Abstract
Background:
Superior capsular reconstruction (SCR) for massive, irreparable rotator cuff tears has become more widely used recently; however, ideal tensioning of the graft and the influence on joint kinematics remain unknown.
Purpose/Hypothesis:
The purpose of this study was to assess the effects of graft tensioning on glenohumeral joint kinematics after SCR using a dermal allograft. The hypothesis was that a graft fixed under tension would result in increased glenohumeral abduction motion and decreased cumulative deltoid forces compared with a nontensioned graft.
Study Design:
Controlled laboratory study.
Methods:
A total of 10 fresh-frozen cadaveric shoulders were tested using a dynamic shoulder simulator. Each shoulder underwent the following 4 conditions: (1) native, (2) simulated irreparable supraspinatus (SSP) tear, (3) SCR using a nontensioned acellular dermal allograft, and (4) SCR using a graft tensioned with 30 to 35 N. Mean values for maximum glenohumeral abduction and cumulative deltoid forces were recorded. The critical shoulder angle (CSA) was also assessed.
Results:
Native shoulders required a mean (±SE) deltoid force of 193.2 ± 45.1 N to achieve maximum glenohumeral abduction (79.8° ± 5.8°). Compared with native shoulders, abduction decreased after SSP tears by 32% (54.3° ± 13.7°; P = .04), whereas cumulative deltoid forces increased by 23% (252.1 ± 68.3 N; P = .04). The nontensioned SCR showed no significant difference in shoulder abduction (54.1° ± 16.1°) and required deltoid forces (277.8 ± 39.8 N) when compared with the SSP tear state. In contrast, a tensioned graft led to significantly improved shoulder abduction compared with the SSP tear state (P = .04) although abduction and deltoid forces could not be restored to the native state (P = .01). A positive correlation between CSA and maximum abduction was found for the tensioned-graft SCR state (r = 0.685; P = .02).
Conclusion:
SCR using a graft fixed under tension demonstrated a significant increase in maximum shoulder abduction compared with a nontensioned graft; however, abduction remained significantly less than the intact state. The nontensioned SCR showed no significant improvement in glenohumeral kinematics compared with the SSP tear state.
Clinical Relevance:
Because significant improvement in shoulder function after SCR may be expected only when the graft is adequately tensioned, accurate graft measurement and adequate tension of at least 30 N should be considered during the surgical procedure. SCR with a tensioned graft may help maintain sufficient acromiohumeral distance, improve clinical outcomes, and reduce postoperative complications.
Keywords: superior capsular reconstruction, rotator cuff, graft, biomechanics, acellular dermal graft, supraspinatus
Along with the rotator cuff, the superior capsule is seen as an important static stabilizer of the shoulder joint, preventing superior migration of the humeral head.3,4,9,24,34,46 In massive rotator cuff tears, repair of these structures often remains challenging due to atrophy, retraction, and fatty infiltration of the remaining rotator cuff.5,22,27 Although arthroplasty and muscle tendon transfers have been described, clinical outcomes have been mixed and significant complications have been reported.16,17 Thus, superior capsular reconstruction (SCR) has emerged as an increasingly popular procedure with initial promising biomechanical results in restoring glenohumeral kinematics.6,33–35,45
As originally described by Mihata et al35 in 2012, SCR involved using a fascia lata autograft rigidly fixed between the greater tuberosity and superior glenoid rim. Since then, the technique has been further evolved, with promising biomechanical and clinical results, as a potential alternative for young patients in the presence of massive, irreparable rotator cuff tears without osteoarthritis.10,14,26,28,30–33,42 With continuous evolution of arthroscopic techniques, Tokish and Beicker49 recently described performing an all-arthroscopic SCR technique using an acellular dermal allograft, thereby reducing donor site morbidity and decreasing soft tissue dissection.6,41,51
To minimize the risk of graft tear, graft thickness and graft tension have been suggested to be important in addition to surgical technique and fixation methods.33,43 As the superior capsule is a well-demonstrated stabilizer against superior humeral head migration, adequate reconstruction is necessary for humeral head centering.34 However, the amount of graft tension needed for the SCR to perform best, especially with dermal allograft, remains in question.33
Therefore, the purpose of the study was to assess the effects of graft tensioning on glenohumeral joint kinematics after SCR using a dermal allograft. We hypothesized that a graft fixed under tension would result in increased glenohumeral abduction motion and decreased cumulative deltoid forces compared with a nontensioned graft.
Methods
Specimens
This study involved 12 fresh-frozen cadaveric shoulders of mean age 66.8 years (range, 64-74 years). All specimens were obtained from Medcure Inc. No ethics approval was required, as deidentified specimens do not constitute human subjects research. Computed tomography scans were performed on all specimens to exclude those with moderate to severe osteoarthritis or bony defects and to measure the critical shoulder angle (CSA).36 The CSA was measured by drawing a line from the superior pole to the inferior pole of the glenoid and a line from the inferior pole to the lateral edge of the acromion in the anteroposterior view.36 Of the 12 specimens, 2 had to be excluded owing to significant degenerative changes, and all data reported in this study pertain to the remaining 10 samples.
