Abstract
Background
Molecular subtyping of triple‐negative breast cancers (TNBCs) via gene expression profiling is essential for understanding the molecular essence of this heterogeneous disease and for guiding individualized treatment. We aim to devise a clinically practical method based on immunohistochemistry (IHC) for the molecular subtyping of TNBCs.
Materials and Methods
By analyzing the RNA sequencing data on TNBCs from Fudan University Shanghai Cancer Center (FUSCC) (n = 360) and The Cancer Genome Atlas data set (n = 158), we determined markers that can identify specific molecular subtypes. We performed immunohistochemical staining on tumor sections of 210 TNBCs from FUSCC, established an IHC‐based classifier, and applied it to another two cohorts (n = 183 and 214).
Results
We selected androgen receptor (AR), CD8, FOXC1, and DCLK1 as immunohistochemical markers and classified TNBCs into five subtypes based on the staining results: (a) IHC‐based luminal androgen receptor (IHC‐LAR; AR‐positive [+]), (b) IHC‐based immunomodulatory (IHC‐IM; AR‐negative [−], CD8+), (c) IHC‐based basal‐like immune‐suppressed (IHC‐BLIS; AR−, CD8−, FOXC1+), (d) IHC‐based mesenchymal (IHC‐MES; AR−, CD8−, FOXC1−, DCLK1+), and (e) IHC‐based unclassifiable (AR−, CD8−, FOXC1−, DCLK1−). The κ statistic indicated substantial agreement between the IHC‐based classification and mRNA‐based classification. Multivariate survival analysis suggested that our IHC‐based classification was an independent prognostic factor for relapse‐free survival. Transcriptomic data and pathological observations implied potential treatment strategies for different subtypes. The IHC‐LAR subtype showed relative activation of HER2 pathway. The IHC‐IM subtype tended to exhibit an immune‐inflamed phenotype characterized by the infiltration of CD8+ T cells into tumor parenchyma. The IHC‐BLIS subtype showed high expression of a VEGF signature. The IHC‐MES subtype displayed activation of JAK/STAT3 signaling pathway.
Conclusion
We developed an IHC‐based approach to classify TNBCs into molecular subtypes. This IHC‐based classification can provide additional information for prognostic evaluation. It allows for subgrouping of TNBC patients in clinical trials and evaluating the efficacy of targeted therapies within certain subtypes.
Implications for Practice
An immunohistochemistry (IHC)‐based classification approach was developed for triple‐negative breast cancer (TNBC), which exhibited substantial agreement with the mRNA expression‐based classification. This IHC‐based classification (a) allows for subgrouping of TNBC patients in large clinical trials and evaluating the efficacy of targeted therapies within certain subtypes, (b) will contribute to the practical application of subtype‐specific treatment for patients with TNBC, and (c) can provide additional information beyond traditional prognostic factors in relapse prediction.
Keywords: Triple‐negative breast cancer, Molecular classification, Immunohistochemistry, Precision medicine
Short abstract
This article describes an immunohistochemistry‐based approach to classification of triple‐negative breast cancers into molecular subtypes for purposes of the translation of TNBC molecular classification into clinical practice.
Introduction
Triple‐negative breast cancer (TNBC) encompasses a subset of breast cancers that lack expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). TNBCs account for 15%–20% of newly diagnosed breast cancer cases 1, 2. Compared with hormone receptor‐positive breast cancer, TNBC is associated with younger patient age, higher incidence of visceral metastases, higher risk of early recurrence, and a poorer prognosis 2, 3, 4.
TNBC has been increasingly recognized as a heterogeneous disease that exhibits substantial differences in terms of pathological features, biological behavior, and gene expression profiles 5, 6. The extreme heterogeneity of TNBC has led to the challenge of finding molecular targets in preclinical studies and the limited benefit from targeted therapies observed in clinical trials for patients with unselected TNBC. Thus, several studies have concentrated on the molecular subtyping of TNBC and the identification of putative targets in TNBC subgroups 5, 7, 8. Our research team has performed multiomic profiling of 465 TNBCs and classified TNBCs into mRNA expression‐based subtypes, which has provided a large data set of comprehensively profiled TNBCs 9. Despite the difference in classification strategies used in these studies, the classification results showed correlations and revealed at least four TNBC subtypes: (a) luminal androgen receptor (LAR), (b) immunomodulatory (IM), (c) basal‐like immune‐suppressed (BLIS), and (d) mesenchymal (MES). Based on the classification results, subtype‐specific molecular biomarkers and potential therapeutic targets were identified, including androgen receptor in the LAR subtype, BRCA1/2 mutations in the BLIS subtype, and immune signaling pathways in the IM subtype 5, 8.
Molecular subtyping of TNBC based on gene expression profiling is essential for understanding the molecular essence of this heterogeneous disease and for guiding individualized treatment. However, it is not currently used to direct therapy in clinical practice, because so far, there are no results from perspective clinical trials supporting the patient benefits from subtyping‐based treatment strategies. The major challenges of carrying out such clinical researches include the high cost, complicated technological process, and potential batch effects of gene expression profiling. Thus, there is a need for a simplified approach to classify TNBCs into molecular subtypes for large clinical trials and ultimately for routine clinical practice. The detection of subtype‐specific highly expressed markers by immunohistochemistry (IHC) may be an alternative, and successful precedents have been set in breast cancer. Analysis of cDNA microarrays has classified breast cancers into four subgroups (i.e., luminal A, luminal B, HER2‐enriched, and basal‐like) 10, 11. An immunohistochemical surrogate panel of five biomarkers (ER, PR, HER2, EGFR, and cytokeratin 5/6) was developed for identifying the basal‐like subtype 12, 13. After that, a simplified classification based on an immunohistochemical panel of ER, PR, HER2, and Ki‐67 was devised as a substitute for the cDNA microarray‐based classification and has been widely adopted in clinical practice 14, 15. In addition, in endometrial cancers, a clinically applicable surrogate method was developed for the molecular classification, based on mismatch repair protein immunohistochemistry, POLE mutation detection, and p53 immunohistochemistry 16, 17.
In our study, by making full use of the mRNA expression data and tumor tissue samples of our established TNBC cohort, we devised an IHC‐based classification method, tested the agreement between our IHC‐based classification and the mRNA‐based classification, and analyzed the prognostic and therapeutic implications of this new classification method.
