Abstract
Background: Unstable distal radius fractures that undergo surgical stabilization have varying complication rates in the literature. Smoking is known to affect bone healing and implant fixation rates but has never been definitively shown to affect postoperative outcomes of surgically managed distal radius fractures. Methods: A retrospective review was performed of patients with surgically treated distal radius fractures at a Level 1 Trauma Center who had at least 6 weeks of follow-up over a 5-year period. Charts were reviewed for basic demographic information, comorbidities, details about the operative procedure, and early complications. Notable physical examination findings were noted, such as wrist stiffness and distal radius tenderness to palpation. Statistical analysis was performed to compare the smoking and nonsmoking groups. To control for confounding differences, a hierarchical multivariable regression analysis was performed. Results: Four hundred seventeen patients were included in the study, and 24.6% were current smokers at the time of surgery. The overall complication rate for smokers was 9.8% compared with 5.6% in nonsmokers. The smoking cohort showed significantly higher rates of hardware removal, nonunion, revision procedures, wrist stiffness, and distal radius tenderness. When controlling for the confounding variables of diabetes and obesity, smokers still had significantly higher rates of the same complications. Conclusion: Patients who smoke have a statistically significant higher rate of postoperative distal radius tenderness, wrist stiffness, nonunion, hardware removal, and revision procedures compared with those who do not smoke in a review of 417 total patients undergoing surgical fixation for distal radius fractures.
Keywords: distal radius, smoking, bone healing, postoperative complications, surgical fixation
Introduction
Distal radius fractures (DRF) are one of the most common fractures seen by physicians in the United States and will likely increase in incidence in the years to come as the population ages.1 Though seen throughout all ages, it is an injury with a spike in incidence in children aged 5 to 14, in addition to being the most common osteoporotic fracture seen in adults.2 Often the result of low-energy falls in the elderly, DRF through osteoporotic bone have been associated with increased risk of subsequent fractures and increased mortality.3 The importance of these fractures spans multiple medical specialties. They are commonly managed in the emergency department by nonorthopedists, make up at least a quarter of all skeletal fractures seen in a primary care setting, and often times benefit from endocrinology specialists for their bone health.1
The treatment patterns of DRF have changed as our understanding of fracture patterns and surgical options has advanced. The American Academy of Orthopaedic Surgeons’ guidelines recommend surgical fixation for fractures with a postreduction radial shortening >3 mm, dorsal tilt >10°, and intra-articular displacement or step-off >2 mm.4 The success of volar locking plates has revolutionized and expanded our indications for surgical management of these fractures, although they are not the only mode of surgical fixation being utilized. Operative fixation, especially in the elderly, provides early benefits of mobility, radiographic stability, and improved grip strength, although this topic remains controversial and more recent studies have found equivalent functional outcomes in the elderly with nonoperative treatment without the risk of surgical complications.5,6,7
While the majority of surgically managed DRF have good to excellent outcomes, it is important to understand the surgical risks of different patient populations. Hardware irritation/failure, carpal tunnel syndrome or other neuropathy, tendon irritation/rupture, infection, wrist stiffness, residual pain, complex regional pain syndrome, and nonunion/malunion all play a significant role in a patient’s postoperative course and their ultimate outcome. The rates of these individual complications vary in the literature and depend on the type of fixation used and the definition of “complication.” Overall complications have been described in the literature from 14% in a large retrospective review to as high as over 30%, including those that required secondary operations.8,9,10-12 As health care costs continue to rise, determining which patients are susceptible to complications is paramount to aid in decision making for definitive management and patient counseling.13
The poorest outcomes of DRF have been described in elderly, female sex, workers’ compensation clients, lower socioeconomic levels, and poor bone health.1 Moreover, smoking has been reported to be a risk factor for osteoporosis, fractures, and impaired fracture healing.14,15,16 Data suggest smoking affects the surgical fixation of bony implants, possibly due to a direct effect of nicotine on osteoblasts.15,17 There is also evidence suggesting detrimental effects of smoking on tendon strength and morphology.18 Being one of the most common modifiable risk factors in patients, the impact of smoking on operative DRF is critical to study but is currently poorly understood.
Our goal was to look at the effect of active smoking on DRF treated with surgical fixation at a high-volume Level 1 Trauma Center. To the knowledge of the authors, no literature directly correlates smoking to specific complications following operative DRF.
