Abstract
Mesenchymal stem cells (MSCs) are widely considered good candidates for cell transplantation therapy. Various central nervous system disorders have been suggested as suitable targets for MSC transplantation therapy. In this context, a great deal of basic and clinical research has been conducted to explore its clinical uses. Although depression is one of the most common diseases in the world, the response rate to the currently available treatment is insufficient and new treatments are much needed. Despite the fact that MSC transplantation therapy has the potential to elicit an antidepressant effect, few studies have been conducted on this topic to date and the underlying mechanism remains poorly understood. To address the development of a new treatment for depression, we evaluated the effect of MSCs using the encapsulation technique and Wistar-Kyoto rats. Encapsulation enables dissection of the complicated underlying mechanism of MSC transplantation therapy. Wistar-Kyoto rats that exhibit treatment-resistant depressive-like behaviors allow us to compare the effect of MSCs with that of conventional antidepressant treatment. In this commentary, we briefly summarize our recent published results and discuss future research prospects.
Keywords: brain-derived neurotrophic factor, ciliary neurotrophic factor, depression, fibroblast growth factor-2, mesenchymal stem cells, transplantation, vascular endothelial growth factor
Comment on: Kin K, Yasuhara T, Kameda M, Tomita Y, Umakoshi M, Kuwahara K, Kin I, Kidani N, Morimoto J, Okazaki M, Sasaki T, Tajiri N, Borlongan CV, Date I. Cell encapsulation enhances antidepressant effect of the mesenchymal stem cells and counteracts depressive-like behavior of treatment-resistant depressed rats. Mol Psychiatry. 2020 Jun;25(6):1202-1214. doi: 10.1038/s41380-018-0208-0. Epub 2018 Aug 14. PubMed PMID: 30108315.
Introduction
Mesenchymal stem cells (MSCs) are considered to be good transplantable cell candidates. The rapid isolation from various tissues and the efficient amplification in culture are the biggest advantages of MSCs for clinical use. Central nervous system (CNS) disorders, such as Parkinson’s disease, traumatic brain injury (TBI), and stroke, are thought to be good targets for MSC transplantation therapy and several clinical trials have been conducted.1
Unlike CNS disorders, MSC transplantation has not been strongly focused on and the literature in the field of psychiatric disorders is limited. Depression, which is one of the most common and leading causes of disability around the world, is not well controlled with currently available treatments. Therefore, a new treatment for treatment-resistant depression is needed and MSC treatment may be a good candidate. Although the intraventricular implantation of MSCs was reported to have an ameliorative effect on depressive-like behavior in a rodent study,2 its effect needs to be compared with that of conventional treatments to determine if it is a good candidate as a new treatment.
Implanted MSCs have the potential to differentiate into neural lineage and to migrate and interact with host cells.1,3 As such, implanted MSCs are thought to exhibit beneficial effects by integrating with and functioning as part of the host tissue. Transplanted cell-secreted factors are also reported to be key factors.4 For example, MSC implantation had a beneficial effect on TBI.5 Stand-alone injections of vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF), both of which are secreted from MSCs, are also beneficial for TBI.6,7 These results suggest that at least some of the effects of MSC transplantation are due to secreted factors. Despite the accumulation of knowledge related to MSC transplantation therapy, one important issue remains unaddressed, namely: whether cell-to-cell contact between transplants and host cells or transplanted cell-secreted factors mediate the beneficial effects of MSCs.
To address these issues, we recently published our latest results on encapsulated MSC implantation to Wistar-Kyoto (WKY) rats.8 In this commentary, we will discuss these results and future research directions. The key factors in this study are “encapsulation” and “WKY rats”. Encapsulation is a technique used to protect graft cells from immune system attack and it may enhance the survival rate of grafted cells. At the same time, it prevents implanted cells from interacting with the host cells. We used this method to dissect the underlying mechanism of its treatment effect. WKY rats are known to exhibit depressive behavior congenitally and to be resistant to conventional antidepressant treatment. A response to electroconvulsive therapy, which is an advanced therapy for pharmacotherapy-resistant depression, has been shown, however.9 These results suggest that WKY rats may be useful in research on the development of new treatments for treatment-resistant depression. Using this animal model, we compared the treatment effect of MSC implantation with that of conventional treatment.