Before dissection, the specimens were thawed overnight at room temperature. The anterior, middle, and posterior aspects of the deltoid tendons were dissected from the muscle belly, and anchor loops were sutured with No. 2 FiberWire (Arthrex) to attach each tendon to an individual shoulder simulator actuator, as previously described.2,15,43,50 The rotator cuff muscles (supraspinatus, infraspinatus, teres minor, and subscapularis) were released from the scapula and sutured to a pulley-strap with No. 2 FiberWire to prevent slippage during load application. The infraspinatus and teres minor were simulated as 1 unit.20 The scapular body was placed in a custom-made rectangular box with the medial border aligned perpendicular to the ground and the glenoid tilted 10° superiorly, and bone cement was added to ensure proper fixation.19,50 Then, a steel rod was screwed into the distal humerus 30 cm from the greater tuberosity and loaded with 1.7 kg, representing the native forearm weight.19,50 The glenohumeral joint capsule was vented to avoid changes during testing.2,15,43
Testing Setup
For biomechanical testing, a validated dynamic shoulder simulator, similar to the testing model developed by Wuelker et al,50 was used.2,13,15,43 The shoulder simulator consisted of 4 linear screw-driven actuators (Bimba) connected to 100-lb load cells (Futek). Actuator position was controlled and recorded in real time throughout the loading cycle while the load cells recorded the forces.15 SiNet Hub Programmer software (Applied Motion Products) was used to create custom motion profiles for each actuator to generate adequate displacement for the supraspinatus and anterior, middle, and posterior deltoid.15 A displacement-controlled setting was used to design the motion profiles similar to a selective cutting scenario.15 Maximum abduction was achieved in the intact specimen to determine the individual amount of displacement for the supraspinatus and the deltoid heads. Then, displacement and speed were adjusted accordingly. These motions were programmed into their respective cylinders to repetitively achieve maximum abduction throughout the testing cycle for the specimen. The displacement pathways were replayed to confirm reproducible, stable, maximum abduction.15
The specimen was mounted to the simulator on a 6 degrees of freedom jig with the scapula in 10° of anteflexion, with 10° superior tilt of the glenoid, resulting in a 110° angle between the scapular spine and vertical axis.50 Correct positing was confirmed under fluoroscopy. The 3-mm steel wires were linked to the FiberWire tendon loops of the deltoid and supraspinatus muscle to connect them with the actuators. Steel wires were restricted to <5 mm of lateral translation along the loading track to prevent wire bending or dislocation during the dynamically changing line of action. Similar to Henninger et al,19 we spread the deltoid pulleys over the lateral edge of the acromion according to the native force vectors (Figure 1A).50 Hence, the anterior deltoid pulley was placed 5 mm lateral to the anterolateral corner of the acromion.19 The middle deltoid pulley was fixed midway between the anterolateral and posterolateral corners of the acromion.19 The posterior deltoid pulley was positioned 5 mm lateral to the posterolateral acromial edge.19 To maintain stability of the specimen at the resting position and during dynamic motion, the subscapularis and infraspinatus were loaded statically with 5 lb.20,50 The static loading conditions for subscapularis and infraspinatus were chosen to maintain joint stability with dynamic loading as the shoulder achieved greater degrees of abduction.20,50
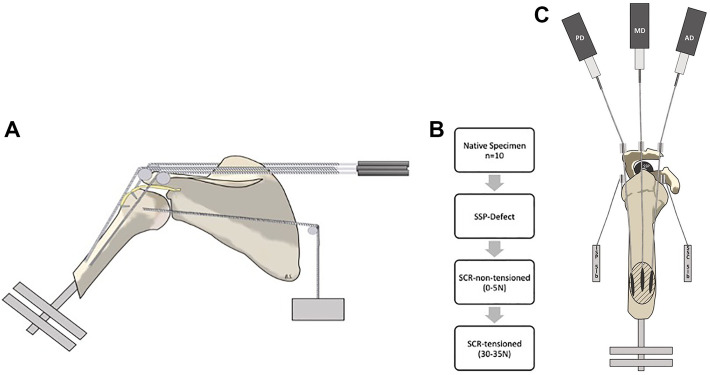
Figure 1.
(A) Testing apparatus viewed in the sagittal plane. The 3 pulleys, corresponding to the tendons of the anterior, middle, and posterior deltoid, were spread over the lateral edge of the acromion according to the native force vectors. Superior capsular reconstruction is indicated as a yellow patch connecting the greater tuberosity with the superior glenoid rim. (B) Flowchart displaying the 4 testing conditions. SCR, superior capsular reconstruction; SSP, supraspinatus. (C) Testing apparatus viewed in the axial view. AD, anterior deltoid; ISP, infraspinatus; MD, middle deltoid; PD, posterior deltoid; SSC, subscapularis.
Motion Analysis
Custom-made optical tracking tripods were placed on the lateral aspect of the distal humerus (moving) and the lateral acromion (fixed) to track 3-dimensional (3D) motion during dynamic testing. MaxTraq 3D (Innovision Systems Inc) software was used for 3D motion analysis.40 MaxTraq data were the primary outcome variable for the abduction angle measurement.
