Abstract
This review paper outlines the definition, pathophysiology, and potential maternal health consequences of cellular fetal microchimerism, the maternal acquisition of intact cells of fetal origin during pregnancy. Increased rates and amounts of cellular fetal microchimerism are associated with several placental syndromes, including preeclampsia and fetal growth restriction. The discovery of cellular fetal microchimerism and methods of detection are briefly outlined, and we present the mechanisms hypothesized to govern pregnancy-related and long-term maternal health effects of cellular fetal microchimerism. Specifically, we discuss the potential implications of cellular fetal microchimerism in wound healing, autoimmunity, cancer, and possibly cardiovascular disease. Cellular fetal microchimerism represents a novel area of research on maternal and transgenerational health and disease, providing exciting opportunities for developing new disease biomarkers and precision medicine with targeted prophylaxis against long-term maternal disease.
Keywords: Fetal microchimerism, placental dysfunction, autoimmune disease, cancer, cardiovascular disease
Cellular fetal microchimerism: One type of microchimerism in women
During pregnancy, fetal and maternal genetic material is exchanged across the placenta.1 This exchange generates cellular fetal microchimerism (cFMC), the maternal acquisition of intact cells of fetal origin.2 The simultaneous, but distinct, maternal acquisition of cell-free fetal DNA (cffDNA) is useful in noninvasive prenatal testing (NIPT).3 Unlike cffDNA, which is rapidly cleared from maternal circulation following delivery,4 cFMC may persist for many years.5 This is reflected in the name, as the term chimerism refers to the long-term presence of genetically foreign material in an individual.
Although cFMC occurs naturally in mammalian pregnancy,6 cellular microchimerism can also arise iatrogenically following blood transfusion, tissue-, cell-, and organ transplantation.2 In cellular microchimerism, the proportion of foreign cells is small (<1 in 10,000 cells).7 cFMC denotes cells of fetal origin transferred to the mother and cellular maternal microchimerism denotes the transfer of maternally originating cells to the fetus. Because of this bidirectional transfer of cells, a woman may host cells of fetal origin from her pregnancies and cells of maternal origin from her own mother (Figure 1).2 Furthermore, the same woman might host other cells transferred from her mother during fetal life, including cells possibly stemming from older siblings, a vanishing twin, or prior maternal termination of pregnancy,2,8,9 and even cells of neither fetal nor maternal origin stemming from maternal blood transfusion.9 Not all papers, however, demonstrate increased cFMC of older sibling origin, and further research is needed to uncover all potential sources of microchimerism in women.10
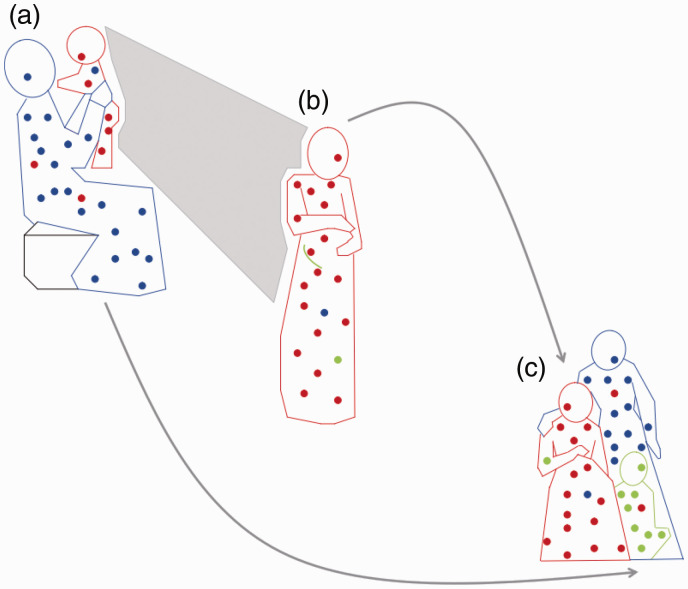
Figure 1.
Microchimerism (Mc) in three generations. (a) Proband as infant (red) exchanges Mc with her mother (blue), resulting in maternal Mc in the infant and fetal Mc in the mother. (b) As an adult, proband (red) still harboring maternal Mc, experiences pregnancy herself (green) and acquires new source of fetal Mc. (c) Later, proband (red), child (green), and proband’s mother (blue), all with persistent Mc from maternal and/or fetal sources.
Note: This figure is available in color in the online version of the journal article.Source: Reproduced with the permission of UPV/EHU Press from Gammill and Nelson (2010).2
The effects of microchimeric cells in a woman’s body likely vary depending on immunological and genetic factors and at what point in her life she obtains these cells, whether during fetal life, childhood, or in adulthood. In transplant medicine, microchimerism originating from the transplant has been shown to correlate with both recipient tolerance and rejection of the graft, the underlying type of microchimerism proposed as a determining factor.11 In the same way, it seems logical that different types of cFMC originating from the fetus during pregnancy would affect how well the woman “tolerates” the pregnancy. In parous women, excessive cFMC appears to mediate both positive and negative effects; it is associated with preeclampsia, wound healing, autoimmune disease, various forms of cancer, and possibly cardiovascular disease (CVD) (Table 1). cFMC represents an exciting new area of research on predictors and possibly mediators of long-term female health and disease.
Table 1.