Materials and Methods
A cohort of 465 patients with TNBC treated at the Department of Breast Surgery of Fudan University Shanghai Cancer Center (FUSCC) was established as described in our previous study 9. We restricted our analysis to the 360 patients whose tumors have had RNA sequencing performed and been classified into four mRNA‐based subtypes. Tumor tissue samples of 210 of these 360 patients were available for making paraffin‐embedded sections. Another two independent cohorts of 183 and 214 patients with TNBC with tumor tissue samples were identified as the validation cohorts for our classification method. None of these tumor tissue samples used for immunohistochemical analysis had been subjected to neoadjuvant therapy.
The workflow for marker selection for IHC‐based classification was shown in supplemental online Figure 1. Immunohistochemical staining was performed on the paraffin‐embedded sections of tumor specimens, and the tumors were classified into IHC‐based subtypes according to the staining results. The agreement between the mRNA‐based classification and IHC‐based classification was analyzed using Cohen's κ statistic. Survival analysis and bioinformatics analysis were carried out to investigate the prognostic and therapeutic implications of our IHC‐based classification. Detailed descriptions of methods can be found in the online supplemental Methods.
Statistical analyses were performed using R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria). All tests were two‐sided, and a p value of <.05 was considered statistically significant.
Results
Selection of Markers for IHC‐Based Classification
See supplemental online Figure 1. To establish a clinically feasible IHC‐based method for the molecular classification of TNBC, we first identified the genes that were highly expressed at the mRNA level in specific subtypes by performing differential expression analysis (supplemental online Tables 1–4). Then, we assessed the correlation of the mRNA expression with the protein expression of these genes and excluded those with a correlation coefficient of <0.5. The accuracy of using each of the remaining genes to identify the corresponding subtype was measured by receiver operating characteristic (ROC) analysis. Supplemental online Tables 5–8 showed the top 10 genes for each subtype ordered by the area under the curve (AUC) values. CD8A and FOXC1 were the top‐ranked genes in the IM and BLIS subtypes, respectively. Androgen receptor (AR) was ranked 5th but was selected as the marker for the LAR subtype owing to its clinical implications and confirmed practicability of detection by IHC. As for the MES subtype, ANK2 and DCLK1 were ranked 1st and 2nd, respectively. We performed immunohistochemical staining for both markers. We observed irregular ANK2 staining in both tumor cells and tumor stroma, which was difficult to quantify. By contrast, the staining of DCLK1 was primarily found in the cytoplasm of tumor cells and was easier to quantify. In addition, DCLK1 was reported to play a metastatic‐promoting role in breast cancer cell lines and was identified as a cancer stem cell marker in several types of cancer, which indicated potential clinical implications of this marker 18, 19, 20, 21. Thus, we chose DCLK1 as the marker for the MES subtype. A positive correlation was observed between each gene's protein expression and mRNA expression (supplemental online Fig. 2). In our FUSCC TNBC data set, all four of these genes were differentially expressed among the four mRNA‐based subtypes (supplemental online Fig. 3A). The ROC curves for using their mRNA expression values to identify the corresponding subtypes are presented in supplemental online Figure 3B. Our previous study extrapolated our mRNA‐based classification to the 158 TNBC cases from The Cancer Genome Atlas (TCGA) data set. This TCGA TNBC data set was used as an independent validation set. We validated the differential expression of these four genes among the mRNA‐based subtypes (Fig. 1A). The AUC of using AR mRNA expression to identify the LAR subtype was 0.912 (95% confidence interval [CI], 0.853–0.972), the AUC of using CD8A mRNA expression to identify the IM subtype was 0.814 (95% CI, 0.738–0.890), the AUC of using FOXC1 mRNA expression to identify the BLIS subtype was 0.718 (95% CI, 0.639–0.797), and the AUC of using DCLK1 mRNA expression to identify the MES subtype was 0.785 (95% CI, 0.699–0.870; Fig. 1B).
Figure 1.
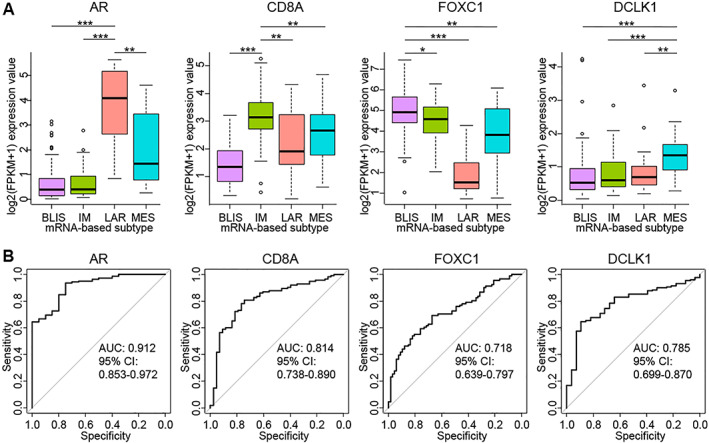
Independent validation of differential expression of AR, CD8A, FOXC1 and DCLK1 among the four mRNA‐based subtypes in the TCGA TNBC data set. (A): mRNA expression of AR, CD8A, FOXC1, and DCLK1 according to the mRNA‐based classification in the TCGA TNBC data set (BLIS, n = 67; IM, n = 43; LAR, n = 20; MES, n = 28). The p values are calculated using the Mann‐Whitney U test. (B): Receiver operating characteristic curves for using the mRNA expression of AR, CD8A, FOXC1, and DCLK1 to identify the corresponding subtypes in the TCGA TNBC data set. The sample number for each ROC curve is 158. ***p < .001, **p < .01, *p < .05.
Abbreviations: AR, androgen receptor; AUC, area under the curve; BLIS, basal‐like immune‐suppressed; CI, confidence interval; FPKM, fragments per kilobase of exon per million mapped fragments; IM, immunomodulatory; LAR, luminal androgen receptor; MES, mesenchymal.
Results of Immunohistochemical Staining and Classification of TNBCs into Subtypes
To examine whether the protein expression of these four genes as assessed by IHC can also be used to identify the corresponding mRNA‐based subtypes, we performed immunohistochemical staining on the paraffin‐embedded sections of 210 TNBCs of known mRNA‐based subtypes. Each gene's protein expression as assessed by IHC showed a positive correlation with its mRNA expression (supplemental online Fig. 4). Similar to the mRNA expression levels, the protein expression levels of the four markers significantly differed among the four subtypes and can be used to accurately identify the corresponding subtypes (Fig. 2A, B). The scanned images of a representative case of each mRNA‐based subtype were shown in supplemental online Data Set.
Figure 2.

