Abstract
Background
The predictive model of postsurgical recurrence for solitary early hepatocellular carcinoma (SE‐HCC) is not well established. The aim of this study was to develop a novel model for prediction of postsurgical recurrence and survival for patients with hepatitis B virus (HBV)‐related SE‐HCC ≤10 cm.
Patients and Methods
Data from 1,081 patients with HBV‐related SE‐HCC ≤10 cm who underwent curative liver resection from 2003 to 2016 in our center were collected retrospectively and randomly divided into the derivation cohort (n = 811) and the internal validation cohort (n = 270). Eight hundred twenty‐three patients selected from another four tertiary hospitals served as the external validation cohort. Postsurgical recurrence‐free survival (RFS) and overall survival (OS) predictive nomograms were generated. The discriminatory accuracies of the nomograms were compared with six conventional hepatocellular carcinoma (HCC) staging systems.
Results
Tumor size, differentiation, microscopic vascular invasion, preoperative α‐fetoprotein, neutrophil‐to‐lymphocyte ratio, albumin‐to‐bilirubin ratio, and blood transfusion were identified as the risk factors associated with RFS and OS. RFS and OS predictive nomograms based on these seven variables were generated. The C‐index was 0.83 (95% confidence interval [CI], 0.79–0.87) for the RFS‐nomogram and 0.87 (95% CI, 0.83–0.91) for the OS‐nomogram. Calibration curves showed good agreement between actual observation and nomogram prediction. Both C‐indices of the two nomograms were substantially higher than those of the six conventional HCC staging systems (0.54–0.74 for RFS; 0.58–0.76 for OS) and those of HCC nomograms reported in literature.
Conclusion
The novel nomograms were shown to be accurate at predicting postoperative recurrence and OS for patients with HBV‐related SE‐HCC ≤10 cm after curative liver resection.
Implications for Practice
This multicenter study proposed recurrence or mortality predictive nomograms for patients with hepatitis B virus‐related solitary early hepatocellular carcinoma ≤10 cm after curative liver resection. A close postsurgical surveillance protocol and adjuvant therapy should be considered for patients at high risk of recurrence.
Keywords: Hepatocellular carcinoma, Early stage, Prognosis, Nomogram
Short abstract
This article reports on novel prognostic models that integrated tumor pathological features with patients' inflammatory variables, underlying liver diseases, and surgical factors to predict the likelihood of recurrence and overall survival of patients with hepatitis B virus‐related solitary early hepatocellular carcinoma after curative liver resection.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide and is the second leading cause of cancer‐related death in China because of endemic hepatitis B virus (HBV) infection [1]. Liver resection is the first‐line treatment option for solitary HCC without portal venous tumor thrombus (solitary early HCC [SE‐HCC]) in patients with well‐preserved liver function [2]. However, the high postsurgical recurrence rate has compromised long‐term survival. The postsurgical 5‐year recurrence rate based on previously published studies ranged from 57.7% to 70% for SE‐HCC [3, 4, 5]. Conventional tumor staging systems are currently incapable of accurately predicting the likelihood of recurrence of SE‐HCC after surgery [6]. Patients with same tumor stage who underwent surgical resection displayed diverse postoperative outcomes, largely because of the heterogeneity that exists among patients and tumors [7, 8]. Furthermore, there are few distinctive prognostic factors that can be identified from conventional clinicopathological data for SE‐HCC.
Recently, molecular signatures such as mRNA [9], DNA methylation [10], and proteogenomic profiles [11] were shown to be good predictors for recurrence of early HCC. However, there are some major issues of molecular signature–based predictors: (a) These profiles were diverse among different studies. Studies based on identical HCC populations with different etiologies may have different aberrant profiles [12]. (b) Different sequencing platforms and software analytic packages are also assuredly contributing to these different profiles. (c) These molecular signature–based biomarkers demand a high level of technology and are expensive, which impedes their application in the current clinical setting.
The development of postsurgical recurrence of HCC is influenced by multiple factors. Tumor clinicopathologic traits, the patient's inflammatory or immune status, underlying liver disease (i.e., cirrhosis related to HBV or HCV), and operative factors are all potential factors contributing to tumor recurrence. Therefore, an ideal postsurgical recurrence predictive model would be generated based on the risk variables selected from the four aspects described above. A nomogram that integrates diverse prognostic and determinant factors is able to generate the individual probability of tumor recurrence or overall survival (OS) in patients with cancer [13].
Considering that nearly 90% of resected HCCs were less than 10 cm [14, 15, 16] and there was no prognostic model for patients with HBV‐related SE‐HCC ≤10 cm after curative hepatectomy, the generation of new models for predicting postsurgical recurrence and survival for this subgroup of patients is urgently required. In this study, we aimed to generate novel prognostic models that integrated tumor pathological features, patient's inflammatory variables, underlying liver diseases, and surgical factors to predict the likelihood of recurrence and OS of patients with HBV‐related SE‐HCC ≤10 cm after curative liver resection.
Materials, Subjects, and Methods
Study Population
From January 2003 to December 2016, 2,462 consecutive patients with preserved liver function (Child‐Pugh A or B class) who underwent liver resection for HCC as their initial treatment in the Department of Liver Surgery, First Affiliated Hospital of Sun Yat‐sen University, were evaluated for this study. Clinical data were entered prospectively in a resectable HCC database in our department and were reviewed retrospectively. Patients with HBV‐related SE‐HCC ≤10 cm were recruited in this study. HCC with etiologies other than HBV (n = 349), tumor size larger than 10 cm (n = 121), multiple tumors (n = 385), macroscopic portal venous tumor thrombus (n = 483), death within 30 days of surgery (n = 6), and R1 resection (n = 37) were excluded. Finally, 1,081 patients were included and randomly allocated to a derivation cohort (n = 811) and an internal validation cohort (n = 270) with a ratio of 3 to 1 based on the data splitting approach [17]. Patient selection is shown in supplemental online Figure 1.