Maternal acquisition of fetal genetic material and associated clinical outcomes. The table indicates which of the maternal (or fetal) outcomes associated with excessive cellular fetal microchimerism (cFMC) and/or cell-free fetal DNA (cffDNA)a are beneficial versus detrimental.
| Beneficial maternal outcomes | Comments |
| Wound healing | Excessive cFMC postpartum is associated with improved wound healing, suggesting a beneficial association.12,13 |
| Detrimental maternal (or fetal) outcomes | Comments |
| Preeclampsia | Excessive cFMC14,15 and cffDNA16a during pregnancy is associated with preeclampsia, suggesting a detrimental association |
| HELLP (hemolysis, elevated liver enzymes, low platelets) | The correlation between excessive cffDNAa and preeclampsia during pregnancy is strengthened in preeclampsia complicated by HELLP16 |
| Fetal growth restriction (FGR) | Excessive cFMC during pregnancy is associated with FGR,17 suggesting a detrimental association |
| Spontaneous preterm labor | Excessive cffDNAa during pregnancy is associated with spontaneous preterm labor,18 suggesting a detrimental association |
| Hashimoto’s thyroiditis, systemic lupus erythematosus, Sjögren’s syndrome | Excessive cFMC in maternal tissue and/or circulation long term is associated with these conditions and appears detrimental (though some studies show conflicting results).19 Pregnancy is associated with flares or reactivation of disease20,21 |
| Systemic sclerosis and primary biliary cirrhosis | Excessive cFMC in maternal tissue and/or circulation long term is associated with these conditions and appears to be detrimental.19 Pregnancy is associated with worsening of certain features or symptoms of disease, but disease remains otherwise stable21,22 |
| Detrimental maternal outcomes long term, beneficial in the short term | Comments |
| Rheumatoid arthritis (RA) | Excessive cFMC in maternal blood and affected organs long term is associated with RA exacerbation.19 During pregnancy on the other hand, high levels of cffDNAa in maternal circulation correlates with low levels of RA activity,23 suggesting a temporary pregnancy-related beneficial effect23,24 |
| Grave’s disease and multiple sclerosis (MS) | cFMC in maternal tissue/and or circulation long term is associated with Grave’s disease and MS,19 suggesting a detrimental association in the long term. Like with RA, these conditions often ameliorate in the short term during pregnancy24 |
| Beneficial maternal outcomes long term, beneficial or detrimental in the short term | Comments |
| Cancer (melanoma, breast cancer, cervical cancer, uterine cancer, ovarian cancer, lung cancer, colon cancer, thyroid cancer) | Excessive cFMC appears to be beneficial in the long term with respect to cancer overall,19,25 but in the short term the correlation appears positive or negative depending on the type and timing of the malignancy6 |
| Unclear whether beneficial or detrimental to maternal outcomes | Comments |
| Cardiovascular disease (CVD) | Excessive cFMC and CVD mortality long term have been linked by a trend.25 However, several studies suggest that enhanced cFMC is associated with tissue repair following cardiovascular damage26–28 |
Distinction between cellular fetal microchimerism (cFMC) and cell-free fetal DNA (cffDNA) is important as the two may invoke different immunological and other responses. cffDNA is considered a marker of apoptotic placental debris shed into the maternal circulation during pregnancy and is rapidly cleared following delivery.24 cFMC denotes intact cells of fetal origin with stem cell-like properties that may persist in the woman’s body for decades, potentially replenishing stem cell niches and continuing to interact with the maternal immune system long term.5,29 cffDNA is more prevalent in maternal circulation during pregnancy than cFMC and is, on average, present in greater amounts1 (see “The physiology of cFMC” section for further details).
The history of cFMC detection
In 1893, George Schmorl published groundbreaking findings demonstrating cells, apparently of placental origin, in the lungs of 17 women who had died of eclampsia.30,31 He speculated that feto-maternal cell transfer occurred in normal pregnancies, though in greater amounts in eclampsia, and was the first to propose a link between eclampsia and placental factors.30 Further supporting his theory, investigators during the 1960s and 1970s detected leukocytes, of presumed fetal origin, in maternal circulation. They used blood samples from women (XX karyotype), who each carried a male fetus (XY karyotype) and isolated lymphocytes of male origin by staining for the Y-chromosome.32–34 The discovery of cFMC represented a paradigm shift: previously, one believed the placenta provided an impenetrable barrier between mother and fetus preventing maternal exposure to fetal antigens. The existence of cFMC disproved that.
A new field of pregnancy-related research opened up with the discovery of cFMC. Today, the most commonly utilized methods of detection are fluorescence in situ hybridization (FISH) and polymerase chain reaction (PCR). With both methods, the Y-chromosome is still often employed as a target for identifying microchimeric cells (Figure 2).2 FISH visualizes the cells in the context of surrounding maternal tissue, but is costly and time consuming. Real-time PCR is simpler and more time efficient.11 With either method, the researcher may compare not only the prevalence of cFMC in different groups but also whether the relative quantities of cFMC differ in each subject (Figure 3). Both parameters may differ significantly between women with disease versus healthy controls.
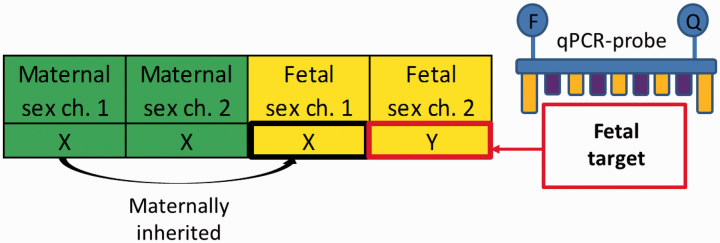
Figure 2.