Materials and Methods
A retrospective review was performed of all consecutive patients who were treated operatively for DRF at a US academic Level 1 Trauma Center between January 2010 and April 2015. Initially using CPT codes (25607, 25608, and 25609) to identify patients, each chart was subsequently identified and reviewed for basic demographic information, comorbidities (smoking status, diabetes mellitus [DM], and body mass index [BMI]), details about the operative procedure, and early complications. All types of fracture fixation (dorsal plates, volar plates, Kirschner wire fixation) were included in the study. The study and database compiled qualified for institutional review board exemption status through our institution and followed all Health Insurance Portability and Accountability Act regulations. All information collected was from a single source and deidentified when compiled. Selected complications were based on those most commonly seen postoperatively in distal radius fracture fixation. Patients with incomplete data or missing information in the medical chart were excluded, as were any patients with less than 6 weeks of documented follow-up.
We defined “smoker” as anyone who reported currently smoking any amount of a nicotine-containing product at the time of surgery. For outcomes data, we recorded several types of adverse outcomes. Follow-up radiographs were routinely obtained at 2 weeks, 4 weeks, 6 weeks, 12 weeks, and 6 months. Patients’ bony healing was monitored for the occurrence of either malunion (unacceptable alignment) or nonunion (failure to achieve radiographic union at 6 months), as well as any evidence of hardware failure. We looked at the incidence of mortality, pulmonary embolus or blood clot, and surgical site infection in the postoperative period. Physical examination findings such as wrist stiffness and distal radius tenderness to palpation, while considered part of the normal postoperative course, were considered clinically relevant if one of the following conditions was stated in the medical record 2 weeks postoperatively: (1) failure to progress in severity; (2) reversal of progress; (3) new diagnosis listed; and (4) newly listed under the problem list.
Initially, the patients were stratified into smoking and nonsmoking groups. Statistical analysis was performed to evaluate for statistical significance of complications, odds ratios, and 95% confidence intervals in the smoking and nonsmoking groups. In addition, to control for confounding variables, a hierarchical multivariable regression analysis was also performed using Statistical Package for the Social Sciences controlling for DM and obesity. Analysis of variance tests were performed, with P <.05 being considered significant.
Results
Four hundred seventeen patients underwent surgical fixation of DRF during the study period queried and met the inclusion criteria. Of all the patients included in the study, 99.3% of the surgeries were performed by one of 4 fellowship-trained hand surgeons. The average age was 49.02 years, and 55.9% were women. Average follow-up was 6.7 months (range: 1.5-65.53 months). At the time of surgery, 24.6% were current smokers, 11.4% were diabetic, and 19.3% has a BMI of greater than 30. The most common treatment method used was volar plating (92.6%). Dorsal plating was used in 4.5% of the cases, and Kirschner wire (K-wire) fixation was used in 9.5% (includes K-wires in isolation and in conjunction with another fixation method).
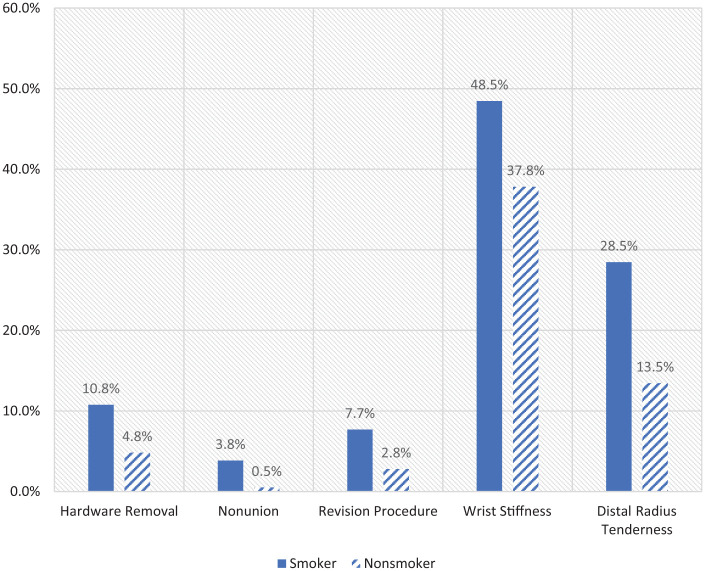
The overall incidence of complications for smokers was 9.8% compared with 5.6% in nonsmokers. The incidence for all of the complications studied is shown in Table 1. The smoking cohort had significantly higher rates of hardware removal (10.8% vs 4.8%; P < .05), nonunion (3.8% vs 0.5%; P < .05), revision procedures (7.7% vs 2.8%; P < .05), wrist stiffness (48.5% vs 37.8%; P < .05), and distal radius tenderness (28.5% vs 13.5%; P < .05) compared with the nonsmoking group as shown in Figure 1.