Encapsulated MSCs ameliorated the behavioral deficit of WKY rats
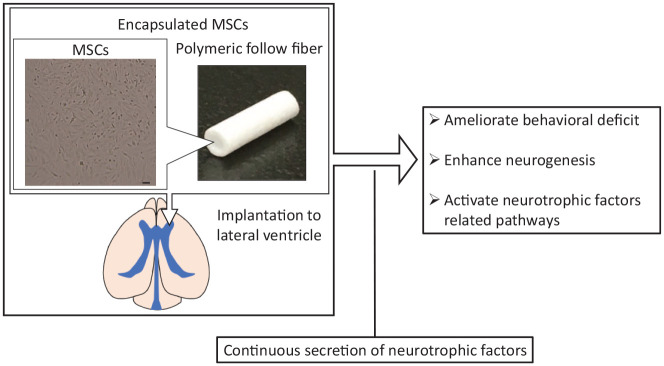
First, we evaluated the effect of MSC implantation with or without encapsulation. The open field test (OFT) and the traditional two-day forced swim test (FST) were used as behavioral tests. WKY rats are less active in a novel field (as measured by the OFT) and spend a longer time immobile in the FST than Wistar rats which are the healthy control.10 Encapsulated MSC implantation to the lateral ventricle showed an ameliorative effect in both the OFT and FST day2 (Figure 1). MSC implantation without encapsulation did not affect the results of these behavioral tests, however.
Figure 1.
Schematic representation of encapsulated mesenchymal stem cell (MSC) implantation. MSCs were encapsulated with polymeric hollow fibers. Encapsulated MSCs continuously secreted vascular endothelial growth factor, brain-derived neurotrophic factor, fibroblast growth factor-2, and ciliary neurotrophic factor. Various treatment effects were exhibited. MSCs, mesenchymal stem cells. Bar: 100 μm.
We checked the survival rate of MSCs without encapsulation and found that they completely disappeared from the lateral ventricle and the brain parenchyma within 1 week. In contrast, encapsulated MSCs survived even 2 weeks after implantation. This means that encapsulation increased the survival rate of the implanted cells. We speculate that encapsulation protects MSCs from immune attack and that this increased survival rate enhanced the treatment effect of MSCs.
Although interaction with host cells is lost in encapsulated MSCs, we observed a robust treatment effect following encapsulated MSC implantation. These results allow us to speculate that cell-secreted factors play a major role in the effect of MSCs.
Encapsulated MSCs enhanced endogenous neurogenesis
Endogenous neurogenesis in the subventricular zone (SVZ) and dentate gyrus (DG) was also evaluated. WKY rats exhibit physiological features that resemble the abnormalities observed in depression.11,12 For example, volume reduction of the hippocampus and neurogenesis in the DG have been reported.10,13 Hippocampal neurogenesis is reported to be enhanced by conventional antidepressant treatment and it may be the key factor underlying its effect.14 In our study, encapsulated MSC implantation increased neurogenesis in these regions (Figure 1). This indicates that one aspect of a physiological feature in WKY rats is ameliorated by encapsulated MSCs.
Various types of neurotrophic factors were secreted from encapsulated MSCs and related pathways are activated
Next, we examine what kinds of secreted factors are responsible for the antidepressant effect of MSCs. To address this, we focused on four neurotrophic factors, namely, VEGF, BDNF, fibroblast growth factor-2 (FGF-2), and ciliary neurotrophic factor (CNTF), and hypothesized that these secreted neurotrophic factors are responsible for the treatment effect of MSCs. All of them are secreted from MSCs and are related to depressive symptoms and behavior in humans and rodent research.8 As the capsules are made of polymeric hollow fibers that consist of semipermeable membranes, encapsulated MSCs were confirmed to secrete these four neurotrophic factors for at least 2 weeks. Implantation of encapsulated MSCs to the lateral ventricle activated the pathway related to these neurotrophic factors (Figure 1). These results support the idea that neurotropic factors are responsible for the antidepressant treatment effect.
Encapsulated MSCs upregulated the expression level of VEGF, CNTF, and their receptors
The intrinsic expression of VEGF, CNTF, and their receptors was upregulated by encapsulated MSCs. Although similar effects were reported in dental pulp stem cell implantation,15 the effects related to nurturing the microenvironment have not been well evaluated or discussed thus far. The intrinsic changes of the VEGF and CNTF pathway itself would result in an ameliorative effect on depression-like behavior and may play a key role in the robust treatment effect of encapsulated MSCs.
Features of encapsulated MSCs and future research prospects
The biggest feature of encapsulated MSCs is that they counteract the treatment-resistant behavioral deficits of WKY rats. This suggests that the antidepressant effect of encapsulated MSCs is stronger than that of conventional antidepressant treatment. Behavioral changes caused by encapsulated MSCs differ from those of conventional antidepressant treatments, however. As conventional antidepressant treatment does not affect the results of the OFT, our findings are difficult to interpret. Other types of behavioral tests may be helpful in the interpretation of the unique effect of MSCs.
The effect of encapsulated MSCs on neurogenesis is also unique. Encapsulated MSCs enhanced neurogenesis in the SVZ and DG. In general, conventional antidepressant treatment enhances neurogenesis only in the DG, although the SVZ is not always evaluated. This unique effect may be a key factor in this novel treatment effect.