In addition, 823 patients with HBV‐related SE‐HCC ≤10 cm underwent curative liver resection at another four tertiary hospitals. Among them, 455 were from the Tumor Hospital of Sun Yat‐sen University, Guangzhou (January 2004 to June 2006); 138 from the Hunan Provincial People's Hospital, Changsha (March 2009 to December 2010); 215 from the Xiehe Hospital of Huazhong University of Science and Technology, Wuhan (January 2012 to April 2013); and 105 from the Gansu Provincial People's Hospital, Lanzhou (January 2008 to December 2009). These data were collected retrospectively and served as the external validation cohort (supplemental online Fig. 1).
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee boards of the five hospitals.
Definition
Liver function reserve was evaluated by the albumin‐to‐bilirubin ratio (ALBI grade) [18]. Although the Child‐Pugh score was applied to evaluate liver function in all patients in clinical practice, we used the ALBI score in data analysis in this study because the ALBI score is more accurate and objective than the conventional Child‐Pugh score [18]. Neutrophil‐to‐lymphocyte ratio (NLR) was obtained by neutrophil count divided by lymphocyte count. Platelet‐to‐lymphocyte ratio (PLR) was obtained by platelet count divided by lymphocyte count. The cutoff value of NLR or PLR that defined a high or low level was determined by the Youden index of NLR or PLR calculated by the receiver operating characteristic (ROC) curves in the derivation cohort. Anatomical resection referred to resection of the tumor‐involved segment/section, together with its portal vein branch, resected en bloc [19]. Major resection was defined as a resection extent of more than three segments. Intraoperative blood transfusion referred to transfusion of packed red blood cells during the operation owing to excessive bleeding that resulted in unstable hemodynamic status or hemoglobin <70 g/L.
Follow‐Up
The patients were followed postoperatively. The follow‐up protocol and management of recurrent HCC was described in our previous study [19]. The end of follow‐up was June 30, 2017. The median follow‐up period was 37.0 months (4–147 months) for the cohort patients from our center and 29.3 months (4–107 months) for the external validation cohort.
Statistical Analysis
The clinical database was established with SPSS for Windows (version 22.0, IBM, Armonk, NY). Continuous data are expressed as means ± SD or median (IQR). The Kruskal‐Wallis analysis of variance (ANOVA) test or ANOVA t test was used to compare continuous data between groups and the χ2 test for discrete data. Cumulative rates of survival were calculated by the Kaplan‐Meier method and compared between groups by means of the log‐rank test. A Cox regression model was used to identify risk factors associated with recurrence‐free survival (RFS) and OS by univariate and multivariate analysis.
The predictive nomograms were constructed based on the results of risk variables associated with RFS and OS identified by Cox multivariate analysis in the derivation cohort using R software for Windows (version 3.3.3, http://www.r-project.org). A final model selection was performed by a backward stepdown process with the Akaike information criterion [20]. The predictive performance of the nomograms was measured by concordance index (C‐index) and assessed by calibration curve comparing nomogram‐predicted versus actually observed Kaplan‐Meier estimates of probability of RFS and OS. Bootstraps with 1,000 resamples were used for calculations [21]. The predictive performance of the nomograms was validated in the internal validation and external validation cohort by calculating C‐indices and assessed by calibration curves.
The discriminatory powers of nomograms were also compared with six conventional HCC staging systems: Barcelona Clinic Liver Cancer staging system (BCLC) [22], American Joint Committee on Cancer Staging (TNM) [23], Japan integrated staging (JIS) [24], Cancer of the Liver Italian Program score (CLIP) [25], Chinese University Prognostic Index (CUPI) [26], and Okuda staging system [27] by analyzing the ROC curves. Values of p < .05 were considered statistically significant.
Results
Baseline Characteristics of Patients
This multicenter study recruited 1,904 patients in total with a median age of 52.0 (IQR, 43.0–60.0) years. Among them, 86.1% (1,639/1,094) were male. Microscopic vascular invasion (MVI) occurred in 24.5% (466/1,904) of patients. Over 90% of patients received regular anti‐HBV therapy postoperatively using nucleotide antiviral drugs. The clinicopathological data, including demographic factors, inflammatory factors, tumor factors and surgical factors of patients, in the derivation, internal validation, and external validation cohorts were summarized in Table 1. The variables among these three cohorts had no significant difference (all p > .05).