Identification of the LAR, IM, BLIS and MES subtypes by the protein expression of AR, CD8, FOXC1 and DCLK1 detected by immunohistochemistry, respectively. (A): Protein expression of AR, CD8, FOXC1, and DCLK1 detected by immunohistochemistry according to the mRNA‐based classification (BLIS, n = 82; IM, n = 45; LAR, n = 55; MES, n = 28). The p values are calculated using the Mann‐Whitney U test. (B): Receiver operating characteristic (ROC) curves for using the protein expression of AR, CD8A, FOXC1, and DCLK1 detected by immunohistochemistry to identify the corresponding subtypes. The sample number for each ROC curve is 210. ***p < .001, *p < .05.
Abbreviations: AR, androgen receptor; AUC, area under the curve; BLIS, basal‐like immune‐suppressed; CI, confidence interval; FPKM, fragments per kilobase of exon per million mapped fragments; IM, immunomodulatory; LAR, luminal androgen receptor; MES, mesenchymal.
Next, we developed our IHC‐based classification method and classified the 210 TNBCs into subtypes. We first determined the order of precedence of the four markers in the classification method according to the AUC of using their protein expression to identify the corresponding subtypes. Then, according to Youden's index, we employed a cutoff of ≥10% positive tumor cells to define AR positivity, FOXC1 positivity, and DCLK1 positivity and another cutoff of ≥20% positive cells to define CD8 positivity (supplemental online Fig. 5). Finally, the 210 TNBCs were classified into the following subtypes: (a) IHC‐LAR: AR‐positive(+); (b) IHC‐IM: AR‐negative(−) and CD8+; (c) IHC‐BLIS: AR−, CD8−, and FOXC1+; (d) IHC‐MES: AR−, CD8−, FOXC1− and DCLK1+; (e) IHC‐unclassifiable (IHC‐UC): AR−, CD8−, FOXC1−, and DCLK1− (Fig. 3A).
Figure 3.
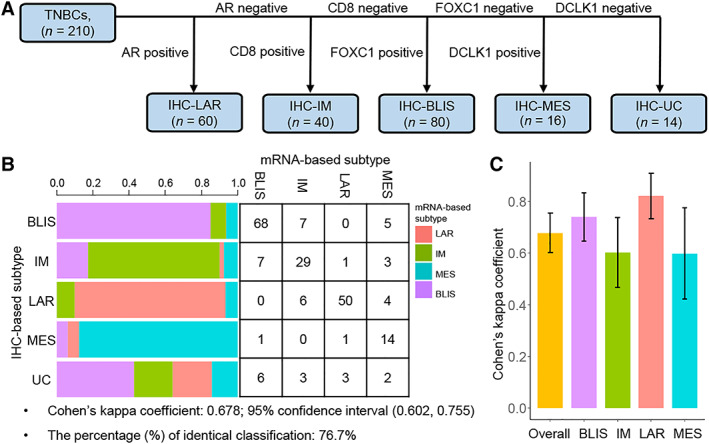
Analysis of agreement between the IHC‐based classification and the mRNA‐based classification. (A): IHC‐based TNBC classification method. (B): Agreement between the IHC‐based classification and the mRNA‐based classification. (C): Agreement between the IHC‐based classification and the mRNA‐based classification for each subtype.
Abbreviations: AR, androgen receptor; BLIS, basal‐like immune‐suppressed; IHC, immunohistochemistry; IM, immunomodulatory; LAR, luminal androgen receptor; MES, mesenchymal; TNBC, triple‐negative breast cancer; UC, unclassifiable.
Agreement Between the IHC‐Based Classification and the mRNA‐Based Classification
We performed κ analysis to evaluate the agreement between the IHC‐based classification and the mRNA‐based classification. The overall Cohen's κ coefficient was 0.678 (95% CI, 0.602–0.755), which indicated substantial agreement between the two classifiers (Fig. 3B). The percentage of samples classified as the same subtype by both classification methods was 76.7%. Additionally, Cohen's κ coefficient for each subtype was calculated. The two classification methods showed the highest agreement in the classification of TNBCs as the LAR subtype (Cohen's κ coefficient [κ] = 0.821; 95% CI, 0.733–0.908), substantial agreement in the classification of TNBCs as the BLIS subtype (κ = 0.739; 95% CI, 0.645–0.833) and the IM subtype (κ = 0.602; 95% CI, 0.467–0.737), and moderate agreement in the classification of TNBCs as the MES subtype (κ = 0.597; 95% CI, 0.421–0.774; Fig. 3C).
Clinicopathological Features and Prognoses of Different IHC‐Based Subtypes
We compared the differences in clinicopathological features among the five IHC‐based subtypes (supplemental online Table 9). Patients with IHC‐LAR TNBC were older than those with TNBC of the other subtypes. The Ki‐67 index was lower in the IHC‐LAR subtype than in the other subtypes. The differences in T category, N category, and tumor grade among the five IHC‐based subtypes were not statistically significant.
We next investigated the prognostic value of our IHC‐based classification. We observed significant differences in relapse‐free survival (RFS) among the five IHC‐based subtypes (log‐rank p = .019; Fig. 4A). After adjustment for other prognostic factors in multivariate analysis, the IHC‐IM (hazard ratio [HR], 0.07; p = .002), IHC‐LAR (HR, 0.18; p = .004), and IHC‐BLIS (HR, 0.26; p = .014) subtypes were associated with better RFS than the IHC‐MES subtype (supplemental online Table 10).
Figure 4.