Table 1.
Overall Incidence of Complications by Smoking Status.
| Smoker (%) | Nonsmoker (%) | Overall (%) | P value (χ2) | P < .05 | OR | CI | |
|---|---|---|---|---|---|---|---|
| Hardware removal | 10.8 | 4.8 | 6.3 | .015 | Y | 2.37 | 1.15-4.87 |
| Infection | 3.1 | 1.3 | 1.7 | .169 | N | 2.44 | 0.64-9.22 |
| Carpal tunnel syndrome | 6.9 | 4.1 | 4.7 | .190 | N | 1.73 | 0.75-4.02 |
| Other neuropathy | 8.5 | 7.6 | 7.8 | .746 | N | 1.1 | 0.42-1.71 |
| FPL rupture | 0.8 | 0.3 | 0.4 | .401 | N | 3.0 | 0.19-49.31 |
| EPL rupture | 1.5 | 0.8 | 0.9 | 0.425 | N | 2.01 | 0.33-12.14 |
| Radiocarpal arthrosis | 4.6 | 1.8 | 2.5 | .073 | N | 2.63 | 0.87-7.99 |
| Malunion | 0.8 | 0.3 | 6.3 | .404 | N | 3.02 | 0.19-48.57 |
| Nonunion | 3.8 | 0.5 | 1.3 | .004 | Y | 7.77 | 1.49-40.54 |
| Entrapment | 0.8 | 0.3 | 0.4 | 0.402 | N | 3.0 | 0.19-48.31 |
| Compartment syndrome | 1.5 | 0.3 | 0.6 | .092 | N | 6.06 | 0.55-67.44 |
| CRPS | 0.8 | 1.3 | 1.1 | 0.614 | N | 0.6 | 0.07-5.14 |
| Revision procedure | 7.7 | 2.8 | 4.0 | .014 | Y | 2.88 | 1.19-6.95 |
| Wrist stiffness | 48.5 | 37.8 | 40.5 | .004 | Y | 1.84 | 1.20-2.82 |
| Distal radius tenderness | 28.5 | 13.5 | 17.0 | <.000 | Y | 3.11 | 1.89-5.10 |
Note. χ2 = χ2 test; OR = odds ratio; CI = 95% confidence interval; Y = yes; N = no; FPL = flexor pollicis longus; EPL = extensor pollicis longus; CRPS = complex regional pain syndrome.
Boldfaced values indicate statistical significance at p < 0.05.
Figure 1.
Statistically significant complications by smoking status. The graph highlights the complication measures that were statistically significant in our smoking group when compared with the nonsmoking group.
In the hierarchical multivariable regression analysis, when controlling for confounding factors of diabetes and obesity, smokers still had significantly higher rates of clinically relevant distal radius tenderness and wrist stiffness, nonunion, revision procedures, and hardware removal (Table 2).
Table 2.
Complication P Values After Multivariable Linear Regression Analysis Between Smoking and Nonsmoking Groups.
| Complication | P value | β value | CI |
|---|---|---|---|
| Distal radius tenderness | <.000* | 0.210 | 0.112-0.296 |
| Nonunion | .003* | 0.156 | 0.019-0.077 |
| Stiffness | .006* | 0.133 | 0.045-0.274 |
| Revision procedures | .016* | 0.143 | 0.025-0.124 |
| Hardware removal | .024* | 0.146 | 0.033-0.155 |
Note. CI = confidence interval.
Statistically significant.
Discussion
The overall postoperative complication rate in active smokers with operative DRF was 9.8% compared with 5.6% in the nonsmoking group. This is slightly lower but consistent with the complication rates reported in other studies, although what is considered a complication varies widely in the literature.8,9,10-12
For operative DRF at our institution, active smokers showed statistically higher rates of hardware removal, nonunion, revision procedures, wrist stiffness, and distal radius tenderness. Reasons for revision surgical procedures included hardware removal for tendon irritation/rupture, hardware loosening, infection, nonunion/malunion, and continued pain. While our higher rates of tendon rupture and infection did not reach statistical significance, the overall number of revision procedures was statistically significant.