Table 1.
Baseline characteristics of the three cohorts of patients
| Characteristics | Derivation cohort (n = 811) | Internal validation cohort (n = 270) | External validation cohort (n = 823) | p value |
|---|---|---|---|---|
| Demographic data | ||||
| Age, years | 51.9 ± 11.4 | 50.6 ± 12.7 | 51.3 ± 11.9 | .245 |
| Sex, male | 700 (86.3) | 240 (88.9) | 699 (84.9) | .257 |
| Antiviral therapy | 749 (92.4) | 245 (90.7) | 766 (93.1) | .450 |
| Cirrhosis | 608 (75.0) | 203(75.2) | 644 (78.2) | |
| ALT, median (IQR), U/L | 35.0 (23.0–54.0) | 33.0 (23.0–50.0) | 37.0 (24–48.0) | .132 |
| Child‐Pugh score | ||||
| 5 score | 713 (87.9) | 239 (88.5) | 740 (90.0) | .713 |
| 6 score | 82 (10.1) | 27 (10.0) | 68 (8.2) | |
| 7 score | 16(2.0) | 4 (1.5) | 15 (1.8) | |
| ALBI grade | ||||
| Grade 1 | 410 (50.5) | 145 (53.7) | 416 (50.6) | .105 |
| Grade 2 | 393 (48.5) | 125 (46.3) | 396 (48.1) | |
| Grade 3 | 8(0.1) | 0 (0) | 11 (1.3) | |
| Hemoglobin (g/L) | 138.2 ± 20.9 | 139.4 ± 18.3 | 138.3 ± 19.0 | .644 |
| Platelet (×109/L) | 188.1 ± 74.6 | 190.0 ± 72.0 | 175.8 ± 73.2 | .714 |
| Inflammatory factors | ||||
| NLR, median (IQR) | 1.9 (1.32–2.63) | 1.9 (1.51–2.72) | 2.0 (1.48–2.79) | .216 |
| PLR, median (IQR) | 94.6 (70.6–123.9) | 95.2 (76.9–138.1) | 96.1 (70.9–135.7) | .423 |
| Tumor factors | ||||
| Size, median (IQR), cm | 5.2 (3.8–8.1) | 5.0 (3.6–8.2) | 5.0 (3.7–8.0) | .183 |
| AFP, μg/L | ||||
| ≤20 | 350 (43.2) | 109 (40.4) | 341 (41.4) | .221 |
| >20, ≤400 | 220 (27.1) | 63 (23.3) | 235 (28.6) | |
| >400 | 241 (29.7) | 98 (36.3) | 247 (30.0) | |
| Tumor capsule | ||||
| Complete | 623 (76.8) | 219 (81.1) | 633 (76.9) | .553 |
| Incomplete | 148 (18.2) | 38 (14.1) | 144 (17.5) | |
| Noncapsule | 40(5.0) | 13 (4.8) | 46 (5.6) | |
| MVI | ||||
| Yes | 210 (25.9) | 72 (26.7) | 184 (22.4) | .167 |
| No | 601 (74.1) | 198 (73.3) | 639 (77.6) | |
| Tumor differentiation a | ||||
| Level 1 | 157 (19.4) | 54 (20.0) | 153 (18.6) | .961 |
| Level 2 | 371 (45.7) | 121 (44.8) | 370 (44.9) | |
| Level 3 | 283 (34.9) | 95 (35.2) | 300 (36.5) | |
| Surgical factors | ||||
| Extent of resection | ||||
| Major | 265 (32.7) | 86 (31.8) | 252 (30.6) | .669 |
| Minor | 546 (67.3) | 184 (68.2) | 571 (69.4) | |
| Resection type | ||||
| Nonanatomic | 554 (68.3) | 193 (71.5) | 543 (66.0) | .221 |
| Anatomic | 257 (31.7) | 77 (28.5) | 280 (34.0) | |
| Resection margin | ||||
| ≤1 cm | 251 (30.9) | 89 (33.0) | 275 (33.4) | .549 |
| >1 cm | 560 (69.1) | 181 (67.0) | 548 (66.6) | |
| Blood loss, median (IQR), mL | 200.0 (150–500) | 200.0 (100–500) | 225.0 (150–600) | .570 |
| No. of blood transfusion | 181 (22.3) | 55 (20.4) | 172 (20.9) | .705 |
Values in parentheses are percentages unless indicated otherwise.
Tumor differentiation: level 1, high + high to moderate; level 2,= moderate + moderate to low; level 3, low + undifferentiation.
Abbreviations: AFP, α‐fetoprotein; ALBI grade, albumin‐to‐bilirubin ratio; ALT, alanine transaminase; IQR, interquartile range; MVI, microscopic vascular invasion; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio.
Construction of RFS and OS Predictive Nomograms
To identify the variables that were applied to build RFS and OS predictive nomograms, Cox univariable and multivariable regression analyses were performed in the derivation cohort (n = 811). Variables selected included age, sex, cirrhosis, antiviral therapy, preoperative alanine transaminase level, Child‐Pugh score, ALBI grade, platelet count, NLR, PLR, tumor size, tumor capsule status, preoperative α‐fetoprotein (AFP) level, tumor differentiation, MVI, type of resection, extent of resection, resection margin, intraoperative blood loss, and intraoperative blood transfusion (supplemental online Table 1). Significant risk factors (p < .05) identified by univariate analysis were entered into the Cox multivariate analysis. The results showed that tumor size, MVI, tumor differentiation, preoperative AFP level, NLR, ALBI grade, and intraoperative blood transfusion were independent risk factors associated with both RFS (Fig. 1A) and OS (Fig. 1B).
Figure 1.
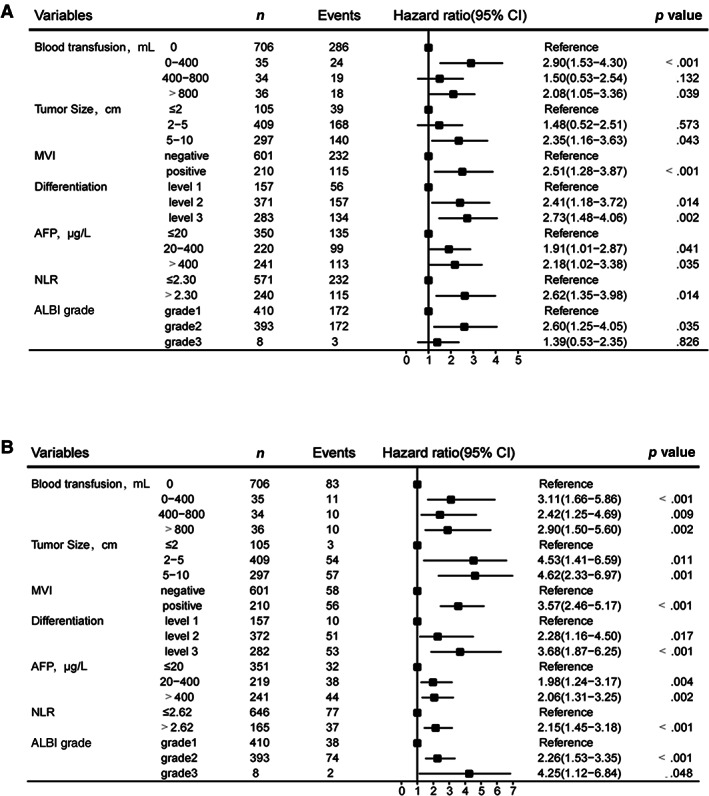
Forest plot to decipher the risk factors associated with recurrence‐free survival and overall survival identified by multivariable Cox regression analysis. (A): Recurrence‐free survival factors. (B): Overall survival factors. Tumor differentiation level 1, high + high to moderate; level 2, moderate + moderate to ‐low; level 3, low + undifferentiation. Abbreviations: AFP, α‐fetoprotein; ALBI grade, albumin‐to‐bilirubin ratio; MVI, microscopic vascular invasion; NLR, neutrophil‐to‐lymphocyte ratio.