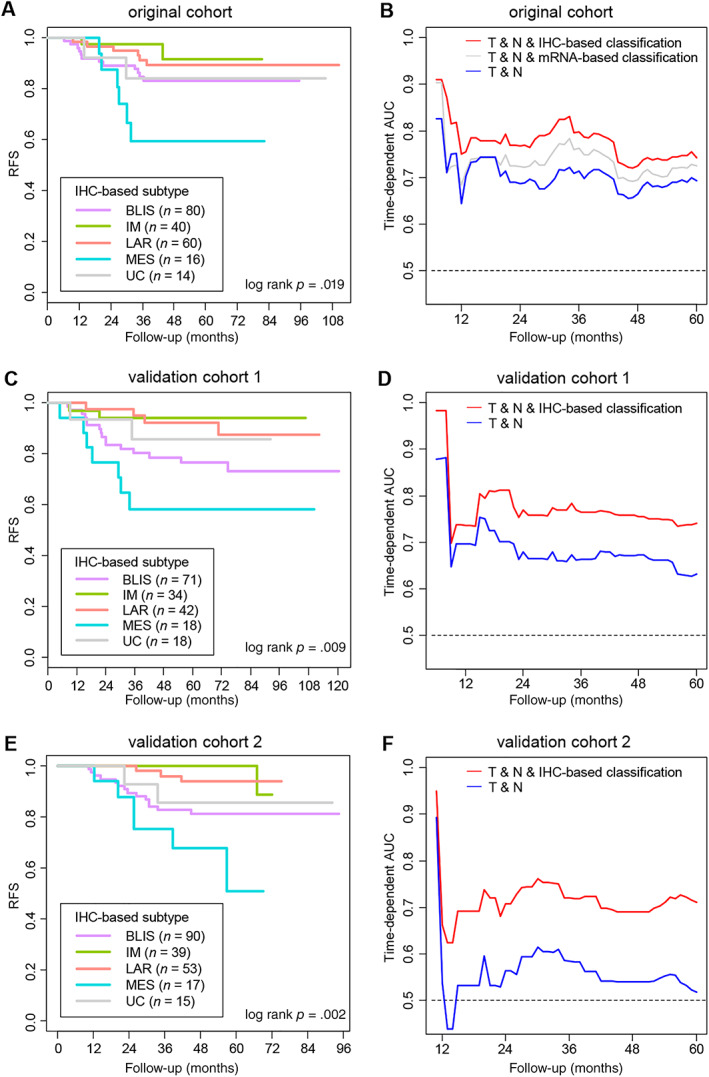
Prognostic value of the IHC‐based classification of TNBC. (A): Kaplan‐Meier curves for RFS according to the IHC‐based classification in the original cohort (n = 210). (B): Time‐dependent AUC of multivariate Cox models to predict relapse in the original cohort (n = 210). (C): Kaplan‐Meier curves for RFS according to the IHC‐based classification in the validation cohort 1 (n = 183). (D): Time‐dependent AUC of the multivariate Cox models established in the original cohort to predict relapse in the validation cohort 1 (n = 183). (E): Kaplan‐Meier curves for RFS according to the IHC‐based classification in the validation cohort 2 (n = 214). (F): Time‐dependent AUC of the multivariate Cox models established in the original cohort to predict relapse in the validation cohort 2 (n = 214). “T” and “N” refer to the pathological T category and pathological N category of tumors, respectively.
Abbreviations: AUC, area under the curve; BLIS, basal‐like immune‐suppressed; IHC, immunohistochemistry; IM, immunomodulatory; LAR, luminal androgen receptor; MES, mesenchymal; RFS, relapse‐free survival; UC, unclassifiable.
We further examined whether the integration of IHC‐based classification and the traditional prognostic factors can add to the accuracy of relapse prediction. We performed time‐dependent ROC analysis and used time‐dependent AUC to measure the accuracy of multivariate Cox models to predict relapse. The model integrating T category and N category and the IHC‐based classification showed higher accuracy than the model only including T category and N category (comparison of time‐dependent AUC: p < .05 during 27–43 months of follow‐up; Fig. 4B; supplemental online Fig. 6A).
To ensure the reliability of our results, we employed two independent validation cohorts. The validation cohort 1 was composed of another 183 patients with TNBC treated at FUSCC during 2007–2014 (supplemental online Table 11). The validation cohort 2 comprised 214 patients with TNBC treated at Harbin Medical University Cancer Hospital during 2011–2014 (supplemental online Table 12). Tumor samples of patients in these two cohorts were classified into IHC‐based subtypes according to our classification method. In both of the validation cohorts, patients with IHC‐MES TNBC had worse RFS than those with TNBC of other subtypes (validation cohort 1, log‐rank p = .009; validation cohort 2, log‐rank p = .002; Fig. 4C, E). In addition, the Cox models established in our original cohort were applied to the validation cohorts. The integrated Cox model still exhibited superior accuracy of relapse prediction (comparison of time‐dependent AUC: validation cohort 1, p < .05 during 24–60 months of follow‐up; validation cohort 2, p < .05 during 11–60 months of follow‐up; Fig. 4D, F; supplemental online Fig. 6B, C).
Relatively High HER2 Pathway Activity in the IHC‐LAR Subtype
We next explored potential therapeutic strategies for different IHC‐based subtypes based on their molecular and pathological features. The IHC‐LAR subtype showed relatively high ERBB2 mRNA expression compared with the other subtypes. The score of an established HER2 signature was also higher in the IHC‐LAR subtype than in the other subtypes (Fig. 5A) 22, 23. In addition, we determined the intrinsic subtypes of TNBCs with RNA sequencing data in our study using the “genefu” package 24. Although all tumors in our study were clinically HER2‐negative according to the guideline for HER2 testing in breast cancer 25, 48.3% of the IHC‐LAR TNBCs were classified as the HER2‐enriched subtype (Fig. 5B). These data suggested relative HER2 signaling pathway activation in the IHC‐LAR subtype despite the negative results of clinical HER2 testing.
Figure 5.
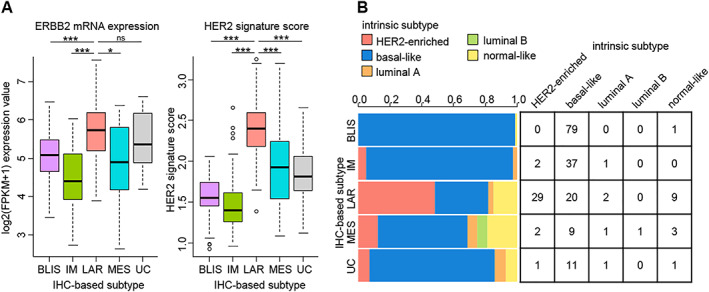
Relatively high HER2 pathway activity in the IHC‐LAR subtype. (A): ERBB2 mRNA expression levels and HER2 signature scores in the five IHC‐based subtypes (IHC‐BLIS, n = 80; IHC‐IM, n = 40; IHC‐LAR, n = 60; IHC‐MES, n = 16; IHC‐UC, n = 14). The p values are calculated using the Mann‐Whitney U test. (B): Distribution of breast cancer intrinsic subtypes in the five IHC‐based subtypes. ***p < .001, *p < .05; ns, not significant.
Abbreviations: BLIS, basal‐like immune‐suppressed; FPKM, fragments per kilobase of exon per million mapped fragments; IHC, immunohistochemistry; IM, immunomodulatory; LAR, luminal androgen receptor; MES, mesenchymal; UC, unclassifiable.