The current literature helps predict and supports our findings. Santiago et al used a rat model to show that exposure to tobacco smoke resulted in delayed fracture healing and callus formation that had decreased density, maturity, and mechanical resistance.19 A systematic review of the nonspine orthopedic literature published in 2013 found higher rates of malunion, delayed union, and nonunion in smokers in 13 of 17 studies reviewed, which supports our findings of increased rates of nonunion in the active smoker group.20 There may also be a role for intervention in the bone health of these patients postoperatively to optimize their chance of union. Vitamin D and calcium levels have been shown to affect fracture healing rates.21,22,23 Vitamin and calcium supplementation has been suggested as low-risk, low-cost interventions to improve bone healing, although more conclusive studies need to be performed to elucidate the impact of supplementation on healing rates in smokers.21,22 Similarly, bone stimulators have been shown to have varying success in the literature, mainly in lower extremity injuries, and have potential as an adjunctive therapy in delayed union or nonunion, but conclusions about their effectiveness in upper extremity fractures and in high-risk populations remain unclear at this time.24,25
While the increased tendon irritation/rupture rates in smokers compared with nonsmokers were not statistically significant, tendon problems were certainly a major cause of hardware removal in the smoking group, the rates of which were statistically significantly higher than nonsmokers in the nonregression analysis and trended toward significance in the regression analysis. To our knowledge, there are no studies investigating the impact of smoking on flexor and extensor tendons of the hand and wrist, but other studies have demonstrated the negative impact that smoking can have on tendon strength and morphology. Ağladıoğlu et al published a case-control analytical study in 2016 that looked at the effects of cigarette smoke on patellar and achilles tendons. They found that the thickness and strain ratio measurements of the tendons were reduced, making the tendons thinner and more brittle.18 The impact of cigarette smoke on the vascularity of tendons has also been described in the literature, which affects the tendon’s strength and ability to heal.26,27 Extrapolating this to the hand and wrist, these type of morphological and biomechanical changes may cause more tendinitis and tendon ruptures, requiring a second surgical procedure and often hardware removal.
Our hardware removal rates in the smoking group were 10.8% compared with 4.8% in the nonsmoking group (P = .004). Previous database studies and large retrospective case reviews have found hardware removal rates from 3% to 10% for their overall population.28,29 While some catastrophic complications necessitate the removal of hardware in the wrist after a distal radius fracture, it is difficult to directly compare rates between institutions because other, more subtle, indications are institution- and surgeon-specific. In a 2015 retrospective case review by Snoddy et al, the most common reasons for hardware removal for volar plates in DRF were pain, tenosynovitis, plate malposition (prominent hardware), and malunion.29 Hardware failure, infection, nonunion, and tendon rupture were less common. Pain was the only significant predictor of hardware removal. Our study did reveal that patients who smoked had significantly higher rates of distal radius tenderness after the first postoperative visit. Preoperative smoking rates have also been linked to worse outcomes and increased pain scores in rotator cuff surgery as well.27 It is possible that the higher rates of pain seen in our study in the smoking group contributed to both higher rates seen in hardware removal and postoperative wrist stiffness. The correlation with smoking and pain, overall, is unclear. Other psychological factors, such as rates of depression and substance abuse, both of which are higher in smokers, may play a role in perception of pain as well.30,31
Hardware failure, including screw loosening or pullout, was also a reason for hardware removal in our study and in other studies looking at removal rates.28,29,10 Similar to fracture healing events, osteointegration of orthopedic implants requires adequate bone density, a hospitable microenvironment, and coordinated events between bone-forming and bone-resorbing cells and signaling cascades to achieve biological stability. A large descriptive review of the literature by Fini et al described that 3 in vitro studies reported cigarette smoke inhibited cell vitality, spreading, and activity on titanium substrates, therefore causing a negative impact of osteointegration on implants.15 It should be noted that this is in addition to the already reported link between smoking and osteoporosis, which is also a risk factor for hardware failure.15
Our study, as is presented, has significant limitations. The most obvious limitation is its retrospective nature. As with all retrospective database/chart review studies, our information was reliant on accurate and consistent medical chart documentation across multiple surgeons. This is especially true when reporting distal radius tenderness and wrist stiffness. Both of these outcomes rely on accurate documentation, and any inconsistency may result in under- or overreporting. Effort was made to standardize the way the information was obtained from the electronic health record, but it is still a potential confounding factor. Prospectively following smoking and nonsmoking cohorts would give a more accurate representation of the risks.