Fetal Y-chromosome used as microchimerism target. The pregnant woman has two X-chromosomes, whereas the male fetus has one maternally inherited X-chromosome and one paternally inherited Y-chromosome. This allows for microchimerism detection by FISH or by qPCR probes targeting the Y-chromosome (as shown here).
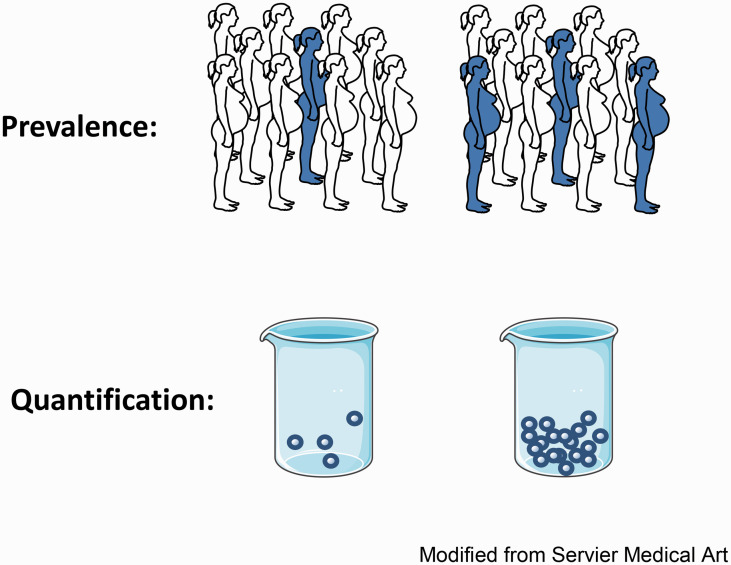
Figure 3.
Determining prevalence and quantity of fetal microchimerism. Using FISH or quantitative PCR, the prevalence and quantity of cFMC may be determined. The prevalence is obtained by identifying the number of women in each group that have detectable cFMC (individuals shown as shaded) relative to the number of women in each group in which cFMC is not detectable (individuals shown as unshaded) and comparing the two groups. The quantity of cFMC is determined by counting the number of fetal microchimeric cells present in a blood or tissue sample from each woman; the beakers represent blood samples from two different women containing different quantities of fetal microchimeric cells.
Although the Y-chromosome is a useful target, it has its limitations.11 Microchimerism arising from a pregnancy with a female fetus is obviously not detectable by this method, since the pregnant woman and fetus would both have the XX karyotype. To distinguish female cFMC from maternal cells, more recently developed PCR probes target HLA-polymorphisms35 and various single nucleotide polymorphisms. These probes are used to target paternally inherited fetal DNA sequences that the mother does not possess (Figure 4), thereby detecting microchimerism from male and female fetuses. Microchimerism derived from different fetuses or other sources of microchimeric cells, such as older siblings or transfusion- or organ donors, are also distinguishable. However, the genotypes of the mother and her child, and ideally, those of other potential microchimerism sources must be obtained, a challenging and often impossible task.
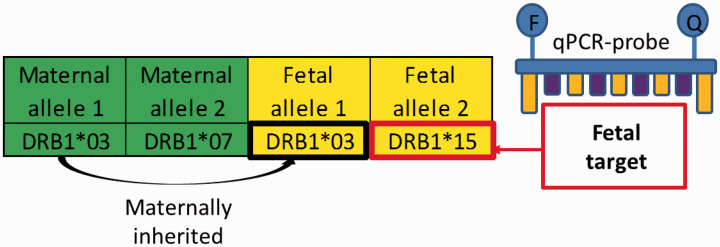
Figure 4.
Fetal HLA-polymorphisms used as microchimerism target. The highly polymorphic HLA Class II genes may be used to detect fetal microchimerism. The pregnant woman has two copies of the DRB1 gene, shown as allele 1 and allele 2. The fetus inherits one of these alleles from its biological mother, and one of the alleles from its biological father. The paternally inherited fetal allele may, as in this example, be different from the pregnant woman’s other allele, thereby providing a target for microchimerism detection.
Although much knowledge has been gleaned from microchimerism studies so far, current methods are limited in their sensitivity and specificity, and provide little information about the gene expression and cell lineage of microchimeric cells.11 Novel methodologies are expected to provide more information: (1) droplet digital PCR, an advanced quantitative PCR method, improves reproducibility,36 (2) cell sorting methodologies enable the study of specific cFMC cell lineages, and (3) studies of RNA transcription patterns will provide information about protein expression in cFMC.11 Information about the function and nature of cFMC will certainly shed more light on its role in the maternal body.