Testing Cycle
Before testing, the native position for the specimen was determined via cameras placed around the testing apparatus to cover a 180° field of view.15 Combined with optical tracking MaxTraq data, a set of 3D coordinates objectively set a reproducible starting position for each testing cycle. Each specimen underwent 3 dynamic motion cycles for each testing condition, with continuous data collection of muscle forces and 3D motion.15 The arm was actively abducted by pulling on the deltoid heads via the pulley systems. Loads were not equally distributed. Individual loading files were created for each shoulder separately because bony anatomic features (eg, acromion shape) differed and therefore deltoid moment arms differed, as described below for each shoulder. First, the distance of the middle deltoid was adjusted (for a maximum abduction), and then the distances of the other 2 deltoid heads were adjusted. Next, the speed and force were adapted to minimize the force needed and to provide a center joint motion under fluoroscopy. Mean maximum glenohumeral abduction and deltoid forces were recorded. Abduction was deemed “maximal” when the greater tuberosity impinged on the acromion or if the shoulder did not increase abduction angle for a period of 5 seconds despite increasing the deltoid force.15 After each motion cycle, the specimen was returned to the starting position, verified with MaxTraq 3D coordinates. A fluoroscopic image in the coronal place ensured that no structural changes to the apparatus had occurred between motion cycles.
Testing Conditions
The specimens remained in the shoulder simulator throughout all testing and surgical repairs. To avoid performance bias, all surgeries were performed by the same surgeon (F.D.). In total, 4 conditions were tested: (1) native, (2) simulated irreparable supraspinatus tear, (3) SCR using a 3–mm thick acellular dermal patch fixed without tension (0-5 N), and (4) SCR using the same acellular dermal patch fixed with a tension of 30 to 35 N. The native shoulder (condition 1) served as the control. A tenotomy of the long head of the biceps tendon was performed in each specimen before the first test so that conditions would not change between tests. After all measurements had been made in condition 1, the irreparable supraspinatus tear was simulated by disconnecting the supraspinatus from the pulley system (condition 2), sharply dissecting the supraspinatus and the superior capsule at their insertion onto the greater tuberosity, and retracting the supraspinatus along with the superior capsule to the glenoid. During condition 3, the SCR (Figure 2, A and B) was performed using a 3–mm thick acellular dermal allograft (ArthroFlex; LifeNet Health Inc) as described in detail below. Graft size was determined based on 4 measurements recorded in 30° of glenohumeral abduction in the scapular plane and neutral rotation: (1) anterior-posterior distance between the glenoid anchors, (2) anterior-posterior distance between the tuberosity anchors, (3) medial-lateral distance between the posterior anchors, and (4) medial-lateral distance between the anterior anchors. For graft fixation, posterior side-to-side suturing was performed, attaching the graft posteriorly to the infraspinatus tendon and underlying shoulder capsule.7,35,37,43 After 3 testing cycles following condition 3 (Figure 2, C and D), the lateral humeral anchor row was removed, and posterior side-to-side sutures were cut to re-tension the graft for condition 4 (Figure 2, E and F). To guarantee similar testing conditions and reduce bias, the graft was pre-tensioned with 30 to 35 N for 30 seconds before testing condition 3. No damage to the graft was noted between testing conditions 3 and 4.
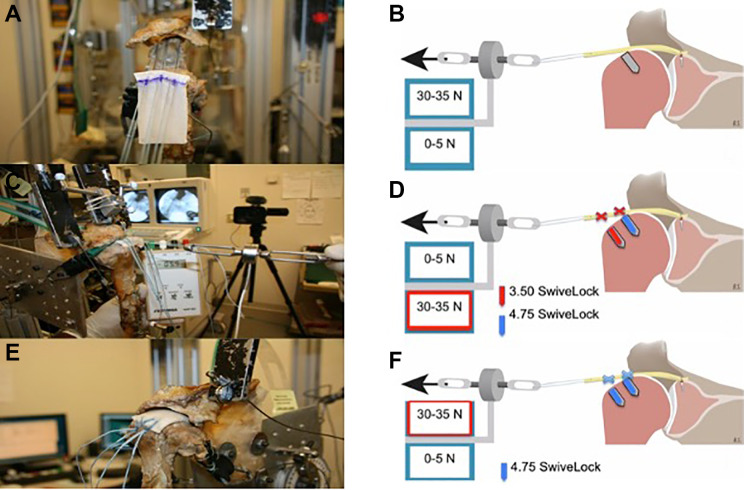
Figure 2.