We used these seven variables to build a predictive RFS‐nomogram (Fig. 2A) and OS‐nomogram (Fig. 2B). The C‐indices of the RFS‐nomogram and OS‐nomogram were 0.83 (95% confidence interval [CI], 0.79–0.87) and 0.87 (95% CI, 0.83–0.91), respectively. Calibration curves based on the seven variables are shown in Figure 3A and D. There was good agreement between actual and nomogram‐predicted probabilities for 1‐, 2‐, and 5‐year RFS and 1‐, 3‐, and 5‐year OS, respectively, in the derivation cohort.
Figure 2.
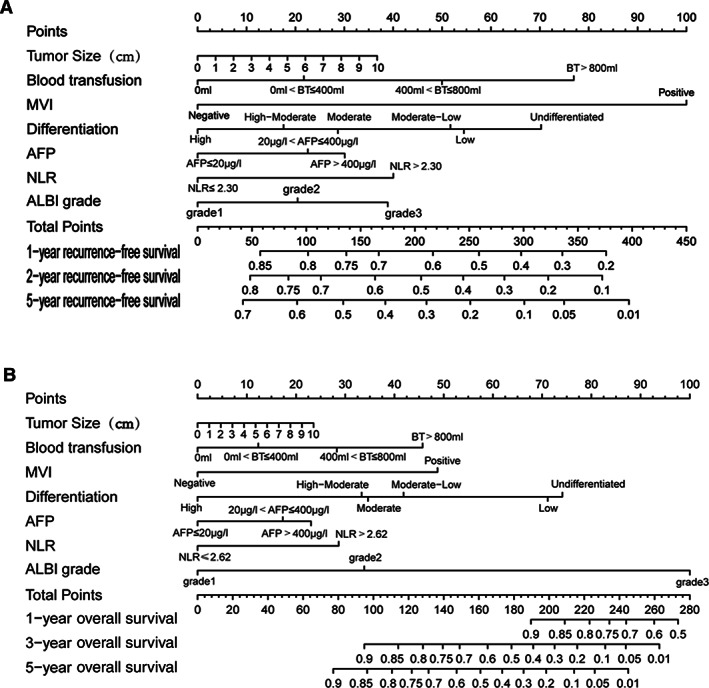
Prognostic nomograms for prediction of postoperative recurrence‐free survival (RFS) and overall survival (OS) for hepatitis B virus‐related solitary early hepatocellular carcinoma ≤10 cm after curative liver resection. (A): RFS predictive nomogram. (B): OS predictive nomogram. To use the nomogram, the value of an individual patient is located on each variable axis, and a line is drawn upward to determine the number of points received for each variable value. The sum of these numbers that is the total score of the patient is located on the Total Points axis, and a line is drawn downward to the survival axes to determine the probabilities of survival rate. Abbreviations: AFP, α‐fetoprotein; ALBI grade, albumin‐to‐bilirubin ratio; BT, blood transfusion; MVI, microscopic vascular invasion; NLR, neutrophil‐to‐lymphocyte ratio.
Figure 3.
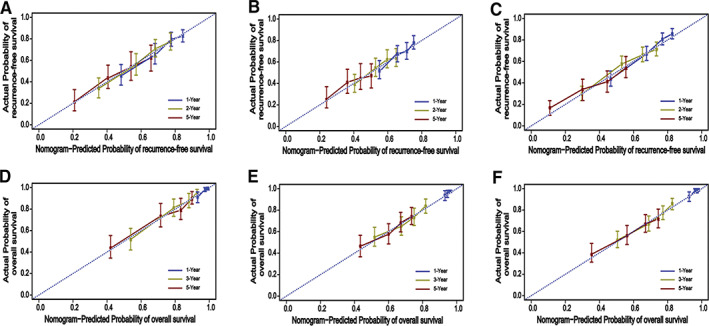
The calibration curves of postoperative recurrence‐free survival (RFS) and overall survival (OS) based on nomogram prediction and actual observation in the derivation, internal validation, and external validation cohort. (A): The 1‐, 2‐, and 5‐year RFS rates in the derivation cohort. (B): The 1‐, 2‐, and 5‐year RFS rates in the internal validation cohort. (C): The 1‐, 2‐, and 5‐year RFS rates in the external validation cohort. (D): The 1‐, 3‐, and 5‐year OS rates in derivation cohort. (E): The 1‐, 3‐, and 5‐year OS rates in the internal validation cohort. (F): The 1‐, 3‐, and 5‐year OS rates in the external validation cohort.
Internal and External Validation of Predictive Accuracy
To validate the accuracy of the predictive performance of the RFS‐nomogram and OS‐nomogram, the probabilities of outcomes were predicted for the internal validation cohort and the external validation cohort. In validation of the RFS‐nomogram, the C‐indices for the internal validation cohort and the external cohort were 0.80 (95% CI, 0.75–0.83) and 0.85 (95% CI, 0.82–0.87), respectively. Calibration plots showed good agreement of actual and nomogram‐predicted probabilities for 1‐, 2‐ and 5‐year RFS in the internal (Fig. 3B) and external cohort (Fig. 3C), respectively. In validation of the OS‐nomogram, the C‐indices for the internal validation cohort and external cohort were 0.85 (95% CI, 0.81–0.89) and 0.89 (95% CI, 0.85–0.92), respectively. Calibration plots also showed good agreement of actual and nomogram‐predicted probabilities for 1‐, 3‐ and 5‐year OS in the internal validation cohort (Fig. 3E) and external cohort (Fig. 3F), respectively.
Comparison of Predictive Powers of Nomograms with Conventional HCC Staging Systems and Other Nomograms Reported in Literature
We compared the predictive powers of the RFS‐nomogram and OS‐nomogram with six conventional HCC staging systems—BCLC, TNM, JIS, CLIP, CUPI, and Okuda—by ROC curve analysis. Our nomograms displayed better discriminatory powers in predicting postoperative RFS and OS in the derivation cohort than those competing models. For the RFS‐nomogram, the C‐index was 0.83 (95% CI, 0.79–0.87), substantially higher than those of BCLC, TNM, JIS, CLIP, CUPI, and Okuda (Fig. 4A; supplemental online Table 2). For the OS‐nomogram, the C‐index was 0.87 (95% CI, 0.83–0.91), significantly higher than those of BCLC, TNM, JIS, CLIP, CUPI, and Okuda (Fig. 4B; supplemental online Table 2). Furthermore, our proposed nomograms had the highest C‐index for RFS and OS compared with those of prognostic nomograms for resectable HCC reported recently in the literature (Table 2).