Second, because of the retrospective nature and not routinely doing functional scores in clinic, our study lacks patient subjective scores to subjectively qualify their outcomes. These scores should be included in future prospective studies to provide more accurate representation of a patient’s functional outcome. While volar plating was the most common surgical treatment method, our study includes all constructs, as well as different plate designs and companies. This was done to capture the most patients and information as possible to attempt to represent the risk of smoking to any surgical intervention for DRF but could be a source of confounding. Our study also does not quantify dose for smoking; we simply had a smoking group and a nonsmoking group because the pack-years was not uniformly reported. Follow-up studies to see whether the complication rate postoperatively is dose dependent would be helpful to add to the body of literature.
In the future, we would like to consider the impact of counseling patients on smoking cessation. While smoking cessation guidance is commonplace in fracture patients, no data exist that suggest how counseling, or smoking cessation in general, affects the postoperative complication rate in DRF. Other postoperative interventions, such as vitamin D, calcium supplementation, and bone stimulator devices, and their effects on the smoking population would also be important to consider. Another avenue to consider is the effect of smoking status on nonoperative management of DRF and compare against the data presented here to see whether these complications are mediated or moderated by surgery.
This study presents evidence that active smokers have significantly higher postoperative complication rates in multiple areas compared with nonsmokers. Studies such as this are important to help counsel patients and to aid with expectations for patients who are active smokers who are being treated surgically. In the future, predicting which patients are more likely to have certain postoperative complications will improve the cost-effectiveness of interventions in those patient populations and provide surgeons with additional information when deciding on surgical versus nonsurgical treatment modalities for DRF.
Footnotes
Ethical Approval: Approval was obtained through our institutional review board and were exempt from informed consent.
Statement of Human and Animal Rights: This study does not directly contain studies of human or animal subjects. This study is a retrospective chart review of outcomes.
Statement of Informed Consent: Informed consent was obtained when necessary.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. MacIntyre NJ, Dewan N. Epidemiology of distal radius fractures and factors predicting risk and prognosis. J Hand Ther. 2016;29(2):136-145. [DOI] [PubMed] [Google Scholar]
- 2. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697. [DOI] [PubMed] [Google Scholar]
- 3. Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301(5):513-521. [DOI] [PubMed] [Google Scholar]
- 4. Lichtman DM, Bindra RR, Boyer MI, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: the treatment of distal radius fractures. J Bone Joint Surg Am. 2011;93(8):775-778. [DOI] [PubMed] [Google Scholar]
- 5. Arora R, Lutz M, Deml C, et al. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93(23):2146-2153. [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Chen X, Li Z, et al. Safety and efficacy of operative versus nonsurgical management of distal radius fractures in elderly patients: a systematic review and meta-analysis. J Hand Surg. 2016;41(3):404-413. [DOI] [PubMed] [Google Scholar]
- 7. Oshige T, Sakai A, Zenke Y, et al. A comparative study of clinical and radiological outcomes of dorsally angulated, unstable distal radius fractures in elderly patients: intrafocal pinning versus volar locking plating. J Hand Surg. 2007;32(9):1385-1392. [DOI] [PubMed] [Google Scholar]
- 8. Arora R, Lutz M, Hennerbichler A, et al. Complications following internal fixation of unstable distal radius fracture with a palmar locking-plate. J Orthop Trauma. 2007;21(5):316-322. [DOI] [PubMed] [Google Scholar]
- 9. Diaz-Garcia RJ, Oda T, Shauver MJ, Chung KC. A systematic review of outcomes and complications of treating unstable distal radius fractures in the elderly. J Hand Surg. 2011;36(5):824-8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thorninger R, Madsen ML, Wæver D, et al. Complications of volar locking plating of distal radius fractures in 576 patients with 3.2 years follow-up. Injury. 2017;48:1104-1109. [DOI] [PubMed] [Google Scholar]