Figure displaying testing conditions 3 and 4. (A) The acellular dermal allograft for superior capsular reconstruction (SCR) with fixation on the glenoid using three 3.0-mm SutureTak anchors. (B) Graft tension was achieved by pulling the graft laterally using a graft-tensioning device. The humeral head was positioned at 30° of abduction and neutral rotation. After proper tension was reached, anchor positions were marked and the graft was fixed at the exact position, while the tension was held until the humeral double-row reconstruction was completed for both testing conditions 3 and 4. (C) Condition 3 using the graft in a nontensioned state (0-5 N) for SCR. (D) In condition 3, lateral row fixation was performed using two 3.5-mm SwiveLock anchors, whereas medial row fixation was performed using two 4.75-mm SwiveLock anchors. (E) In condition 4, the graft was fixed in a tensioned state (30-35 N) after removal of the lateral humeral anchor row. (F) Double-row fixation (condition 4) using two 4.75-mm SwiveLock anchors.
Superior Capsular Reconstruction Technique
The SCR was performed in accordance with existing literature using a human acellular dermal patch with average dimensions of 4.0 × 7.0 cm and 3.5-mm thickness.6,14,43,49 With the shoulders in 30° of glenohumeral abduction, the defect size was measured so that the graft could be adequately prepared to allow a 2-cm overhang on the lateral end of the graft in order to connect the tensioning device. Next, glenoid preparation was performed, followed by fixation of the graft via three 3.0-mm SutureTak anchors (Arthrex). For humeral fixation, a double-row construct was placed into the greater tuberosity at the articular margin. Before humeral fixation, an additional suture was placed into the lateral side of the graft and connected to a custom-built tensiometer. This tensiometer consisted of a hook, a 50-lb Futek Loadcell (Futek Advanced Sensor Technology), and a handheld strain gauge indicator (Omega HHP-SG). The device was used to control graft tension at the time of humeral fixation by pulling the graft laterally.
During condition 3, a tension of 0 to 5 N was applied to the graft; for condition 4, a tension of 30 to 35 N was applied.12,21 Because no previously gathered data were available for optimal SCR graft tension, 0 to 5 N of tension was chosen to represent the exactly measured graft tension when the graft was placed at 30° of abduction. Subsequently, 30 to 35 N of tension was chosen because pilot testing before this study demonstrated that a force of 30 to 35 N was needed to sufficiently tension the graft without risking pullout from the glenoid fixation.12,21
For each shoulder, condition 3 (nontensioned graft) was performed first, followed by condition 4 (tensioned graft). A micro SutureLasso (Arthrex) was used to ensure the exact same location penetrated on the graft, placed directly over the medial anchor row anchor.
At the same time, the designated graft tension was controlled with the handheld strain gauge indicator. Each tape was separately passed through the graft and subsequently tied down with 2 SwiveLock anchors (Arthrex) on the lateral aspect of the greater tuberosity, according to a double-row rotator cuff reconstruction. In condition 3, two 3.5-mm SwiveLock anchors were used. After the measurements on condition 3 were finished, the lateral anchors were removed, sutures were pulled out from the graft, and graft tension was adjusted by pulling on the graft. In condition 4, sutures were passed through the graft right above the anchor and tied using two 4.75-mm SwiveLock anchors for the lateral row. While the lateral row was performed, graft tension (30-35 N) was continuously controlled. No lateral anchor failures due to the refixation or graft damage during condition 4 were noted.
Statistical Analysis
A power analysis was performed to determine detectable differences in the dependent variables given estimated standard deviations.2,43 For the glenohumeral abduction angle, an error variance of 1° across all conditions with a correlation of 0.3 between measurements was assumed. A sample size of 6 specimens would provide 80% power to detect a 1° difference in shoulder angle at an α level of .05. Linear mixed-effects regression was used to examine change in maximum abduction angle and deltoid muscle force over time. Random effects were obtained for each shoulder specimen to account for the association between repeated measurements and paired shoulders. Two separate hypotheses were tested: (1) whether there were differences in maximum abduction and deltoid force between the native state and each test condition and (2) whether these differences occurred between the nontensioned and tensioned SCR. If any significant differences were detected, a Bonferroni adjustment was used to correct for multiple comparison. Correlations were analyzed using the Pearson test (R), and significance tests were performed. The α level for all statistics was set at .05. All statistical analysis was performed using Stata 14 (StataCorp).
Results
In the native condition, the 10 shoulders achieved a mean ± SE maximum glenohumeral abduction of 79.8° ± 5.8°, requiring a mean 193.2 ± 45.1 N total deltoid force. Compared with the native condition, maximum abduction decreased significantly by 32% after supraspinatus tears (54.3° ± 13.7°; P = .04). A significant increase was seen in total deltoid forces of 23% (252.1 ± 68.3 N) compared with the native condition (P = .04). The nontensioned SCR did not demonstrate significant improvement in shoulder function compared with the supraspinatus tear state (mean maximum abduction, 54.0° ± 16.0°; mean deltoid force, 277.8 ± 39.8 N). However, compared with the supraspinatus tear state, a tensioned SCR significantly improved shoulder function during biomechanical simulation, reaching a mean abduction angle of 65.0° ± 12.6° (P = .04). A tensioned SCR restored a maximum abduction of 81% of the native condition; however, this difference was still statistically significant (P = .04). Deltoid forces also remained significantly elevated in the tensioned group compared with the intact joint (282.3 ± 47.9 N; 146% compared with intact; P = .01) (Tables 1 and 2).