Figure 4.
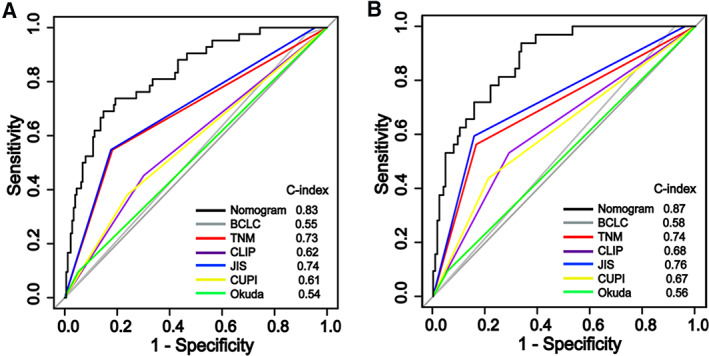
Comparison of predictive accuracy between nomograms and the six conventional hepatocellular carcinoma staging systems. (A): The RFS‐nomogram. (B): The OS‐nomogram. Abbreviations: BCLC, Barcelona Clinic Liver Cancer staging system; CLIP, Cancer of the Liver Italian Program score; CUPI, Chinese University Prognostic Index; JIS, Japanese Integrated Staging; TNM, American Joint Committee on Cancer staging.
Table 2.
Typical postsurgical outcome‐predictive nomograms for HCC reported in recent literature
| Author, year | Region | Patients, n a | Patient criteria | Variables recruited in nomogram | C‐index | External validation | |||
|---|---|---|---|---|---|---|---|---|---|
| Tumor factor | Liver/patient factor | Inflammatory factor | Surgical factor | ||||||
|
Cho [28] 2008 |
U.S. | 184 | BCLC 0‐C | Size, satellites, AFP, vascular invasion | Age | No |
Margin, estimated blood loss |
0.67 (RFS) 0.74 (OS) |
No |
|
Shim [29] 2015 |
Korea | 760 | BCLC 0‐B |
Tumor volume, MVI |
Age, platelet, albumin |
No | No |
0.69 (RFS) 0.66 (OS) |
No |
|
Li [30] 2015 |
China | 310 |
≥10 cm, single or multiple, or with PVTT |
Size, number, differentiation, vascular invasion, capsule | HBV‐DNA level | No | No | 0.78 (OS) | Yes, single center |
|
Yang [31] 2016 |
China | 540 | Multiple HCC | Size, number, MVI, capsule, local invasion, AFP | HBV‐DNA load, MELD score | No | Anatomic resection | 0.80 (OS) | Yes, single center |
|
Li [32] 2016 |
China |
1,328 |
Within Milan criteria |
Tumor number, size, capsule, AFP, MVI |
HBeAg, HBV‐DNA |
No | Surgical margin |
0.76 (RFS) 0.79 (OS) |
Yes, single center |
| Shen [33] 2016 | China | 618 | Single or multiple tumor, PVTT | Tumor number, size, PVTT, AFP, MVI | No | NLR | No |
0.75 (RFS) 0.75 (OS) |
No |
| Torzilli [34] 2016 | Eastern & Western Network | 2,046 | BCLC 0‐C |
Number, size, macrovascular invasion |
Cirrhosis, esophageal varices, total bilirubin | No | No |
0.61 (RFS) 0.62 (OS) |
No |
|
Fu [35] 2017 |
China | 734 | BCLC 0‐B |
Size, number, MVI, AFP |
GGT | No | No |
0.65 (RFS) 0.7 (OS) |
No |
|
Ma [36] 2019 |
Hong Kong | 291 | Within Milan criteria |
Number, MVI, AFP |
Prothrombin time |
No | Magnitude of hepatectomy |
0.67 (RFS) 0.67 (OS) |
No |
| Kim [37] 2019 | Korea | 420 |
HBV‐related, BCLC 0‐C |
Number, PVTT, PIVK‐II, satellites, hemorrhage |
Albumin, ALP |
No | Resection margin |
0.71 (RFS) 0.82 (OS) |
No |
| The present study | China | 811 | HBV‐related, solitary, ≤10 cm, no PVTT |
Size, MVI, AFP, differentiation |
ALBI grade | NLR | Blood transfusion |
0.83 (RFS) 0.87 (0S) |
Yes, four centers |
Number of patients in the derivation cohort.
Abbreviations: AFP, α‐fetoprotein; ALBI grade, albumin‐to‐bilirubin ratio; ALP, alkaline phosphatase; BCLC, Barcelona Clinic Liver Cancer staging system; GGT, γ‐glutamyl transpeptidase; HBeAg, hepatitis B‐virus E antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; MELD, model for end‐stage liver disease; MVI, microscopic vascular invasion; NLR, neutrophil‐to‐lymphocyte ratio; OS, overall survival; PIVK‐II, protein induced by Vitamin K absence‐II; PVTT, portal venous tumor thrombus; RFS, recurrence‐free survival.
The Nomogram Score Could Clearly Classify the Patients into Subgroups with Different Risk of Recurrence or Postoperative Mortality
Based on the RFS‐nomogram's score, patients could be divided into low risk (score ≤ 100), intermediate risk (100.1–200), and high risk (>200) of recurrence. The 2‐year RFS rates of the three subgroups from the derivation cohort could be markedly discriminated (Fig. 5A). Similar results were obtained from the internal validation cohort (Fig. 5B) and the external validation cohort (Fig. 5C).
Figure 5.