High Expression of Immune Checkpoint Molecules and High Percentage of the Immune‐Inflamed Phenotype in the IHC‐IM Subtype
Similar to the IM tumors according to the mRNA‐based classification, IHC‐IM tumors also showed high levels of both stromal tumor‐infiltrating lymphocytes and intratumoral tumor‐infiltrating lymphocytes (Fig. 6A). The expression of several immune checkpoint molecules and immunostimulatory molecules was also higher in the IHC‐IM subtype than in the other subtypes (Fig. 6B).
Figure 6.
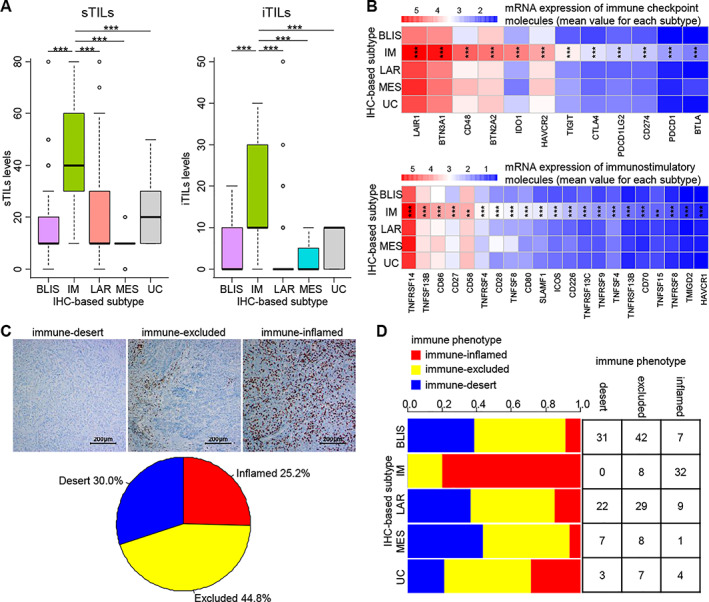
Potential efficacy of immune checkpoint inhibitor therapy for the IHC‐IM subtype. (A): Tumor‐infiltrating lymphocyte levels in the five IHC‐based subtypes (IHC‐BLIS, n = 80; IHC‐IM, n = 40; IHC‐LAR, n = 60; IHC‐MES, n = 16; IHC‐UC, n = 14). The p values are calculated using the Mann‐Whitney U test. (B): The mRNA expression of immune checkpoint molecules and immunostimulatory molecules according to the IHC‐based classification (IHC‐BLIS, n = 80; IHC‐IM, n = 40; IHC‐LAR, n = 60; IHC‐MES, n = 16; IHC‐UC, n = 14). The p values are calculated using the Kruskal‐Wallis test. (C): Immune phenotypes (desert, n = 63; excluded, n = 94; inflamed, n = 53) of TNBC defined by the CD8 immunohistochemical staining (at ×100 magnification). (D): Distribution of immune phenotypes in the five IHC‐based subtypes. The p values are calculated using Pearson's chi‐square test. ***p < .001.
Abbreviations: BLIS, basal‐like immune‐suppressed; IM, immunomodulatory; IHC, immunohistochemistry; iTIL, intratumoral tumor‐infiltrating lymphocyte; LAR, luminal androgen receptor; MES, mesenchymal; PD‐1, programmed cell death 1; sTIL, stromal tumor‐infiltrating lymphocyte; UC, unclassifiable.
Most human solid tumors exhibit one of three immune phenotypes: immune‐desert, immune‐excluded, and immune‐inflamed 26. The tumor's immune phenotype has been revealed to be closely associated with the tumor response to immunotherapy 26, 27, 28, 29. Based on CD8 immunohistochemical staining, we classified the 210 TNBC tumors into immune phenotypes 28 and illustrated the distribution of immune phenotypes according to the IHC‐based subtype (Fig. 6C, D). A total of 80.0% of IHC‐IM tumors exhibited the immune‐inflamed phenotype, whereas tumors of the other subtypes mainly showed the immune‐excluded phenotype (distribution of immune phenotypes, p < .001).
High Homologous Recombination Deficiency Scores and High Expression of a VEGF Signature in the IHC‐BLIS Subtype
In our previous study, we reported relatively high homologous recombination deficiency (HRD) scores in the mRNA‐based BLIS subtype 9. This feature was preserved in the IHC‐BLIS subtype (Fig. 7A). In addition, we calculated a VEGF signature score 30 and found that this score in the IHC‐BLIS subtype was higher than in the other subtypes (Fig. 7B). This result suggested that antiangiogenesis therapy might be a choice for patients with IHC‐BLIS TNBC.
Figure 7.
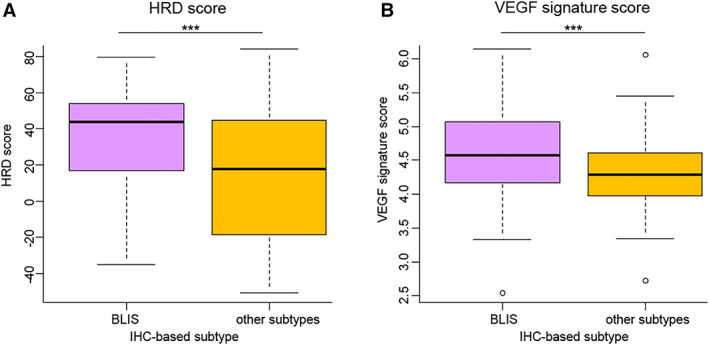
HRD scores and high expression of a VEGF signature in the IHC‐BLIS subtype. (A): HRD scores in the IHC‐BLIS subtype (n = 72) and in the other subtypes (n = 101). The p values are calculated using the Mann‐Whitney U test. (B): VEGF signature scores in the IHC‐BLIS subtype (n = 80) and in the other subtypes (n = 130). The p values are calculated using the Mann‐Whitney U test. ***p < .001.
Abbreviations: BLIS, basal‐like immune‐suppressed; HRD, homologous recombination deficiency; IHC, immunohistochemistry.
Stem Cell‐Like Characteristics of the IHC‐MES Subtype
In all of our three cohorts, patients with IHC‐MES TNBC had poorer RFS than those with TNBC of other subtypes. Gene set enrichment analysis demonstrated enrichment of stem cell‐related signatures in the IHC‐MES subtype (supplemental online Fig. 7A). We further examined the JAK/STAT3 signaling pathway, which is known to play an important role in the growth of stem cell‐like human breast cancer cells 31. We found that the IHC‐MES subtype showed higher mRNA expression of both IL6 and JAK1, which are crucial upstream activators of the JAK/STAT3 pathway 32. In addition, we calculated a phosphorylated STAT3 signature score defined by Sonnenblick et al. 33. The score was also higher in the IHC‐MES subtype than in the other subtypes (supplemental online Fig. 7B). These data indicated stem cell‐like characteristics of IHC‐MES TNBCs.