TABLE 1.
Maximal Glenohumeral Abduction for Each Testing Conditiona
| Intact | Rotator Cuff Tear | Nontensioned (0-5 N) Graft | Tensioned (30-35 N) Graft | ||||
|---|---|---|---|---|---|---|---|
| Degrees | % | Degrees | % | Degrees | % | Degrees | % |
| 79.8 ± 5.8 | 100 | 54.3 ± 13.7b | 68 | 54.0 ± 16.0b | 68 | 65.0 ± 12.6c | 81 |
aValues for abduction are expressed as mean ± SE or as percentage. The percentage of abduction was calculated by dividing each value by the value for condition 1 (native).
bSignificant difference compared with condition 1.
cSignificant difference compared with condition 2, simulated supraspinatus tear.
TABLE 2.
Total Deltoid Forces for Each Testing Conditiona
| Intact | Rotator Cuff Tear | Nontensioned (0-5 N) Graft | Tensioned (30-35 N) Graft | ||||
|---|---|---|---|---|---|---|---|
| Newtons | % | Newtons | % | Newtons | % | Newtons | % |
| 193.2 ± 45.1 | 100 | 252.1 ± 68.3b | 123 | 277.8 ± 39.8b | 144 | 282.3 ± 47.9b,c | 146 |
aForce values are expressed as mean ± SE or as percentage. The percentage of force was calculated by dividing each value by the value for condition 1.
bSignificant difference compared with condition 1.
cSignificant difference compared with condition 2, simulated supraspinatus tear.
CSA averaged 34.0° ± 2.4° (range, 30.6°-39.1°) within the study population. The CSA was positively correlated with the functional results of the tensioned SCR when the maximum abduction angle was re-created (r = 0.685; P = .02). Hence, the greater the CSA, the higher the maximum abduction angle after SCR (for CSA 39.1°, the maximum abduction angle was 78.5°; for CSA 30.6°, maximum abduction was 59.2°). No significant correlation was seen between CSA and reduction of maximum abduction angle for the supraspinatus tear condition (r = 0.321; P = .33) or any of the deltoid force measurements (P > .05).
Discussion
The most important finding of this study was that in a dynamic biomechanical shoulder model, SCR with the graft fixed under tension significantly increased maximum abduction angle compared with a simulated irreparable rotator cuff tear. However, the tensioned SCR did not completely restore the maximum abduction angle to the intact state. In comparison, SCR with a nontensioned graft improved neither maximum abduction angle nor cumulative deltoid force, thus resulting in a shoulder function similar to that of a simulated irreparable rotator cuff tear.
Massive rotator cuff tears can lead to limitation in active shoulder range of motion, pain, and muscle weakness.8,11,31,45 This is in part due to abnormal superior humeral head translation and consequential narrowing of the subacromial space.7,38,39,47 To elevate the arm in a patient with rotator cuff tear, greater compensatory forces are required by both the deltoid and the intact remaining muscle-tendon units of the rotator cuff.15,17 Based on recent biomechanical studies, reconstruction of the superior capsule improves shoulder function by reversing superior humeral head migration.29,33–35,43 In addition, Burkhart et al6 proposed the “reverse trampoline” effect (tenodesis effect) from the graft on the humeral head, thereby restoring a stable fulcrum for glenohumeral motion. Thus, maintenance of this fulcrum may provide the added benefit of optimizing the remaining intact force couple.
As described by Mihata et al,33 graft thickness and arm position between 15° and 45° of shoulder abduction are major aspects for sufficient reconstruction of the superior capsule. However, the optimum graft tension for best results of SCR remains unclear. Depending on graft tension, SCR can potentially prevent superior translation of the humeral head by 2 mechanisms: the aforementioned tenodesis effect and/or the spacer effect.33–35,45 Preventing the humeral head from superior migration seems to be the key factor, regardless of the mechanism. The data from this study show that compared with a nontensioned graft, a tensioned graft may act as humeral head depressor, which is of great importance for initiating abduction in the first 30° and improving shoulder function.45 However, at higher abduction angles, the resultant force vector from the deltoid may be directed more horizontally into the glenoid, providing a concavity compression force and preventing superior head migration.44 As a result, the graft may be superfluous at higher abduction angles and thus may contribute only as a subacromial spacer.44
In the present study, the nontensioned graft did not increase maximum abduction angle, nor did it reduce acquired deltoid force compared with the torn condition. This may be due to failure to restore the normal kinematics of the shoulder or failure to act as an adequate subacromial spacer, thus not preventing superior head migration. The graft thickness was only 3 mm and therefore may not have been sufficient to function as a spacer to properly retain the humeral head. Under this tensionless condition, patients may benefit from SCR because of the pain relief of an interposition arthroplasty and the prevention of painful direct bone-to-bone contact. However, this may not be sufficient to reverse true pseudoparalysis.48 Deltoid muscle force required to produce active shoulder motion is also an important factor when considering the potential effect on long-term SCR performance. Similar to reverse total shoulder arthroplasty, SCR relies on the deltoid for arm elevation; however, large increases in muscle may lead to deltoid-related pain and chronic muscle fatigue.1,16
Clinically, graft retears occur and are associated with worse outcome results, especially when thinner and more elastic grafts are used.14,23 This complication may be seen less frequently when facia lata grafts are implanted compared with dermal grafts,25 although further study on this subject is warranted.