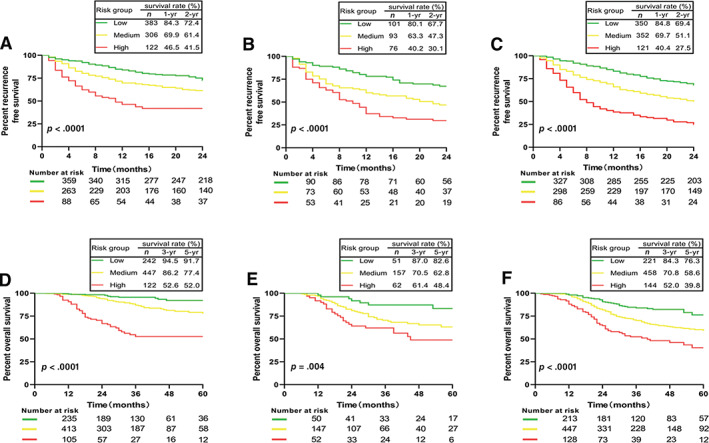
Survival curves for subgroup of patients with different risk of postsurgical recurrence or mortality stratified by nomogram score. (A): Recurrence‐free survival (RFS) curves in derivation cohort. (B): RFS curves in the internal validation cohort. (C): RFS curves in the external validation cohort. (D): Overall survival (OS) curves in the derivation cohort. (E): OS curves in the internal validation cohort. (F): OS curves in the external validation cohort.
As to the OS‐nomogram, patients could also be classified into low risk (score ≤ 75), intermediate risk (75.1–150), and high risk (>150) of postsurgical mortality. The 5‐year OS rates of these three subgroups were 91.7%, 77.4%, and 52.0%, respectively, in the derivation cohort (p < .001; Fig. 5D). Similar results were observed in the internal validation cohort (Fig. 5E) and the external validation cohort (Fig. 5F).
Discussion
To date, the postsurgical prognostic model for HBV‐related SE‐HCC ≤10 cm is not established. In the present study, we constructed a RFS‐nomogram and OS‐nomogram to predict postoperative recurrence and OS for these patients based on seven conventional clinicopathological and surgical variables that are easily obtained, allowing for the nomograms to be conveniently used in the real clinical world. The nomograms showed excellent performance to predict postoperative RFS and OS for an individual who had undergone curative liver resection for HBV‐related early HCC. Compared with the six conventional HCC staging systems, the two nomograms displayed better discriminatory power in prediction of outcomes.
The postsurgical recurrence of HCC is multifactorial. The full coverage of survival‐related risk factors may potentially increase the predictive accuracy of nomogram. Our nomograms were generated by seven significant risk variables that derived from tumor traits (tumor size, MVI, differentiation, AFP), underlying liver function (ALBI grade), patient's inflammatory factor (NLR), and surgical factors (intraoperative blood transfusion), thereby yielding higher C‐indices compared with those previously published nomograms (Table 2).
Of the tumor clinicopathologic traits, tumor size, MVI, differentiation, and AFP were identified as independent risk factors associated with RFS and OS by multivariable Cox regression analysis (Fig. 1). These factors are well‐known potential risk factors related to postsurgical recurrence of HCC and affect long‐term survival of the patient [5, 38, 39, 40, 41]. Tumor size is a critical survival predictor for HCC [5, 38]. Current staging systems do not depict this stepwise increment with respect to tumor size. Notably, we did not categorize the tumor size by a cutoff value (i.e., 5 cm) but used the continuous increment of a 1‐cm interval in our nomograms. This could make the nomogram's score for each patient more accurate. Another pivotal prognostic factor for HCC is MVI [39, 41]. In the derivation cohort of the present study, the recurrent risk of patient with MVI was 2.5‐fold higher than that without MVI (Fig. 1A). As shown in the RFS‐nomogram, MVI was the prominent factor contributing to recurrence (Fig. 2A). The occurrence of MVI ranged from 15% to 57.1% [41]. It was 24.5% in the present cohort of 1,904 patients (Table 1). There is a positive relationship between tumor size and the likelihood of MVI [5, 41].
The patient's inflammatory or immune status is one of the critical factors contributing to tumor recurrence [42]. Peripheral blood NLR is a simple index reflecting the systemic inflammation status of the tumor host [43, 44]. Numerous pieces of evidence show that high level of the NLR is a risk factor of HCC recurrence after curative resection [45, 46]. Therefore, inclusion of NLR might improve the predictive performance of a nomogram for HCC.
The two nomograms contained one liver function variable: ALBI grade. The ALBI grade was equally applicable in patients with HCC with underlying HBV‐related or HCV‐related cirrhosis or without cirrhosis and gave clear discrimination of survival in each grade [47]. In the Cox univariable analysis, the Child‐Pugh score was a risk factor that affected OS (supplemental online Table 1); however, in multivariable analysis, it was ALBI grade but not Child‐Pugh score that was the risk factor associated with RFS and OS (Fig. 1). Therefore, the ALBI grade was more reliable than the Child‐Pugh score in the outcome prediction for HCC patients.
Intraoperative blood transfusion was the only surgical variable entered in the models. Surgical factors are lacking in the currently available HCC staging systems. Many studies showed that intraoperative blood transfusion was a risk factor that negatively influenced long‐term survival of patients with HCC after curative liver resection [48, 49]. In the Cox multivariable analysis, we demonstrated that intraoperative blood transfusion was a significant risk factor associated with both RFS and OS in patients with SE‐HCC ≤10 cm (Fig. 1). Liver resection for HCC carries a high risk of intraoperative bleeding because of underlying cirrhosis. In the U.S., the nationwide blood transfusion rate in HCC resections performed from 2005 to 2007 was 28.7% [50]. The blood transfusion rates were 22.3%, 20.4%, and 20.9% in the derivation cohort, internal validation cohort, and external cohort, respectively (Table 1). Reducing intraoperative blood transfusion by minimizing intraoperative blood loss may improve outcomes of patients with HCC.
The two nomograms were validated by an internal validation cohort of separate patients from our center and the external validation cohort of patients from another four tertiary hospitals in different geographic areas in mainland China. The C‐indices were 0.80 and 0.85 of the two validation cohorts for predicting RFS and were 0.85 and 0.89 for predicting OS. The calibration plots showed good agreement of actual and nomogram‐predicted probabilities for RFS and OS in the internal validation cohort and external validation cohort, respectively (Fig. 3). To date, most of the reported nomograms for HCC lack external validation, especially multicenter validation (Table 2). Our multicenter external validation data showed that the proposed nomograms display good predictive performance for patients from different areas of China. Thus, they are suitable for national application in actual clinical practice.
The proposed nomograms could clearly divide patients into three subgroups with different risk of recurrence or mortality (Fig. 5). Thus, it may help surgeons to adopt close surveillance protocol and design postoperative therapeutic clinical trials for those with high risk of recurrence.