The physiology of cFMC
Studies performed to date have shown that maternal acquisition of fetal genetic material occurs commonly in healthy human and other mammalian pregnancies.6 It therefore appears to be a physiological phenomenon. The fetal genetic material acquired by the pregnant woman is comprised of intact cells (of placental and/or other fetal cell origin) as well as genetic material shed by the growing placenta, including cffDNA.2 Maternal acquisition of fetal genetic material appears to begin early on in pregnancy, as fetal genetic sequences have been detected in maternal blood at as early as 4–5 weeks of gestation.37 At this early stage of pregnancy, the uteroplacental circulation has not yet been established and any cFMC that potentially arises is therefore likely placental in origin.37 Accordingly, microchimeric cells of a placental phentoype have been detected in maternal blood.38–40 Later in pregnancy, once the circulation at the materno-placental interface has developed, cFMC may also encompass cells originating in the fetus transferred directly from the fetal to the maternal circulation.37 The fetus and the placenta stem from the same zygote and therefore generally have the same genetic makeup, hence why the term “cellular fetal microchimerism (cFMC)” encompasses cells originating in both the fetus and the placenta. In the maternal body, cFMC appears to have pluripotent stem cell-like properties allowing differentiation into various cell types: cFMC has been detected amongst various phenotypic subsets of hematologic cells in the maternal circulation39,40 as well as in various organs, including the heart, lungs, brain, breast, thyroid, liver, gallbladder, kidney, spleen, lymph nodes, cervix, endometrium, colon, and skin.2,6,29
As pregnancy progresses, the concentration of cFMC in maternal circulation increases with increasing gestational age.39,41 Quantitative studies in a population of women with healthy pregnancies at term show that fetal cells make up 0.6–7.6 cells per 10,000 nucleated maternal blood cells, while cffDNA accounts for about 1.4–5.4% of total DNA in maternal plasma.1 The prevalence of circulating cFMC is reported to range from 6 to 50% in blood,14,42 whereas cffDNA is detectable in 100% of all pregnancies.1 The discrepancy in amount and prevalence between cFMC and cffDNA may suggest that transfer of intact fetal cells is either less common than shedding of non-cellular fetal genetic material or it could reflect the sequestration of cFMC in maternal tissue. The size and makeup of the populations studied, differences in sampling techniques, and the sensitivity and specificity of the assays used for detection may explain the wide range in the cFMC prevalence reported.
After parturition, cFMC remains in the maternal circulation,5 whereas cffDNA is cleared from maternal circulation with 100% clearance by one day postpartum.4 To distinguish between the two when testing blood from a woman with an ongoing pregnancy, the sample is centrifuged and different compartments of the blood are tested; cffDNA remains suspended in plasma, whereas cFMC aggregates together with maternal white blood cells and platelets. As a result, NIPT based on cffDNA is specific for the current pregnancy; prior pregnancies will not likely affect the results.4 cFMC, on the other hand, can persist in maternal blood, tissues, and organs for decades,5,29 potentially for life. As a result, one woman might hypothetically carry fetal cells from all of her prior pregnancies. Surprisingly, a study by Gammill et al. demonstrated that parity does not appear to affect prevalence or quantity of circulating cFMC.43 One potential explanation proposed by the authors is that cFMC derived from different offspring may compete for predominance in the woman’s circulation.43 They suggest that this mechanism may resemble the graft-graft competition observed in bone marrow transplant recipients who receive umbilical cord blood stem cells from two different donors, a procedure known as double unit umbilical cord blood transplantation.44 Gammill et al. suggest that in the context of cFMC in multiparous women, fetal cells from each pregnancy may act like individual grafts and that one fetal graft may reject other fetal grafts, thereby eradicating fetal cells from all other pregnancies.43 However, it has also been suggested that fetal microchimeric cells may migrate to maternal bone marrow, there awaiting signals of tissue damage,45 a phenomenon that could potentially “make room for” the fetal cells of younger siblings entering maternal circulation during subsequent pregnancies.
But why do fetal cells get transferred during pregnancy? Evolutionarily speaking, cFMC should, in theory confer benefit, especially to the child. Boddy et al. hypothesize that cFMC may affect lactation, maternal thermoregulation, and mother–child bonding.6 Firstly, they cite a study showing that fetal microchimeric fibroblasts from mice differentiate into mammary epithelial cells when exposed to lactation hormones in vitro46 and another study showing that 56% of healthy parous women carry cFMC in their mammary tissue.47 Secondly, they present evidence that cFMC persists after pregnancy in maternal thyroid tissue48 and speculate that fetal cells may upregulate maternal body temperature in women with newborn infants.6 Finally, based on evidence that fetal cells in maternal mouse brains can differentiate into mature neurons49 and that cFMC may be found in the female human brain,50 they propose an evolutionary role for cFMC in strengthening the emotional tie between mother and child.6
The impact of cFMC in inducing immunotolerance during pregnancy and temporary secondary effects on autoimmune disease
In addition to modulating maternal body function postpartum, maternal acquisition of fetal genetic material has been proposed to play a role in inducing materno-fetal tolerance during pregnancy, a factor potentially contributing to why certain autoimmune diseases, like rheumatoid arthritis (RA), are temporarily ameliorated during pregnancy.24 In 1993, a study by Nelson et al. looked into fetal–maternal disparity in HLA class II molecules,51 a concept illustrated in Figure 4. They found that the more disparate the non-shared fetal and maternal HLA-II alleles were, the more likely arthritis was to improve during pregnancy. When the non-shared alleles were similar, arthritis remained active or even worsened.51 Later on, a significant inverse correlation was found between levels of cffDNA in maternal serum and activity of RA in pregnancy23 (Table 1). Adams et al. propose that the amelioration of the disease may be due to disparate fetal HLA-antigens inducing maternal tolerance of the pregnancy that extends to maternal tolerance of the antigens central to RA.24 A similar mechanism may apply in multiple sclerosis (MS) and Graves’ thyroiditis, which also appear to ameliorate during pregnancy.24 Adams et al. propose a link between this temporary state of tolerance and the temporary presence of cell-free fetal genetic material in the maternal circulation observed in pregnancy.24
Conversely, systemic sclerosis, an autoimmune disease in which some features tend to worsen during pregnancy,21 is more common in women who have given birth to children with greater similarity in the non-shared allele of the HLA-II, DRB1 gene.52 These findings suggest that the degree of materno-fetal HLA-(dis)similarity may be linked to different maternal autoimmune diseases by different pathophysiological mechanisms during pregnancy versus long term. Accordingly, the mechanisms proposed to govern short-term immunotolerance during pregnancy24 differ from the mechanisms proposed to link cFMC to autoimmunity in general.53 In this setting, the distinction between cffDNA and cFMC is important. cffDNA is rapidly cleared from maternal circulation following pregnancy.4 In contrast, cFMC persists and has the potential to engraft and continue to interact with the maternal immune system long term.5 Further studies on fetal–maternal HLA-disparity, maternal acquisition of cFMC and other cell-free fetal genetic material, are required in order to understand the molecular mechanisms relating these phenomena to maternal autoimmune disease and materno-fetal tolerance during pregnancy.