An interesting additional finding of this study was the influence of the CSA, as described by Gerber et al18 and Moor et al.36 In this biomechanical simulation, maximum shoulder abduction motion was greater after SCR in shoulders with higher CSA (>35°) compared with smaller CSA (<33°). However, CSA was not correlated with loss of range of motion after the supraspinatus defect was created, as a predictor for motion loss. The power and sample size of the study were not strong enough to further evaluate this observation in detail, but future work may delineate its relationship.
This study has several limitations. In this biomechanical model, only rotator cuff and deltoid muscles were simulated, and the pectoralis major and latissimus dorsi tendon, which may play a role in superior glenohumeral stability, were unloaded. Isolated, irreparable supraspinatus tendon tears were created; however, irreparable defects of the rotator cuff are often massive tears including the subscapularis or infraspinatus tendon. Although the present results support higher tension for graft fixation during SCR using dermal patch, we did not examine the effects of different graft material, cyclic loading on the construct, or the load-to-failure capabilities of the graft. We investigated only nontensioned and tensioned SCR states, so the influence of different tension states on shoulder kinematics after SCR remains unclear. However, during pilot testing, it was noted that 30 to 35 N was the force needed to sufficiently tension the graft without risking pullout from the glenoid fixation.12,21 Additionally, superior head migration was not measured, and flexion was not assessed. Further, only 1 loading rate was investigated, and equal loads through each deltoid head were assumed. Finally, because this was a biomechanical cadaveric study, all data represented a time-zero condition, precluding any effect of biological healing and graft incorporation.
Conclusion
SCR using a graft fixed under tension demonstrated a significant increase in maximum shoulder abduction compared with a nontensioned graft; however, maximum shoulder abduction remained significantly less than the intact state. Further, the nontensioned SCR showed no significant improvement in glenohumeral kinematics compared with the supraspinatus tear state.
Footnotes
Final revision submitted July 10, 2020; accepted July 30, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: The University of Connecticut Health Center/UConn Musculoskeletal Institute received direct funding and material support for this study from Arthrex. The company had no influence on study design, data collection, or interpretation of the results or the final manuscript. K.B. is a paid consultant for Arthrex. C.R.A. is an employee of Arthrex. A.D.M. has received research grants from Arthrex, consulting fees from Arthrex and Astellas Pharma, royalties from Arthrex, and honoraria from Arthrosurface. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval was not sought for the present study.
References
- 1. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221–1230. [DOI] [PubMed] [Google Scholar]
- 2. Adams CR, Comer B, Scheiderer B, et al. The effect of glenohumeral fixation angle on deltoid function during superior capsule reconstruction: a biomechanical investigation. Arthroscopy. 2020;36(2):400–408. [DOI] [PubMed] [Google Scholar]
- 3. Adams CR, DeMartino AM, Rego G, Denard PJ, Burkhart SS. The rotator cuff and the superior capsule: why we need both. Arthroscopy. 2016;32(12):2628–2637. [DOI] [PubMed] [Google Scholar]
- 4. Apreleva M, Hasselman C, Debski R, Fu F, Woo SL, Warner J. A dynamic analysis of glenohumeral motion after simulated capsulolabral injury: a cadaver model. J Bone Joint Surg Am. 1998;80(4):474–480. [DOI] [PubMed] [Google Scholar]
- 5. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894–1908. [DOI] [PubMed] [Google Scholar]
- 6. Burkhart SS, Denard PJ, Adams CR, Brady PC, Hartzler RU. Arthroscopic superior capsular reconstruction for massive irreparable rotator cuff repair. Arthrosc Tech. 2016;5(6):e1407–e1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611–616. [DOI] [PubMed] [Google Scholar]
- 8. Burkhart SS, Hartzler RU. Superior capsular reconstruction reverses profound pseudoparalysis in patients with irreparable rotator cuff tears and minimal or no glenohumeral arthritis. Arthroscopy. 2019;35(1):22–28. [DOI] [PubMed] [Google Scholar]
- 9. Cain PR, Mutschler TA, Fu FH, Lee SK. Anterior stability of the glenohumeral joint: a dynamic model. Am J Sports Med. 1987;15(2):144–148. [DOI] [PubMed] [Google Scholar]