There are some limitations of this study. First, only patients with HBV‐related HCC were recruited in this study. Whether the nomograms can be used for those with non–HBV‐related HCC needs further validation. Second, the nomograms were generated to predict postoperative RFS and OS based on the data of patients with SE‐HCC ≤10 cm undergoing curative liver resection; they may not be suitable for those with intermediate or advanced stage HCC after hepatectomy or those with early stage HCC receiving nonsurgical treatment. Third, this is a retrospective study. Patient selection bias was unavoidable.
Conclusion
We generated two conveniently available nomograms that could accurately and objectively predict postoperative recurrence and OS for patients with HBV‐related SE‐HCC ≤10 cm after curative liver resection. We could discriminate patients from different risk of recurrence by the nomogram's score. A close surveillance protocol and postsurgical adjuvant therapy is considered for those patients with high risk of recurrence.
Author Contributions
Conception/design: Xiao‐Hui Wang, Shao‐Qiang Li
Provision of study material or patients: Xiao‐Hui Wang, Cai‐Xue Tu, Cai‐Ling Xiang, Sheng‐Hua Hao, Xian‐Hai Mao, Xiao Yue, Xiao‐Ming Qiu, Xiao‐Jun Yang
Collection and/or assembly of data: Xiao‐Hui Wang, Bing Liao, Cai‐Xue Tu, Cai‐Ling Xiang, Sheng‐Hua Hao, Xian‐Hai Mao, Xiao Yue, Xiao‐Ming Qiu, Xiao‐Jun Yang
Data analysis and interpretation: Xiao‐Hui Wang, Bing Liao, Wen‐Jie Hu, Bao‐Gang Peng, Shao‐Qiang Li
Manuscript writing: Xiao‐Hui Wang, Ming Kuang, Shao‐Qiang Li
Final approval of manuscript: Xiao‐Hui Wang, Bing Liao, Wen‐Jie Hu, Cai‐Xue Tu, Cai‐Ling Xiang, Sheng‐Hua Hao, Xian‐Hai Mao, Xiao‐Ming Qiu, Xiao‐Jun Yang, Xiao Yue, Ming Kuang, Bao‐Gang Peng, Shao‐Qiang Li
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1
Supplementary Tables
Acknowledgments
We thank Professor Fu‐Tian Luo from the Statistics Department of Sun Yat‐sen University for his help with statistical analyses. This work is supported by a grant from the National Natural Science Foundation of China (grant 81472254) and from the Science and Technology Planning Project of Guangdong Province, People's Republic of China (grant 2016A020215064).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver; European Organization for Research and Treatment of Cancer . EASL‐EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 2012;56:908–943. [DOI] [PubMed] [Google Scholar]
- 3. Pompili M, Saviano A, de Matthaeis N et al. Long‐term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol 2013;59:89–97. [DOI] [PubMed] [Google Scholar]
- 4. Roayaie S, Obeidat K, Sposito C et al. Resection of hepatocellular cancer ≤2 cm: Results from two Western centers. Hepatology 2013;57:1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hwang S, Lee YJ, Kim KH et al. The impact of tumor size on long‐term survival outcomes after resection of solitary hepatocellular carcinoma: single‐institution experience with 2558 patients. J Gastrointest Surg 2015;19:1281–1290. [DOI] [PubMed] [Google Scholar]
- 6. Maida M, Orlando E, Cammà C et al. Staging systems of hepatocellular carcinoma: A review of literature. World J Gastroenterol 2014;20:4141–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin DC, Mayakonda A, Dinh HQ et al. Genomic and epigenomic heterogeneity of hepatocellular carcinoma. Cancer Res 2017;77:2255–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hlady RA, Robertson KD. Genetic and epigenetic heterogeneity in normal liver homeostasis and its implications for liver disease and hepatocellular cancer. Semin Liver Dis 2018;38:41–50. [DOI] [PubMed] [Google Scholar]
- 9. Cai J, Tong Y, Huang L et al. Identification and validation of a potent multi‐mRNA signature for the prediction of early relapse in hepatocellular carcinoma. Carcinogenesis 2019;40:840–852. [DOI] [PubMed] [Google Scholar]
- 10. Qiu J, Peng B, Tang Y et al. CpG methylation signature predicts recurrence in early‐stage hepatocellular carcinoma: Results from a multicenter study. J Clin Oncol 2017;35:734–742. [DOI] [PubMed] [Google Scholar]
- 11. Gao Q, Zhu H, Dong L et al. Integrated proteogenomic characterization of HBV‐related hepatocellular carcinoma. Cell 2019;179:561–577. [DOI] [PubMed] [Google Scholar]
- 12. Shen J, Wang S, Zhang YJ et al. Genomewide DNA methylation profiles in hepatocellular carcinoma. Hepatology 2012; 55: 1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balachandran VP, Gonen M, Smith JJ et al. Nomograms in oncology: More than meets the eye. Lancet Oncol 2015;16:e173–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim C, Mise Y, Sakamoto Y et al. Above 5 cm, size does not matter anymore in patients with hepatocellular carcinoma. World J Surg 2014;38:2910–2918. [DOI] [PubMed] [Google Scholar]
- 15. Chang YJ, Chung KP, Chang YJ et al. Long‐term survival of patients undergoing liver resection for very large hepatocellular carcinomas. Br J Surg 2016;103:1513–1520. [DOI] [PubMed] [Google Scholar]
- 16. Wakayama K, Kamiyama T, Yokoo H et al. Huge hepatocellular carcinoma greater than 10 cm in diameter worsens prognosis by causing distant recurrence after curative resection. J Surg Oncol 2017;115:324–329. [DOI] [PubMed] [Google Scholar]
- 17. Steyerberg EW, Harrell FE Jr. Prediction models need appropriate internal, internal‐external, and external validation. J Clin Epidemiol 2016;69:245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson PJ, Berhane S, Kagebayashi C et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence‐based approach—the ALBI grade. J Clin Oncol 2015;33:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li SQ, Huang T, Shen SL et al. Anatomical versus non‐anatomical liver resection for hepatocellular carcinoma exceeding Milan criteria. Br J Surg 2017;104:118–127. [DOI] [PubMed] [Google Scholar]
- 20. Harrell JFE, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 21. Steyerberg EW, Harrell FE Jr, Borsboom GJ et al. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J Clin Epidemol 2001;54:774–781. [DOI] [PubMed] [Google Scholar]