Discussion
By taking full advantage of the gene expression profiling data and tumor tissue samples of a large cohort of patients with TNBC, we developed a surrogate method based on IHC for the molecular classification of TNBC and assessed the agreement between the IHC‐based classification and the mRNA‐based classification. Further analyses demonstrated that the IHC‐based classification had considerable prognostic value and suggested subtype‐specific therapeutic strategies.
The selection of immunohistochemical markers is a crucial step in the development of IHC‐based classification method. We identified candidate molecules through differential expression analysis, mRNA/protein correlation, and ROC analyses. In addition to performing these statistical analyses, we considered the feasibility of immunohistochemical detection and potential clinical implications. Immunohistochemical detection of AR has been performed in numerous studies. More importantly, several studies have indicated the potential of androgen blockade as a treatment option for AR‐positive TNBC 34, 35. CD8 is an extensively studied immune marker, and its expression is associated with patient prognosis and tumor response to immunotherapy 27, 28. FOXC1 has also been detected by IHC in basal‐like or triple‐negative breast cancers in several studies, and high FOXC1 expression is associated with aggressive tumor biological behavior and poor prognosis 36, 37. One previous study reported cytoplasmic immunoreactivity of DCLK1 in breast cancer tumor cells but not in surrounding stroma 38. This was consistent with our staining results. Additionally, in several types of cancer, DCLK1 has been recognized as a marker of cancer stem cells that may serve as a potential therapeutic target 19, 20, 21. Among these four markers, only DCLK1 shows a cytoplasmic staining pattern, which poses some challenges in the interpretation of staining results. A unified method for immunohistochemical staining of DCLK1 in clinical laboratories and for staining results interpretation should be developed before this marker can be applied in clinical practice.
One recent study established a surrogate IHC panel for the molecular classification of TNBC and examined the relationship between the IHC‐based subtypes and patients’ clinicopathological features 39. However, in that study, the researchers did not perform gene expression profiling on the samples. Thus, they were unable to assess to what extent their IHC‐based classification agreed with the “gold standard” (i.e., the mRNA‐based classification). By contrast, our study was based on a large data set of comprehensively profiled TNBCs. We selected the classification markers by analyzing the mRNA expression data of 360 TNBC samples. The κ statistic indicated substantial agreement between our IHC‐based classification and the mRNA‐based classification (κ = 0.678). Although the current Cohen's κ coefficient is satisfactory for research, additional work is required before wide clinical implementation of our IHC‐based classification. First, a following study should be conducted to assess the analytical validity of our immunohistochemical panel by testing its reproducibility and robustness. Second, the cutoff values of the immunohistochemical markers may be further optimized by applying our immunohistochemical panel to more TNBC samples in larger scale study.
Our IHC‐based TNBC classification can help stratify patient prognosis. RFS differed significantly among the IHC‐based subtypes. Multivariate survival analysis indicated that the IHC‐based classification was an independent prognostic factor for RFS. In addition, the use of IHC‐based classification in combination with T category and N category can improve the accuracy of relapse prediction. It should be noted that although there is difference between the original cohort and the validation cohorts, especially validation cohort 2, in clinicopathological features including tumor grade, T category, and N category, our IHC‐based classifier can help stratify patient prognosis in all three cohorts. This indicates that the prognostic value of our classifier can hold up across different clinical settings.
Most molecular features and therapeutic implications of different mRNA‐based subtypes were preserved in the corresponding IHC‐based subtypes. The IHC‐based classification also provided some novel insights to advance the understanding and precision treatment of TNBC. Tumors of the IHC‐LAR subtype were identified by positive immunohistochemical staining of AR, a nuclear transcription factor that regulates gene expression and affects cellular proliferation and differentiation 40, 41. Interestingly, despite the negative results of HER2 testing by IHC and fluorescence in situ hybridization, nearly half of IHC‐LAR TNBCs were categorized as the HER2‐enriched subtype according to the intrinsic subtyping of breast cancer. This result was consistent with the finding of a previous study by Santonja et al., in which they classified TNBC tumors into intrinsic subtypes and found that 4 out of 10 LAR tumors were categorized as the HER2‐enriched subtype 42. In addition, the IHC‐LAR subtype exhibited relatively high levels of ERBB2 mRNA expression and HER2 pathway activity. These data indicated that some IHC‐LAR tumors may respond to certain HER2 tyrosine kinase inhibitors (e.g., lapatinib) 43.
Tumors of the IHC‐IM subtype were characterized by high levels of CD8+ T cells. CD8+ T cells are a crucial component of adaptive immune system and play a major role in antitumor immunity 26. The IHC‐IM subtype showed relatively high expression of immune checkpoint molecules such as CTLA4, CD274 (PD‐L1), IDO1, and PDCD1 (PD‐1). Immunohistochemical staining of CD8 could be used not only to identify tumors of the IHC‐IM subtype but also to show the pattern of immune infiltration and to classify tumors into immune phenotypes 27, 28. The majority of IHC‐IM tumors showed an immune‐inflamed phenotype characterized by the infiltration of CD8+ TILs into the tumor parenchyma. These features suggest that patients with IHC‐IM TNBC are more likely to benefit from immune checkpoint inhibitor therapy 44.
Tumors of the IHC‐BLIS subtype were discriminated by high expression of FOXC1. FOXC1, a member of the forkhead box family, regulates several biological processes, including cell growth, proliferation, differentiation, migration, and survival 45, 46. It has also been reported to be a possible regulator in several types of cancer 47, 48, 49. In our study, in addition to a high HRD score and high genomic instability 9, the IHC‐BLIS subtype was also characterized by high expression of a VEGF signature, which was associated with tumor angiogenesis and poor prognosis 30. This subtype might be sensitive to angiogenesis inhibitors, such as apatinib and bevacizumab.
Tumors of the IHC‐MES subtype were identified by positive DCLK1 staining. DCLK1 is a microtubule‐associated gene and plays an important role in early neurogenesis 50, 51. It has also been reported as a tumor stem cell marker 20, 21, 52. The IHC‐MES subtype was characterized by the enrichment of stem cell‐related gene signatures. The activated JAK/STAT3 pathway may be a potential therapeutic target.
In each of the three cohorts, approximately 5%–10% samples were negative for all the four immunohistochemical markers and were therefore classified as the IHC‐unclassifiable subtype. The failure of classifying these tumors into specific subtypes may be attributed to the false‐negative results of immunohistochemical detection of certain markers.