Placental dysfunction and acquisition of cFMC in pregnancy
There are indications that acquisition of fetal genetic material not only affects maternal health during pregnancy but is also affected by the health of the pregnancy, specifically by placental dysfunction (Table 1). cFMC is detected more frequently and in greater quantities in preeclampsia than in gestational-age matched uncomplicated pregnancies.14,15 Preeclampsia is a syndrome in which placental cellular stress plays an important role.54,55 Relative to uncomplicated pregnancies, cFMC is also more common in fetal growth restriction (FGR) in pregnancies with impaired uteroplacental perfusion,17 a condition related to preeclampsia. Similarly, cffDNA in maternal circulation appears to correlate with placental dysfunction. It is associated with preeclampsia and to an even greater degree with HELLP (hemolysis, elevated liver enzymes, and low platelets), a more severe form of the disease.16 cffDNA is also increased in pregnancies complicated by spontaneous preterm labor.18
Two distinct processes have been suggested that could explain why an increase in circulating cFMC and other fetal genetic material is observed in pregnancies with placental dysfunction: one is increased cell transfer, the other is diminished maternal clearance.2 A third option is diminished cell migration into maternal tissues. These processes may occur independently or together. Further research is required to determine what mechanisms underlie such processes. In addition, the potential role of cFMC in mediating the development of the excessive systemic maternal vascular inflammation that occurs in preeclampsia55 has yet to be explored. Further investigation of these mechanisms may also shed light on the links between autoimmune disease and pregnancy outcomes.
cFMC and long-term maternal health and disease
In non-pregnant, parous women, cFMC has been associated with both long-term maternal disease benefits and risks19,25 (Table 1). Potential benefits include renewal of stem cell niches and enhanced maternal tissue repair.19 With respect to the maternal immune system, cFMC may have beneficial or adverse effects depending on the setting, potentially acting either as triggers or as effector cells in a maternal inflammatory reaction.53
In wound healing, cFMC has been suggested to be restorative. Cells of fetal origin have been found more frequently in inflamed maternal wounds than in healthy tissues, where they appear to participate in maternal angiogenesis and inflammation.13 cFMC identified by Mahmood and O’Donoghue in Caesarean section scars express cytokeratin, collagen I and III, and TGF-beta3, a sign that these cells participate in wound healing.12 Progenitor cells of fetal origin seem to be recruited to the site of injury in response to maternal signals.12,13
In autoimmune disease, the long-term effects of cFMC appear to be detrimental. Non-pregnant parous women with Hashimoto’s thyroiditis, Grave’s disease, systemic sclerosis, systemic lupus erythematosus, Sjögren’s syndrome, RA, MS, and primary biliary cirrhosis have enhanced levels of cFMC in circulation and/or in diseased tissues, suggesting that cFMC may worsen or initiate such conditions.19 However, results are conflicting, with some studies showing no difference in prevalence of cFMC amongst subjects with autoimmune disease versus healthy controls.56–59 One mechanism proposed to induce autoimmunity is that fetally derived HLA peptides, foreign to the mother, may trigger a maternal alloimmune response similar to that observed in chronic organ transplant rejection.53 Alternatively, fetal T-cells might react to allogeneic non-shared maternal antigens in a mechanism resembling graft-versus-host disease.60 Theoretically, such alloimmune reactions would be clinically indistinguishable from autoimmunity. The suggestion that cFMC contributes to autoimmune disease is consistent with the observation that autoimmunity is more prevalent in women than in men. However, the fact that men and children can also develop autoimmune disease, though less frequently than women, indicates that cFMC is not the sole contributor to this type of disease process.
In cancer, fetal cells are detectable at multiple tumor sites including in melanoma, and breast-, cervical-, uterine-, ovarian-, lung-, colon-, and thyroid cancer.19 The role of cFMC in such cancers is likely complex. For example, in certain types of breast cancer, cFMC has been suggested to have a protective effect,61 possibly by way of allosurveillance and tissue repair.19 This fits with the observation that young parous women have a lower lifetime risk of breast cancer than nulliparous women or women with a first pregnancy after the age of 35 years.62 However, epidemiological studies also show a temporarily increased risk of breast cancer directly following childbirth,63 indicating that immunogenetic interactions involved in breast cancer development may change over time. Further complicating the matter, breast cancer represents a heterogeneous set of diseases, each of which may relate to cFMC differently. That being said, in the long term, and with respect to cancer overall, cFMC appears to be protective.19 Kamper-Jorgensen et al. recently found an association between circulating cFMC and a reduced hazard ratio of female cancer death.25 They also found a trend linking circulating cFMC to a reduced hazard ratio of female all-cause mortality, though this association was not statistically significant.