- 22. Bruix J, Reig M, Sherman M. Evidence base diagnosis, staging and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016;150:835–853. [DOI] [PubMed] [Google Scholar]
- 23. Edge SB, Byrd DR, Compton CC et al. AJCC Cancer Staging Manual. 7th ed New York: Springer, 2010. [Google Scholar]
- 24. Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): Its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol 2003;38: 207–215. [DOI] [PubMed] [Google Scholar]
- 25. A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients: The Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28:751–755. [DOI] [PubMed] [Google Scholar]
- 26. Leung TW, Tang AM, Zee B et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: A study based on 926 patients. Cancer 2002;94:1760–1769. [DOI] [PubMed] [Google Scholar]
- 27. Okuda K, Obata H, Nakajima Y et al. Prognosis of primary hepatocellular carcinoma. Hepatology 1984;4:3S–6S. [DOI] [PubMed] [Google Scholar]
- 28. Cho CS, Mithat Gonen M, Shia J et al. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J Am Coll Surg 2008;206:281–291. [DOI] [PubMed] [Google Scholar]
- 29. Shim JH, Jun MJ, Han S et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg 2015;261:939–946. [DOI] [PubMed] [Google Scholar]
- 30. Li Y, Xia Y, Li J et al. Prognostic nomograms for pre‐ and postoperative predictions of long‐term survival for patients who underwent liver resection for huge hepatocellular carcinoma. J Am Coll Surg 2015;221:962–974. [DOI] [PubMed] [Google Scholar]
- 31. Yang P, Qiu J, Li J et al. Nomograms for pre‐ and postoperative prediction of long‐term survival for patients who underwent hepatectomy for multiple hepatocellular carcinomas. Ann Surg 2016;263:778–786. [DOI] [PubMed] [Google Scholar]
- 32. Li J, Zhou J, Yang PH et al. Nomograms for survival prediction in patients undergoing liver resection for hepatitis B virus related early stage hepatocellular carcinoma. Eur J Cancer 2016;62:86–95. [DOI] [PubMed] [Google Scholar]
- 33. Shen J, He L, Li C et al. Prognostic nomograms for patients with resectable hepatocellular carcinoma incorporating systemic inflammation and tumor characteristics. Oncotarget 2016;7:80783–80793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Torzilli G, Donadon M, Belghiti J et al. Predicting individual survival after hepatectomy for hepatocellular carcinoma: A novel nomogram from the “HCC East & West Study Group.” J Gastrointest Surg 2016;20:1154–1162. [DOI] [PubMed] [Google Scholar]
- 35. Fu YP, Yi Y, Huang JL et al. Prognostic nomograms stratify survival of patients with hepatocellular carcinoma without portal vein tumor thrombosis after curative resection. The Oncologist 2017;22:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ma KW, She WH, Tan T et al. Validated nomogram for the prediction of disease‐free survival after hepatectomy for hepatocellular carcinoma within the Milan criteria: Individualizing a surveillance strategy. Surg Today 2019;49:521–528. [DOI] [PubMed] [Google Scholar]
- 37. Kim JM, Kwon CHD, Joh JW et al. Nomograms in hepatectomy patients with hepatitis B virus‐related hepatocellular carcinoma. J Gastrointest Surg 2019;23:1559–1567. [DOI] [PubMed] [Google Scholar]
- 38. Goh BK, Teo JY, Chan CY et al. Importance of tumor size as a prognostic factor after partial liver resection for solitary hepatocellular carcinoma: Implications on the current AJCC staging system. J Surg Oncol 2016;113:89–93. [DOI] [PubMed] [Google Scholar]
- 39. Sumie S, Nakashima O, Okuda K et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol 2014;21:1002–1009. [DOI] [PubMed] [Google Scholar]
- 40. Yang SL, Liu LP, Yang S et al. Preoperative serum α‐fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg 2016;103:716–724. [DOI] [PubMed] [Google Scholar]
- 41. Pawlik TM, Delman KA, Vauthey JN et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 2005;11:1086–1092. [DOI] [PubMed] [Google Scholar]
- 42. Ji J, Eggert T, Budhu A et al. Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. Hepatology 2015;62:481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang J, Jia Y, Wang N et al. The clinical significance of tumor‐infiltrating neutrophils and neutrophil‐to‐CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J Transl Med 2014;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hiramatsu S, Tanaka H, Nishimura J et al. Neutrophils in primary gastric tumors are correlated with neutrophil infiltration in tumor‐draining lymph nodes and the systemic inflammatory response. BMC Immunol 2018;19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Okamura Y, Sugiura T, Ito T et al. Neutrophil to lymphocyte ratio as an indicator of the malignant behaviour of hepatocellular carcinoma. Br J Surg 2016;103:891–898. [DOI] [PubMed] [Google Scholar]
- 46. Yang T, Zhu J, Zhao L et al. Lymphocye to monocyte ratio and neutrophil to lymphocyte ratio are superior inflammation‐based predictors of recurrence in patients with hepatocellular carcinoma after hepatic resection. J Surg Oncol 2016;115:718–728. [DOI] [PubMed] [Google Scholar]
- 47. Toyoda H, Lai PB, O'Beirne J et al. Long‐term impact of liver function on curative therapy for hepatocellular carcinoma: Application of the ALBI grade. Br J Cancer 2016;114:744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu L, Wang Z, Jiang S et al. Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: A meta‐analysis. PLoS One 2013;8:e64261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wada H, Eguchi H, Nagano H et al. Perioperative allogenic blood transfusion is a poor prognostic factor after hepatocellular carcinoma surgery: A multi‐center analysis. Surg Today 2018;48:73–79. [DOI] [PubMed] [Google Scholar]
- 50. Aloia TA, Fahy BN, Fischer CP et al. Predicting poor outcome following hepatectomy: Analysis of 2313 hepatectomies in the NSQIP database. HPB (Oxford) 2009;11:510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1
Supplementary Tables


