release are required for corneal cold nociception. Nat Commun 2019; 10: 5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C-C, Yang W, Guo C, Jiang H, Li F, Xiao M, Davidson S, Yu G, Duan B, Huang T, Huang AJW, Liu Q. Anatomical and functional dichotomy of ocular itch and pain. Nat Med 2018; 24: 1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y-Y, Li H, Liu Z-L, Li Q, Qiu H-W, Zeng L-J, Yang W, Zhang X-Z, Li Z-Y. Activation of STAT3-mediated CXCL12 up-regulation in the dorsal root ganglion contributes to oxaliplatin-induced chronic pain. Mol Pain 2017; 13: 1744806917747425–1744806917747425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han JH, Ahn M-H, Jung J-Y, Suh CH, Kwon JE, Yim H, Kim HA. The levels of CXCL12 and its receptor, CXCR4, as a biomarker of disease activity and cutaneous manifestation in adult-onset Still’s disease. Clin Exp Rheumatol 2019; 37: 67–73. [PubMed] [Google Scholar]
- 26.Nishiguchi MA, Spencer CA, Leung DH, Leung TH. Aging suppresses skin-derived circulating SDF1 to promote full-thickness tissue regeneration. Cell Rep 2018; 24: 3383–3392.e3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willyard C. Unlocking the secrets of scar-free skin healing. Nature 2018; 563: S86–S88. [DOI] [PubMed] [Google Scholar]
- 28.Jing P-B, Cao D-L, Li S-S, Zhu M, Bai X-Q, Wu X-B, Gao Y-J. Chemokine receptor CXCR3 in the spinal cord contributes to chronic itch in mice. Neurosci Bull 2018; 34: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]