Although Kamper-Jorgensen et al.’s results indicate that cFMC might improve maternal health overall, they also found a trend towards increased CVD mortality among women harboring cFMC.25 They conclude that the role of cFMC may vary by cause of death, and that in the setting of CVD, cFMC may be detrimental. Interestingly, women with pregnancies complicated by preeclampsia have a twofold risk of experiencing major cardiovascular events (e.g. stroke and myocardial infarction) later in life, and a fourfold risk of hypertension and heart failure relative to women with uncomplicated pregnancies.64–66 The association follows a “dose–response” relationship, whereby in preterm preeclampsia with FGR, the risk of maternal CVD increases 7–8 fold.66 This, combined with the evidence that enhanced cFMC is associated with preeclampsia and FGR, lends support to the hypothesis that cFMC may be involved in the pathophysiological pathways linking these placental syndromes to maternal CVD.
Mouse studies, on the other hand, suggest a beneficial role for cFMC in CVD. One study showed that fetal cells homed to injured tissue after induced cerebral ischemic stroke and that multiparous female mice had better outcomes than nulliparous females and displayed signs of immunosuppression in the brain.28 Another mouse study found that fetal cells home to injured maternal hearts and differentiate into endothelial cells, smooth muscle cells, and cardiomyocytes, thereby potentially contributing to tissue repair.27 This is consistent with a study in humans showing that differentiated fetal cells were found in the hearts of two women with cardiomyopathy.26 However, further study of cFMC, maternal–fetal histocompatibility, and CVD is required to determine whether cFMC plays a restorative or detrimental role in the pathophysiology of human female CVD.
Future research
The presence of cFMC is associated with a number of medical conditions in women, but the mechanisms governing the genesis of cFMC and the short- and long-term positive or negative implications for maternal health are still unclear. The effects of cFMC may vary over time in the same woman, depending on immunogenetic relationships across generations and number of pregnancies. Further research is needed to investigate the origins and functional capacity of cFMC, factors impacting the amount of cell transfer during pregnancy, as well as to investigate the consequences of cFMC in maternal circulation and tissues. Novel insights in this field may provide tools for identifying women in need of preventive and therapeutic interventions for a range of diseases, as well as establishing new targets for or modes of treatment in these women. Long-term research in this field has the potential to improve women’s health outcomes.
Acknowledgements
None.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: HESF is an employee of Oslo University Hospital and receives a PhD salary for the FETCH study (fetal microchimerism and maternal cardiovascular health) from the Regional Health Authority of South-Eastern Norway (Reference number: 2017007).
Ethical approval
Not applicable.
Informed consent
Not applicable.
Guarantor
ACS.
Contributorship
HESF researched literature and wrote the first draft of the invited review article, revised it and submitted the final version. GMJ and ACS provided guidance in the review process and concepts, and contributed text to the first draft of the article. All authors reviewed and edited the manuscript and approved the final version of the article.
ORCID iD
Heidi ES Fjeldstad https://orcid.org/0000-0002-8538-3922
References
- 1.Lo YM, Lau TK, Chan LY, et al. Quantitative analysis of the bidirectional fetomaternal transfer of nucleated cells and plasma DNA. Clin Chem 2000; 46: 1301–1309. [PubMed] [Google Scholar]
- 2.Gammill HS, Nelson JL. Naturally acquired microchimerism. Int J Dev Biol 2010; 54: 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo YM, Tein MS, Lau TK, et al. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. Am J Hum Genet 1998; 62: 768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo YM, Zhang J, Leung TN, et al. Rapid clearance of fetal DNA from maternal plasma. Am J Hum Genet 1999; 64: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi DW, Zickwolf GK, Weil GJ, et al. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A 1996; 93: 705–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boddy AM, Fortunato A, Wilson Sayres M, et al. Fetal microchimerism and maternal health: a review and evolutionary analysis of cooperation and conflict beyond the womb. Bioessays 2015; 37: 1106–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monaco AP. Chimerism in organ transplantation: conflicting experiments and clinical observations. Transplantation 2003; 75: 13s–16s. [DOI] [PubMed] [Google Scholar]
- 8.Guettier C, Sebagh M, Buard J, et al. Male cell microchimerism in normal and diseased female livers from fetal life to adulthood. Hepatology 2005; 42: 35–43. [DOI] [PubMed] [Google Scholar]