Our study has several limitations. First, our IHC‐based classification does not achieve perfect agreement with the mRNA‐based classification because of the discrepancy between the markers’ mRNA expression and protein expression and because of spatial tumor heterogeneity. Second, therapeutic implications of our IHC‐based classification are primarily indicated by data analysis and need to be validated by experimental research or prospective clinical studies. Our center has carried out a phase Ib/II clinical trial (FUSCC Refractory TNBC Umbrella trial, FUTURE trial, NCT03805399) to treat patients with advanced TNBC with subtype‐specific therapy according to the IHC‐based classification of their target lesions. We will use the data from the FUTURE trial to further validate the clinical relevance of our IHC‐based classification. Third, an independent cohort with both RNA sequencing data and tumor tissue samples may be required to validate the agreement between our IHC‐based classification and the mRNA‐based classification.
Conclusion
We developed a clinically applicable method based on IHC to classify TNBCs into molecular subtypes. We demonstrate that the result of our IHC‐based classification exhibits substantial agreement with that of the mRNA expression‐based classification. Our IHC‐based classification allows for subgrouping of patients with TNBC in large clinical trials and evaluating the efficacy of targeted therapies within certain subtypes. It will contribute to realizing the subtype‐specific treatment for patients with TNBC.
Author Contributions
Conception/design: Shen Zhao, Wen‐Tao Yang, Yi‐Zhou Jiang, Qing‐Yuan Zhang, Zhi‐Ming Shao
Provision of study material or patients: Shen Zhao, Ding Ma, Yi Xiao, Xiao‐Mei Li, Jian‐Li Ma, Han Zhang, Xiao‐Li Xu, Hong Lv, Wen‐Hua Jiang, Qing‐Yuan Zhang, Zhi‐Ming Shao
Collection and/or assembly of data: Shen Zhao, Ding Ma, Yi Xiao, Xiao‐Mei Li, Jian‐Li Ma, Han Zhang, Qing‐Yuan Zhang, Zhi‐Ming Shao
Data analysis and interpretation: Shen Zhao, Ding Ma, Yi Xiao
Manuscript writing: Shen Zhao, Ding Ma, Yi Xiao, Xiao‐Mei Li, Jian‐Li Ma
Final approval of manuscript: Shen Zhao, Ding Ma, Yi Xiao, Xiao‐Mei Li, Jian‐Li Ma, Han Zhang, Xiao‐Li Xu, Hong Lv, Wen‐Hua Jiang, Wen‐Tao Yang, Yi‐Zhou Jiang, Qing‐Yuan Zhang, Zhi‐Ming Shao
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supplementary Methods
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81874112, 81502278, 81874113, and 81572583), Shanghai Pujiang Program (18PJD007), the Training Plan of Excellent Talents of Fudan University Shanghai Cancer Center (YJYQ201602), and the Shanghai Key Laboratory of Breast Cancer (12DZ2260100). The funders had no role in the study design, data collection and analysis, or manuscript preparation.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Contributor Information
Wen‐Tao Yang, Email: yangwt2000@163.com.
Yi‐Zhou Jiang, Email: yizhoujiang@fudan.edu.cn.
Qing‐Yuan Zhang, Email: zhma19650210@163.com.
Zhi‐Ming Shao, Email: zhimingshao@yahoo.com.
References
- 1. Bauer KR, Brown M, Cress RD et al. Descriptive analysis of estrogen receptor (ER)‐negative, progesterone receptor (PR)‐negative, and HER2‐negative invasive breast cancer, the so‐called triple‐negative phenotype: A population‐based study from the California cancer Registry. Cancer 2007;109:1721–1728. [DOI] [PubMed] [Google Scholar]
- 2. Criscitiello C, Azim HA Jr., Schouten PC et al. Understanding the biology of triple‐negative breast cancer. Ann Oncol 2012;23(suppl 6):vi13–vi18. [DOI] [PubMed] [Google Scholar]
- 3. Carey LA, Perou CM, Livasy CA et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492–2502. [DOI] [PubMed] [Google Scholar]
- 4. Liedtke C, Mazouni C, Hess KR et al. Response to neoadjuvant therapy and long‐term survival in patients with triple‐negative breast cancer. J Clin Oncol 2008;26:1275–1281. [DOI] [PubMed] [Google Scholar]
- 5. Lehmann BD, Bauer JA, Chen X et al. Identification of human triple‐negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Metzger‐Filho O, Tutt A, de Azambuja E et al. Dissecting the heterogeneity of triple‐negative breast cancer. J Clin Oncol 2012;30:1879–1887. [DOI] [PubMed] [Google Scholar]
- 7. Burstein MD, Tsimelzon A, Poage GM et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple‐negative breast cancer. Clin Cancer Res 2015;21:1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu YR, Jiang YZ, Xu XE et al. Comprehensive transcriptome analysis identifies novel molecular subtypes and subtype‐specific RNAs of triple‐negative breast cancer. Breast Cancer Res 2016;18:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang YZ, Ma D, Suo C et al. Genomic and transcriptomic landscape of triple‐negative breast cancers: Subtypes and treatment strategies. Cancer Cell 2019;35:428–440.e5. [DOI] [PubMed] [Google Scholar]
- 10. Perou CM, Sørlie T, Eisen MB et al. Molecular portraits of human breast tumours. Nature 2000;406:747–752. [DOI] [PubMed] [Google Scholar]
- 11. Sørlie T, Perou CM, Tibshirani R et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001;98:10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nielsen TO , Hsu FD, Jensen K et al. Immunohistochemical and clinical characterization of the basal‐like subtype of invasive breast carcinoma. Clin Cancer Res 2004;10:5367–5374. [DOI] [PubMed] [Google Scholar]
- 13. Cheang MC, Voduc D, Bajdik C et al. Basal‐like breast cancer defined by five biomarkers has superior prognostic value than triple‐negative phenotype. Clin Cancer Res 2008;14:1368–1376. [DOI] [PubMed] [Google Scholar]
- 14. Cheang MC, Chia SK, Voduc D et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldhirsch A, Wood WC, Coates AS et al. Strategies for subtypes‐‐Dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Talhouk A, McConechy MK, Leung S et al. A clinically applicable molecular‐based classification for endometrial cancers. Br J Cancer 2015;113:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Talhouk A, McConechy MK, Leung S et al. Confirmation of ProMisE: A simple, genomics‐based clinical classifier for endometrial cancer. Cancer. 2017;123:802–813. [DOI] [PubMed] [Google Scholar]