- 10. Catapano M, de Sa D, Ekhtiari S, Lin A, Bedi A, Lesniak BP. Arthroscopic superior capsular reconstruction for massive, irreparable rotator cuff tears: a systematic review of modern literature. Arthroscopy. 2019;35(4):1243–1253. [DOI] [PubMed] [Google Scholar]
- 11. Chung SW, Kim JY, Kim MH, Kim SH, Oh JH. Arthroscopic repair of massive rotator cuff tears: outcome and analysis of factors associated with healing failure or poor postoperative function. Am J Sports Med. 2013;41(7):1674–1683. [DOI] [PubMed] [Google Scholar]
- 12. Davidson PA, Rivenburgh DW. Rotator cuff repair tension as a determinant of functional outcome. J Shoulder Elbow Surg. 2000;9(6):502–506. [DOI] [PubMed] [Google Scholar]
- 13. Debski RE, McMahon PJ, Thompson WO, Woo SL, Warner JJ, Fu FH. A new dynamic testing apparatus to study glenohumeral joint motion. J Biomech. 1995;28(7):869–874. [DOI] [PubMed] [Google Scholar]
- 14. Denard PJ, Brady PC, Adams CR, Tokish JM, Burkhart SS. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy. 2018;34(1):93–99. [DOI] [PubMed] [Google Scholar]
- 15. Dyrna F, Kumar NS, Obopilwe E, et al. Relationship between deltoid and rotator cuff muscles during dynamic shoulder abduction: a biomechanical study of rotator cuff tear progression. Am J Sports Med. 2018;46(8):1919–1926. [DOI] [PubMed] [Google Scholar]
- 16. Ek ETH, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22(9):1199–1208. [DOI] [PubMed] [Google Scholar]
- 17. Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg Am. 2006;88(1):113–120. [DOI] [PubMed] [Google Scholar]
- 18. Gerber C, Snedeker JG, Baumgartner D, Viehöfer AF. Supraspinatus tendon load during abduction is dependent on the size of the critical shoulder angle: a biomechanical analysis. J Orthop Res. 2014;32(7):952–957. [DOI] [PubMed] [Google Scholar]
- 19. Henninger HB, Barg A, Anderson AE, Bachus KN, Tashjian RZ, Burks RT. Effect of deltoid tension and humeral version in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21(4):483–490. [DOI] [PubMed] [Google Scholar]
- 20. Hurschler C, Wulker N, Mendila M. The effect of negative intraarticular pressure and rotator cuff force on glenohumeral translation during simulated active elevation. Clin Biomech (Bristol, Avon). 2000;15(5):306–314. [DOI] [PubMed] [Google Scholar]
- 21. Kim DH, Jang YH, Choi YE, Lee H-R, Kim SH. Evaluation of repair tension in arthroscopic rotator cuff repair: does it really matter to the integrity of the rotator cuff? Am J Sports Med. 2016;44(11):2807–2812. [DOI] [PubMed] [Google Scholar]
- 22. Kooistra B, Gurnani N, Weening A, van den Bekerom M, van Deurzen D. Low level of evidence for all treatment modalities for irreparable posterosuperior rotator cuff tears. Knee Surg Sports Traumatol Arthrosc. 2019;27(12):4038–4048. [DOI] [PubMed] [Google Scholar]
- 23. Lee S-J, Min Y-K. Can inadequate acromiohumeral distance improvement and poor posterior remnant tissue be the predictive factors of re-tear? Preliminary outcomes of arthroscopic superior capsular reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):2205–2213. [DOI] [PubMed] [Google Scholar]
- 24. Leschinger T, Besch K, Aydin C, et al. Irreparable rotator cuff tears: a biomechanical comparison of superior capsuloligamentous complex reconstruction techniques and an interpositional graft technique. Orthop J Sports Med. 2019;7(8):2325967119864590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lim S, AlRamadhan H, Kwak J-M, Hong H, Jeon I-H. Graft tears after arthroscopic superior capsule reconstruction (ASCR): pattern of failure and its correlation with clinical outcome. Arch Orthop Trauma Surg. 2019;139(2):231–239. [DOI] [PubMed] [Google Scholar]
- 26. Makovicka JL, Chung AS, Patel KA, Deckey DG, Hassebrock JD, Tokish JM. Superior capsule reconstruction for irreparable rotator cuff tears: a systematic review of biomechanical and clinical outcomes by graft type. J Shoulder Elbow Surg. 2020;29(2):392–401. [DOI] [PubMed] [Google Scholar]
- 27. Melis B, Wall B, Walch G. Natural history of infraspinatus fatty infiltration in rotator cuff tears. J Shoulder Elbow Surg. 2010;19(5):757–763. [DOI] [PubMed] [Google Scholar]
- 28. Mihata T, Lee TQ, Fukunishi K, et al. Return to sports and physical work after arthroscopic superior capsule reconstruction among patients with irreparable rotator cuff tears. Am J Sports Med. 2018;46(5):1077–1083. [DOI] [PubMed] [Google Scholar]
- 29. Mihata T, Lee TQ, Hasegawa A, et al. Superior capsule reconstruction for reinforcement of arthroscopic rotator cuff repair improves cuff integrity. Am J Sports Med. 2019;47(2):379–388. [DOI] [PubMed] [Google Scholar]
- 30. Mihata T, Lee TQ, Hasegawa A, et al. Five-year follow-up of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. J Bone Joint Surg Am. 2019;101(21):1921–1930. [DOI] [PubMed] [Google Scholar]