- 9.Muller AC, Jakobsen MA, Barington T, et al. Microchimerism of male origin in a cohort of Danish girls. Chimerism 2015; 6: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamper-Jorgensen M, Mortensen LH, Andersen AM, et al. Predictors of male microchimerism. Chimerism. 2012; 3: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eikmans M, van Halteren AG, van Besien K, et al. Naturally acquired microchimerism: implications for transplantation outcome and novel methodologies for detection. Chimerism 2014; 5: 24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmood U, O’Donoghue K. Microchimeric fetal cells play a role in maternal wound healing after pregnancy. Chimerism 2014; 5: 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nassar D, Droitcourt C, Mathieu-d’Argent E, et al. Fetal progenitor cells naturally transferred through pregnancy participate in inflammation and angiogenesis during wound healing. Faseb J 2012; 26: 149–157. [DOI] [PubMed] [Google Scholar]
- 14.Gammill HS, Aydelotte TM, Guthrie KA, et al. Cellular fetal microchimerism in preeclampsia. Hypertension 2013; 62: 1062–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzgreve W, Ghezzi F, Di Naro E, et al. Disturbed feto-maternal cell traffic in preeclampsia. Obstet Gynecol 1998; 91: 669–672. [DOI] [PubMed] [Google Scholar]
- 16.Swinkels DW, de Kok JB, Hendriks JC, et al. Hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome as a complication of preeclampsia in pregnant women increases the amount of cell-free fetal and maternal DNA in maternal plasma and serum. Clin Chem 2002; 48: 650–653. [PubMed] [Google Scholar]
- 17.Al-Mufti R, Lees C, Albaiges G, et al. Fetal cells in maternal blood of pregnancies with severe fetal growth restriction. Hum Reprod 2000; 15: 218–221. [DOI] [PubMed] [Google Scholar]
- 18.Leung TN, Zhang J, Lau TK, et al. Maternal plasma fetal DNA as a marker for preterm labour. Lancet 1998; 352: 1904–1905. [DOI] [PubMed] [Google Scholar]
- 19.Fugazzola L, Cirello V, Beck-Peccoz P. Microchimerism and endocrine disorders. J Clin Endocrinol Metab 2012; 97: 1452–1461. [DOI] [PubMed] [Google Scholar]
- 20.Galofre JC, Haber RS, Mitchell AA, et al. Increased postpartum thyroxine replacement in Hashimoto’s thyroiditis. Thyroid 2010; 20: 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy RA, Mendoza Pinto C, Domingues V, et al. Systemic autoimmune diseases and pregnancy In: JM Anaya, Y Shoenfeld, A Rojas-Villarraga, et al. (eds) Autoimmunity: from bench to bedside. Bogota, Colombia: El Rosario University Press, 2013, pp. 455–474. [PubMed] [Google Scholar]
- 22.Trivedi PJ, Kumagi T, Al-Harthy N, et al. Good maternal and fetal outcomes for pregnant women with primary biliary cirrhosis. Clin Gastroenterol Hepatol 2014; 12: 1179–1185.e1. [DOI] [PubMed] [Google Scholar]
- 23.Yan Z, Lambert NC, Ostensen M, et al. Prospective study of fetal DNA in serum and disease activity during pregnancy in women with inflammatory arthritis. Arthritis Rheum 2006; 54: 2069–2073. [DOI] [PubMed] [Google Scholar]
- 24.Adams KM, Yan Z, Stevens AM, et al. The changing maternal “self” hypothesis: a mechanism for maternal tolerance of the fetus. Placenta 2007; 28: 378–382. [DOI] [PubMed] [Google Scholar]
- 25.Kamper-Jorgensen M, Hjalgrim H, Andersen AM, et al. Male microchimerism and survival among women. Int J Epidemiol 2014; 43: 168–173. [DOI] [PubMed] [Google Scholar]
- 26.Bayes-Genis A, Bellosillo B, de la Calle O, et al. Identification of male cardiomyocytes of extracardiac origin in the hearts of women with male progeny: male fetal cell microchimerism of the heart. J Heart Lung Transplant 2005; 24: 2179–2183. [DOI] [PubMed] [Google Scholar]
- 27.Kara RJ, Bolli P, Karakikes I, et al. Fetal cells traffic to injured maternal myocardium and undergo cardiac differentiation. Circ Res 2012; 110: 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritzel RM, Patel AR, Spychala M, et al. Multiparity improves outcomes after cerebral ischemia in female mice despite features of increased metabovascular risk. Proc Natl Acad Sci Usa U S A 2017; 114: e5673–e5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khosrotehrani K, Johnson KL, Cha DH, et al. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA 2004; 292: 75–80. [DOI] [PubMed] [Google Scholar]
- 30.Lapaire O, Holzgreve W, Oosterwijk JC, et al. Georg Schmorl on trophoblasts in the maternal circulation. Placenta 2007; 28: 1–5. [DOI] [PubMed] [Google Scholar]
- 31.Schmorl CG. Pathologisch-anatomische Untersuchungen uber Puerperal-Eklampsie. Leipzig, Germany: Verlag FCW Vogel, 1893. [Google Scholar]
- 32.Walknowska J, Conte FA, Grumbach MM. Practical and theoretical implications of fetal-maternal lymphocyte transfer. Lancet 1969; 1: 1119–1122. [DOI] [PubMed] [Google Scholar]
- 33.Schroder J, De la Chapelle A. Fetal lymphocytes in the maternal blood. Blood 1972; 39: 153–162. [PubMed] [Google Scholar]
- 34.Schroder J, Tiilikainen A, De la Chapelle A. Fetal leukocytes in the maternal circulation after delivery. I. Cytological aspects. Transplantation 1974; 17: 346–354. [PubMed] [Google Scholar]
- 35.Maloney S, Smith A, Furst DE, et al. Microchimerism of maternal origin persists into adult life. J Clin Invest 1999; 104: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George D, Czech J, John B, et al. Detection and quantification of chimerism by droplet digital PCR. Chimerism 2013; 4: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas MR, Williamson R, Craft I, et al. Y chromosome sequence DNA amplified from peripheral blood of women in early pregnancy. Lancet 1994; 343: 413–414. [DOI] [PubMed] [Google Scholar]
- 38.Mueller UW, Hawes CS, Wright AE, et al. Isolation of fetal trophoblast cells from peripheral blood of pregnant women. Lancet 1990; 336: 197–200. [DOI] [PubMed] [Google Scholar]