- 18. Liu H, Wen T, Zhou Y et al. DCLK1 Plays a Metastatic‐Promoting Role in Human Breast Cancer Cells. Biomed Res Int 2019;2019:1061979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kantara C, O'Connell M, Sarkar S et al. Curcumin promotes autophagic survival of a subset of colon cancer stem cells, which are ablated by DCLK1‐siRNA. Cancer Res 2014;74:2487–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakanishi Y, Seno H, Fukuoka A et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet 2013;45:98–103. [DOI] [PubMed] [Google Scholar]
- 21. Bailey JM, Alsina J, Rasheed ZA et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology 2014;146:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Desmedt C, Haibe‐Kains B, Wirapati P et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res 2008;14:5158–5165. [DOI] [PubMed] [Google Scholar]
- 23. Desmedt C, Zoppoli G, Gundem G et al. Genomic characterization of primary invasive lobular breast cancer. J Clin Oncol 2016;34:1872–1881. [DOI] [PubMed] [Google Scholar]
- 24. Gendoo DM, Ratanasirigulchai N, Schroder MS et al. Genefu: An R/Bioconductor package for computation of gene expression‐based signatures in breast cancer. Bioinformatics 2016;32:1097–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolff AC, Hammond MEH, Allison KH et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018;36:2105–2122. [DOI] [PubMed] [Google Scholar]
- 26. Chen DS, Mellman I. Elements of cancer immunity and the cancer‐immune set point. Nature 2017;541:321–330. [DOI] [PubMed] [Google Scholar]
- 27. Herbst RS, Soria JC, Kowanetz M et al. Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mariathasan S, Turley SJ, Nickles D et al. TGFβ attenuates tumour response to PD‐L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim JM, Chen DS. Immune escape to PD‐L1/PD‐1 blockade: Seven steps to success (or failure). Ann Oncol 2016;27:1492–1504. [DOI] [PubMed] [Google Scholar]
- 30. Hu Z, Fan C, Livasy C et al. A compact VEGF signature associated with distant metastases and poor outcomes. BMC Med 2009;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marotta LL, Almendro V, Marusyk A et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(‐) stem cell‐like breast cancer cells in human tumors. J Clin Invest 2011;121:2723–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Balko JM, Schwarz LJ, Luo N et al. Triple‐negative breast cancers with amplification of JAK2 at the 9p24 locus demonstrate JAK2‐specific dependence. Sci Translatl Med 2016;8:334ra353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sonnenblick A, Brohée S, Fumagalli D et al. Constitutive phosphorylated STAT3‐associated gene signature is predictive for trastuzumab resistance in primary HER2‐positive breast cancer. BMC Med 2015;13:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bonnefoi H, Grellety T, Tredan O et al. A phase II trial of abiraterone acetate plus prednisone in patients with triple‐negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12‐1). Ann Oncol 2016;27:812–818. [DOI] [PubMed] [Google Scholar]
- 35. Traina TA, Miller K, Yardley DA et al. Enzalutamide for the treatment of androgen receptor‐expressing triple‐negative breast cancer. J Clin Oncol 2018;36:884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jensen TW, Ray T, Wang J et al. Diagnosis of basal‐like breast cancer using a foxc1‐based assay. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ray PS, Wang J, Qu Y et al. FOXC1 is a potential prognostic biomarker with functional significance in basal‐like breast cancer. Cancer Res 2010;70:3870–3876. [DOI] [PubMed] [Google Scholar]
- 38. Liu YH, Tsang JY, Ni YB et al. Doublecortin‐like kinase 1 expression associates with breast cancer with neuroendocrine differentiation. Oncotarget 2016;7:1464–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim S, Moon BI, Lim W et al. Feasibility of classification of triple negative breast cancer by immunohistochemical surrogate markers. Clin Breast Cancer 2018;18:e1123–e1132. [DOI] [PubMed] [Google Scholar]
- 40. Giovannelli P, Di Donato M, Galasso G et al. The androgen receptor in breast cancer. Front Endocrinol (Lausanne) 2018;9:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tan MH, Li J, Xu HE et al. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin 2015;36:3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Santonja A, Sánchez‐Muñoz A, Lluch A et al. Triple negative breast cancer subtypes and pathologic complete response rate to neoadjuvant chemotherapy. Oncotarget 2018;9:26406–26416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leary A, Evans A, Johnston SR et al. Antiproliferative effect of lapatinib in HER2‐positive and HER2‐negative/HER3‐high breast cancer: Results of the presurgical randomized MAPLE trial (CRUK E/06/039). Clin Cancer Res 2015;21:2932–2940. [DOI] [PubMed] [Google Scholar]
- 44. Schmid P, Adams S, Rugo HS et al. Atezolizumab and nab‐paclitaxel in advanced triple‐negative breast cancer. N Engl J Med 2018;379:2108–2121. [DOI] [PubMed] [Google Scholar]
- 45. Lehmann OJ, Sowden JC, Carlsson P et al. Fox's in development and disease. Trends Genet 2003;19:339–344. [DOI] [PubMed] [Google Scholar]
- 46. Lam EW, Brosens JJ, Gomes AR et al. Forkhead box proteins: Tuning forks for transcriptional harmony. Nat Rev Cancer 2013;13:482–495. [DOI] [PubMed] [Google Scholar]
- 47. Peraldo‐Neia C, Migliardi G, Mello‐Grand M et al. Epidermal Growth Factor Receptor (EGFR) mutation analysis, gene expression profiling and EGFR protein expression in primary prostate cancer. BMC Cancer 2011;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sizemore ST, Keri RA. The forkhead box transcription factor FOXC1 promotes breast cancer invasion by inducing matrix metalloprotease 7 (MMP7) expression. J Biol Chem 2012;287:24631–24640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xia L, Huang W, Tian D et al. Overexpression of forkhead box C1 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Hepatology 2013;57:610–624. [DOI] [PubMed] [Google Scholar]
- 50. Westphalen CB, Quante M, Wang TC. Functional implication of Dclk1 and Dclk1‐expressing cells in cancer. Small GTPases 2017;8:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shu T, Tseng HC, Sapir T et al. Doublecortin‐like kinase controls neurogenesis by regulating mitotic spindles and M phase progression. Neuron 2006;49:25–39. [DOI] [PubMed] [Google Scholar]
- 52. Chandrakesan P, Yao J, Qu D et al. Dclk1, a tumor stem cell marker, regulates pro‐survival signaling and self‐renewal of intestinal tumor cells. Mol Cancer 2017;16:30. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supplementary Methods