- 11. Wichlas F, Haas NP, Disch A, et al. Complication rates and reduction potential of palmar versus dorsal locking plate osteosynthesis for the treatment of distal radius fractures. J Orthop Traumatol. 2014;15(4):259-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williksen JH, Husby T, Hellund JC, et al. External fixation and adjuvant pins versus volar locking plate fixation in unstable distal radius fractures: a randomized, controlled study with a 5-year follow-up. J Hand Surg. 2015;40(7):1333-1340. [DOI] [PubMed] [Google Scholar]
- 13. Moses H, Matheson DHM, Dorsey ER, et al. The anatomy of health care in the United States. JAMA. 2013;310(18):1947-1963. [DOI] [PubMed] [Google Scholar]
- 14. Cusano NE. Skeletal effects of smoking. Curr Osteoporos Rep. 2015;13(5):302-309. [DOI] [PubMed] [Google Scholar]
- 15. Fini M, Giavaresi G, Salamanna F, et al. Harmful lifestyles on orthopedic implantation surgery: a descriptive review on alcohol and tobacco use. J Bone Miner Metab. 2011;29(6):633-644. [DOI] [PubMed] [Google Scholar]
- 16. Hopper JL, Seeman E. The bone density of female twins discordant for tobacco use. N Engl J Med. 1994;330(6):387-392. [DOI] [PubMed] [Google Scholar]
- 17. Pereira ML, Carvalho JC, Peres F, Gutierres M, Fernandes MH. Behaviour of human osteoblastic cells cultured on plasma-sprayed titanium implants in the presence of nicotine. Clin Oral Implants Res. 2008;19(6):582-589. [DOI] [PubMed] [Google Scholar]
- 18. Ağladıoğlu K, Akkaya N, Güngör HR, et al. Effects of cigarette smoking on elastographic strain ratio measurements of patellar and achilles tendons. J Ultrasound Med. 2016;35(11):2431-2438. [DOI] [PubMed] [Google Scholar]
- 19. Santiago HAR, Zamarioli A, Sousa Neto MD, et al. Exposure to secondhand smoke impairs fracture healing in rats. Clin Orthop. 2017;475(3):894-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel RA, Wilson RF, Patel PA, et al. The effect of smoking on bone healing: a systematic review. Bone Jt Res. 2013;2(6):102-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Childs BR, Andres BA, Vallier HA. Economic benefit of calcium and vitamin D supplementation: does it outweigh the cost of nonunions? J Orthop Trauma. 2016;30(8):285-288. [DOI] [PubMed] [Google Scholar]
- 22. Gorter EA, Hamdy NAT, Appelman-Dijkstra NM, et al. The role of vitamin D in human fracture healing: a systematic review of the literature. Bone. 2014;64:288-297. [DOI] [PubMed] [Google Scholar]
- 23. Gorter EA, Krijnen P, Schipper IB. Vitamin D status and adult fracture healing. J Clin Orthop Trauma. 2017;8(1):34-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cook JJ, Summers NJ, Cook EA. Healing in the new millennium: bone stimulators: an overview of where we’ve been and where we may be heading. Clin Podiatr Med Surg. 2015;32(1):45-59. [DOI] [PubMed] [Google Scholar]
- 25. Schofer MD, Block JE, Aigner J, et al. Improved healing response in delayed unions of the tibia with low-intensity pulsed ultrasound: results of a randomized sham-controlled trial. BMC Musculoskelet Disord. 2010;11:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carbone S, Gumina S, Arceri V, et al. The impact of preoperative smoking habit on rotator cuff tear: cigarette smoking influences rotator cuff tear sizes. J Shoulder Elbow Surg. 2012;21(1):56-60. [DOI] [PubMed] [Google Scholar]
- 27. Mallon WJ, Misamore G, Snead DS, et al. The impact of preoperative smoking habits on the results of rotator cuff repair. J Shoulder Elbow Surg. 2004;13(2):129-132. [DOI] [PubMed] [Google Scholar]
- 28. Lutsky KF, Beredjiklian PK, Hioe S, et al. Incidence of hardware removal following volar plate fixation of distal radius fracture. J Hand Surg. 2015;40(12):2410-2415. [DOI] [PubMed] [Google Scholar]
- 29. Snoddy MC, An TJ, Hooe BS, et al. Incidence and reasons for hardware removal following operative fixation of distal radius fractures. J Hand Surg. 2015;40(3):505-507. [DOI] [PubMed] [Google Scholar]
- 30. Gass JC, Morris DH, Winters J, et al. Characteristics and clinical treatment of tobacco smokers enrolled in a VA substance use disorders clinic. J Subst Abuse Treat. 2018;84:1-8. [DOI] [PubMed] [Google Scholar]
- 31. Secades-Villa R, González-Roz A, García-Pérez Becoña ÁE. Psychological, pharmacological, and combined smoking cessation interventions for smokers with current depression: A systematic review and meta-analysis. Plos One. 2017;12(12):e0188849. [DOI] [PMC free article] [PubMed] [Google Scholar]