- 31. Mihata T, Lee TQ, Hasegawa A, et al. Arthroscopic superior capsule reconstruction can eliminate pseudoparalysis in patients with irreparable rotator cuff tears. Am J Sports Med. 2018;46(11):2707–2716. [DOI] [PubMed] [Google Scholar]
- 32. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459–470. [DOI] [PubMed] [Google Scholar]
- 33. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical effect of thickness and tension of fascia lata graft on glenohumeral stability for superior capsule reconstruction in irreparable supraspinatus tears. Arthroscopy. 2016;32(3):418–426. [DOI] [PubMed] [Google Scholar]
- 34. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423–1430. [DOI] [PubMed] [Google Scholar]
- 35. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248–2255. [DOI] [PubMed] [Google Scholar]
- 36. Moor BK, Bouaicha S, Rothenfluh DA, Sukthankar A, Gerber C. Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint? A radiological study of the critical shoulder angle. Bone Joint J. 2013;95(7):935–941. [DOI] [PubMed] [Google Scholar]
- 37. Nesbitt RJ, Herfat ST, Boguszewski DV, Engel AJ, Galloway MT, Shearn JT. Primary and secondary restraints of human and ovine knees for simulated in vivo gait kinematics. J Biomech. 2014;47(9):2022–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nové-Josserand L, Edwards TB, O’Connor DP, Walch G. The acromiohumeral and coracohumeral intervals are abnormal in rotator cuff tears with muscular fatty degeneration. Clin Orthop Relat Res. 2005;433:90–96. [DOI] [PubMed] [Google Scholar]
- 39. Paletta GA, Jr, Warner JJ, Warren RF, Deutsch A, Altchek DW. Shoulder kinematics with two-plane x-ray evaluation in patients with anterior instability or rotator cuff tearing. J Shoulder Elbow Surg. 1997;6(6):516–527. [DOI] [PubMed] [Google Scholar]
- 40. Pauzenberger L, Heuberer PR, Dyrna F, et al. Double-layer rotator cuff repair: anatomic reconstruction of the superior capsule and rotator cuff improves biomechanical properties in repairs of delaminated rotator cuff tears. Am J Sports Med. 2018;46(13):3165–3173. [DOI] [PubMed] [Google Scholar]
- 41. Pennington WT, Bartz BA, Pauli JM, Walker CE, Schmidt W. Arthroscopic superior capsular reconstruction with acellular dermal allograft for the treatment of massive irreparable rotator cuff tears: short-term clinical outcomes and the radiographic parameter of superior capsular distance. Arthroscopy. 2018;34(6):1764–1773. [DOI] [PubMed] [Google Scholar]
- 42. Petri M, Greenspoon JA, Millett PJ. Arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e751–e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scheiderer B, Kia C, Obopilwe E, et al. Biomechanical effect of superior capsule reconstruction using a 3-mm and 6-mm thick acellular dermal allograft in a dynamic shoulder model. Arthroscopy. 2020;36(2):355–364. [DOI] [PubMed] [Google Scholar]
- 44. Singh S, Reeves J, Langohr GDG, Johnson JA, Athwal GS. The subacromial balloon spacer versus superior capsular reconstruction in the treatment of irreparable rotator cuff tears: a biomechanical assessment. Arthroscopy. 2019;35(2):382–389. [DOI] [PubMed] [Google Scholar]
- 45. Singh S, Reeves J, Langohr GDG, Johnson JA, Athwal GS. The subacromial balloon spacer versus superior capsular reconstruction in the treatment of irreparable rotator cuff tears: a biomechanical assessment. Arthroscopy 2019;35(2):382–389. [DOI] [PubMed] [Google Scholar]
- 46. Terry GC, Hammon D, France P, Norwood LA. The stabilizing function of passive shoulder restraints. Am J Sports Med. 1991;19(1):26–34. [DOI] [PubMed] [Google Scholar]
- 47. Thompson WO, Debski RE, Boardman ND III, et al. A biomechanical analysis of rotator cuff deficiency in a cadaveric model. Am J Sports Med. 1996;24(3):286–292. [DOI] [PubMed] [Google Scholar]
- 48. Tokish JM, Alexander TC, Kissenberth MJ, Hawkins RJ. Pseudoparalysis: a systematic review of term definitions, treatment approaches, and outcomes of management techniques. J Shoulder Elbow Surg. 2017;26(6):e177–e187. [DOI] [PubMed] [Google Scholar]
- 49. Tokish JM, Beicker C. Superior capsule reconstruction technique using an acellular dermal allograft. Arthrosc Tech. 2015;4(6):e833–e839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wuelker N, Wirth CJ, Plitz W, Roetman B. A dynamic shoulder model: reliability testing and muscle force study. J Biomech. 1995;28(5):489–499. [DOI] [PubMed] [Google Scholar]
- 51. Zastrow RK, London DA, Parsons BO, Cagle PJ. Superior capsule reconstruction for irreparable rotator cuff tears: a systematic review. Arthroscopy. 2019;35(8):2525–2534. [DOI] [PubMed] [Google Scholar]