- 39.Adams Waldorf KM, Gammill HS, Lucas J, et al. Dynamic changes in fetal microchimerism in maternal peripheral blood mononuclear cells, CD4+ and CD8+ cells in normal pregnancy. Placenta 2010; 31: 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bianchi DW, Flint AF, Pizzimenti MF, et al. Isolation of fetal DNA from nucleated erythrocytes in maternal blood. Proc Natl Acad Sci U S A 1990; 87: 3279–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujiki Y, Johnson KL, Tighiouart H, et al. Fetomaternal trafficking in the mouse increases as delivery approaches and is highest in the maternal lung. Biol Reprod 2008; 79: 841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo YM, Lo ES, Watson N, et al. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood 1996; 88: 4390–4395. [PubMed] [Google Scholar]
- 43.Gammill HS, Guthrie KA, Aydelotte TM, et al. Effect of parity on fetal and maternal microchimerism: interaction of grafts within a host? Blood 2010; 116: 2706–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gutman JA, Riddell SR, McGoldrick S, et al. Double unit cord blood transplantation: who wins-and why do we care? Chimerism 2010; 1: 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bayes-Genis A, Roura S, Prat-Vidal C, et al. Chimerism and microchimerism of the human heart: evidence for cardiac regeneration. Nat Rev Cardiol 2007; 4: S40–S45. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Zhang J, Geng Y, et al. Plasticity of the response of fetal mouse fibroblast to lactation hormones. Cell Biol Int 2003; 27: 755–760. [DOI] [PubMed] [Google Scholar]
- 47.Dhimolea E, Denes V, Lakk M, et al. High male chimerism in the female breast shows quantitative links with cancer. Int J Cancer 2013; 133: 835–842. [DOI] [PubMed] [Google Scholar]
- 48.Cirello V, Recalcati MP, Muzza M, et al. Fetal cell microchimerism in papillary thyroid cancer: a possible role in tumor damage and tissue repair. Cancer Res 2008; 68: 8482–8488. [DOI] [PubMed] [Google Scholar]
- 49.Zeng XX, Tan KH, Yeo A, et al. Pregnancy-associated progenitor cells differentiate and mature into neurons in the maternal brain. Stem Cells Dev 2010; 19: 1819–1830. [DOI] [PubMed] [Google Scholar]
- 50.Chan WF, Gurnot C, Montine TJ, et al. Male microchimerism in the human female brain. PLOS One 2012; 7: e45592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson JL, Hughes KA, Smith AG, et al. Maternal-fetal disparity in HLA class II alloantigens and the pregnancy-induced amelioration of rheumatoid arthritis. N Engl J Med 1993; 329: 466–471. [DOI] [PubMed] [Google Scholar]
- 52.Nelson JL, Furst DE, Maloney S, et al. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet 1998; 351: 559–562. [DOI] [PubMed] [Google Scholar]
- 53.Adams Waldorf KM, Nelson JL. Autoimmune disease during pregnancy and the microchimerism legacy of pregnancy. Immunol Invest 2008; 37: 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Redman CW, Sargent IL, Staff AC. IFPA Senior Award Lecture: making sense of pre-eclampsia – two placental causes of preeclampsia? Placenta 2014; 35: S20–S25. [DOI] [PubMed] [Google Scholar]
- 55.Redman CW, Staff AC. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol 2015; 213: S9.e1, S9–S11. [DOI] [PubMed] [Google Scholar]
- 56.Mosca M, Curcio M, Lapi S, et al. Correlations of Y chromosome microchimerism with disease activity in patients with SLE: analysis of preliminary data. Ann Rheum Dis 2003; 62: 651–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murata H, Nakauchi H, Sumida T. Microchimerism in Japanese women patients with systemic sclerosis. Lancet 1999; 354: 220. [DOI] [PubMed] [Google Scholar]
- 58.Selva-O’Callaghan A, Mijares-Boeckh-Behrens T, Prades EB, et al. Lack of evidence of foetal microchimerism in female Spanish patients with systemic sclerosis. Lupus 2003; 12: 15–20. [DOI] [PubMed] [Google Scholar]
- 59.Yan Z, Lambert NC, Guthrie KA, et al. Male microchimerism in women without sons: quantitative assessment and correlation with pregnancy history. Am J Med 2005; 118: 899–906. [DOI] [PubMed] [Google Scholar]
- 60.Scaletti C, Vultaggio A, Bonifacio S, et al. Th2-oriented profile of male offspring T cells present in women with systemic sclerosis and reactive with maternal major histocompatibility complex antigens. Arthritis Rheum 2002; 46: 445–450. [DOI] [PubMed] [Google Scholar]
- 61.Gadi VK. Fetal microchimerism in breast from women with and without breast cancer. Breast Cancer Res Treat 2010; 121: 241–244. [DOI] [PubMed] [Google Scholar]
- 62.Subramani R, Lakshmanaswamy R. Pregnancy and breast cancer. Prog Mol Biol Transl Sci 2017; 151: 81–111. [DOI] [PubMed] [Google Scholar]
- 63.Nichols HB, Schoemaker MJ, Cai J, et al. Breast cancer risk after recent childbirth: a pooled analysis of 15 prospective studies. Ann Intern Med 2019; 170: 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Staff AC, Redman CW, Williams D, et al. Pregnancy and long-term maternal cardiovascular health: progress through harmonization of research cohorts and biobanks. Hypertension 2016; 67: 251–260. [DOI] [PubMed] [Google Scholar]
- 65.Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2017; 10. DOI: 10.1161/CIRCOUTCOMES.116.003497 [DOI] [PubMed] [Google Scholar]
- 66.Zoet GA, Koster MP, Velthuis BK, et al. Determinants of future cardiovascular health in women with a history of preeclampsia. Maturitas 2015; 82: 153–161. [DOI] [PubMed] [Google Scholar]