Our results demonstrated that encapsulation enhanced the survival rate and enabled a continuous supply of a cocktail of exogenous neurotrophic factors. We believe that encapsulated MSC transplantation is thus a great candidate for the treatment of depression. To realize a clinical translation, research is needed to clarify the ingredients of MSCs in more detail. We focused on four trophic factors that were selected based on the results of previous studies and were not evaluated separately. Clarifying the role of each factor will be the theme of future research. The continuous injection of a specific factor may be more valuable than the implantation of encapsulated MSCs. Detailed adjustment of the dose may facilitate the treatment effect. The effect of other secretions, such as exosome, must also be evaluated.
The long-term effect of encapsulated MSCs remain unknown. We evaluated the effect of encapsulated MSCs only 2 weeks after implantation. Despite the fact that MSCs survived in the capsule 2 weeks after implantation, there are trends related to decreased survival in the capsule and the lower secretion of neurotrophic factors. Microenvironmental nurturing may, however, compensate for these changes. In general, depression requires long-term treatment. Any changes in treatment effect over time would be important to consider.
Conclusions
In Kin et al., we demonstrated that encapsulation enhanced the survival rate of implanted MSCs and succeeded in providing a continuous supply of a cocktail of exogenous neurotrophic factors. The results of the OFT and FST demonstrated that encapsulated MSCs attenuated behavioral deficits. These ameliorative effects were accompanied by activation of the relevant pathway associated with VEGF, BDNF, FGF-2, and CNTF. Encapsulated MSCs may be great candidates for new treatments for depression, and future research is needed to clarify the mechanism responsible for these treatment effects.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contributions: KK wrote this commentary. TY and ID edited the manuscript.
ORCID iD: Kyohei Kin  https://orcid.org/0000-0002-5445-5647
https://orcid.org/0000-0002-5445-5647
References
- 1. Yasuhara T, Kawauchi S, Kin K, et al. Cell therapy for central nervous system disorders: current obstacles to progress. CNS Neurosci Ther. 2020;26:595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tfilin M, Sudai E, Merenlender A, Gispan I, Yadid G, Turgeman G. Mesenchymal stem cells increase hippocampal neurogenesis and counteract depressive-like behavior. Mol Psychiatry. 2010;15:1164-1175. [DOI] [PubMed] [Google Scholar]
- 3. George S, Hamblin MR, Abrahamse H. Differentiation of mesenchymal stem cells to neuroglia: in the context of cell signalling. Stem Cell Rev Rep. 2019;15:814-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Badyra B, Sułkowski M, Milczarek O, Majka M. Mesenchymal stem cells as a multimodal treatment for nervous system diseases [published online ahead of print June 23, 2020]. Stem Cells Transl Med. doi: 10.1002/sctm.19-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Das M, Mayilsamy K, Mohapatra SS, Mohapatra S. Mesenchymal stem cell therapy for the treatment of traumatic brain injury: progress and prospects. Rev Neurosci. 2019;30:839-855. [DOI] [PubMed] [Google Scholar]
- 6. Siddiq I, Park E, Liu E, et al. Treatment of traumatic brain injury using zinc-finger protein gene therapy targeting VEGF-A. J Neurotrauma. 2012;29:2647-2659. [DOI] [PubMed] [Google Scholar]
- 7. Khalin I, Alyautdin R, Wong TW, Gnanou J, Kocherga G, Kreuter J. Brain-derived neurotrophic factor delivered to the brain using poly (lactide-co-glycolide) nanoparticles improves neurological and cognitive outcome in mice with traumatic brain injury. Drug Deliv. 2016;23:3520-3528. [DOI] [PubMed] [Google Scholar]
- 8. Kin K, Yasuhara T, Kameda M, et al. Cell encapsulation enhances antidepressant effect of the mesenchymal stem cells and counteracts depressive-like behavior of treatment-resistant depressed rats. Mol Psychiatry. 2020;25:1202-1214. [DOI] [PubMed] [Google Scholar]
- 9. Kyeremanteng C, MacKay JC, James JS, et al. Effects of electroconvulsive seizures on depression-related behavior, memory and neurochemical changes in Wistar and Wistar-Kyoto rats. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:170-178. [DOI] [PubMed] [Google Scholar]
- 10. Kin K, Yasuhara T, Kameda M, et al. Hippocampal neurogenesis of Wistar Kyoto rats is congenitally impaired and correlated with stress resistance. Behav Brain Res. 2017;329:148-156. [DOI] [PubMed] [Google Scholar]
- 11. Rittenhouse PA, López-Rubalcava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar–Kyoto rat. Psychoneuroendocrinology. 2002;27:303-318. [DOI] [PubMed] [Google Scholar]
- 12. Pardon MC, Gould GG, Garcia A, et al. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002;115:229-242. [DOI] [PubMed] [Google Scholar]
- 13. Tizabi Y, Hauser SR, Tyler KY, et al. Effects of nicotine on depressive-like behavior and hippocampal volume of female WKY rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104-9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang AH, Snyder BR, Cheng PH, Chan AW. Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells. 2008;26:2654-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]